From CALIFORNIA INSTITUTE OF TECHNOLOGY
Deep-sea corals reveal why atmospheric carbon was reduced during colder time periods
We know a lot about how carbon dioxide (CO2) levels can drive climate change, but how about the way that climate change can cause fluctuations in CO2 levels? New research from an international team of scientists reveals one of the mechanisms by which a colder climate was accompanied by depleted atmospheric CO2 during past ice ages.
The overall goal of the work is to better understand how and why the earth goes through periodic climate change, which could shed light on how man-made factors could affect the global climate.
Earth’s average temperature has naturally fluctuated by about 4 to 5 degrees Celsius over the course of the past million years as the planet has cycled in and out of glacial periods. During that time, the earth’s atmospheric CO2 levels have fluctuated between roughly 180 and 280 parts per million (ppm) every 100,000 years or so. (In recent years, man-made carbon emissions have boosted that concentration up to over 400 ppm.)
About 10 years ago, researchers noticed a close correspondence between the fluctuations in CO2 levels and in temperature over the last million years. When the earth is at its coldest, the amount of CO2 in the atmosphere is also at its lowest. During the most recent ice age, which ended about 11,000 years ago, global temperatures were 5 degrees Celsius lower than they are today, and atmospheric CO2 concentrations were at 180 ppm.
Using a library of more than 10,000 deep-sea corals collected by Caltech’s Jess Adkins, an international team of scientists has shown that periods of colder climates are associated with higher phytoplankton efficiency and a reduction in nutrients in the surface of the Southern Ocean (the ocean surrounding the Antarctic), which is related to an increase in carbon sequestration in the deep ocean. A paper about their research appears the week of March 13 in the online edition of the Proceedings of the National Academy of Sciences.
“It is critical to understand why atmospheric CO2 concentration was lower during the ice ages. This will help us understand how the ocean will respond to ongoing anthropogenic CO2 emissions,” says Xingchen (Tony) Wang, lead author of the study. Wang was a graduate student at Princeton while conducting the research in the lab of Daniel Sigman, Dusenbury Professor of Geological and Geophysical Sciences. He is now a Simons Foundation Postdoctoral Fellow on the Origins of Life at Caltech.
There is 60 times more carbon in the ocean than in the atmosphere–partly because the ocean is so big. The mass of the world’s oceans is roughly 270 times greater than that of the atmosphere. As such, the ocean is the greatest regulator of carbon in the atmosphere, acting as both a sink and a source for atmospheric CO2.
Biological processes are the main driver of CO2 absorption from the atmosphere to the ocean. Just like photosynthesizing trees and plants on land, plankton at the surface of the sea turn CO2 into sugars that are eventually consumed by other creatures. As the sea creatures who consume those sugars–and the carbon they contain–die, they sink to the deep ocean, where the carbon is locked away from the atmosphere for a long time. This process is called the “biological pump.”
A healthy population of phytoplankton helps lock away carbon from the atmosphere. In order to thrive, phytoplankton need nutrients–notably, nitrogen, phosphorus, and iron. In most parts of the modern ocean, phytoplankton deplete all of the available nutrients in the surface ocean, and the biological pump operates at maximum efficiency.
However, in the modern Southern Ocean, there is a limited amount of iron–which means that there are not enough phytoplankton to fully consume the nitrogen and phosphorus in the surface waters. When there is less living biomass, there is also less that can die and sink to the bottom–which results in a decrease in carbon sequestration. The biological pump is not currently operating as efficiently as it theoretically could.
To track the efficiency of the biological pump over the span of the past 40,000 years, Adkins and his colleagues collected more than 10,000 fossils of the coral Desmophyllum dianthus.
Why coral? Two reasons: first, as it grows, coral accretes a skeleton around itself, precipitating calcium carbonate (CaCO3) and other trace elements (including nitrogen) out of the water around it. That process creates a rocky record of the chemistry of the ocean. Second, coral can be precisely dated using a combination of radiocarbon and uranium dating.
“Finding a few centimeter-tall fossil corals 2,000 meters deep in the ocean is no trivial task,” says Adkins, Smits Family Professor of Geochemistry and Global Environmental Science at Caltech.
Adkins and his colleagues collected coral from the relatively narrow (500-mile) gap known as the Drake Passage between South America and Antarctica (among other places). Because the Southern Ocean flows around Antarctica, all of its waters funnel through that gap–making the samples Adkins collected a robust record of the water throughout the Southern Ocean.
Wang analyzed the ratios of two isotopes of nitrogen atoms in these corals – nitrogen-14 (14N, the most common variety of the atom, with seven protons and seven neutrons in its nucleus) and nitrogen-15 (15N, which has an extra neutron). When phytoplankton consume nitrogen, they prefer 14N to 15N. As a result, there is a correlation between the ratio of nitrogen isotopes in sinking organic matter (which the corals then eat as it falls to the seafloor) and how much nitrogen is being consumed in the surface ocean–and, by extension, the efficiency of the biological pump.
A higher amount of 15N in the fossils indicates that the biological pump was operating more efficiently at that time. An analogy would be monitoring what a person eats in their home. If they are eating more of their less-liked foods, then one could assume that the amount of food in their pantry is running low.
Indeed, Wang found that higher amounts of 15N were present in fossils corresponding to the last ice age, indicating that the biological pump was operating more efficiently during that time. As such, the evidence suggests that colder climates allow more biomass to grow in the surface Southern Ocean–likely because colder climates experience stronger winds, which can blow more iron into the Southern Ocean from the continents. That biomass consumes carbon, then dies and sinks, locking it away from the atmosphere.
Adkins and his colleagues plan to continue probing the coral library for further details about the cycles of ocean chemistry changes over the past several hundred thousand years.
###
The study is titled “Deep-sea coral evidence for lower Southern Ocean surface nitrate concentrations during the last ice age.” Coauthors include scientists from Caltech, Princeton University, Pomona College, the Max Planck Institute for Chemistry in Germany, University of Bristol, and ETH Zurich in Switzerland. This research was funded by the National Science Foundation, Princeton University, the European Research Council, and the Natural Environment Research Council.

How does CO2 decide whether to be cause or effect? Flip a coin?
It checks which way the political winds are blowing and then goes with the floe 😉
LOL! +1
Poor opening line…. We know a lot about how carbon dioxide (CO2) levels can drive climate change,……IMO.
“We know a lot about how carbon dioxide (CO2) levels can drive climate change”
What do you mean, “we”? I certainly don’t.
I thought the same at first. Then thought, “Whoops! The research grant money …
Quick! – think up a good $$$ opening line.”
Sorry, but you are wrong. There are a lot of theories and hypotheses, but none have been proven and most data does not match the models (by a big margin). In fact other effects besides CO2 may have a much larger effect on climate change.
Relative to biology, its both, which as CO2 increases, biology gets more robust and as biology gets more robust, CO2 increases. Relative to temperature, it’s also both, but the IPCC exaggerated the magnitude of the effect CO2 has on temperature by at least a factor of 3-4.
Can anyone explain to me how a paper studying, and titled – “Deep-sea coral evidence for lower Southern Ocean surface nitrate concentrations during the last ice age.” has anything at all to do with “WHY” CO2 levels were lower during the last Ice Age?
I mean seriously….Is there a mechanism in which CO2 concentrations in the atmosphere have something to do with the amount of nitrate available on the ocean’s surface? Or even better, a mechanism in which nitrate levels in the surface ocean affects the amount of CO2 in the atmosphere? What am I missing?
Correlation is not causation….that’s still a “thing” right?
The authors seem to be discussing why iron deficiencies in the South Pacific might explain the relative concentrations of 16N vs 15N in fossil corals.
I don’t see that it has much to do with CO2 and I further suspect that their suppositions concerning the availability of iron could be conjecture. But I didn’t read the paper.
Henry’s law!!!!!
Precisely, colder water absorbs more CO2 and that CO2 drives more life in the oceans. They once again have cause and effect backwards.
What do you call it When the vey first sentence of an article is backward, PROPAGANDA! RR
The very first sentence is incorrect!
Study: why CO2 levels are higher during global warm periods
….all this crap over 1 degree
Astounding that they can write the entire account without even mentioning that colder waters absorb more of all gas species.
The bottom line of some wild speculation about winds bringing more iron into the water just about rounds it off. It seems the main aim of this “study” is an attempt to provide an alternative explanation and thus undermine the importance out-gassing / absorption phenomenon, without actually naming it.
Yet more pseudo-science.
No. The overturning is too slow, the deep oceans too stratified for the mixing you need for a Henry’s Law to adequately explain observations/proxies results. Stop looking for simple explanations in a complex system of systems; interconnected systems that operate on many different time scales.
Do you have reliable over-turning data for all ocean currents over the last few glaciations… over maybe just one glacial interglacial cycle even ?
If the authors had even estimated and compared the two to it may have been more credible.
If the overturning were too slow, how can you explain what happens to the roughly 8 kg of carbon-14 created in the upper atmosphere every year? Given that 1 in 8000 carbon-14 atoms decay every year, there must be 64,000 kg of carbon-14 on Earth for equilibrium, and there’s only 800 kg in the atmosphere. Almost all of it is transferred to the oceans at the rate it is created, where the concentration is 0.8 ppt compared to 1.0 ppt in the atmosphere. This gives us an exchange with the deep ocean of 40 Pg/yr so that 40 kg of carbon-14 enters the deep ocean and 32 kg comes out, making a net flow of 8 kg/yr into the deep ocean. When we apply the Van t’ Hoff correction to Henry’s law, which we can deduce using the enthalpy of dissociation of CO2 from the ocean and Boltzmann’s equation, we find that CO2 release from the ocean increases with temperature, while absorption, which is exothermic, remains unchanged. The concentration of CO2 in the atmosphere must increase to balance the faster release from the ocean. This calculation gives us a relationship between temperature and atmospheric CO2 concentration that matches almost exactly the ice core records. See link below for math.
http://homeclimateanalysis.blogspot.com/2016/01/carbon-cycle-correlation-between.html
The ice core record is, as urederra suggests, the result of the temperature-dependence of Henry’s law.
“Stop looking for simple explanations in a complex system of systems”
You mean like the belief that manmade CO2 is the one and only driver of climate?
Kevan,
Not sure of the ocean surface fractionation rates for 14C, but there is significant fractionation through the surface interface for 13C. Basically it is +2PDB fractionation from the atmosphere to the ocean and -10 from the ocean to the atmosphere. These surface film fractionations are separate from the thermal dictates of Henry’s law.
gymnosperm, I have not looked into the fractionation of carbon-13. It is a stable isotope that makes up 1% of our carbon. Looking it up just now, it appears that your +2 PDB means the ratio of c13/c12 is a factor of 1.002 higher than a standard sample, while -10 PDB is a factor of 0.990. That’s very interesting, but I’m not sure what to make of it, because I have not studied it. The carbon-14 study is immediately compelling, however, because the total inventory is known, and the flow into the deep ocean is easy to deduce. The ocean surface carbon-14 concentration is around 96% of atmospheric, deep ocean 80%, vegetation 99%. In order for a net 8 kg of carbon-14 created by cosmic rays to be transported from the atmosphere into the deep ocean, we need 40 Pg/year flow of carbon-12 both ways between atmosphere and deep ocean. If we were suppose that the 8 kg flows through the ocean surface on its way to the deep ocean, the exchange with the ocean surface would have to be 200 Pg/yr. If we assume that the carbon-14 remains in the ocean surface, the surface must contain a total inventory of 63,200 kg of carbon-14, and therefore around 63,000 Pg of carbon, which it does not, so that can’t be so. If we were to assume that the carbon-14 goes into the biomass, the exchange would have to be 8000 Pg/yr, which cannot be true because this is ten times as much carbon as is in the entire atmosphere, and roughly the mass of carbon that exists in the entire biosphere. So I conclude the 8 kg of carbon-14 must be descending into the deep ocean. Kevan
We do? Perhaps you should attach your observations showing how ‘climate change’ is ‘driven’ by carbon dioxide. It doesn’t appear the case from historic measurements nor from current day measurements. Even the mechanism seems to be less than clear.
