Guest Post by Willis Eschenbach
My business card gives my job title as “Generalist”. Let me give you an example of why this is an advantage in climate science. I worked for a while in the field of low-tech renewable energy. One of the things I did was to work with inexpensive solar water heating. Using solar energy to heat water can be extremely cost-effective. One of the reasons it can work cheaply is that it doesn’t require pumps. The water can be circulated while it is being heated using the principle of the thermosyphon. Here’s a diagram of how a thermosyphon works:
 Figure 1. Principle of the thermosyphon. Image Source
Figure 1. Principle of the thermosyphon. Image Source
The reason the thermosyphon works is because a cold fluid is denser than a warm fluid. As a result, you get a pressure difference in the two legs of the system. This pressure difference works to constantly circulate the water. The water sinks on the cold denser side, and rises on the warm less dense side. Thermosyphon water systems are great in the developing world because they can be built very cheaply, using plastic pipe and 55-gallon drums.
If you’ve worked much with thermosyphon systems, you may have noticed that the system shown in Figure 1 is missing a critical component for successful operation. To work efficiently, the system needs a one-way valve to keep the circulation from running in reverse.
The reason it needs a one-way valve is that at night, the solar collector reverses function, and it becomes a thermal radiator. It radiates away the heat towards outer space. This makes the “Return” leg of the circuit (shown in red) colder and therefore denser than the “Advance” leg of the circuit (shown in blue). And absent a one-way valve, this of course reverses the circulation entirely.
As a result, during the night-time, the circuit as shown takes warm water from the top of the tank and circulates it to the thermal radiator. There it is cooled by radiation to space and returned to the bottom of the tank. It is a reverse thermosyphon system, which will run as long as the water in the tank is warmer than the thermal radiator.
Now, what does a reverse thermosyphon system have to do with the climate?
To elucidate that connection, consider the following situation shown in Figure 2.
 Figure 2. Incoming solar radiation and outgoing thermal radiation, Pacific Ocean. The north and south poles are at the right and left ends of the diagram, and the equator is in the middle.
Figure 2. Incoming solar radiation and outgoing thermal radiation, Pacific Ocean. The north and south poles are at the right and left ends of the diagram, and the equator is in the middle.
In Figure 2, the sun is warming the surface layer of the ocean at the Equator. At the poles, on the other hand, very little solar energy is absorbed by the surface. Instead, the poles are areas of net radiation to outer space.
Now, considering what we know about reverse thermosyphon systems, in Figure 2 what would we expect in the way of natural thermal circulation?
Since the water is cooled at the poles it will be denser, while the sun-warmed tropical surface waters will be less dense. As a result, the water will sink at the poles and rise at the equator, as shown schematically in Figure 3.
 Figure 3. Simplified overall circulation pattern, Pacific Ocean. The north and south poles are at the right and left ends of the diagram, and the equator is in the middle.
Figure 3. Simplified overall circulation pattern, Pacific Ocean. The north and south poles are at the right and left ends of the diagram, and the equator is in the middle.
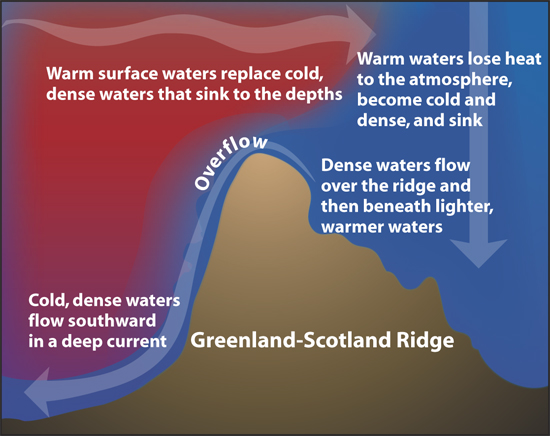
Of course, nature is never that simple. In addition to the temperature difference, the circulation is also driven by the salinity difference. Salty water is denser than fresh water, and the polar waters are salty. Since the circulation is driven by both temperature and salinity differences, it is called “thermohaline circulation”. (The circulation is also driven in part by the wind, although that is not included in the name.)
This salinity difference only increases the strength of the circulation shown above. People sometimes ask why the oceans stay so cold when they are always being warmed by the sun. It is because there is a constant stream of very, very cold water being added to the bottom of the ocean by the thermohaline circulation.
To further complicate matters, there is a very small addition of geothermal heat moving upwards through the sea floor. Estimates put this warming on the order of a tenth of a watt per square metre (W/m2).
My back of the envelope number for ocean heating is as follows: one watt per square metre (W/m2) applied for one year will raise a cubic metre of sea water by about eight degrees C. Rough, but useful.
Again using approximate numbers, the overturning of the ocean occurs over something on the order of five hundred years. A tenth of a watt over a hundred years will raise the temperature of the bottom hundred metres of water by eight-tenths of a degree. In five hundred years, it would raise the temperature of the bottom hundred metres by no less than four degrees. In this manner, the icy polar water is very gradually warmed as it moves equatorward.
Now, with all of that as prologue, here’s the question of interest. It has been said that the reason that the warming is currently stopped is because the “missing heat” is hiding in the depths of the ocean … but that the surface layers have not warmed significantly. Many skeptics have said that this is simply not physically possible. They argue that because the ocean is heated from above, the heating would perforce be greater nearer to the surface. They claim there is no possible mechanism by which the deeper layers could warm independently of the surface.
So my question is, given the situation and circulation shown in Figure 3, what would be the effect on the average ocean temperature of a slight warming at the poles?
Well, if the water that is descending at the poles is slightly warmer than in the past, then there will be less cold water added to the bottom of the ocean. With less cold water added to the bottom, on average the ocean depths would warm slightly compared to the past … and the interesting point is, the ocean would warm from the bottom up.
So that is how the ocean depths could warm separately from the surface. And that is how an understanding of low-tech renewable energy systems can assist our understanding of the climate … and why my business card says “Generalist”.
Regards to you all,
w.
A Final Disclaimer: No, I do not think that the current plateau in the warming is caused by “missing heat” hiding in the ocean. And in any case I don’t think that we have the data to measure the ocean that accurately.
I’m just pointing out that yes, it is possible for the ocean to warm from the bottom up—you just need to turn down the volume or turn up the temperature of the polar leg of the thermohaline circulation.
The Consistent Request: If you disagree with someone, please quote their exact words so we can all understand your objection.
If the solar gatherer gets cold then the densest fluid is at the lowest part of the system and will not rise. No valve is needed to prevent ‘reverse’ cycling.
And if the water in the radiator gets colder than the water in the bottom? Why wouldn’t it lower and displace that water back towards the tank and pull on the hot water, making the system cycle backwards?
No valve needed, as long as the tank is above the collector.
Tank location has long been a hindrance to acceptance of these systems stateside. Nobody really wants a water tank in the attic.
Up north, where you have freezing, you need a heat exchanger system with antifreeze in the collector loop. This makes a more complicated system, and one prone to leakage of antifreeze into the drinking water.
@ Mike McM ” .. prone to leakage of antifreeze into the drinking water.”
The ‘hot’ water storage tank contains water for washing, bathing etc, NOT drinking.
Not entirely on topic but interesting.
In the UK the traditional domestic water system is different from the rest of Europe, and North America by the sound of it. Water from the mains comes into the house and is stored in a tank in the attic, well insulated these days. There is normally a second tank for storing hot water (on an upper floor normally but not always), either heated by an internal electrical heat element or by a gas or solid fuel boiler in the way Willis describes in Fig 1. There are various safety features to prevent hot water tanks exploding and sending boiling water to the floors below. In the case of the boiler option the water in the hot tank normally cools gradually depending the insulation.
This system does have an advantage over the European system that in the event of power and water cuts there is a reservoir of both hot and cold water which can be rationed until normal service is resumed.
I would hope the hot water we use for washing and bathing is as uncontaminated as that which we use for drinking.
I do recall from my London layovers that the hot water systems in the UK were a tad different. AGA stoves maybe for cooking and heating? Dual purpose boilers, heating and hot water? I had the impression that the extra tanks were for leveling out the pressure in some of the city water supplies.
I always picked up the latest of the self-build magazines and was surprised at the differences in British homebuilding from the US. Some odd ideas, too. Timber (lumber to us Yanks) was regarded as untried and risky (try to get a loan on a wood framed house). But then, some wood framed houses could be expected to last 60 years (or even more! if well cared for). Timber has more embodied energy than fired brick and mortar. Double glazed windows were louder than single glazed, because the second pane resonated and amplified the street noise. The front door must open into a hallway, not a room. Forced air heat spreads germs.
British houses are built to last, I discovered. Which makes them too expensive to tear down and replace with more energy efficient construction. Taking the train in from Gatwick, one sees an endless sea of chimney pots on row houses that I doubt have much more insulation now than they had when Victoria ruled. < /rant >
@Mike McM
The cold water tank in the attic in UK houses was to comply with water company bylaws that cold water feed to lavatory cisterns, baths, wash-hand basins had no direct connexion to the mains supply to prevent possible back siphoning of dirty water. Usually the only direct mains supplied cold tap was at the kitchen sink, and this was the only water considered safe to drink as the attic tank could be contaminated with dust or vermin.
Interestingly attic tanks became a major problem during Winter… supply pipes bursting and flooding the house… during the 1970s following a Government programme to encourage energy conservation with grants for loft insulation. It was the escaping heat which had kept those lofts above freezing and water pipes, and people’s homes, safe.
Those unintended consequences at which Governments excel.
Typically houses had coal fires with back-boilers (in place of the solar panels in the figure above) and a tank…. cold feed from the attic tank which thus served as an expansion tank to avoid explosions… usually upstairs. There were no non-return valves since the back-boiler was relatively small and heat loss once the fire was out was minimal and in the good old days tanks were not insulated, nor the fire out for long even in Summer.
joe public You don’t have two lines of water supply to your house one for water to be warmed and another to run for drinking water if there is a danger of mixing then mixed it will be.
“To work efficiently, the system needs a one-way valve to keep the circulation from running in reverse.”
The example shown is of course the one layout that does not require a valve, as noted above. There is a small caveat which is that if the incoming water is below the temperature of the collector, the water will flow through the circuit but only when the tank cools, but is is not a loss, it’s a slight gain.
For those who, inspired by the idea, plan to build your own, there is an important design parameter you should know. In order to maximise the net heat gained per day the volume of the tank should be circulated through the collector exactly once during a heating cycle, typically 8 hours. The collector size and pipe size should be matched to the volume of the tank to achieve this flow rate (or a pump capacity).
This optimisation was worked out from first principles in 1982 by Prof Bernhard Scheffler, a physicist at the University of Pretoria. He also patented a polypropylene ‘floating ball’ check valve that has an extremely low forward flow resistance. It is used when the top of the collector is at or above the bottom of the storage tank. The floating ball has a density just slightly greater than that of water so that it seats when the flow rate drops to zero preventing back flow.
I grew up in a house that had a gravity system for heating the house. As noted elsewhere, the temperature gradient needs to be fairly high t provide enough force for circulation, and the pipes need to be big, or friction will prevent circulation or slow it down too much.
It was a big house, and when built it used coal, but this was switched out the heating oil when my dad bought the house.
It was very interesting that all of the light fixtures were gas lamps that had been converted to electric lamps. The house had been built a few years prior to Edison’s invention of the light bulb, and subsequent wiring of cities with electric.
Clever they were, just pulled wires into the gas lines. It is amazing to consider the rapidity with which entire cities were wired for electricity in those years after Edison’s invention.
Of course, Philadelphia itself was a big giant gravity fed water system, one of the first. There was a reservoir where the Philadelphia Museum of Art is now located, and it fed water to what at the time was the entire city. Eventually, the areas east of Broad Street came to be supplied by water from the Delaware, but the west side of Center City still gets it’s water from the Schuylkill River. The old water works are still right there below and in back of the art museum.
One way valves (we call the check valves, and they are cheap and reliable with a few design types, gate and spring, in common usage.
A small pump is the way to go. I am right now very involved with water…pumps, motors, designs and applications, control circuit.
I am thinking that there must be a pump design waiting to be invented which uses ambient heat for power. Have to think on that
40% by volume ethanol in water freezes at -23.3 C. I would definitely take the risk of a leak like that. In fact, I make it happen at least a couple of times a week.
For the life of me, I can’t figure out why the water heated by the equatorial sun waits until it reaches the poles before it radiates to space.
Seems to me that while the water in the tropics is hotter, it could actually radiate much faster than it will after it reaches the cold polar regions.
I guess this free circulation process of yours Willis wouldn’t work on Kevin Trenberth’s isothermal planet.
I think that’s why I don’t like his diagram.
G
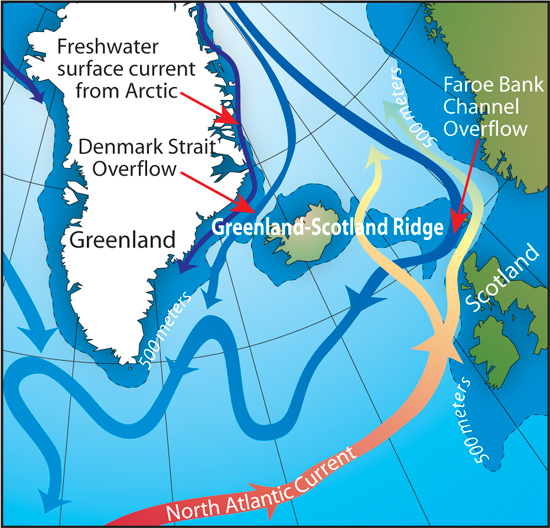
The fact it doesn’t wait probably explains why the Gulf Stream/North Atlantic Drift is cooler by the time it gets to Scotland than it is when it leaves the Gulf. Although still warm enough to influence the climate in Scotland. Hence Inverewe gardens almost as far north as Eggers Island
http://en.wikipedia.org/wiki/Inverewe_Garden
Hi Mr. Smith
It does, else British would have been Eskimos, but once it reaches the Nordic Seas, cold Arctic winds strip the surface currents of any heat left.
http://www.vukcevic.talktalk.net/NIJ.gif
a keyhole to N. Atlantic natural variability.
