
Guest essay by Eric Worrall
Professor Phillip Williamson, NERC Science Coordinator, University of East Anglia has written a long whiny piece in The Conversation, complaining that the British Government didn’t do enough to silence James Delingpole’s criticism that the Ocean Acidification scare is nonsense.
Science loses out to uninformed opinion on climate change – yet again
Ocean acidification is an inevitable consequence of increasing carbon dioxide in the atmosphere. That’s a matter of fact. We don’t know exactly what will happen to complex marine ecosystems when faced with the additional stress of falling pH, but we do know those changes are happening and that they won’t be good news.
The journalist James Delingpole disagrees. In an article for The Spectator in April 2016, he took the sceptical position that all concerns over ocean acidification are unjustified “alarmism” and that the scientific study of this non-problem is a waste of money. He concluded that the only reason that the study of ocean acidification was ever funded at all was because there was insufficient (and decreasing) evidence for global warming and it acted as a “fallback position”.
Having had the role of science coordinator for the UK Ocean Acidification research programme and being involved in relevant national and international projects for around ten years previously, I know such claims – which Delingpole presented as facts – to be false. I also spotted a range of other errors and inaccuracies in his piece.
…
At the end of a long and frustrating process IPSO’s final ruling was published on January 5 and it doesn’t seem we are much further forward. My complaint was rejected on the basis that the article was “clearly a comment piece” and that it was not IPSO’s role to resolve conflicting evidence for contentious issues.
…
The Delingpole article which triggered this complaint;
Ocean acidification: yet another wobbly pillar of climate alarmism
A paper review suggests many studies are flawed, and the effect may not be negative even if it’s real
There was a breathtakingly beautiful BBC series on the Great Barrier Reef recently which my son pronounced himself almost too depressed to watch. ‘What’s the point?’ said Boy. ‘By the time I get to Australia to see it the whole bloody lot will have dissolved.’
The menace Boy was describing is ‘ocean acidification’. It’s no wonder he should find it worrying, for it has been assiduously promoted by environmentalists for more than a decade now as ‘global warming’s evil twin’. Last year, no fewer than 600 academic papers were published on the subject, so it must be serious, right?
First referenced in a peer-reviewed study in Nature in 2003, it has since been endorsed by scientists from numerous learned institutions including the Royal Society, the National Oceanic and Atmospheric Administration and the IPCC. Even the great David Attenborough — presenter of the Great Barrier Reef series — has vouched for its authenticity: ‘If the temperature rises up by two degrees and the acidity by a measurable amount, lots of species of coral will die out. Quite what happens then is anybody’s guess. But it won’t be good.’
No indeed. Ocean acidification is the terrifying threat whereby all that man-made CO2 we’ve been pumping into the atmosphere may react with the sea to form a sort of giant acid bath. First it will kill off all the calcified marine life, such as shellfish, corals and plankton. Then it will destroy all the species that depend on it — causing an almighty mass extinction which will wipe out the fishing industry and turn our oceans into a barren zone of death.
Or so runs the scaremongering theory. The reality may be rather more prosaic. Ocean acidification — the evidence increasingly suggests — is a trivial, misleadingly named, and not remotely worrying phenomenon which has been hyped up beyond all measure for political, ideological and financial reasons.
…
The key findings of the ruling;
Findings of the Complaints Committee
19. The article was written in the first person, and sought to challenge what it made clear was the consensus view on ocean acidification. Before the article set out its criticisms, it referred to there being an extensive academic literature on the subject, and made clear that the theory had been endorsed by scientists from a number of institutions. The article referred to the author as being one of a group of “sceptics”, and a “denier”, and the final sentence of the article suggested it was “time our supposed ‘conspiracy theories’ were taken more seriously”. The article was clearly a comment piece, in which the author was expressing sceptical views on ocean acidification, and describing sceptical views expressed by others, that were contrary to the academic consensus. The Committee’s role is not to make findings of fact or to resolve conflicting evidence in relation to matters under debate. Rather, it assesses the care taken not to publish inaccurate, misleading or distorted information, and establishes whether a distinction is clearly made between comment, conjecture and fact, in determining whether the Code has been breached.
20. The Committee noted the complainant’s position that no experts in the field had expressed concern that ocean acidification could cause a “mass extinction”. However, it was not in dispute that many considered ocean acidification to be a matter of concern, and some believed it posed a serious threat to marine life. In this context, the claims the article made in support of its position that it was a “scaremongering” theory were not significantly misleading. The Committee noted the complainant’s position that the evidence did not “increasingly suggest”, that ocean acidification was “trivial”. The article went on to make clear what this evidence was, which the author was entitled to select in support of his position. In addition, the article made clear that this view was contrary to the consensus. The article was not misleading on this point.
21. The Committee noted the complainant’s position that the article misrepresented the paper reviewing the academic literature on ocean acidification. It was not misleading to claim that the paper was a “review of all the papers published on [ocean acidification]”, in circumstances where the paper described itself as “providing a brief overview of the history of research on [ocean acidification]”. The paper in question did refer to there being a publication bias towards papers which report negative effects of ocean acidification, and referred to a paper which highlighted methodological problems in research in the area. The manner in which the article presented the author’s interpretation of the paper was not significantly misleading.
22. The article reported that two named individuals had omitted historical data on oceanic pH from their research on ocean acidification, but that another named individual had incorporated this data into his own chart. The fact that the article misdated one of the charts referred to in this debate was not a significant inaccuracy in this context. While the Committee noted that the complainant agreed with the decision to omit this data, such that he considered the conclusions derived from its use to be invalid, the article was not a significantly misleading report of this scientific debate. It was not significantly misleading for the article to express the view that the omission of this data represented a flaw.
23. In support of the position that ocean acidification “wouldn’t be a disaster”, the article referred to reasons put forward by Patrick Moore. The Committee noted that the complainant disagreed with these reasons, and referred to research by other scientists which suggested that ocean acidification would harm the marine eco-system. The article had previously made clear that many were concerned by the possible consequences of ocean acidification, and it was not misleading for it to describe the alternative point of view, as put forward by Mr Moore. It was not disputed that this individual had been involved in the early days of Greenpeace movement, and whether or not he was “co-founder” was not significant in the context of the article.
24. It was not in dispute that the ocean acidification research programme had received public funding. Which government department had provided this funding, and whether it was provided directly, or via a research council, was not significant. The article’s claim that it looked “increasingly to be the case” that global warming theory was a “busted flush”, the claims about the reasons why research has been conducted on ocean acidification, and the claim about the ease with which the issue of ocean acidification could have been “resolved”, were matters of comment, and were clearly presented as the author’s opinion. The Committee did not establish that the article failed to clearly distinguish between comment and fact. It did not establish that the article contained any significant inaccuracies or misleading statements, such as to demonstrate a failure to take care over the accuracy of the article under the terms of Clause 1 (i), or such as to require correction under the terms of Clause 1 (ii). There was no breach of Clause 1.
Read more: https://www.ipso.co.uk/rulings-and-resolution-statements/ruling/?id=08168-16
In my opinion this entire sorry episode goes straight to the heart of the difference between the way alarmists like Williamson see the world, and the way normal people view the world.
Alarmists seem to want their models, theories and opinions to be accepted as established fact. But the reality is their shaky theories are full of poorly supported conjecture and extrapolation.
Nothing bad has happened to the oceans due to alleged ocean acidification, and given vast and rapidly changing natural variations in ocean pH in key marine environments such as continental shelves, it seems unlikely that any plausible change in average ocean pH will ever have any noticeable impact on marine ecosystems.
By complaining about an article in a relatively obscure paper all the complainant has achieved is to raise the profile of that article. Good result.
The Barbara Streisand effect at work.
…and yet few people have a positive opinion of Babs because of her hoity-toity attitude, so let it happen.
Actually, the Spectator is probably the premier Conservative weekly Politics & Current Affairs magazine in the UK – the article will certainly have been read by the right-wing movers and shakers. But any controversy is obviously good publicity…
The Spectator is a weekly British conservative magazine. It was first published on 6 July 1828,[2] making it the oldest continuously published magazine in the English language
https://en.wikipedia.org/wiki/The_Spectator
CO2 levels were as high as 7000ppm in the past, at a time when most life existed in the oceans, and the oceans did not acidify. And at 7000ppm the oceans didn’t boil away in a runaway greenhouse effect either.
Global warminger “climate change” is a leftist scam.CO2 levels are just not high enough to impact ocean pH…and it won’t be when it’s doubled either
Ocean waters already have 99% of free CO2 in it. How can a dribble more make that much difference?
“Global warming er “climate change” is a leftist scam.”
Indeed. They actually admit that in their own company:
green-agenda.com
Yes, the continued willful ignorance of the Earth’s climate history is astounding from those who fancy themselves as “scientists.” Observation Trumps theory. (I’ll continue to use that one with glee, and think the USA’s new President should adopt it as his climate change catch phrase).
What causes sudden deaths in marine life are many hazards such as volcanic eruptions that add lots of sulfur and screen of gases and volcanic debris that cuts down sunlight.
If it ain’t on the other side of neutral it ain’t acid.
That’s too complex for the Prof
Aye, Mr Scott. He doesn’t seem to use the same pH scale I was taught. Maybe he grew up reading i.t.a. alphabet…
http://imgur.com/ONmBABz
The correct description would be to say that the ocean is becoming “slightly less caustic”.
It is just possible though, that accuracy doesn’t sound scary enough.
Dictionary definition of caustic is “capable of burning or corroding by chemical action”.
It is not generally synonymous with the word basic.
It particularly is used to refer to burns of living tissue, which is why it usually is used in regard to strongly basic solutions.
Likewise, one will find that every dictionary definition of “acidification” is “to make or become an acid”.
Becoming slightly or somewhat less basic is not acidification in any usage outside of climate alarmism.
If it is rightfully called “Acidification.” Then Chemically pure water is orders of magnitude more “Acidic” than Ocean water!
Dihydrogen monoxide, which kills directly a large number of people yearly by inhalation, is acidic enough to kill some marine life!
“If it ain’t on the other side of neutral it ain’t acid.”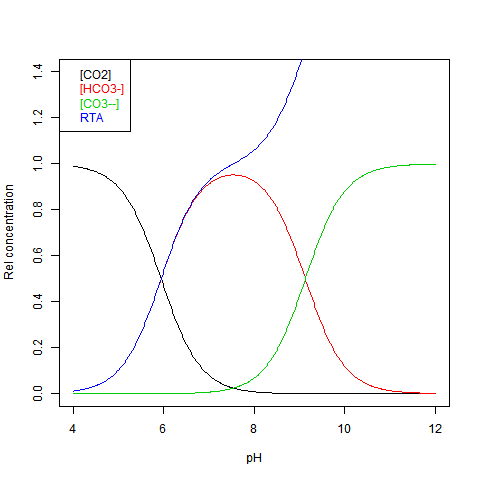
People speak a lot of buffers here. But they don’t seem to know what that means. A buffered system is one with an acidic species A and a complementary base B, both present in significant quantity. That generally has to mean that the acid and base are weak. The neutral point, at which both A and B are present in equal quantity, is called the pKₐ.
