
From the UNIVERSITY OF ALASKA FAIRBANKS, and the “at least they didn’t blame climate change” department:
A research team led by the University of Alaska Fairbanks and Colorado College has solved a century-old mystery involving a famous red waterfall in Antarctica. New evidence links Blood Falls to a large source of salty water that may have been trapped under Taylor Glacier for more than 1 million years.
The team’s study, published in the Journal of Glaciology, describes the brine’s 300-foot path from beneath Taylor Glacier to the waterfall. This path has been a mystery since geoscientist Griffith Taylor discovered Blood Falls in 1911.
Lead author Jessica Badgeley, then an undergraduate student at Colorado College, worked with University of Alaska Fairbanks glaciologist Erin Pettit and her research team to understand this unique feature. They used a type of radar to detect the brine feeding Blood Falls.
“The salts in the brine made this discovery possible by amplifying contrast with the fresh glacier ice,” Badgeley said.
Blood Falls is famous for its sporadic releases of iron-rich salty water. The brine turns red when the iron contacts air.
The team tracked the brine with radio-echo sounding, a radar method that uses two antenna — one to transmit electrical pulses and one to receive the signals.
“We moved the antennae around the glacier in grid-like patterns so that we could ‘see’ what was underneath us inside the ice, kind of like a bat uses echolocation to ‘see’ things around it,” said co-author Christina Carr, a doctoral student at UAF.
Pettit said the researchers made another significant discovery – that liquid water can persist inside an extremely cold glacier. Scientists previously thought this was nearly impossible, but Pettit said the freezing process explains how water can flow in a cold glacier.
“While it sounds counterintuitive, water releases heat as it freezes, and that heat warms the surrounding colder ice,” she said. The heat and the lower freezing temperature of salty water make liquid movement possible. “Taylor Glacier is now the coldest known glacier to have persistently flowing water.”
Pettit said she enlisted Badgeley as an undergraduate student to help with the overall mission of understanding the hydrological plumbing of cold-based glaciers.
“Jessica’s work is a perfect example of the high level of work undergraduate students can do when you give them a challenge and set the expectations high,” she said.
###
The National Science Foundation sponsored the research.
If freezing releases heat that can be trapped under ice, how would this affect the ice core samples? What I mean is who do we know that, as they were formed over time, the heat release affected ice layers above sufficiently to bollox up things like gas trapped within the ice.
The water was already frozen when it fell as snow on top of the glacier.
No freezing involved, just compressing snow into ice.
But with added compression from high hydrostatic pressures…water melts without needing to increase temperature.
The liquid caused by this compression is what allows glaciers to flow.
rocketscientist,
Depends of the temperature of the ice… You can’t skate on ice of -20°C, because no liquid water is formed at such pressure and temperature. Still there is some liquid-like water present at the ice-air border of included air bubbles by some less ordered molecules (a few molecules thick), but at the crystal borders there is no liquid water, as long as no salt/dust inclusions are present.
Glaciers don’t need liquid water to deform: ice is plastic and behaves as a thick liquid. See, already from 1908:
https://www.jstor.org/stable/93090?seq=1#page_scan_tab_contents
Ferdinand,
This may come as some surprise but I have skated on ice well below -20 °C. It can be brutal on the toes and nose but if you keep moving you’ll stay warm.
Whether your skates glide across the ice at -20 °C or not depends on how much you weigh and how wide are the blades of your skates. If you concentrate the load sufficiently (hence increase the local pressure), water will liquefy under the pressure. It is this phase change ability with pressure that allows ice to behave plastically regardless of the temperature.
If you suspend a weight from a thin cable that is wrapped around an ice block and maintain the ice block below freezing, the cable will slowly cut right through the ice block without ever spilling a drop and without cutting the ice block in twain. The water, liquefied by the local pressure concentration under the weighted cable, will simply flow from the high pressure side of the cable to the unpressurized side of the cable where it will refreeze allowing the cable to slowly pass through the block.