George,
We have discussed this before. The ocean is a liquid surface covered by the full mass of the atmosphere. The polar ice caps are solid surfaces at an average elevation of 2,100 m for Greenland and 2,500 m for Antarctica. The icecaps are therefore less well insulated from space by the atmosphere than the oceans. A solid surface at 2,500 m elevation and covered by thin dry air is an effective thermal radiator.
George,
It doesn’t. Most of the energy absorbed in the tropics goes to evaporate water. That evaporated water condenses as it rises and cools. Those water droplets in the top of tropical thunder clouds radiate to space. So the rate of OLR at TOA in the tropics is likely controlled by the rate moisture is delivered to TOA. To further confuse the issue, CO2 being emitted at the surface is being pumped out the top of those clouds were it can radiate to space rather than back radiate to the surface. This mechanism is in addition to what Willis is discussing.
vukcevic’s image is not actually what happens with the deep water ocean circulation.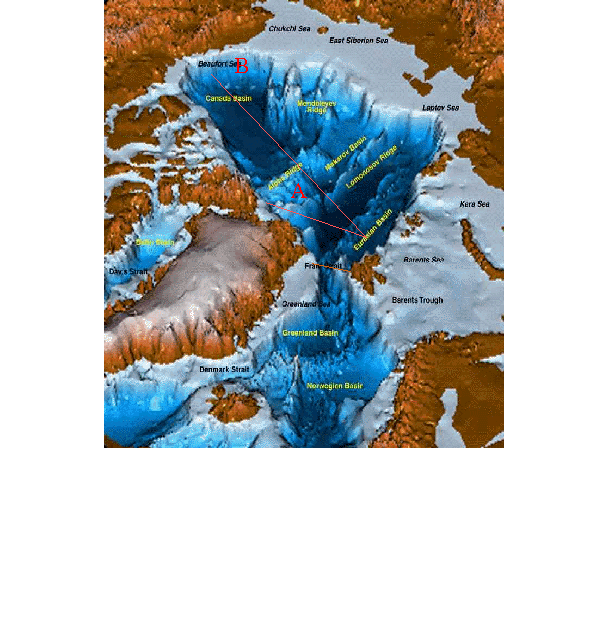
The deep water from the Arctic in the thermohaline ocean circulation system actually forms under the sea ice throughout the entire Arctic Ocean basin (not around Iceland) and it overflows from there at various chokepoints between Greenland and Europe. (the warmers have even distorted the deep water ocean formation so that it fits the agenda, but that is not what really happens.)
The Denmark Strait Overflow, for example, is the biggest waterfall on the planet with its -0.5C dense cold water overflowing from the Arctic ocean basin, and it plunges 3000 metres to form the Arctic Deep Water in the Atlantic. The ocean water at the surface here is +5.0C to +10.0C so it is NOT the Arctic Deep Water that is part of the thermohaline ocean circulation system.
It takes a long time for this cold dense water in the middle of the Arctic to eventually migrate and then overflow at the Denmark Strait. Hundreds of years.
http://ars.els-cdn.com/content/image/1-s2.0-S0012821X15001442-gr001.jpg
To answer Willis’ point, the deep water in the Arctic ocean basin is the coldest densest water on the planet getting to temperatures approaching -1.5C. If it sank at -1.0C rather than -1.5C, nothing would really change because it would still be the coldest, densest water on the planet and the ocean circulation system would just continue circulating.
Mr. Illis
Thanks for your comment. The key phrase: “keyhole to N. Atlantic natural variability”
CET- Natural variability
N. Atlantic SST
..
Well I never would have guessed that my tongue in cheek post would have garnered so many responses.
So I should thank those who responded; Sandy, Vukcevic, Phillip, and fhHaynie; well of course Bill Illis too.
I really wanted to draw attention to two aspects of earth climate discussions that constantly bug me.
First of course is the notion promulgated by Kevin Trenberth (is he really a fellow Kiwi transplant), et al that earth can be represented as a static isothermal object, in which Willis’s heat siphon couldn’t work.
The second is the notion that somehow, energy arrives on earth from the sun, at the tropics, and finally leaves as a consequence of that marvelous cooling system at the top and bottom of the planet. Well what better place to put a waste disposal system, than some place that is quite unlivable for ordinary mortals.
In my view, earth is being cooled most efficiently and most rapidly, in the hottest middle part of the day and at the hottest and driest tropical desert areas of the earth; well also barely livable for that reason.
S-B says that total radiant emittance increases as T^4, and that makes places like the Summer Sahara, almost 12 times better coolers, than places like winter midnight at Vostok Station.
The poles do not cool the earth. They are that cold, because they get such a pitiful amount of energy from the sun; so little in fact, that they need the exported heat (noun) from the tropics, just in order to maintain the low Temperatures that they do have.
If somehow we could stop the heat exports from the tropics; then you would really see just how cold it could get on earth .
Now I have adopted a rule (just for my own personal use) regarding the use of terms like “hot , cold , warm, cool ” and others of that ilk.
I take the range of Temperature that separates “hot” from “cold” to be that amount of Temperature rise, that has got the entire united nation’s panties in a bunch; namely one degree Fahrenheit or about 0.6 deg. C if you like (I do).
The warmulans say we have to reverse that amount of change, even if we have to turn off the entire earth economy; well 2 deg. C is tops and initiates our departure from earth to find some more livable place.
So from 0.5 to 2.0 deg. C or thereabouts is known to result in worrysome “forcing”, presumably in both directions (up or down).
It follows from that rule, that the 20 to 30 deg. C tropical oceanic water Temperatures DO radiate LWIR EM radiant energy a heck of a lot faster than the roughly zero degree ocean waters of the Arctic, or Antarctic Southern oceans.
Yes; I didn’t come down in the last shower, so I do know that oceans evaporate, and conduction and convection transport heat energy to the upper atmosphere.
But please; can we stop trying to make it appear that the gulf stream is cooling this planet by conveying vast amounts of “heat” (AKA thermal energy) , To some magic heat disposal facility at the poles.
Yes I do believe that those currents transport vast amounts of heat to the poles, (and to Scotland too). Blimey, think how ornery they would be, if it didn’t ! But it is the lack of sunshine that makes those places cold; not hot water from the tropics.
g Yes, I know it’s just my opinion; so don’t expect to read it in any peer reviewed papers, and don’t use it for your PhD dissertation.
But earth would cool much faster if that hot water stayed in the tropics where it can radiate more efficiently.
Bill,
Thanks!
Imagine the Arctic Basin as it has been during most of the Pleistocene and Holocene, with no Pacific inflow thanks to the now shallow Bering Strait’s being dry land.
I almost asked this same question on the previous post written by Willis, where he said “I found this most fascinating, as it shows the great oceanic heat transport systems that move the energy from the tropics, where there is an excess, to the poles where it is radiated to space. ”
Isn’t the key question an extension of this though – “How does CO2 at 400 ppm on a background of water vapor at 40,000 ppm change this from CO2 at 280 ppm on a background of 40,000 ppm of water vapor” ?
And as a footnote; I do like the pictorial schematics that Bill Illis brings us from time to time. they really do say more than a thousand words. (you too vuk)
G
Mr Illis
Two images are from the same Source:
www’.whoi.edu/cms/images/oceanus/Dickson_map_550_52088.jpg
www’.whoi.edu/oceanus/feature/a-newfound-cog-in-the-ocean-conveyor
(delete apostrophe in the links)
except that the one I quoted is part of their more recent in depth study of a newly discovered current (2011); to find out more google North Icelandic Jet current.
To Billy Illis and Willis
Regarding the “coldest and densest” waters:
The coldest water is NOT the densest water. The flow pressures are therefore not as shown in the maps. Water is densest at 4 deg C which one of the miracles of water. The region of maximum density lies well outside the Arctic. It a category error to apply to the coldest water flows an attribute that only applies to water above 4 degrees C, i.e. linear expansion with an increase in temperature.
As the water warms from -1.5 to 0.0 and then to +4 C the density increases, not decreases. Thus water spilling over the edge and ‘running down to the equator’ increases in density as it warms on the journey.
Because the volume decreases as it warms the laughable part of ‘global warming’ with respect to the warming of, let’s say, the whole Arctic Basin, is that sea level will DROP as the Arctic Ocean warms.
Remember that because gravity levels the ocean, a change in density without a change in mass means the sea level can change without a change in volume. Sea level can drop in the Arctic, only, as the ocean warms.
As the average temperature of the deep oceans is 3 C, raising it to 4 will drop the volume. This is a physical fact. Warming of really cold ocean water, reduces the depth. The fact that sea level is presently rising can be attributed to warming of water above 4 deg C, or the cooling of water below that temperature.
Given the lack of relevant data and the continuing spread of sea ice in the Antarctic and it’s recovery in the Arctic I see no guarantee that it is not the former case. Sea level rise may be an indication of cooling oceans.
Warmists may need to take that with a grain of salt.
A question for Crispin in Waterloo: I agree with you, that water is densest at 4 deg C. Fresh water is densest at 4 deg C.
However, the current discussion is about average ocean water. I have looked for information on density of ocean water vs temperature, and a short search does not reveal much. I did find one table that seemed to indicate that density of ocean water seems to keep increasing with lower temperatures.
So, is there an appropriate URL that you could point me to, that would show the density of ocean water with decreasing temperatures below 4 deg C? I found:
http://www.bing.com/images/search?q=Density+of+Seawater+and+Freshwater&view=detailv2&&&id=AD1E512581D800EF9DD80CBBEBB8CE58AAEFF3B6&selectedIndex=29&ccid=zlvYfBmZ&simid=608023836166586868&thid=JN.USfm4sXdoF6%2bLLxPeZlENw&ajaxhist=0
which shows that there is an increase of density in water with dissolved salt below the 4 deg C point, with that density increasing to lower temperatures with more dissolved salts.
“Colder water is not the densest”
Very true, and good catch.
And of course the salinity skews everything even more. Case in point is the Mediterranean Sea, where evaporation exceeds inflow from rivers, leading to a thermo-halocline (Is that a word? It is now). Atlantic water constantly pours into the sea through the Straits of Gibraltar at the surface, travels Eastward, warming and becoming saltier.
Below the surface though, there is a constant Westward flow out of the straits below the surface flow. This water is warmer but saltier, and different enough from the Atlantic water for it to persists as an identifiable stream below the surface of the Atlantic, for thousands of miles!
So, in the Mediterranean, there is a layer of warmer water under the surface, travelling in the opposite direction.
As for unusual expansion curve of water, remember from basic studies that this is why the unit called a gram was originally defined as the mass of one cubic centimeter of water at 4 degrees C.
It is also why ice floats. Almost unique among chemical compounds, the fact that solid water floats has, of course, profound implications for the climate of the earth, for the biosphere, and perhaps even for life itself.
Were this not the case, everything would be different. Likely earth would be a block of ice.
Janice, as sea water freezes, the salt is excluded from the growing crystalline structure. Good thing, that.
Hey, been wondering, is this fact used to desalinate water for drinking anywhere…beside the poles of course?
Menicholas, I am aware that salt is excluded from ice. True of most material as it goes from a liquid state to solid, and a concept used for zone-refining of materials. However, that salt appears to go into solution with the liquid water, thus making the ocean under the ice quite salt-laden. The salt-laden water does become denser as it gets colder, within the temperatures normally seen in the ocean waters. Thus, if we are talking about pure water, 4 deg C gives us the most dense pure water. In general, the vast majority of ocean water is not pure water, thus the 4 deg C is irrelevant to the discussion. As a matter-of-fact, the Denmark Strait Overflow would probably not sink 3000 m, being at -0.5 C, unless the ocean water becomes more dense at that temperature, as compared to the surface water which is at +5.0 to +10.0 C. I am just a bit confused as to why information about pure water is being injected into this part of the discussion, and looking for clarification.
Once you introduce salinity to water, as in anything close to the salinity of the majority of the world’s oceans, the density increases as it gets colder until it reaches about -1.9C.
Normal salinity ocean water is densest at -1.9C. If there is less salinity as in the Amazon delta, the Mackenzie River delta, or the Laptev/East Siberian Sea in the Arctic, the density is maximized at about -1.3C, but it almost never gets higher than this ANYWHERE.
Since salinity mixes reasonably well throughout the world’s oceans, the biggest determinator of “density” is the temperature. The colder the water gets, until it gets to -1.9C, the denser it gets. When it gets colder than -1.9C, it starts to freeze, and then it becomes ice which is the least dense water there is, so it floats.
In practise, the least dense water is the frozen water,or sea ice at the top. There will generally be a strata of warmer water next, and then the densest deepest water will be at the bottom of the ocean. -1.5C in the Arctic, -1.0C around Antarctica and 1.0C in the deepest darkest parts of the oceans at mid-latitudes and the equator.
This is really as cold as ocean water gets, even in the ice ages (maybe -0.5C colder) because if it got any colder, it would freeze and become sea ice, floating to the surface instead.
Water is a very interesting substance. Almost every other chemical becomes more dense as it turns from a liquid to a solid. But not water. It becomes less dense. If water acted like almost every other chemical, the Earth would be a frozen snowball and every other planet in the universe with water would be the same. ie, there would be no life anywhere.
Thank you, Bill. I appreciate the good information about ocean water density vs temperature. I’ve copied that into my random notes subdirectory. As a side note, there are three metals that I know of that expand as they freeze: Bismuth, antimony, and plutonium.
Is this what you are looking for, how the density curve changes as the salinity varies?
http://linkingweatherandclimate.com/ocean/figs/density2.png
And for fresh water, of course, the standard curve:
http://www1.lsbu.ac.uk/water/images/maximum_density.gif
This is a very interesting subject Janice. Thank you for bringing it up. I for one had never thought about it.
“Another interesting characteristic of the density of salt water is that as salinity increases, the temperature of maximum density decreases. This is shown by the red line in the figures above and below. It should also be noted that the freezing point for water decreases with increased salinity (blue line in the figure below). This is why putting salt on roads helps melt snow and ice faster. In the figure below, the freezing point and the maximum density point are shown to intersect at 24.7 psu. The area between the red and blue lines indicates the range where ice would form more rapidly”
http://linkingweatherandclimate.com/ocean/waterdensity.php
You need to look at it in relation to this:
http://www.astecor.com.br/astecor/wp-includes/pomo/salt-water-freezing-point-table-768.jpg
http://www.astecor.com.br/astecor/wp-includes/pomo/salt-water-freezing-point-table-761.jpg
Menicholas, Bill and Janice
What a wonderful sets of contributions. I knew the salt would make a difference but I couldn’t find the informative charts you guys did. Great explanations.