Show your working.
That’s about where I had to stop reading unfortunately. Can’t stand the meme.
Agree. It has been a lot of this kind of junk sci. lately, is the deadline for AR6 coming up soon?
Assert. Then draw preferred conclusions.
It’s how climate science works.
Andrew
No. Make up preferred conclusions. THEN assert.
Like all so-called “climate scientists”, they have it a$$=backwards. We know a lot about how temperature affects CO2 in the air. Not so much about the effect of CO2 on temperature in the actual climate system.
They’re also wrong about glacial and interglacial CO2 levels. They can go lower than 180 ppm during glacial phases and higher than 280 in interglacials. IIRC, stomata data show 330 ppm during the Eemian (the previous interglacial), without benefit of a Neanderthal industrial age, as I wrote in other comments recently. During longer, warmer interglacials of the past million years, CO2 might well have been even higher. The prior range could be from ~150 to ~350 ppm.
It’s funny how you make the claim that *all* climate scientists have things ass-backwards, yet your only evidence of that is produced by… climate scientists.
Marcus Holm-“It’s funny how you make the claim that *all* climate scientists have things ass-backwards, yet your only evidence of that is produced by… climate scientists.”
It appears you need a translator. In conducting actual, real science, the only evidence that matters is actual evidence-empirical, naturally occurring, observable evidence. Anything “produced” by human formulation or manipulation or modeling is not actual “evidence” of anything unless it matches and replicates the “measurements” and “mechanisms” of nature, exactly.
Since the cause/effect thing has not been established conclusively, and there are still “unknowns” regarding the actual natural mechanisms, the statement that “We know a lot about how CO2 levels can drive climate change” could not come from an actual, credible scientist. Most likely, being a press release, it was written by someone who thinks they are a “climate scientist”…a “so-called climate scientist”.
Chimp,
Forget stomata data for accurate absolute CO2 levels. Stomata (index) data are derived from plants that by definition grow on land, where one can measure 180-550 ppmv CO2 within a day. They reflect the average local CO2 level over the previous growing season, not “background” CO2 as ice cores do. The main advantage is that stomata data have a better resolution than ice cores. The main disadvantage: a local bias above background and more local variability. Therefore stomata data are calibrated against… ice cores and direct measurements over the past century.
The main problem is that no one can know how the local bias changed by huge land (use) changes in the main wind direction and even changes in the main wind direction itself (MWP vs. LIA)…
Thus if there are differences between the average CO2 levels of stomata and ice cores over periods longer than the resolution of the ice cores, then the stomata data need to be recalibrated, not reverse… And huge variability of the stomata index data reflects local CO2 variability, not necessarily global variability…
Aphan wrote ” In conducting actual, real science, the only evidence that matters is actual evidence-empirical, naturally occurring, observable evidence. Anything “produced” by human formulation or manipulation or modeling is not actual “evidence” of anything unless it matches and replicates the “measurements” and “mechanisms” of nature, exactly.”
This is not how science actually works. Direct measurements are very often either impossible or impractical, and I wager that 99.9% of all empirical evidence in science is produced by a combination of “manipulation” and modelling. For some reason, maybe because it’s the only science where they look at the details, some people think climate science is somehow worse in this respect than other sciences, and therefore less trustworthy. It’s not.
I work with people in every field from molecular biologists to astrophysicists, and the climate people are fundamentally doing the same sort of work as everyone else in the natural sciences. I trust them as much as the people developing new medicines and nuclear waste storage schemes. Arguments like the one I quoted are rooted in ignorance.
modeling combined with a reasonable level of observational derived measurements is considered semi-empirical, which IS NOT empirical!
Not Even Close!
“…the climate people are fundamentally doing the same sort of work as everyone else in the natural sciences.” (M Holm)
I will assume you are referencing researchers like Judith Curry, Roy Spencer, John Christy and Bob Carter.
By the way, I don’t trust researchers who display confirmation bias in any field of study.
Marcus Holm
March 15, 2017 at 9:58 am
“Climate scientists” in quotes aren’t scientists. They are computer gamers motivated by ideology.
Stomata data are gathered by real scientists, if nowadays they need to spin to get grants. Paleoclimatology used to be a science, as did climatology, but those fields have been corrupted by the crooks in quotes.
Ferdinand Engelbeen
March 15, 2017 at 11:00 am
Ice core “data” if anything have even worse problems. Paleoclimatologists need to look at a wide range of sources of information, not just ice and stomata but sediment cores, fossils and species assemblages.
Chimp,
Ice core data were and are collected by real scientists, as good as stomata data and sediment records. There are far less problems with ice core data, as these are direct measurements of CO2 in the enclosed air, as good as in the atmosphere.
Etheridge measured CO2 in the atmosphere, at several depths of the firn from in the pores and in the ice with the same GC and found the same levels in enclosed bubbles in ice as in still open pores at the same depth. Thus there is no shift in the CO2 levels at closing time.
Diffusion of CO2 in ice cores is so low that it is not directly measurable. One has deduced the migration near a melt layer where extra CO2 was found. In “warm” (-22°C) coastal ice cores (Siple Dome), that gives a theoretical broadening from the resolution from 20 to 22 years at medium depth, up to 40 years at full depth:
http://catalogue.nla.gov.au/Record/3773250
In the much colder (-40°C) inland cores like Vostok and Dome C that is practically zero.
Thus ice cores show direct measurements of ancient atmospheres, with one drawback: it is always an average of several years, from 10 years over the past 150 years to 560 years for the past 800,000 years.
Stomata data, as already said are proxies, not direct measurements and reflect local CO2 levels over land. Not “background” CO2 levels in the bulk of the atmosphere. The only advantage is that they have a better resolution. But neither the variability and surely not the absolute height are accurate for the past CO2 levels…
You beat me to it, Ian.
They don’t “know”, they “assume”, CO2 levels can drive climate change.
but at least this group also-“knows” or “assumes” that “climate change can cause fluctuations in CO2 levels”.
Which is a good thing
And JPL isn’t exactly jumping up and down and shouting “We know the answer!” They have been rather quiet about what, if anything, they are learning from the OCO-2 satellite. The last thing I saw showed vertical distribution of CO2, but obfuscated what was happening at ground/sea level. Everything I have seen to date from OCO-2 doesn’t support the claim that Man is primarily responsible for the increases in atmospheric CO2. However, it does show quite clearly the massive outgassing in the oceans.
Agree completely, Ian W
When I read that line in the lead-off sentence, I figured this was something from The ONION (it’s a satire broadsheet)
But I guess it was “peer reviewed”, so it must be sooper-good
According to their attached chart, climate warming is caused by “something” that happens about every 100,000 years give or take 10-20,000. I’m danged if I can remember what I or my relatives were doing that far back, but I’m human so I’m sure it was my fault!
Last time I looked at AR4, WG1, 0.04% ofthe Earth’s atmosphere was CO2, Man’s emissions were approximately 4% (it was just an estimate after all) of that per annum! The numbers in this piece seem to contradict the UNIPCC data!
Witch! Witch!
Earth’s average temperature has naturally fluctuated by about 4 to 5 degrees Celsius over the course of the past million years.
Their own graphs show some much larger fluctuations.
And warmer past interglacials.
Yup. Glacial intervals have been more than ten degrees C colder than now and past interglacials perhaps three degrees C warmer, for a fluctuation range possibly as great as 15 degrees C.
They do say “average temperature” not the peaks.
I think research like this is on the good side of the leger. New and innovative ways to study the actual past workings of the wporld.
The problem comes when biologists start to make conclusions about climate interactions. Their job is to present the facts.
They do say “average temperature” not the peaks
I take your point, but I thought they were graphing averages over some pretty large time periods to begin with in order to get a smooth curve. So a swing of 9 deg over 10,000 to 20,000 years would be a “fluctuation in average temp”? – Not that it really matters.
Average temperature is meaningless in this context.
Their statement is worded horribly.
Polar amplification is not some alarmist jargon-talking point. Polar amplification is real and has a straightforward thermodynamic explanation.
Those temp plots are of Antarctic ice core proxies, i.e. Polar region.
Same as we see today. Global temps are slowly rising (much less than alarmists claim though). But the poles are the energy balancing system where much of the heat is funneled back to space.
Energy (as SW) enters mostly at equatorial and mid-latitudes. Energy (as LWIR) leaves via the polar radiators to space.
Math exercise: Do the math (calculus) on the areas involved.
Surface area of the SW absorbing surface: -55 deg to +55 deg latitude.
Surface area of the LWIR radiating surface: (-65 deg to -90 deg lat)+(+65 deg to +90 deg lat)
The ratio of those two areas is a ROM estimate of polar amplification, both when global temps are rising and declining.
Polar amplification is not some alarmist jargon-talking point. Polar amplification is real and has a straightforward thermodynamic explanation.
Those temp plots are of Antarctic ice core proxies, i.e. Polar region.
The problem with “polar amplification” is that it is not “bipolar” so your “ie.” does not work and thermodynamic explanation would require it be occurring at BOTH poles. Where is your data showing “polar amplification” in Antarctica, how much has that continent warmed apart from the tip of the antarctic peninsula?
The thermodynamic explanation would also require the presence of the mid tropo, tropical hot-spot which has proven so illusive.
So, yes. polar amplification is not some alarmist jargon-talking point.
Polar amplification IS some alarmist jargon-talking point. The reason that the Arctic air is warmer is lack ice cover allows more heat to leave the ocean.
Agreed that Energy In enters mostly in the Equatorial/tropical zone, and whilst I agree that LWIR leaves at the poles, of course most LWIR leaves in the warmest regions of the planet, not the poles, in view of the Stefan Boltzmann Law wherein radiance is proportional to the fourth power of the temperature.
Perhaps what you intended to infer is that more energy (LWIR out) leaves the poles than energy comes in (SW) to the poles. Warm currents distribute energy (in the form of heat) from the Equatorial/Tropical areas of the planet polewards such that the polar regions have more energy/warmth than the received directly from incoming solar irradiance, Hence the polar regions, to the extent that they are not capped by ice, are net emitters.
Further to my above comment, perhaps i should also have pointed out that most of the evaporation and convection driving the water cycle also takes place at the Equatorial/Tropical region of the planet. This is where huge amounts of energy are lifted high into the atmosphere where the energy can be radiated to space.
JPeden,
Antarctic ice temperature proxies (based on D/H and 18O/16O ratio’s) reflect mostly Antarctic temperatures. Global temperature changes are about half of that, due to the “polar enhancement effect”.
During the Eemian, global temperatures were average 2 K higher than today, but SIberia and Alaska were 5-10 K warmer, with as result trees growing up to the Arctic Ocean, where nowadays only tundra is…
Thanks!
FE, agree with observations. Eemian Arctic 5-8C higher than now (ice cores), temperate ocean proxies (Ca/Mg ratio, forams types, alkenones..) about 2C, tropics about 1C. Confirmed by Arctic treelines.
Ferdi, I think you will find that Alaska and Siberia border the Arctic , not the Antarctic. So you are citing evidence of ARCTIC amplification, not “polar amplification.
There is currently no warming in Antarctica so this phenomenon can not be correctly attributed as “polar”.
