Public Release: 14-Aug-2017
American Geophysical Union
WASHINGTON D.C. — The Montreal Protocol, the international treaty adopted to restore Earth’s protective ozone layer in 1989, has significantly reduced emissions of ozone-depleting chemicals from the United States. In a twist, a new study shows the 30-year old treaty has had a major side benefit of reducing climate-altering greenhouse gas emissions from the U.S.
That’s because the ozone-depleting substances controlled by the treaty are also potent greenhouse gases, with heat-trapping abilities up to 10,000 times greater than carbon dioxide over 100 years.
The new study is the first to quantify the impact of the Montreal Protocol on U.S. greenhouse gas emissions with atmospheric observations. The study’s results show that reducing the use of ozone-depleting substances from 2008 to 2014 eliminated the equivalent of 170 million tons of carbon dioxide emissions each year. That’s roughly the equivalent of 50 percent of the reductions achieved by the U.S. for carbon dioxide and other greenhouse gases over the same period. The study was published today in Geophysical Research Letters, a journal of the American Geophysical Union.
“We were surprised by the size of the decline, especially compared with other greenhouse gases,” said Lei Hu, a researcher with the Cooperative Institute for Research in Environmental Sciences (CIRES) working at NOAA and lead author of the new study.
Hu added that the benefits of the Montreal Protocol on greenhouse gas emissions would likely grow in the future. By 2025, she projects that the effect of the Montreal Protocol will be to reduce U.S. greenhouse gas emissions by the equivalent of 500 million tons of carbon dioxide per year compared with 2005 levels. This reduction would be equivalent to about 10 percent of the current U.S. emissions of carbon dioxide.
Previous studies have demonstrated that the Montreal Protocol has been more effective at curtailing global greenhouse gas emissions than any other international effort – even though climate change was not a consideration during the initial treaty negotiations in the late 1980s.
The new analysis, based on data collected by NOAA’s atmospheric monitoring network, confirms that the Montreal Protocol has been highly successful in the U.S. in its primary goal – reducing emissions of manufactured chlorine-based chemicals that, in addition to depleting ozone world-wide, create a hole the size of the continental U.S. in the Earth’s protective ozone layer over the Antarctic each September and October.
Those chemicals–chlorofluorocarbons (CFCs) and hydrochlorofluorocarbons (HCFCs), and their substitutes, the hydrofluorocarbons (HFCs)–have been widely used as refrigerants, foam blowing agents, aerosol propellants, fire retardants, and solvents. Chlorine from CFCs was first identified as capable of destroying stratospheric ozone in 1974. The Montreal Protocol has controlled the production and consumption of these chemicals since the late 1980s.
Implementation of the Montreal Protocol in the United States, largely through the Clean Air Act, led to a near complete phase-out of U.S. production and consumption of chlorofluorocarbons (CFCs) beginning in 1996 and a 95 percent decline of hydrochlorofluorocarbons (HCFCs) production since 1998.
As a result, total emissions of CFCs in the U.S. have decreased by two-thirds from 2008 to 2014, while emissions of HCFCs declined by about half, the study authors said.
Another indication of the treaty’s impact is increasing U.S. emissions of ozone-friendly chemicals, such as hydrofluorocarbons or HFCs. However, some HFCs are also potent greenhouse gases, and their increased use is offsetting some of the climate benefit of the Montreal Protocol, said Stephen Montzka, a researcher at NOAA and co-author of the new study.
Countries adhering to the Protocol, including the U.S., agreed to limit future production and consumption of HFCs in 2016.
“This shows what can be achieved by concerted and thoughtful international effort,” said Scott Lehman of the Institute of Arctic and Alpine Research at the University of Colorado Boulder and co-author of the new study. “Hopefully, the Protocol can serve as a model of the international cooperation that we need to tackle the real problem – carbon dioxide.”
###
The American Geophysical Union is dedicated to advancing the Earth and space sciences for the benefit of humanity through its scholarly publications, conferences, and outreach programs. AGU is a not-for-profit, professional, scientific organization representing 60,000 members in 137 countries. Join the conversation on Facebook, Twitter, YouTube, and our other social media channels.
Notes for Journalists
This research article is open access for 30 days. A PDF copy of the article can be downloaded at the following link: http://onlinelibrary.wiley.com/doi/10.1002/2017GL074388/pdf.
Journalists and PIOs may also order a copy of the final paper by emailing a request to Lauren Lipuma at llipuma@agu.org. Please provide your name, the name of your publication, and your phone number.
Neither the paper nor this press release is under embargo.
Title: “Considerable Contribution of the Montreal Protocol to Declining Greenhouse Gas Emissions from the United States”
Authors: Lei Hu, Colm Sweeney, Debra J. Mondeel, David Nance: Cooperative Institute for Research in Environmental Sciences, University of Colorado-Boulder and NOAA Earth System Research Laboratory, Global Monitoring Division, Boulder, Colorado, U.S.A.;
Stephen A. Montzka, Arlyn E. Andrews, Kirk Thoning, John B. Miller, James W. Elkins, Bradley D. Hall, Pieter P. Tans: NOAA Earth System Research Laboratory, Global Monitoring Division, Boulder, Colorado, U.S.A.;
Scott J. Lehman: Institute of Arctic and Alpine Research, University of Colorado-Boulder, Boulder, Colorado, U.S.A.;
David S. Godwin: Stratospheric Protection Division, Office of Atmospheric Programs, Office of Air and Radiation, U.S. Environmental Protection Agency, Washington, District of Columbia, U.S.A.;
Benjamin R. Miller: Cooperative Institute for Research in Environmental Sciences, University of Colorado-Boulder, NOAA Earth System Research Laboratory, Global Monitoring Division and Institute of Arctic and Alpine Research, University of Colorado-Boulder, Boulder, Colorado, U.S.A.;
Caroline Siso: Cooperative Institute for Research in Environmental Sciences, University of Colorado-Boulder, NOAA Earth System Research Laboratory, Global Monitoring Division, Boulder, Colorado, U.S.A. and Stratospheric Protection Division, Office of Atmospheric Programs, Office of Air and Radiation, U.S. Environmental Protection Agency, Washington, District of Columbia, U.S.A.;
Thomas Nehrkorn, Marikate Mountain: Atmospheric and Environmental Research, Lexington, Massaschusetts, U.S.A.;
Marc L. Fischer: Environmental Technologies Area, Lawrence Berkeley National Laboratory, Berkeley, California, U.S.A.;
Sébastien C. Biraud: Earth and Environmental Sciences Area, Lawrence Berkeley National Laboratory, Berkeley, California, U.S.A.;
Huilin Chen: Cooperative Institute for Research in Environmental Sciences, University of Colorado-Boulder, Boulder, Colorado, U.S.A. and Centre for Isotope Research, University of Groningen, Groningen, The Netherlands.