When water sets up to freeze, it forms what look like polymers – chains of H2O molecules like worms. That is why pure water expands as it cools from 4. The salt must interfere with that process, or else the freezing takes time, or happens gradually. In the last case as the fresh water frozen out of the water the local salinity would rise, lowering the freezing temperature, but the fresh water ice would be solid and remain so.
The freezing temperature vs salinity curve is amazing. I never saw that before. I would like to pursue this further to see where water is and the condition and the influence of ice cycles.
Thanks
The actual chemistry is call Freezing-point Depression. There is a nice article in Wikipedia about it. They even post the formulas for calculating freezing-point depression, which I remember having to do in a chemistry course many years ago. One formula is for an ideal solution, the other one is when you need very precise values.
For Janice:-
Here is a link to a Water Density Calculator which may also help.
Crispin in Waterloo:
“The freezing temperature vs salinity curve is amazing. I never saw that before. I would like to pursue this further to see where water is and the condition and the influence of ice cycles.”
Yes, very interesting indeed. Boiling point elevation/freezing point depression is of course a very extensively covered subject in most chemistry and physics courses, and indispensable knowledge when covering physical chemistry.
It does seem to have been overlooked I many of these discussions, which, in retrospect, is odd.
I found one more chart that gives a better perspective, since the temp vs freezing point chart is in different units than the other chart.
Since ice does form at the surface, it must be the case, as you point out, that the process is rather more complicated, and that as the freezing progresses, salt is excluded gradually and allows the ice to remain on the surface.
(Unless one is to think that the ice forms at the bottom, or somewhere in the water column, and floats up to the surface, which I have seen no evidence to believe is the case)
Otherwise, water would sink before it froze and sea ice would have a hard time ever forming in open water, no matter the temp. It would seem that the whole water column would get very cold and then freeze all at once from top to bottom!
Here it is, and I agree that this needs a closer look.
http://www.fondriest.com/environmental-measurements/wp-content/uploads/2014/01/360x300xwatertemp_salinity.jpg.pagespeed.ic.yheIMC6nSN.jpg
*Somewhat off topic, but interesting:
I do know from various chemistry classes than getting certain substances to crystalize can be very difficult, and have heard some very strange tales of people attempting for years to get crystallization started.
As most students have experienced, some organic samples will tend to “oil out” instead of crystalizing.
I recall one technique was to scratch the glass vessel, which will sometimes get crystal growth started.
Sometimes a small seed crystal is needed, which upon being introduced the whole sample will abruptly crystalize.
One such anecdote involved a researcher who was unable to get the process going in a novel new compound, but heard that a colleague in another building had succeeded in doing so. He walked to this colleague’s office with the sample in hand and, as he entered building which housed the lab where a small amount of the crystalized sample was kept, the sample in his hand, which had resisted all attempts for a very long time (I do not remember how long, but I am thinking months or years), suddenly crystalized! Just from being in the same building!
This and many other great stories came from my undergraduate Organics instructor, a gentleman by the name of T.W. Graham Solomons, who wrote many widely used textbooks, mostly on organic chemistry.
Learned a lot from him, that is for sure.
We are now juggling three concepts about water. There is the density, along with the salinity, and the temperature. As to freezing, there has to be an additional concept added, which is pressure. The pressure on the ocean bottom forces the water to remain fluid, and not freeze. Therefore, the temperature will remain quite constant. The water is very cold, and very dense, but will not freeze. Nor will the temperature change very much, as the inflow of new cold water is very slow. It is a super-cooled liquid, and there is a lot of it, so there is an enormous thermal mass.
The sea ice forms on the surface of the ocean, because it is at the right temperature and pressure to do so. It does not form below the surface and float upwards, at least as far as I know. The pressure component is what contributes to our earth having a molten core, or at least an zone of molten material. Same situation with water.
In your freezer, ice forms in ice-cube trays on the surface of the tray, and the top surface of the water. The tray allows for nucleation points, so the ice crystals can form on those surfaces. If you have contaminants in your water, and if the ice cube is very clear, you can see the contaminants at the very center of the ice cube, where they were pushed as the pure water froze out around them. The original zone refinement.
Interesting Janice,
I wonder how salt effects the phase diagram of water? I am still looking for a chart.
This diagram seems to indicate that the freezing point is affected very little by pressure, all the way up to about ten mega-pascals, which I think is roughly the pressure at the deepest point of the ocean.
There seems to be some disagreement on this thread about how cold the water at the bottom of the ocean is or can get.
http://upload.wikimedia.org/wikipedia/commons/thumb/0/08/Phase_diagram_of_water.svg/700px-Phase_diagram_of_water.svg.png
So, as far as I can find from some quick checking, the coldest ocean water is between -2 and -3 C, which according to the chart is about the freezing point.
I have not been able to find out if salt affects the shape of the phase diagram, but it appears that for fresh water, pressure has little affect on freezing point until the pressure gets near that at the deepest trenches.
Again, his is from quick checking, not exhaustive research.
So, if pressure does NOT keep the water down there from freezing, and there certainly is a lot of it, and it is very near the freezing point…this could be very important in understanding how ice ages may start or end.
Because at least one theory seems to be that during ice ages the arctic is open water, which allows for huge amounts of snow to fall up there.
We know that ice ages begin fairly rapidly, and end even more quickly.
I wonder what would happen if the water at the bottom of the ocean suddenly became cold enough and froze?!
I think that if a large enough quantity of sea water at the bottom of the ocean got cold enough to freeze, and it excluded the salt as it does at the surface, the mass of resulting ice would pop up to the surface like a enormous cork, and cause an incredible amount of turbulence, no?
And, adding salt to water is an endothermic process, right? So the excluded salt could lower other water in the vicinity enough to cause it to freeze. It could be a runaway chain reaction sort of event, causing the ocean to suddenly flip!
Now, what could initiate such an event? A large amount of freshwater somehow getting down there? Lower the salinity enough to cause the freezing point to go up above the ambient temp?
Or maybe some other endothermic reaction or event.
Hmmm. Now I am really wondering.
Anybody know of anyone already considering this/these question/questions?
Impossible!
LOL. Care to elaborate?
Menicholas, on your chart (posted at 3:48), there is something quite interesting. The depth of the Mariana Trench is about 11 miles. The pressure at that depth is about 15750 psi, which converts to about 110E6 Pascals. On the chart, there is a “nose” that comes out from the green Liquid area, which happens at just over 100 MPa, and corresponds to about -20 C. That is why the deep ocean water does not freeze. It would have to drop a lot further down in temperature to freeze at that pressure.
Janice,
Several things. The trench is 11 kilometers deep, not 11 miles, (Somewhat deeper than Mount Everest is high…about 6 miles) and it is only this deep in a very small portion of it.
And the trench does not contain the coldest water, perhaps because it is cut off from the thermohaline circulation and is warmed by heat flowing through the thin crust at the subduction boundary.
The coldest water is near Antarctica and in the Atlantic and Arctic…since the Bering Straits is too shallow to allow deep water to enter the Pacific there, there is no flow from pole to pole in the Pacific.
And yes, I was looking at that nose, and was wondering later why it is there, and what it signifies. I started wondering if the phase diagram of other substances is equally bumpy (for lack of a better word).
Certainly water has many unusual properties, and this seems to be one of them. The hiccup in density near the freezing point is well described and understood as being due to hydrogen bonding holding water molecules further apart in hexagonal ice, but the nose peaking at 205 mPa is very strange, and harder to find material on as well.
But note, the line is very straight and does not bend to the left until well up past 10 mPa, and only lowers 5 degrees or so at 50 mPa
http://upload.wikimedia.org/wikipedia/commons/thumb/0/08/Phase_diagram_of_water.svg/700px-Phase_diagram_of_water.svg.png
OK six hours later now. I have been reading a lot on this all day and evening, had had more to say, but wanted to find out the exact cause of this nose.
There is a lot of good information on the exact process by which surface water freezes, first into frazzle ice and some other forms with unusual properties, like being ale to bend without breaking around waves and such.
But, yet to find anything regarding why deep water will not freeze.
One thing is clear: It is incorrect to refer to the freezing point of water. Better to refer to the melting point of ice, because water can be readily supercooled, and is thus less dense than warmer water around it…being that the hump in the density curve remains, but peaks at the freezing point in sea water.
It is stated that supercooled water can remain so indefinitely, but normally only if undisturbed will it not freeze eventually.
Surface water in the process of freezing typically is supercooled a few degrees.
Another interesting tidbit is that the water at the bottom of the ocean is near freezing even in the tropics.
Here is what I wanted to be sure of…The vast majority of the ocean is no where near the pressure you mention. Only in the deepest trenches is it so, and these are tiny in relation to the size of the ocean
Also, notice the pressure scale is logarithmic on the phase diagram. The peak of the nose is at
over 200 mega pascals.
Most of the ocean bottom, the abyssal plains, is more like 6 kilometers and thus about 60 mPa.
Anyway, it seems likely that adding salt to water is only endothermic if the salt is in solid form when added. The change in enthalpy is due to breaking the ionic bonds when the salt is dissolved…so call off the runaway chain reaction from that cause.
And, making the remaining water saltier will continue to decrease the melting point.
But the water down there is near the freezing point, and if, if I say, a large quantity of water did freeze for any reason, it would rise to the top with tremendous force.
Seems like a coiled spring to have so much water so close the freezing point, and under pressure.
Could some event cause a sudden reduction in pressure…briefly but long enough to initiate rapid freezing of supercooled bottom water? Maybe related to methane clathrates? It is mostly water, those clathrates, and if dissiociated from the methane, what are the thermal and salinity altering effects?
So many questions.
One thing I am more sure of than ever…there is more to be concerned about regarding a return to ice age conditions than some catastrophic warming of our frozen wastelands by a few degrees.
If you or anyone has reading material to link to regarding phase change of sea water under pressure, I would like to see it. The phase diagram is what it is. The question is, does salt alter the shape of those curves, or just shift them left and right? Might it smooth out the nose? Salt does alter the maximum density in relation to freezing point, after all.
This conversation gave me a reason to read a lot of material I was not about to read anyway, and I also found a whole lot of very interesting maps and graphs and charts. I learned a lot today, not sure how accurate it all is…much is official “climate science”. But the P Chem parts I am fairly certain are on the level.
Thanks, and TTYL.
http://www.srh.noaa.gov/jetstream/ocean/images/layers.jpg
http://www.chartgeek.com/wp-content/uploads/2012/04/lakes_and_oceans_large1.png
As Charles, mentions; no valve required as the cold, dense fluid in the collectors will not rise at night on a passive thermosiphon system. The one-way/check valve IS required on an active (pumped) system where the collectors are higher than the storage tank. Without a check valve, at night when the collectors cool, then the hot water in the storage tank wants to migrate to the roof while the cold fluid in the collectors descends to the lowest point setting up a reverse thermosiphoning process.
Tony B,
You don’t mention that you have ever built one of these systems. Willis has.
I have never built one of these either, but what Willis says sounds right to me. The circulation is not driven by “hot water rising” or “cool water descending”. It is caused by the fact that the water in the colder branch is heavier than the water in the warmer branch. As a result, there is a difference of pressure at the bottom; that creates a flow from high pressure to low pressure. So if there is a difference in temperature between the two branches, you will get a flow and the direction of flow will be determined by which branch is colder.
The same process drives the winds and ocean currents.
Mike, you said:
So what part of your correction disproves that hot water rises and cold water descends (we’ll leave out what ice does because it’s a tad strange)? Let’s simplify the problem by turning it into just a column of water. Hot water is less dense and will sit at the top of the column. Cold water is denser and will sit at the bottom. If I heat the bottom of the column that water will rise to the top because of gravity. If I cool the bottom of the column that water will, um, stay put because it’s already denser and at the low point of the system.
Tsk, Tsk,
Right. It mixes; it does not produce a net flow in either direction.
I think some of this conversation is overlooking an important factor, and that is the temperature differential needed to overcome friction in the pipes. If water is flowing too slowly to be useful, …it is not…um…useful.
High differences in temp/density are needed to cause water to flow through narrow diameter pipes, and this gets worse the more bends and such there are.
I do design and build systems which must maximize performance in some cases, and optimize costs in others. Large pipe is more expensive, and often there are physical restrictions that make large pipes impractical or impossible. And physical space may necessitate many turns and fittings and distance between collector and tank.
Of course, if the discussion is a theoretical one, and no concern must be given to making such a system work, and making it cost efficient, and optimizing performance, then the point I make is moot.
“Right. It mixes; it does not produce a net flow in either direction.”
A pot of water heating on your stove does mix, but it mixes by means of the hot water in contact with the bottom of the pot rising up and cooling, whereupon it cools and descends.
A Lava Lamp is a very good way to visualize exactly what happens in situations like pot of water, the atmosphere during conditions that cause convection, etc. Due to surface tension, blobs of the warmer fluid periodically pinch off and rise, displacing the cooler material above it. If the heating is very gradual, the blobs may tend to be larger and more cohesive.
In fact, I think it is helpful to imagine the atmosphere as a very large and complicated Lava Lamp…sort of. Or one of those wave machine dealios…sort of. But more complicated and with more different processes happening at once.
Mike M.: I’ve installed the systems for many years now. And you’ll might be surprised at how little hot and cold water don’t mix when left undisturbed. Regular electric water heaters rely on relatively undisturbed water. Active solar water heater storage tanks also rely on it. The cold inflow into the better tanks is often designed to disturb the stored water as little as possible. There is another issue with Willis’ drawing, but relatively minor and probably just the way it is drawn for illustrative purposes. The collector is shown with horizontal piping. Doesn’t work nearly as well as vertical piping for thermosiphon systems.
Mike: it’s only a matter of time before a hard freeze busts your collector. Maybe you have a dribble valve, which is sufficient in areas of light freezes, but a moderate to heavy freeze will get you on a thermosiphon eventually unless you have some sort of drain down arrangement..