Greg,
There is amplification near both poles, as the Antarctic Peninsula is warming too. Most of Antarctic mainland isn’t, which the climate models don’t reflect. There may some hints by the isolation of Antarctica due to the circumpolar vortex mainly in winter, but nobody really knows why,,,
Over the past 800,000 years, there seems to be Antarctic amplification by comparing sediments at lower latitudes with the ice core temperature proxies.
Could be + or – average which is about right. The latest warming doesn’t seem outrageous.
From the article:
“A healthy population of phytoplankton helps lock away carbon from the atmosphere.”
Phytoplankton consume atmospheric CO2. Without CO2, there are no living phytoplankton. Why would a ‘healthy’ population want to ‘help’ ‘lock away’ their own food source?
Most phytoplankton get their CO2 from the water rather than the air.
So, their ‘lock away’ comment means ‘prevents the co2 from returning to the air.’
Steve Fraser says: “So, their ‘lock away’ comment means ‘prevents the co2 from returning to the air.’”
My take is that their comment means to ‘lock away’ carbon from the Carbon Cycle of life. Not something that any carbon based life form should strive for.
Locking away might be like Prince Albert’s Social Security “lock box”.
Not all phytoplankton get eaten, but even those which die and fall to the ocean floor are rarely locked away effectively forever. They decay or become part of rock formations which will get lifted up or reworked by seafloor spreading, subduction or other geological processes.
It’s not whether phytoplankton *want* to lock away carbon, it’s an unavoidable side effect of phytoplankton growing, living, and dying. With more phytoplankton, there’s more food, which means more animals, which means more dying, which means more carbon sequestration.
Moreover, the population of phytoplankton is not limited by the availability of carbon (dioxide). The piece itself clearly says that *iron* is the limiting nutrient. So even if phytoplankton were sentient and “wanted” things, they wouldn’t much mind contributing to carbon sequestration because it’s not a problem for them.
“Moreover, the population of phytoplankton is not limited by the availability of carbon (dioxide).”
Yet, if there were no carbon dioxide, there would be no phytoplankton. So, contrary to your comment, phytoplankton is indeed limited by the availability of CO2.
Carbon Dioxide is the base of the food chain for all carbon based life forms. You’re saying that life wouldn’t mind limiting their own food source.
The availability of Carbon Dioxide is not a problem here on Earth? Consider three sister planets:
Venus – atmosphere 95% CO2
Earth – atmosphere 0.04% CO2
Mars – atmosphere 95% CO2
One of these planets currently supports carbon based life forms that consume CO2 – perhaps Carbon sequestration is not something that life should strive for.
Thomas Homer,
Plankton takes its CO2 from the surrounding waters, which contain abundant (for the plants) CO2 in different forms (mostly bicarbonates). That indeed is not the limiting factor.
What they use as CO2 or bicarbonates (for shell formation in ehux) makes that the pCO2 (the equilibrium pressure with the atmosphere) lowers and more CO2 is removed out of the atmosphere. Thus even making the atmospheric level still lower, down to 180 ppmv during glacial periods. That is at the edge of survival for C3 cycle plants (like all trees and many other plants)…
https://wattsupwiththat.com/2017/03/11/about-those-devastating-epa-budget-reductions/comment-page-1/#comment-2448843
[excerpts}
I started writing about “CO2 Starvation” circa 2008 – the following post is from January 2009.
I later changed 200ppm to ~150ppm and added comments about C3, C4 and CAM photosynthesis, but the problem remains much the same.
I have corresponded about this subject with several parties, including Patrick Moore, who later wrote an important paper about it.
Here is a question for you:
Since “CO2 lags temperature at all measured time scales”, as I proved in January 2008, how is it that the mainstream global warming debate between warmists and skeptics is STILL about “the magnitude of climate sensitivity to CO2”, in effect “By how much can the future cause the past?”
I know it is a bit more complicated than I stated above, but not much. This seems to be a huge and voluntary “blind spot” for the climate science community. One is reminded of the controversies of “continental drift”, or the “bacterial origin of stomach ulcers”. Why is this subject not openly and rationally debated? It is controversial, but is it really that scary?
[P.S. to Ferdinand – NO sidetracks into your favorite mass balance argument please – it is irrelevant!)]
Regards, Allan
https://wattsupwiththat.com/2009/01/30/co2-temperatures-and-ice-ages/#comment-79524
(Plant) Food for Thought (apologies – written too late at night)
Background:
http://www.planetnatural.com/site/xdpy/kb/implementing-co2.html
1. “As CO2 is a critical component of growth, plants in environments with inadequate CO2 levels – below 200 ppm – will cease to grow or produce.”
http://en.wikipedia.org/wiki/Carbon_dioxide_in_the_Earth's_atmosphere
2. “The longest ice core record comes from East Antarctica, where ice has been sampled to an age of 800 kyr BP (Before Present). During this time, the atmospheric carbon dioxide concentration has varied by volume between 180 – 210 ppm during ice ages, increasing to 280 – 300 ppm during warmer interglacials…
… On longer timescales, various proxy measurements have been used to attempt to determine atmospheric carbon dioxide levels millions of years in the past. These include boron and carbon isotope ratios in certain types of marine sediments, and the number of stomata observed on fossil plant leaves. While these measurements give much less precise estimates of carbon dioxide concentration than ice cores, there is evidence for very high CO2 volume concentrations between 200 and 150 myr BP of over 3,000 ppm and between 600 and 400 myr BP of over 6,000 ppm.”
Questions and meanderings:
According to para.1 above:
During Ice ages, does almost all plant life die out as a result of some combination of lower temperatures and CO2 levels that fell below 200ppm (para. 2 above)? If not, why not?
Does this (possible) loss of plant life have anything to do with rebounding of atmospheric CO2 levels as the world exits the Ice Age (in combination with other factors such as ocean exsolution)? Could this contribute to the observed asymmetry?
When all life on Earth comes to an end, will it be because CO2 permanently falls below 200ppm as it is permanently sequestered in carbonate rocks, hydrocarbons, coals, etc.?
Since life on Earth is likely to end due to a lack of CO2, should we be paying energy companies to burn fossil fuels to increase atmospheric CO2, instead of fining them due to the false belief that they cause global warming?
Could T.S. Eliot have been thinking about CO2 starvation when he wrote:
“This is the way the world ends
Not with a bang but a whimper.”
Regards, Allan 🙂
Allan, the fact that CO2 lags behind temperature in antarctic ice is of course interesting, but it doesn’t mean what you think it means. Interglacial periods during ice ages, occurring about every 100,000 years, are due to slow and regular variations in Earth’s orbit (Milankovitch cycles). Knowing this, why would we expect to see CO2 rise and fall *before* the start and end of an interglacial? The role of CO2 in interglacial periods is primarily that of a feedback effect, increasing the warming caused by increased insolation. This doesn’t mean that, in another context, a change in atmospheric CO2 won’t act as a forcing in its own right.
Allan, you’re really off the deep end when you get to this point: “When all life on Earth comes to an end, will it be because CO2 permanently falls below 200ppm as it is permanently sequestered in carbonate rocks, hydrocarbons, coals, etc.?
Since life on Earth is likely to end due to a lack of CO2, should we be paying energy companies to burn fossil fuels to increase atmospheric CO2, instead of fining them due to the false belief that they cause global warming?”
The answer to the first question is “no”. CO2 won’t ever be permanently sequestered, because the planet is tectonically active and volcanoes continually replenish the CO2 sequestered into rock. On geological timescales, chemical weathering (and other mechanisms of sequestration) and volcanism result in an equilibrium between atmosphere and rock.
This means that the second question is entirely vacuous. As long as the Earth is tectonically active and has an atmosphere, it is 100% certain that life on Earth will not end due to a lack of CO2.
Marcus,
I think you are wrong.
Atmospheric CO2 has been declining for many millions of years, as it is sequestered in carbonate rocks. It is clear that the rate of CO2 sequestration is greater than the rate of CO2 volcanic replenishment, otherwise atmospheric CO2 would not be declining over geologic time, and it is.
Allan, in the very long term you’re right, but the time frame is hundreds of millions, if not billions, of years. As the Earth cools, its geological processes will calm down and yes, inevitably the atmosphere will run out of carbon. There is a slight imbalance, but it is very slight. If the sun dimmed and ice sheets covered the world, chemical weathering would stop and volcanic CO2 would in a few million years melt the ice, and CO2 levels would stop rising. I call that some kind of equilibrium.
In terms of how much of the end of atmospheric carbon should impact our decisions today, and on the timescales relevant to the article we’re commenting on, this is about as important as dealing with the sun turning into a red giant. A curious tangent at best.
Marcus,
I think we could be looking at an instant of geologic time. Even if I am out by a few million years, it still points out how utterly foolish it is to worry about CO2 being too high – it is clearly too low – and CO2 abatement schemes are utter nonsense.
Atmospheric CO2 is inexorably declining as it is being sequestered in carbonate rocks. In the last Continental Last Ice Age, atmospheric CO2 declined to about 180 ppm – next time it could drop lower, even closer to the extinction point of C3 plants at about 150-160 ppm.
It is a bit more complicated – a few plants (less than 1%) use the C4 photosynthesis pathway, including corn and sugar cane – but I doubt terrestrial life could survive for long on Sugar Frosted Flakes – notwithstanding the persistent rumour that “They’re Great!” 🙂
There are also CAM plants, so we can look forward to having pineapple with our Sugar Frosted Flakes.
Regards, Allan
__________________
Dr. Patrick Moore, a co-founder of Greenpeace, has also written on this subject:
https://wattsupwiththat.files.wordpress.com/2016/06/moore-positive-impact-of-human-co2-emissions.pdf
Executive Summary
This study looks at the positive environmental effects of carbon dioxide (CO2) emissions, a topic which has been well established in the scientific literature but which is far too often ignored in the current discussions about climate change policy. All life is carbon based and the primary source of this carbon is the CO2 in the global atmosphere. As recently as 18,000 years ago, at the height of the most recent major glaciation, CO2 dipped to its lowest level in recorded history at 180 ppm, low enough to stunt plant growth.
This is only 30 ppm above a level that would result in the death of plants due to CO2 starvation. It is calculated that if the decline in CO2 levels were to continue at the same rate as it has over the past 140 million years, life on Earth would begin to die as soon as two million years from now and would slowly perish almost entirely as carbon continued to be lost to the deep ocean sediments. The combustion of fossil fuels for energy to power human civilization has reversed the downward trend in CO2 and promises to bring it back to levels that are likely to foster a considerable increase in the growth rate and biomass of plants, including food crops and trees. Human emissions of CO2 have restored a balance to the global carbon cycle, thereby ensuring the long-term continuation of life on Earth.
***********************************
Marcus you wrote:
“If the sun dimmed and ice sheets covered the world, chemical weathering would stop and volcanic CO2 would in a few million years melt the ice, and CO2 levels would stop rising.”
Did you seriously write that or was that satire?
There is NO chance that yours statement is correct – increasing CO2 has NO significant impact on global temperature. ECS is near-zero.
Furthermore, CO2 lags temperature at all measured time scales. Precedence studies prove it is CO2 is primarily an effect, not a cause.
Temperature drives CO2 much more than CO2 drives temperature.
For your hypos to be correct, you need a time machine – the future cannot cause the past.
Allan,
Without invoking the mass balance, there are a few straightforward answers:
1. That CO2 in pre-industrial times (and for current seasonal to year by year variability) lags temperature changes does not exclude a (small) response of temperature to CO2 changes. That is simply a matter of total fortifying effect. As long as the combined fortifying factor is less than unity, there is no runaway effect. Here for an arbitrary effect of T on CO2 and with or without an arbitrary effect of CO2 on T, with a lag of CO2 in both cases:
http://www.ferdinand-engelbeen.be/klimaat/klim_img/feedback.jpg
All what happens is that both end somewhat higher where CO2 clearly has an influence on temperatures, despite that it lags.