Contact information for the authors: Lei Hu: +1 (303) 497-5238, lei.hu@noaa.gov
AGU Press Contact:
Lauren Lipuma
+1 (202) 777-7396
NOAA Press Contact:
Theo Stein
+1 (303) 497-6288
Disclaimer: AAAS and EurekAlert! are not responsible for the accuracy of news releases posted to EurekAlert! by contributing institutions or for the use of any information through the EurekAlert system.
And yet, in 2015 “The Antarctic ozone hole widened to one of its largest sizes on record earlier this month, the U.N.’s World Meteorological Organization (WMO) announced Thursday.”
https://www.usatoday.com/story/weather/2015/10/29/antarctic-ozone-hole-largest-ever/74812810/
I guess “normal” is not one of their words
Yikes 20 authors, that’s a BS factor of 400 ( N^2 ) .
If the data doesn’t fit, the hypothesis you must acquit.
The “science” on which the Montreal Protocol was based was fabricated by a “scientist” paid by Dupont Chemical. Dupont then ran a huge campaign and lobbied in Washington to have the CFC banned. The goal was to ban their own refrigerant that was out of patent and becoming cheap; they happened to have another refrigerant all patented, more expensive, and ready to go. 20 years later, with the replacement refrigerant out of patent, the “scientist” admitted to the fraud.
We know now that it is nitrogen gas and UV from solar input that breaks down ozone at very cold temperatures. CFC’s are innocent. However, the myth persists and now there is a movement to ban HFCs just because they are similar to CFCs, but not because they do any bad chemistry.
The idea of greenhouse gases is an exercise in misdirection. These gases are more accurately called “radiative gases”. During the day, CO2 and water vapor are saturated, converting IR to heat and heat to IR, effectively have no effect. It is during the night that these gases, with no solar input, convert heat to IR and chill the atmosphere. That is why it cools down so quickly after sunset and why little breezes kick up so quickly in the shadows of clouds on a partly cloudy day.
For that matter, the “science” of the “global warming by CO2 emitted by man” says that the upper tropical troposphere is warming faster than anywhere else and warming Earths’ surface. They now try to ignore the fact that 35 years of searching for this reputed “hotspot” has failed to locate even a hint of what they say. In fact, NASA has reported that this region of the atmosphere has been cooling slowly for 35 years. So, the alarmists like to talk generally and try to hide the failure of their entire “scientific” model.
Furthermore, even though NO, methane, and CFCs are more powerful radiative gases than CO2 and water, their concentrations in the atmosphere are minuscule, even minuscule compared to CO2 and water vapor. For example, methane is 20-times the radiative gas compared to CO2, but it is 1/400th of the concentration of CO2. Yawn, a nothing burger.
Bottom line, the greenhouse gases they want all to panic over actually cool the planet and have no detectable warming effects, but clearly detectable cooling effects.
The question is about how long chlorine resulting from the breakdown of CFCs lasts in the upper atmosphere.
The chlorine is supposed to last a long time in the upper atmosphere, which is why they say we still have a rather large ozone hole.
My question for all the chemists out there is this: Given that chlorine gas has a specific gravity about two and a half times that of air, and given that it has a low concentration in the lower atmosphere so we can’t blame vapor pressure for keeping it up there, why doesn’t the chlorine sink fairly rapidly into the lower atmosphere? This link leads me to believe that it should.
Are they invoking pixie dust to keep the chlorine up there for decades after we quit producing CFCs?
commieBob, up in the stratosphere, because of the ultraviolet radiation, chlorine is mostly chlorine atoms, not chlorine molecules. A fair bit of it gets adsorbed onto ices.
The kicker of the ozone issue, though, is that sea weed produces and releases easily ten times the halogenated hydrocarbons emitted by humans. The halogenated hydrocarbons are produced by an enzyme called vanadium haloperoxidase, which is widespread among seaweeds.
Maybe someone has done work showing marine halogenated hydrocarbons don’t make it to the stratosphere, and contribute little of the chlorine there, but I’ve not seen it.
Chlorine is a very reactive chemical, whether it is dissociated or molecular. I would expect the reactive half life (until it reacted into another compound) to be relatively short. At that point it would be even heavier and sink more quickly (unless there is a heck of an updraft).
How big is the ozone ‘hole’ now compared to then?
About the same.
Yes, it is not clear if this study is an attempt to find another excuse for the Montreal protocol when it is becoming obvious that the link to ozone depletion is tenuous, or to find an excuse for why global temperatures won’t comply with GCM computer predictions.
It’s truly amazing how “greenhouse” gases and CFCs emitted in the northern hemisphere magically migrate and concentrate over the south pole. A miracle! A miracle!
who told you there was a glass wall around the equator?
Aerosols from El Chichon and Mt Pinatubo spread to both hemispheres, why to you think gases cannot mix and travel too?
Ozone, for example, is not attracted to a magnetic pole.
OK, so you are no longer claiming “magically migrate” then. So who claimed that CFCs were “concentrating” at the south pole? Which “greenhouse” gases were you referring to other than CFCs?
Maybe if you dropped the heavy sarcasm and stated clearly what you think is wrong it would help.
Ozone was reduced by 5-8% as a result of Mt Pinatubo according to NASA. It is fair to assume a similar effect from El Chichon. Much of the loss of ozone was natural causes that was falsely attributed to CFCs by those who can not do more that fit a straight line to any data they look at and then draw false correlations to the rise human activity.