Actually, since it’s a closed loop (ignoring the inlet from the water supply and the outlets to the residence), you do need a valve to prevent “reverse” cycling.
When the sun is shining, the water in the solar collector warms up and becomes less dense. Since it’s now lighter than the water in the rest of the system, and in particular the water in the pipes coming down from the tank on the roof, there’s a net upward force as the heavier water from the pipes moves in to displace the lighter warm water.
At night, the water in the collector radiates heat to space with no counterbalancing flow of heat from the sun. This water becomes colder, and eventually colder than the rest of the water elsewhere in the system. As it becomes colder, it becomes denser and will flow to displace the water in the pipes coming down from the water tank. It’s precisely the opposite of the effect seen when the sun is shining.
(And it occurs to me such a system could be used in warm climates to “store cold” for use in cooling the house during the heat of the day.)
“If you’ve worked much with thermosyphon systems, you may have noticed that the system shown in Figure 1 is missing a critical component for successful operation. To work efficiently, the system needs a one-way valve to keep the circulation from running in reverse.”
Well I have designed and installed several solar water heater systems and I’m affraid this is back to front.
It is the more usual arrangement where the panel is on the roof and the tank lower in the building that a one-way value is required to prevent reverse flow the night. Often the water pump which is required in that configuration is sufficent to prevent that so no extra valve is needed.
The other point about thermosyphon is that it is less efficient than a pumped system. The low-tech , no external power set-up is attractive but it takes a significant temperature difference to start and drive the syphon. It can be particularly slow to kick in. This means additional heat loss throught the panel window and lost production before it starts up.
The ideal is to keep the working fluid turning fast enought to keep the panel output just a couple of degrees warmer than the input. Beyond some point the turbulent losses in the fluid mean that extra pumping becomes counter productive. In my system the 8W pump is enough to keep the panel dT around 2 deg C. When it drops to 0.5 deg C I cut the pump.
Sometimes the simplicity and independence may justify the reduced efficiency.
My real time monitoring system shows 50.5 deg C in the 200l tank. I’m off for a shower. 😉
BTW it is possible to make a panel which can run without anti-freeze but it needs some careful design work. This system works on tap water, it freezes several times each winter without busting pipes.
http://upload.wikimedia.org/wikipedia/commons/2/29/Autoconstruction_panneau_chauffe-eau_solaire_CESI.jpeg
Willis, plus this idea.Replace your solar panel with a heat exchanger fitted to the inlet/outlet gas manifold of your air conditioner outdoor unit. Instead of running a fan, the water extracts the heat. a win win. Both these ideas could save up to 20% of electrical demand.
http://www.ecogen.com.my/airlink.html
Add a geothermal leg to make it even more efficient.
To really make best use of the available energies, a small circulation pump and a set of control circuits wired to a microprocessor. A few solenoid valves, and some insulation on the tank.
And make the AC a heat pump.
If everyone had such a set up, or as many parts of it as their location would allow, a lot of energy could be saved.
Working on a setup like this for my new house.
Indeed rough numbers, greater savings than all the draconian co2 measures and money actually saved.
Menicholas,
I had thought the same thing re: a circulating pump. We don’t live in a dirt-poor area, so we can afford to add a small pump. It wouldn’t take much at all — a pump powered by a single small solar panel would be enough.
A pump would greatly add to the efficiency. Many years ago I had an apartment building with hot water continually circulating, so when a tenant turned the faucet he didn’t have to wait for hot water; it was always right there. One day the pump went out, and for various reasons it took a week to get a replacement. The only effect on the residents was waiting for hot water to start flowing, but it gave me a chance to compare utility bills on the ‘house meter’.
There was no apparent change. So even though it ran 24 hours a day, the circulating pump required very little electricity. Adding a small pump to your setup would make it much more efficient. And now they sell all sizes of solar panels, from 1 watt on up. The right size would power a pump motor, and …profit!
The minds-eye image of a solar powered circulating pump is too simplistic. The actual circuit would need to be a solar re-charged marine quality lead acid battery which then supplies current to the pump. The losses within the charging system will be significant. And, if the motor is DC to avoid the need for an inverter, the whole system will need to wired with large gauge connections to prevent further loss. If an inverter is used so that the pump works more efficiently – the inverter will need to be quite expensive to accommodate the motor start current.
However, a circulating pump for standard mains current should be an AC pump and probably synchronous. A synchronous motor is extremely efficient… which is probably why dbstealey saw very little change in mains power consumption.
“using approximate numbers, the overturning of the ocean occurs over something on the order of five hundred years.”
I’m just an ignorant bystander, but if the North Atllantic Drift had a speed of only 1knot, that’s 10,000 miles per year, which suggests to me a “lap time” on the order of 2 years.
It is less than one knot then, right?
The whole ocean moving at one knot is a lot of energy. These are not currents, like we normally think of them.
One knot is a little over .5 meters per second. This is over 27 miles a day.
Thermohaline circulation is more on the order of one or a few centimeters per day.
One knot is of the same order of magnitude of most of the major surface currents.
Thermohaline flow is very gradual compared to these,
By the time it gets to the north of the UK the current is doing about 1mph a lot faster when it exits the Gulf.
Yes, about one knot was what I recalled as figure but I don’t know how typical that is.
Some oil co. wanted to sink an old petrol platform off Hebredises IIRC saying the pollution would not travel far because of the very slow drift currents. Greenpeace challenged this and were able to establish that the deep trench currents were more like one knot. The dumping was abandonned.
But talk of ballpark figure of the order of several hundred does not seem applicable here.
I think solar water heaters are a much more efficient way of using the sun’s heat than solar panels to generate electricity. They should be compulsory in warm areas. In fact I think they are in parts of Australia.
If the waters at the poles warm slightly, then the temperature difference is less and so the water at the poles is slightly less dense. This should reduce the flow and make any flow of heat to the depths take longer. But maybe more heat at a slower pace is the same amount of energy as less heat at a faster pace. So not a lot of difference and still no quick mechanism for storing heat in the depths.
Arctic may have slightly warmer water than usual, but Antarctic colder than usual (over last 30 years). So again no net gain in deep heat.
I’d add that if the tropical surface water warmed, it would increase evapo(u)ration, so only a portion of the increased warmth would reach the poles.
No need for the ‘(u)’ – the English spelling of evaporation is the same as the US spelling. 🙂
Well solar (PV) panels don’t use the sun’s heat; they use the sun’s EM radiation energy to directly generate electricity.
And storing high Temperature “heat” energy is somewhat more difficult than storing electricity.
Current top brand solar panels are pushing 25% conversion efficiency, and advanced multiband gap, multi-junction cells can get close to 45% conversion efficiency.
Commercially available heating panels can warm your swimming pool and that’s about it. How would you cook with solar themal collectors ??
Look up solar ovens. They don’t use the hot water system but they cook quite well.
… including cooking your retinae.
Well where I live, the sun is usually set or setting, before dinner gets cooked, and I normally have my breakfast poached eggs on toast several hours before the sun rises.
So electricity does it for me; or gas would work in some other locations.
I don’t usually eat hot food in the middle of the day, when it might be cloudy.
How to cook using solar?
One way is to buy a magnifying glass, and use it to ignite charcoal, thereby liberating the stored solar energy in the briquettes.
Thing is, we still need regular water heater. Even here in South Florida, just this past winter there were weeks on end with little sun, and cold at night.
Low sun angle means only a few hours of substantial heating if ones house has a lot of trees about (Mine does), and if it is cloudy you do not even get warm water. Few will tolerate no hot water, even occasionally.
So it is supplementary, saving money at times.
Add up the cost of such a system, and it has to be sunny much of the year to make it economical. Have an attic water tank spring a leak, and the damage will cost way more than you ever saved.
I have a fairly large and open lot, but from late October to late February (within two months of the solstice), the sun is behind trees all morning
I like geothermal myself. The ground only one or a few tens of feet down is always near the average yearly temperature for a given location. Farther north, the deeper than constant tem depth becomes.
Here in my area, ground water is about 75 F. That is warm enough to increase heat pump efficiency on a cold night, and it is cool enough to help save money on AC in summer.
I live in southwest Florida. Years ago when I bought my house I removed trees on my southern exposure that would block winter sun. Then I planted trees on my western exposure that would shade my house in summer. Just an easy way to help with the heating and cooling costs. I did leave the large oak tree that shades my driveway all year long. Keeps the car from being in the sun all day but I have to clean the bird crap off a couple of times a week.
Try living in Canada. Staying cool in the summer is easy compared to staying warm in the winter here. This past winter saw several days in a row below -20oC and a few below -30 (where I live).
I had a geothermal system installed last year – 2 units based on an open loop water source using a known aquifer 120 feet below surface. In mid-winter the water temperature dropped from 55oF to 38oF. No one mentioned this would happen and it reduced the efficiency of the units, causing them to run continuously. I still saved a bundle in propane costs and will recover my investment in under 5 years.
But staying warm is the real problem. AC is a convenience not a life necessity and so is warm water.
Tom in Florida: Have you tried using Irritape (amazon it)? Supposedly scares birds away. I bought some to use in my garden this year.
Tom, I think that my cooling costs are so much higher than any heating cost in Winter, that I would not have cut down any trees for the reason you mention. At least half of years I never turn on the heat even once.
Although I have to say, there are some days that I needed to use heat and AC in the same day! Not many, but it happens. It tends to be very warm ahead of the cold fronts. And warm up very rapidly once the high moves off to allow an easterly component to the wind.
I would cut down or prune any trees that I thought might be a hazard in a hurricane or tropical storm. And I have a very large mango tree that shades my solar pool heater in the winter…when I need it most, so I cut the top off of it. This also has the added benefit of allowing me to have the fruit where I can pick it more easily.
It grows so darn fast though that I need to cut it again, this time more severely. I also now have a very wide mango tree, which I found produces more fruit anyway…since the flowers only form on the ends of branches, more branches equals more flowers and hence more fruit.
“They should be compulsory in warm areas”
No, they should not be COMPULSORY anywhere. Choose it if they want it.
Solar water heaters like the Ivanpah solar energy facility in California?
The one which is blinding airline pilots, incinerating birds in midflight, and performing at about half of what was expected?
Original plans had to be scaled back, but the financial backers of that facility have said they are not even considering building another.
I introduced a friend to a similar very cheap system for his swimming pool that was using upwards of $480 per month to keep warm and because of that cost only used it for 2 months of the year. One 125 foot coil of black 1.5″ poly on top of his garage coupled with his filter system (needed anyways), voila no costs, 4-5 months of pool use and he has to turn it off because the water in the pool got too hot in July and August!!!
asybot,
I had a system like that. You need to live where there’s no wind, or the coil of PVC will act as a radiator. I replaced it with solar collectors that had black anodized copper under glass, so the wind didn’t matter. They worked much, much better. But the coil of PVC was very cheap by comparison.
My problem is snow. Glass might take the snow load if well supported but if say if a lower area clears and heats up, the glass would likely crack if any snow slid down over it from above. I think I’d have to use polycarbonate sheeting but it so much more expensive.
polycarbonate works well. It’s slightly opaque so somewhat less efficient than glass and may yellow and need changing in 5-10 years. A minimal cost. The photo I linked above is PC covered.
The three layer bee-hive kind of PC sheet gives some good “double glazing” effect that notably reduces window heat loss, especially in very cold regions. This largely compensates for the loss of input due to the opacity.
It’s a shame that your friend didn’t put a reversing valve into the system. I had an acquaintance here in Houston who did the same thing, but with a reversing valve. In the middle of Summer his pool was a delightful 78 F, thanks to the 74-77 F nights in Houston. Re-radiation works.
“One 125 foot coil of black 1.5″ poly on top of his garage coupled with his filter system (needed anyways), voila no costs, 4-5 months of pool use and he has to turn it off because the water in the pool got too hot in July and August!!!”
Nice, but next time, try using 1/2 inch poly tubing. Far more cost effective, and far more efficient at gathering available heat. This tubing is widely available for use as irrigation lines, and since it is produced in large quantity, it is cheaper.
Put it in a black frame, and cover with transparent sheeting of some sort. Just clear plastic will work, but you can make it more durable using DBs methods/materials suggestions.
I like the idea of using a preassembled heat exchanger. Posting a link to a source for what I have in mind. Expensive, but durable. Copper pipe with aluminum fins soldered onto them. Baseboard heating units work very well, and will work as well at gathering as at radiating.
Here is one guy showing how, and if you follow the links of related videos, you can see dozens if not hundreds of alternate ways to do this:
http://www.amazon.com/22×25-Water-Exchanger-Outdoor-Furnace/dp/B00B1GRMBC/ref=sr_1_5?ie=UTF8&qid=1431817087&sr=8-5&keywords=heat+exchanger+water
Hi
I have a 10m by 5m pool and Solar heating consisting of 3 x 2.5 metre domes constructed with approx 15mm black poly in ever decreasing circles I do not know the total length of piping used to create each of the 3 domes but each of my domes (diametre) 2.5 metres is much bigger than the panel shown in the video.
The system does not work very well because poly is a heat insulator and therefore a silly medium with which to constrct a heat exchanger. It is more than 10 years since I have used it because it works so poorly.
For the last 12 or so years I have thought about replacing the system with back painted copper tube (say 22mm) but I have never got around to it. I have not done any research on how copper tubing stands up to the usual pool chemicals.
I live in quite a sunny climate. My pool has sun on it all day long. It is nearly 8pm and my pool is just over 26 degrees. In the height of summer, it gets to between 35 to 37 degrees, but the Solar heating using poly piping is not in my opinion worth the cost. .
Hi Richard,
Not sure why you have such poor results. A lot of people have them in Florida. This time of year, it will heat my spa up to 110F in an hour or so, If I leave it on for more than two hours, it will soften the PVC pipes in my pump assembly so much something will come apart…usually the union fails.
It makes it extremely hot.
My rooftop unit was on the house when I bought it, it is not the homemade type, but the concept is the same. It is two inch pipes with a line of thin tubes which extend down to another two inch pipe. The design is modular, so the systems can be matched to available roof size and heating needs using standardized premanufactured parts.