2. The current CO2 levels lead with 110 ppmv above the long term T-CO2 equilibrium as established in 800 kyear ice cores and confirmed by over 3 million samples for the ocean surface per Henry’s law. The long term steady state for the current (weighted) average ocean surface temperature is 290 ppmv, not the measured 400 ppmv:
http://www.ferdinand-engelbeen.be/klimaat/klim_img/antarctic_cores_001kyr_large.jpg
Similar changes over the past ~200 years are available for CH2 and N2O and δ13C changes, which all point to the human influence.
Still short term T variability leads short term CO2 changes over seasons to 1-3 years, but that is not more than 1.5 ppmv (4-5 ppmv/K) noise around the 110 ppmv trend caused by human emissions.
3. The laboratory measured influence of CO2 on T is ~1 K for 2xCO2 (at 280 ppmv base). The real life effect may be lower or higher, but you can’t say it is (near) zero, if you have no proof.
Thank you Ferdinand, and greetings.
Ferdinand you wrote above:
“1. That CO2 in pre-industrial times (and for current seasonal to year by year variability) lags temperature changes does not exclude a (small) response of temperature to CO2 changes. That is simply a matter of total fortifying effect. As long as the combined fortifying factor is less than unity, there is no runaway effect.”
I generally agree up to this point, My conclusion is that “temperature, among other factors (which could include fossil fuel combustion, deforestation etc.), drives atmospheric CO2 much more than CO2 drives temperature.” And yes, both temperature and atmCO2 could be driving each other, but it is absolutely clear that temperature is dominant over atmCO2.
I cannot understand your first plot, which suggests about 2 time units (what are they?) of CO2 lag after temperature. I am aware of a ~9 month lag of atmospheric CO2 after LT global temperature, and a ~13 month lag of CO2 after Nino 3.4 SST. I know of no lag of 2 time units, whatever units they are.
Ferdinand you wrote above:
“2. The current CO2 levels lead with 110 ppmv above the long term T-CO2 equilibrium as established in 800 kyear ice cores and confirmed by over 3 million samples for the ocean surface per Henry’s law. The long term steady state for the current (weighted) average ocean surface temperature is 290 ppmv, not the measured 400 ppmv:”
Point 2 can be confusing. You say that there is a QUANTITATIVE lead of atmospheric CO2 ahead of “current (weighted) average ocean surface temperature (of) 290 ppmv”. Let’s assume you are correct, but that does not change the ~800 year lag of atmospheric CO2 after temperature in the same ice core records. So OK, provided the conditions in my previous sentence are included. You are saying there is a strong correlation between the increase in fossil fuel combustion and the increase in atmCO2 for the past ~150 years. OK again, as I wrote above in point 1 – fossil fuel could be the primary driver, as could deforestation – huge areas of forest land in North and South America were cleared in the past 150 years and deforestation continues today in South America and Asia. But maybe you are correct and fossil fuel combustion is dominant. Whatever the cause, the atmCO2 increase is entirely beneficial to humanity and the environment, and CO2 abatement and sequestrations schemes are costly nonsense.
Ferdinand you wrote above:
”3. The laboratory measured influence of CO2 on T is ~1 K for 2xCO2 (at 280 ppmv base). The real life effect may be lower or higher, but you can’t say it is (near) zero, if you have no proof.”
I understood the “laboratory influence” estimate of [ECS = ~1K/(2xCO2)], but it is small-scale, theoretical, and ignores all the greater factors that drive temperature.. The reason I think ECS is much less than 1C, say [0C +/- 0.3C], is because of several observations:
There is the lag of CO2 after temperature, at all measured time scales.
We have a full-scale test of the hypothesis occurring right now on this planet – an Earth–scale test is more meaningful than lab-scale or physical arguments. While fossil fuel combustion and atmospheric CO2 both increased strongly since about 1940, global temperatures decreased from ~1940 to ~1975, increased to ~2000 and has been flat since – so there is a negative correlation of temperature with CO2, a positive one, and a zero one.
The evidence suggests that “ECS = near-zero” is the correct answer – CO2 is NOT a significant driver of global temperatures and the alleged global warming (CAGW) crisis is false.
Earth-scale tests trump lab tests. There is NO credible observational evidence that increasing atmospheric CO2 has a significant impact on global temperature.
The global cooling that occurred from ~1940 to ~1975, during the time that fossil fuel combustion (and allegedly atmospheric CO2 concentration) strongly accelerated, essentially DISPROVES the catastrophic humanmade global warming (“CAGW”) hypothesis.
Consider IF we had a similar situation starting about now:
Hypothetically, let’s say from 2020 to 2055 there was continued fossil fuel combustion and a significant increase in atmospheric CO2, and yet average global temperature cooled by ~0.5C.
We would conclude that ECS is near-ZERO and that the CAGW hypo is false.
But this has already happened, which is why the warmists have “adjusted” the temperature record in order to minimize this ~35-year past cooling.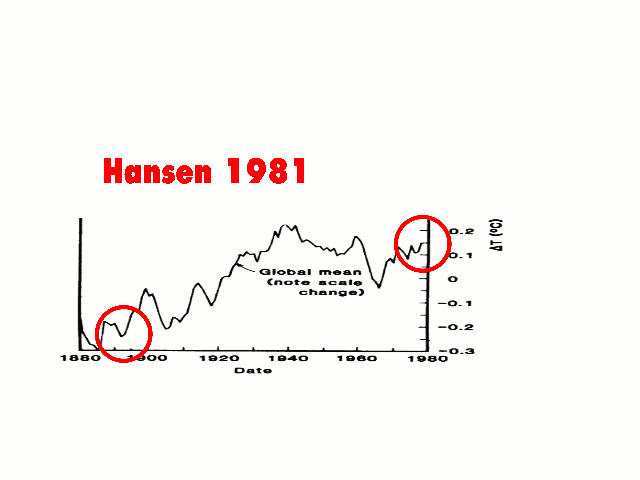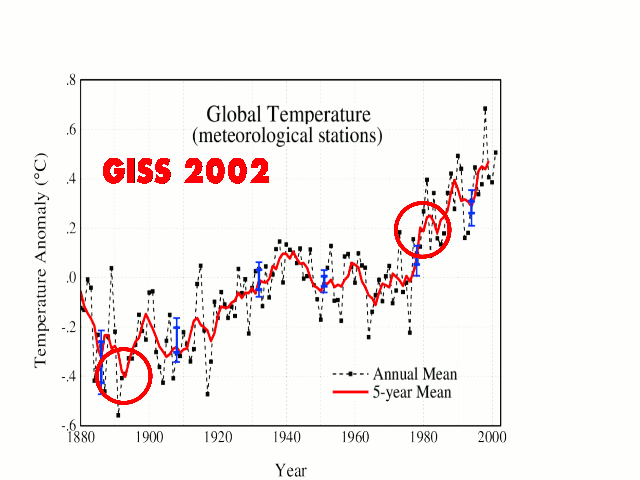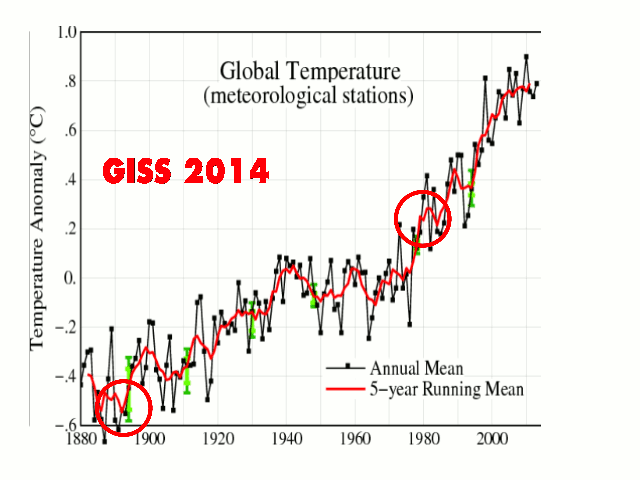
In fact, we already have a good bound on the magnitude of ECS, which is NEAR-ZERO, and strong evidence that the CAGW hypothesis is false. Do we need another 35 years of data to reproduce this same result? We don’t.
Best personal regards, Allan
nawww…they are just saying the oceans are too clean
dirtier oceans….more phyto
“However, in the modern Southern Ocean, there is a limited amount of iron–which means that there are not enough phytoplankton to fully consume the nitrogen and phosphorus in the surface waters.”
I was under the impression that huge animals like whales thrived down there and consume large amounts of “Krill” which ( to me ) feed on life like phytoplankton to thrive. Frankly, I am really confused by this .
Don’t they know CO2 is a regulated substance? Geez, get with the political advocacy program.
“Adkins and his colleagues collected coral from the relatively narrow (500-mile) gap known as the Drake Passage between South America and Antarctica…” Climate work aside, I’m guessing they have some interesting WEATHER stories from their time in this area…
I cannot find this article in PINAS. Can someone please send me precise details? Thank you.
http://www.pnas.org/content/early/2017/03/14/1615718114
There’s no “I” in it.
The simplest explanation for a lower atmospheric CO2 level during a period of lower temperatures is that CO2 solubility in oceans increases as temperature decreases and conversely solubility decreases as temperature increases, inceasing the atmospheric CO2 level. Doubt whether biological ocean processes have any significance to this.
Yes. And supported by the CO2 lag time. And the Southern Ocean is not semi barren as hypothesized. See comment below.
Alan and Ristvan,
Does this research give a (contadicting?) answer to the strange difference in lags between a glaciation and a deglaciation?
If the (deep) oceans are warming, CO2 lages with 800 +/- 600 years.
If the (deep) oceans are cooling, CO2 lags with several thousands years (up to 5000 years after the Eemian).
CO2 from warming oceans simply will follow the warming deep ocean turnover time, while during cooling one would expect a similar lag, but that is not the case and the above findings make it even more contradictory…
Here’s an interesting quote from an article on Adkins research done in 2014-
“The researchers found that the deep ocean started warming before the start of a rapid climate change event about 14,600 years ago in which the last glacial period—or most recent time period when ice sheets covered a large portion of Earth—was in the final stages of transitioning to the current interglacial period.
‘We found that a warm-water-under-cold-water scenario developed around 800 years before the largest signal of warming in the Greenland ice cores, called the ‘Bølling–Allerød,'” explains Adkins. “CO2 had already been rising in the atmosphere by this time, but we see the deep-ocean reorganization brought on by the potential energy release to be the pivot point for the system to switch from a glacial state, where the deep ocean can hold onto CO2, and an interglacial state, where it lets out CO2.’ ”
Read more at: https://phys.org/news/2014-07-corals-clues-climate.html#jCp
Sounds like Adkins might be a real scientist after all. Ocean warming started BEFORE the rapid climate change (surface) events, 14,600 years ago…the pivot point for switch from glacial to interglacial….in which the oceans LET OUT CO2.
And this quote from the above article-
“The overall goal of the work is to better understand how and why the earth goes through periodic climate change, which could shed light on how man-made factors could affect the global climate.”