… and a bee can’t fly because it would break the laws of aerodynamics. -20° isn’t much below 0° F. When I was a kid I spent a lot of time skating on outdoor rinks at that kind of temperature. Here’s a link to a guy who says he skated at -50°C.
rocketscientist April 25, 2017 at 3:21 pm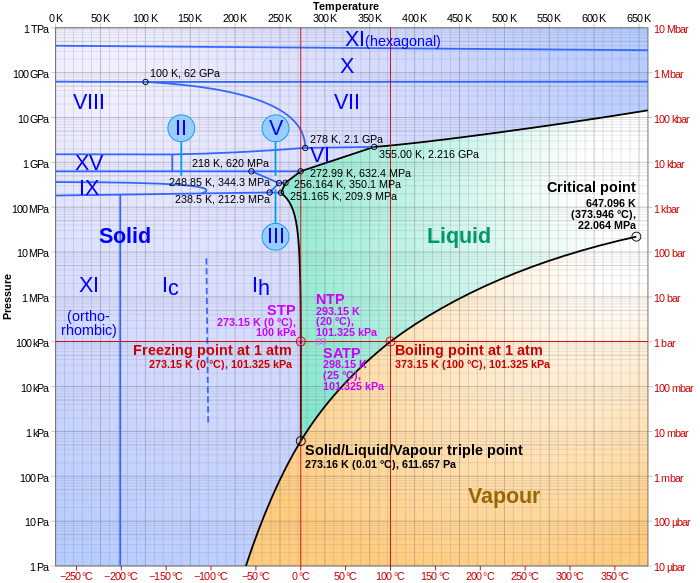
Ferdinand,
This may come as some surprise but I have skated on ice well below -20 °C. It can be brutal on the toes and nose but if you keep moving you’ll stay warm.
Whether your skates glide across the ice at -20 °C or not depends on how much you weigh and how wide are the blades of your skates. If you concentrate the load sufficiently (hence increase the local pressure), water will liquefy under the pressure. It is this phase change ability with pressure that allows ice to behave plastically regardless of the temperature.
Liquid water doesn’t exist below 251K (-22ºC)
“Pitt physics professor explains the science of skating across the ice”. Pittsburgh Post-Gazette. December 23, 2012. It used to be thought … that the reason skaters can glide gracefully across the ice is because the pressure they exert on the sharp blades creates a thin layer of liquid on top of the ice… More recent research has shown, though, that this property isn’t why skaters can slide on the ice… It turns out that at the very surface of the ice, water molecules exist in a state somewhere between a pure liquid and a pure solid. It’s not exactly water — but it’s like water. The atoms in this layer are 100,000 times more mobile than the atoms [deeper] in the ice, but they’re still 25 times less mobile than atoms in water. So it’s like proto-water, and that’s what we’re really skimming on.
rocketscientist So have I grew up in Northern Minnesota if one could not skate below 20C they would be very little skating in either Northern United States or most of Western Canada, where I grew up twenty of thirty days of high temperatures not breaking 0 F is not uncommon. One thing is certain one cannot form a snow ball or make a snowman(or sorry that not PC correct snowperson, sorry my bad snowperson still too dated, i believe the modern therm is snowthem or something gender neutral) but is digress any way at not to far below -5 C those temperatures snow particles no long sticks together.
You can’t skate on ice of -20°C
????? Tell that to high school hockey players in the Yukon.
You can rollerskate in a herd of scientists.
I find skating below -15C is very difficult because of wind chill brrr…
One must realize as the pressure applied to ice is increased the local temperature will increase, unless you are removing heat from the system. Because water is a rather poor conductor of heat I suspect that increased local pressures increase the local temperature thereby allowing the phase change.
Sorry,
I never skated on any ice, so I was wrong for the right reason…
Below -22 degrees C there is no water formed from ice under any pressure. That was my point. That you still can skate on ice is probably because the water molecules at the ice/air border have an unordered liquid-like structure, which bonds may easily be broken, so these smear the glide…
“The water was already frozen when it fell as snow on top of the glacier.”-MarkW
It is a presumption to think that glaciers only receive water from precipitation. I include the possibility that ground water springs may contribute water sub-glacier. This includes the possibility of brine springs.
An example of springs in a location previously covered by glacier ice in the recent geologic past:
“Twenty-six brine springs discharging from Paleozoic carbonate deposits were sampled along the eastern edge of the Alberta Basin from two regions, Fort McMurray and Wood Buffalo National Park. Geochemical data indicate that the springs originate as an influx of meteoric and glacial waters that have come into contact with, and dissolved, halite deposits.”…
Brine springs of northern Alberta. Available from: https://www.researchgate.net/publication/279653428_Brine_springs_of_northern_Alberta [accessed Apr 26, 2017].