During years with a lot of sun, which is most years here in SW Florida, I can keep my pool very warm (75-85 F) just by running my filter pump through the roof unit during the warmest part of the day…usually around 11:00 AM to 3:00 or 4:00 PM, during the lowest sun months. It will heat my 20,000 gallon pool about two degrees per day on a sunny winter day. I have never measured how much it would at this time of year…the pool is already very warm…about 90. In winter I used a thermal blanket on the pool.
The ones I have, and most people I Florida seem to have the same sort, have no enclosure, or covering at all. Just tubes sitting on the roof. I have a white concrete tile roof.
http://solarsource.net/wp-content/uploads/2014/01/Heliocol-Solar-Pool-System-on-a-Barrel-Tile-Roof.jpg
Rod ,May 15, 2015 at 10:44 pm
Re solar hot water.
Theoretically sound but practically imperfect seems to apply to solar hot water in our experience, and we’re in an area with enough sun to make them work.
And, when the nearest plumber (electrician etc) is around 100km or more away, reliability is needed and self help is a necessity when feasible. As a friend who moved to the city puts it “I never realised how much time you spend looking after yourself in the bush”
Firstly Willis hasn’t mentioned the effects of frost, so he can look to do some plumbing with a system like that in winter. Might even find the non-return valve (and others) split as we have – on the roof and somewhat insulated.
For us the payback period is around 20 years. In that time we’ve burnt the bum out of two commercial systems. Ran the first one on well water, the second on rain water when we had it – and there is plenty of sacrificial anode left in the shell of that one.
Decided that they’re a very expensive hobby and have gone to a Rinnai on-demand gas system as they have stood the distance around here. Plumber who did the installation knows of only one solar system left in the district. And warned us to avoid heat pump systems because of problems already showing up
Sounds about right Ian.
In the UK I am waiting for the wailing and gnashing of teeth in the UK, when the super (subsidised) solar panels need to be replaced and disposed of responsibly (perhaps the poor can be forced to pay for that as well as the original insulation and grid subsidy!)
Also never seen much on decommissioning costs of the masses of pretty useless “bird blenders” which have been erected in the once beautiful North Wales seascape (where I live).
These things will get problematic and costly when the gear boxes start to fail (about 8 years) due to “brinelling” in the gear box bearings.
The profiteers and the crop of politicians who lined their pockets, will be nowhere to be seen.
Technology waste, even from so called recycling programs in the UK, already finds it’s way to the poor, particularly Africa for dismantling and extracting metals etc. There is a BBC documentary series, which I confident you’ve seen, called “Blood, Sweat and T-Shirts” which documents how poor workers around the world in various industries are being fully exploited, underpaid and poisoned.
Some friends of mine who recently renovated their house ripped out their electric hot water tank and solar heating “top up” system in favour on an on-demand gas boiler. Seems to be a growing trend in Aus.
I made comment and suggested they should have installed the larger model boiler in the range as the one installed just doesn’t seem to be powerful enough and was “told off” for not being a plumber. Well, that’s true, I am not a plumber but I can read a technical specification sheet, count the number of systems that require hot water and do “sums” to have reached that conclution. It’s not rocket science after all!
Indeed, I didn’t want to comment on posts above, as one can get into a lengthy discussion (and it’s the weekend!), but HW solar panels are a waste of money. The trouble is pesky maths (or ‘math’ to Americans!).
Your hot water solar system costs £4,000 (according to the Energy Savings Trust)
Your typical hot water usage costs you £300 a year
That is NOT a 13 year payback, because it won’t provide all your hot water. The Energy Savings Trust say that, in fact, you will be lucky if it gives you a quarter of your hot water needs. And that’s even before you account for maintenance. Add on lost interest and you actually NEVER get your money back! Stunning, isn’t it? But you can’t argue with maths.
Quote
Your hot water solar system costs £4,000 (according to the Energy Savings Trust)
Your typical hot water usage costs you £300 a year
Unquote
That seems way, in our house alone hot water accounts for 60% of our bill. look at my link above much lower cost than you quoted. Are all Americans ripped off cost wise?
My system cost me about 150 euros to build. Most of that was good quality pump.
8W for about 5h per day running cost under a cent per day. Been running about 6years now.
I’ve no idea about America, I’m English, and live in England. The figures are from the British Energy Savings Trust. If your hot water is 60% of your energy bill, then you must be somewhere warm, or you must have very many grown-up children. Which is it?
Hot water 60% of bill?
I have never broken it out, but mine is nothing like that.
Air conditioning is the biggest part, and well pump for watering the landscape and lawn is second.
(I am a plant and tree collector, working on building a botanical garden/park for posterity.
Being a long time plant nut, I am always pushing the envelope of what I can grow, and so must be very careful on cold nights to protect stuff. Planning microclimates is where I start, but sometimes you need to put something that will die from cold out in the open and away from structures and trees. Besides for some ornamentals from tropical Africa, Madagascar and Indian Ocean Islands, I grow my own bananas, pineapples and am working on coconuts. Mangos, guava, and all types of citrus are fine unprotected, but there is a reason that commercial banana and pineapple growers do not have plantations in Florida. But with care it can be done.)
AC is very low in winter, but water goes way up, being the dry season here. This past year I had to use heat, which is unusual for SW Florida.
In summer, it rains nearly every day, and so well costs go down very low, but AC skyrockets.
Pool pump is probably number two or three most of year. Hot tub/spa (what can I say, I love a hot soak before work and before bed, and about every other time) heater (heat pump) cost me nearly zero in winter of 2013/14, because it was sunny every day, and the rooftop solar pool heater did the trick, but this past winter, it was very cloudy (el nino? I can recall el ninos where there was a persistent stream of high clouds from the southwest for months on end. Greenhouses do not heat up nearly as much when cloudy 🙁 ), so spa costs went way up.
Oh, well, at least I have ground water here. At last place, was city water, and watering the lawn at $30 per thousand gallons was galling. They charge for sewer same as water usage, and I am pretty sure none of he landscape water goes into it.
As for a solar hot water system for home hot water…if you are not doing a lot or all of it yourself, I agree that it is a losing proposition. This is a project for people who have the know how and time to do it themselves, and then you only pay for the materials. If you are clever and resourceful, it can be done very inexpensively.
I am still working on a plan for how to integrate everything and make it actually a money saver.
As for the rooftop solar pool heater, it is more properly called a heat exchanger. It is a heater if it is on during sunny and hot periods, but turn it on at night, and it will cool very efficiently. Here in Florida, during the high sun season, it is impossible to tell if and when it will rain when leaving the house in the morning, and if you have the pool water going to the roof in a thunderstorm, it will dissipate a lot of heat instead of warmig your pool. Same if it gets cloudy and windy.
The answer is a control circuit. I devised one that uses a circuit board designed for a boiler, monitors temp in pool and on roof, opens a solenoid valve when roof is hotter than pool, closes it when it is not.
But, often overlooked is the solar blanket for the pool. It is a big sheet of bubble wrap like plastic, and if you have a reel setup, it just rolls up when pool is needed, pull it back onto the pool when not. With rooftop solar heater and thermal blanket, one can keep pool open far longer than having one or the other…or neither.
Get the expensive one…UV will ruin the cheap ones pretty quick. The ones with a layer of aluminized plastic trap a ton of heat. Just for a lark, every once in a while I will keep the water going to the roof and the blanket on the pool when it is hot but dry out (like now), and get the entire swimming pool up to 104 degrees (max safe temp).
Everyone knows what a hot bath feels like, but I can tell you, until you have dived into a pool of 104 degree water, you cannot possibly imagine the bliss. Even on a hot day, it feels amazing. But only for an occasional thing…chlorine evaporates very fast out of hot water.
Solar pool heaters
have a fitting which allows air into the system when pump shuts off. Gravity then allows the water to drain out into the pool while the pipes and tubes fill with air.
There are other solutions as well. My first year here, I was not paying attention, and had the valves closed, which prevented the water draining, and the system survived a few nights well below freezing with heavy frost with no damage…not sure how. The tubes are kind of a heavy material, and rather rubbery. Heavy enough that gravity holds them down even with the water drained out, end a very strong wind. I have been through a few tropical storms and one near miss from a weak hurricane.
My wife is from high in the mountains in the Philippines and we visit her family once every few years. About 10 years ago we were there and although the house is not bad, on the south side of the mountain it is rather primitive. Cooking is done with a wood fire and/or bottled gas. There is electrically sometimes but often cut off for weeks at a time and really only used for lighting and keep cell phones charged.
The water supply is from a mountain spring which is gravity feed to a large water tank on the flat roof of the house. The water is ice cold and no fun to wash with. After a day or two off this I had her bother pick up some paint and we painted then then silver water tank black. Now as long as you wait till about 3pm you can have a nice warm shower and the dishes clean up a lot better. All this for $15 of black paint. On my return trip a few years later I noticed all the water tanks in the village are now painted black.
Watch out for nasty bacteria in those tanks…water is at the perfect temperature for reproduction.
Good point but it is used for washing. Drinking water is all boiled, mostly used for tea that grows there.
CNC,
I’ll bet you’re treated like royalty when you visit the mountain. They probably plan for weeks for the arrival of their ‘rich’ relatives to a location like that. ☺
I’ve seen many of these black, roof-top tanks in Mexico.
I’m confused by this… I see how warm water at the poles is drawn down to the depths. That’s well known.
But as most sunlight is at the equator, wouldn’t most of the heating from CO2 and water vapour be at the equator – a tropical hotspot?
So wouldn’t the surface waters need to be warmer to transport the heat to the Poles before they sink?
Is the idea that the warming is so small it can’t be detected before it’s tidied away from the weather systems down below?
Yes. The heat still has to make its way from the tropics to the polar regions, either in the upper ocean or in the atmosphere. It should be measurable in both cases.
Salinity also affects density and tends to mke it rather less simple in our oceans.
Also:
“The reason the thermosyphon works is because a cold fluid is denser than a warm fluid. As a result, you get a pressure difference in the two legs of the system. This pressure difference works to constantly circulate the water. The water sinks on the cold denser side, and rises on the warm less dense side”
Sounds rather like adiabatic convective overturning in the atmosphere which I have proposed as a variable method of countering the thermal effects of radiative gases by running faster / slower or by rising higher / lower against gravity.
Ah, just noticed that Willis did cover the salinity aspect.
Yes. Salinity was partially addressed. However, if the poles melt – that’s fresh water added to the system. The melt water would therefore decrease the salinity of the polar ocean water, which should slow the thermohaline flow. So the model presented is much too simplistic to justify a plausible ‘heat hiding in the depths’ reasoning…
In an atnosphere the one way valve is provided by the decline in density and pressure with height so the system can never run in reverse.
Stephen
The one way valve is precipitation. The condensation of water vapour releases latent heat into the rising cloud. The removal of the largest droplets from the cloud by gravity assisted rainfall means that the warmed air aloft cannot lose the latent heat by re-evaporation of cloud droplets (the water is now missing). The warmed and now dry air is effectively held aloft and has to radiate its excess heat to space, thereby cooling, becoming denser and so able to descend again.
Without the decline in density and pressure with height would there be any precipitation?
“Without the decline in density and pressure with height would there be any precipitation?”
Good thing for gravity, eh?
But really, there are quirks of physical chemistry that make it seem like a very fortunate coincidence that much rain is ever falling.
Coalescence in a cloud is very inefficient. Most rain starts as ice crystals…snow. And they forms rapidly due to the vapor pressure curves of water in it’s various phases doing what they do at the temperatures and vapor pressures which prevail in certain sizes and types of clouds.
This is the sort of thing I studied when I took first year meteorology/physical geography way back when
“The Wegener–Bergeron–Findeisen process (after Alfred Wegener, Tor Bergeron and W. Findeisen), (or “cold-rain process”) is a process of ice crystal growth that occurs in mixed phase clouds (containing a mixture of supercooled water and ice) in regions where the ambient vapor pressure falls between the saturation vapor pressure over water and the lower saturation vapor pressure over ice. This is a subsaturated environment for liquid water but a supersaturated environment for ice resulting in rapid evaporation of liquid water and rapid ice crystal growth through vapor deposition. If the number density of ice is small compared to liquid water, the ice crystals can grow large enough to fall out of the cloud, melting into rain drops if lower level temperatures are warm enough.”
http://en.wikipedia.org/wiki/Bergeron_process
Philip,
You seem to be under the presumption warm (unstable) air ‘warms’ as it rises? That is not correct. The adiabatic process ‘cools’ the air as it rises (around 6F/1000ft). The latent heat process from condensation just reduces the cooling rate (around 3F/1000ft), but it is still cooling non the less. Look up dry/moist adiabatic lapse rate for details.
“..The warmed and now dry air is effectively held aloft and has to radiate its excess heat to space, thereby cooling, becoming denser and so able to descend again.”
Tops of thunderstorms (especially in the tropics where the tropopause is generally higher, thus cooler) are from -40F to -80F specifically because of the adiabatic process – there is very little ‘excess heat’ to radiate to space compared with the temperature it was at when it started it’s journey upwards (75-90F?).
Also, the adiabatic lapse rate works the same way (but opposite) when air descends…the parcel warms.
@Stephen Wilde
“…Without the decline in density and pressure with height would there be any precipitation?.”
Sure! Convection is not the only lifting mechanism in the atmosphere. There is also orographic lift (mountains/upslope flow), isentropic lift (warmer air forces up over cooler air as in a Warm Front) and kinematic lift (jet stream/linear & radial divergence aloft). All these mechanisms force what may be stable air vertically.
*Now* this all presumes there is still a vertical thermal gradient even though there is no density/pressure gradient in your ‘atmosphere’.
Gromitdog,
“vertical thermal gradient ”
This is usually referred to are the ELR- Environmental Lapse Rate.
It is distinct, of course, from the adiabatic lapse rates, both the dry rate and the wet rate.
I have seen many conversation on this and other sites where some commenters seem to have not learnt this, ignore it, or have forgotten it.
The difference between the ELR and the DAR rate determines whether (And, here it South Florida, it determines weather.) the atmosphere is stable, unstable, or conditionally instable.
As such, these parameters are key principles in meteorology and weather forecasting.
Measuring the ELR is one reason for the long time use of those radiosonde devices, even before all this climate kerfuffle began.
