Guest essay by Leo Goldstein
While we are watching the 21st episode of the Clowns on Parade series (COP21), we should remind the leading participants of a couple of facts that even a clown apprentice can understand. The US, Western Europe, Canada, and Australia (the only countries in the world that engage in the climate masochism) release less than one-third of the anthropogenic CO2, an even smaller part of other “greenhouse gases” (a misleading name), and almost no black soot. This fact is not in dispute, but the alarmists usually reply that these countries are “historically responsible” for the most CO2 emissions.
Well, this claim is incorrect, too, and not only because CO2 release is beneficial because of its large fertilization effect and small and slow warming effect. When the sinks are properly taken into account, only 33% of the surplus CO2 in the atmosphere is attributable to the US and other Western countries. The rest is attributable to China and the rest of the world, as shown in Table 1.
Table 1. Surplus CO2 attribution by country or group of countries, 2012.
| Country or group of countries | Surplus CO2 attribution |
| USA | 20% |
| Western Europe | 10% |
| Canada + Australia | 3% |
| China | 15% |
| Rest of the World | 51% |
This analysis uses data and methodology from the most official IPCC aligned authors, including AR lead authors and heads of the Global Carbon Project. The data is known to be skewed against the US, Western Europe, Canada and Australia. The methodology is from Raupach et al, 2014. Figure 1 shows the evolution of the countries’ contributions to the surplus CO2 levels over time, since 1880.
Fig. 1. Anthropogenic CO2 accumulation in atmosphere, calculated using methodology from Raupach et al., 2014. To obtain the surplus CO2 concentration in ppm, divide GtC by 2.13.
This graph shows surprisingly low surplus CO2 levels from 1880 to 1940. This may be because the IPCC models are wrong. Nevertheless, I performed the same analysis using Halperin, 2015 (H2015), and arrived at the same attribution result (with the differences within 2%). The resulting graph of contributions over time is shown in Figure 2.
Fig. 2. Anthropogenic CO2 accumulation in atmosphere, calculated using H2015 and Comment #1 to H2015. To obtain the surplus CO2 concentration in ppm, divide GtC by 2.34.
This looks more reasonable, doesn’t it?
BTW, I updated the best estimate of the surplus atmospheric CO2 half-life to be 35 years (±5 years), based on my subjective corrections to the official data, made after the H2015 had been published (and announced on WUWT first). H2015 has not been retracted or corrected, because its main result is that surplus CO2 concentration drops exponentially, rather than the exact half-life in the exponential decay formula. Figure 2 shows the results obtained with the updated estimates (but not the corrected data).
Let me also say a few words about the carbon cycle for those who are not familiar with the topic. Carbon is one of the most prevalent elements in the Earth’s crust. In the atmosphere, almost all carbon exists in the form of carbon dioxide (CO2). There is a continuous exchange of carbon between the atmosphere and two other pools: ocean and land (biomass and detritus). The industrial revolution (1760-1840) brought about a sharp improvement in human conditions, starting in Europe and spreading to the rest of the world. Decreases in epidemics and child mortality rates caused a population increase from one billion in 1800 to more than seven billion today. By converting forests and other natural ecosystems into agricultural lands (land use change) and burning coal and other fossil fuels, humans started releasing growing amounts of CO2 into the atmosphere. In accordance with the La Chatelier’s principle and other laws of nature, the increased (over putative equilibrium) concentration of CO2 in the atmosphere caused its accelerated removal to other pools, which became known as sinks. IPCC went to extraordinary lengths to confuse both scientists and the broader public on this topic. But even using IPCC’s own models, one can conclude that only a small fraction of the extra CO2, released 100 years ago, is still in the air. The calculations take into account the dynamic nature of the exchange (e.g. if a molecule of CO2 entered the ocean but another one popped out from the ocean into the atmosphere, the net effect is zero). Figure 3 below shows how little impact old CO2 release has had on contemporary CO2 concentrations. For example, only 11% of the CO2 released in 1900 should be counted toward the atmospheric CO2 in 2012 (according to H2015 and H2015#1).
Fig. 3. Percentage of annual CO2 release remaining in the atmosphere. The solid line shows values according to H2015 and Comment #1 to H2015.
The “clowns” referenced at the beginning of this article are only the delegations of the western countries, and those who spend their own money and time on worries over CO2. Many other national delegations who are visiting COP21 in Paris are simply there to protect their nations from this progressive madness. On the other hand, a majority of NGOs and activists going there are simply profiteers and haters. Ultimately, we want to prevent an international conflict over hot air, foisted upon the world by IPCC, UNFCCC, UNEP, and their likes.
References and Supporting Information are available here.



What surplus CO2? Experiments and farming practices have demonstrated that vegetation grows best with at least 1000 ppm of CO2 in the atmosphere. Gaia is still in CO2 deprivation.
….. “What surplus CO2?”
Exactly right Kevin !
The idea of some surplus, comes from the fantasy that
there is an “ideal” amount of CO2 in the low 200s of parts
per million, put about by the late Maurice Strong and his
pals at UNEP. That’s what gives rise to fatuous “research”
like this which is not only meaningless, but a waste of time.
I agree with Wun. Not only an “ideal” amount of CO2, but one that has never been DECLARED…along with an ideal CLIMATE, and ideal AVERAGE TEMPERATURE…and also lacking a clear statement of variation from those ideals. Hence the raging lunatic “ten of the warmest years are in this century” talking point…in comparison to what? 1998? And by how much? 0.02 degree KELVIN? It should be in Kelvin. and if that is the case, that imperceptible 0.02 degree, calculated from incomplete and tortured data, is only 0.02/288, a 0.007 percent increase on the heat content, as measured by a host of dodgy instruments and agenda-driven scientists. Like Wanki Moon said, “we can’t feel it, but we need to fix it!!!!!!!!!!!!!
Kevin Lohse:
There is no “surplus CO2” because the sinks are observed to NOT be overloaded. And the half- life of a ‘pulse’ of CO2 into the atmosphere is observed to be less than one year (n.b. much, much less than the decades suggested by the models of Halperin, IPCC and Engelbeen).
Increased CO2 emissions from oceanic plants provided a ‘pulse’ of 9.3Gt of additional CO2 into the atmosphere and this additional 9.3Gt of CO2 was added to and withdrawn from the atmosphere in the three years 1989 to 1991.
A half-life of 6 months reduces a ‘pulse’ by 98% within three years.
I explain this in a post to this thread that has ‘vanished’ but I hope will be found by the Mods, and I also provide a brief explanation in this thread here.
Richard
As many people have noted in the past, there is a factor to consider in comparing bomb CO2 to natural CO2 and their residence times or their half lives in the atmosphere.
In a one-step radioactive decay like C-14 to C-12, once a decay has happened, the atoms concerned are no longer part of that particular radioactivity scheme.
In CO2 half-life comparisons, an atom that goes from air to ground is not out of the equation because it can go back into the air.
Euan Mearns did a recalculation of residence time with correction for this recycling effect.
“Every year the oceans exchange approximately 90 Gt C with the atmosphere. 92 Gt go in and 90 Gt comes out again. Surface ocean waters contain about 1020 Gt C and so what happens is that 92 Gt goes in, mixes with 1000 Gt and what comes out again is not the same CO2 molecules that went in. What we are trying to measure using the bomb 14C data is the rate at which that notional 2 Gt difference is sequestered. The bomb 14C data can only be used to measure that if the CO2 exhaled had the exact same 14C composition (14C/12C) as that inhaled and this will clearly not be the case.”
http://euanmearns.com/whats-up-with-the-bomb-model/
It might be that currently accepted residence time models incorporate this effect. Maybe they do not. I have not chased it up, so this is merely a flag.
Geoff Sherrington:
I made no mention of “bomb CO2” so I fail to understand why your response to my comment is about details of “comparing bomb CO2 to natural CO2 and their residence times or their half lives in the atmosphere”.
I stated that in 1989-1991 (i.e. decades after”bomb CO2″) a ‘pulse’ of additional 9.3 Gt of ‘natural’ CO2 from oceanic biota was withdrawn from the atmosphere in the three years, and I explained that this demonstrates a half-life of less than a year for atmospheric CO2.
Richard
The article above is about CO2 in the atmosphere but the amounts are expresses in GtC. Why not, as you do, refer to Gt of CO2. The danger with referring to GtC is that people think that we are putting carbon in the air or talk about carbon pollution.
Geoff,
Richard doesn’t see that different processes are at work: some are highly temperature dependent, some are highly pressure dependent and some are both. The process that removed the extra CO2 pulse in the years 1991-1992 was a combination of a temperature drop (ocean solubility) and light scattering (on photosynthesis by the Pinatubo aerosols). That ends if the temperature increases and/or the aerosols are dropping out in 1-3 years.
That is completely independent of the removal of an extra injection of ~55 ppmv then (110 ppmv now) above steady state in the atmosphere…
Further, the main difference for the 14C bomb spike decay and the 13C/12C ratio decay is in the exchange with the deep oceans of ~40 GtC/year: what goes into the deep is the isotopic composition of today. What comes out is the composition of ~1000 years ago… That makes that the 14C spike decay rate is at least a factor 3 faster than of a 12CO2 spike…
Chris Schoneveld,
Almost all scientific works use GtC or nowadays PgC, as it is surely CO2 in the atmosphere, but only 1% CO2 in the oceans, 90% bicarbonate and 9% carbonate. In plants it is a host of cellulose, starch, sugars and many other carbon containing molecules… Carbon makes it easy to follow the fluxes, but indeed it can be confusing…
That short lifetime is a result of an active sink that exists seasonally. The longer lifetimes are the results with consideration that over an average year, natural sinks and sources balance out.
Ferdinand Engelbeen and Donald L. Klipstein:
You each make the same untrue assertion.
Ferdinand Engelbeen says
Nonsense!
The mechanisms of the sinks are NOT relevant. The sinks – whatever they are – are observed to NOT be overloaded in 1989 to 1991 because they sequestered all of the additional 9.3 Gt of of ‘natural’ CO2 within 3 years.
If the sinks were not overloaded for the additional 9.3 Gt of CO2 emitted from ‘natural’ sources then they were not overloaded for the CO2 emitted from ‘anthropogenic’ sources in the same period.
Donald L. Klipstein says
No! In each year the annual rise is the residual of the seasonal rise and fall of CO2 within the year. If the seasonal sink (or sinks) could sequester all of the additional 9.3 Gt of CO2 emitted from ‘natural’ sources then there would not have been an increase of 9.3 Gt of CO2 in the atmosphere.
In other words, if the seasonal sink (or sinks) could sequester – so were not overloaded – for the additional 9.3 Gt of CO2 emitted from ‘natural’ sources then they were not overloaded for the CO2 emitted from ‘anthropogenic’ sources in the same period.
You are both claiming the sinks magically know if CO2 is from a ‘natural’ or ‘anthropogenic’ source and they sequester the two types of CO2 differently.
Richard
Richard Courtney,
See my response here
Ferdinand Engelbeen:
I understand that it is unpleasant for you that your narrative is refuted by observation of reality which shows that the sinks for atmospheric CO2 are not overloaded and that the half-life of additional CO2 in the atmosphere is less than a year.
I went to your link downthread and found your twaddle that it contains so I replied by saying:
The sinks have the the same effect(s) on sequestration of ALL CO2 and not only on non-anthropogenic CO2. This remains true if the ability of the sinks to sequester CO2 changes.
Richard
That number can be easily verified by the co2 produced, the volume of air and how many ppm/v the co2 goes up each year. At least half if not more is absorbed every year. NOAA. To make things worse for CAGW is the tonnage released from 1750 to 2011, if anthropogenic was adding co2, then the co2 levels in 2011 should have been 325 ppm/v, not in the 390s. The difference would have to be more than doubled the production. I guess they’ll doctor the mining records as well.Those are not speculative numbers but real numbers. That’s from the same people who say co2 stays in the atmosphere 1000 years.
For those that are interested or don’t believe it. Do the math.
I can show that there were 365 bmt released from 1750 to 2011, by two different methods it comes out 46 ppm and 45 ppm. So if they are starting at a base of 280 ppm, then how did it get to 390 +/- in 2011? I was surprised that both ways gave me such close results. I don’t have to go looking at decay rates or speculate what the isotope ratios are.
rishrac:
You conclude your post saying of recent rise in atmospheric CO2
I don’t dispute your claims because you have not provided sufficient information for your claims to be assessed.
Please provide an outline of how you conducted your estimates together with a reference or preferably a link to a description sufficient for replication.
I ask this because I am genuinely interested in your claims.
Richard
Richard Courtney:
The sinks have the same effect(s) on sequestration of ALL CO2 and not only on non-anthropogenic CO2. This remains true if the ability of the sinks to sequester CO2 changes.
Yes they have the same effect, I didn’t say or imply anything different.
Extra natural emissions 1988-1991: 1.2 ppmv or 0.4 ppmv/year
1.2 ppmv extra removed in 2 years 1991-1992: 1.2 ppmv or 0.6 ppmv/year
Natural sinks 1988-1992: 1.5-2.5 ppmv/year
Natural sinks were far higher than the extra natural emissions in 1988-1991 or the extra sinks in 1991-1992, thus the natural sources didn’t overload the natural sinks. If there were no human emissions at all, CO2 levels in the atmosphere would have dropped in every year of that period.
Human emissions in the same years: 3.0 ppmv/year, (far) higher than the natural sinks, thus human emissions did overload the natural sinks in every year of the period 1988-1992.
Not that difficult, you see…
rishrac,
Aren’t you confusing between releases counted as Gt / Pg CO2 and counted as GtC / PgC? That is a factor 44/12 difference…
CO2 releases in the past and current are about twice the increase in the atmosphere: 110 ppmv increase in the atmosphere or ~230 GtC are from ~370 GtC emissions since ~1750…
Richard C,
I agree with you (and Tom Quirk) that there are big natural C fluxes not yet accounted for, but nearly everyone agrees that current atmospheric CO2 is much higher than any time of equivalent temperature in the Pleistocene. Also, during the recent crawl in temperature increase, atmospheric CO2 increase is not yet showing any sign of slowing.
So on every pre industrial timescale temperature and CO2 are in lockstep with temperature (almost) always leading–except for today.
The plankton or other sources are not new. We are the new player.
gymnosperm:
Your assertions about temperature and CO2 being in “lockstep” are supported by the ice core data but are refuted by the stomata data. The difference is because the ice core data lack sufficient temporal resolution.
And we are not really the only “new player”. We increase the burning of fossil fuels but fossil fuel fires have always existed, and each new volcano is a “new player”.
Human activities are not the ‘centre of the universe’.
Richard
Ferdinand:
You say
I do not understand how you can conduct such doublethink.
You said
If that process removed the ‘pulse’ then it would have had similar effect on the anthropogenic emission but you say “That is completely independent of the removal of an extra injection of ~55 ppmv then (110 ppmv now) above steady state in the atmosphere…”.
n.b. “completely independent of” and NOT ‘the same’.
You are claiming the sinks magically know if CO2 is from a ‘natural’ or ‘anthropogenic’ source and they sequester the two types of CO2 differently so they have different ‘half-lives’ in the atmosphere. THEY DON’T.
The ‘pulse’ demonstrates that the half-life of additional CO2 in the atmosphere is less than a year which is much less than the 35 years your model says.
Richard
Geoff, C-14 does not decay to C-12. It is generated in the atmosphere by bombardment of a Nitrogen isotope and it decays to another N isotope.
the mention of surplus caught my attention also–for a surplus to exist, wouldn’t one have to define the proper amount to begin with. I don’t believe there is a person alive who can actually do that.