I have read before that deep ice core samples may be misleading in prehistoric CO2 levels, due to extreme pressures at the bottom of the ice sheet changing or damaging that particular ice/CO2 content in the ice core in the process of turning to solid ice under immense pressures. Therefore the CO2 count, especially after the sample is decompressed and melted for analysis, may not be what the original atmospheric composition actually was in those original snow flakes. Considering that original snow/ice may be hundreds of thousands of years old, and under immense pressures all these eon’s.
Not sure of the exact process how this could work, and have no links to post, but would be interested in hearing if anyone else has detailed knowledge of these claims and how it works, if indeed there is any truth to any of this? If there is any truth to this, then it could throw out the ice core proxies for climate throughout the ice ages. A big deal if true…but I have no proof to offer. But a very interesting question?
It is indeed an important question Ron. Anybody out there have any information or links?
Try https://wattsupwiththat.com/2011/10/31/little-bubbles-part-1/ and https://wattsupwiththat.com/2011/11/01/little-bubbles-part-2-firn-the-great-equalizer/ for some amazing facts about the veracity of ice core dating problems.
I’ve often suspected that conditions like solid state diffusion and ice sublimation would compromise the accuracy of the atmosphere bubbles in ice cores.
Ron,
What happens under pressure is that water and CO2 forms clathrates, solid forms of CO2 hydrates, which did give problems at measuring time. Today´s cores are allowed to relax for at least a year in cold conditions at te surface and are measured either by grating under vacuum or complete sublimation and cryogenic freezing and separation. No CO2 can hide then anywhere.
The old method of melting the ice is completely abandoned for CO2, That method did give lots of extra CO2 in Greenland ice cores due to the acid dust from Icelandic volcanoes mixing with (carbonate) sea salts which sets CO2 free in situ and even more with melting. Antarctic ice cores have only sea salts as inclusion…
fthoma,
Seems that I have missed that story… Anyway, look at the reaction of Willis Eschenbach at that time, his reaction is the one I should have given too.
The CO2 levels found in the bubbles indeed are a mixture of several years, but mainly from the last years before bubble closing. How many years depends of the snow accumulation rate. That gives average 10 years for coastal Law Dome to 600 years for inland Vostok. Including 20 years of overlap (1960-1980) with direct measurements at the South Pole for the Law Dome cores…
The average gas age at bubble closing depth – 72 m – of Law Dome is only 7 years older than at the surface, while the surrounding ice is already 40 years old…
“Antarctic ice cores have only sea salts as inclusion…”
Definitely not true. There is both volcanic ash layers (mostly from West Antarctic volcanoes) and mineral dust (mostly from Patagonia) in antarctic ice cores, even in the inland cores from East Antarctica:
http://onlinelibrary.wiley.com/doi/10.1029/2008GL033382/full
https://www.researchgate.net/publication/223479372_Characteristics_and_sources_of_tephra_layers_in_the_EPICA-Dome_C_ice_record_East_Antarctica_Implications_for_past_atmospheric_circulation_and_ice_core_stratigraphic_correlations
So if that is the explanation for the stronger variation in CO2 in Greenland ice cores it presumably applies in Antarctica too.
tty,
I stand corrected. Probably a matter of quantities. Coastal ice receives more sea salts and inland ice relative more finest volcanic dust in Antarctica. In Greenland it is both as the distances to see and volcanoes is much shorter.
Anyway, it doesn´t help to explain the low CO2 levels during glacial periods, as any mix of dusts should increase the CO2 levels as is seen in Greenland ice cores.
tty,
Another point is the type of volcanic dust: Icelandic volcanoes are from deep magma spots, where the magma is highly acidic (less carbonates, more HCl and HF, highly toxic for grazing animals), while subduction volcanoes include lots of carbonates, thus releasing far more CO2 (ten times the deep magma volcanoes) and probably turn more alkaline, so don´t set CO2 free with sea salts.