Water is a weak buffer. Its acid is H³O⁺, and base is OH⁻. The pKₐ is 7. But here we don’t have pure water. It’s a different buffer system. The (Lewis) acid is CO₂ or H₂CO₃ and the base is CO₃⁻⁻. The Bjerrum plot is here:
As you see, we are slightly on the alkali side of the pKa, which is 7.53. But it is in the zone where adding CO₂ depletes CO₃⁻⁻. That is the unwanted outcome. And it has nothing to do with pH 7.
Nick, here is a post with a lot of recent science research on this overblown topic:
The ‘Ocean Acidification’ Narrative Collapses Under The Weight Of New Scientific Evidence
“The ocean “acidification” narrative that claims humans are gradually lowering pH levels in sea water with their CO2 emissions may rest on presumptions, hypotheticals, and confirmation bias — not robust, observational scientific evidence.
A paper by Wei et al. (2015) published a year ago in the Journal of Geophysical Research effectively illustrates the vacuousness of the ocean “acidification” paradigm.
In the paper, the authors assert that “model calculations” (yes, calculations from modeling) have indicated oceanic pH levels may have decreased (i.e., lowered pH = less alkaline = more “acidic”) since the 1800s by a total of about 0.1 as consequence of the rise in anthropogenic CO2 emissions. This overall pH-lowering “trend” of less than 0.1 since the industrial era began is “predicted” to “potentially threaten the existence and development of many marine calcareous organisms”. Again, it’s the 150-year -0.1 trend in pH-lowering — which the authors admit is subject to “large errors” in measurement — that threatens the oceanic biosphere according to modeled predictions. In contrast, large natural pH drops of -0.2 to -0.5 occurring on 10-year timescales do not threaten “marine calcareous organisms.” Here are the key points from the paper:”
http://notrickszone.com/2016/12/29/the-ocean-acidification-narrative-collapses-under-the-weight-of-new-scientific-evidence/#sthash.o0EcULCJ.dpbs
Sea water is NOTHING like pure water Nick.
Your science is in the trough with your climate swill.
“A paper by Wei et al. (2015) published a year ago”
The paper by Wei et al was not a general study of the W Pacific. It was a study of coral reef markers on the east side of the island of Hainan. The location was subject to the variable effects of river water. It showed a degree of pH variability that was quite high (range about 0.4) compared with ocean. I don’t see how this helps here.
Keep reading Nick,there is a lot more to that paper,you have not commented on.
Nick… suggest you look a little closer at the Title of the Y axis. It is Relative concentration. Since this is a buffered system and the pH changes very little with addition of CO2, guess what… as CO2 goes up… so does CO₃⁻⁻. It is RELATIVE. The ratio stays constant at constant pH. So contrary to your claim, adding CO2 does NOT lower CO₃⁻⁻.
Alcheson,
” It is Relative concentration.”
Yes, it’s relative to DIC. This does go up as you add CO₂, so it slightly exaggerates the reduction of CO₃⁻⁻ in absolute terms. The blue curve is relative TA; TA does not change with adding CO₂. So if you mentally divive by the blue curve (which has max 2) you’ll see it right. We know CO₃⁻⁻ does diminish by the overall reaction. Each added CO₂ takes out a CO₃⁻⁻, except for what remains unreacted; the Bjerrum plot shows that is very little.
Nick/
please enlighten me on how you measured the pH of sea water in the oceans?
[sampling areas/frequency/calibration techniques, etc]
the way I remember doing pH, is calibrating each day and being happy if the error is smaller than 0.1 [in subsequent checks of the buffer solutions]
I am not sure but from what I have read I believe the order of alleged acidification is in the order of a few 0.01….which fall largely within the error of measurement [0.05]
Henry,
“please enlighten me on how you measured the pH of sea water”
Well, I didn’t measure it. There is nowadays fancy instrumentation for doing it directly with good accuracy. But in older days, sensible people measured total alkalinity (TA, titration) and dissolved inorganic carbon (DIC, gravimetric) and worked pH out from equilibrium relations (as eg with this calculator).
Nick you say
Well, I didn’t measure it. There is nowadays fancy instrumentation for doing it directly with good accuracy
Henry says
Now, could you let me know how much delta pH we are talking about here globally and to which investigations you are referring alleging some alarmism about this topic?
I doubt someone sat there doing titrations for days/months/years in all 5 oceans of the world ???
Henry,
There is a huge literature on ocean electrolytes. And yes, there are measures of DIC and TA in all five oceans over long periods. Go find out.
Henry says
Now, could you let me know how much delta pH we are talking about here
Nick says
go find out
Henry
you are clueless….
“Having had the role of science coordinator for the UK Ocean Acidification research programme and being involved in relevant national and international projects for around ten years previously,I know…”
I am at risk of losing a great paying government job, that requires no personal accountability, and carries added perks of international travel on taxpayer funded per diem. It’s a great gig and Delingpole is screwing this all up for me.
James Delingpole fairly represented the alarmist position.
He said that the alarmist position was the scientific consensus.
He selected evidence that contradicted the consensus position.
James Delingpole bent over backwards to be fair and was found to have done so.
Professor Phillip Williamson should be glowing red with embarrrassment but he’s probably incapable of understanding how badly he just got smacked down.
I am absolutely terrified that if ocean acidification continues, the ocean may become as acidic as distilled water.
Not likely.
Not even possible.
O, the huge manatees!
+1
Has the pH of the ocean actually dropped so much that an alarming phrase like “Ocean Acidification” (spellcheck doesn’t even recognise the word) is justified? I hate to think what pHillip Williamson’s salary is and who’s paying it.
The ocean surface has -theoretically- dropped 0.1 pH unit from ~8.1 to ~8.0 over 165 years with some of the man-made CO2 absorbed. In the past 30 years it was actually measured at a few fixed places (longer by sporadic surveys) and indeed it is dropping with a few hundreds over that period.
To make it sound more scary for laypeople: the “acidity” (H+ concentration) increased with 30%… But they “forget” to tell you that the pH still is basic. And they forget to tell you that the pH difference between poles and equator is about a full pH unit. And they forget to tell you that the pH within the coral reefs can change with a full pH unit within a day. And they forget to tell you that fish can swim into much larger pH differences without harm and that corals and coccoliths did evolve and grow in periods with much higher temperatures and CO2 levels than today…
FE
I was thinking of commenting but I couldn’t put it better than you have.
Anyone who has maintained a reef tank can attest to normal pH variations and not much worry in an established tank.
And, “they” forget to mention that natural upwelling along the continental shelf results in large changes in pH over a short period of time. The Monterey Bay Aquarium in California has been monitoring their water intake for years and records are available on the internet.
Lets not forget that its a 0.1 drop in the ocean pH calculated rather than directly measured.
+100
good comment
Many rivers are acidic, and where they flow into oceans are generally highly biologically productive ecological zones.
If you do the maths you will see if the oceans continue absorbing CO2 at the current rate, then their ‘evil gas’ levels will rise by just over one part per million over the next century.
Ocean acidification by CO2 is total and complete alarmist BS, especially when you consider varying pH levels in any individual part of the ocean, during any one year, far exceed anything CO2 could ever achieve.
A pity that Professor Phillip Williamson doesn’t apply such scrutiny to the wild claims of alarmists.
I believe his salary depends on him not applying scrutiny.
Excellent point. If he applied scrutiny to the wild claims of the alarmists, he might have to start polishing up his resume’. They all know where their paycheck comes from.
The University of East Anglia!!! Where else?
Where is Anglia, anyway? It sounds Like fishing heaven!
Now I remember, the place with the motor oil hate-crime art.
East Anglia is full of twats! (another name for a pregnant fish)
Located to the east of England and often called the Fenlands
http://www.stedmundsburychronicle.co.uk/qgismaps/EA_peatandhillshade.jpg
Lots of good fishing
http://news.bbcimg.co.uk/media/images/56957000/jpg/_56957812_391558f5-95b7-485b-91f8-442ffccae722.jpg
Twats, I can relate to (in an alternate sense). I’ve known some enigmatic ones.
As to fishing, that does seem to be the scientific approach at UREA (University Representing East Anglia)
Perhaps the Pope will rename it East Anglican.
I was born there.
It has been a singular embarrassment to see the science being produced by the people of my birth.
The Norfolk Rifles, from East Anglia, were slaughtered in the retreat to Dunkirk during WW2.
http://www.wartimememoriesproject.com/ww2/allied/regiment.php?pid=1535
We have an earned right for absolute disclosure of data as a free people.
The corollary is open scientific debate.
Don’t try and shut down those you disagree with, give a written answer and get the Spectator to publish it.
Then expect further debate.
The cover ups in East Anglia were shown with Climategate.
Now this.
It comes down to a problem of trust.
In a buffered sea water environment, against archaic change that allowed corals to thrive,
with evidence that they survive nuclear obliteration at Bikini Atoll
https://wattsupwiththat.com/2016/08/18/more-evidence-of-coral-reef-resilience/,
why should we trust models?
IIRC, East Anglia was hypothesized as the landing point for a German invasion in an influential alarmist 1903 novel by Ernest Childers, The Riddle of the Sands.
“Non Angli, sed angeli”
Pope Gregory I
University of Easy Access … it shows in the quality of their staff!
Nullius in verba. Take nobody’s word for it. The motto of the Royal Society.
Professor Phillip Williamson clearly does not understand that the word ‘nobody’ refers to him as much as to anyone else. He seems to be “an example of the shortfall of narrow education.” (h/t Dan Pangburn)
Do you know where that clip comes from?
I think it’s from the 2004 film version of Around the World in 80 Days…
Yes
Good one!
“Ocean acidification is an inevitable consequence of increasing carbon dioxide in the atmosphere. That’s a matter of fact. We don’t know exactly what will happen to complex marine ecosystems when faced with the additional stress of falling pH, but we do know those changes are happening and that they won’t be good news.”
“That’s a matter of fact.”
followed by:
“We don’t know exactly what will happen to complex marine ecosystems when faced with the additional stress of falling pH”
followed by:
“but we do know those changes are happening and that they won’t be good news.”
I’m sorry, but this idiot claims to be an effing scientist!!!???
Whilst I’m not a scientist, I’m conversant with criminal law, and evidence, albeit not an expert. However, at its most fundamental level, a contention similar to the one proposed by this clown would have had an entire criminal case thrown out of court before it had even been seen by a judge.
How is it possible this pompous, ignorant balloon is allowed by his employers to associate their name with the ridiculous contention that ‘we know for a fact; but we don’t know; but we’re sure the results will be catastrophic’?
WTF is happening to our scientific community when it is represented by these morons. Is there no facility whereby these imbeciles can be ‘struck off’ as other professionals can when proven to make false, misleading or reckless statements?
A scientist is anyone who applies the scientific method. So I would say that you are more of a scientist than the Clown Prof at UEA.
Yes, it’s amazing, isn’t it? Claim that there are ongoing changes due to CO2 lowering pH and that they are bad, but then admit that you don’t know what the effects of lowering pH are.