CO2 has been well below 1000 for 30 million years or so, and below 500 for more than 10 million. Many modern ecosystems of the world (e.g. savannas and grasslands) have largely evolved under low CO2, hence the rise of C4 grassesin the tropics. C4s perform best below 300 ppm (e,g, many cereals inc maize), and as low as 180 ppm, so your statement above is incorrect. Most experiments show that crops don’t benefit much above 700 ppm at most. The world above 1000 ppm is a fundamentally different world.
@frank, who wrote:
It would be interesting for you to produce a source for this, as it goes against literally hundreds of experiments that say otherwise.
===|==============/ Keith DeHavelle
Surplus compared with natural background. Surplus CO2 in the air is beneficial, I know.
“Send in the clowns” Verse 3
“Don’t you love farce?
………………………………
But where are the clowns?
Quick, send in the clowns.
Don’t bother, they’re (t)here.”
not possible to attribute changes in atmos CO2 to fossil fuel emissions because of uncertainties in natural flows.
http://papers.ssrn.com/sol3/papers.cfm?abstract_id=2642639
http://papers.ssrn.com/sol3/papers.cfm?abstract_id=2654191
Jamal–
Enjoyed your second link above. You use the IPCC’s own estimates of uncertainty to arrive at the following conclusion:
“Given its level of uncertainty, the carbon budget of the IPCC AR5 is unable to
discriminate between a world with fossil fuel emissions and one without fossil fuel emissions.”
Well stated, sir!
I seem to recall that the energy budget suffers under a similar inability to distinguish between a net increase or decrease in heat–What was it?–0.6 +- 17 or something like that.
Jamal.
Your calculation may be mathematically right, but the real world shows that the variability is less than +/- 1.5 ppmv (less than +/- 3 GtC) around a trend of over 70 ppmv over the past 55+ years:
http://www.ferdinand-engelbeen.be/klimaat/klim_img/dco2_em2.jpg
Thus sorry, the variability in natural flows is far too small to prevent the attribution of the increase above the temperature controlled dynamic equilibrium between atmosphere and oceans/vegetation…
Moreover, there are lots of other observations which show the influence of human emissions:
http://www.ferdinand-engelbeen.be/klimaat/co2_origin.html
Ferdinand,
I don’t suppose you could run that graph back to 1930 and forward to 2015?
All the graphs I have seen show zero impact from the extra CO2 emitted during the war years of the 1940’s and CO2 is now apparently leveling off despite the rapid increase in emissions from China and India.
Nice graph.
The increase per year varies from about 0.5 to 3 ppm/year within a decade.
Any ideas on the 6 fold variability over a relatively short time period?
The emissions don’t vary that much, indicating there is a lot of much bigger processes going on.
Ian W,
Last official figures of CO2 emissions are from 2012, but I can use the unofficial one’s, be it that 2014-2015 weren’t higher than 2013…
Before 1959 we have only CO2 levels from ice cores, where the best resolution is a decade, thus removing the year by year variability in sink capacity. Assuming that the CO2 levels in the atmosphere and the CO2 emissions inventories were accurate:
http://www.ferdinand-engelbeen.be/klimaat/klim_img/acc_co2_1900_1959.jpg
Which shows that the ratio between CO2 remaining in the atmosphere and total emissions (~57%) was about the same as in recent decades (~53%).
In recent years there is a drop in the ratio (~40%), but that did happen in the past too (around the Pinatubo outburst). Anyway, there is no sign of saturation of the deep ocean removal of CO2 as the IPCC expects in its Bern model, it looks like more that the biosphere is an increasing sink for CO2…
J,
The variability around the trend is mostly caused by the influence of temperature changes on tropical vegetation: El Niño gives too high temperature and drought in some regions, floods in other ones. The net effect is that a lot of plant debris / wildfires emit more CO2 than there is uptake by plants.
That the temperature influence on plants is the main driver can be seen in the opposite CO2 and δ13C changes, following the temperature changes with several months:
http://www.ferdinand-engelbeen.be/klimaat/klim_img/temp_dco2_d13C_mlo.jpg
If the oceans were the main driver, the CO2 and δ13C changes would parallel each other.
The influence of temperature changes like El Niño is temporarily and levels out to (below) zero in 1-3 years. Vegetation is a net, increasing sink for CO2 over periods longer than 3 years, at least since 1990…
Ferdinand,
Thanks for your reply.
But the vegetation can make a 6 times difference over the span of a decade (0,5 to 3) ppm/year?
Do we see six times the plant growth changes from year to year????
J,
That is a six fold change in rate of change from one year to the next, but in absolute change it is not more than +/- 1.5 ppmv around a trend of over 70 ppmv since 1958…
That is the danger of looking at derivatives: you remove most of the trend and inflate the noise…
Here the real variability shown around the trend, as caused by temperature variability on the Pinatubo + El Niño period:
http://www.ferdinand-engelbeen.be/klimaat/klim_img/temp_co2_1990-2002.jpg
Where the temperature variability was enhanced with a factor 8, still more than what is observed in the CO2 trend…
Author’s correction: the graphs and the supplemental information for the article have a serious error. See the corrected article and supplemental information (the same link as in the text).
“Table 1. Surplus CO2 attribution by country or group of countries, 2012.
Country or group of countries Surplus CO2 attribution
USA 20%
Western Europe 10%
Canada + Australia 3%
China 15%”
Since China at least twice the CO2 as the US, how long after 2012, will it take for China to exceed
the US.
And factoring in the “only 11% of the CO2 released in 1900 should be counted toward the atmospheric CO2 in 2012 ” factor.
Is possible by end of 2015?
Or will take longer, say by end of 2025?
And you include a conservative estimate of future CO2 emissions, what will India and China total Surplus CO2 attribution be as compared to US, Western Europe, Canada + Australia in 5 years time [2020]?
CO2 is a trace gas because it is taken in by plants on a constant basis, yet has a half life of 35 years? These two things cannot both be true.
Possibly, Mooloo. Factors to consider, absorption of co2 by younger plant life, vs more mature. Density in any given area, and rate of absorption by other “sinks” . As long lived plants adapt to higher levels will heir ability to use even more co2 become apparent?
This is a thought which we may need to entertain, if we get plant life accustom to co2 levels of say 600 ppm or even 1000 ppm, what happens if we can’t maintain it? Make no mistake such a increase for plants will follow over to animal life. You may just create a different biosphere. Healthier larger plants may equal larger animal life. But all boats may not rise with the tide, there is likely going to be winners and losers.
I am curious. It is late for me and i am wondering down strange paths.
michael
Mooloo,
Spring uptake and fall release by vegetation is enormous, but all plants together and all releases from decay/feed/food together give only ~1 GtC extra uptake in more permanent storage (humus, peat, browncoal,…
See: http://www.sciencemag.org/content/287/5462/2467.short
and
http://www.bowdoin.edu/~mbattle/papers_posters_and_talks/BenderGBC2005.pdf
Ferdinand says:
Still citing that “biological impossibility” to justify your “junk science” claims, …. HUH?
Your denial of the fact that the civilized world is totally committed to the use of refrigeration for preserving their dead biomass “food” resources ….. is utterly amazing, …… disingenuous and verges on intentional dishonesty.
People should believe their nose ….. far, far more than any of your purty colored plotted graphs.
Samuel,
I did show you that significant amounts of CO2 were released from under a snow deck at -20°C in Alaska. Who is here the d*niar?
Shur nuff, Ferdinand,
And you have convinced yourself that there are sufficient numbers of snow decks in Alaska that are outgassing enough CO2 into the atmosphere to cause the bi-yearly 6 ppm increase in atmospheric CO2.
Ferdinand, do you, your wife or another family member own and use a refrigerator and/or freezer?
If so, ….. just what the hell is that refrigerator and/or freezer used for, …. Ferdinand?
Testify, Ferdinand, ….. testify, …… the scientific world wants to know.
Samuel,
Lots of land in the NH have hardly and freezing days and simply emit lots of CO2 from inside the compost heap every winter, where the inside temperature can reach high temperatures. Good for animals like toads who like it there…
Ferdinand says:
http://www.mapsofworld.com/world-maps/image/wether/average-temparature-january-enlarge.gif
Here is how the EPA made CO2 a pollutant: http://www.wsj.com/articles/SB124001537515830975
Remember that we are discussing a cabin * cycle*.
It is the deviation from equilibrium caused by an individual pulse of CO2 that has a half-life of 35-41 years.
Is this guy saying that CO2 is actually in surplus for the first time on hundreds of thousands of years.
Sorry but, NO, NO, NO.. !!
The atmospheric CO2 level is STILL PERILOUSLY LOW.
MORE is needed…. MUCH MORE.
To maintain the future food supply to the growing world population we need to go 700ppm +
.. preferably 1000ppm+
Then we could all live in the jungles of Nebraska again 🙂
It’s OK with me, I’ll be long dead before we reach 1000+ ppm CO₂ To each his own I say!
If the alarmists have their way and reduce atmospheric CO2 back down to below 280ppm..
.. a large proportion of the world’s population will be dead.
I very much doubt that the world’s population can be fed at 280ppm or below.
You posed a vital question. How much of world’s agricultural production is based on the anthropogenic CO2? What would happen if we suddenly managed to drop the CO2 back to 280? I guess the production could drop by two-numbered percents, possibly setting up conditions prone to a serious food crisis. It could be avoided only by making agriculture much more efficient. Efficiency is loathed by the Green how like romantic ‘biological’ crops at what expense ever.
Also Ari, it would be very interesting to see a projection to say, year 2030 on atmospheric CO2 contributions by country.
Hugs, a 280 PPM CO2 atmosphere would reduce food production by 15% to 20%, quickly leading to WW three. I am disappointed that the US only gets 20 percent credit for feeding an extra billion people.
Hugs: an online British textbook once explained how acres of wheat crops would cause a regional drop in CO2 to levels that were insufficient for growth in the afternoon of high-growing days. Alas, that paragraph has been removed and I did not have a screen capture of it. I don’t think it was removed for nefarious reasons, but simply because the now higher CO2 levels is enough to correct the situation.
The point I want to bring out is that lower levels of CO2 begin directly affecting the growth of crops long before the global average becomes critically low. I didn’t appreciate what the impact of hundreds of acres of crops would have on the local levels of CO2.
Bart L
Agree.
+ half a dozen.
I am puzzled, a bit, about this CO2.
I know it’s the Watermelons’ weapon of choice.
I also know that there are very old trees – a few, certainly, but not zero – that have lived throughout the Christian [=Common] Era:
The Glastonbury Thorn, allegedly; probably some British Yew trees, even an oak or two perhaps; certainly several American Bristlecone pines of three species, and giant Sequoias like the General Sherman tree.
These individual organisms have lived through – adapted to – the local climate, and CO2 levels – pertaining over at least a couple of thousand years.
How accurately do we know CO2 levels for – say – the last five thousand years?
These trees have survived two, three – even five – thousand years, so CO2 @ – say – 331 ppm [thanks https://www.random.org/ ] may not be their only experience.
Possibly!
Auto
AndyG, great observation.
I once asked a warmy if he would push the “God button” to instantly restore CO2 to 280ppm if he had access to it. He said he would.
“Congratulations – food production just fell 18%, and you just killed 1 billion people.”
Andy,
Plants evolved in much higher CO2 levels than today, but that doesn’t mean that there is no surplus in the current earth, compared to the current temperature. Over the past few million years, there was a dynamic equilibrium between temperature and CO2 levels in the atmosphere. In the past 165 years, humans emitted more CO2 than the earth could cope with, with as result increasing CO2 levels. That is the point of discussion here…
Nature does things slowly.
The biosphere is expanding, now that it is off starvation rations.
Humans have NOT emitted more CO2 than the Earth can cope with…. that is a truly moronic suggestion.
The Earth’s history shows that nature can cope with one heck of a lot more CO2 than we currently have.
“Over the past few million years, there was a dynamic equilibrium between temperature and CO2 levels in the atmosphere.’
yes a basic “can we survive scenario”.
Have you ever studied predator/prey scenarios?
CO2 graphs over the last few million years provides a classic example of basic survival.
See how they drop to 180ppm at regular intervals…… that is a “near death’ experience for the world’s biosphere.
If you don’t believe me.. try growing something at 180ppm CO2 !!
You now have a task that you have set yourself, to prove me wrong.
You must build a greenhouse that uses only CO2 at 180ppm.. and survive using only that food.
Me, if I didn’t like meat so much, would be quite content to live on food produced at 1000ppm.
But meat needs vegetation too.. and those dips to 180ppm CO2.. not going to happen.
Humans have SAVED THE WORLD with their release of fossil fuel CO2 that was once in the atmosphere but got inconveniently sequestered.
Ferdinand Engelbeen writes: “In the past 165 years, humans emitted more CO2 than the earth could cope with”
Ferdinand, would you venture this statement is based on a theoretical model of past climate? That it is in fact an educated guess?
I love those saw-tooth graphs that dip to 180 and peak at 280..
An total and absolute proof that the world can only just survive at 280ppm.
And that the biosphere starts to die and release CO2 back into the atmosphere at 180ppm.
The planet is truly thankful that man came along and used fossil fuels to regenerate the atmosphere.
However…..much more is needed.
Think about it, Ferd..
Its not the first burger/beer that goes to the waist…… . its repeated burgers/beer……
And the biosphere is lovin’ it !!
It is now no longer anorexic.
Thanks to fossil fuels , we can look forward to times of prosperity, food-wise……
(unless the sullen sun takes us back down to another LIA.)
“Ferdinand Engelbeen
December 12, 2015 at 12:54 am
Over the past few million years, there was a dynamic equilibrium between temperature and CO2 levels in the atmosphere.”
Equilibrium in a dynamic (Chaotic) system? Seriously? And for your next (Nature) trick…
Ferdinand
I’m kinda bored with your ‘ man is increasing CO2 in the atmosphere meme’. Can you move onto something else? You’re into this shit more than I am, perhaps you have nothing better to do. I am curious about the oxygen level now and in the past, also the overall atmospheric pressure now and in the past. Maybe that will give you something else to research. I’ll send you $1 for your effort. I’m not being rude. I am just used to using subcontractors for menial tasks. You’ll love it.
Please don’t attack Mr Engelbeen. He’s one of the few here who actually can read. Err no, that’s too bad. He’s one of the few here who can read a scientific paper, if not write one. He’s very reasonable and does not need to be compared to Dr Mann.
Equilibrium is a relative thing, so add the word ‘relative’ or ‘global’ when needed. Currently the atmospheric CO2 concentration is far from equilibrium with the oceanic concentration. It is never is in a perfect equilibrium.
“I’m kinda bored with your ‘ man is increasing CO2 in the atmosphere meme’”
I’m not…… I think its totally essential for the continued survival of the human species on Earth.
AndyG55
I’m ok with CO2. Not ok with the idea that man is producing more than ‘NATURE’ can handle. In this current environment of reason against emotion, I give no ammunition about CO2 levels being anthropogenic. Say to a ‘greenie’ that levels of CO2 have increased due to man and she will be dancing in the street. I used she on purpose because there will be 6 idiot men (nerds) who want to lay her and will agree with anything she says (human nature).
Humanity is so stupid.
Ferdinand, since the climate, the oceans, the biosphere are all doing okay, please explain how we have emitted more CO2 than Earth can cope with.
Wow, more reactions than I expected…
Here some background explanation:
In the far past, CO2 levels in the atmosphere were in dynamic equilibrium with CO2 in the oceans (and the biosphere) at much higher levels than today: several thousands of ppm. That is the time that most land plants evolved: trees, lots of grasses and other plants. Some of that CO2 was taken out of the atmosphere by plants and buried as coal. A much larger part was and is buried as carbonate rock, especially during the Cretaceous period of about 120-60 million years ago by microscopic plants with a carbonate shell in the oceans: coccolithophores.
Since then, CO2 levels in the atmosphere slowly got lower, with in the past few million years in the pre-industrial period between 180 and 300 ppmv, following temperatures with a long lag of several centuries.