Moreover, as far as I remember, confirmed by the first reference, for inland ice cores most dust peaks are during glacial periods, that should give CO2 peaks at the same depths as where the highest dust peaks are found, which is not the case…
tty,
Sorry for the late reply, was travelling and has a bad Internet connection with lots of “timeouts”…
I have looked at the dust inclusions at Vostok: between zero and 5 ppm, the latter mainy during cold periods (less precipitation, more wind,…). Even if that was all carbonates and completely transfered into CO2, that is 0-5 ppmv CO2, mostly in glacial periods, where the lowest values of around 180 ppmv were measured. Doesn’t seem a problem in Antarctica.
In contrast, CO2 levels in the Greenland ice cores can go up with hunderds of ppmv at high dust inclusions and did keep increasing with the old method of melting all ice and measuring CO2 under vacuum.
Hmmmm, I wonder if it might be possible to make homemade ice cream, using this discovery.
“While it sounds counterintuitive, water releases heat as it freezes”
.. and absorbs it when it melts right? yay the world is saved, all the melting glaciers are cooling the planet…
🙂
Not any more than leaving the refrigerator door open cools the kitchen.
Melting ice will “cool” the surrounding fluid from which it is absorbing heat (ocean/air), but the overall heat in the system does not change.
There is that pesky 2nd law…..
The second law:
You can’t get ahead.
Hi rocketscientist,
you wrote
“One must realize as the pressure applied to ice is increased the local temperature will increase, unless you are removing heat from the system.”
Where did you “learn” this rocketscience?!!?
Why does it sound counterintuitive ??
It’s called latent heat.
G
Actually you have it backwards.
Water freezes when it loses heat. So the heat loss (first) is necessary for freezing.
G
Yes, George, you have to cool water to its freezing point or below to get it to freeze, but at the moment the water changes state from a liquid to a solid, the water molecules slow down and release energy in the form of latent heat. For example, when temperatures are expected to reach freezing in an orange grove, they may spray the trees with a fine mist so that the freezing process can release heat and prevent the fruit from freezing. Another example is a sodium acetate reusable heat pack. It gives off heat and warms your hands as the sodium acetate freezes inside. The freezing process giving off heat is what makes it seem counter intuitive.
“all the melting glaciers are cooling the planet”
Much too little melting to matter except locally.
However the release of heat at altitude when water vapor condenses and freezes is the main mechanism for transporting heat away from the surface. IR radiation is actually much less important, though it is given star rating in CAGW.
Link to full text of article:
https://www.cambridge.org/core/services/aop-cambridge-core/content/view/B5C197906AD54619AEA26068AD92989A/S0022143017000168a.pdf/an_englacial_hydrologic_system_of_brine_within_a_cold_glacier_blood_falls_mcmurdo_dry_valleys_antarctica.pdf
This is old news, and not complete news. It has already been published that bacteria species that reduce iron and sulfur compounds are responsible for extracting the iron from the underlying rock and releasing it into the briny water.
“The biogeochemical data presented here imply that the bacterial assemblage below the Taylor Glacier can grow chemoautotrophically or chemoorganotrophically by harvesting energy from bedrock minerals or the assemblage may grow heterotrophically on ancient marine organics by respiring Fe(III) or SO42−.”
https://www.ncbi.nlm.nih.gov/pmc/articles/PMC1932727/
And of course many of the microbes that are using these reactions are using them to produce energy. Their reactions are often exothermic and the heat given off prevents the water from melting. Some lakes covered by often hundreds of metres of ice have warm water (plus 20 degrees centigrade) and where these have been sampled they contain communities of anoxic microbe ‘slimes’.
I certainly would not like to walk on that ice, were I came from that can happen also but most of the time it from a spring, in northern Minnesota spring water is 48 degrees, Walking frozen lakes in areas where the springs are is not wise even in the coldest of winters. Ice can also be reduced from fish movement also, my brother set up a net to catch spawning fish, when he set it he had eighteen inches of ice the next day it was six. That was early winter and the air temperature never got above freezing.
Spot on, Brad. The freezing point is also lowered when dealing with a brine which this is. What you are in fact witnessing is of course acid rock drainage in all its glory. This is the same phenomena is in existence at Golden Mountain National Park in northern Taiwan. The Taiwanese turned into a National Park and it is now a huge tourist attraction.
The other factor is that increased pressure will also cause water to “melt”. The negative slope of the liquid /solid phase curve give water a unique triple point. This phenomena is what allows glaciers to “flow” in the first place. It’s what keeps ice less dense than liquid water and allows it to float. Thank goodness for that or our polar oceans would be frozen all the way to the bottom with no hope of ever thawing.
re ice cores: I have always been rather skeptical of ice core proxy data and the ability to accurately age samples due to the nature of ice to melt under high pressure and reconfigure deposited strata.