At least with pH, they are probably right about the direction. When it comes to hurricanes, tornadoes, snow, rain, etc, they flip back-and-forth.
Beautifully put HotScot, my sentiments exactly. If I had made similar silly assertions to my boss (in the petroleum industry) I would have run off by the end of the day. Not so in the global warming industry.
Policy based evidence making. h/t Tim Worstall
We don’t know exactly what will happen…, but we do know those changes are happening and that they won’t be good news.
You don’t know, but you do know? Riiiight… I guess we should just keep quiet and send you more money.
“You don’t know, but you do know?”
That’s the whole foundation of the progressive science movement. It has a lot to do with the semantic of feeling so strongly that you “just know”.
“We don’t know exactly what will happen…, but we do know those changes are happening and that they won’t be good news.”
You beat me to it.
If you quoted just the “We don’t know” part, he’d squeal “I was taken out of context! Report the whole quote, please.” As if.
What does the professor consider “good news?” Even if something happens and can be shown to be a direct result of human-produced CO2 [good luck on that proof!], I’d say the chances are at least 50-50 that most people would consider that result to be just fine. Here I sit, in the southern state of Virginia, with eight inches of snow on the ground (that I had to shovel yesterday and today) and the temperature expected to dip below zero F tonight, or about -18 C. A bit of warming–CO2-induced or no–might be welcome to an old codger like me.
Good news? Nothing to do with Science, I’m sure. More likely the “dictatorship of the proletariat.”
no evidence that ocean acidification is related to fossil fuel emissions.
https://papers.ssrn.com/sol3/papers.cfm?abstract_id=2669930
Jamal, you make the same error in that paper as in previous papers: the uptake of CO2 by the ocean surface is hardly influenced by yearly emissions, it is influenced by the total extra CO2 pressure in the atmosphere, that is the accumulated CO2 from all previous years in the atmosphere.
Moreover, it doesn’t make any sense to look at the detrended series, as the cause and effect is in the trends, not in the year by year variability of the trends, as that is looking at the noise around the trends…
Not clear from your report is if you have separated the ocean surface data from the deep ocean data. Only the surface oceans – the “mixed layer” – of a few hundred meters is directly influenced by CO2 from the atmosphere with a half life time of less than a year. The deeper oceans are mixed at much slower rates, taking ~35 years half life time for the removal of excess CO2 out of the atmosphere and a return time of ~1000 years for the effect in the deep oceans, due to its enormous mass and the slow deep ocean current (THC).
If you have used all the measured depths, you have averaged out most of the CO2 influence…
Due to ocean buffer chemistry the increase of DIC (all inorganic carbon species) in the ocean surface is about 10% of the increase in the atmosphere. That is the Revelle factor. That gives a slight decrease of pH, which is measured at some six fixed places in the oceans, here for the Bermuda’s:
http://www.biogeosciences.net/9/2509/2012/bg-9-2509-2012.pdf
Further you say:
This correlation is a pre-condition to the anthropogenic ocean acidification hypothesis which holds that the annual rate of human emissions causes annual changes in oceanic CO2 concentration (Scripps, 2013) (NOAA-1, 2015).
There is no such statement in the Scripps reference, that is a misinterpretation of yours. They talk about longer term uptake, not year by year uptake.
Yearly emissions do influence yearly uptake, but the influence is very small, much smaller than the natural seasonal to year by year variability. It takes longer time spans to show the correlation in the trends.
Thanks for this comment!
Thank you Ferdinand for a well written and informative treatment of the subject.
Bartleby:
I write to correct your post. When corrected it would say,
‘Thank you Ferdinand for a well written and misleading treatment of the subject’.
There is no need to thank me for providing the correction. The provision was my pleasure because the item from Ferdinand is very, very flawed.
Ferdinand’s post misleads in too many ways for full rebuttal in this thread, but the following example is sufficient to demonstrate the falseness of Ferdinand’s assertions.
There is insufficient sampling to provide meaningful ocean surface layer data or deep ocean data. And the exchange rates between ocean surface layer and deep ocean are completely unknown for water, for carbon and for CO2.
Indeed, the complete lack of knowledge of exchanges between ocean surface layer and deep ocean enables warmists to claim their ‘missing heat’ has been transported to deep ocean despite there being no place(s) where such flows are observed.
Richard
Richard,
Happy to see you back again. I hope your health is improving. So we can have a new fight now and then…
The response of Jamal Munshi is what I was discussing, as he looks at the correlation between the variability of human CO2 emissions and variability in pH change after detrending.
That is like saying that you should have a correlation beween yearly seawater temperatures and sealevel change by detrending both and looking at the momentary waves and tides which “proves” that there is no influence of higher seawater temperatures on sea levels…
By detrending he simply removed the correlation, which is in the trends over longer periods, not in the year by year emissions and year by year pH changes…
CO2 uptake by the oceans is directly proportional to the total extra CO2 pressure in the atmosphere, not the yearly emissions. As the pH change is small (0.01 pH unit per decade), you need very accurate measurements (which are nowadays colorimetric) and longer time periods, before a trend is emerging out of the “noise”.
Indeed there are few data of the oceans, compared to its enormous surface and volume. That would be a problem if there were lots of contradictory data. Except nearby river discharges or enclosed seas, all sampling at the same coordinates over time point in the same direction: increasing DIC (total inorganic carbon) and slightly decreasing pH. The measured increase in DIC and decrease in pH is totally in line with the theory for the measured CO2 increase in the atmosphere. That is only possible if CO2 is average moving from the atmosphere into the oceans, not reverse. That is independently confirmed by over 3 million sea surface pCO2 samples, which show that the atmosphere is in average 7 μatm higher in partial CO2 pressure than the ocean surface pCO2… See:
http://www.pmel.noaa.gov/pubs/outstand/feel2331/exchange.shtml
While there is insufficient knowledge about the water – and heat – flux between surface and deep oceans, the CO2 flux is known from two independent sources: the dilution of the human 13C/12C fossil fuel “fingerprint” by the deep ocean – atmosphere exchanges and the speed of the 14C decline from the peak in atomic bomb tests. Both give a deep ocean – atmosphere and back CO2 cycle of around 40 GtC/year:
http://www.ferdinand-engelbeen.be/klimaat/klim_img/deep_ocean_air_zero.jpg
Ferdinand;
Thanks for your good wishes. Sadly, my thought that I was ‘winning’ over my health problems was dashed when I suffered a stroke (which proves life is not fare). Now, correcting voice recognition requires typing which is difficult for me and, therefore, making a contribution on the web is so difficult for me that I need good reason for me to make the effort.
You now admit you were plain wrong when you claimed to know CO2 flux between deep ocean and ocean surface layer because you write
but you pretend the CO2 flux is known from isotope ratio changes when you write
NO! The isotope changes do NOT concur with your assertions, but that discrepancy can be accounted by adopting an assumption of dilution. Anything can be made to fit anything by adoption of selected assumptions but that is NOT evidence for the assumptions being correct.
I would like to know why atmospheric CO2 concentration has been rising since the end of the Little Ice Age (LIA).
I think the atmospheric CO2 rise is probably a response to the temperature rise which has been happening over the centuries since the LIA and that the small anthropogenic CO2 emission has trivial effect, but I am willing to admit the available information is not sufficient to resolve the matter.
You like to claim the atmospheric CO2 rise is a direct result of the small anthropogenic CO2 emission and you assert that your assumptions are evidence for your claim: they are not.
And you attempt to bolster your assumptions by asserting
NO! CO2 uptake by the oceans is directly proportional to the total extra CO2 pressure in the atmosphere and that pressure varies throughout the year as the emissions and sequestrations vary: see the variation at Mauna Loa. That reality does not concur with your claims so – in your misleading post that I refuted – you said it should be ignored when you wrote
That “noise” is the varying “total extra CO2 pressure”. It results from the total CO2 emission of which the anthropogenic CO2 emission is a very small contribution.
Considering the seasonal variation in atmospheric CO2 emission at Mauna Loa as being solely a result of emissions (regardless of sequestratrions) provides a very, very conservative indication of total CO2 emission. The accumulation rate of CO2 in the atmosphere (~1.5 ppmv/year which corresponds to ~3 GtC/year) is equal to almost half the human emission (~6.5 GtC/year). However, this does not mean that half the human emission accumulates in the atmosphere, as is often stated by you and some others. There are several other and much larger CO2 flows in and out of the atmosphere. The total CO2 flow into the atmosphere is at least 156.5 GtC/year with 150 GtC/year of this being from natural origin and 6.5 GtC/year from human origin. So, on the average, at most 3/156.5 = 2% of all emissions accumulate. But you say to ignore that and you wrote
The cause and effect is in the variations in emissions and sequestrations that you deliberately ignore so remove by detrending!
As I said to Bartleby, you have provided a well written and misleading treatment of the subject.
Richard
Richard,
We have been there many times before…
1. Human emissions are known with reasonable accuracy. Without any CO2 exchange with other reservoirs, that would have reduced the δ13C level of the atmosphere down to -11 per mil.
Because there are huge exchanges between the reservoirs, CO2 is redistributed between all the reservoirs, and so is the isotopic ratio. For the ocean surface and vegetation, that is going fast with redistribution half lifes of less than a year. Ocean surface and leaves show the same δ13C patterns as the atmosphere. The deep oceans are different: the isotopic composition of today (minus some isotopic shift) is going into the deep, but the isotopic composition of ~1000 years ago (minus some isotopic shift) is coming back out of the deep. As the (deep) oceans have a higher δ13C level than the atmosphere and much higher than of human emissions, that “dilutes” the δ13C “fingerprint” of human emissions. According to the calculations, some 40 GtC/year from the deep oceans is necessary. That can be simple circulation or (partly) additional, but 40 GtC/year it is, independently confirmed by the 14C decline from the bomb tests.
Still the oceans can be (part of) the cause of the increase, but that is difficult to fit: humans add ~9 GtC/year as CO2″, but the increase in the atmosphere is only 4.5 GtC/year. Any extra addition by the oceans (or vegetation) would give a total increase in the atmosphere larger than 9 GtC/year.
The δ13C decline is actually measured in the ocean surface via coralline sponges over the past 600 years and they show a decline in complete lockstep with human emissions. It would be a hell of a coincidence that some natural process starts exactly at the same moment and in exact ratio with human emissions, while there is no such large isotopic shift in 800,000 years measured in ice core CO2:
http://www.ferdinand-engelbeen.be/klimaat/klim_img/sponges.jpg
If you have another explanation for the δ13C changes, I am all ear…
2. There is zero evidence that temperature is the main driver of the CO2 increase in the atmosphere. Ice cores show a very strict ratio between temperature and CO2 levels in the pre-industrial era of ~16 ppmv/K. Henry’s law gives 4-17 ppmv/K for seawater in equilibrium with the atmosphere.
Thus maximum 16 ppmv for 1 K warming ocean surface since the LIA. That is all. The current (dynamic) equilibrium between oceans and atmosphere is 290 ppmv for the current average ocean surface,
Oceans are a proven sink for CO2: the average pCO2 of the atmosphere is 7 μatm higher than of the oceans.