There was a rather fixed linear ratio between temperature and CO2 levels in the atmosphere of ~16 ppmv/°C. For the current temperature, the dynamic equilibrium between atmosphere, oceans and vegetation would be around 290 ppmv.
The real measured pressure in the atmosphere now is 400 ppmv. The pressure difference between 400 and 290 ppmv is what pushes ~0,5 ppmv/year extra in plants and ~1.65 ppmv/year in the oceans. That is not enough to remove all human emissions of ~4.5 ppmv/year. Thus currently nature can’t cope with human emissions.
That is the current uptake process, regardless of what is better for plants or not.
I am pretty sure that plants love more CO2 in the atmosphere, reason why greenhouse owners supply a lot of CO2 into their greenhouses and (organic) gardeners use mulching (plant rests) between growing crops…
Ferdinand, I think you are right to stress that humans have changed the balance but you are wrong to think that the equilibrium we had before the industrial revolution was an equilibrium between temperature and CO2; it was an equilibium between sinks and sources. Humans have now increased the source and earth has yet to respond (biologically) to the higher levels before the greater sinks can balance the source.
Chris Schoneveld,
In fact you are right that it is a matter of dynamic equilibrium between sinks and sources if you look at very long term, but in the past 800,000 years (ice cores), or 2 million years (foraminophores), there is a nice correlation between temperature and CO2 levels in the atmosphere: about 8 ppmv/K local in Antarctica, about 14-20 ppmv/K globally. In the 420,000 year period of the Vostok ice core that is quasi-linear:
http://www.ferdinand-engelbeen.be/klimaat/klim_img/Vostok_trends.gif
Where most of the deviations are caused by the (long) lags of CO2 after temperature changes…
Ferdidnand,
I presume the 14ppmv/K is the equilibrium concentration of CO2 as a function of temperature and not the equilibrium temperature as a function of CO2 concentration? Think the comment about CO2 lagging temperature suggests the former versus the latter.
“Over the past few million years, there was a dynamic equilibrium between temperature and CO2 levels in the atmosphere”?????
“Equilibrium-a state in which opposing forces or influences are balanced.”
Since when have temperature and CO2 levels “opposed” each other?
erikemagnuson
Think the comment about CO2 lagging temperature suggests the former versus the latter.
Indeed temperature changes clearly drive CO2 changes over the many millennia in the past. That doesn’t exclude the opposite influence, as long as that is modest, but the historical influence was small. Most of the time there is an overlap between T and CO2 changes during a glacial – interglacial transition, so it is difficult to separate the influences. After the previous interglacial (the Eemian), temperatures and CH4 levels were already at a new minimum, while CO2 levels remained high. After that CO2 levels started to drop ~40 ppmv without much influence on temperatures:
http://www.ferdinand-engelbeen.be/klimaat/klim_img/eemian.gif
Where delta-18O in the atmosphere (measured in N2O) is a measure for ice sheet growth and wane, here reverted to align with the other variables. Higher values mean less land ice and vv.
The CO2 lag is not an artifact of a dating error, as both CH4 and CO2 are measured in the gas phase and CH4 follows the temperature proxy (dD in the ice phase) almost immediately, while CO2 lags with hundreds (during warming) to thousands (during cooling) of years…
Aphan:
Equilibrium-a state in which opposing forces or influences are balanced
Not the same as a “dynamic” equilibrium or “steady state”: that is where opposing fluxes are balanced. In the case of CO2: as much CO2 is entering the atmosphere as is leaving the atmosphere when the “steady state” is reached and CO2 levels in the atmosphere don’t go up or down if averaged over a year, despite that over the seasons 20% of all CO2 in the atmosphere is exchanged with CO2 from other reservoirs…
Of course, if you change the temperature of the ocean surface or change the CO2 pressure in the atmosphere, the steady state is disturbed and a disequilibrium between CO2 influxes and outfoxes tries to restore the dynamic equilibrium: either by increasing the CO2 influx and decreasing the outflux for a temperature increase until these are back in equilibrium at ~16 ppmv/K or by increasing the outflux and decreasing the influx for any increase in CO2 pressure in the atmosphere, whatever the cause…
Look at the title of the post! Man is increasing it. How much and for how long is the question.
I’m bored with a bunch of aggressive people who pop up to throw slurs.
Not aggressive, just bored with the same old shit. I also haven’t just ‘popped up’. I have looked at the title and have thought ‘so what -your opinion’. I read these things and file them away. I take what I feel is right and discard the rest. Just like a normal human. Stop being a ‘fan boi’ and let Ferdinand defend his position on his own. I actually don’t have anything against him
Ari writes: “This analysis uses data and methodology from the most official IPCC aligned authors”
Ari, the Clowns have been taken to the woodshed so many times for generating absurd claims it’s very difficult to believe anything written that quotes them as an authority. I’d suggest locating a credible source?
What I mean to say is the message is interesting, but I wouldn’t believe the IPCC if 97% of their “scientists” told me the sky was blue.
Why is a blue ball, blue? 😉
It tends to absorb red?
What does it reflect?
Blue.
Does the sky reflect blue ?
Well. Mostly blue. I take it we’re in agreement?
No, the sky does NOT reflect blue..
The sky passes blue.
I hope you can see the intrinsic difference 🙂
I was clarifying my first statement about the Blue ball, not your question about the sky. The sky, like the sea, when viewed from space, absorbs red and reflects mostly blue. This is due to water vapor in the atmosphere. As you note, the sky passes blue, which is why the planet appears mostly blue from space.
During the time between 1:20 and 1:22, I was typing and had not read “Does the sky reflect blue ?” while I was writing a PS to my earlier reply, “Blue”, which was intended to be humorous. I’m a retired IR astronomer and have a little background, I thought you and I were toying with the absurd and exchanging jokes.
Sorry, but the sky does NOT reflect blue. What you see from space is the 70% water reflecting blue.
If the sky reflected blue, the land surface would appear blue from space
nb.. by “blue” I mean light in the blue frequency range.
And I was typing and missed your reply at 1:34
All is good . 😉
You guys could be the person to answer the question why sunlight is roughly white?
(No, the answer is not the black body radiation in certain temperature added with atmospheric absorption and reflection, but rather why it is essential to humans to perceive normal light as white instead of say, deeply coloured?)
Hugs..
Many species are presumed to see “light” differently that we do. sometimes deeply coloured.. or even uncoloured. (some humans also see “differently”)
You have to remember that our whole definition of what light is and what we see, is defined on the basis of what we see.
Science is just our current interpretation of what “is”.
This is the thing that many people still fail to “get”..
Science is OUR definition of OUR understanding of how things work… be it right or wrong.
If we ever meet an actual alien intelligence, if they exist…
…. it would be highly instructive to see their definitions as opposed to ours for similar physical phenomena.
So the answer.. in a round about way.. is that white is white because we have defined it that way.
All is swell . . ; )
The sky scatters blue. You are seeing blue light scattered down while red goes straight on. Red is passed, blue is scattered, nothing is absorbed or reflected.
AndyG55 said, “No, the sky does NOT reflect blue..The sky passes blue.”
Actually, the sky scatters blue (and UV). It is called Rayleigh scattering. The sun probably looks green outside of the atmosphere because the peak emission is in green. However, it tends to look yellow to us because blue light is removed from the path of the sunlight.
When one considers the historical emissions, the developing world are living off the back of those emissions, and should therefore be deemed to have a share in them.
Hence when the developing world go forward, they do not need to re-invent the wheel. They do not need to re-invent all the technological advances that everyone takes for granted and/or those in the developing world will take for granted as and when they develop, eg., cars, washing machines, cookers, fridges, microwave, computers, mobile phones, tablets, aircon, aeroplanes, trains etc. The developing world’s future emissions will be less because of the historic emissions of the developed world.
Since the developing world will make use of the discoveries and inventions that the developed world has created and which has of course come about by the historic consumption of fossil fuels, the developing world should be deemed to have a share in the historic emissions.
The developing world should not receive the benefit of all past advances free of the historic share in the CO2 that was emitted in creating and obtaining those advances/technologies etc.
Obviously some sort of carbon tax on the developing world is in order? No free rides? I have to agree with your impeccable logic. There’s no other fair solution.
Carbon tax is a massive stupidity..
A tax on the world’s ONLY supply of food..
DUMB !!!
But, if we need to tax carbon, we should tax the developing nations. After all, the patents on the internal combustion engines have all run out.
This is all sarcasm by the way Andy. I expect you understand but wanted to make it clear since I just hate being yelled at 🙂
Nobody “needs” a carbon tax.. except to raise revenue.
And you are NOT going to raise much revenue from the developing countries…… QED !!
Not yelling at you. Yelling at the moronic stupidity of the AGW agenda
Sorry.. I use caps for emphasis, because I can’t be bothered with html to get bold.
It’s OK, I got that part too.
And I’m sorry if I got your back up. Also apologies to Richard if any offense was taken. I grew up with Monty Python. It’s my only excuse.
Chuckle.. you haven’t seen me with my back up.. 😉
That was a friendly, robust conversation. 🙂
RV
Fully agree with the last paragraph. I and very many others have been stating this for some time. Without the West and even colonialism, which brought with it direct development benefits, the vast majority of developing countries would still be largely technologically and scientifically, and hence development wise, stuck in the state they were in in the 17-18th Century. There are very similar historical precedences of local benefits from colonialism in other Imperial regimes – the Romans, the Greeks, the Ottomans and even the Chinese. The Developing World equally owes its present developed state to the West’s fossil fuelled Industrial Revolution and its on-going scientific and technological development followed by post-independence technological transfers and on-going western education opportunities – something the UN and most of the media don’t ever mention.
BTW, that was intended as humor also. I don’t want to be misinterpreted. It was a sarcastic reduction to the absurd. I agree with all of your points.
Ari Halperin,
The half life time of ~35 years seems correct, but the decay is quite linear, not exponential. For a linear process, the e-fold decay rate = extra pressure in the atmosphere / net sink rate. That makes:
In 2012:
110 ppmv / 2.15 = 51.2 years or a half life time of 38 years.
The figures for 1988 (from Peter Dietze):
60 ppmv, 1.13 ppmv/year, 53 years, half life time 39 years
In 1959:
25 ppmv, 0.5 ppmv/year, 50 years, half life time 37 years
Looks very linear to me, widely within the borders of accuracy of the emission inventories and natural sink capacity variability…
Until ~1900 human emissions were widely in the natural noise, from 1900 on the increase in the atmosphere is above the noise…
But is it the driver of climate change and will that change be dangerous (Selfishly for polar bears. No-one cares for scorpions, rattle snakes or funnel webs)?
Ferdinand Engelbeen:
No, the half-life of a ‘pulse’ of CO2 added to the atmosphere is observed to be less than a year.
I explain the matter in a post to this thread which has vanished but I hope will be found by the mods. Briefly, the issue is as follows.
Tom Quirk has analysed a ‘pulse’ of 9.3Gt of CO2 in the atmosphere and said
Quirk used isotope analysis to determine that the 1989 – 1991 ‘pulse’ of 9.3 Gt of additional CO2 derived from oceanic plants.
It is very important to note that the sinks for CO2 sequestered the 9.3 Gt of additional CO2 so it was “withdrawn from the atmosphere” in three years. This sequestration rate demonstrates that the sinks are NOT overloaded.
The total sequestration of the 9.3 Gt of additional CO2 also demonstrates that – whatever the form by which surplus CO2 concentration drops – the half-life is less than one year (n.b. much, much less than the decades suggested by the models used by you, Alperin and IPCC):
a half-life of 6 months reduces a ‘pulse’ by 98% within three years.
I have explained this to you in the past but your response is to claim the sinks magically know what CO2 is ‘natural’ and what CO2 is ‘human emitted’ and the sinks sequester the two types differently.
Richard
Patrick MJD,
The influence of more CO2, whatever the cause, is certainly not extreme. The longer the “pause” gets, the lower the sensitivity of temperature for the CO2 increase… 95% of all climate models already fail reality today, let be in 2050 or 2100, thus that is not a concern…
Richard Courtney,
We can repeat the same discussion here again until eternity, but that has no interest for anyone.
Here my point of view again in short for anyone interested and then it stops for me:
9.3 Gt of additional CO2 in 1989-1991= 2.54 GtC = 1.2 ppmv CO2 = 0.4 ppmv/year
1.2 ppmv extra removed in 2 years, 1991-1992 = 0.6 ppmv/year.
Human emissions in the same years: 3.0 ppmv/year
Net sink rate, including the extra removal in the same years: 2.0 ppmv/year.
Whatever the origin of the CO2 molecules that are grabbed out of the atmosphere by the sinks.
Human emissions overloaded the net sink rate in all years since 1958, including the extra source of CO2 from ocean plants in the period 1989-1991 and the extra sinks in the period 1991-1992 (Pinatubo).
Ferdinand:
There is no “discussion” when I cite observed reality and you spout assertions based on your imagination.
It is an observed fact that the sinks sequestered a 9.3Gt ‘pulse’ of CO2 into the atmosphere so the pulse was removed from the atmosphere in the three years 1989 to 1991.
That fact demonstrates
(a) the sinks are NOT overloaded
and
(b) a ‘pulse’ of additional CO2 into the atmosphere has a half-life in the atmosphere of less than a year (a half-life of 6 months removes 98% in three years).
Your response is to ignore that observed reality and to say
Sequestration of the pulse in 1989-1991 is observed in 1990 and did not fail to occur until there were were “extra sinks” in the period “1991-1992”.
There were no “extra sinks” in the period “991-1992.
Volcanoes emit CO2 and are not ‘sinks’ for CO2. Pinatubo was a volcano.
etc.
Ferdinand, only you is fooled by your excuses for why reality does not agree with your imagination.
Richard
Richard Courtney:
There were no “extra sinks” in the period “1991-1992.
Volcanoes emit CO2 and are not ‘sinks’ for CO2. Pinatubo was a volcano.
The average human emissions 1989-1992 were 3.0 ppmv/year with little variability.
The average sink rate in the period 1989-1992 was -2.0 ppmv/year with a variability between -1.5 and -2.5 ppmv/year.
In my (rusty) math, every year human emissions exceeded the capacity of the variable sink rate.
Volcanoes indeed emit CO2, but the measurements show that even the emissions of the Pinatubo where smaller than the effect of the temperature drop and the effect of scattered light on photosynthesis (leaves normally part of the day in the shadow of other leaves)…
See: https://climate.agry.purdue.edu/climate/dev/publications/j65.pdf
Roderick et al. [2001] and Gu et al. [2003] proposed that the volcano eruption-derived sulphate aerosols in the upper troposphere enhanced solar radiation scattering that ultimately increased the terrestrial carbon sink by increasing plant productivity.
Talking about volcano CO2 emissions a paper came out last year showing that global CO2 emissions from volcanos have been underestimated and are actually around 0. 5 gigatonnes of carbon/year, which works out at about 1.8 gigatonnes of CO2, which is about 5% of human CO2 emissions of 36 gigatonnes, or 10% of our emissions in the early 1990’s. I think the paper was called ‘Global volcanic CO2 fluxes have been underestimated due to neglect of light scattering’. Since we began emitting CO2 in 1850 the sinks have been taking up about 50% of our emissions each year (or so we’re told), which begs the question: Why weren’t the emissions from volcanos at 1.8 gigatonnes/year accumulating in the atmosphere before 1850? Wouldn’t CO2 just increase at the rate of 50% of 1.8 gigatonnes every year?
Richard (not Courtney),
0.5 GtC/year is about 0.25 ppmv/year. If we may assume that volcanic emissions are relative constant besides huge outbreaks like the Pinatubo (larger than all volcanic outbursts over the past century together), then that figure would increase the CO2 levels in the atmosphere with 0.25 ppmv/year minus the pressure difference dependent sink rate, until the sink rate reaches 0.25 ppmv/year. For a decay rate of ~51 years, that is when the atmospheric increase is at 0.25 * 110 / 2.15 = 12.8 ppmv above steady state.