Only an idiot would trust estimates of ancient atmosphere composition that were obtained from ice cores. And only a bigger idiot would use those estimates to make policy decisions which affect national economies & people’s lives.
That may well be the case, but, can you tell us why?
I think the physics of the process is more complex than presented: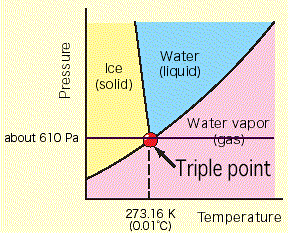
Ice has 9% volume larger than liquid water, hence if ice melts inside an air tight glacier a vacuum will occur, decompression causing evaporation of some of the water. At the triple point there is presence of all three: ice, liquid water and the water vapour.
My knowledge is limited to give precise account of the process.
But if you notice, 610 pascals is very low pressure. The pressure within the glacier is WAY above that. Decreasing the pressure within the glacier would actually be a negative feedback, working to refreeze the water. Furthermore, the phase diagram is three dimensional, you could possibly add a fourth dimension (ion concentration), but I’m not sure if that would change too much.
*two dimensional…could be three dimensional by adding salt content.
Actually, a phase diagram IS three-dimensional (pressure, volume, temperature). The diagram above is a projection onto two dimensions.
The compression needs to persist if the water is to remain liquid without adding heat. If the structural arrangement of the overlying ice reacts the forces the ice will not change phase because the pressure isn’t applied. However, if the pressure is applied and causes the ice to melt the overlying ice must move to account for the reduced volume. If the overlying ice cannot move then it is supporting its own weight and therefore not able to squeeze the ice below it, hence no additional pressure and stoppage of pressure melt.
A more detailed picture is here from Wiki:
https://en.wikipedia.org/wiki/Pressure_melting_point#/media/File:Phase_diagram_of_water.svg
You need a lot of pressure (~210 MPa that is ~2000 bar) to lower the melting pressure of ice to -20°C, that is around the temperature of the coastal ice cores of Antarctica. -40°C is the temperature of Vostok and other inland ice cores, no pressure on earth can melt that,,,
You forget that geothermal heat warms the ice from below. There is large lake underneath your “-40 C” ice at Vostok.
http://www.pnas.org/content/113/50/14249/F1.expansion.html
Dear Ferdinand,
you mentioned 2000bar pressure needed to get the ice (at -20°C) melting:
For this you need “only” 21 km ice “on top”. (For the rocketscientist approx. 70 000 ft)
I guess: not on this planet.
(I knew that this is not new for your Ferdinand).
BR
So, no mention of heat flux from the bedrock below it. No mention of volcanism detected beneath the glacier recently and other offshore and onshore volcanic activity a bounding in the region. There is a lake with microbiota swimming around in it below a couple of miles of ice that haven’t see the open air for millions of years in east Antarctica. Also, the geochemistry of the red water may show it carries basemetal and other element content. It could give a clue as to origin. I’m betting on volcanic activity.
Or simply left over geothermal from long extinct volcanic flows.
Moving magma of active volcanism would produce measurable seismic tremors.
No signs of volcanic activity in the Dry Valleys immediate area which is very well explored geologically, though there is tephra layers from volcanoes further away, e. g. on Ross Island about 100 km away.
Interesting line –
“New evidence links Blood Falls to a large source of salty water that may have been trapped under Taylor Glacier for more than 1 million years.”
So how high was the sea level when this salty water was in a position to be trapped under the glacier?
Brines aren’t necessarily sea-water-derived.
Correct. There are even largish hypersaline lakes in the Dry Valleys (Lake Vida and Lake Vanda), though much of the salt is probably ultimately derived from sea-water derived salt dust. The Dry valleys have persisted virtually unchanged in their present form for about 15 million years. Quite a lot of salt dust can accumulate in that time, even though the sea is 100 km away.
Many years ago Hem produced some interesting graphs showing the solubility of iron in relation to pH and Eh. They could be useful in explaining this phenomenon.