The existence of various layers in the atmosphere with different ELR values makes for some interesting and notable weather conditions. One such is the “cap” that can exist at certain altitudes at certain times of year.
Warm air is prevented from rising very high due to subsidence (or whatever reason…the ELR is lower than the DAR), but only up to a point. Convection continues as heat builds during the day, but is suppressed from reaching a height which would allow clouds or thunderstorms to develop.
So a very deep layer of warm accumulates. At some point, either an impulse moves in which lowers the cap or enough heat is built up to punch through it, and all the trapped air can now escape upwards like through a chimney.
Thunderstorm formation can be nearly instantaneous, and severe weather can form out of a clear sky within minutes. Not moving in from somewhere else, which can be seen coming, but developing overhead faster than can be reacted to by boaters or travelers.
Wish I had my list of links of videos…better ones are around:
GromitDog,
Rising air cools and descending air warms, I agree, but why does this happen? Moist air rising to form cloud cools more slowly than rising dry air and similarly descending cloudy air warms more slowly than descending dry air, so why is that?
Now what about the Chinook wind? A moist air mass starting at sea level in the Northeast Pacific, rises over the Rockies and on doing so cools to form cloud and rain. This now dry cold air at elevation then descends eastward onto the Alberta plains and warms to a higher temperature than it originally had at Pacific sea level. How is that possible? The air has both increased its potential energy, being lifted 2,000 feet onto the plains AND has increased its kinetic energy, being warmer than at its original sea level start point. Where does the heat come from that makes it possible for this change to happen?
Thank you Willis for the concise presentation.
Well, provided my understanding of the official sermon is correct, nearly 1 molecule of anthropogenic origin in about 10000 other atmospheric molecules overrules everything else. A personalized fiery place without air-conditioning awaits the heretics.
Volcanic activity e.g. along the tectonic plates in the ocean floor, Sun’s radiation directly warming e.g. the ocean’s surface and the thermal radiators e.g. at the poles are inconvenient details. A bit like thermosyphon and Coriolis effects in general.
Does an atmospheric or tectonic circulation similar to thermohaline already exist?
http://www.ocean-sci.net/5/203/2009/os-5-203-2009.pdf
Geothermal heating, diapycnal mixing and the abyssal circulation
Abstract. The dynamical role of geothermal heating in abyssal circulation is reconsidered using three independent arguments. First, we show that a uniform geothermal heat flux close to the observed average (86.4 mW m−2) supplies as much heat to near-bottom water as a diapycnal mixing rate of ∼10−4 m2s−1 – the canonical value thought to be responsible for the magnitude of the present-day abyssal circulation.This parity raises the possibility that geothermal heating could have a dynamical impact of the same order. ….
…For strong vertical mixing rates, geothermal heating enhances the AABW cell by about 15% (2.5 Sv) and heats up the last 2000 m by ∼0.15◦C, reaching a maximum of by 0.3◦C in the deep North Pacific.Prescribing a realistic spatial distribution of the heat flux acts to enhance this temperature rise at mid-depth and reduce it at great depth, producing a more modest increase in overturning
than in the uniform case. In all cases, however, poleward heat transport increases by ∼10% in the Southern Ocean. The three approaches converge to the conclusion that geothermal heating is an important actor of abyssal dynamics, and should no longer be neglected in oceanographic studies.
“The case is hereby made that geothermal heating is an important actor of abyssal dynamics. We recommend its inclusion in every model dealing with the long-term ocean circulation, for it substantially alters bottom water mass characteristics and generates a non-negligible circulation in the present-day climate.”
Has nobody stuck a reliable thermometer in the mud on the ocean floor?
Yeah, but it kicked up so much sediment they could not find it again.
But seriously, the ocean crust is different ages, and thicknesses and hence temperatures.
And then their is heat flux from the ridges themselves, the spreading centers, black smokers and volcanoes of various sizes.
And my understanding is that these are sporadic in output and poorly understood, because they are so hard to get to. We have the maps, but little actual explorations going on.
I have heard it said we have more knowledge of the surface of the moon and Mars than of the ocean bottom.
Or the water column, for that matter.
By the way…I do know their from there, and even they’re.
I just do not know how to type.
Autocorrect!
Willis, surely if the poles were running warmer, the surface water which carried the heat to the poles would also have to be warmer? So the surface would warm as well?
Yes of course the surface has to warm first Eric. And it has to become significantly warmer in the equatorial regions first to get the energy to the poles.
If the surface water at the poles was warmer, it wouldn’t sink as fast and the system would stop flowing. The flow rate and direction is dependent on the absolute temperature differentials.
Willis is wrong about the ocean heating from the bottom up. But I am sure he knows that, this post is a test : )
What if the various heat transport mechanisms became more or less efficient for various reasons and at various times?
There could be times and circumstances that more heat is transported to the poles because of fluctuations in the transport mechanisms, independent of weather the tropics were warming or not.
Or, as energy entering the system in the tropics increased, perhaps the transport speeds up, rather than the temperature rising?
This could explain the contention that the tropics have been stable to within a degree for a very long time.
Please read up on Willis’ emergent thermostatic hypothesis as has been discussed on WUWT previously. It is hard to argue against. Sorry I don’t have a link handy but it’s a must read.
Will do.
Thx
i’sonu2
So, with out getting too scientific about it. With a 500 year time lag, any warming supposedly found in the deep ocean now is a result of the warmth in the Medieval warm period and we have to wait until 2515 to get any deep ocean heat from any purported arctic/antarctic warming now. Seems fair. Has anybody told Trenberth and his fan club ?
Ivor Ward,
Very good point. But if you tell it to Trenberth, he will just make up an ad hoc excuse/explanation on the spur of the moment, and continue on his merry way to the next grant proposal.
Trenberth and his fan club believe that Godzilla is a fire breathing monster that will arise from the deep ocean and fry the world.
I’d also like to know from Travesty Trenberth how there will be positive water vapor feedback to heat in the deep oceans ? I think that is probably not going to be by simple physics (smiley icon)
Of course there is also day and night to consider. What happens in a solar water heater, also happens in the Oceans!
As to the theoretical heating from underneath, chicken and egg: to get slightly warmer water descending from the poles, the poles would first need to receive warmer water arriving from the equator.
wwf; As surface water near the poles cools, it “sinks” and “draws” nearby (warmer) surface water to replace it. The “sinking” is what drives circulation, not warm surface water “pushing” toward the poles; similar to tectonic subduction.
“..what would be the effect on the average ocean temperature of a slight warming at the poles?”
A drop in temperature in the north, as the Atlantic meridional overturning circulation slows down when there are oceanic warming pulses to the AMO and Arctic.
And in the Pacific, the Thermohaline circulation goes in the reverse direction with the lower cold feed moving polewards.
Willis, the geothermal heat is a key piece of the puzzle. I bring it up once in a while because, as far as I know, the ocean reanalysis and the ocean circulation models don´t account for it properly. The sea floor´s heat flux, and its areal distribution, ought to be resolved (I suspect the heat flux is higher over the mid Atlantic ridge and other spreading centers). On the other hand, it ought to be much lower 200 km offshore Brazil.
I noticed your approximate value of 500 years to complete the ocean thermohaline circulation, and couldn’t help thinking of the average 800 years lag time (based on ice-core data) for a rise in the earth’s atmospheric temperature to lead to a rise in atmospheric CO2. Suppose a more detailed calculation for the time needed to complete the ocean circulation gave 800 years rather than 500 years. Then, a very simple model would have the earth’s atmospheric temperature rise at the same time that extra heat was entering the ocean’s surface waters. These surface waters will then descend to the ocean floor and return, slightly hotter than expected, to the surface 800 year’s later. The slightly hotter-than-expected surface water would then release more CO2 into the atmosphere — giving us the 800 year lag in the ice core data — and also cause the earth’s average atmospheric temperature to rise slightly more than it would have otherwise.
Just for grins, subtract 800 years from 2000AD and get 1200AD. So, by this model, the current slight rise in the earth’s temperature AND the rise in atmospheric CO2 could be due to the slightly warmer water of the medieval climate optimum returning now to the ocean’s surface. This makes the increase in the earth’s atmospheric temperature mostly an echo of the medieval climate optimum. Coming a few centuries from now, cooling temperatures and less atmospheric CO2 as the slightly colder waters from the little ice age start returning to the ocean’s surface …
Thanks, Willis
However the story just starts here, we need to take it yet more general. How do you get thermal energy IN the ocean? By direct visible sunlight and perhaps some near IR. Far IR does not penetrate water more than a few micron and most of that energy is redirected to evaporization. I know there have been huge discussion on that, but doesn’t change the physics (engineers know that and have develloped infrared evaporizers). Therefore increased temps in the oceans is not from enhanced greenhouse effect, instead, it’s a direct result of less cloudiness and which can be verified here:
http://www.climate4you.com/images/CloudCoverTotalObservationsSince1983.gif
source:
http://www.climate4you.com/ClimateAndClouds.htm
And then there is latent heat of evaporation.
(internal links seem dead)
It doesn’t matter how far IR penetrates the ocean. What matters is that is absorbed. And when radiation (of any wavelength) is absorbed, its energy is also absorbed. Energy cannot be created or destroyed. This results in warming of the ocean. Some of this warming may be used to provide Latent Heat of Evaporation. But only a small proportion, because the amount of evaporation possible is limited by the humidity level allowed for the ocean temperature.
You say most of it goes in evaporation. Let’s check that. What goes up most go down. Water that evaporates eventually returns as rain. So do some calculations.
What is the annual rainfall worldwide? LOOK IT UP.
How much energy is required to evaporate that amount of water? WORK IT OUT.
Now compare it to the amount of LWIR incident on the ocean (and elsewhere). LOOK IT UP.
These are steps that laymen miss out or don’t bother to do, and this is why they become fixed on ridiculous conclusions.
MikeB
You say
OK. I get that.
You did not bother to do those steps so the ONLY thing your post says is that you claim to be a “layman”.
Richard
richardscourtney “so the ONLY thing your post says”
Quite a while back I looked up total annual rainfall and calculated the total amount of energy it represents as convected latent heat. I only remember that it DWARFS the amount “trapped” heat from CO2 by such a large amount that only a slight increase in rainfall obliterates the effect of ALL the CO2 in the atmosphere.
Water vapor has not and cannot be modeled yet but IT is what controls our climate not CO2.
http://www.ssd.noaa.gov/goes/east/eaus/wv-animated.gif
Mike,
Rainfall rates are greatly different in different regions so global rainfall is missleading indicator of rate of energy transfer. I have done some calculations on the tropical Pacific and those calculations are what I have based my statements on. I’m presently refining my calculations to establish confidence limits on the numbers. If you would like to do your own calculations, make a comment on my blog, http://www.retiredresearcher.wordpress.com. I can e-mail you the data files that I am analyzing.
Thank you fhhaynie , but we already know this. There are a number of peer-reviewed studies providing this information, one of which is well known, or infamous, depending on your viewpoint.
http://climateknowledge.org/figures/Rood_Climate_Change_AOSS480_Documents/Kiehl_Trenberth_Radiative_Balance_BAMS_1997.pdf
From global annual rainfall figures, the amount of energy lost from the surface via evaporation is calculated to be about 80 watts per square metre.
The global average for downward LWIR is around 325 watts per square metre, so only a small proportion of this powers evaporation (without even taking the solar input into consideration).
I’m not talking about global average calculations, I’m talking about calculations at the big source of evaporation (equatorial Pacific).
MikeB, you said:
because the amount of evaporation possible is limited by the humidity level allowed for the ocean temperature.
I’m afraid, as a layman, I cannot understand that. Temperature is merely equivalent to kinetic energy on molecular level. When water molecules get highly agitated by absorbing infrared energy, and collisions with other molecules, they may gain enough kinetic energy to break the surface and leave the water, evaporating. Thus leaving behind less energetic molecules, “cooling” the surface. This process is not depending on the humidity level above the water surface. It just happens as is. However, there is a dynamic equilibrium
http://preparatorychemistry.com/Bishop_Study_Guide_14.pdf
If more water molecules escape into the air, other water molecules will cling together, releasing the latent energy by condenstation and fall back into the water; that’s dynamic equilibrium. However for every gram of water evaporizing some 2500 joules of latent energy is removed from the water, and the returning condensing water in equilibrium release 2500 joules per gram into the atmosphere by doing so.
So what more infrared effectivily does, much more than heating the water, is heating the boundary layer above the water by this latent energy conveyor belt of dynamic equilibrium. This in turn, induces convection in the atmosphere that moves the energy and water vapor to higher levels, where the energy can be radiated out to space much more effectivily.
fhhaynie – global rainfall is missleading indicator of rate of energy transfer. –
I didn’t mention rate, I wrote total amount of energy per year. The only variable is how high the rain fell from.
Wow, what a stunningly inaccurate comment. Assumes that there are no differences between rainfall falling on land and oceans, that longwave infrared is the only energy which evaporates water, assumes that water evaporated from land in the same manner as it is over oceans, assumes no transpiration from plants and animals, assumes none of the energy is used to generate winds and other energy transfers etc etc. Talk about missing steps!!!
AP Wow, what a stunningly inaccurate comment. Assumes that there are no differences between rainfall falling on land and oceans
There’s nothing “inaccurate” about recognition of a total and the ONLY assumption being made is the accuracy of the estimate itself. All the other things you wrote are subterfuge to that point, heat energy is heat energy. My remark ONLY concerned the TOTAL.
Mike B,
You would do well to read here at WUWT and leave the keyboard alone. You appear silly.
There is a major problem with DWLWIR and the oceans.
About 60% of DWLWIR is absorbed within just 3 microns of the oceans. It is likely that even more than this is absorbed in the first 3 microns due to the omni-directional nature of DWLWIR such that a considerble proportion of all downwelling LWIR is striking the oceans at a grazing angle of say 25deg or less so you can imaging the effect of this on vertical penetration.
I think that Nick Stokes in response to one of my comments once did a calculation based upon the average DWLWIR figure used by K&T and worked out how much energy is absorbed in the first 3 microns. It is sufficient to drive some 15 metres of annual rainfall!
So the issue here is if DWLWIR has sensible energy capable of performing sensible work, how can that energy be dissipated to depth (and thereby diluted) at a rate quicker than the rate that DWLWIR is being absorbed in the top 3 microns?