As volcanoes are around for millions of years, that difference with the temperature dictated steady state was accounted for a very long time ago…
The 50% is just coincidence as that is the result of the slightly quadratic emissions and therefore slightly quadratic increase in the atmosphere and net sink rate. If human emissions would stay at the same level, the CO2 levels in the atmosphere would go up to a fixed level in the atmosphere.
Ferdinand Engelbeen:
You really must be desperate to ask me upthread to address the twaddle you have addressed to me in this sub-thread.
As I said upthread
Nothing you have said in this sub-thread changes that.
The sinks have the the same effect(s) on sequestration of ALL CO2 and not only on non-anthropogenic CO2. This remains true if the ability of the sinks to sequester CO2 changes.
Richard
Richard Courtney,
The sinks, whatever they may be or whatever they are, have sequestered 1.5-2.5 GtC/year in two years, 1991-1992. Human emissions in the same years were 3 GtC/year.
If that is not overloading the sink capacity, then nothing is overloading the sink capacity in your mind. My impression then is that you need to go back to first grade to learn that 3 is larger than 2.5…
As far as I know, I never said or implied that the sinks are making a differentiation between natural and human released CO2. What you don’t understand is that both go into the total extra, which was ~60 ppmv above steady state in the years 1991-1992. The extra injection from vegetation 1998-1991 was 1.2 ppmv, which gives an extra 2% increase in sink rate caused by the extra pressure in the atmosphere. That is all.
The real extra sink rate was from the Pinatubo scattering of sunlight, but that is a different, independent process, which you reject as “non existing”. That only shows that you don’t like anything that contradicts your narrative.
Ferdinand Engelbeen:
You say to me
NO! CO2 emissions from several sources (e.g. the oceans) were much larger than the anthropogenic emissions and that does NOT mean any of them was overloading the sinks.
You need to understand that when reality refutes your model then YOUR MODEL IS WRONG.
And you say
You do it repeatedly and in this post I have quoted you doing it again.
You claim that
(a) anthropogenic CO2 overloads the sinks
but
(b) the sinks are not overloaded for ‘natural’ CO2 increases (e.g. such as the ‘pulse’ in 1989-1991).
Furthermore, you claim
(1) the sinks adjust to sequester ALL ‘natural’ increases to natural emissions
but
(2) the sinks adjust to sequester only ABOUT HALF of anthropogenic emissions.
Ferdinand, the sinks do NOT know what is ‘natural’ CO2 and what is anthropogenic CO2: your claims are magic and not science.
All your excuses are attempts to ‘handwave away’ why reality does not agree with your model (epicycles). Abandon your model and adopt one that fits with reality such as the model we suggested in Rorsch et al. 2005: Salby did.
Richard
Richard Courtney,
If you don’t understand that different processes are at work, then any further discussion is of no use. Seasonal changes are huge but end with small differences, only half human emissions after a full cycle. 1-3 year changes are small and zero out after 1-3 years. Both largely caused by seasonal and year by year temperature changes.
Human emissions are twice the residuals after seasonal changes and twice the 1-3 years variability. These are one-way additions increasing the CO2 pressure in the atmosphere. The increased pressure in the atmosphere causes a small increase in ocean surface waters and in more permanent storage in vegetation and somewhat more in the deep oceans. All together about half human emissions. Thus human emissions are overloading the sink capacity of nature. If you can’t understand that or don’t want to understand that, that is up to you, not my task to convince you after too many years of discussion.
Further:
(a) anthropogenic CO2 overloads the sinks
but
(b) the sinks are not overloaded for ‘natural’ CO2 increases (e.g. such as the ‘pulse’ in 1989-1991).
Again you simply don’t want to understand what I wrote:
Anthropogenic CO2 overloads the sinks, including the natural CO2 increases and natural sinks whatever they may be and wherever they may be. If you still can count to 3…
(1) the sinks adjust to sequester ALL ‘natural’ increases to natural emissions
but
(2) the sinks adjust to sequester only ABOUT HALF of anthropogenic emissions.
As repeatedly said to you: you willfully change the meaning of what I wrote by omitting two essential words: “as mass”. Moreover, I never said that the sinks “adjust” to sequester half the human emissions. The sinks adjust to the increased pressure in the atmosphere, whatever the cause of the increase: biomass, volcanoes or human emissions. Again a willful change of the meaning of what I wrote. Pure manipulation from your side.
The total CO2 mass sequestered is more than the increase in natural emissions alone, but less than anthropogenic emissions. Thus the sinks are not overloaded by the natural emissions, they are overloaded by human emissions.
As proven before, to no avail: only human emissions fit all observations, several of the Rörsch e.a. possible solutions violate one or more observations, thus are simply wrong and Dr. Salby is wrong on several essential points…
Ferdinand Engelbeen:
You say to me
No, Ferdinand, I say to you if you don’t understand that at all times THE SAME processes are at work on ‘natural’ and ‘anthropogenic’ CO2, then no discussion is possible.
You wrote
You are claiming the sinks magically know if CO2 is from a ‘natural’ or ‘anthropogenic’ source and they sequester the two types of CO2 differently so they have different ‘half-lives’ in the atmosphere. THEY DON’T.
The ‘pulse’ demonstrates that the half-life of additional CO2 in the atmosphere is less than a year which is much less than the 35 years your model says.
Richard
Ferdinand:
And the ONLY model that is refuted by observations is the ‘sink overloading’ model adopted by you, Halperin and IPCC.
The argument you and I are having is because the 1989-1991 atmospheric CO2 ‘pulse’ is observed to refute your model.
Richard
Richard,
It is near impossible to have a discussion with someone who doesn’t want to understand what his opponent says. For the last time:
All different sink processes react the same way to human and natural CO2 alike. Nobody said or implied anything else.
What you refuse to see is that different processes have different time constants and different limits. The response of the ocean surface and the biosphere to temperature changes is fast but limited. The response of the deep oceans to pressure changes is slow but near unlimited.
You are claiming the sinks magically know if CO2 is from a ‘natural’ or ‘anthropogenic’ source and they sequester the two types of CO2 differently so they have different ‘half-lives’ in the atmosphere. THEY DON’T.
This is simply obstinacy from your side: I said several times to you that I never said or implied that the sinks were making any differentiation between natural CO2 and human CO2. You only use that “argument” because you have no arguments at all.
All what I said was that the 1991-1192 sink rate caused by temperature and the Pinatubo influence on photosynthesis was more than the increase 1988-1991 caused by the ocean biosphere. In figures (all ppmv within that year):
http://www.ferdinand-engelbeen.be/klimaat/klim_img/pulse_1988_1992.jpg
As you can see: the biological pulse is peanuts compared to human emissions.
The sinks caused by the extra pressure in the atmosphere, whatever its cause, were higher than the extra sources caused by the bio-pulse and temperature in the first year.
Human emissions were higher than total natural sinks in every year. All sinks together weren’t enough to remove all human emissions (as mass, whatever the origin of the individual molecules!) in every year of the past 55+ years, including in the period 1988-1992.
The ‘pulse’ demonstrates that the half-life of additional CO2 in the atmosphere is less than a year which is much less than the 35 years your model says.
Which is completely refuted by the above figures:
The half life of a temperature response is very short, but the bio-life response is limited: it zeroes out in 1-3 years and is negative over longer periods: the biosphere currently is a net, but small sink for CO2. In the period 1988-1992 it was near neutral.
The sinks were not overloaded by the biological release neither by the temperature uptick. They were overloaded by human emissions, in every year of the period in question.
Ferdinand:
You have the gall to say to me
Say what!? This argument in this thread began when you wrote
n.b. YOU WROTE “different processes are at work” and “The process that removed the extra CO2 pulse in the years 1991-1992 was …” and “is completely independent of the removal of an extra injection of ~55 ppmv then (110 ppmv now) above steady state in the atmosphere”.
You are claiming the sinks magically know if CO2 is from a ‘natural’ or ‘anthropogenic’ source and they sequester the two types of CO2 differently so they have different ‘half-lives’ in the atmosphere. THEY DON’T.
The ‘pulse’ demonstrates that the half-life of additional CO2 in the atmosphere is less than a year which is much less than the 35 years your model says.
The problem with this debate is that you keep contradicting yourself and claiming you said other than you did as and when you are shown to be plain wrong. What you call my “obstinacy” is my consistency which contrasts with your inconsistency.
Richard
Richard Courtney,
Indeed I wrote: different processes are at work. I didn’t write different sink rates for human and natural CO2 are at work. That is what your prejudice makes of what I wrote…
A process may differentiate for what kind of CO2 is released for specific processes, but a process never differentiates between natural and human CO2 molecules when it takes CO2 away out of the atmosphere, it may make a differentiation between 12CO2 and 13CO2 for natural and human CO2 alike. That is all.
You are claiming the sinks magically know if CO2 is from a ‘natural’ or ‘anthropogenic’ source and they sequester the two types of CO2 differently so they have different ‘half-lives’ in the atmosphere. THEY DON’T.
You are completely confused between “processes” and the origin of what is sequestered: the processes have different half lives and different limits, no matter the origin of the increases themselves or the origin of the molecules which are at that moment in the atmosphere. The two processes work independent of each other and grab any CO2 which is in the neighborhood, whatever their origin, when they remove CO2 out of the atmosphere.
The -small- CO2 release as described by Tom Quirk, was from a biological source, partly caused by a temperature uptick, an all natural CO2 release, completely dwarfed by human emissions in the same years.
Your fast half life for the increase and removal of CO2 is for the influence of temperature and the Pinatubo on ocean surface and bio-life: fast, but limited in quantity and duration.
The removal of any extra pressure above steady state in the atmosphere, whatever the cause, is quasi-independent of temperature and is a much slower process that involves the removal of CO2 out of the atmosphere (whatever its composition) into the deep oceans: slow, but quasi unlimited in quantity and duration.
As I said: different processes at work, not different preferences…
Ferdinand Engelbeen:
Could you explain what those numbers are?
The 2012 numbers, for example, are 110 ppmv and 2.15. I assume the 110 ppmv is the difference between the 2012 atmospheric CO2 concentration and what the atmospheric concentration would be if it were in equilibrium with oceanic CO2 at the current ocean temperature and carbon content. But I’m not sure.
As to the 2.15, I’m in the dark. Judging from how you use it, it would seem to be a decay in the atmospheric CO2 concentration. But I don’t see that concentration decaying. So maybe it’s somehow the difference between “excess” emissions, whatever that may be, and the rate of atmospheric-carbon-dioxide-concentration increase. But I’m guessing it’s something else.
Could you explain that further?
Joe Born:
110 ppmv indeed is the difference between observed CO2 level and what the theoretical steady state (dynamic equilibrium) between oceans (and vegetation) and the atmosphere should be for the current temperature. That is the level found in ice cores over the past 800,000 years and it is in the ball park of Henry’s law for the solubility of CO2 in seawater (4-17 ppmv/K in the literature).
Human emissions are X ppmv/year, based on sales inventories and burning efficiency. The increase is measured in the atmosphere and with the law of conservation of mass, the difference is what is absorbed by the sinks, wherever and whatever they may be. Currently (2012) that was 2.15 ppmv/year.
Henry’s law says that the uptake or release of any gas in solution is directly proportional to the pressure difference between the partial pressure in the atmosphere and in the liquid. If the pressure difference doubles, the uptake (or release) doubles. That seems the case here too.
Peter Dietze explains why the IPCC’s Bern model is wrong. I only used his calculation of the sink rate to compare with the current one, but if you like:
I don’t know what the Oeschger eddy diffusion ocean model is, but if that assumes that the ocean sink is caused by the diffusion of CO2 from the ocean surface into the deep oceans, it is certainly wrong: the atmosphere – deep oceans exchange is not by diffusion but by direct ocean water exchanges near the poles (sinks) and equator (upwelling) largely bypassing the rest of the ocean surface. Mixing at the ocean surface with the atmosphere is mainly a matter of wind, which is sufficiently present near the poles and the sink rate is directly proportional to the pCO2 difference, which is huge there: 400-150 = 250 μatm (~ppmv). That is the THC (Thermohaline Current), a continuous (down) flux of water caused by wind and salt content/temperature/density.
The main difference with the Bern model is that it assumes a rapid saturation of the deep oceans per Revelle/buffer factor. That indeed is true for the ocean surface, but not for the deep oceans, as the sink places are largely under saturated and once removed from the surface, the Revelle/buffer factor plays no role at all…
Ferdinand Engelbeen:
Thanks a lot for the number clarification.
And thanks for the diffusion discussion, too, although my grasp is still a little tenuous.
Here’s my take on it. Although the ocean cannot absorb CO2 without increasing its average concentration, the effect we see at the surface is negligible because on sub-millennial time scales there’s a time-delay effect: the higher-atmospheric-partial-pressure-caused concentration increase in the arctic downwelling flow in essence disappears for something on the order of a millennium, after which it reappears at the upwelling sites. That transport mechanism vastly overwhelms any diffusion.
This means that for a given temperature the ocean exhibits an essentially fixed partial pressure at its surface. Therefore, the atmospheric-concentration decay is indeed essentially linear for the time horizon we’re interested in: for that time period we have the simple exponential decay that is characteristic of a one-box model, so we can use that model to infer response time.
Ferdinand Engelbeen:
I confess that I have not completely followed the Dietze post to which you referred, so the following question may not make much sense. But I hope we will be able to communicate anyway.
Dietze says, “The Oeschger eddy diffusion ocean model suggestion that the decay will work faster at the beginning and take much longer at the end (363*ln 2 = 251 years), is illogical. Such impulses are continuously injected into the atmosphere and nature treats them all equally, as it cannot distinguish between ‘old’ and ‘new’ CO2.”
I see no reason why we wouldn’t expect carbon dioxide (and whatever it’s in equilibrium with in the ocean) to propagate by something analogous to diffusion from the surface to the depths, and that would indeed suggest a diffusion response (i.e., a response faster at the beginning, and more slowly later, than that of a simple “one-box” model). Analogously to my argument in “Is the Bern Model Non-Physical, moreover, I see no reason why Dietze thinks that diffusion implies some segregation among sources. (That post showed four “boxes,” while diffusion can be thought of as performed by an infinite number of infinitesimal “boxes.”)
So any light you can shine on why Dietze dismisses the possibility of diffusion would be welcome.
Again, my perusal of the Dietze post may not have done it justice, and I apologize if as a consequence my objection is not well formed. But I hope you will nonetheless give it consideration, since I may not be the only person whom that post confused.
Manmade CO2 over decades is a decades-long series of an infinitely large number of infinitely small pulses of CO2. If each year’s human contribution is counted as a pulse and the Bern model (maybe with a correction of its parameters) is applied to each one, and the results of the yearly pulses are added together, then the results should be close enough to reality. The non-constant half-life of a pulse of CO2 comes from CO2 gradient in the top of the ocean from a pulse of CO2 decreasing as the CO2 is absorbed/mixed deeper into the ocean.
Ferdinand Engelbeen’s calculations look like ones used to calculate rate of exponential decay. The increase of atmospheric CO2 is (nearly enough) the sum of each year’s emissions after they have been reduced by a corrected Bern model, although using exponential decay instead approximates this usefully.
I agree that in the short period of our observations so far it would be difficult to distinguish between a simple “one-box” response and the higher-order Bern Model. But my understanding of Mr. Engelbeen’s view, which I take from his 7:36 AM response, is that the massive Arctic downwelling so dominates the CO2 exchange between ocean and atmosphere that on sub-millennial time scales the ocean partial pressure (modulo temperature changes) can be considered constant: a one-box model is superior to the Bern Model.