There was a drop in lower stratospheric temperature and ozone levels following both eruptions. The recovery in ozone is due to the lack a major eruptions since, NOT Montreal regulations.
So why isn’t there an equally large hole over the Arctic?
scoreboardweb August 15, 2017 at 8:44 pm
So why isn’t there an equally large hole over the Arctic?
Because it isn’t as cold.
“Because it isn’t as cold.”
So it’s temperature that’s causing the large hole, not CFC’s?
scoreboardweb asked: “So why isn’t there an equally large hole over the Arctic?
Destruction of ozone by chlorine radicals is assisted by polar stratospheric clouds which usually only form below -80 degC. Only the Antarctic is usually cold enough to produce these clouds. Occasionally some form in the Arctic and there is some ozone depletion there.
Explain how ozone cannot be attracted to a magnetic pole.
scoreboardweb asked: “So it’s temperature that’s causing the large hole, not CFC’s?”
No. Darkness causes the ozone hole. Ozone, in order to exist in the upper atmosphere, requires lots of UV light to continually break apart O2 (and O3) molecules to create the free radical O molecules needed for bonding with O2 (in the presence of UV light) to create O3. In the long dark polar winter, the air is naturally depleted of both O and O3.
The Antarctic ozone hole is larger, even though the periods of darkness over the two poles are approximately equal. However, this is only because of the unique geography of Antarctica and its totally surrounding ocean currents and associated steering winds create a more effective barrier to some air from lower latitudes vcmixing with the “native” O and O3 depleted air than does Arctic geography. A better way to say this is that the ozone hole over Antarctica is larger because Antarctica is protected from mixing air by the same geography that makes it colder than the Arctic.
Once polar spring occurs, the creation of O3 via UV begins again, but counter intuitively, the ozone “hole” doesn’t reach its peak size until AFTER the UV light returns. That’s because the UV immediately begins breaking apart remaining O3 molecules, but it takes a while for enough free oxygen radicals to repopulate the depleted air for O3 creation to happen on the same scale. But once there are enough free O radicals, the O+O2=O3 reactions begin to occur in large numbers again, and O3 returns, and the ozone hole closes, like clockwork. In other words, in the spring, UV based O3 production slightly lags UV based O3 destruction. And that is my hypothesis.
@ scoreboardweb – August 15, 2017 at 9:00 pm
“NO”, it’s not the temperature that’s causing the large Ozone hole.
It is caused by the lack of UV radiation from the Sun making contact with the O2 in the atmosphere above Antarctica during the Southern Hemisphere wintertime.
https://qph.ec.quoracdn.net/main-qimg-0994483fb9d3f4d2990e4e4028f83c50-c
Six (6) months of darkness (no UV radiation) below the Antarctic Circle at the South Pole.
And no UV radiation ……. = no Ozone formation ….. = big Ozone Hole.
Patrick:
Ozone is made entirely of oxygen. Oxygen is repelled by a magnetic field. It is ‘paramagnetic. An industrial quality oxygen detector is a “paramagnetic oxygen cell’. It has a magnetic field that drives the Oxygen to one side away from the magnet where it is detected.
Can someone tell when the “hole” was made? Could it have been there 10,000 yrs ago?
“Crispin in Waterloo but really in Ulaanbaatar August 16, 2017 at 8:35 am
Patrick:
Ozone is made entirely of oxygen. Oxygen is repelled by a magnetic field.”
Yes I know this. So how does O3 get to the poles if repelled by a magnetic field then to go on to form a hole given we know O3 is created and destroyed continuously with UV? Simple, and obvious answer, it can’t. Anyway, Greg was talking about aerosols from volcanoes (Sulphur etc etc). I was talking about “greenhouse” gasses and ozone. Not the same. Greg missed the quotes.
Mickey Reno August 16, 2017 at 5:38 am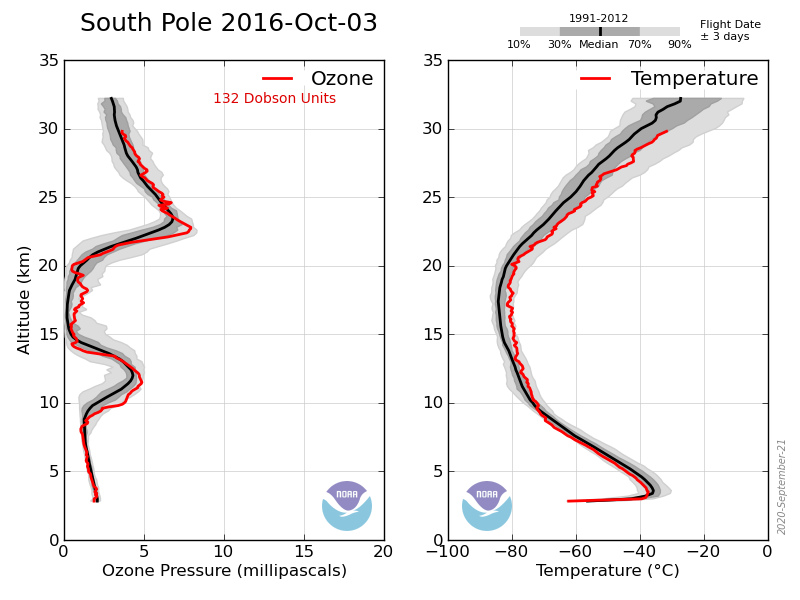
scoreboardweb asked: “So it’s temperature that’s causing the large hole, not CFC’s?”
No. Darkness causes the ozone hole. Ozone, in order to exist in the upper atmosphere, requires lots of UV light to continually break apart O2 (and O3) molecules to create the free radical O molecules needed for bonding with O2 (in the presence of UV light) to create O3. In the long dark polar winter, the air is naturally depleted of both O and O3.
During winter over the South Pole O3 stays constant because there is no UV to photolytically destroy it.
The Antarctic ozone hole is larger, even though the periods of darkness over the two poles are approximately equal. However, this is only because of the unique geography of Antarctica and its totally surrounding ocean currents and associated steering winds create a more effective barrier to some air from lower latitudes vcmixing with the “native” O and O3 depleted air than does Arctic geography. A better way to say this is that the ozone hole over Antarctica is larger because Antarctica is protected from mixing air by the same geography that makes it colder than the Arctic.