Understanding how and why the earth goes through periodic climate change….what a perfectly rational, incredible, intelligent thing to have as one’s goal!!! And understanding that “COULD” shed light on how man-made factors “COULD” affect the global climate…??? YES! It COULD…not the obligatory WILL. It might just be me, but I sense “skeptical code” being written into the text of this man’s work. Kind of like morse code blinked during filmed interrogations…:)
Ferdinand…here is a possible additional explanation for the lag other than colder waters being more soluble. This popped into my mind as I read the post. As the oceans recede from the onset of glaciation then exposed coastal sediments which had been under water will dry out and then be readily carried by local surface winds strong or normal. Plus the high tides will also sweep the exposed dry material out into the currents to be carried out to where they would feed increased levels of iron to the phytoplankton. Thus as the sea levels drop the food available is slowly and steadily increased which means more CO2 being deposited out of the system as the creatures die. The reverse would also then hold true as rising sea levels would steadily shut off the food supply. The south coast of Australia {iron rich?} and points east which would rise out of the receding waters would be a potential main contributor as well as the southern tips of South America and Africa. Possible?
Reading one of these articles is like reading a novel. Well maybe a historical novel where certain facts are known and everything else is extrapolated. Sometimes even the facts aren’t real. Unelievable. And then the stories are repeated so many times they seem to become reality. It’s fiction fiction fiction. But we all ready know that on this forum.
Color me very skeptical. The Southern ocean in not barren. Its seasonal pH fluctuation is 0.5. By comparison, the barren Station Aloha (where ‘acidification’is detectable because there is little biological pump) season variation is 0.5. Maybe it is even more fertile during glaciation, but the observation still wrecks the 14N/15N metric.
It seems pretty clear from other observations that the main glacial/interglacial CO2 driver is simply Henry’s law. The well established lag between d18O temperature inference and CO2 concentration in ice cores is ~800 years, temperature leading. Not coincidentally, that is the time frame for one complete thermohaline circulation. Dissolved CO2 sunk into the abyss as a consequence of winter sea ice formation, slat exudation, and sinking cold extrasaline water cannot reenter the atmosphere until it re-emerges in surface waters
The Southern Ocean is home to the animal species with the greatest biomass on earth, exceeding humans: Antarctic krill.
http://www.coolantarctica.com/Antarctica%20fact%20file/wildlife/krill.php
A fantastically successful species.
http://www.coolantarctica.com/Antarctica%20fact%20file/wildlife/krill1.jpg
“Adkins and his colleagues collected coral from the relatively narrow (500-mile) gap known as the Drake Passage between South America and Antarctica (among other places). Because the Southern Ocean flows around Antarctica, all of its waters funnel through that gap–making the samples Adkins collected a robust record of the water throughout the Southern Ocean.”
Pity we have no quoted details of the proportion of samples collected here and how these samples differed from samples collected elsewhere, since one could reasonably argue that the Drake Passage is a somewhat atypical area of the Southern Ocean with exceptional extreme conditions which, given the narrower gap probably existing in colder periods due to increased sea ice, water locked up as ice on land and resultant lower sea levels , possibly produced even more atypical conditions to those found in other areas.
John, UK, I’d like to add to your comment. The lack of samples is one thing but I also think that the “flushing” effect in the Drake Passage. leads me to believe that sampling time frames may make a large difference. I mean that as the circulation through the passage was sampled was that during spring , summer or fall? .
If the oceans are 270x the mass of the atmosphere, wouldn’t that mean a 270ppm increase in our atmosphere should only correlate to a 1ppm increase in concentration in the ocean once equilibrium is reached? And if that’s the case, how is that going to acidify the oceans as much as they’re predicting?
Not how it works. Henry’s law requires the partial pressures of atmospheric and dissolved oceanic gasses equilibrate. The dissolved partial pressure is water temperature dependent (colder holds more gas, so for a given volume of dissolved gas colder means a lower dissolved partial pressure so will absorb more. 270 times more ocean mass just says 270 times more in the ocean than in the atmosphere.
How much would a 120 meter lower ocean change the atmospheric pressure on the surface ?
Almost nothing at all. But itnwould change exposed land. The Sundra Strait would be dry land. The area of Australia would be larger (the entire GBR woild be dry land). And that would affect Southern Ocean iron fertilization, since its iron fertilizer comes mainly from wind borne Australian dust.
http://austhrutime.com/last_glacial_maximum_australia.htm
@ Robertvd…thanks for the link. I have recently become interested into expanding my understanding of how the Southern Hemisphere fits into the global climate picture. A conversation with a NZer on FB made me wonder if the Maoris had any myths/legends from their past which might add insights to how the SH behaves during shorter term climate changes in relation to the NH, such as during GSM events or the longer term D.O or Heinrich events. There were some intriguing possibilities from that. I also read a post at Climate etc recently which had been written by Javier, and which sparked some connections in the picture which I see of the climate system.
Pete,
As ristvan already said, that is a matter of equilibrium, not of mass. It doesn’t make a difference if you shake a 0.5, 1.0 or 1.5 liter bottle of Coke from the same batch at the same temperature: the pressure under the screwcap will be (practically) the same.
It makes a difference if one (volcanoes, humans) add some extra CO2 into the atmosphere beyond the equilibrium. That will ultimately redistribute between atmosphere and deep oceans in a 1:50 ratio (that is the difference in CO2 mass, not the water mass).
Our emissions of ~400 GtC since 1850 indeed will give a ~1% increase in the deep ocean CO2 (and derivatives), which leads to ~1% increase in the atmosphere or ~3 ppmv.
The main point is how much time the redistribution will need.
According to the IPCC ranging from a few years to many millennia for different compartiments with different saturation levels.
According to real life measured sink rates, about 35 years half life time, no saturation in sight,
Thus if we cease all emissions today, half of the extra 110 ppmv in the atmosphere will be gone in 35 years, 3/4 in 70 years,… Much shorter than the IPCC expects with their Bern model…
FE, agree again. The Bern model assumes saturating sinks. We know experimentally that is NOT true for oceans. Support data: North Atlantic coccolithophores have increased 10x in abundance last 30 years. (Coccoliths are a semipermanent sink as the form a carbonate exoskeleton- think White Cliffs of Dover chalk.)
Ferd Engelbeen, I always read your comments because I learn things.
Thanks millions for all your posts and comments here.
Same goes for you, Rud.
Thanks Pop!
Always trying to find out things for myself first and then trying to explain that to others in an understandable way. Not always that simple for highly technical subjects…
The mass of CO2 has to partition into the the oceans from the atmosphere to establish equilibrium, however, so while i get what you are saying, mass balance must be achieved, i.e., total CO2 mass = ocean volume x ocean [CO2] + atmosphere volume x atmosphere [CO2], the mass in each phase will be dependent on the ratio of the phases, ignoring dynamic sinks/sources.
Typically the concentrations are expressed as mass/unit volume because of the volume ratio relationship but of course can this is all interchangeable given the phase densities. The distribution coefficient is temperature dependent as per Henry.
Of course these physical processes are not so easily modeled when life is thrown into the mix and this wouldn’t be a topic of discussion if that were not the case.
But, what is the “correct” equilibrium?
@Rud Istvan, “We know experimentally “, I hope they aren’t models 😉
Jeff Alberts,
Based on Henry’s law, the current equilibrium is around 290 ppmv for the current (area weighted) ocean surface temperature. Confirmed by over 3 million on the spot seawater samples. Including the biological pump and working as good for one sample in a closed bottle as for the full dynamics of the total ocean surface…
The remarkable point is that despite the variability of all the different ins and outs like the biological changes, 1991 Pinatubo, 1998 and other El Niño’s,… the net sink is in average highly linear in ratio with the extra pressure of CO2 in the atmosphere above that equilibrium.
“Based on Henry’s law, the current equilibrium is around 290 ppmv for the current (area weighted) ocean surface temperature.”
Nonsense. The oceans are not a static pool of water, and you cannot determine the equlibrium level based on short term observations. The oceans hold vastly more CO2 than the atmosphere, and equilibrium cannot occur until the entire distribution from the surface to the depths has adjusted itself to static forcing conditions.
Bart,
As I have shown you many times, Henry’s law shifts the steady state with exactly the same level (16 ppmv/K) for the whole dynamic ocean – atmosphere system as for a single sample. That is confirmed by 800,000 years of ice core CO2 measurements and temperature proxies.
It doesn’t matter one ppmv if you take a seawater sample within a closed flask, shake it and measure CO2 in the above air or if you measure CO2 in the global atmosphere above the oceans: for the same average temperature of the ocean surface, the same CO2 pressure in the atmosphere will be measured at steady state, no matter how much CO2 is exchanged between the atmosphere and the deep oceans.
The only part where the (much colder) deep oceans play a role is when for any reason the steady state shifts (with ~16 ppmv/K) due to temperature changes of the ocean surface or when an external source (volcanoes, humans) inject additional CO2 into the atmosphere above the steady state. That is redistributed between the atmosphere and the deep oceans with a half life of 35 years. Again confirmed by observations over the past 57 years…
Based on ocean surface temperature, emissions, CO2 level in the atmosphere above steady state and the above 35 years half life time, one can calculate the increase in the atmosphere, which is midst the temperature caused noise in vegetation uptake:
http://www.ferdinand-engelbeen.be/klimaat/klim_img/dco2_em6.jpg
You have not “shown” any such thing. You have merely asserted it. And, you are wrong. Trivially so. You cannot establish equilibrium across the entire depth and height of the oceans in a matter of just a few years.
Bart,
You cannot establish equilibrium across the entire depth and height of the oceans in a matter of just a few years.
The temperature – CO2 steady state is between ocean surface and the atmosphere and has an equilibrum rate half time of less than a year. That is where Henry’s law works. Not for the deep oceans. If that was the case, the CO2 levels in the atmosphere would be around 180 ppmv at average 5°C deep ocean temperatures, as they were during glacial times (or worse for C3 plants).
Temperature changes in the ocean suface need ~800 years to influence the deep oceans and as result CO2 levels followed the deep ocean changes with the same lag. Still with ~16 ppmv/K. Some 100 ppmv in 5,000 years or 0.02 ppmv/year. That is the CO2 response speed of the deep oceans to temperature changes at the surface. Not really fast.
Our emissions are currently some 4.5 ppmv/year or 200 times faster.
There isn’t any indication that the deep oceans are the cause of the current increase in the atmosphere, to the contrary, they are a large part of the ~35 years half life time of the current excess in the atmosphere above steady state…
Bart, can you tell us where in upwelling ocean water has the concentration of CO2 been measured as being higher than surface concentrations?
Ferdinand:
“Still with ~16 ppmv/K.”
Incorrect. There is a continual influx of CO2 from upwelling waters. If it does not downwell at precisely the same rate, it will accumulate at the surface. This is a complex time varying transport problem, and your model is hopelessly simplistic.
David:
It’s not a question of it being higher. It is a question of dynamic balance between inflow and outflow to the surface system in response to changing boundary conditions. I presented a toy model to describe what is happening here.
Bart, you can take your “model” and stick it where the sun don’t shine. If you are claiming that the CO2 increase in the atmosphere is coming from upwelling ocean waters, please provide us with physical measurements of the concentration of CO2 in upwelling water. Until you provide concrete data, your supposition/conjecture is mere speculation. You should know better, actual measurements supersede any “model” you posit.
Bart doesn’t have to give you measurements. It’s a mathematical, known formula. Deep ocean cold water being brought to the surface by upwelling currents is rich in CO2. The closer it gets to the surface, the more it warms, the more it warms, the less CO2 it can retain. That released CO2 has to go somewhere. ‘
“CO2 solubility in water: 0.08g/kg/degree C below 20C. Above 20C the solubility drops by half to about 0.04g/kg/°C.
The ocean surface area is 360 million sq. kilometers, or 360 trillion sq. meters. The top meter is 360 trillion tons of water. A change of temperature of one degree for cold water changes the solubility by:
360 X 1012 tons = 360 X 1015 kg X 0.08 g/kg = 28.8 X 1015 g CO2 or 28.8 Petagrams CO2. A tenth of a degree temperature change changes solubility by 2.88 Petagrams CO2. This is about 780 Gigatons carbon equivalent. (3.7 grams CO2 = 1 gram carbon.)”