As I said above, though, I’m not positive I got it right.
Donald and Joe,
For the moment there is no appreciable difference between the Bern model and a single decay rate model (in fact a triple decay rate model). In the case of independent sink processes, one can simply sum the different decay rates:
1/τ = 1/τ1 + 1/τ2 + 1/τ3 + … …+ 1/τn
Where the fastest is always dominant. The less fast only help to increase the total decay rate.
In the case of a triple decay rate: the fastest is the ocean surface with a tau of less than a year, but a limit in uptake at about 10% of the change in the atmosphere, the limit is in the buffer capacity of seawater for CO2. The ocean surface is rapidly in equilibrium with the atmosphere and follows the increase with 2-3 years delay. The third fastest is already slow: ~170 years for the uptake by vegetation (according to the Bern model). Maybe right, as the increase of ~110 ppmv above long term steady state is good for only ~1 GtC extra uptake. But on the other side there is no limit in uptake: we are burning now what was buried in the far past as coal…
The middle range is the most interesting: what goes into the deep oceans is mixed with the gigantic amount of C there and what returns is the composition of about the total mix. The decay rate of that part is around 40 years half life time. If we forget for one moment the limited uptake by the ocean surface and vegetation, then there is the main discussion between the Bern model and the one-decay model:
The Bern model assumes a saturation of the deep ocean sinks due to the Revelle/buffer factor. That plays a role for the surface, but at the sink places the sinking waters are largely undersaturated for CO2. Once in the deep oceans, the Revelle/buffer factor plays no role at all, as that only is important for the surface-atmosphere equilibrium reactions.
Until now it is impossible to know which one is right: the Bern model or the one-decay model. Anyway, there is no sign of weakening of the sinks, even an increase in sink capacity by vegetation…
Ferdinand Engelbeen:
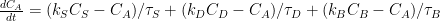
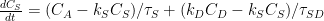

 ,
,
I see that I have not comprehended your explanation. The difficulty, as it almost always is in these discussions, is that no one (including me) disambiguates his verbal description with math.
The best I can do with your explanation is the following:
where the C’s are concentrations of carbon dioxide or carbon in the atmosphere, ocean surface, ocean depths, and biosphere, the k’s are coefficients of some sort, and the tau’s are time constants.
The first equation’s first right-side term is the exchange between the atmosphere and the ocean surface, the second is the exchange between the atmosphere and the ocean at locations where there’s upwelling from the well-mixed depths, and the third is the atmosphere’s exchange with the biosphere.
The second equation says the ocean surface conducts exchanges not only with the atmosphere but also with the ocean depths.
The third equation just mirrors in the depths the exchanges defined in the previous two equations, and the last equation mirrors the atmosphere-biosphere exchange that the first equation included.
This doesn’t reflect your statement that “the fastest is the ocean surface with a tau of less than a year, but a limit in uptake at about 10% of the change in the atmosphere, the limit is in the buffer capacity of seawater for CO2” because I didn’t comprehend it. That sounds a little like what is in some circles referred to as a slew-rate limit, but, still, I don’t quite get it. This is likely because I haven’t really grokked the “Revelle/buffer factor.”
I also didn’t comprehend the apparent inconsistency between “tau of less than a year” and the “2-3 year delay,” although that may merely have meant that a step response would come withing 1/e^5 of its final value in 2-3 years.
And, obviously, there’s no attempt to have this reflect temperature and seasonal dependencies.
From time to time I think about writing a computer model for your block diagram. It would be a simple matter if that diagram were intended to represent just the linear system the equations above define, but your comments about buffer capacity suggest that it isn’t. It may be helpful if sometime you were to set your block diagram forth in terms of differential equations like those above. Or just express in terms of those quantities what your statement about buffer capacity mean.
I recognize that there was a lot of math in the thread that accompanied your last head post, but a lot of that dealt with temperature and seasonal dependencies, and, in any event, the attempts at discrete-time math, and the distribution through so many comments, made it too obscure for me to make sense of.
Joe Born,
You can make it a little easier:
– You can drop the second part of the second equation, as most of the deep ocean – atmosphere is direct at the source/sink places, largely bypassing the rest of the surface. Moreover the exchanges between surface and deep oceans are indirectly from the atmosphere, thus ultimately are included in the atmosphere – deep oceans exchanges…
– Then you can also drop the second part of the third equation.
– The limit in the ocean’s surface needs some explanation: Henry’s law says that for a given temperature, the solubility of a gas in a liquid is directly proportionally to the partial pressure of the gas in the atmosphere above it. That is true for free CO2 in water as gas, not for bicarbonates or carbonates. As free CO2 is less than 1% of total carbon (90% is bicarbonate, 9% is carbonate), a doubling of CO2 in the atmosphere only doubles free CO2 in water: from 1% to 2% of total carbon in seawater. Thanks to a chain of reactions, bicarbonate and carbonate also increase, but don’t double. The total dissolved inorganic carbon content of the ocean surface (DIC) increases with about 10% for a 100% increase in the atmosphere.
– For the deep oceans, you don’t need to take into account the difference in mass: what goes in never comes out, at least not on short term.
– For the biosphere you need to take into account the difference in total carbon: ~800 GtC in the atmosphere, ~550 GtC in (land) biomass. No need to take ocean biomass into account as that doesn’t react on CO2 changes in the atmosphere as much as land plants do, as CO2 is not a limiting factor in the oceans.
– For the ocean surface you need to take into account the difference in total carbon: ~800 GtC in the atmosphere, ~1000 GtC in the ocean surface. To make it yourself easy, you can assume that the ocean surface only contains ~100 GtC at equilibrium with the atmosphere and thus is easily saturated with a fast exchange rate, as that includes the ~10% buffer factor…
The “tau of less than a year” and the “2-3 year delay,” are a matter of continuous inflow of human emissions. If it was a one-time pulse, the step response of the ocean surface would be within 2-3 years back in equilibrium, but as human emissions and the increase in the atmosphere are continuously going on, the 2-3 years delay also will continue.
Indeed all these equations are dealing with the increased pressure in the atmosphere due to human emissions (or any other one-way addition to the atmosphere), not with temperature changes.
There is no need to introduce temperature for seasonal or short term (1-3 years) variability, as that zeroes out after a year or a few years. Only the longer term increase in temperature should be taken into account, which is positive for the oceans (both surface and deep exchanges) and negative for the biosphere. As that is about 10% of the current increase, not taking into account the temperature increase gives already a good approximation of what happens with human emissions…
Ferdinand Engelbeen:
Thank you for your detailed reply. If I may, I’d like to deal with it piecemeal. This comment is directed to your comment that:
As far as the second equation goes, that seems fair enough; as you say, the second equation then becomes simpler:
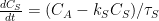
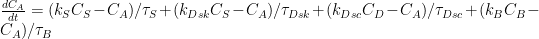
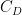 , I infer from your comments that we can safely consider it a constant for sub-millennial time horizons. But for the time being I will retain the third equation but have it (1) reflect the other changes and (2) include a time delay. This results in the following form, which I caution is questionable for a reason I will get to directly:
, I infer from your comments that we can safely consider it a constant for sub-millennial time horizons. But for the time being I will retain the third equation but have it (1) reflect the other changes and (2) include a time delay. This results in the following form, which I caution is questionable for a reason I will get to directly:
![\frac{dC_D}{dt} = [C_A-k_{Dsc}C_D(t-t_{trans})]/\tau_{Dsc}+(C_A-k_{Dsk}C_S)/\tau_{Dsk}](https://s0.wp.com/latex.php?latex=%5Cfrac%7BdC_D%7D%7Bdt%7D+%3D+%5BC_A-k_%7BDsc%7DC_D%28t-t_%7Btrans%7D%29%5D%2F%5Ctau_%7BDsc%7D%2B%28C_A-k_%7BDsk%7DC_S%29%2F%5Ctau_%7BDsk%7D&bg=ffffff&fg=000&s=0&c=20201002)
 ago, where
ago, where 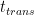 is probably on the order of a millennium. That term makes me a little queasy because it indicates that a high enough atmospheric concentration at the source locations could add to the depth concentration. It could, of course, but only by also affecting the surface, which the equations isolate from the source and sink exchanges in accordance with what I believe you said.
is probably on the order of a millennium. That term makes me a little queasy because it indicates that a high enough atmospheric concentration at the source locations could add to the depth concentration. It could, of course, but only by also affecting the surface, which the equations isolate from the source and sink exchanges in accordance with what I believe you said.
(I haven’t reached the buffering issues, which will no doubt require further changes, but let’s take one step at a time.)
Although that made the second equation simpler, your statement also suggests that the first equation should become more complicated. Specifically, the exchange rate at the source locations should be proportional to the difference between the (differently weighted) atmosphere and ocean-depth quantities, whereas the difference upon which the rate at the sink locations depends would, it seems to me, have to be one between (again, appropriately weighted quantities at) the atmosphere and the surface rather than between atmosphere and the depth quantities.
I.e., the process at the downwelling, sink locations can’t know what’s going on at the depths, whereas conditions at the upwelling, source locations could reflect depth conditions. (Incidentally, it’s probably best if the C’s be considered total carbon mass, or the difference of that quantity from some reference value, rather than concentration, since these equations suggest that the changes in those quantities balance.) This changes the first equation into the following mess:
The right-hand side’s second term represents the (surface-content-dependent) sink exchange between the atmosphere and the depths, whereas its third term represents the (depth-content-dependent) source exchange.
As to the depth quantity
The right-hand side’s second term represents the sink exchange, which, as I argued above, depends on the surface content. Particularly questionable is the right-hand side’s first term, which represents the source exchange. I have so written it that the rate depends among other things on the source concentration, which in turn depends on what the depth quantity was
I have other questions about your reply, and I hope I will be able to reach them before the lights go out at this thread. But I’m not sure that will happen.
Ferdinand Engelbeen:
I’m afraid my ignorance of chemistry is holding me back here.
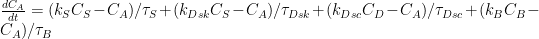
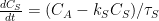
![\frac{dC_D}{dt} = (C_A-k_{Dsc}C_D)/\tau_{Dsc}+[C_A-k_{Dsk}C_S(t-t_{trans})]/\tau_{Dsk}](https://s0.wp.com/latex.php?latex=%5Cfrac%7BdC_D%7D%7Bdt%7D+%3D+%28C_A-k_%7BDsc%7DC_D%29%2F%5Ctau_%7BDsc%7D%2B%5BC_A-k_%7BDsk%7DC_S%28t-t_%7Btrans%7D%29%5D%2F%5Ctau_%7BDsk%7D&bg=ffffff&fg=000&s=0&c=20201002)

It sounds at first as though you’re saying there’s and equilibrium reaction occurring in the ocean by which carbon so switches among free CO2, bicarbonate, and carbonate as to keep 1% as free CO2, 90% as bicarbonate, and 9% as carbonate. That being the case, if doubling of atmospheric CO2 causes a doubling of ocean CO2, then bicarbonate and carbonate would both have to double, too, in order to maintain the 1% – 90% – 9% allocation. Yet you say, “The total dissolved inorganic carbon content of the ocean surface (DIC) increases with about 10% for a 100% increase in the atmosphere.” Since the 100% increase in atmospheric CO2 would necessitate a 100% free-CO2 increase in the ocean to balance the partial pressures, the 1% – 90% – 9% allocation in the ocean would no longer prevail if bicarbonate and carbonate increased by only 10% rather than 100%.
What am I missing?
By the way, I meant to put the updated tentative model in one place but failed to in my last comment:
Joe Born,
Sorry for the delay, I am on a cable line, normally extremely fast, but in the evening someone is streaming whole films I suppose, which makes horrible typing on WordPress. Yesterday night I had to give up…
Ocean chemistry indeed doesn’t make it simple…
At the current carbon levels in the atmosphere, the ratio is about 1:90:9 for CO2:bicarbonate:carbonate at the current pH in the ocean surface.
If you double CO2 in the atmosphere, that doubles free CO2 in the ocean surface, which increases bicarbonate and carbonate, but also H+: the pH gets slightly lower. That gives that the equilibrium isn’t maintained at 1:90:9 but shifts to 2:89.1:8.9 or something like that. That makes that while free CO2 doubles, bicarbonates and carbonates don’t double… That can be seen in the “Bjerrum” plot:
https://en.wikipedia.org/wiki/Bjerrum_plot
The Revelle buffer factor is a measure for that effect. For the calculation, you can use that effect as a 10% increase in total carbon in the ocean surface for a 100% increase in the atmosphere. As the ocean surface is a minor player and rapidly saturated, any error in that part has little effect on the overall sink rate.
The atmosphere – deep ocean exchange may be simplified by assuming that the source quantity is constant for periods shorter than a millennium, only modulated by temperature and the sinks do all the work for the extra pressure in the atmosphere. In reality the sources are suppressed and the sinks increase with increased CO2 pressure in the atmosphere. For the levels in atmosphere and deep oceans that makes no difference. So you can make the third term in the first equation a constant and so for the first term in the third equation (let’s number the equations to make it easier to follow…).
I need to learn Latif, but so little time and so much still wanting to do…
Ferdinand Engelbeen: , but insert “latex” after each initial (but not final) dollar sign.
, but insert “latex” after each initial (but not final) dollar sign.
Thanks for the further reply. I think I’ll probably be able to incorporate that into the equations, which should then be pretty close to reflecting your model. Unfortunately, I won’t be able to reach that until sometime tomorrow, but I hope you’ll stay tuned.
In the interim, I’ll mention that your LaTeX learning curve might be flattened by using an application such as TexMaker, which helps you write it and displays the results. To put an equation into your comment, all you have to do is copy the resultant code, e.g., “$\frac{dC_S}{dt}$” for
Joe Bron,
If the threat closes, you can Google my name and send a mail, or via Facebook (but I seldom look at that)…
Ferdinand Engelbeen:
![\rho_{CO2_S} = \rho_{CO2_S}(1850)+0.1[\rho_{C_S}-\rho_{C_S}(1850)]](https://s0.wp.com/latex.php?latex=%5Crho_%7BCO2_S%7D+%3D+%5Crho_%7BCO2_S%7D%281850%29%2B0.1%5B%5Crho_%7BC_S%7D-%5Crho_%7BC_S%7D%281850%29%5D&bg=ffffff&fg=000&s=0&c=20201002)
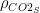 is the concentration of free aqueous carbon dioxide in the ocean’s surface layer and
is the concentration of free aqueous carbon dioxide in the ocean’s surface layer and  is that layer’s concentration of total dissolved inorganic carbon. Perhaps I’ll have a chance later to come back to that later and base it explicitly on the Bjerrum relationship.
is that layer’s concentration of total dissolved inorganic carbon. Perhaps I’ll have a chance later to come back to that later and base it explicitly on the Bjerrum relationship.