Once polar spring occurs, the creation of O3 via UV begins again, but counter intuitively, the ozone “hole” doesn’t reach its peak size until AFTER the UV light returns. That’s because the UV immediately begins breaking apart remaining O3 molecules, but it takes a while for enough free oxygen radicals to repopulate the depleted air for O3 creation to happen on the same scale. But once there are enough free O radicals, the O+O2=O3 reactions begin to occur in large numbers again, and O3 returns, and the ozone hole closes, like clockwork. In other words, in the spring, UV based O3 production slightly lags UV based O3 destruction. And that is my hypothesis.
The warming up of the stratosphere over the pole causes the PSC to evaporate thereby releasing Cl compounds which catalyst the destruction of the O3, it is due to the S polar stratosphere being cold enough to form the PSCs that leads to the enhanced destruction which causes the Ozone hole. Most years nearly all the O3 in the 14-22km range is destroyed but not above or below that altitude.
Previously the O3 concentration at ~17km peaked at ~17mPa.
A grave situation, no doubt.
As Mickey Reno says above, the waxing and waning of ozone around the south pole is driven by variation in sunlight through the seasonal cycle. Any effect of halogenated molecules is completely secondary and essentially immaterial.
Abundant observational data show that the “ozone hole” grows and fades naturally – – no need to invoke human causation
What really ticks me off, in addition, is this BS claim that CFC/HFC/CHFC compounds are “10,000 times more potent greenhouse gases” than CO2. That’s based on laboratory measurements of long-wavelength absorption and BS assumptions about long residence times in the atmosphere
But, if a molecular species is present in infinitessimally small concentrations, then its absorptive effect is also infinitessimally small. Period.
And, BTW, it is now abundantly clear that CO2 does NOT correlate with temperature (1880-1940 heating without change in CO2; 1940-1975 cooling with large CO2 increases), this entire discussion (sources and effects of CFCs, etc) is also immaterial
A Sheep in Wolves clothing [yes that was intentional].
The baseline measurements of the “ozone hole” were rather dodgy, as the rule to reduce purportedly ozone affecting gasses went into effect quite soon after.
“Hopefully, the Protocol can serve as a model of the international cooperation that we need to tackle the real problem – carbon dioxide.”
Yikes! My waders got wet inside on that one. Ozone is not human controlled any more than CO2.
Wow! now I know why the models do not agree with observations. The forgot to factor in the ozone gases!
Cheers
Roger
http://www.rogerfromnewzealand.wordpress.com
” The study’s results show that reducing the use of ozone-depleting substances from 2008 to 2014 eliminated the equivalent of 170 million tons of carbon dioxide emissions each year. That’s roughly the equivalent of 50 percent of the reductions achieved by the U.S. for carbon dioxide and other greenhouse gases over the same period.”
Now wait a minute here….that’s not the way we measure our CO2 output or reductions.
Does this mean we’ve actually reduced our CO2 emissions by another 50%?
I think what they are trying to suggest is that the reduced GHE from having less CFCs etc is the equivalent of half the REDUCTIONS in CO2, not half the total emissions.
Lqtitude
Looks to me like a path to claim that the ozone thingy was not important it is the CO2 equivalent that matters! Reducing Ozone is the ‘real purpose’ as it eventually sinks in that the Montreal Protocol accomplished nothing to do with Antarctic ozone.
To make any claim related to CO2-equivalence one has to have a GHG heating value for CO2 and that gas or particle. Well, prove it. The heating value, net, of CO2 is highly disputed.
If some gas has 10,000 times the heating value of CO2, why isn’t it used as a thermal radiation insulator? Such a claim implies that CO2 is ineffective at intercepting IR and an insulation wimp.
The Montreal Protocol was a stalking horse for the CAGWGlobal Warming Alarmsm Scare. The Montreal Protocol was quite ineffective, as is witnessed by the continual waxing and waning of the Ozone Hole where it is business as usual. The CAGW Alarmism implementation was much more damaging as it enlisted legislation to attempt to regulate industry and transport in western Countries. These regulations were severely damaging to civilisation, but were equally useless as the climate continued on its merry way, waxing and waning as it always had, quite independently of the measured amount of CO2 in the atmosphere.
As Pop Piasa said, “Ozone is not human controlled any more than CO2”. However the attempts to limit ‘Ozone affecting gases’ are much less damaging than those to control CO2.
Yes, I recall the “ozone hole” scare in the 70s. They started measurements and discovered a “hole”. The obvious assumption for a climatologists is that there would never be a “hole” if it were not for humans. Case closed, let’s make an international treaty to “save the planet”.
Yes. That’s exactly as I saw it too, Greg.
Greg, the ozone hole was first measured by Dobson in 1954 or 56. The units of measure are called Dobson units. Just using better equipment for measuring made the hole bigger. We have only 60 yrs of size measuring. There is a volcano in Antarctica that puts out chlorine gas.
I’m no climate scientist. But who cares about the amount of CFC’s in the atmosphere when your goal is to shrink the ozone hole? Shouldn’t they be focusing on the end goal? Not that I agree with it; the ozone scare was, as many have noted, just a warm-up for CAGW (pun intended).
I’m just an old country engineer, but it seems the CAGW and Montreal Protocol are not ends in themselves, but means to an end. The M-P outlaws, or establishes a procedure to autlaw, useful industrial solvents and other chemicals, while CAGW “solutions” involve the elimination of cheap, abundant energy. The end game appears to me to be the destruction of western civilization. I can only wonder what they have in mind to replace it.
I think we may be experiencing a glimpse of what is on store.
“I can only wonder what they have in mind to replace it.”
Look at Venezuela for a start.
“ the ozone scare was, ”
The ozone scare was a carefully planned and orchestrated “flim-flam scam” to scare the bejesus out of the public in order to get CFC production and sales banned …… because Freon is a CFC and Dupont’s exclusive patent for producing and selling it was about to expire.
False. The patent expired years before.
OK, after reading the following cited article I now think I am better informed on the subject.