The biosphere is a proven sink for CO2: more oxygen is produced than consumed by decay and feed/food
The rest of the 110 ppmv above the long-time equilibrium is from human emissions.
3. The seasonal CO2 exchanges are all temperature dependent, not pressure dependent. Nevertheless, if you assume that these are important, then the actual global variability is ~2.5 ppmv each side for a global temperature change of ~0.5 K each side. As we are ~110 ppmv above long time equilibrium per Henry’s law, the seasonal variability is good for + and – 2.5% of the total CO2 pressure in the atmosphere above equilibrium. After a full year cycle, the influence is near zero.
What you didn’t take into account is that temperature has opposite CO2 effects on ocean surface and vegetation (and between the hemispheres): some ~60 GtC is absorbed in spring/summer by vegetation and released again in fall/winter. Some ~50 GtC is released by the ocean surface in spring/summer and absorbed again in fall/winter. The net result is ~10 GtC (~5 ppmv) less CO2 in summer with higher (!) temperatures than in winter.
Human emissions on the other hand are ~4% additional, to the extra pressure already in the atmosphere, each year again. As only half of that quantity (as total mass, not only from human emissions) is absorbed by oceans and vegetation (due to the total extra pressure in the atmosphere), the difference accumulates over time…
4. The cause and effect is in the variations in emissions and sequestrations
As the variations are less that 2.5% around the trend and are all temperature dependent, not pressure dependent, and these level out to zero after 1-3 years, this is just nonsense.
It is like looking at waves and tides and declaring that there is no influence of temperature on sea level change. The difference in that case is that you need at least 25 years of data to see a (statistical) sea level change, while for CO2 three years of data is sufficient…
Professor ! Have you taken your ox to the Vet ? I fear it may be bleeding !
Or is it your wallet ?
Inquiring people want to know !
His ox is a sacred cow, to him.
If the ocean becomes this lifeless toxic soup of acids I won’t have to bottom-paint the boat every year. See; there’s always a silver lining.
But seriously folks; it the oceans are warming, as we’re constantly being told by alarmists, and consequently outgassing CO2 aren’t they becoming more alkaline not less?
Snort! Good one Chris.
Chris,
It should get more alkaline if temperature was the only influence, but as human emit more CO2 than the oceans can emit for the current surface temperature, DIC levels (CO2+bicarbonate+carbonate) increase and pH goes a little bit down. See Fig. 5 for Bermuda over time:
http://www.biogeosciences.net/9/2509/2012/bg-9-2509-2012.pdf
LOL once again the Eco-fascists have it both ways. What else is new.
5 year old citation:
https://wattsupwiththat.com/2012/01/09/scripps-paper-ocean-acidification-fears-overhyped/
This covers the topic of ocean acidification quite well.
I’d like top think that this is also worth a re-read: http://wattsupwiththat.com/2015/09/15/are-the-oceans-becoming-more-acidic/
I think most of the marine life forms we know exist now evolved in times when atmospheric CO2 was over 1000 PPM. What was the marine PH then? What is there about fossil fuel CO2 that will do something to the ocean that “natural” CO2 couldn’t? I suspect that most of the CO2 we are setting free now was in the atmosphere when corals developed.
DMA,
Based on a talk I recently attended at Miami University (OH), different organisms appear to have different optimum pH requirements for shell construction that probably reflect the predominant pH during the time that the organisms evolved. However, they have also evolved mechanisms for coping with variations from their optimum. Also, many organisms that build shells then protect the calcite/aragonite with mucous. The primary impact is that the farther the pH is from the optimum, the more energy the organism has to expend to build the shells. But, they really aren’t in any danger of suddenly dissolving because of lowered pH.
If the atmosphere is 1000 ppm then the rain will be be slightly more acidic, leaching more salts in the run off, which are a natural buffer when they wash out to the ocean. It’s the reason that oceans and lakes without outlets (Saltlake, Dead Sea, Salton Sea) are all salty.
Eric,
I still find your use of labels a wee bit problematic;
“Alarmists seem to want their models, theories and opinions to be accepted as established fact. But the reality is their shaky theories are full of poorly supported conjecture and extrapolation.”
(“normal people” . . : )
“Alarmists” . . please be careful about giving alarmists in general a bad rep ; )
Why?
Because alarmists don’t all act like they are entitled to be unconditionally believed, and because sometimes things worthy of some alarm sounding happen . .
So John, what you’re saying is something like:
Because Patrick was correct about alarmist issue X, we shouldn’t lambast Phillip when he is alarmist about Y?
JohnKnight You mean things with the climate might sometimes be alarming?
Ha.
Watch out for that river!
Why?
Because it might change course !
When’s that going to happen?
About 20-50 years !
Yeah. I’ll keep an eye out.
Actually I was thinkng of Mr, Trump in particular . .
Having spoken to 2 chemical engineers, 2 professors of chemistry & 3 professors of ocean science who read Williamson’s paper (June 2016 with 376 Reads) –
https://www.researchgate.net/publication/304324178_Carbon_dioxide_and_ocean_acidification_observations_in_UK_waters_Synthesis_report_with_a_focus_on_2010_-_2015?channel=doi&linkId=576be75d08aead4e3add04a1&showFulltext=true
the 97% consensus is…. Ocean acidification is total & utter bollox !
One suggested he’d got his chemistry training serving behind the counter in a pharmacy on a Saturday morning (that’s a peer review).
Williamson is big on “Take-home messages” ….all entail more funding
3.2 Results
3.2.1 Long-term trends
Take-home messages
3.2 The new UK measurements confirm that pH is highly variable, there-
fore it is important to measure consistently to determine any long term
trends
4.1 Concluding remarks 1. High-quality observations of ocean acidification should continue to
be supported, with adequate resources
I particularly enjoyed this part:
which is then followed with:
So which is it Phil? The question isn’t whether or not you could find anyone else to measure it for you, it’s why you didn’t bother measuring it.
Anyone can go to a pet store that sells aquarium supplies and purchase a crude but fairly accurate pH test kit. People who keep expensive fish need to test the water daily to avoid going too far one way or the other and killing off all their pets. With the relatively tiny volumn of water in an aquarium, it can go dangerously acidic or alkaline in a short time. This is basic science, you don’t even need maths to do a simple color comparison. This I know because it is now a part of my field kit when on the littoral; four tests today, four happy ocean pH readings between 8 and 8.5. We are going digital in a few weeks, can’t be accused of failing to differentiate between bright blue and pale yellow….
PMK
Hmmm, aquarium…air pump…bubbles…absorbed CO2…canary in cage in mine….did the fish die yet because of the CO2 acidification of the aquarium?
Hmm.. intriguing point. First I thought your comment a bit of a red herring, but wait..The pump will accelerate the equilibration of carbonate in the water, and will be drawing air from the air in the room. Likely the air in a small, occupied room would contain definitely more CO2 than the outside atmosphere and could easily be more than double the 400 ppm outside. Maybe you can do some ‘ocean acidification’ experiments in your living room.
Great point Pamela, one that’s so easily lost in the fray of “scientific” discussion; we overlook the obvious right in front of us (was that an unintentional rhyme?)
We think “well then, this is over my head. I feel a little depressed now. Maybe I’ll go look at the fish for a bit.”
What a wonderful observation you’ve made. Thanks.
James Delingpole is excellent. He knows extremely well how to skewer an alarmist without falling foul of any press standards issues. Having laid out the acidification story he presents the other side, including the damning case shown by Mike Wallace (alarmists showed data only from 1988 but when Wallace plotted up information from 1910, there is no evidence of anything untoward regarding pH). Philip Williamson’s response to that is truly puerile, complaining amongst other things that one study was published in 2006 rather than 2004, and that Defra was the main funder rather than NERC, despite his listing NERC before Defra in his bio.Tut tut, as if that makes any difference. ha ha.
But truly awful is the fact that Williamson somehow exonerates Feely and co-workers for omitting over 1.5 million datapoints that were available and which go the opposite way to their “scenario”.
see here https://wattsupwiththat.com/2014/12/23/touchy-feely-science-one-chart-suggests-theres-a-phraud-in-omitting-ocean-acidification-data-in-congressional-testimony/
Keith,
That article lumps everything together, including measurements not ready for the task in a wildly variable medium. If you look at time series at fixed places, there is an increase of DIC (total inorganic carbon) and a small decrease in pH over time…
See my response here…
Talk about fake news: omit masses of data that say the opposite of the point being made, and make alarmist claims repeatedly in 2004, 2006, 2008 et seq.
Apparently, Williamson has never heard of the well-established concept of buffering. Any A-Level student of Chemistry can tell him that carbonate and Bicarbonate ions in seawater react with added hydronium ions to maintain the pH where it was.
The oceans can only become acidic if the earth runs out of rocks
Correct, and it gets even better when the borate buffer is included. Borate is the orphan buffer. Repeating a comment I made on WUWT, “IPCC on acid. . .”, September 25, 2013:
Neil Jordan September 25, 2013 at 2:00 pm
Re Sabertooth says: September 25, 2013 at 11:43 am
The pH ceiling of 8.3 is explained in Emerson & Hedges Chemical Oceanography, which also explains a pH floor of 7.6, also alkaline:
http://courses.washington.edu/pcc588/readings/EH_IV_CarbSys.pdf
This reference also includes borate buffering in addition to the carbonate and bicarbonate buffering that are customarily used to describe seawater buffering. According to Frankignoulle (1994):
http://www.co2.ulg.ac.be/pub/frankignoulle_1994.pdf
borate buffering accounts for 30% of the global buffering effect in seawater.
“Apparently, Williamson has never heard of the well-established concept of buffering.”
Sounds like you haven’t heard of Lewis acidity (c 1923). Hydronium ions are barely relevant here, as is pH buffering. That just reflects that H⁺ and OH⁻ are very rare species where the main reaction is between CO₃⁻⁻, HCO₃⁻ and CO₂. And the key reaction is the depletion of carbonate, causing more CaCO₃ to dissolve.
And no, the buffer doesn’t keep the pH constant. It just changes rather gradually as the CO₃⁻⁻ is depleted.
You really have very little idea of what you are talking about, Mr. Stokes…and your statement here makes that 100% obvious to any chemist.
LOL Menicholas is right Nick…this time you blew it
“You really have very little idea of what you are talking about”
OK, tell us your version. Here’s the Bjerrum plot:
You might like some help from the calculator here.
Neil Jordan: Thank you for that information. I didn’t know that borate ion also plays a role in buffering seawater.
Nick Stokes: And the problem with that is what, exactly? Increase in partial pressure of CO2 leading to a transient increase in hydronium ion concentration. A tiny amount of the abundant carbonate ion in seawater or in rock reacts with the hydronium ions to form an increased concentration of bicarbonate ion, bringing the pH back to where it was before.
I really don’t understand why you brought up Lewis acids.
I tried your calculator, and it doesn’t work.
“I really don’t understand why you brought up Lewis acids.”