![\dot{A} = [(P_S - P_A)/ \tau_S + (P_{sink} - P_A) / \tau_{sink} + (P_{source} - P_A) / \tau_{source}]r + (k_{AB} B - A) / \tau_B + E](https://s0.wp.com/latex.php?latex=%5Cdot%7BA%7D+%3D+%5B%28P_S+-+P_A%29%2F+%5Ctau_S+%2B++%28P_%7Bsink%7D+-+P_A%29+%2F+%5Ctau_%7Bsink%7D+%2B+%28P_%7Bsource%7D+-+P_A%29+%2F+%5Ctau_%7Bsource%7D%5Dr+%2B+%28k_%7BAB%7D+B+-+A%29+%2F+%5Ctau_B+%2B+E&bg=ffffff&fg=000&s=0&c=20201002)
 is the rate of change of the atmospheric carbon mass, the P’s are carbon-dioxide partial pressures,
is the rate of change of the atmospheric carbon mass, the P’s are carbon-dioxide partial pressures,  is the atmospheric ratio of carbon mass to carbon-dioxide partial pressure,
is the atmospheric ratio of carbon mass to carbon-dioxide partial pressure,  is human carbon emissions,
is human carbon emissions, 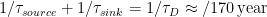 ,
, 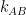 is an assumed equilibrium ratio of atmospheric carbon and to carbon in vegetation, and
is an assumed equilibrium ratio of atmospheric carbon and to carbon in vegetation, and

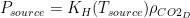
I got a little stuck on the surface carbon-dioxide concentration, so I just abstracted what you said to adopt this relatioship:
where
Also, I decided it may be less confusing to base things on total carbon mass, so I,m adopting the notation that A, S, D, and B stand for that mass in the atmosphere, ocean surface, the ocean depths, and the biosphere, respectively. Then the first equation becomes:
where
$P_{sink}=K_H(T_{sink})\rho_{CO2_S}$,
where $K_H(T)$ is the Henry’s-Law coefficient at temperature $T$ and $T_{sink}<T_S<T_{sink}$. Note that the source and sink pressures are based on different concentrations.
We’re treating the deep ocean’s total dissolved organic solids D as constant, which leaves just two further differential equations.
$\dot{S}=(P_A – P_S)/r \tau_S $
$\dot{B}=(A-k_{AB}B) / \tau_B $
Is that close to your model? If so, I’ll translate it into an R script after Christmas.
What is the rate for sequestration and deposition of oceanic CO2 into the shells of the surface biosphere, and ultimately the seabed? Is it significant enough to merit a place in your formula? The DOE Center for Research on Ocean Carbon Sequestration suggests that this accounts for more than 2Gt per year.
===|==============/ Keith DeHavelle
Ferdinand Engelbeen:
![\dot{A} = [(P_S - P_A)/ \tau_S + (P_{sink} - P_A) / \tau_{sink} + (P_{source} - P_A) / \tau_{source}]r + (k_{AB} B - A) / \tau_B + E](https://s0.wp.com/latex.php?latex=%5Cdot%7BA%7D+%3D+%5B%28P_S+-+P_A%29%2F+%5Ctau_S+%2B++%28P_%7Bsink%7D+-+P_A%29+%2F+%5Ctau_%7Bsink%7D+%2B+%28P_%7Bsource%7D+-+P_A%29+%2F+%5Ctau_%7Bsource%7D%5Dr+%2B+%28k_%7BAB%7D+B+-+A%29+%2F+%5Ctau_B+%2B+E&bg=ffffff&fg=000&s=0&c=20201002)
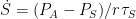
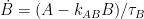 ,
,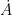 is the rate of change of the atmospheric carbon mass, the P’s are carbon-dioxide partial pressures,
is the rate of change of the atmospheric carbon mass, the P’s are carbon-dioxide partial pressures,  is the atmospheric ratio of carbon mass to carbon-dioxide partial pressure,
is the atmospheric ratio of carbon mass to carbon-dioxide partial pressure, 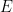 is human carbon emissions,
is human carbon emissions, 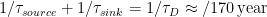 ,
, 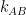 is an assumed equilibrium ratio of atmospheric carbon to carbon in vegetation, and
is an assumed equilibrium ratio of atmospheric carbon to carbon in vegetation, and

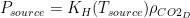
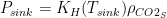 ,
,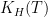 is the Henry’s-Law coefficient at temperature
is the Henry’s-Law coefficient at temperature 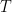 and
and  . Note that the source and sink pressures are based on different concentrations.
. Note that the source and sink pressures are based on different concentrations.
I see I didn’t get the equations to come out right. Let me try the three differential equations again:
where
where
On a very closely related matter –
BBC is presently showing, as background to its feed on the Paris agreement, an animation of global CO2 concentrations which looks very like the OCO-2 animations, but clearly shows great swirls of orange and red emanating from the northern industrial nations, India and China. It shows no high concentrations anywhere in the southern hemisphere or the tropical oceans. This is very unlike the OCO-2 data releases that we have had so far, and if it is from OCO-2, has clearly been selected and processed to demonstrate the ‘guilt’ of the industrial nations. This animation appears on the screen as a background to the chat, is not referred to or explained, and no attribution is made. Subtle but massively effective.
I think we need to get to the bottom of this – anyone know where to start?
mothcatcher:
I suspect the animation you mention is this NASA computer model graphic. It predates the OCO-2 data and – as you say – is very different from the OCO-2 data.
At 3pm UK time this afternoon BBC1 is scheduled to show a somewhat better computer model graphic titled ‘Toy Story’.
Richard
Richard – I am sure you are right. The NASA model graphic that you link to does not seem quite so dramatic as the BBC version (that may be wrong memory on my part of course) but the features are the same. Thanks for the link. I do remember seeing this before, but was surprised to learn there is a 2014 version, as I had thought it much older.
Anyway, seems to me there is a strong case that the BBC presentation can be demonstrated to be propaganda, and they should be called to account specifically on this point.
Sorry if this is duplicated – my earlier reply to you seems to have vanished.
mothcatcher:
I would be grateful if anybody were able to tell me how the BBC can be “called to account” for any of the BBC’s pro-AGW propaganda. Please see this and also read its links.
Richard
Richard –
You are depressing me. Yes, I’m aware every day of the wall that BBC has constructed.
It just occurred to me, here is something very specific that can be demonstrated. But who can publicise it?
Inconvenient truth is not allowed in the CO2 discussion, if the discussion is controlled by the CO2 obsessed. BBC, NPR, and other state controlled media have self censorship down to a fine art,
data from the Japanese IBUKU climate satellite shows you have overestimated the human contribution. you give a figure of 33 percent yet IBUKU puts the human contribution from humans in the northern hemisphere as zero! As the CO2 is emitted it is immediately reabsorbed in the northern hemisphere.. Oc ean de-gassing and plant decomposition(southern hemisphere) are the sources of CO2 in the atmosphere. The human contribution is small if not negligible. (agreed by both Lindzen and by Salby)
Terry,
Human emissions are ~4.5 ppmv/year, of which ~2.3 ppmv/year remains in the atmosphere. That is 0.006 ppmv/day. Even concentrated in 10% of the surface, IBUKU couldn’t detect that. OCO-2 has a better accuracy and can focus on specific areas, but even then it will be a hell of a job to detect human emissions in the natural noise..
Further, CO2 levels increase and 13C/12C ratios decrease first in the NH, which points to a low-13C source of CO2 in the NH.
Lindzen doesn’t agree with you, while Dr. Salby does, but he is wrong…
AndyG55, Bartleby:
Looking at whole earth images from space, it is quite easy to see: yellowy/reddish hot regions of land, shades of green for vegetation/jungle/forest. and a loverly blue for sea water.
Nowhere do I see a ‘blue sky’ when looking at the earth from space.
I would suspect that if the atmosphere only passed/reflected blue/red as you both appear to suggest, that a whole range of satellite imaging techniques would never have worked!!!!
Does the earth look blue from space because we have very large oceans?
http://pics-about-space.com/earth-from-space-nasa?p=1#img3078181329541982023
Steve –
The ocean appears blue because water absorbs in the red spectrum and reflects in the blue. It’s no more complicated than that. Divers experience the effect directly, with a loss of red in the first 30 to 40 feet below the surface. Underwater photographers are sensitive to this and use filters or artificial light to enhance red for this reason.
The atmosphere also tends to absorb red light due to water vapor, which is why infrared astronomers place their instruments as high above water vapor possible. The Mauna Kea observatory is probably the premier example of a land based IR observatory. During the 70’s and 80’s NASA operated the Kuiper Airborne Observatory (KAO), a Lockheed C-141 Starlifter equiped with a 1 meter IR telescope with a service ceiling of 48,000 feet above Mean Sea Level for the purpose of getting above 99% of the water vapor in Earth’s atmosphere. It has since been replaced by the SOPHIA platform equipped with a 3 meter telescope flown on a Boeing 747 “stretch”, used for the same purpose. Of course the Hubble is well above the tropopause (the boundary layer of water vapor in Earth’s atmosphere) and capable of much better performance in the IR spectrum.
The KAO was successfully used by Dr. Charles Townes (inventor of the MASER) of Lawrence Livermore National Laboratories in the late 1970’s to map the central parsec of our galaxy in the IR spectrum and was also used to image the re-entry of the maiden flight of the Columbia (STS-1) in 1981 under the direction of Dr. Debra Strange of Martin-Marietta Aerospace.
It’s possibly interesting to readers of this blog that IR astronomers weren’t particularly concerned about the absorption spectra of CO₂ during the operations of the KOA; water vapor was the enemy and we did everything we could to get above it.
Bartleby, your question about what the pre:climate obsession scientists were concerned about is fascinating. Thanks.
I have never suggested that the atmosphere only passes blue. Where did you read that ?
Andy writes: “I have never suggested that the atmosphere only passes blue”
I know. That’s why I wrote my post-script.
And now, of course, any humor that might have existed in my previous exchange with Andy is lost by explanation. Thanks Andy 🙂
How does your calculated half-life of 35 years explain the “bomb curve” that describes how fast 14C dissappearded from the atmosphere since the
Gösta Petterson (http://www.false-alarm.net/paper-5/) derives a e-folding time of 14 years (half-life aprx 10 years). 35 years might be far way from what IPCC claim but it is still far too high to explain what we actually can observe.
Does you analasis leave any room for the natural increase in C02 concentration as a result of waming ocean?
More info on the Gösta Pettersson analysis
http://wattsupwiththat.com/2013/07/01/the-bombtest-curve-and-its-implications-for-atmospheric-carbon-dioxide-residency-time/
The “bomb curve” is direct proof that CO2 never accumulates in the atmosphere, from any source. Just as water does not “accumulate” in a river.
One-half of the entire mass of CO2 in the atmosphere in 1965 was gone by 1975. That curve is measured and confirmed continuously. By 2015, only 1/32 of the 1965 CO2 remains.
Most of the 2015 level of atmospheric CO2 is derived from deep sources that have very long geochemical cycle periods. Anthropogenic CO2 is about 3 percent of the current 400 ppm, based on annual flux proportions. Segalstad represents the basic isotope analysis on his web page. http://www.co2web.info/
Comparing the southern hemisphere bomb curve to measuring stations in the NH show a slightly longer period of about 12 years for the mass 1/2 life. The so-called e-folding time is calculated directly from the observed 14C decline, with a value of 16 years for the NH and maybe 20 years for the SH.
The OCO-2 observations directly show the tropical and SH exchange of carbon from huge surface pools exchanging with the atmosphere above. That satellite was designed to be able to observe the anthropogenic portion of CO2 addition to the atmosphere. If it were actually finding that, then we would already be seeing the analysis being screamed out by the alarmists. Just the opposite is occurring.
Johan Montelius and BW,
I had a firm discussion about that with Gösta Petterson, as he didn’t take into account the deep ocean return: what goes in the deep oceans is the isotopic composition of today, what comes out is the isotopic composition of ~1000 years ago, long before the 14C bomb spike. That makes that much less 14C returns than 12C and the 14C decay rate is at least 3 times faster than for any 12CO2 peak…
See the scheme here…
Reblogged this on Centinel2012 and commented:
COP21 is only about taxes and the redistribution of wealth: the politicians have bamboozled some “scientists” into actually being that this is a real problem and the drive to get their hands on more and more money feeds the system. Its a political problem more than a science problem. But I guess we are stuck with it for now and just have to wait until nature proves them wrong.
I would change the word bamboozled to bribed.
Ari Halperin:
Your above essay says
True, but that ignores my post in the thread below – and in response to – your original WUWT article .
My linked post explained that Tom Quirk has analysed a ‘pulse’ of 9Gt of CO2 in the atmosphere and said
Quirk used isotope analysis to determine that the 1989 – 1991 ‘pulse’ of 9.3 Gt of additional CO2 derived from oceanic plants.
It is very important to note that the sinks for CO2 sequestered the 9.3 Gt of additional CO2 so it was “withdrawn from the atmosphere” in three years. As I said to you in my post in the previous thread (which I have linked from this post), this sequestration rate demonstrates that the sinks are NOT overloaded.
The total sequestration of the 9.3 Gt of additional CO2 also demonstrates that – whatever the form by which surplus CO2 concentration drops – the half-life is less than one year (n.b. much, much less than the decades your model suggests): a half-life of 6 months reduces a ‘pulse’ by 98% within three years.
I repeat the conclusion of my previous and linked post to you
That is also true for the Bern Model used by IPCC, Engelbeen’s model, and all other models based on an assumption that the sinks are overloaded when observations show the sinks are NOT overloaded. And if the sinks are not overloaded then CO2 emissions from human activities are not overloading them so there is NO surplus atmospheric CO2.
The observed and continuing rise in annual atmospheric CO2 concentration probably results from a response to altered equilibrium state of the carbon cycle. Some processes of the system are very slow with rate constants of years and decades. Hence, the system takes decades to fully adjust to a new equilibrium state. The CO2 emissions from human activity may be altering the equilibrium state of the carbon cycle but natural effects are more likely to be the cause of the alteration; e.g. the temperature rise from the Little Ice Age.
(ref. Rorsch A, Courtney RS & Thoenes D, ‘The Interaction of Climate Change and the Carbon Dioxide Cycle’ E&E v16no2 (2005) )
Richard
The issue I have with Ferdinand’s model and his equilibrium time of 50 years above (or half-life of 38 years) is that it is based on the assumption that anthropogenic sources are driving the increases in atmospheric CO2. But it cannot also prove that anthropogenic CO2 is driving the atmospheric increases. That would be circular logic. It is also based on the assumption that the pre-industrial atmospheric CO2 concentration was 280ppmv and that the system must be out of equilibrium by 110ppmv. But none of these things are known with any certainty and even the pre-industrial atmospheric CO2 concentration is still a matter of debate. And the idea that the system must be out of equilibrium by 110ppmv is not a known fact. His equilibrium time of 50 years is arrived by taking today’s CO2 concentration of 400ppmv and then subtracting 290ppmv (the assumed equilibrium CO2 level + 10ppmv for Henry’s law) and then assuming the system must be out of equilibrium by 110ppmv and then dividing that by how much he assumes the sinks are absorbing of our emissions every year (2.15ppmv). The whole thing just seems rather assumption-based.
Richard (not Courtney),
The 290 ppmv for the current area weighted average temperature is based on two points: ice cores which show a rather linear ratio between temperature and CO2 levels over the past 800,000 years and Henry’s law which shows that for ~15°C the pCO2 of ocean waters is about 290 μatm or ~290 ppmv in equilibrium with the atmosphere. That is confirmed with currently over 3 million pCO2 measurements of seawater.
Any linear process reacts proportionally on disturbances: if the pressure difference doubles, the sink rate doubles. We didn’t know that the process was linear, but the data show it: there is no change in half life time between 1958 and 2012 (within the accuracy of emissions and increase rate), despite a quadrupling of the increase in the atmosphere: the net sink rate quadrupled too.
Without assuming that humans are responsible for the increase, the net sink rate is the difference between human emissions and what remains in the atmosphere. Whatever the natural sinks and sources may do. That is a matter of conservation of mass…
The only exception that humans are not responsible for the increase is if the natural carbon cycle also increased a fourfold, fully synchronized with the fourfold increase of human emissions. For which is not the slightest indication…
Richard:
I agree all that you say about Ferdinand’s model.
However, my point was – and is – that all the models (i.e. Bern, Engelbeen, Halperin, etc.) which assert the anthropogenic emission is overloading the sinks are wrong because it is observed that in the real world the sinks are NOT overloaded.
Observation of the real world demonstrates that the sinks are not overloaded and “surplus” atmospheric CO2 is sequestered with a half-life of less than a year.
Models can indicate anything happens. Observation of reality reveals what is happening in reality.