Here is an excerpt from that article, to wit:
I knew Dupont people who were involved. Dupont threw in the towel because they were being beat up in the press – ‘destroying the environment.’ Fighting the green state fails. It wasn’t a science decision, it was a marketing decision. Marketing in that the company name was being devalued by the press attacks.
Corporations learn that it’s better to go along with the fascist state than to try to fight them. Chrony capitalism is a rational response.
Interesting how volcanoes are so easily ignored in the Ozone Hole “movement”.
Yep, fit a linear trend to any and all data and ignore the actual variations in the data which may tell you something about the cause. This has been the cornerstone of climatology for 30 years or more.
Greg, is the mechanism of how volcanoes reduce upper atmosphere ozone known? If so, even if only a theory, would you please briefly describe it?
SO2 reacts with water vapour to form sulphuric acid aerosols. These aerosol initially block incoming solar causing the warming of the stratosphere and tropospheric cooling. They also cause breakdown of ozone.
Chlorine is also involved and also present in volcanic emissions.
Initial work by Solomon which attributed ozone depletion to anthopogenic CFCs ignored close-by volcanic eruptions which ejected chlorine.
Thanks Greg. Ignoring entirely the provenance of this paper (if you can), would you have any comments on its accuracy or soundness, as far as the data an science go? Among other things, the paper draws attention to the chlorine emitted in volcanic eruptions.
http://www.larouchepub.com/eiw/public/1989/eirv16n24-19890609/eirv16n24-19890609_018-cfcs_are_not_depleting_the_ozone.pdf
Regardless of Rogelio Maduro’s work, is there any evidence that the ozone hole is being depleting by CFCs, HCFCs and HFCs as opposed to other sources of chlorine?
Bromine s strongly involved in the destruction of ozone. A lot of it comes out of the oceans. GCR’s are also involved, and the chemistry has been reproduced in the lab.
See Prof Lu, Univ of Waterloo. Find three papers and also read the hilarious discussion on Eli Rabbet’s blog as ‘Eli’ tries to disprove it.
Bill Illis: You are correct. See:
http://cfc.geologist-1011.net/
It’s all in how you interpret …… and report …. the data.
I’ve come to expect regular propaganda about the Montreal Protocol, and how it reduced the ozone hole. I used to believe this crap, but I’ve changed my mind.
The “ozone hole” was unknown until it was detected by satellites. The photochemistry of halogen compounds, including CFCs and other refrigerants accelerate the ozone decomposition is undeniable. What is seldom said is this. The ozone in the atmosphere is made and decomposed by solar UV radiation. Whether man made chemicals play a role in the process is quite unproven.
Ozone is a heat-trapping gas so I wonder what is the net effect of more ozone but less of the chlorofluoro carbons.
It isn’t possible to trap heat.
Got to get your quota of nits.
Why not? I don’t think you are right about that, unless you are talking about something very specific. Otherwise we we wouldn’t have insulation. Also, at a molecular level, heat can be kinetic motion of the molecules or it can be “trapped” in the molecule itself when photons excite electrons to jump out of their normal orbitals, which is later released the photon as radiant heat.
I don’t know, I am not a professional chemist – maybe I misunderstood my high school chemistry teacher.
Gotta agree: insulation doesn’t trap heat. It doesn’t retain it either, though there are retained heat cookers. They should rather be called ’embedded heat cookers’.
Insulation is all about give and take, or give and get.
If I could trap heat, I would become rich.
The fun part is trying to figure out how a chemical that’s supposed to stay in the upper atmosphere for a full century suddenly stopped staying in the atmosphere after only a third of that time – while there was enough bootleg CFC production out of China and India to make up for the amount that was supposed to be disappearing. We should have almost the same amount of ozone-depleting chemicals in the air as we did when Reagan was President.
If CFCs have decreased significantly in the last 30 years, it means the scientists were completely wrong about the chemistry of the stuff…
Chad Irby August 15, 2017 at 8:22 pm
If CFCs have decreased significantly in the last 30 years, it means the scientists were completely wrong about the chemistry of the stuff…
The CFC concentrations have decreased in line with the chemistry prediction, you should read the report more carefully, it says “significantly reduced emissions of ozone-depleting chemicals”.
http://cdiac.ornl.gov/oceans/images/nhemispherecfcs5.png
Mean mid-year tropospheric CFC-11, CFC-12, CFC-113, carbon tetrachloride (CCl4), sulfur hexafluoride (SF6) concentrations in the northern (NH) and southern (SH) hemispheres for the period 1920.5 to 2015.5 The concentrations are expressed as the mixing ratio (mole fraction) of the trace gas in dry air and are reported in parts-per-trillion (ppt).
Phil: The first evidence for an ozone hole was seen in the mid-1980s, when CFC levels were lower than they are today. We don’t have very many observations of “normal ozone levels” in the Antarctic spring from before the mid-1980’s, so we don’t know how low CFCs must go to return to “normal”. Most of the early measurement were made from the coast of Antarctica, which is on the edge of today’s large ozone hole. IIRC, one set of early measurements from the 1970s was made from the South Pole and didn’t find anything like today’s large hole. Then CFC levels were half of present levels. The biggest change the Montreal Protocol has brought is a halt to the rise of CFCs. Otherwise we might be nearing 1000 ppt, (Developing countries were allowed to produce CFCs until about 2010 and China did so.)
First reports of an ozone hole were from International Geophysical Year (IGY) of the 1950s.
Nice attempt at changing the subject Phil.
You even quote the portion where Chad says that CFC concentrations have gone down. Then you accuse him of not knowing that the concentrations have gone down.
This issue is the effect of the CFCs. Even though the concentrations have gone down, the ozone hole remains as large as ever.
The problem, as I mentioned, is that the concentrations _have_ gone down, Despite the CURRENT chart from the report (see above), we were told that the concentrations _would_not_go_down_ by an appreciable amount for another 30 years or so, because CFCs were so pernicious.
Which, again, means they were wrong when they first predicted this.
You’re doing the same thing with ozone that the warmists did with their temperature predictions. Make an apocalyptic prediction, then 30 years later (when the actual prediction collapsed in a tangle of reality), pretend they didn’t make a prediction at all. Then, to top it off, point to a completely different set of numbers and yell, “SUCCESS!”