It cannot be by conduction because at the very top of the oceans the energy flux is upwards (the very top being cooler than the top millimeters below) and energy cannot swim against the flux.
It is unlikely to be by the action of wind, waves or ocean overturning since those are all slow mechanical processes. Ocean overturning may be a diurnal phenomena, and consider the position where prevailing conditions are say BF3 or below with little wind and little wave action.
I have asked Willis many times to explain how DWLWIR effectively heats the oceans, how it can cause dew to evaporate, how it works in crater lakes (where there is little in the way of wind due to the topography of the location). Willis merely argues that the oceans would freeze but for DWLWIR relying upon the gross energy flows, but such argument is circuitous.
We are very fortunate that the absorption charcteristics of solar is so different with that being absorbed predominantly in say the top metre (but some going to penetrate well below 10 metres).
The upshot is that according to K&T solar impacting the oceans is only about half that of DWLWIR. The energy from Solar is absorbed in a volume approximately million times greater than that in which DWLWIR is absorbed. This allows Solar to gently heat the oceans.
As for DWLWIR, who knows, but there certainly is fundamental problems with it and the notion that it adds anything to ocean temperatures.
Richard Verney:
Aren’t the important (for anthropogenicity) parameters to be found between atmospheric levels of 280 ppm CO2 and further Beer Lambert Law-neutered CO2 levels of 400 ppm, both on a background of 40,000 ppm water vapor (in the tropics)?
re: “No, I do not think that the current plateau in the warming is caused by “missing heat” hiding in the ocean. ”
Even if it were, the question would then be why there seems to be a unjustified leap of faith among warmists that it will sometime soon stop hiding there and impact the atmosphere instead.
Doesn’t the formation of sea ice play a big part in the thermohaline circulation? Would the increase in antarctic sea ice also increase the high-salt water dropping to the ocean bottom, and possibly taking warmer water with it?
Yes, high saline concentrations are denser than fresh water. It is the freezing at the surface (separating out the salt) that gets the flow going.
If the water doesn’t freeze the relatively warm surface water would just pool at the poles and the current would stop.
This is why ice extent can be a proxy for temperature.
No it would not. If it did, you have demonstrated that their is no need to dam rivers just run a salt water hose into them. The currents are driven by the earths rotation, wind and then only at the polar terminal boundaries by density and temperature
Grey, obviously density is something you don’t understand very well? Yes the wind and earths rotation drive the surface layers but they do nothing to mix the ocean layers.
It so happens though that the Atmosphere is the big heat pump, not the ocean. The ocean (water vapor) is just the power source for the atmosphere.
Hurricanes, particularly large ones, can and do cause significant mixing of surface layers in the oceans.
Also very interesting, but have not read much about it recently, so called Abyssal “Storms” on the ocean bottom.
Not sure if the sinking water at the poles could be warmer than it is — it has to cool to just about 0C (and be salty) to be dense enough to sink in the near-freezing polar waters. This is evident by the temp of the deepest water (4C) — the slightly above freezing temp due to Willis’ geothermal heating over time.
IOW, if it doesn’t cool to just above freezing, it won’t sink, unless those vast polar waters are somehow no longer near freezing. Presumably, if the polar waters warm a bit, the sinking may simply occur further poleward than it does now.
The ~4C abyssal ocean water is that temp because that is the maximum density of seawater. Colder is less dense. As is warmer. Nothing to do with geothermal heat input.
In the illustration above (vukcevic May 16, 2015 at 1:49 am) a little known current
North Icelandic Jet is shown. It is thought to be the ocean’s densest water (σθ more than 28 kg/m3).
Not for seawater:
http://www.ewoce.org/gallery/index.html
Could you be more specific? The link you provided contains hundreds of sub-links.
Sir, you seem to not be taking into account that the density curve changes as the salinity does:
http://linkingweatherandclimate.com/ocean/figs/density2.png
Vuk:
– Could you be more specific? The link you provided contains hundreds of sub-links.
Yes, you can see many ocean temperature profiles from different places. In everyone, the bottom water is far below 4ºC.
Do you ever read anything that is posted here ??
Up above, we have been treated to the most informative dissertations on the freezing of water and salinity, that I have ever seen,
None of the plotted data above has ever been seen by me before, and I have known for many decades, that sea water is not maximally dense at 4deg C.
Why don’t you go back and read this whole thread again, and learn something , that some fellow visitors dug out of oblivion for us.
@Willis
Your tthermosyphon syphon is a gravity-driven system, where the colder water running downhill creates a pressure difference and pushes the warmer water up and closing the circuit:
But that’s not how thermo-haline circulation (THC) works, is it? It’s not a matter of cold-water running “downhill”, but rather cold-water spreading to replace warmer water pushed north by other mechanisms such as wind-driven currents (e.g. Gulf Stream)
http://www.fisica.edu.uy/~barreiro/papers/Rahmstorf_EQS_2006.pdf
So I think you may have created a beautiful, simple but incorrect gravity-driven explanation of THC
The one thing we never seem to get from our warmista friends is any form of mechanism which can stop the heat showing in the atmosphere, then bypass the troposphere and the upper 700m of Ocean, to appear in the deep ocean. Conversely they never provide any mechanism by which this process will reverse. Is it magic perhaps? Does physical reality suddenly change at the turn of each century? Or is it the reduced solar output? Did God get out of bed on the wrong side and decide to heat the deep ocean not the air just to make the humans look stupid?
The High Priests of the CAGW religion have been praying hard for that one to be true, Ivor.
The CO2-based CAGW Model Prayer
Our CO2 which art in heaven,
Carbon be Thy name.
Thy Heat shall come,
our world undone
on land and sea and air.
Give us this day our Daily Grant,
and banish all doubts from the 3% that doubt against us.
And lead us all to a One World Government,
but deliver us from The Pause.
For Thou art the Magic Gas of Everything forever and ever.
Amen
In terms of “green” energy, various types of wind and solar are useful for the end-user but not for a power grid. A small wind turbine or solar panels or solar water-heating can decrease a household’s power drain from the grid and may even donate energy to the grid, but it cannot be a major part of a grid.
YOU CANNOT MAKE A RELIABLE ENERGY SUPPLY FROMUNRELIABCLE ENRGY SOURCES.
that has not stopped our legislators in Vermont from decreeing that 75% of all energy must come from renewables by 2032 (not 2031 or 2035) of course they didn’t make it in math and were streamed into social studies and astrology so they could succeed, avoid welfare, and enter politics …
You’d better have lots of rivers suited for dams in Vermont.
If this is the mechanism that can “heat the ocean from the bottom up” and it runs at a speed of about 500 years per cycle, it doesn’t explain the “missing heat” from greenhouse gases suddenly deciding to go into the ocean depths in the 1990s and supposedly heating it measurably at the depths in just a couple decades. There hasn’t been enough time for anthropogenic global warming to have this effect throughout the oceans yet.
Incoming solar radiation is absorbed in the first 100 meters or so of the oceans. It thermally diffuses to greater depths. There are decades long time lags between 700 meter and 2000 m for which temperature gradients have been measured and heat contents calculated. With surface temperatures failing to rise for a few decades, the temperature of the oceans below 700 m will continue to rise from heat that arrived while surface temperatures were increasing. It is simple thermal lag that allows heat to continue to accumulate in the depths. Doesn’t matter. It will never return to the surface to bother us.
Pumps are cheap. Cheap to run. They are now quiet, They enable control.
But the popular GHE is radiative flux only, SWIR, LWIR, microwave, gasses only and does not include the powerful effects of water vapor, latent heat of evaporation, 970 Btu/lb. Even the sensible heat of water, 1 BTU/lb-F, is 4 times the heat capacity of air at 0.24 Btu/lb-F.
http://www.writerbeat.com/articles/3713-CO2-Feedback-Loop
Was hoping the geothermal thing would be developed a bit more. Seemingly neutral to the convective/thermosiphon thing as no obvious relation to latitude. Back of envelope surprisingly high.

The ultimate reason the polar oceans (and thence the entire ocean) cannot be warmed as you suggest is that the polar oceans remain much warmer than the polar atmosphere.
Absolute (not anomaly) data is hard to find but with Bob Tisdale’s help in finding these worldwide series allow direct comparison of absolute ocean and atmospheric temperature from Jan. 2000 to present. After converting from Kelvin it is clear that in the polar bottom water formation areas the ocean was 4-5 degrees warmer than the atmosphere in Jan. 2000.
I have compared each month in the series and there is not one single instance anywhere on earth where the ocean is not warmer than the atmosphere on an average monthly basis.
I think your comment has one of the keys to demolishing the argument, however I spot a flaw with the diagram. Isn’t 0C = 273K?
According to the temperature chart, my home was roughly 0C in Jan 2000. Seems wrong. I don’t think brightness temperature is thermometer temperature.
“The brightness temperature is a measurement of the radiance of the microwave radiation traveling upward from the top of the atmosphere to the satellite, expressed in units of the temperature of an equivalent black body.”
Wow, this from the RSS site:
“Each product measures the mean temperature of the atmosphere in the thick layer.”
So I think the “brightness” defines an actual temperature, trouble is, NOBODY will tell you the altitude of the top of TLT nor at what altitude the mean might actually be measured.
In an abundance of caution working from memory I understated the difference between ocean surface and TLT in the bottomwater formation areas. It is actually 12 deg C, so at a lapse rate of 2 deg C/1000′ the argument holds to a mean TLT measured at 6000′.
It makes nothing but sense that the atmosphere be colder in the bottom water formation areas because these are most often at the edge of the ice and benefiting from the extra density of brine rejection. Why else would the water be freezing?
Anyway, no way to be sure without more information, and thanks for pointing this out.
“The heat is hiding in the deep ocean” is a nice bit to speculate and chew on but how do you prove it? The laws of gravity are proven due to their predictable nature allowing us to get a probe to Mars.
The heat in the ocean hypothesis was after the fact not predicted. In solving crimes, the prosecutor develops theories of how and who committed the crime, the prosecutor does not predict crimes but explains them after the fact. Science is used in that way but science does not progress in that way. If a hypothesis is not predictable, it is not provable or falsifiable and therefore invalid.
Now we have a prediction; it is claimed warming will continue when the oceans release the stored heat. Except it is not testable. Even if a warming trend continued after this flat temperature period, there is no way to prove it was heat released from the oceans and not due to another reason. It is too generalized to be provable. If a cooling trend occurred it is just as facile to claim the oceans are absorbing heat at a greater rate.
Creating new explanations in response to new observations and discoveries is how science works. The issue in climate science is they have yet come up with a predictable explanation of anything. Evolution predicts what archaeologists will find, and evolutionary predictions have consistently been proven true. Climate science behaves like crime fighters who explain observations and events after the fact, not like scientists whose explanations predict observations. In a word, it’s lame science.
Thermal incline.
At 4° water is is dense as it gets.
Blow 4° it rises.
Look at the 4° ice-so line over time
You may find an answer
In the Arctic, the freezing surface concentrates salts in the water below it. That process increases the density much more than changing temperature. That denser water sinks. The process is likely to be the pump action that contributes to the deep ocean “convayor belt”.
Yes, that’s an important factor that Willis left out.
Water reject almost all the salt as it freezes That water sinks drawing more warmer water from the Atlantic. That sounds like yet another negative feedback in the system.
4 is as dense as fresh water gets. Salt water gets much denser. So do some people, apparently.
Add wind, spotty ocean floor heating (very hot to virtually none from place to place), evaporative cooling, clouds, land mass blockages and fresh water additions in various places at various times and one has a very complex system which may behave nothing like what one might expect from the broad generalities expected based upon the averages of the various data. In a word, chaotic. Not to diminish the fact that oceans on a 70% water world are, undoubtedly, major determinants of climate, which, not surprisingly, is also chaotic. They have the ability to accumulate and disseminate heat over long periods of time in a very indeterminate manner.
Is the efficiency of a thermosiphon enhanced by greater temperature differentials between the hot and cold circuits? If so, wouldn’t a warming of seawater at or near the poles reduce the temperature differential between them and the Equator and thus the circulation rate? Additionally, would warmer seawater at the poles have a reduced salinity (due to expansion?), and thus a reduced salinity differential as well, thereby also slightly offsetting the thermohaline circulation? (Of course, increased evaporation may offset any reduced salinity, but it seems likely that Equatorial regions would experience at least some increased evaporation as well.) Moreover, with a turnover rate of 500 years is it likely we’d be seeing an increase, however minuscule, of deep ocean temperatures this early in the game, especially in the presence of confounding factors?
Willis,
Your use of the language is different than mine.
“the water that is descending at the poles is slightly warmer than
in the past”
“the oceans would warm from the bottom up”
In this sequence, isn’t the surface warming first? How do I misunderstand?
When does the water sink? When it’s denser than the water below. When it reaches 4 C it will be, because that’s the temperature at which the density of water is an absolute maximum. So it’s at least plausible that water will always sink at the same temperature, or perhaps less absolutely, the sinking temperature might be stabilized by this factor. However, it might sink at a different place if cooling is slower.
That latter idea seems consistent with what’s been happening in the arctic, where the position of recent decades ice loss appears generally consistent with the gulf stream currents getting further north, possibly as it cools slower in warmer temperatures.
R.
Nice simplified illustrations, Willis. As to the hiatus/pause, the number of papers attempting to explain it away now exceeds fifty according to Anthony. They each do it in their own way and obviously they can’t all be right. I love the ones that are looking for the lost heat in the ocean bottom. Don’t be surprised if despite what you said they will start quoting you out of context on that. The existence of the hiatus is what is being questioned but these people don’t even know about the other hiatus in the eighties and nineties that is being covered up by fake warming. It lasted 18 years, same as the current hiatus today. I discovered this when doing research for my book and it is shown as figure 15 in it. The false warming covering it up has been called “late twentieth century warming” and is one of the mainstays of their global warming argument. I don’t have good data on the fifties and sixties but there is a possibility of yet another hiatus from the fifties to the seventies. If we accept this it would make the entire global temperature curve from the fifties till the present a series of three hiatus platforms where no warming takes place. They would be connected with one another by short step warming periods that raise the global temperature just before a new hiatus begins. The last such step warming started in 1999, raised global temperature by a third of a degree Celsius, and then stopped in 2002. That established the temperature level for the current hiatus platform. This, and not an imaginary greenhouse effect is what makes twenty-first century temperatures higher than the twentieth was. The previous step warming connection would have been about 1976 and would have raised global temperature to the level of the eighties and nineties hiatus/pause. But that is not the global temperature history we are taught by the wise men of IPCC. Goes to show that high pay for climate “scientists” results in low quality temperature history.