Richard
The statement that the “US, Western Europe, Canada, and Australia ([are] the only countries in the world that engage in the climate masochism)” is in error. New Zealand (which you can think of as Australia’s Canada) part of that group also. We have an Emissions Trading Scheme that puts up energy prices. See http://www.climatechange.govt.nz/emissions-trading-scheme/about/basics.html
Richard,
“Australia’s Canada”! I like that.
Except that NZ has about a fifth of Oz’ population, while Canada’s is only around a tenth of the USA’s (less than California’s, even without factoring in illegal immigrants).
In New Zealand (NZ), there is a 15% GST on EVERYTHING (Except certain sanitary items). The ETS in NZ added about 8% to energy costs to consumers and although power is about 80% from renewables in NZ…IT IS VERY EXPENSIVE! Top rate income rate in NZ is 33% (I think, could be 36%), ACC levy 1.2%. So at NZ$60k…you have already been taxed 34.2% (In tax bands). After that…15% GST on everything! Almost 50% tax!
Sorry for missing NZ!
Could someone produce a chart (or point me to a link) showing the percentage of man made CO2 emissions by decade, of total emissions ever? Ie so one can say x% of all CO2 ever emitted by man was post 2000? Its a killer fact I’d like to chuck at people in arguments about AGW but can never find a link to the exact figures. Eyeballing the chart above suggests 25-30% of all man’s CO2 emissions have come post 2000, maybe more, especially as it only goes up to 2010.
Data for human CO2 emissions can be found here: http://cdiac.ornl.gov/ftp/ndp030/global.1751_2011.ems
Adding it together is a chore. Units are in ‘million metric tonnes’ carbon. To convert carbon into CO2 multiply by 3.67 and then to convert million metric tonnes into gigatonnes divide by 1000. You can then convert gigatonnes to ppmv by dividing by 7.8.
I already added the total amount of CO2 up a while ago and this is what I got (the trends for the time-periods were from the Phil Jones BBC interview).
Period———-Length in Years————–Trend/Decade———-CO2 Emitted
1860-1880—————21———————–0.163ºC—————12 gigatonnes
1910-1940—————31———————–0.150ºC—————110 gigatonnes
1975-1998—————24———————–0.166ºC—————480 gigatonnes
1975-2009—————35———————–0.161ºC—————770 gigatonnes
Thank you, thats a very helpful link.
The source I saw and use for anthropogenic co2 is 365 bmt from 1750 to 2011. That number was widely quoted on a number of websites. Also the years from 1860 to 1880 is the same amount of co2 released in 1965. Total co2 increase per ppm in 1965 was 1 ppm. Does that make sense that 1 ppm in 20 years contributed to a rise in temperature when the same amount didn’t in 1 year? I know CAGW likes to throw confusion in their with units of measure. I keep in the metric realm and plainly spelled out, a billion metric tons.
If you ask about this at a climate confrence, they like to play word games. Because I don’t know the difference between an English ton and a metric one, or carbon ton..
Basically every year after 1965 should have went up 0.163 C every year at least. In any of the last 5 years , in one year the temperature should have risen 0.489 C . I don’t think any of us would be talking about whether co2 is a problem. Just from the trend lines it isnt. Thanks for those new numbers.. something else to look at.
Jim writes: “Could someone produce a chart (or point me to a link) showing the percentage of man made CO2 emissions by decade”?
Oh come on Jim. You’re starting to sound like a scientist. Get down on your knees and BELIEVE!
From the quoted report: “All emission estimates are expressed in million metric tons”
And don’t forget to convert those “estimates” to pure fantasy before converting them to gigatons. OK?
Most of the time human CO2 emissions are expressed in gigatonnes Bart, that’s why I converted them. Just thought it might be more helpful.
Chipster, what would be more helpful would be actual measurements.
As the clowns toast to their climate change summit, surely they are not toasting with champagne, soda, or “sparkling water”!
Ari Halperin:
That statement is just wrong.
Yeah… Not even in the top 10 by percentage…
http://hyperphysics.phy-astr.gsu.edu/hbase/tables/elabund.html
#10, according to Wikipedia table (reference #3)
Are the quantities discussed and graphed here Gt C or Gt CO2?
(The sky is blue because air is blue.)
A Gt or Pg C produces 3.67 Gt or Pg of CO2. Convert Pg C to ppmv by a factor of .4694. Convert Pg CO2 to ppmv by a factor of .1291
That is why I asked the question.
You left out Russia/USSR and Japan. They contributed far more than Canada and Australia. Lumping them in with ROW pants a distorted picture.
http://www.wri.org/sites/default/files/uploads/historical_emissions.png
No, I included them in the Rest of the World (ROW)
Ari Halperin and Geoff Sherrington,
For me it looks like that in Fig. 1 the whole increase from 1750 to the present day would be anthropogenic by nature. This amount today is about 250 GtC. This is also the claim of IPCC that the measured CO2 increase is totally anthropogenic, which means that the anthropogenic portion would be about 28 %. The direct measurements show that the C13/C12 ratio is -8.4 permill corresponding the anthropogenic CO2 portion of 7.7 % (=65 GtC). What is actually your figure for the anthropogenic CO2 in the atmosphere is not clear for me.
I have simulated the recycling process between the atmosphere, the ocean and the biosphere and the results show that the anthropogenic portion in the atmosphere is the same as the measured value. The simulations for the future show that the mean residence time for the anthropogenic CO2 in the atmosphere is 15 years and that of the total CO2 increase in the atmosphere is 32 years.
I think that Geoff Sherrington has a point in doubting that the radioactive 14C residence time measurements are not the same as the anthropogenic CO2 for the reasons he shows. In my simulations the year 2013 fluxes show that the anthropogenic CO2 flux into the ocean from the atmosphere is 7.9 GtC/y but at the same time the anthropogenic recycle CO2 flux from the ocean’s mixing layer into the atmosphere is 3.2 GtC.
aveollila
You need to take into account the “thinning” effect of the deep ocean circulation on both the 14C level and the 13C/12C ratio: what goes into the deep oceans is the isotopic composition of today, what comes out is the isotopic composition of ~1000 years ago, long before human emissions and atomic bomb tests… That makes that the human “fingerprint” both for 14C and 13C/12C ratio is at least a factor 3 too small…
The deep ocean circulation is taken into effect. What goes down is depending on concentration and temperature today and what comes up is depending on concentrations ~1000 years ago. This is why 14C from the bomb tests disappeared as it did – and the same thing happens with any CO2 that we add to the atmosphere.
With a e-folding time of 14 years we can ignore any emissions that happened more than 30 years ago.
Johan M,
The difference between any excess 12CO2 drop and the 14 C bomb spike drop is much larger than you expect:
– CO2 return in 1960 at the height of the 14C bomb spike was about 97% of the CO2 sink in the deep oceans. As 12CO2 is ~99% of all CO2, about 97% of 12CO2 returns,
– The 14C bomb spike doubled the “background” 14C level in the atmosphere. Thus the 14C return is 97% (mass) x 45% (concentration – radio decay) of the 100% spike. Which gives a much faster decay rate than for a 12CO2 spike…
A half life of 35 years means that the current excess of 110 ppmv gets 55 ppmv in 35 years, 27.5 ppmv in 70 years… and negligible in 5 * 35 = 175 years…
Most CO2 coming out of the ocean is CO2 that was recently absorbed by the surface, rather than CO2 coming out from upwelling deep water – which is a CO2 sink because it dates back to when the atmosphere had less CO2 and it is usually colder.
Ferdinand Engelbeen
I can’t follow you.
The flow from the ocean to the atmosphere is determined by the temperature of and concentration in the oceans. This flow does not increase just because we add C02 to the atmosphere, it increases when the concentrations in the oceans increase (which will take time) or when the temperature change (and this we have seen).
If you add 10 Gt of C-14 C02 to the atmosphere it will be only 5 Gt left in ten years time. It has not been replaced with 5 Gt of C-12 CO2, it is gone (absorbed by the oceans, resulting in a tiny increase of the flow from the oceans to the atmosphere).
Donald L. Klipstein,
That is seasonal, and largely in equilibrium: there is very little difference between what goes in the ocean surface and what comes out: about 50 GtC/season influx in the atmosphere and 50.5 GtC/season outflux: about 10% of the change in the atmosphere after a full seasonal cycle.
There is a permanent flux of ~40 GtC between equatorial upwelling and polar sinks: about 40 GtC/year influx and ~43 GtC/year outflux. Thus while both fluxes are quite large, the deep ocean cycle is the main sink for the extra CO2 in the atmosphere…
Johan M,
The ocean – atmosphere exchange is not static: there are huge CO2 flows between the two over the seasons and continuous between the equator and the poles. If the water temperature increases, that results in more influx at the equator and less outflux at the poles, the CO2 levels increase, but that increase reduces the influx and increases the outflux. At about 16 ppmv/K that is back in equilibrium: influx and outflux are again equal…
Your 14C level doesn’t disappear like that: before the bomb tests about 90% of the 14C which did sink into the deep oceans returned ~1000 years later out of the deep + what was newly formed by cosmic rays was more or less in equilibrium.
When humans started to emit 14C-free fossil fuels, that did give problems for radiocarbon dating: from ~1870 on one needed correction tables. The nuclear tests in the 1950’s were the opposite problem: 14C levels doubled in the atmosphere. Thus in 1960 at the peak of 14C, only some 45% returns from what was going into the deep oceans, not zero and not 90%. That makes that the decay rate of 14C is not the same as for 12CO2, but much faster…
In graph form:
http://www.ferdinand-engelbeen.be/klimaat/klim_img/14co2_distri_1960.jpg
Ocean surface and vegetation play much less role in this case, as what is absorbed in one season is returned in the next season. There is only some redistribution of 14C over the different reservoirs, which is small (10%) in the ocean surface and very slow in vegetation.
Ferdinand Engelbeen
So what your saying is that if we emit 10 Gt of 14C C02 in the atmosphere we would only have 5 left in 10 years but …. and this is where I don’t follow you, we would have an additional say 3 Gt of 12C C02 so that the exes of C02 was still 8 Gt?
In you diagram, if we emit so much C02 that we in one pulse double the amount of C02 in the atmosphere the amount of C02 going into the ocean would double: 82 to the deep ocean and 100 into the surface ocean. The surface ocean would after some years increase its concentration of C02 and increase the flow to the atmosphere. How much would the up going flow from the deep ocean change? It is dependent only on the concentration of C02 in the deep ocean and this will not change significant.
If your model of the ocean was correct, should we then not see a two phased decline in the bomb curve? Initially the amount of 14C in the atmosphere would decline rapidly but as the 14C increase in the surface ocean it starts to level off and then only declines at the rate allowed by the deep ocean. There of course many layers in the ocean but to describe how C02 is absorbed it is sufficient to use a model with a single well mixed ocean.
Johan M,
If you instantly double total CO2 in the atmosphere, that wouldn’t change anything in the decay rate of 12CO2: slightly over 50 years or 35-40 years half life time. That is because the double pressure will increase the sink rate a lot as mass of CO2 and depress the source rate a lot as mass, but still 99% of all CO2 going in and coming out of the oceans is 12CO2. The decay rate of 12CO2 is only a decay in CO2 mass in ratio to the CO2 level above steady state.
Not so for 14CO2: an instant doubling doubles the 14C sink rate near the poles, but what returns still is the old concentration of ~1000 years ago: less than half what goes into the oceans.
The 12CO2 decay rate is a mass decay rate.
The 14CO2 decay rate is a concentration decay rate.
If both happen at the same time:
The 14CO2 decay rate is a mass x concentration decay rate.
The latter thus is much faster than a mass only decay rate of 12CO2…
Ferdinand Engelbeen
“Not so for 14CO2: an instant doubling doubles the 14C sink rate near the poles, but what returns still is the old concentration of ~1000 years ago: less than half what goes into the oceans.”
And the same is true for 12CO2.
It is not the time per se that is important its the size of the reservoir. The bomb curve gives a good estimate on the size of the reservoir and the size of the flow from the atmosphere to the oceans. It also tells us that to since it follows a mono phased exponential decay the oceans do not have to be modeled as divided up into surface and deep oceans.
As concentrations in the atmosphere increase the flow from the atmosphere to the oceans increase proportionally. The flow in the opposite direction is determined by the concentration in the oceans (and temperature). This concentration will only change marginally and this is why the e-folding time is so short.
If you want to have a long e-folding time you need to argue for a much lower flux between the atmosphere and the oceans, or at least with the deep ocean. But if you do this then this would be contradicted by the bomb curve, no?
Johan M,
And the same is true for 12CO2.
No, what goes in the oceans with a CO2 doubling still is 99% 12CO2 in and out. The quantities is what matters in that case: the current 110 ppmv extra pressure in the atmosphere is good for ~2.15 ppmv/year extra uptake by all sinks together. A doubling since pre-industrial to 560 ppmv gives 270 ppmv above current steady state (~290 ppmv, including warmed oceans) or 270 * 2.15 / 110 = 5.3 ppmv/year.
Still for the same over 50 years e-fold decay rate for CO2 mass/pressure above steady state, while the concentration drop of 14CO2 has an e-fold decay rate of ~14 years…
What is right is that you can make an estimate of the deep ocean – atmospheric carbon cycle with the 14C bomb spike decay: that is about 40 GtC/year. The same ~40 GtC/year is found in the “thinning” of the human emissions “fingerprint” in the δ13C level:
http://www.ferdinand-engelbeen.be/klimaat/klim_img/deep_ocean_air_zero.jpg
Here too, ocean surface and vegetation play a minor role in δ13C level changes: again the deep ocean exchanges are what causes the difference between the δ13C level if all human emissions would remain in the atmosphere and what is observed…
The residence time of a pulse of atmospheric CO2 is longer than the mean residence time of a molecule of atmospheric CO2. Absorption of molecules of CO2 by the ocean changes the equilibrium (or lack thereof) between the ocean and the atmosphere, and causes the ocean to gas out other molecules of CO2.
IPCC AR5 Figure 6.1 displays the global carbon stores and fluxes before, during, and after 1750 through 2011, the increase following the Keeling hockey blade. The amount of anthropogenic carbon added between 1750 and 2011 is twice the Keeling curve increase. How embarrassing. So exactly 57%, not 50% or 60%, is sequestered by never before existing sinking processes in the ocean and vegetation to exactly create and match the 43% net anthropogenic sources. This is akin to the mobster who hides his proceeds from gambling, drugs, and prostitution in used car lots, horse racing, and spas.
Why waste time and effort disputing ice caps, sea ice, sea levels, extreme weather, S-B back radiation, and thermodynamics when CAGW falls apart on its indefensible mass and heat balances?
Nicholas,
You need to look up at what a dynamic equilibrium or “steady state” means: a process where inflows and ouflows are equal. If such a process is disturbed by e.g. a pressure change, the process reacts by trying to remove the disturbance, That is called Le Châtelier’s principle.
In this case the ocean’s react on human emissions – or volcanoes alike: an increased pressure in the atmosphere gives less CO2 influx and more CO2 outflux. In some years that is 10% of the emissions, in other years it is 90%. In some decades it is 60%, in others 40%. In average 57%, but that is no law. If it is 40 % tomorrow, so what?
That it is relative constant over the years is because human emissions increased slightly quadratic over time. So did the increase in the atmosphere and so did the net sink rate…
One can discuss a lot of things in (C)AGW, but that humans are responsible for the increase is rock solid and one of the worst argument skeptics can use: a completely lost debate, even before you start…
Is that your profile on LinkedIn or your evil twin?