– See more at: http://notrickszone.com/2013/10/08/carbon-dioxide-and-the-ocean-temperature-is-driving-co2-and-not-vice-versa/#sthash.tOjHIvfD.dpuf
Dirk asked-
“Bart, can you tell us where in upwelling ocean water has the concentration of CO2 been measured as being higher than surface concentrations?”
It’s been mapped a lot. Google “mean annual sea-air flux” and “maps”
http://www.ldeo.columbia.edu/res/pi/CO2/carbondioxide/image/annfluxgmm2u2windmap.jpg
http://www.pmel.noaa.gov/pubs/outstand/feel2331/images/fig06.jpg
Aphan, you say that ” It’s a mathematical, known formula. ” But Aphan, mathematics is not science. Then you say, “Deep ocean cold water being brought to the surface by upwelling currents is rich in CO2.” All I ask you is for proof of this statement with physical measurements. Without said physical measurements, your statement is simple conjecture.
…
Then Aphan posts maps. They are labeled “Air-Sea Flux.” Those maps do not measure the concentration of CO2 in THE UPWELLING WATER Please post a map of the requested data, not something you think is relevant. What one would need is CO2 measurements at surface, and 100 meter increments BELOW the surface. Then one would highlight areas of upwelling and downwelling movement of sea water.
….
Until you can provide this data, the conjecture that the source of the rise in atmospheric CO2 from upwelling sea water is pure conjecture. Your “maps” don’t help.
David Dirkse-
You ignored this previously-Its a known FACT that colder water holds more CO2 than warmer water.
“CO2 solubility in water: 0.08g/kg/degree C below 20C. Above 20C the solubility drops by half to about 0.04g/kg/°C.
“The ocean surface area is 360 million sq. kilometers, or 360 trillion sq. meters. The top meter is 360 trillion tons of water. A change of temperature of one degree for cold water changes the solubility by:
360 X 1012 tons = 360 X 1015 kg X 0.08 g/kg = 28.8 X 1015 g CO2 or 28.8 Petagrams CO2. A tenth of a degree temperature change changes solubility by 2.88 Petagrams CO2. This is about 780 Gigatons carbon equivalent. (3.7 grams CO2 = 1 gram carbon.)”
Temperature changes, change CO2 concentrations in ocean water. It’s KNOWN. Well, except apparently by you.
https://www.liverpool.ac.uk/~ric/lfs/pdf/climatedynamics_2016_lwmm.pdf
“Increased residual upwelling drives elevated atmospheric CO2 at a rate of typically 1–1.5 parts per million/10 6 m3 s−1, by enhancing the communication between the atmosphere and deep ocean. This metric can be used to interpret the longterm effect of Southern Ocean dynamics on the natural carbon cycle and atmospheric CO2, alongside other metrics, such as involving the proportion of preformed nutrients and the extent of sea ice cover.”
And this article-
http://oregonstate.edu/ua/ncs/archives/2008/may/new-study-finds-increasing-acidification-pacific-ocean%E2%80%99s-continental-shelf
“During the past 50 years, atmospheric CO2 levels have gradually increased to a level of about 380 parts per million. (2008)
…These atmospheric CO2 levels form the beginning baseline for carbon levels in ocean water. As water moves away from the surface toward upwelling areas, respiration increases the CO2 and nutrient levels of the water. As that nutrient-rich water is upwelled, it triggers additional phytoplankton blooms that continue the process.”
“The research team used OSU’s R/V Wecoma to sample water off the coast from British Columbia to Mexico. The researchers found that the 50-year-old upwelled water had CO2 levels of 900 to 1,000 parts per million making it “right on the edge of solubility” for calcium carbonate-shelled aragonites, Hales said.”
Do you get that? Water that 50 years prior to 2008 ago was roughly 310 ppm at the surface, sank, became more enriched with CO2, and 50 years later when it “upwelled” back to the surface, it had CO2 levels of 900-1,000 ppm.
Do you grasp that if the 2008 amount of CO2 in our atmosphere was 380 ppm, that there is NO WAY that the surface water measured by researchers that had CO2 levels between 900-1000 ppm could POSSIBLY have come from the atmosphere into the water? It had to be sequestered CO2 coming UP from the water? (Because air doesn’t sequester CO2)
Oh, and by the way, those MAPS, represent areas where the OUTGOING CO2 from the ocean exceeds the INCOMING CO2, or the incoming CO2 exceeds the outgoing CO2 from the atmosphere every year. I’m just SURE that NOAA and NASA etc have ways to actually MEASURE that for you. They also define the word flux for you.
https://wattsupwiththat.com/2012/07/23/new-research-in-antarctica-shows-co2-follows-temperature-by-a-few-hundred-years-at-most/
“He explains that one of the theories is that when Antarctica warms up, there will be stronger winds over the Southern Ocean and the winds pump more water up from the deep bottom layers in the ocean where there is a high content of CO2 from all of the small organisms that die and fall down to the sea floor and rot. When strong winds blow over the Southern Ocean, the ocean circulation brings more of the CO2-rich bottom water up to the surface and a portion of this CO2 is released into the atmosphere. This process links temperature and CO2 together and the new results suggest that the linking is closer and happens faster than previously believed.”
(Article can be read here-http://www.clim-past.net/8/1213/2012/cp-8-1213-2012.html)
Aphan,
The answer is provided by the same source as for the CO2 exchange maps:
http://www.pmel.noaa.gov/pubs/outstand/feel2331/exchange.shtml
DIC (= CO2 + bicarbonates + carbonates) levels in tropical waters around 1850 µmol/kg in polar waters around 2150 µmol/kg.
While the upwelling waters initially are richer in CO2 (+ derivatives), the upwelling is cooler and contains lots of nutrients which cause abundant life and drawdown the total C content of the oceans at the surface in the upwelling zones. As there is further warming of the upwelling waters at the equator, another part is going into the atmosphere.
Near the poles, the opposite happens and CO2 from the atmosphere is pushed into the oceans. The balance between these two is what changes CO2 in the atmosphere, not the height of the fluxes either way,
Based on the bomb tests 14C decay rate and the dilution of the human δ13C “fingerprint” from fossil fuel use, the global CO2 flux between the upwelling and downwelling sites is ~40 GtC/year. Based on the pCO2 difference measurements between atmosphere and ocean surface from the above source, the deep oceans absorb ~3 GtC/year more near the poles than they release from the upwelling zones near the equator. Thus Bart is completely wrong on this.
Over the past 800,000 years, the steady state (dynamic equilibrium) between ocean surface (inluding upwelling and absorption by the deep oceans) was ~16 ppmv/K. Currently we are 110 ppmv above steady state for the current average ocean surface temperature.
If you warm the oceans everywhere with 1 K, that gives a temporary increase of 16 μatm CO2 pressure (pCO2) in the ocean waters. That increases the outflux at the upwelling zones and decreases the uptake at the polar zones. The net effect is an increase of the CO2 level/pressure (pCO2) in the atmosphere. When the pCO2 pressure in the atmosphere increased with 16 μatm (~= ppmv), the driving force for any CO2 exchange (that is the ΔpCO2 between ocean surface and atmosphere) is restored everywhere and the original in and out CO2 fluxes again are the same as before the temperature increase. Only the CO2 level in the atmosphere increased for all the dynamics of the global oceans as good as for a single static sample per Henry’s law:
http://www.ferdinand-engelbeen.be/klimaat/klim_img/upwelling_temp.jpg
Thus the increase of 110 ppmv is not caused by temperature or more or less upwelling.
BTW, your solubility parameters are for fresh water. In seawater free CO2 is only 1% of all carbon species and seawater can contain far more CO2 in form of bicarbonates and carbonates. The difference between polar and equatorial seawater in total carbon species is not comparable to CO2 only (99%) in fresh water.
If you give a reference to NotricksZone, please also read the comments, I had lot of comments there…
that gives a temporary increase of 16 μatm CO2 pressure (pCO2) in the ocean waters
Of course the increase in pCO2 is not temporary, it is permanent as long as the temperature increase of the ocean surface is there. What is temporary is the increased pCO2 difference between the warmer ocean surface at the equator and the atmosphere and the decreased pCO2 difference between the atmosphere and the warmer polar ocean surface. That leads to more CO2 release at the equator and less uptake near the poles, until the atmospheric pCO2 increased with the same pCO2 of 16 μatm/K as the oceans warmed.
FE,
I’m not responding to Bert’s entire argument. Merely the part I endorsed and that David Dirkse demanded proof of:
I had said: “Deep ocean cold water being brought to the surface by upwelling currents is rich in CO2.”
DAVID replied: “All I ask you is for proof of this statement with physical measurements. Without said physical measurements, your statement is simple conjecture.”
It’s absolutely known, and stated, in almost every scientific paper that talks about “upwelling”.
My entire response to David is based on his disbelief that (colder)upwelling ocean water contains more CO2 than (warmer) ocean surface water does. AND that we have maps SHOWING where surface ocean waters have increased amounts of CO2 due to upwelling.
So when David said;
“Bart, can you tell us where in upwelling ocean water has the concentration of CO2 been measured as being higher than surface concentrations?”
I posted the maps. If not from upwelling, CO2 rich deep water, where exactly does David think the “higher than normal surface amounts of CO2” in those areas comes from? Aliens? CO2 balloons falling out of the sky? Floating CO2 concentrators?
Bartemis, Bart, not Bert. Autocorrect demons.
Aphan, you post: “I’m not responding to Bart’s entire argument.” which is where you’ve made your mistake. Bart rejects the mass balance argument, and contends that the increase in atmospheric CO2 is coming from the oceans. He can’t provide evidence of that claim. Ferdinand has spent countless hours arguing with Bart, to no avail. I suggest you in fact respond to Bart’s entire argument, so that we know where you stand on the source of the increasing CO2 in our atmosphere. Remember, that for every cubic meter of upwelling water, there is a cubic meter of downwelling water….all you need to do is show that the total out-gassed CO2 is greater than Ferdinands calculations.
David-
“Remember, that for every cubic meter of upwelling water, there is a cubic meter of downwelling water”
Remember, that every cubic meter of downwelling water is often (if not always) a different temperature and chemical/nutrient make-up than every cubic meters of upwelling water. This isn’t about volumes of water trading places. It’s about how the components in those volumes react to pressure changes, depth changes, temperature changes, salinity changes, and the difference between being in the air/ocean boundary vs out of it.
“all you need to do is show that the total out-gassed CO2 is greater than Ferdinands calculations.”
You’ve moved the goalposts. That is not the original statement(s) you made, to which I responded. 🙂
Humans are definitely adding CO2 to the atmosphere. But the idea that the amount of CO2 that natural components add and remove from the atmosphere never, ever changes is ridiculous.
The goalposts have not moved, we’re discussing Bart’s argument, and you still have not responded to Bart’s entire argument. You post: “But the idea that the amount of CO2 that natural components add and remove from the atmosphere never, ever changes is ridiculous.” That is not what is being “argued” (which by the way is how YOU are moving those darn goal posts. What is being argued is that Bart’s entire argument is not supported by real data.
David,
Here’s how I communicate. I say specific things, usually very logically and clearly. I expect other people to do the same thing, unless or until they prove they are not clear, logical people. If I respond to one statement, out of many, it is irrational and illogical to assume that I was referring to, alluding to, supporting or disagreeing with something other than what I said. You insinuating that I NEED to respond to Bart ‘s entire argument is your opinion. Not mine.
I responded to YOUR unreasonable demand for proof of a given, specific, known fact.