I don’t know if this phenomenon is a rare as this is made out to be. Although the place noted in the article may be an excessive edge case. I took the photo below in Antarctica in another geological era, (1993).
charles the moderator
April 25, 2017 at 2:01 pm
You are a fine photographer!
Algae on snow-ice likely.
Reply: Could be ~ctm
Beautiful photograph.
Also it is well known that liquid water can exist in fine clays at ~-70C adding a dimension of weirdness in construction on permafrost. Sometimes the ground isn’t as solid as it should be. When you go to the expense of a trip to Antarctica make sure you gather everything and study every thing you can.
In doing geological fieldwork in the 50s – 70s, there was an admonition to only have to visit an outcrop once. In addition to mapping the geology, we corrected minor topographic stuff, estimated the cloud cover in ‘tenths’ in the morning, noon and end of day, estimated the head on any large streams with rapids and falls, noted tree species, sizes, extent, and animals and birds encountered, ice break up on lakes and rivers and the first frosts of late summer.
Some did a more thorough job than others but they wanted this stuff for 30 day work months, at wages of $100 /month, everything found including tobacco but no alcohol. I could pay my next year’s university tuition and books and live some style! None of us would have returned from Antarctica with just instrument readings and pails of dirty water.
Noting tree species is not much of a hardship in Antarctica. But there are other odd things to study, like 50,000 years old frozen bird poop in Snow Petrel colonies.
Is there any report on how many (if any) halophiles, acidophiles and chemophiles have been found in that water?
The earth is bleeding because meanyhead humans are cutting and hurting it!
GAIA BLEEDS! SHE BLEEDS!!! PREPARE THE SACRIFICES!!!
Just a thought on my part, not meant to to direct/influence this excellent thread. With consideration of the psychometric (hope proper term) charts of H2O, triple points and such and the extremely high elevations of the land mass in Antarctica. With 2 or 3 miles of ice over a land mass that most likely releases “some” heat to the overburden of ice mass, could it be possible that a 10,000 ft (+/-) head pressure (again pos or likely neg) affect the melting or freezing at the triple point or other critical point in the underlying H2O?
This is a most interesting thread!
Just a larger glacial perspective picture here. Blood Falls is at the end of one of the small glaciers flowing away from Taylor Dome near the McMurdo Antarctic station. Blood Falls is very near sea level in the Dry Valleys (indeed, there are sea lion carcasses right next to Blood Falls that have not decayed due to how dry it is).
In the background on the right, one can see the rise to the main ice-sheet of Antarctica in this region. Not far beyond this perspective is Taylor Dome which is 2,400 metres above sea level. The iron rich brine can be coming from anywhere out to 50 miles under this dome. The ice at the bottom of Taylor Dome is likely in the -5.0C range so saltiness could very well make it liquid and it would be squeezed out from the bottom of the ice-sheet to eventually flow into the ocean (although it is so dry here it probably does not make it).
http://querosaber.com.pt/media/galeria_multimedia_v2/offline/15564.0.original.jpg
Could it (salt water) be sucked in to a vacuum?
Here is another story describing the process:
http://factslegend.org/30-interesting-blood-falls-facts-a-glacier-that-bleeds/3/
Wait, they observed a physical phenomenon, designed an experiment to collect data, and developed a reasonably hypothesis to explain the process? How did they get published?
It is basic fishplant knowledge, extrapolated. Automated brine, slurry, controlled, but in nature.
Reaction?
The fluid coming out of the glacier is a brine which means there will be freezing point depression. By looking at the actual composition of the fluid the actual freezing point (or range) can be calculated. However, the indication that the fluid is flowing out from under the glacier might tell us that the freezing point hasn’t been reached.
The process of ‘freeze distillation’ has long been used for various purposes. When water freezes to ice, it tends to exclude dissolved matter from the crystal matrix. In this way the ice formed as salt water freezes contains less salt than the water which has not frozen, depending on how slowly the water has been allowed to freeze. If the source of salt water is isolated and the process is continued long enough, the unfrozen water becomes a brine, with a freezing point mush lower than the usual freezing point of water. The zero point of the Fahrenheit thermometer was originally set as the temperature at which a saturated brine would freeze: 0°F or about -18°C.
This process is known as “brine rejection” and is important for the creation of the very cold, salty and dense Antarctic Bottom Water (AABW) which is an important part of the global thermohaline circulation.