Simple. The main reaction is
CO₃⁻⁻+CO₂+H₂O ⇌ 2HCO₃⁻
It’s a Lewis acid-base reaction, and neither H⁺ nor OH⁻ is a reagent. That means that they don’t have a place in the equilibrium relation, which is why pH is of small importance, and pH 7 quite irrelevant.
The problem is that every extra CO₂ removes one CO₃⁻⁻, and that is likely to dissolve one CaCO₃. That probably doesn’t matter if it’s rock, but marine life may be what is to hand.
The calculator works fine, and shows the result also on the Bjerrum plot.
In your equilibrium reaction below presumably the CO2, when driven from the ocean by outgassing due to heating of the surface layer, with predicted surface heating, would have a CaCo3 sparing effect.
In shallow waters around coral reefs one could hypothesise that the warming would drive off CO2 sufficient to protect organisms with calcareous exoskeletons,as shallow water heats fast.
When corals are highly adaptive species from the past, why not hypothesise they are the same now and are tuned for adaptation?
Nick, please don’t go down this road, you are badly mistaken:
The ‘Ocean Acidification’ Narrative Collapses Under The Weight Of New Scientific Evidence
http://notrickszone.com/2016/12/29/the-ocean-acidification-narrative-collapses-under-the-weight-of-new-scientific-evidence/#sthash.o0EcULCJ.dpbs
“Narrative Collapses Under The Weight”
As I’ve said elsewhere, that is a nonsense article. The paper he is misrepresenting was a study of a coral reef in a particular location on Hainan. It agrees with other papers on reef and other bio environments, and has nothing to do with broad ocean acid-base..
I see that Nick is going ignore the other TWENTY TWO published papers,that also discusses “acidification” claims in other areas of the world.
You really that dense,Nick?
“other TWENTY TWO published papers”
That’s how it goes. Someone, often Richards, produces a long list of papers that are supposed to prove something or other. The other day we had 160, or 283 or something, papers supposed to show the global cooling scare. So one gets nominated. No, it doesn’t show it. But never mind, there are SO MANY others.
How about we find just one?
Nick, this is why you have little traction with many here, because you ignored what those other papers said. Here is what Kenneth Richards said to that hapless SOD,who make similar baloney comment you did here:
“Kenneth Richard 29. December 2016 at 7:35 PM | Permalink | Reply
sod: “It is always the same: you have found 2 or 3 papers who mention the term ‘acification’ and claim little effect. so you generalise to ‘acification does not exist’”.
It’s a little worse than that, sod. There are not “2 or 3” papers referenced here. There are 23. Close, though. And these are just samples of what’s available in the scientific literature.
Here are a few others that show that varying pCO2 by 285 ppm to 4,568 (pH range 8.1 to 7.1) over a 4-week period did not affect the growth and calcification of corals.
—–
http://link.springer.com/article/10.1007%2Fs00338-014-1241-3
This study investigated the response of the gorgonian coral Eunicea fusca to a range of CO2 concentrations from 285 to 4,568 ppm (pH range 8.1–7.1) over a 4-week period. Gorgonian growth and calcification were measured at each level of CO2 as linear extension rate and percent change in buoyant weight and calcein incorporation in individual sclerites, respectively. In general, growth and calcification did not stop in any of the concentrations of pCO2… These results highlight the susceptibility of the gorgonian coral E. fusca to elevated levels of carbon dioxide but suggest that E. fusca could still survive well in mid-term ocean acidification conditions expected by the end of this century, which provides important information on the effects of ocean acidification on the dynamics of coral reef communities.
—–
And another that varied seawater CO2 to levels of 430, 907, 1,865, and 3,247 ppm over the course of 6 months, but found corals were “largely unaffected” by each incremental CO2 change.
—–
http://www.biogeosciences.net/11/1581/2014/bg-11-1581-2014.html
Calcifying foraminifera are expected to be endangered by ocean acidification; however, the response of a complete community kept in natural sediment and over multiple generations under controlled laboratory conditions has not been constrained to date. During 6 months of incubation, foraminiferal assemblages were kept and treated in natural sediment with pCO2-enriched seawater of 430, 907, 1865 and 3247 [ppm] pCO2. The fauna was dominated by Ammonia aomoriensis and Elphidium species, whereas agglutinated species were rare. After 6 months of incubation, pore water alkalinity was much higher in comparison to the overlying seawater. Consequently, the saturation state of Ωcalc was much higher in the sediment than in the water column in nearly all pCO2 treatments and remained close to saturation. As a result, the life cycle (population density, growth and reproduction) of living assemblages varied markedly during the experimental period, but was largely unaffected by the [ppm] pCO2 treatments applied.
—–
And by the way, sod, it’s not “acification”, it’s acidification. I’d assume you’d want to use the proper scientific terminology.
http://notrickszone.com/2016/12/29/the-ocean-acidification-narrative-collapses-under-the-weight-of-new-scientific-evidence/#comment-1155091
Nick,writes this silliness since it is clear you didn’t read them:
“The other day we had 160, or 283 or something, papers supposed to show the global cooling scare. So one gets nominated. No, it doesn’t show it. But never mind, there are SO MANY others.
How about we find just one?”
Here it is Nickola boy:
International Team of Specialists Finds No End in Sight to 30‐Year Cooling Trend in Northern Hemisphere
“An international team of specialists has concluded from eight indexes of climate that there is no end in sight to the cooling trend of the last 30 years, at least in the Northern Hemisphere.
In some, but not all cases, the data extend through last winter. They include sea surface temperatures in the northcentral Pacific and north Atlantic, air temperatures at the surface and at various elevations as well as the extent of snow and ice cover at different seasons.
In almost all cases it has been found that the year‐to‐year variations in climate are far more marked than the long‐term trend. The long‐term trend often becomes evident only when data from a number of years are displayed.
The report, prepared by German, Japanese and American specialists, appears in the Dec. 15 issue of Nature, the British journal. The findings indicate that from 1950 to 1975 the cooling, per decade, of most climate indexes in the Northern Hemisphere was from 0.1 to 0.2 degrees Celsius, roughly 0.2 to 0.4 degrees Fahrenheit.”
http://www.nytimes.com/1978/01/05/archives/international-team-of-specialists-finds-no-end-in-sight-to-30year.html?_r=1
Don’t continue your feeble replies here,ok? I lived though that decade as a Teenager who read some of these reports. Where were you,in a coma during that time?
Don’t lie about the global cooling concerns that were discussed in science research,the magazines and the networks anymore.
Kenneth,in reply to the hapless David Appell answers:
“Kenneth Richard 31. December 2016 at 6:52 AM | Permalink | Reply
Um, no, I didn’t change the subject. Assuming you agree with the claim that human CO2 emissions have directly caused a pH lowering of -0.1 in the last 150-200 years, you suggested I need to “disprove” this claim. Disproving your claim implies that it has already been scientifically proven as true, that it is a verifiable fact. Can you point to the experimental scientific evidence that shows human CO2 emissions cause oceanic pH levels to decrease on centennial scales, but that the natural factors and variations which contribute to + or – 0.2 to 0.5 decadal-scale changes in pH do not have any influence whatsoever on detectable centennial-scale trends? In other words, provide the physical, observational evidence that shows natural variability contributes nothing to centennial-scale trends.
But speaking of publishing in Nature and Science, it looks as though this has already been done.
http://www.nature.com/news/ocean-calamities-oversold-say-researchers-1.16714?WT.mc_id=TWT_NatureNews
The state of the world’s seas is often painted as verging on catastrophe. But although some challenges are very real, others have been vastly overstated, researchers claim in a review paper. The team writes that scientists, journals and the media have fallen into a mode of groupthink that can damage the credibility of the ocean sciences. The controversial study exposes fault lines in the marine-science community. Carlos Duarte, a marine biologist at the University of Western Australia in Perth, and his colleagues say that gloomy media reports about ocean issues such as invasive species and coral die-offs are not always based on actual observations. It is not just journalists who are to blame, they maintain: the marine research community “may not have remained sufficiently sceptical” on the topic.
http://www.nature.com/ismej/journal/v6/n9/full/ismej201219a.html
We found that pH did not have a significant impact on the composition of associated microbial communities in both coral species. In contrast to some earlier studies, we found that corals present at the lower pH sites exhibited only minor physiological changes and no microbial pathogens were detected. Together, these results provide new insights into the impact of ocean acidification on the coral holobiont.
http://www.nature.com/nclimate/journal/v2/n8/full/nclimate1473.html
Using a model of pH regulation combined with abiotic calcification, we show that the enhanced kinetics of calcification owing to higher temperatures has the potential to counter the effects of ocean acidification.
http://www.sciencemag.org/content/344/6186/895.abstract
Reef corals are highly sensitive to heat, yet populations resistant to climate change have recently been identified. To determine the mechanisms of temperature tolerance, we reciprocally transplanted corals between reef sites experiencing distinct temperature regimes and tested subsequent physiological and gene expression profiles. Local acclimatization and fixed effects, such as adaptation, contributed about equally to heat tolerance and are reflected in patterns of gene expression. In less than 2 years, acclimatization achieves the same heat tolerance that we would expect from strong natural selection over many generations for these long-lived organisms. Our results show both short-term acclimatory and longer-term adaptive acquisition of climate resistance. Adding these adaptive abilities to ecosystem models is likely to slow predictions of demise for coral reef ecosystems.”
http://notrickszone.com/2016/12/29/the-ocean-acidification-narrative-collapses-under-the-weight-of-new-scientific-evidence/#comment-1155421
There are simply too many published science research showing that CO2 has little to no harmful effects on Corals and other marine organisms.
Sunsettommy,
“Here it is Nickola boy”
That isn’t a scientific paper, it’s a newspaper report. It’s talking about a paper, but doesn’t name the authors or give proper citing information. How about citing and quoting the paper? It seems to be just another one documenting recent temperatures.
And your quote from Richards thread does not reveal more papers about acidification. It describes how some particular organisms respond to high CO₂.
Nick, you’re not helping again. I know you’ve been warned about this in the past. Obfuscation happens accidentally in scientific writing, it’s to be expected. Par for the course as it were. But when it becomes a trend, a “habit”, it gets to be annoying. We’re all wrong most of the time, but not all the time. You need to bring up your average.
I suppose we all do. But it would be easier on the rest of us if you expressed a bit more humility. When you present your speculation with such firm sincerity, you are likely to mislead the less informed. As a scientist, you have a moral obligation to avoid doing that. Of course, that’s just my opinion, but then it’s also the subject of the article.
Nick,who is now in desperate mode, tries a deflection when he says this:
“That isn’t a scientific paper, it’s a newspaper report. It’s talking about a paper, but doesn’t name the authors or give proper citing information. How about citing and quoting the paper? It seems to be just another one documenting recent temperatures.”
However in the article he whines over sn a newspaper article written in 1978 by WALTER SULLIVAN,was credible and contained these statements:
“¶Average surface air temperatures recorded at 358 stations north of latitude 20 degrees south from 1951 to 1975 have been analyzed by Drs. R. Yamamoto and T. Iwashima of Kyoto University in Japan on regional and season bases. A general cooling is evident with “an intensive cooling episode” from 1961 to 1964.