Hunter: “First reports of an ozone hole were from International Geophysical Year (IGY) of the 1950s.”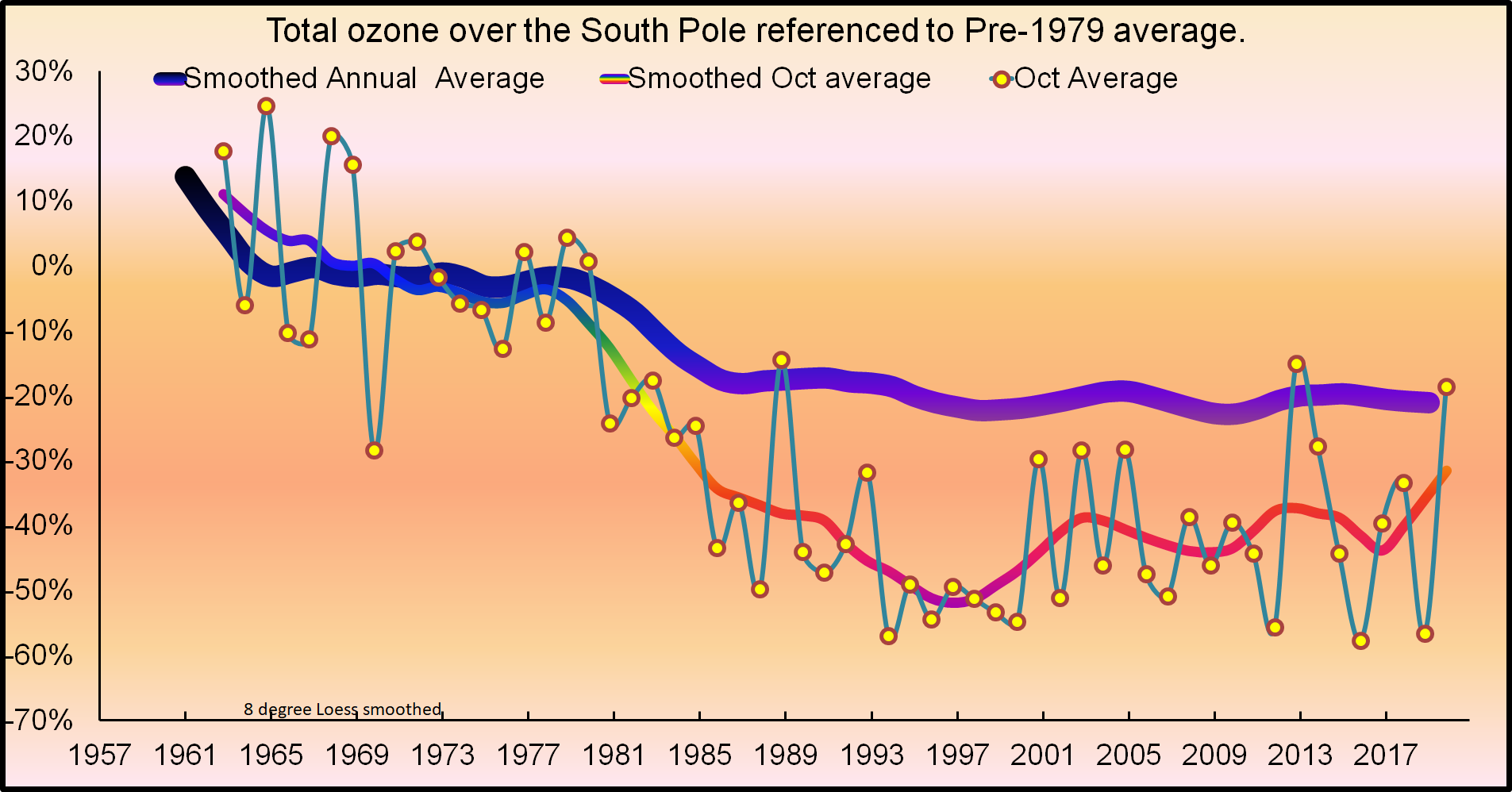
First measurements made in the late 1950’s, but there was no obvious hole back then. The graph from below is from the South Pole. Total column ozone measurements from Brewer and Dobson spectrophotometers at four Antarctic stations (Faraday (previously Argentine Islands) in 65.3°S since 1957; Halley in 73.5°S since 1957; Syowa in 69°S since 1961; and South Pole in 90°S since 1961). These are measurement of the total ozone overhead and look quite different from the hole measured from space at the altitude of greatest depletion. The other stations are on the edge of the continent and near the edge of the ozone hole, so the absence of a depletion there isn’t as definitive as at the South Pole.
https://www.esrl.noaa.gov/gmd/dv/spo_oz/ozdob.html
Chad Irby August 16, 2017 at 12:01 pm
The problem, as I mentioned, is that the concentrations _have_ gone down, Despite the CURRENT chart from the report (see above), we were told that the concentrations _would_not_go_down_ by an appreciable amount for another 30 years or so, because CFCs were so pernicious.
As mentioned above you appear to be confusing emissions and concentration. The paper in the original post refers to emissions not concentrations:
Implementation of the Montreal Protocol in the United States, largely through the Clean Air Act, led to a near complete phase-out of U.S. production and consumption of chlorofluorocarbons (CFCs) beginning in 1996 and a 95 percent decline of hydrochlorofluorocarbons (HCFCs) production since 1998.
As a result, total emissions of CFCs in the U.S. have decreased by two-thirds from 2008 to 2014, while emissions of HCFCs declined by about half, the study authors said.
We were told that the concentrations of the CFCs would go down slowly after the phase out of their production for two reasons: continued release by leakage from existing equipment (referred to in the paper) and the fact that the lifetime of the CFCs in the atmosphere was long (50-130 yrs depending on species).
As shown in the figure I posted the gradual reduction in CFC concentrations is consistent with that expectation. (Molina and Rowland’s (1974) estimate for 111 and 112 lifetimes was 40-150 years and modern values are not very different).