I said cold upwelling water contains more co2 than warmer surface waters. It’s a known characteristic of ocean water. You replied I’d have to prove it. Already been done scientifically. FE even agrees in this thread. You asked Bart WHERE this rich in CO2 water shows up at higher values than regular air. I showed you WHERE. I said ZERO about, nor even hinted, that such realities prove that human CO2 is not causing an increase in the atmosphere. THAT is fact.
You asked where I stood on atmospheric CO2 concentrations, at which point I clarified my stance. LOGICALLY, that alone should have given you reason to know I DO NOT agree with Bart’s entire theory. BUT, since you don’t seem to believe in the chemical composition of upwelling deep ocean waters vs surface water composition, then WHERE does the obviously measured and observed higher CO2 concentration in certain areas of the oceans COME FROM??
Goal post moving:
My original statement: “Deep ocean cold water being brought to the surface by upwelling currents is rich in CO2.”
You replied: “All I ask you is for proof of this statement with physical measurements. Without said physical measurements, your statement is simple conjecture.”
NOW you day “all you need to do is show that the total out-gassed CO2 is greater than Ferdinands calculations.”
Goal post moved.
Again, you seem to have presumed, assumed I was defending something I never stated, or even insinuated that I was. And now you want me to change my level of participation to the level you demand. Sorry. Just not that interested.
Yes, Pete, basically that’s right. Chemistry is a proportionate science, which is why the frauds who try to infest it with their bullsh** are so swiftly trapped and ridiculed out of the field.
‘colder climates experience stronger winds’ Why ?
Wind results from a pressure gradient, flowing from higher pressure ( a fair weather ‘high) to lower pressure (a stormy ‘low’). So wind flows ‘downhill’ across pressure isobars on a weather map. Colder average temperate regions have the ability to create larger pressure gradients due to greater thermal contrast (think a cold clear winter high working against a cloudy warmer low), and over larger regions. So windier. Warmer more tropical regions can still have strong winds (local thunderstorms, cyclones) but on average are less windy because there are fewer and weaker highs and lows. My personal experience of this is Chicago versus Fort Lauderdale.
But if all the Earth is colder the range between high and low would be the same. From 100 to 60 is the same range as from 85 to 45. The ‘downhill’ flow is the same. So why would wind blow stronger ?
See below.
How does this person state that we know co2 levels drive climate change.
Please supply facts
We know a lot about how carbon dioxide (CO2) levels can drive climate change, but how about the way that climate change can cause fluctuations in CO2 levels? New
The statement, “We know a lot about how carbon dioxide (CO2) levels can drive climate change…” is code for “We are not Climate Deniers™, so please continue reading and/or please continue the funding.”
‘colder climates experience stronger winds’ Why ?
One distinction:
It’s probably not ‘colder climates’ but rather ‘ice age climates’ that experience stronger winds, for two reasons that come to mind:
1. The ‘katabatic winds'( winds that came from cold air masses ‘falling off’ from the tops of the kilometers high glacial sheets. These things still occur in Antarctica, and they scream.
2. In the larger scale, the ice albedo feedback, coupled with the orbital influence meant a much greater pole-to-equator radiative as well as thermal gradient. The thermal gradient is what determines the wind.
This certainly coincides with the dustiness of the glacials.
Now, the glacials were in fact colder climates than present, but it was not the global average temperature, but rather the gradient of temperature that determined the wind.
There is a corollary to this with modern global warming – it’s not the global mean temperature that matters but rather the gradients. Most models indicate a decrease of thermal gradient with global warming. This would appear to be small but the effect is more stable and less violent climate.
Wind speeds:
Jupiter’s Little Red Spot: 384 mph
Saturn’s equator: 1118 mph
Uranus: 560 mph
Neptune: 1500 mph.
Colder generally is windier.
It is purely due to Henry’s law. Were they paid to confirm this?
Certainly not by a soft-drink company.
“We know a lot about how carbon dioxide (CO2) levels can drive climate change,” In reality there is no evidence that CO2 has any effect on climate. There is no such evidence in the paleoclimate record and there is plenty of scientific rational to support the idea that the climate sensivity of CO2 is zero. A most important task for the IPCC has been to make an accurate determination of the climate sensivity of CO2. In their first report, the IPCC published a wide range of guesses as to the climate sensivity of CO2 and in their last report the IPCC published the exact same range. So after more than two decades of study the IPCC has learned nothing that would allow them to narrow the range of their guesses. One researcher has pointed out that the initial radiometric calculations of the climate sensivity of CO2 neglected the fact that doubling CO2 in the Earth’s atmosphere would cause a slight decrease in the dry lapse rate in the troposphere which is a cooling effect. The decrease in the lapse rate reduces the climate sensivity of CO2 by more than a factor of 20. The IPCC also assumes that H2O provides a positive feedback to CO2 but they neglect the fact that besides being the primary so called greenhouse gas, H2O is a major coolant in the Earth’s atmosphere as evidenced by the fact that the wet lapse rate is significantly higher than the dry lapse rate which is a cooling effect. The feedback also has to be negative for the Earth’s climate to have been stable enough for life to evolve. We are here. The radiametric greenhouse effect upon which the AGW conjecture depends has not been observed anywhere in the solar system rendering the AGW conjecture as science fiction.
What if instead of this as an explanation “…likely because colder climates experience stronger winds, which can blow more iron into the Southern Ocean from the continents. …”. That another explanation is that falling sea levels means that offshore coastal sediments exposed by the falling seas dry out, and are then readily carried by local surface winds, and by high tides which will float the dry sediments out into the ocean currents.
That’s the best answer they can give ? Or the only answer they are allowed to give. I wouldn’t have put my name on that report. The first 3 paragraphs are standard climate science meme. I did read the rest, but I really didn’t have to.
First they are wrong about the the lower and upper levels of co2 during ice ages and warming eras.
Second, nowhere did they address the 800 year lag in co2 levels behind temperature.
And third, there are different kinds of plankton with some performing a warmer water and some performing cold. That is easy to see when the climate changes and one group replaces the other in the drilling logs.
I’m sure some warmist will drag it out as proof, but it’s another report for the garbage bin. The only thing that might be useful is the n14 and n15. Much like your body can distinguish between radioactive elements and calcium. Oh look here comes an n14 now. ( sarcasm if you missed it on the useful part )
Before we can consider any of this relevant, we need to have
a reliable CO2 baseline. The OCO 2 satellite shows much lower
CO2 readings at the poles than closer to the equator.
During periods of glaciation, the distance from the major areas of
emission to the core sites would be even greater.
Stomata would be very hard to use because the CO2 emitted by
the topsoil varies greatly. The richness of the topsoil can be a
visual guide, but actual measurements of CO2 readings
proximate to the plant would have to be made to calibrate for
that plant, as has been observed above.
The CO2 readings at ground level would be about the same as they
are now, if you make readings eliminating ambient contribution.
The total CO2 in the atmosphere would have been less because
the amount of soil and ocean exposed would be dramatically
less and Henry’s law would have been at work.
Below are some articles on some of the other problems with
ice core readings.
http://www.kin152.org/climatologie/CO2.pdf
http://www.warwickhughes.com/icecore/
Jerry
“Before we can consider any of this relevant, we need to have
a reliable CO2 baseline. The OCO 2 satellite shows much lower
CO2 readings at the poles than closer to the equator.”
I dont know what OCO-2 images that you refer to, but the 30 sequential images released with NO fanfare by NASA eleven months ago (15th April 2016) show quite the opposite at certain times of the year. From late May to November there is a massive transport of atmospheric bearing CO2 to the southern hemisphere.
They are the most revealing factual account of CO2 accumulation and transport that has been provided. To date there has been NO comment, to them apart from my own efforts. This includes the scientific community, science blogs, and blog commenter’s.
I read the comments above from various individuals in this post and previous regarding CO2 and carbon cycles and wonder what it will take for someone to either.
1, provide a summary of what is happening with OCO2 images – i.e. progress the discussion
2. call them ridiculous with supportive data.
There is also no comment on the accelerating accumulations up to and above 100km altitude
There may be some very valid points made above, but I have difficulty taking them seriously if the visual evidence showing something else is occurring is so compelling. When looking at the rapid and sudden accumulation in the southern hemisphere remember, only 8% of emmisions are in the southern hemisphere, it is winter – the coldest period, and no supporting evidence that the ocean is outgassing or adsorbing.
On a previous post, an outdated original release OCO-2 image was used. So I politely offered the updated one and was condemned for providing a fresh baseline.
I look forward to polite comment.
Kind regards.
Ozone, the first release from OCO 2 was consistent with what I know and believe.
None of the later releases were. I have no proof, but the first was very slow in
coming out.
Ozone
Oct 1 to Nov. 11, 2014 is the photo I referred to. I should have been specific.
https://wattsupwiththat.com/2015/10/04/finally-visualized-oco2-satellite-data-showing-global-carbon-dioxide-concentrations/
Jerry
Go to the link below.
I see that there have been a lot in the sequence released again without any notification at all. For such an important topic globally, I wonder why the low profile. This will bring the total of sequential images to well over 40.
Make them into a slide show that moves quickly for a visualisation.
https://oco.jpl.nasa.gov/galleries/gallerydataproducts/
Also, my own comments using the images here.
http://www.blozonehole.com/blozone-hole-theory/blozone-hole-theory/carbon-cycle-using-nasa-oco-2-satellite-images
They confront most things that were assumed about the carbon cycle.
Regards
Jerry
Fortunately I downloaded the original large size set to Feb 2016 when they were released. They have now been altered to allow for recalibration as the concentrations increase, and the highlights that show atmospheric transport are gone. But the annual transport is evident and explained in my conclusions.
Further work I am underatking will provide greater clarity on a number of issues.
Also have a look at the straight line annual increase in CO2 at the Antarctic surface stations
Ozone
I will contact you through your site.
Bravo ozonebust. This is most interesting.
The data seems to me to be consistent with previously-available data that I viewed years ago. I thought then and I think now that the CO2 concentrations appears to be overwhelming naturally-driven, It seems that natural seasonal CO2 fluxes overwhelm the impacts of fossil-fuel combustion and other human causes, with the possible exception of land use changes like major deforestation.
It seems that we are “busting our humps” trying to insist that what we see is manmade,
Maybe I’m just confused… 🙂
Regards, Allan
Here is my post from 2009:
http://blogs.chron.com/sciguy/archives/2009/09/the_more_i_study_climate_science_the_more_confused.html
We know a lot less about CO2 than we think.
Please examine the 15fps AIRS data animation of global CO2 at
http://svs.gsfc.nasa.gov/vis/a000000/a003500/a003562/carbonDioxideSequence2002_2008_at15fps.mp4
It is difficult to see the impact of humanity in this impressive display of nature’s power (unless you believe that most of our industrial capacity is located in the high Arctic).
Still, annual CO2 concentration keeps increasing at ~1.5ppm/year – even as CO2 fluctuates by up to 16ppm/year in its natural seasonal sawtooth pattern.
Questions:
1. IF atmospheric CO2 declines in the coming years contemporaneous with global cooling (or soon thereafter), what does this demonstrate, if anything?
2. IF atmospheric CO2 continues to increase in the coming years contemporaneous with global cooling, what does this demonstrate, if anything?
3. IF CO2 drives temperature as the IPCC alleges, how is it that the only signal apparent in the data is that CO2 lags temperature by ~9 months? See
http://icecap.us/images/uploads/CO2vsTMacRae.pdf
I still don’t buy this biomass and iron fertilization argument, for atmospheric CO2 reductions.