¶Generally similar trends are evident in temperatures of the lower 18,000 feet of the atmosphere as charted by Dr. Horst Dronia of the Weather Office in Hannover, West Germany. For the period from 1949 to 1976, he has calculated, for 220 points in the Northern Hemisphere, the average temperature of the atmosphere from the separation between the pressure levels near the surface (at 1,000 millibars) and one high up (at 500 millibars). An increase in separation indicated expansion and hence warming. A decrease, for example, of 20 meters (66 feet) was taken to mean atmospheric shrinking, indicating a cooling in that case of I degree Celsius (almost 2 degrees Fahrenheit).
¶Observations extending higher into the atmosphere confirmed the trend. The authors were Drs. J. K. Angell and. 1. Korshover of the National Oceanic and Atmospheric Administration Laboratories. in Silver Spring, Md.
¶North Pacific water temperatures compiled by the same agency’s Marine Fisheries Service have been analyzed by Dr. Jerome Namias of the Scripps Institution of Oceanography at La Jolla, Calif. The original source was temperature readings of cooling water intake made by ships at a rate of more than 20,000 a month. The data, plotted for 153 locations, show a gradual cooling broken by a sharp warming in 1967‐68.
¶A similar study based on data from weather ships in the North Atlantic has been done by Dr. Martin Rodewald, former head of the Oceanic Division of the German Weather Service. Since the seven American weather ships were withdrawn in 1973 only two have remained, but observations of a cooling trend have continued.
¶A gradual increase in area of the northern circumpolar vortex, the massive flow of frigid air around the Arctic, has been recorded by Drs. Angell and Korshover. In 1976 its southern’ extent was the greatest in 10 years and last winter it was 1 percent larger than in any previous winter observed.
¶Snow and ice cover in the Northern Hemisphere have varied greatly but there has been a net increase according to a satellite photograph analysis by Dr George J. Kukla of Columbia University’s Lamont‐Doherty Geological Observatory. This has been most marked in the spring when so highly reflective a cover returns much solar energy into space at a time of intense solar radiation.
¶Antarctic sea ice coverage, after increasing to 1972, has been shrinking.
The observations come, at a time when a warming trend could have been expected from the increase of carbon dioxide in the atmosphere due to extensive fuel burning. The gas inhibits the escape of solar heat from the earth. Dr. Kukla, in a telephone interview this week, said that the cause of the apparent cooling remained unknown and that no scientific attempt to predict whether the trend would continue was possible. Monitoring of the various indexes is continuing, he added.”
As for the Richards essay about the feebleness of the acidicfication propaganda are numerous examples of ocean BIOTA gladly using locally elevated CO2 levels in the water to build structure.
Here is but two examples you missed from Kenneth Richards post:
“http://link.springer.com/article/10.1007%2Fs00338-014-1241-3
This study investigated the response of the gorgonian coral Eunicea fusca to a range of CO2 concentrations from 285 to 4,568 ppm (pH range 8.1–7.1) over a 4-week period. Gorgonian growth and calcification were measured at each level of CO2 as linear extension rate and percent change in buoyant weight and calcein incorporation in individual sclerites, respectively. In general, growth and calcification did not stop in any of the concentrations of pCO2… These results highlight the susceptibility of the gorgonian coral E. fusca to elevated levels of carbon dioxide but suggest that E. fusca could still survive well in mid-term ocean acidification conditions expected by the end of this century, which provides important information on the effects of ocean acidification on the dynamics of coral reef communities.”
or this one,
“http://www.biogeosciences.net/11/1581/2014/bg-11-1581-2014.html
Calcifying foraminifera are expected to be endangered by ocean acidification; however, the response of a complete community kept in natural sediment and over multiple generations under controlled laboratory conditions has not been constrained to date. During 6 months of incubation, foraminiferal assemblages were kept and treated in natural sediment with pCO2-enriched seawater of 430, 907, 1865 and 3247 [ppm] pCO2. The fauna was dominated by Ammonia aomoriensis and Elphidium species, whereas agglutinated species were rare. After 6 months of incubation, pore water alkalinity was much higher in comparison to the overlying seawater. Consequently, the saturation state of Ωcalc was much higher in the sediment than in the water column in nearly all pCO2 treatments and remained close to saturation. As a result, the life cycle (population density, growth and reproduction) of living assemblages varied markedly during the experimental period, but was largely unaffected by the [ppm] pCO2 treatments applied.”
There are dozens more like this showing that high CO2 levels doesn’t shut down or kill organisms.It is clear you are disagreeing with many published scientists on this topic. Maybe YOU should publish a paper demonstrating their many errors,that you have yet mentioned here.
You are rapidly becoming a joke,Mr Stokes!
People also claim that the motive for alarmism is funding / money / noble cause corruption. Google scholar shows some of the 2004, 2006, 2008 et seq papers by Feely, Sabine and co-workers have been cited thousands of times. No doubt that is good for the next research grant, but also aggrandises their reputation, despite their omission of data that does not support their thesis.
“Alarmists seem to want their models, theories and opinions to be accepted as established fact. But the reality is their shaky theories are full of poorly supported conjecture and extrapolation.”
Very well put.
Yup, sounds like AGW in a nutshell.
I have yet to see an explanation of how an ocean can be both warming AND becoming less alkaline. A warming ocean GIVES OFF CO2, it doesn’t absorb it. That’s why there’s a delay in rising CO2 when the temps go up.
We can see the effects of the MWP in the rise of CO2 from the 1800’s – smack on the time delay needed for oceans to begin releasing CO2.
“I have yet to see an explanation of how an ocean can be both warming AND becoming less alkaline.”
As water warms, the ratio between [CO₂] in water and pCO₂ in air diminishes. But if pCO₂ rises faster than this ratio, through our emissions, then more CO₂ dissolves, despite the warming.
Nick, quit digging.
Stan, he seems to forget that the Ocean waters already has 99% of free CO2 in the system,a dribble more isn’t going to destroy the highly buffered chemistry that has been around for hundreds of Million years.
He doesn’t understand the dumb propaganda that “acidification” is.
Thanks for putting it so clearly – ‘saved me the trouble….!
The number of “scientists” who get this so wrong should be a worry for educators the world over.
See Don J Easterbrook’s testimony to the US Senate, where he explains in one sentence what he thinks of the “acidification” scare: “… thats a fraudulent statement…”
Now that it’s clear where the bulk of the atmospheric CO2 comes from (the oceans – not cars, factories or aircraft) and why the changing CO2 levels FOLLOW the varying temperature by about hundreds of years, it seems that “climate science” follows different rules to those of real science…
airbornedata,
The CO2 levels followed temperature with different lags, from a few months (seasonal and ENSO) to decades (MWP-LIA) and hundreds to thousands of years (galcial-interglacial and reverse), but that is not the case for the current increase. The current equilibrium level for the current average seawater surface temperature is ~290 ppmv in the atmosphere. The rest of the 400 ppmv in the atmosphere is caused by ~200 ppmv human emissions since the start of the industrial revolution. The net flux of CO2 is from the atmosphere into the oceans, not reverse, as DIC in the oceans increases everywhere (and pH decreases), while it should decrease (and pH increase) by warming oceans. Here for Bermuda and Hawaii:
http://www.biogeosciences.net/9/2509/2012/bg-9-2509-2012.pdf
http://www.pnas.org/content/106/30/12235.full.pdf
Which doesn’t imply that there are any negative effects from more CO2 in the atmosphere or oceans…
“Dissolution of CaCO3 in equatorial Pacific sediments has intensified during the late Holocene, having now reached an intensity that is comparable to that which occurred during the onset of each of the late-Pleistocene periods of glaciation. Extrapolating from the robust relationship that has characterized at least the past 500 kyr, we conclude that the ocean’s carbonate chemistry has already made the transition that would lead into the next period of continental ice sheet growth.”
Modern CaCO3 preservation in equatorial Pacific sediments in the context of late-Pleistocene glacial cycles, R.F. Anderson, M.Q. Fleisher, Y. Lao and G. Winckler
Marine Chemistry xx (2007) xxx–xxx
http://www.ldeo.columbia.edu/~winckler/Publications_files/Anderson_et_al_mar_chem_2007.pdf
Not much to add to these perspectives. Perhaps one. The natural diurnal/seaonal/ecosystem variation in ocean pH exceeds by about 10x that posited by AGW. So the whole ‘acidification’ false alarm is bogus. Some referenced examples in essay Shell Games in ebook Blowing Smoke. A subset thereof available free at guest post on CE about three years ago. You only miss the corals example.
I’ll bet that the vertical profile of ocean pH also varies by more than what AGW fears – particularly if taken over a vent.
Yup. And does not require a vent. Ocean is vast. The mixed zone is very biologically active.
What is really being said here is “James Delingpole is far more convincing than the experts are”. Every time I read these things (like Russia throwing our election—meaning Russia was more convincing and credible than our Democrat candidate), I think how really clueless these people must be not to realize they are saying their position is weak and unconvincing.
It comes down to people telling the truth being more convincing and believable than people spouting alarmist gobbledygook.
Most people know hogwash when they smell it, even if they do not have a strong background in the relevant scientific disciplines.
One often judges the product by the sales representative.
Just Curious since I have no knowledge of the Veracity of the English University System; Does ANY valid Science come out of the University of Eco Alarmism?
No.
But they’re good at Modern Literature.
Only by accident.
Even the worst soldier can hit the center of a target if he fires enough shots.
Or if he’s from Texas, shoot once and paint the target around the hole. 😉
During one of my recent climate change presentations a member of the audience broke into the middle with an obvious challenge about the Great Barrier Reef dying because of ocean acidification. Rather than go off track I expressed my doubts saying that’s not what I recalled and tabled it until the end. I had some understanding about the GBR, but had to do more research. The claim is wrong on two points.
Point 1)
Above a pH of 7.0 a solution is alkaline and becomes more or less alkaline.
Below a pH of 7.0 a solution is acidic and becomes more or less acidic.
HS chemistry class.
The ocean’s pH is about 8.0. That’s alkaline. Variations are more or less alkaline, not more or less acidic. The obvious reason for incorrectly using the term “ocean acidification” is a propaganda gambit to scare the gullible and uninformed who associate acid with bad, like alien blood and spit.
Highly alkaline compounds such as caustic soda can be just as dangerous as acidic compounds, e.g. concentrated bleach, sodium hypochlorite, pH 9 to 13. On the other hand: rain has a pH of 4.5, lemon juice has a pH of 2.0, tomatoes a pH of 4.5, and vinegar a pH of 2.2. If they get on your hands the flesh doesn’t melt and they don’t burn a hole in the kitchen counter.
Improperly using the term “acidification” to scare the public over bogus CAGW is a disgrace to science. Spit out the Kool-Aid and grow a backbone.