Had emissions not been curtailed by the Montreal Protocol we would by now have substantially higher CFC concentration. Note that the convergence of the NH and SH values indicates much lower emissions since emissions primarily occur in the NH.
Chad Irby August 15, 2017 at 8:22 pm
We should have almost the same amount of ozone-depleting chemicals in the air as we did when Reagan was President.
If CFCs have decreased significantly in the last 30 years, it means the scientists were completely wrong about the chemistry of the stuff…
Whereas we have substantially more than when Reagan was president.
The new study is the first to quantify the impact of the Montreal Protocol on U.S. greenhouse gas emissions with atmospheric observations. The study’s results show that reducing the use of ozone-depleting substances from 2008 to 2014 eliminated the equivalent of 170 million tons of carbon dioxide emissions each year. That’s roughly the equivalent of 50 percent of the reductions achieved by the U.S. for carbon dioxide and other greenhouse gases over the same period.
Just as Jim Hansen’s 1988 paper said it would be.
Who can tell me the baseline concentration of ozone (ppm). Then, what is the delta ppm that represents a hole?
The Montreal folks sure picked a good scare word when they chose “hole”. For the unwashed masses it evokes an absence, nothingness, not just a reduction.
Which is exactly what is observed between 14km and 22km altitude.
Does this explain “The Pause”?
gallopingcamel: Or can it explain the warming of the 80’s ??(Not sure when there was a peak of CFC’s emissions?)
clearing of the stratosphere by the two eruptions can probably explain much of the the warming of the 80s and 90s … and thus the reduced of warming since Y2K.
https://climategrog.wordpress.com/tls_icoads_70s-20s/
Greg, how is it that volcanoes can cause warming? i can see how it might cause cooling and then give back the warming that was lost. But, make things warmer than before an eruption? (help me out here, thanx)…
There is a link above the image. I you want to understand more, read it, that will help you out. That is why I put there. It links to further articles on the subject. like this one: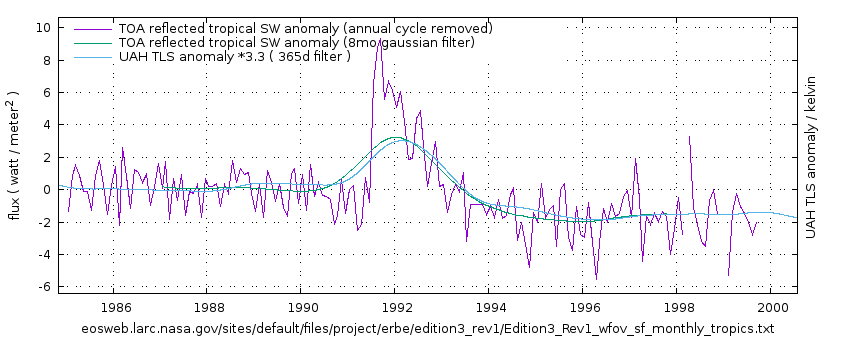
https://climategrog.wordpress.com/erbe_sw_tls/
Studying feedbacks in the energy budget to the Mt Pinatubo eruption (ERBE data) I found that climate response continued well after the initial aerosols had dispersed. That warming effect ran to the end of the data.
https://climategrog.wordpress.com/tropical-feedback/
What a pity it isn’t possible to trap heat.
Never noticed that clear nights tend to be colder?
Next time it’s cold out, try wearing a coat , let us know how you get on. Most people understand as soon as they are old enough to wear mittens.
Phillip is attempting to change the subject via nit picking.
Greg – “Never noticed that clear nights tend to be colder?”
So do Clear Nights have a lower CO2 concentration or a lower Water Vapor concentration?
Guess this comment shows which is the Control Knob.
Agree with you about heat – but per your volcano article. I thought they covered that in this blog a few weeks ago and discovered they used a massive sample of two volcanoes over the last 100 years, and ignored El Chicon, which didn’t exhibit the behavior, with a similarly large eruption.
sorry marque2 , don’t follow. What they, what volcanoes? Say something coherent or link.
El Chichon did have similar effect on TLS , it is shown inverted in the first of the three image above, but if it’s clearer on its own:
PB,
Why bother trapping heat when it is more fun trapping gullibles?
These authors are addressing gullibles in now-trendy gullible-speak, a product of the 21st century that puts it apart from proper science.
e.g. their claim that the Montreal Protocol was more effective than other ways to reduce GHGs is wrong. Modestly, the team I worked with from 1970 discovered and mined the Ranger uranium deposits which subsequently produced 120,000 tonnes of Uranium oxide and at times nearly 10% of global uranium production. This substituted for very large quantities of fossil fuels that would otherwise have been consumed for electricity. Far more than the claimed MP effect.
Importantly, claims are made of chemicals thousands of times more potent for global warming than CO2. They are in ppb to ppt concentrations in the air. Is that enough for them to be active? If there were only a few molecules of any of them, they could not contain enough energy effect to allow detection of any atmospheric heating they might produce. Raise the concentration and maybe you might get a detectable heat. The question is, what concentration is needed to give a measurable ellect? I have not seen papers estimating these threshold concentrations and would be pleased for references.
Until then it is merely hypothetical pie in the sky, not good science.
Geoff
I remain skeptical of this claim too but I have not had time to read the paper. Whether each individual species is detectable is not important, it should be calculable. Very low concentrations could be in the linear range rather than near saturated end of the logarithmic response like CO2. So changes more significant.
Yet during the era of highest CFC production, use and emissions….. Global temps fell on average according to their own temp records…. The 40’s to the 70’s shows a decline in global average temps.
I dunno. Everything is so squirrely with these CAGW mob. 10 000 times stronger than CO2, they say, but no observational impact during the highest and unregulated use of the CFC era…. wuwt?
10,000 times stronger but 100,000 times less present 😉
Wasn’t there a study that documented that the Montreal protocol had no impact on the ozone hole?
There is no Ozone hole from late December to early August. It is a seasonal phenomenon and the Ozone is not getting destroyed, it is getting swept out to mid-latitudes by the winter polar vortex. By the end of December, the Ozone moves back in and there is no hole.
At the peak of the hole in late September, the 50S latitude has the highest Ozone levels of anywhere on the planet for any time of year.