Nicholas,
I don’t know of any twin and a look-alike between not more than 150 Engelbeen’s worldwide (most in Flanders/Belgium) is rather questionable, so that is my profile…
I got the same numbers as Nicholas and used a completely different method. In fact even today or the last 5 years the rate of sinking is greater than 50 %. How do explain 3 of the 6 years from 1998 were below 2 ppm? In the last 5 years close to 100 billion metric tons has been sunk. And somehow co2 rates went up before the 20th century? I just commented about the numbers of 12 bmt from 1860 to 1880, that was the same amount released in one year 1965. So in 1965 the co2 ppm went up 1 ppm. That seems to be about right as co2 rises with every 12 bmt, 6 bmt makes its way into the atmosphere and 6 bmt gets sunk. So in those 20 years the handling system of the earth couldn’t sink that small amount of co2? There are no negative numbers. Are you aware that 1998 remains the highest record for co2 ppm at 2.93ppm? It’s been 17 years and production levels have gone one way, up.
Your logic falls apart here Ferinand. The increase by humans is not rock solid. To go from 280 ppm to 380 ppm would require a production on 1200 bmt or more.
rishrac,
You make too much from the variability in sink rate: that is largely caused by temperature variations, besides other points like light scattering by volcanic eruptions (Pinatubo) and other unknown factors. Temperature only explains 60% of the variability in sink rate, the rest is largely unknown.
All we know for sure is that in every year of the past 55+ years, human emissions were larger than the increase in the atmosphere. One can think of many ways that human emissions are not responsible for the increase, but all alternative explanations I have heard of violate one or more observations, while human emissions fit all observations…
Further, as said before to others: looking at the derivatives obscures most of the real cause: by taking the derivative, you remove most of the trend and inflate the noise. All the variability in rate of change is good for +/- 1.5 ppmv around the trend of ~70 ppmv. That is all. Even if the increase one year is negative and next year 150% of human emissions, that says next to nothing about the cause of the trend, which is (near) 100% caused by human emissions, as long as the longer term increase is between 0-100% of human emissions…
http://www.ferdinand-engelbeen.be/klimaat/klim_img/acc_co2_1960_cur.jpg
Only a small contribution of warming oceans (about 10 ppmv since the LIA) is added to the increase.
Forgot to add:
Increase in the atmosphere currently is 110 ppmv, or ~~230 GtC. Human emissions in the same period were ~370 GtC…
Alarmists will never, ever let facts get in their way.
It’s not about climate or saving the world. For politicians, it’s about taxes and power.
For the spear carriers, it’s about eliminating capitalism in favor of a more “fair” and “equitable” system: socialism. Never mind that it has never, ever worked. The fact that the poor under socialism are much worse off then the poor under capitalism doesn’t matter. They want what they want because they “know” better than all the stupid people who disagree.
There are figures for half-life a little longer than 35 years posted to WUWT earlier this year.
Willis Eschenbach came up with a decay time constant tau of 59 years, which means half-life of 41 years, assuming the decay is exponential, in:http://wattsupwiththat.com/2015/04/19/the-secret-life-of-half-life/
This is with assuming the decay is exponential. However, the decay of a pulse of CO2 into the atmosphere decays more quickly at first and more slowly later on as the pulse of CO2 is absorbed into deeper layers of the ocean and the CO2 gradient in the upper level of the ocean decreases. The actual decay is the Bern model, after a correction for warming by CO2 (which decreases solubility of CO2 in water) being less than the amount IPCC considers most likely.
No. IPCC’s Bern model is a fraud.
How does one insert an Excel table or jpeg image without trashing the formatting?
Nicholas,
For a jpeg image simply give the full URL to where it resides on the net (I don’t think a direct insertion works). For an Excel table, make it an image by copying it into an image program and save it as an jpeg image…
Thanks, Ari Halperin, for a good article.
Since the vegetable world has no voice, let me thank world industry for the food on their behalf.
Now, if you are worried of global temperature rise, please look at ENSO, the El Niño/La NIña irregular cycling that, seems to me, control the Earth’s climate through water vapor abundance in our atmosphere. Water vapor turns to rain and a little additional “greenhouse” warming.
What’s not to like?
FE et. al.
I am basing the following comments on IPCC AR5 Figure 6.1.
Before 1750 there is a net sink of -1.2 Pg C/y between atmosphere and earth.
In 2011 there is a net source of 2.8 Pg C/y for total difference of 4.0 Pg C/y.
8.9 total anthro – 4.9 newly sunk – 4.0 residual
You et. al. claim (if I understand correctly) that such an increase from natural variability is not reasonable therefore it must be anthropogenic.
My contention is that considering the magnitudes of the uncertainties as shown in Table 6.1 not only is such natural variability reasonable and possible no one has any way of knowing.
I need more than esoteric theoretical, hocus pocus, isotopes, proxies, models, etc. to convince me.
Nicholas,
The observed natural variability in the past 55+ years is not more than +/- 1.5 ppmv (+/- 3 GtC). Besides the extremes like the 1991 Pinatubo and 1998 super El Niño even not more than +/- 1 ppmv around a trend which is over 70 ppmv in the same period, while human emissions in the same period were over 130 ppmv.
Temperature can’t be the cause, as that gives not more than 10 ppmv extra since the LIA.
Vegetation can’t be the cause, as that is a proven sink for CO2, based on the oxygen balance.
The oceans can’t be the cause, as these have a too high 13C/12C ratio, while we see a firm drop in ratio.
Human emissions fit all observations. No other huge source is known and all other explanations violate one or more observations…
If you know of any huge natural source that fits all observations and at the same time explains the disappearing of all human emissions, you may have a good argument…
“It is ten times as likely that atmospheric CO2 is coming from natural sources, namely the warming ocean surface, as it is likely that it is coming from anthropogenic sources. The changes in CO2 track ocean surface temperature, not global carbon emissions. Burning fossil fuels is not increasing atmospheric CO2. Recovery from the Little Ice Age, driven by the sun, is causing the oceans to release CO2. It is temperature driving CO2 release, not the other way around. Just as it has always been.”
http://notrickszone.com/2013/03/02/most-of-the-rise-in-co2-likely-comes-from-natural-sources/#sthash.cG6WEl3r.dpbs
“One gets such wholesale returns of conjecture out of such a trifling investment of fact.”
Mark Twain
Nicholas,
If the laws of solubility (Henry’s law, established in 1803) show not more than 16 ppmv/K for ocean waters, confirmed by over 3 million field seawater measurements, what the hell is someone saying that the 110 ppmv increase in the atmosphere is caused by warming oceans? Did the oceans warm by 8°C since the LIA? Did more that a third of land vegetation burn down – without regrowth? Did human emissions meanwhile disappear in space?
Some elementary thinking is lacking in several skeptics sites…
Further from that page: by taking the derivative, you effectively remove much of the trend, which is caused by human emissions which are all trend and little variability, while inflating the variability which is largely caused by the effect of temperature variability on vegetation: all variability and little trend (even negative over periods longer than 3 years)…
Sorry this is piece meal. Taking a break from shoveling climate change off the driveway.
“The observed natural variability in the past 55+ years is not more than +/- 1.5 ppmv (+/- 3 GtC).”
ppmv/y? How is this measurement possible? Data mining? Statistical hallucinations? This level of uncertainty contradicts Table 6.1. Net anthro is 1.8/y, 4 Gt. Same order of magnitude, could easily go either way.
Ice core sampling, which is key to the isotope analysis, is a bit iffy in my book. Lots of yet undetermined uncertainty, lot of known unknowns.
Sources of uncertainty in ice core data
A contribution to the Workshop on
Reducing and Representing Uncertainties in High-Resolution Proxy Data
International Centre for Theoretical Physics, Trieste, Italy, June 9 – 11, 2008
Eric J. Steig, University of Washington Seattle, WA 98195
The natural variability ranges you cited above, I assume Pg C/y, are of the same magnitude as anthropogenic. And then there are the uncertainties.
Per IPCC AR5 Table 6.1, 1750- 2011, the uncertainty in the ocean-atmosphere flux is +/- 30 Pg C. Compare that to total anthropogenic source of 8.9 Pg C. FF & cement is also +/- 30. Net land use is +/- 80 PgC. Residual land sink is +/- 90.
In a sense you are telling me, with all the above uncertainties, that you know with precise certainty how many of the CO2 molecules dancing on a pin head are due to anthropogenic sources. I’m just not buying that.
Nicholas,
The variability is ppmv around the trend, no matter if you look at monthly or yearly data. Human emissions were according to WG1, AR5, chapter 6, Fig 6.1: 7.8 +/- 0.6 PgC/year. Except for a few extreme outliers, natural variability is +/- 1 ppmv or +/- 2.13 PgC around the trend. That is less than halve human emissions.
Land use changes are additional, but I never use them in calculations, as they are too uncertain. What the individual natural cycles do is not of the slightest interest: we know human emissions with reasonable accuracy, we know the increase in the atmosphere with high accuracy (+/- 0.1 ppmv or +/- 0.2 PgC). Thus the difference is known with reasonable accuracy, whatever the natural carbon cycles are or were of how they changed over the years…
The difference is the net sink rate: as long as the conservation of mass holds. That is around half human emissions +/- 2.13 PgC.
Only 0.003% is from man of the total CO2. To distinguish percent by country is to distinguish that percent of 0.003% them. At what point is it meaningless.
Can you show me where you got that figure? I’d like to use it myself
This earlier WUWT article by Erik Swenson is interesting. Figures 3 through 9 show CO2 concentrations throughout the year graphically.
http://wattsupwiththat.com/2015/10/04/finally-visualized-oco2-satellite-data-showing-global-carbon-dioxide-concentrations/
In northern hemisphere winter there is a definite elevation in CO2 concentrations. In summer the excess disappears and all CO2 generated appears to be absorbed in situ. I would say that this indicates the residence time for anthropogenic CO2 is on the order of a year or less.
The definite elevation shouldn’t be happening either. CAGW went to great lengths to prove that co2 is a well mixed gas in the atmosphere. .. I readily agreed with the 40 years on here because that’s a whole lot easier to prove. I thought it was 20 or under. One year is a maybe, I’m not discounting it. One thing for sure, it’s not a thousand years.
pochas94,
What you see over a year is the seasonal changes, which in the NH are dominated by the extra-tropical forests. The fluxes involved are huge: 60 GtC in and out for the biosphere, 50 GtC out and in the ocean surface, countercurrent of each other. That goes in both directions with temperature over the seasons. That has zero influence on the CO2 levels in the atmosphere, as long as ins and outs are equal.
Human emissions are one-way additional and influence the ins and outs of oceans and biosphere somewhat. That is a much slower process than the temperature influence: 35-40 years half life time to remove the extra CO2.
Two independent decay times, one is the reaction of nature to temperature, the other the reaction of nature to an extra pressure in the atmosphere…
I highly doubt it.
pochas94,
Human emissions are 90% in the NH, increase of CO2 is in the NH first:
http://www.ferdinand-engelbeen.be/klimaat/klim_img/co2_trends_1995_2004.jpg
Human emissions have a low 13C/12C ratio, decrease is in the NH first
http://www.ferdinand-engelbeen.be/klimaat/klim_img/d13c_trends.jpg
Human emissions are about twice the residual increase in the atmosphere, overall change after a full cycle:
http://www.ferdinand-engelbeen.be/klimaat/klim_img/seasonal_CO2_d13C_MLO_BRW.jpg
No problem with any of that , Ferdinand. The question is, is the secular (decadal) rise in CO2 anthropogenic, natural, or other. The seasonal CO2 disappears so fast that something else must be involved in the secular rise.
pochas94,
The huge seasonal changes are temperature controlled. They end with about half human emissions after a full cycle. The smaller 1-3 years variability is temperature controlled, less than half human emissions and zero out in less than 3 years. And a small part of the increase (~10 ppmv per Henry’s law for the solubility of CO2 in ocean waters) is temperature controlled.
Human emissions are twice the increase in the atmosphere and fit all observations. If you can find a natural alternative that does increase a fourfold in the atmosphere, in the same time frame as human emissions did, without violating any observation, we can have a discussion about the origin of the increase. If you haven’t, a non-human origin of the increase is about the worst argument one can use in any debate with the other side as that is a lost case before you even start a debate…
Why is the sink today 150% larger than in 1965?
Well, to mention some possibilities, it could be the secular temperature trend, it could be acidification. are either of these considered?
Richard from above
“Period———-Length in Years————–Trend/Decade———-CO2 Emitted
1860-1880—————21———————–0.163ºC—————12 gigatonnes ”
In that 20 years is the same as the amount emitted in 1965. the rise in 1965 was 1.02 ppm which means that half of the 12 gigatons in 1965 was sunk. In 2014 the rise was 2.13 ppm, that year 38 gigatones with 19 gigatons sunk. I’m not mixing amounts or different carbon amounts up.
That’s a 3100% increase in sink from 1860 – 1880? and there was an increase in co2 during that time. the eco system couldn’t handle 12 in 20 years. ??
Pochas94 and Rishrac,
Have a look at the total emissions since 1900 and the increase in the atmosphere:
http://www.ferdinand-engelbeen.be/klimaat/klim_img/temp_emiss_increase.jpg
The red line is the measured increase in high resolution (~10 years) ice cores up to 1960 and the atmosphere at Mauna Loa (or any other station, they differ with only a few ppmv worldwide) thereafter.
According to Henry’s law the CO2 level in the liquid (in this case the oceans) is directly proportional to the partial CO2 pressure in the atmosphere above it. The sink rate therefore is directly proportional to the difference in CO2 pressure (pCO2) between atmosphere and oceans. The pCO2 in the atmosphere (~=ppmv, ppmv is in dry air, pCO2 is in wet air) shows a slightly quadratic increase over the years, thus the sink rate increases slightly quadratic too.
For the current average seawater temperature, the dynamic equilibrium (“steady state”) between atmosphere and oceans would be at 290 ppmv in the atmosphere. Between 1958 when the accurate measurements in the atmosphere started and today, the pCO2 difference between the atmosphere and the sea surface increased a fourfold. So did the sink rate: a fourfold increase in 55+ years. That is pure mechanical: a fourfold increase in pressure difference gives a fourfold uptake – if no other chains of events happens. In the case of CO2 in seawater, there are other (buffer) reactions involved, but these have no influence on the linear ratio between pressure difference and uptake.
Before 1900, the amounts were too small to have much influence, smaller than the “noise” caused by temperature variability. Since 1900 the trends are clear.
Why the increase remains around 50% of human emissions is just coincidence: human emissions increased slightly quadratic over time, so did the increase in the atmosphere and thus the net sink rate. If human emissions would stay the same, the atmospheric CO2 would rise and the sink rate would increase asymptotically until human emissions and sink rate were equal… at a new “steady state”.
Temperature plays a secondary role over time and is good for about 10% of the increase, but is responsible for over 60% of the variability around the increase.
The main point is not the momentary emissions in each year but what remains in the atmosphere as extra pressure difference and the speed at which the extra pressure in the atmosphere above steady state is removed: about 40 years half life time, which makes the removal rate too slow to remove all human emissions the year that they were released…
It will be interesting to see what correlations/models/conclusions will be drawn when all of these charts are modified to pull out the inappropriate “adjustments” made for political reasons.
This graph, for example, shows 2000 much higher than 1940. While that is now the accepted standard, there is good evidence to suspect that the actual temperatures for the two decades were about the same. They made the mid-century temperature drop go away; what if it were to be allowed to exist again?
The odd thing is that, once the models are “retrained” on more realistic temperature representations, they may actually get closer to reality. But some of this will simply come from the fact that once the variation de-adjusted and is no longer extreme, the models will be more correct en ensemble even if none of them get the actual events right.
And everyone will be on to the next issue to use to demand Global Governance.
===|==============/ Keith DeHavelle
I am away on travel but to put in an appearance, just let me say nothing has changed in the outlook. Atmospheric CO2 is not significantly affected by humans. Will have more analysis next time.
Hello Bart,
Missed your comments already…
See you next time.
Apologies for not responding to all comments, addressed to me – too busy these days.