Point 2)
Bleaching is not dying. There have been numerous bleaching incidents in the past. Bleaching is caused by, among other causes, warm water temperatures especially those caused by El Ninos and NOT pH. The GBR has always mostly recovered. Long term damage or death has been relatively minor. The polyps and other life that inhabit the coral structure die when the water gets too hot or too cold or too fresh and when the conditions revert they recolonize. That’s the way it has been going for millions of years.
Also during the post presentation the same person stated another BS MSM sound bite and another member of the audience pronounced it BS whereupon they got their undies in a knot and stomped out of the room like a six year old possibly to go whine at their mommy.
If you thin skinned uppity millennial snowflakes don’t want your BS challenged quit believing the MSM and green web site sound bites & do your own homework and thinking!!! Yes, saving the world makes one all warm and fuzzy. Too bad it’s not real.
BTW per GBRMPA over 75% of the GBR is just fine. 22% is badly damaged, 85% of that is in the northern sector and El Nino was at fault.
http://www.gbrmpa.gov.au/media-room/latest-news/coral-bleaching/2016/the-facts-on-great-barrier-reef-coral-mortality
http://www.gbrmpa.gov.au/media-room/coral-bleaching
Would you prefer de-alkalization?

“Bleaching is not dying.”
No but it’s not a large step from one to the other.
“There have been numerous bleaching incidents in the past.”
And also some dying events.
“and El Nino was at fault”
Oh, nothing to see, move along.
You forgot the sarc tag Tony !
And That’s not all…
Great Barrier Reef Almost Drowned 125,000 Years Ago
…studying the impact a warming climate had on the Great Barrier Reef during the Last Interglacial period over 125,000 years ago.
Published in the journal Global and Planetary Change, the study led by researchers from the University of Sydney found the natural wonder off Australia’s east coast almost drowned and died at the time. The Last Interglacial was marked by higher temperatures, which led to melting of glaciers and polar ice sheets, raising sea levels. While both the temperatures and sea levels then were higher than they are now, scientists consider it a significant comparative period because Earth might head in that direction if carbon emissions don’t reduce soon.
…
The research paper, titled “The evolution of the Great Barrier Reef during the Last Interglacial Period,” found that the reef began growing once again after sea levels stabilized, a testimony to its resilience. However, the modern-day shallow reef is the last layer of a much thicker reef system, and it grew on top of the previous layer. But the current human-induced climate change and pollution threatens its existence.
It survived being drowned and survived being left high and dry during the last glacial maximum but is doomed by a bit of El Nino bleaching. Yeh Right!!!
Link:
https://www.yahoo.com/news/great-barrier-reef-almost-drowned-061225252.html
Crown of thorns starfish and other imported pests are not helping either.
Previous Delingpole victory over the oppressor class.
https://wattsupwiththat.com/2011/04/09/james-delingpole-beats-a-press-complaint-from-uea/
Censorship, name calling, appeal to ‘authority’, sophist distraction instead of addressing key points…. Phil Williamson seems to be one unsettled NERC science coordinator!
Why has no one mentioned the margin for error that’s used when measuring ocean ph , last I heard ph of the ocean was 8.1 , not acidic .
It does vary naturally 8.3 is considered to be normal or heathy so taking the error margin / fudge factor into account 8.1 is ok .
Anyone using the words “ocean acidification ” cannot be an expert in any field except maybe journalism or politician.
It would be useful if there was an accepted standard method for measuring oceanic pH. At the moment everybody just does it the way they want to so nothing compares adequately with any other method.
Still, I suppose some people take refuge in ambiguity. It happens a lot, this mixing up of methodologies.
Nearly every river that has run into the world’s oceans for millions and millions of years as been neutral or on the acidic side of neutral pH… sometimes down to pH 5.5 or even lower.
Yet the oceans have remained steadfast around the 8.1pH +/- a bit.
Carbonic acid as formed from CO2 and H2O can only exist for milliseconds and the resulting carbonates are used by crustaceans etc to build their shells.
Sea water is MASSIVELY buffered by salts, and carbonates, being surround and contained within carbonate rocks and sands.
Any one that thinks a tiny change in atmospheric CO2 will have the slightest effect , has put their brain to sleep, or has drunk so much Klimate Kool- Aide as to need a massive stomach and brain pump.
What you say about acidic river water is absolutely true of course, but there is another source of acidity in seawater, namely underwater hydrothermal vents on spreading zones, which must be pumping millions of tonnes of SO2, HCl, and H2S into the oceans every year.
Nick Stokes: Re: Lewis acids. Your equation is still very much a Bronsted-Lowry acid/base reaction as it omits the reaction of CO2 with water to make carbonic acid (H2CO3). Carbonic acid plus carbonate gives two moles of bicarbonate. As I said, absolutely nothing to do with Lewis acids. The Lewis definition of acids (electron pair acceptors) and bases (electron pair donors) is subsumes the Bronsted-Lowry definition (proton donors and acceptors, respectively). Thus, all protic acids are Lewis acids, but not all Lewis acids are protic acids, and ditto for bases.
Graemethecat ,
“As I said, absolutely nothing to do with Lewis acids. The Lewis definition of acids (electron pair acceptors) and bases (electron pair donors) is subsumes the Bronsted-Lowry definition”
That is rather contradictory. You can always regard it as Lewis acid-base. Your alternative view assumes that H₂CO₃ must be formed first. I don’t know whether that is true, but the Lewis view lets you be agnostic about it.
Anyway, the key thing is that you can write the overall equilibrium without protons:
CO₃⁻⁻+CO₂+H₂O ⇌ 2HCO₃⁻
You can write subsidiary equilibria with protons, such as
CO₃⁻⁻+H⁺ ⇌ HCO₃⁻
and that determines pH, but the reaction can’t go anywhere while pH stays around 6 or more.
When the earth was at 3000 ppm CO2, life in the ocean, including corals, and on land was thriving. To think that now, with CO2 levels barely above the minimum level to support life, the oceans are going to become an acid bath and most marine life will become extent lacks any common sense.
What’s that old saying? “Common sense is not so common.” ESPECIALLY in “Klimate Science.”
I might have something utterly wrong here, but this is how I think of it..
The Ocean is, on average, 4,000 meters deep.
On average, every year, just over 1,000mm (1 metre) of rain falls on it. (~ 770mm rain falls on the land)
Every drop of rain that falls through an atmosphere containg *any* amount of CO2 will hit the ground/water at about 30mph and with a pH of about 5.5
So, every 4,000 years, the ocean effectively evaporates and is replaced by the same amount of water with a pH of 5.5
Its nowhere near as simple as that but….
Now, do we say that The Ocean is 4,000 million years old? Keeps the maths simple for the Good Professor (and Griff)
Therefore, the entire ocean has been evaporated and replaced by acidic water about 1 million times since the start of history.
So Professor, why is The Ocean still alkaline?
meters. metres smeters, mtrees. sigh
tink its time UK folks gave up on the Europe Thing
oh, wait……….
Peta,
Youi forgot the other side of the balance: where water evaporates, mostly near the equator, also CO2 is emitted. That means that the deep ocean-air carbon cycle (~40 GtC/year) between the warm waters and cold uptake is about in equilibrum, as most of that water + CO2 sinks near the poles and returns ~1000 years later near the equator…
Currently slightly more CO2 is going into the deep than returns, due to the increased CO2 pressure in the atmosphere…
IPSO is not an arm of Uk govt -it is an independent regulator for press standards -see here:
https://www.ipso.co.uk/
It does not shut down freedom of information -rather it protects it.
It seems to agree or at least not dispute Delingpole is utterly wrong -but argues that misrepresenting the science is OK because its just ‘opinion’
utterly wrong
The technical term for the rubbish spouted by the Grauniad and the Biased Broadcasting Corporation on this subject…
The oceans are becoming more acidic at the fastest rate in 300m years, due to carbon dioxide emissions from burning fossil fuels
Alex Rogers, professor of biology at Oxford University, said: “The health of the ocean is spiralling downwards far more rapidly than we had thought. We are seeing greater change, happening faster, and the effects are more imminent than previously anticipated. The situation should be of the gravest concern to everyone since everyone will be affected by changes in the ability of the ocean to support life on Earth.”
https://www.theguardian.com/environment/2013/oct/03/ocean-acidification-carbon-dioxide-emissions-levels
Utterly wrong
The researchers say that by 2020, ten percent of the Arctic will be inhospitable to species that build their shells from calcium carbonate. By 2100 the entire Arctic will be a hostile environment.
http://www.bbc.co.uk/news/science-environment-24904143
Utterly wrong – again
Williamson doesn’t even have a page in Connollypedia
Griff has made his prediction (Ice) in the other thread. Won’t have to wait long for a credibility check…
He has not misrepresented any science, computer modelling is not science. Much of the impact stuff takes place in labs where they place shellfish in a flask, add acid and note that its shell dissolves.
Even worse as many tests were done with strong acids and a sudden supply to get the right pH change, but that doesn’t say anything about a slow change over time with increasing CO2 levels in the atmosphere where the shellfish has time to adapt…
Exactly, so the tests were not models of the situation in nature.
http://www.nature.com/news/crucial-ocean-acidification-models-come-up-short-1.18124
Yet according to a survey published last month by marine scientist Christopher Cornwall, who studies ocean acidification at the University of Western Australia in Crawley, and ecologist Catriona Hurd of the University of Tasmania in Hobart, Australia, most reports of such laboratory experiments either used inappropriate methods or did not report their methods properly
To assess the use of appropriate experimental design in ocean acidification research, 465 studies published between 1993 and 2014 were surveyed, focusing on the methods used to replicate experimental units. The proportion of studies that had interdependent or non-randomly interspersed treatment replicates, or did not report sufficient methodological details was 95%.
95% were not fit for purpose
Kip Hansen covered this here https://wattsupwiththat.com/2015/09/04/ocean-acidification-trying-to-get-the-science-right/
Further, “the number of experimental units used per treatment in studies was low (mean = 2.0).” Think about that — imagine doing a medical study, an RCT, but using only 2 patients per cohort. Then consider that there are obvious co-confounders with the two patients, such as being siblings! No journal would touch the resultant paper – it would have no significance at all. Granted, one might get away with reporting it as a Case Study, but it would never be considered clinically important or predictive. And yet that is precisely the situation we find generally in OA research – very small numbers of experimental units poorly isolated, often with co-confounders that obfuscate or invalidate treatment effects.
Since “acidification” is mis-leading, and “neutralisation” is boring, why don’t we take a leaf out of the alarmists book and call it “de-caustification”
Because “neutralisation” is correct.
It looks as if the Independent backs UEA:
http://www.independent.co.uk/voices/science-loses-out-to-uninformed-opinion-on-climate-change-yet-again-a7515711.html
Well that’s a huge shock. /s
You can’t hide the decline – of the UAE’s reputation.
But it’s still…
The University of Easy Access.
A long time ago I used to go out with a girl from UEA , she was ‘Easy Access’ but then so was I…..happy days.
I heard from a U of East Anglia climate researcher on NPR (National Public dogma-Radio) that anthropogenic CO2 emissions have been steadily increasing at the rate of 3% per year, leading me to wonder:
At the current ~400PPM count, it would seem we’re running lower than expected given that we were at ~300PPM in 1950. ?? At 3%/yr, starting at 300 in 1950, mathematically, it doesn’t work out.