It is just the way the atmospheric circulation organizes itself and it has probably always been like this.
Bill Illis August 16, 2017 at 4:03 am
There is no Ozone hole from late December to early August. It is a seasonal phenomenon and the Ozone is not getting destroyed, it is getting swept out to mid-latitudes by the winter polar vortex. By the end of December, the Ozone moves back in and there is no hole.
At the peak of the hole in late September, the 50S latitude has the highest Ozone levels of anywhere on the planet for any time of year.
You are mistaken, the stratospheric ozone moves south from the topics but is prevented from reaching the South Polar region because of the presence of the winter polar vortex, therefore it accumulates outside the vortex. Over the pole O3 is neither created nor destroyed because of the absence of UV radiation there during the winter. In the spring the sun raises the stratospheric temperature thus removing the polar stratospheric clouds and releasing the chlorine compounds which destroy the O3 in the 14-22 km altitudes thereby creating the ozone hole.
No. Ozone is created from Oxygen by UV radiation. O3 molecules are unstable in the absence of UV and thus dissipate during the dark, sunless wintertime. The hole is nothing new, it was observed long before human emission of chlorine compounds could be considered a contributing factor.
Art August 17, 2017 at 8:19 am
No. Ozone is created from Oxygen by UV radiation. O3 molecules are unstable in the absence of UV and thus dissipate during the dark, sunless wintertime.
Not true, here’s the last few years’ data measured over the S Pole, note that during the winter the level is around 250DU, the drop of O3 to around 100DU occurs early October, i.e. spring not winter.
The hole is nothing new, it was observed long before human emission of chlorine compounds could be considered a contributing factor.
Again not true, the ‘hole’ started to form in the early 80s at the same time as atmospheric CFCs were rapidly growing and were at about 60% of their peak.
How come no one is talking about the intensity of UVB rays? Weren’t we all supposed to die of skin cancer because UVB-blocking ozone was being depleted? I have never found a source showing UVBs had increased as ozone was depleted. I have not researched this in a long time, but I recall finding that there has been no meaningful increase in skin cancer rates, after allowing for the fact that people now spend more time outside than they did in the past.
Need a lot more info on this graph. Number of Data points, measurement info, +/- ranges. Interesting that change is negative at the pole and positive most elsewhere. Hope to hear more about this.
https://www.nasa.gov/topics/solarsystem/features/uv-exposure.html
http://onlinelibrary.wiley.com/doi/10.1029/2009JD012219/pdf
The ozone depletion occurs at the poles, mainly during winter, and even when not winter the region is subject to lower sun angle than the regions closer to the equator.
Not many people sun bathe or expose bare skin at the poles during winter (and there is minimal sunshine if they do). Skin cancer and increased cataracts alarmism are just part of the normal scare tactics
Taphonomic August 16, 2017 at 8:36 am
The ozone depletion occurs at the poles, mainly during winter, and even when not winter the region is subject to lower sun angle than the regions closer to the equator.
During the spring not the winter.
Greg in Houston August 16, 2017 at 4:29 am
How come no one is talking about the intensity of UVB rays?
The Dobsonmeter which measures the ozone concentration uses the relative intensity of UVB.
“This shows what can be achieved by concerted and thoughtful international effort,” said Scott Lehman of the Institute of Arctic and Alpine Research at the University of Colorado Boulder and co-author of the new study. “Hopefully, the Protocol can serve as a model of the international cooperation that we need to tackle the real problem – carbon dioxide.”
These are real great idiots. They really compare in many respects harmful synthetic gases with for plant growth necessary an essential CO2 and therefore essential also for the life of all animals and humans.
Against the statements of these idiots one can really only say: Think first before you speak.
It was thanks to this agreement that the fridge that exploded causing the Grenfell Tower fire in London had butane in rather than a CFC based coolant.
James Bull
From what little I’ve read, the whole ozone depletion scare remains controversial.
The reason it was easy to implement the treaty and emissions was because all that was required was to substitute one chemical refridgerant for another at the factories. No requirement to exchange all of the R12 in vehicles already on the road or in existing refridgerators and freezers. It didn’t cost any govt much, if any, money to pass the laws and in general the manufacturers had no particular reason to object to the refridgerant switch. AC units didn’t work as efficiently using the new stuff, which offset some of its advantages with respect to emissions,as cars burned more gasoline to cool the vehicle.
I seriously doubt the figures claimed here – the people writing this are enthusiastic true believers, and therefore completely biased and apt to see benefits where few exist –
Once upon a time I asked this hypothetical question of my staff, who at the time were researching a environmental issue that had never been studied before. How did we know there was NOT a hole in the ozone layer over the Antarctica before people began staying there or before we put a satellite up? Both basically happened since the middle of the 20th Century. At the other end, what is the first thing a Indian villager buys when his village gets reliable electricity and he saves enough money? A refrigerator! Just like carbon dioxide emissions it is doubtful that India will reduce its output of “older” refrigerants anytime soon.
Trivia: Why do people often sneeze when they look towards bright sun? UV solar radiation converts oxygen to ozone which is highly reactive and irritating to the nasal lining. This causes sneezing. So back to the explanations about solar UV and ozone – low solar UV in winter along with low atmospheric temperature and high level cloud leads to reduced ozone, which we are supposed to be afraid of because the ozone is supposed to protect us from the solar UV… except that the ozone is low exactly when and where the UV is low. Another example of a natural self regulated system that we should just be appreciative of, rather than trying to change the climate/atmosphere through human rituals.
It is an interesting idea that has been mentioned re: the Montreal Protocol /Ozone Hole scare being a dry run for Global Warming. If this were true – does it give us any pointers as to how the current scare might end?
““This shows what can be achieved by concerted and thoughtful international effort,” said Scott Lehman of the Institute of Arctic and Alpine Research at the University of Colorado Boulder and co-author of the new study. “Hopefully, the Protocol can serve as a model of the international cooperation that we need to tackle the real problem – carbon dioxide.”
Yeah, but controlling CFC’s didn’t cost us hundreds of billions of dollars and blight the landscape with windmills like eliminating CO2 would do. Besides, there is no evidence that CO2 in the atmosphere is harmful.