The problem is that during each interglacial warming period, all that CO2 is released back into the atmosphere within 5,000 years. And the biomass argument fails to explain how CO2 locked into coal, oil, or carbonates, can suddenly liberate itself back into the atmosphere. It is not possible. And until they fully explain how CO2 concentrations suddenly rise again, the whole idea is a non-starter.
The easiest explanation is that cooler oceans absorb more CO2, via Henry’s Law. But for that argument to work we need a mechanism to involve the whole ocean, and not just the surface layers. That simplistic mechanism is not accepted at present, because it is claimed that oceanic overturning is too slow.
But there needs to be a better theory than biomass ‘locking away’ atmospheric CO2.
Ralph
Ralf, the CO2 accumulates and concentrates as depths increase. Very little,
if any comes out of the ocean. Mostly, it precipitates, a great deal of it as CACO3.
Photo of deep pool in link.
https://phys.org/print356288318.html
Oxidized hydrocarbons are the source of the CO2. Accumulation of CO2 in the
atmosphere depends on the ability of hydrocarbons to rise when so much of
the earth is frozen and the oceans are cold and Henry works very efficiently.
Why would oil fields suddenly leak and catch fire, just because the tropics warmed by a few degrees? And remember that this response is almost instantaneous. The world only needs to warm by a polar half degree (a tropical 0.2 degree) and CO2 concentrations start to rise. Are you saying that a 0.5ºc rise in polar temperatures will make oil fields leak and catch fire?
You will need to do better than that.
Ralph
According to CLIMAP during the LGM much of the SE Pacific was as warm or warmer then its is today.
Now if this warming was linked to increasing amounts of blocking over this area during ice ages as l believe. Then not only was there warmers waters, but just as importantly there would also have been a increase in the amount of sunshine falling over this area. Now l believe it this that would have lead to the increase in biomass during the ice ages in the southern oceans.
The scary thing about those graphs is they show the end of the current interglacial is overdue.
http://www.sciencedirect.com/science/article/pii/S0009281916300551
This article is correct in that soil is a source of CO2. The microbes oxidize
the upwelling methane. When methane hits the atmosphere, it does not
sink, it rises. Soil is not a methane sink as the USEPA would have you
believe.
http://www.techtimes.com/articles/17607/20141010/methane-hotspot-in-us-southwest-considerably-underestimated-study.htm
The methane in this photo represents a picture of low/no/oxidized methane
in the dark blue pixels. The lighter blue to red pixels represents unoxidized
methane.
The hotspot near Four Corners is a very arid spot, therefore; there is not
enough moisture in the soil to support the microbes required to oxidize the methane.
Spots in the photo which are yellow have either less methane or less moisture
than is required to support the microbial culture sufficient to oxidize it.
Oxidized hydrocarbons are the main source of CO2.
Volcanoes are a minor source.
“He is now a Simons Foundation Postdoctoral Fellow on the Origins of Life at Caltech.” Yes, I’m also curious on where the current life at CalTech might have originated.
This concept (warming driving CO2 in the atmosphere) was explored by Ohio State’s Professor of Energy Conservation Robert Essenhigh in 2001 in a research Viewpoint: http://researchnews.osu.edu/archive/nowarm.htm
They beclowned themselves in the first paragraph. There has been no definitive proof that CO2 has any more than a tertiary effect on the climate, so they begin with a big nothingburger fail in the first sentence. I am afraid I didn’t read past that. I may go back and finish it when I need a good belly laugh!
when i was very young i discovered a cold beverage holds its co2 and after they warm they go “flat” because the co2 gets released……logical our oceans would work in a similar way…..of course this means the temperature is what drives the co2 levels and matches the actual historic record of co2 LAGGING behind the temperature moves…..strange a layman can use simple basic science and see the truth.
Aw Man there goes my next climatology paper Bill. (Taylor)
I was gonna prove how the hotter it got, the more CO2 a soda held, until it exploded; but only ”soon”
and at the same time, my model program was going to show that
climate math indicated more was coming out,
than was ever put in.
Like the Green House Gas Effect, where a rock can’t be gotten warm enough by heating it with fire,
in a vacuum, with one mode of energy in, and one mode out.
It’s put into a bath of cold nitrogen and oxygen;
and when that still doesn’t get it warm enough,
refractory insulating media are suspended in the bath
so less and less of the light from the fire
gets to the firelight warmed rock.
Which of course makes more and more light come out of the rock,
as less and less light, gets in.
Paleo data shows no relationship between co2 and temp for the last 600 mm years. Look it up. http://www.biocab.org/carbon_dioxide_geological_timescale.html
Here is another possible cause for more plankton growth in the far southern ocean during glaciation.
Glacial periods are associated with low insolation in the far northern hemisphere due to Earth’s orbital cycles. During those times the southern hemisphere is receiving maximum solar insolation. And plankton likes sunlight.
Land vegetation also plays a part in this.
For one thing, the forests die back (which reduces the annual cycle in and out of Carbon) but then ALL that extra C4 grassland replaces the forests.
Grass is actually a more effective Carbon sink because when it dies each winter, a large portion of the Carbon gets stored into the soil. Each acre of grass sequesters about 0.3 tons of Carbon each year (700 pounds).
Take the current extent of grassland and expand that by 500% and how much extra Carbon does the soil pack away each year. Some equilibrium is then reached until the planet warms up again and CO2 levels start going back up and the forests take back the grassland and soak up the Carbon and it re-enters the annual in and out of the Carbon cycle.
No climate scientist is going to get into this because they get fired if they bring it up.
Maybe CO2 is a cause for the 1 or 2 degree ocean warming over the last 100 years. But perhaps… might the 1 or 2 degree ocean warming caused the increased CO2? We need to look at other things like land use change on albedo and heat capacity/transmissivity effects?
“As the sea creatures who consume those sugars–and the carbon they contain–die, they sink to the deep ocean, where the carbon is locked away from the atmosphere for a long time. This process is called the “biological pump.
[…]
That biomass consumes carbon, then dies and sinks, locking it away from the atmosphere”
=====================
This kind of fictional story telling is a legacy of the deeply flawed fossil fable.
Anyone familiar with how life works–people who open their eyes and look, for example–know it isn’t true.
quote:
* * * * * * * *
“This is the freshly dead carcass of a thirty ton grey whale
It’s only been on the bottom for 6 weeks, but already…
* * * * * * * *
But we know that it IS true over long time scales, otherwise we would not have limestone hills and coal deposits.
However, the real question, is can this process work over just 80,000 years? And then go into reverse and release all that CO2 again within 5,000 years. That, is the real problem.
R
OK it’s only a press release, not even an abstract, but as press releases go, it appears to have been written by someone who actually read the paper and more or less understood it (that’s a novelty!)
First they start off with CO2 drives temperature (well of course they had to)
Then they present some good data that is probabaly new, about nitrogen isotopes.
Then they conclude that temperature drives atmospheric CO2.
Wonders will never cease. They are denialists pretending to be warmists. Sheep in wolves’ clothing (or whatever)
Their careers may suffer if their colleagues bother to read past the first line.
Smart rock, you are probably right. However, I’ve reread it 3 or 4 times, if you read it one way, that’s true and another, it sounds like they are hedging, but that could be as you say , sheep in wolves clothing.
Is the 1st 3 paragraphs standard for getting something published ? If so, I’ll copy and paste that.
Is this phenomenon becoming increasingly common? If so, it would imply the crackup of the paradigm.
“It is critical to understand why atmospheric CO2 concentration was lower during the ice ages. This will help us understand how the ocean will respond to ongoing anthropogenic CO2 emissions,”
This (nonsensical) statement shows a complete lack of understanding of how control loops and feedback operate.The author needs to do a little study on either industrial process control or electronics, to gain an understanding of how cause and effect apply to any system involving a feedback loop.
Geologic iron dust isn’t bioavailable. Iron oxide is fairly tough, weathered iron oxide even more. What makes iron bioavailable is the sulfate anion.
Now, about ocean carbon sequestration..the greatest volume of carbon is in methane clathrate. And these deposits derive their stability from hydrostatic pressure, not just temperature. Falling sea levels from continental glaciation would cause dissociation of clathrates which would increase carbon dioxide levels.
Remember, methane clathrates have 10 times as much carbon as all petroleum ever extracted and will be extracted in the future.
Henry’s Law is a Physical Law, not a political consensus of ill-informed opinion that calls itself a ‘law’. For those non-scientists in the room, it means that the warmer the water, the more easily dissolved gases can escape from solution. Conversely, cooling the water allows it to hold more dissolved gases. Temperature is the driver. It does NOT mean that adding gases to water will lower the temperature.
tadchem,
You made me smile even as I considered the irony of you having to state this in writing. 🙂
The reason he has to state it in writing is because the same DemonicRats running the scam also run the Education System,
and they simply stopped teaching children gas mechanics – the simplest phase of matter – when the wackos at the head of the climate bureaus, running the scams, decided they didn’t want to talk about Venus’ instrument-laden probes landing and proving the bullshit story an utter fabrication of ridiculous nature,
and they didn’t want any kids or adults in the education system,
talking about gases and vapors and the LAW that rules over them: the Ideal Gas Law.
Spoken by a long-time atmospheric chemist and applied natural chemist.
Oh I know why, but thank you for elaborating. It’s just that here, in discussions amongst the very smart, and the not so smart, it’s ironic that the most basic principles often get ignored as if they don’t matter.
My study of climate change has been focused on how it influenced the evolution of the horse from 55 to 60 million years ago to present. The most complete book on this subject is German paleoanthropologist, Dr.Jens Lorenz Franzen, whose work has been closely associated with mammalian fossils at the Messel Pit in Germany.
I did not read anything about the size of the glaciers or their movement across the landscape, sometimes moving rocks, sediment, and other debris for hundreds of miles during the discussion of cold periods. The truth is that the surface land during these periods was largely covered with ice, in some places, the ice was nearly a mile and a half thick. As a result of the amount of water on land, the oceans were much shallower, at least around land. Most grazing herbivores used these coastal grasslands for habitat. The modern horse that evolved in N. America around 1.7 mil years ago, has teeth that are specialized to allow grazing of coast grasses as well as more tender ones.
I think it is important when discussing CO2 levels during glacial periods to keep in mind that most of the grass and plant growth is found in coastal grasslands around the continents. These low-lying areas did not get the rainfall that the higher areas where glaciers formed. Areas around the East Coast are surrounded by barrier islands and shallow water lay between the mainland and the barrier islands. In some areas, these coastal grazing lands extended almost to the continental shelf.
I am interested in finding out if these low levels of CO2 could reflect the probability that after long periods of glaciation (or stadials) plus the deposit of rocks, sediment and other debris on top of what was previously top soil could have led to long periods where very little plant or animal life could survive until birds as well as small and midsize animals could deposit seeds. How long a process would this be? And could the barrenness of the land help to explain the low levels of CO2, even after the interstadial period had been in process for a few thousand years?
Scientists believe that the large megafauna survived during the stadials and hot interstadials, and perished during the periods after abrupt, rapid global warming occurred melting sea ice and flooding coastal grasslands. Apparently normally paced (?) transitions between stadial and interstadials during the time before humans arrived in N. America had only led to a few minor migrations and re-emigrations over millions of years. The horse always returned to N. America. This last time, the Spanish just returned him a few hundred years ahead of schedule.
I know that you are interested in CO2 stored in the atmosphere and the concentrations found in the oceans. However, the types of plants and animals that are found in a an area during a particular period of climate history, if the timing can be determined to the same level of accuracy used in other measures might help explain some of the mystery of Co2 as well as what really caused a species who shares 70% of our DNA to disappear. sometime between 10,000 and 7,600 years ago.
I hope that you could consider my question and provide some sense of the likelihood that this could occur.
Christie Finn