67 years later?
So they can’t do Maths, Physics or Chemistry…a top notch Uny !!!
Glass and glass lined steel vessels are widely used in the chemical industries for their resistance to chemical corrosion in acid conditions particularly hydrochloric acid. hot caustic soda will however attack glass.
If it is a matter of fact that “ocean acidification is an inevitable consequence of increasing carbon dioxide in the atmosphere” then can there be no circumstance where increasing carbon dioxide in the atmosphere does not have that consequence?
What about the warming period at the transition from ice age to interglacial?
OA is a deliberately scary-named meme to prop up global warming / climate change in the absence of any actual warming. File alongside catastrophic sea level rise, collapsing sea ice, collapsing glaciers, acid rain, storms, hurricanes and the crocodile under my bed. All b*ll*cks.
In spite of so many scientific works-made on the basis of models, assumptions, measurement, and who knows what else, to date there is no real evidence of who caused climate change on our planet. Each of these articles carries little hint of the real causes, but it is only one dot in relation to the overall picture of these causes.
Once again I have to, again, draw the attention of everyone involved in this research, that almost all new way to deceive “knowing” the truth, using models and mathematics. Almost no one uses logic and consciousness, which are associated with the “warehouse” of all causes and knowledge of the true causes of any phenomenon.
If using logic and natural law, then it must reject the assertion that climate change and global warming resulting from human factors.
Climate changes are the consequences of interaction between the planet and the sun. But how ? That you should explore !!
Here, my help: change the magnetic fields of the planets and their variations caused by changes in temperature and planets themselves and their wrappers. Again, I should know how and why. If anyone is interested, we can bring about discussion.
If this does not happen, it means that everyone staying in positions for which no truth can get more money than the truth. Why? Therefore, the truth is one and few believed in it. There is much more money on combinatorics unknown quantities, as used by “experts” who have given today and wrote several million “evidence, valued at approximately $ 45 billion in the last 20 years (around 2 billion). Only set, and I submit that millions of not stating the truth, because you will lose profits if the truth wins
Can we put some hard numbers into this? The surface oceanic CO2 level in the 1970’s was 2012 micromoles of CO2 per kilogram of seawater, according to the GEOSECS program, which actually went out and measured CO2 levels. (This, to my knowledge, is the last time anyone actually went out and did a thorough measurement across the major oceans at all depths.) Atmospheric level at the time was about 330 ppm by volume. Converting the atmospheric figure to a concentration by weight, the oceanic/atmospheric ratio of CO2 concentration at the time works out to about 147:1.
Since that time the atmospheric level has increased to 400 ppm, i.e. about a 20% increase. Since the oceanic concentration is so much greater, the effect on it of this increase would be minimal, significantly less than a 1% change.
The oceanic pH was measured by GEOSECS to be in the region of 8.0, but varied significantly from place to place and time to time. If it maintained a comfortably alkaline pH with such a relatively massive amount of CO2, the effect on its pH with such a minor increase in CO2 levels would be drowned (pun intended) by the normal variation in pH. In effect, looking for pH changes in the ocean due to atmospheric CO2 changes is looking for a very small signal drowned in noise. I suspect very few of the oceanic acidification mavens have any experience in dealing with very small signal-to-noise ratios.
The whole concept of ocean acidification may have arisen from an egregious numerical error in a technical paper by Takahashi et al. (https://dge.carnegiescience.edu/SCOPE/SCOPE_16/SCOPE_16_1.5.07_Takahashi_271-286.pdf) written at the end of the GEOSECS program. In this paper they calculated the average CO2 concentration in the world’s oceans then multiplied this number by the volume of the oceans to obtain the total CO2 content. However, they figure they used for the volume of the oceans was 1370 cu.km, (see p.279 of the paper) whereas the actual figure is 1370,000,000 cu.km, so their figure for the total CO2 content was too low by a factor of a million. To compound this error, the erroneous total CO2 content was given in the summary at the top of the paper but not the (correct) average concentration.
Based on this erroneous figure, the atmosphere contains about 20,000 times as much CO2 as the oceans, so it would be reasonable to assume that any increase in atmospheric CO2 would have a significant effect on oceanic pH. However, put the missing factor of a million back in and the whole problem disappears.
Roger,
Besides the (possible?) calculation error, you need to make a differentiation between the ocean “mixed layer”, the upper few hundred meters of the ocean in direct contact with the atmosphere by wind and waves, and the deep oceans which are quite isolated from the atmosphere. The exchanges of the surface are fast: half life time less than a year, while the deep oceans – atmosphere exchanges have half life times of ~35 years.
While the deep oceans have a carbon content of ~28,000 Gt, the surface has only ~1,000 GtC, comparable to the atmosphere at ~800 GtC. Due to buffer chemistry, a change of 100% in the atmosphere only gives a change of 10% in the ocean surface and a small change in pH. That is measured at a few fixed stations over the past decades. That makes that the surface is readily saturated.
The absorption by the total oceans is near unlimited and indeed all human emissions up to now would give some 1% increase in the deep oceans and atmosphere when back in equilibrium, but because of the limited exchange fluxes, that will take a lot of time…
What would happen if CO2 levels went the other way and decreased? How would carbonate based shells be grown? How would seaweed grow without CO2? An ocean without CO2 would be in much worse shape. Adding CO2 to sea water is like adding CO2 to a greenhouse, the algae, plankton and innumerable other CO2 dependent creatures would be very thankful.
I don’t think we have to worry about that. The oceans are where the vast bulk of CO2 resides, and anything that happens in the atmosphere isn’t going to change it.
If they were serious about this they would study highly productive estuaries and highly reduced sediments, both which are full of life. Organisms have ways of either walling off or modifying the environment either externally or internally. As noted above acid rivers can affect low salinities, but salt water chemistry still dominates. I recall measuring 6.9. There are a few papers on this, but historically not much interest. Would like to see a correlation attempted between productivity or just biomass against both pH concentrations and variations. It would be misleading, but perhaps instructive.
We’re all doomed.
Ocean ACIDIFICATION:
Calcium carbonate bubbling away.
The Oceans look like a can of boiling club soda.
The white cliffs of Dover collapse into the sea only to froth even more.
Oh bother! Oh worry! Oh fret.
That does it, I’m spending my 401K now.
I have a lot of trouble seeing how CO2 is a danger to coral or shellfish in general. The main ingredient used in building shell is CO2. The Great Barrier Reef is built from billions and billions of tons of CO2. Without CO2 would would be no coral.
It isn’t like the oceans are actually acidic. They are alkaline and heavily buffered. Inorganic chemistry says it will be very, very difficult to make the oceans acidic. You would need to precipitate vast quantities of salt.
If anything, the salt that would precipitate if you add CO2 to the oceans is limestone, CaCO3. And this is the principle ingredient in shell. So if anything, adding CO2 should make it easier for organisms like coral to make shell.
It sounds to me like people have gotten caught up in the issue of acid dissolving limestone, but they have failed to consider that this ONLY WORKS works on land, where CO2 and water can interact over centuries to form limestone caves.
In the oceans this DOES NOT HAPPEN. Seawater does not dissolve limestone. Rather, limestone is formed from seawater.
Speaking of acid and corals, came across this. Worth repeating. Always knew some students were smarter than their professors.
“With such low pH levels, it’s a wonder there are still reefs left in Palau to study. But, as Barkley discovered during her most recent trip, these conditions have had no discernable impact on the corals. Nor did the hot-tub-like temperatures appear to have an impact, since there was little evidence of coral bleaching.
Not only were the reefs seemingly unaffected, but certain ones—the lagoon reefs in particular—appeared to be doing well. They were saturated with the vivid, splashy colors that recreational divers dream about, and they sheltered a huge and diverse population of fish. “
From http://www.whoi.edu/oceanus/feature/coral-crusader
Honest observer number 1
“Acidification” As said several times before, this nonsensical term for the pH change is analogous to claiming that if one slows down one’s pace it means walking more backwards. And 600 papers scientific written with that terminology! Shameful.
If decreasing basicity really is a problem leading to decreasing calcification in ocean creatures I’m sure they will adapt; just as it has in the past. The scare – that ocean life will be decimated – in unjustified.
Congratulations to James on the free speech point.
On the science, unlike many of the diverse fields related to climate, there are actually a number of researchers in the marine field who are questioning the alarmist claims and are getting published (see http://icesjms.oxfordjournals.org/content/73/3/529.1.abstract ). The whole journal issue is concerned with the issue and many of the papers are open access.
as a general note, to all of you,
on my experience with pools and cooling towers
[I have had a pool for more than 24 years]
I have noted that pH7.4
I am not exactly sure why.
perhaps a biologist can tell me?
perhaps primitive organisms like acid?
you tell me
last comment came out wrong? puzzles me/
as a general note, to all of you,
on my experience with pools and cooling towers
[I have had a pool for more than 24 years]
I have noted that pH smaller than 7 produces more growth and algae than pH greater than 7.4
I am not exactly sure why.
perhaps a biologist can tell me?
perhaps primitive organisms like acid?
you tell me
@Nick & others
I think I figured it out/
I mean the answer to my own question…
If we talk about fresh water, I think you will find as the pH declines, salinity increases. It is not the decrease in pH that primitive live seems to prefer, it is the increase in salinity and other waste [from humans and factories] that the algae etc. thrive on….
hence, for example, by experience, I have stopped using calcium hypochlorite in my pool, since I noticed that although the chlorine [from the granules] initially does work to kill,
I found that some kind of algae were thriving on the calcium being added to the water.
I must say that I remember standing at some cooling towers not believing the incredible persistence to the right of life by these creatures. I think I tried to add chlorine and or acid to sort the problem… Maybe if I had realized what I know now I could have done something else about the problem….
Hence, the end of this discussion on ocean acidification [we are talking about one or two hundredth of a pH unit], is exactly the same as the end the of the carbon dioxide discussion
more carbon dioxide and acid/salt is better for life
it is the dung that we throw around that actually promotes life…
“some kind of algae were thriving on the calcium”
Use sodium hypochlorite
“more carbon dioxide and acid/salt is better for life “
Which acid do you prefer?
Nick says
“some kind of algae were thriving on the calcium”
Use sodium hypochlorite
“more carbon dioxide and acid/salt is better for life “
Which acid do you prefer?
Henry says
Most algae thrive on sodium as well…
I use the tri-CL-cyanuric acid
compensating pH to >7.4 with bi-carbonate
My pool is always crystal clear.
I am toying with you…
“Professor Phillip Williamson, NERC Science Coordinator, University of East Anglia has written a long whiny piece in The Conversation, complaining that the British Government didn’t do enough to silence James Delingpole’s criticism that the Ocean Acidification scare is nonsense.”
Those hypocrites.
A lot of water bombs and other heavy weponry lies sunk since WWII both in the north sea as in the Baltic Sea.
Seems no problem for environmentalists. Or their ‘marine life’.
Main concern is Boy’s holidays on that touristic juwel barrier reef.