The Arctic does not have a land mass underlying it like the Antarctic does. It would seem that this is another major factor that determines water circulation and may partially explain why the Antarctic stores more water (as ice) than the Arctic.
The south pole is around 2,700 meters (~9,000 ft) above sea level whereas the North pole is around 0 metres (0 ft) above sea level.
Willis,
You do an excellent job of provoking thought and discussion with your seemingly simple forays of musings. Please keep it up, generally. 😉
Rert,
“When does the water sink? When it’s denser than the water below.”
So when does the water near the bottom rise? When it is less dense than the water above. So the water both rises and sinks in the same place. That is convective mixing. A difference in density between the top and bottom does not produce a net flow in either direction.
There is a net flow downward when water is flowing towards the area at the surface and away at depth. It is the horizontal flows that cause the vertical flows. There are two factors that contribute: winds push around the water at the surface and pressure differences (due to difference in average density in the water column) push around the water at depth. The wind is the larger effect; which is why oceanographers usually do not use the term thermohaline circulation. They call it the meridional overturning circulation or just MOC.
“When it reaches 4 C it will be, because that’s the temperature at which the density of water is an absolute maximum”.
That is the case for pure water. It is irrelevant for the ocean. The density of seawater decreases as temperature rises for all temperatures.
Not so fast Mike, the curve changes, but there is still a “hump”, above the freezing point.
See charts I posted above.
Thanks both for this, both make sense. The effect of salinity changes on density profile was news to me,
The point I was floundering to make properly was that there is no guarantee that the descending water in an ocean circulation will be warmer, just because surface conditions are warmer. The pattern of circulation might compensate, and that seems consistent with the sea ice movements in the Arctic, particularly around the NE extreme of the Gulf stream.
So though I think Willis’s post is a very interesting insight, my comment was that it is (obviously) much more complicated than that.
Willis wrote” “Well, if the water that is descending at the poles is slightly warmer than in the past, then there will be less cold water added to the bottom of the ocean. With less cold water added to the bottom, on average the ocean depths would warm slightly compared to the past … and the interesting point is, the ocean would warm from the bottom up.”
I find this confusing and it seems like others do too. I think a better description is the following.
Over most of the planet, the surface ocean is much warmer than the deep ocean. So there must be a flow of heat from the surface to the deep. Since the deep ocean stays cold, there must be a return flow of heat to the surface. That can only happen in places where the surface is colder than the deep. If you now warm the surface water slightly in those places, there is less return of heat to the surface. So the deep will start getting warmer. Then the flow of heat down over most of the ocean starts to decrease, and the flow up in the cold surface regions starts to increase until a new balance is established.
I’m just really glad ice floats or we wouldn’t even be here.
The ocean floor is about a half mile thick, or thin, depending on your point of view. Not much heat resistance between the hot core and deep ocean.
Nickreality,
I think you need to brush up on oceanic crust. It is generally less than 10 km, but not uniform, and as thin as you say is rare.
Oceanic Crust:
“Composition[edit]
Although a complete section of oceanic crust has not yet been drilled, geologists have several pieces of evidence that help them understand the ocean floor. Estimations of composition are based on analyses of ophiolites (sections of oceanic crust that are preserved on the continents), comparisons of the seismic structure of the oceanic crust with laboratory determinations of seismic velocities in known rock types, and samples recovered from the ocean floor by submersibles, dredging (especially from ridge crests and fracture zones) and drilling. Oceanic crust is significantly simpler than continental crust and generally can be divided in three layers.
Layer 1 is on an average 0.4 km thick. It consists of unconsolidated or semiconsolidated sediments, usually thin or even not present near the mid-ocean ridges but thickens farther away from the ridge. Near the continental margins sediment is terrigenous, meaning derived from the land, unlike deep sea sediments which are made of tiny shells of marine organisms, usually calcareous and siliceous, or it can be made of volcanic ash and terrigenous sediments transported by turbidity currents.[3]
Layer 2 could be divided into two parts: layer 2A – 0.5 km thick uppermost volcanic layer of glassy to finely crystalline basalt usually in the form of pillow basalt, and layer 2B – 1.5 km thick layer composed of diabase dikes.
Layer 3 is formed by slow cooling of magma beneath the surface and consists of coarse grained gabbros and cumulate ultramafic rocks. It constitutes over two-thirds of oceanic crust volume with almost 5 km thickness.”
http://en.wikipedia.org/wiki/Oceanic_crust
Check, just a little exaggeration. 7.5 km +/-. The continental crust ranges from 30 to 50 km (3,200 ft/km +/-). Guess which crust will transfer the most heat easiest and fastest. My point is that ocean floor geothermal heat flux could be pumping gazillion Btu’s into the ocean, venting CO2, raising the temperature slightly (that’s a lot of water at 1 Btu/lb-F) and outgassing CO2. IPCC admits not knowing below 2,000 meters which is half the ocean. Pretty extensive “Don’t know!”
Yes, I hear you.
It has been opined that we know more about the surface of the moon than we do about the deep ocean.
I was going to comment on this part of the discussion, but it seems that everyone has left.
The gist of the point I was going to make is just that some of the commenters seems to overlook a few things in some of their comments. No big deal, we all do, and this stuff gets complicated, so a lot to keep in mind.
One is that thermal stratification of water is inherently very stable, as the cold water is more dense, and so can only rise is either heat is added, of if colder water descends and forces some of the bottom water to rise.
From what I have been able to determine over the past few days, it seems that the upward leg of the thermohaline circulation is thought to be much more diffuse than the places where very dense water forms and descends to depth. It seems there may be no upwards currents, just a very spread out upward movement over large areas as new dense water descends.
Also overlooked is that parcels of water with a certain temp and density will resist mixing with parcels that have different characteristics.
Regarding heat from the earth warming the bottom water, it seems to me we need to get some good data on the rate of thermal flux from below, and how much of an effect this has.
I wonder if the rocks and sediment at the bottom may still be warming up after being cooled by the ice age ocean for tens of thousands of years.
Consider that even a small pond retains winter cold water at the bottom all Summer, and even after the bottom water warms, the sediment and rock underneath the bottom water will only then begin to warm.
Where is the no-return valve in the oceans? So why doesn’t the heat flow reverse towards the equator, equalising in and out heat transfer? So why should the oceans heat up?
Ken Mourin
Is it true that as the area of Antarctic sea ice grows the area of under ice melting, increasing the volume of cold dense South Polar water that flows northward? This increased cold water volume must have an effect on climate over time in noth north and south hemisphere. Or, so it seems logical to me.
Willis, there is a substantial difference between the systems shown in Fig. 1. &. 3. respectively. In the thermosyphon the solar collector is at a lower elevation than the water tank. On the other hand, in the climate system sea surface is at the same gravitational potential at both tropics and poles. Therefore in the latter case no thermodynamically driven overturning is possible. Or, because solar radiation penetrates into the ocean a bit, it would be a very shallow overturning cell, not at all akin to the complete in-depth overturning observed. True, geothermal heating happens at the bottom, but that only accounts for a negligible fraction of fluxes measured in meridional overturning circulation.
The reason MOC still works is the complete lack of cost effectiveness in its design. Unlike in case of a thermosyphon, it does require a pump to run it. The pump is called deep turbulent mixing and it is a process driven by pure mechanical energy input, that is, energy at a low entropy, which has nothing to do with thermodynamics. It is provided by winds and tides. They generate internal waves in the bulk of oceans, which eventually break at specific sites like rugged bottom features or continental margins of complex geometry, resulting in vigorous turbulence and mixing different oceanic layers together. In doing so it replenishes buoyancy to the abyss by mixing some warmer/fresher water downward.
That’s a critical step. Without it eventually the abyss would get saturated with dense, cold water of high salinity and downwelling in polar regions would grind to a crawl. Neither geothermal heat flux acting at the bottom is powerful enough to change that, nor thermal conductivity of seawater is high enough to transport heat downwards anywhere close to the required rate. And diffusivity of salts is even less than that, by some two orders of magnitude.
In a world with neither tides nor winds, the deep ocean is locked in a stale state and becomes anoxic eventually, with next to no overturning whatsoever. The mechanical energy input driving deep turbulent mixing may be minuscule compared to the huge amount of heat moved around, still, it is indispensable to keep the engine running.
Even rate of vertical turbulent mixing is measured to be far too small over much of the open ocean to do the job, it really happens at poorly known specific sites, intermittently, when this rate increases by several orders of magnitude locally, for a while. And no, there is no such thing as “upwelling”, ever. There is no power on Earth that could raise a dense, cold, salty, undisturbed water parcel to the surface against the gravitational gradient.
The energy needed to run this pump is provided by tides and winds on a roughly fifty-fifty bases. One needs a full exeligmos (54 years &. 33 days) to have a similar tidal configuration over any specific region and winds clearly have an annual cycle, so the pump does run in a somewhat haphazard way. By the way, most of vertical turbulent mixing (about 80% of it) happens in the Southern ocean, so that’s the engine behind the Great Conveyor Belt, not temperature or salinity differences.
Seawater is a curious substance, it is very different from freshwater indeed. As everyone knows density of pure water is highest around 4 degrees centigrade, that is, well above freezing. That’s why overturning happens during the fall and spring seasons in lakes at temperate regions and stops in the winter, because liquid water next to the water/ice interface is too light to go down. However, seawater is not like that in this respect. At high enough salinity density of water is highest just above freezing, so a water parcel only has a chance to go down if it is close to the ice/water interface, which holds for the entire ocean except for some brackish pockets and marginal seas. It is exacerbated by the phenomenon of brine exclusion, that is, as ice crystals do not like salts very much, upon partial freezing the salinity of the liquid phase increases and with that its density as well.
Now, the pump may operate far away from the poles, but as it makes room in the abyss for dense parcels to sink by replenishing buoyancy there, the sites where actual downwelling would occur are those where density of seawater is highest. According to the previous paragraph, it is somewhere along the ice margin. The density is influenced by two factors, temperature and salinity.
In the downwelling region surface temperature is determined by the physical properties of water, not climate, at least until the ice margin is gone entirely, which, under the current configuration of continents, is not projected even by the wildest cAGW scenarios. It is the exact location of the ice margin, which is influenced by climate, but that’s another topic.
However, that’s only true at atmospheric pressure, that is, close to the surface. With increasing pressure freezing point of seawater decreases. If the ice/water interface goes down to some depth, as it does around Antarctica, but not in the Arctic, temperature of liquid water next to the ice can decrease well below its freezing point on the surface, and with that increases its density. Therefore water parcels of the highest density are formed in a different way around the two poles. Salinity of Antarctic bottom water is somewhat less than that of North Atlantic deep water, but its temperature is lower and with that its density is a bit higher. When the two water masses meet at the bottom somewhere around the equator, Norh atlantic deep water forms a layer above Antarctic bottom water.
In another geologic epoch, when polar regions were covered by open ocean and there was no ice/water interface whatsoever, deep water formation worked in an entirely different way. Then water masses of the highest salinity went down at the horse latitudes, where evaporation exceeds precipitation by a wide margin. It made abyssal temperatures much higher, around 20 centigrade, as opposed to 3 today. It still happens in marginal seas like the Red sea or restricted areas of the Caribbean, but it is not a dominant process and can only form intermediate water masses.
In some other marginal seas, like the Black sea, there is an ice margin in the winter at the Northern edge, but surface waters are much too brackish to sink into the salt water below, which forms a stable anoxic mass, with much dissolved methane and highly toxic hydrogen sulfide in it.
The upshot is temperature, and with it heat content of deep waters is not regulated by climate, but by physical properties of seawater and configuration of continents. Heat content can only increase, if salinity also increases with it, which means an increasing ratio of North Atlantic deep water relative to Antarctic bottom water. I do not think this is what’s happening.
All those words and he has never heard of the earths rotation!!!!!!!!!!!!!!!!!!!
Berenyi, I think you may underestimate the pumping capacity of the Antarctic vortex. This whirling dervish accelerates both air and water north of it from at least the troposphere to the abyssal ocean. It is this pump that renders what would otherwise be meridional overturning a truely global thermohaline circulation.
Your points that thermal/density are wildly insufficient to drive this circulation as a heat engine are well taken.
I love that we’re talking thermal and solar
If we are going to measure the Earth’s temperature, should it be measured in the overflow at the Denmark Straights gap? Isn’t everything else just weather.
It’s about time that readers of WUWT get wised up, that oceanic waters (containing salts) DOES NOT have it’s maximum density at 4 deg. C
The data is readily available on line the average ocean salinity is about three times as much as the salinity needed to guarantee that sea water continues to increase in density right up to the point where it freezes, which is somewhat colder than zero deg. C
Pure fresh water has its maximum density at 4 deg C but salt water does not.
Sea water contains about 3-4% of dissolved salts.
For salt water containing more than about 1.5% (as I recall), the density increases right down to the freezing Temperature which is sub zero.
This drum has been beaten here at WUWT for years, as it is a key issue in climate effects and the freezing cycle of water.
So let’s not hear any more of this 4 deg. C stuff.
Yes it is the cause of the turnover of fresh water lakes, but it doesn’t turn over the ocean.
Mr. Smith,
Got any references to back that up?
I have only recently begun to look at this closely, but it seems to not be just as you describe either:
http://linkingweatherandclimate.com/ocean/figs/density2.png
Mr. Smith,
Never mind that last question. I see that you are correct. I was misreading what the chart seemed to be indicating.
24 pcu seems to be the critical level, and sea water is usually 35 or more pcu.
Good on ya!
Here is a simple graph that sums it nicely:
http://www.fondriest.com/environmental-measurements/wp-content/uploads/2014/01/360x300xwatertemp_salinity.jpg.pagespeed.ic.yheIMC6nSN.jpg
The heat capacity of the ocean is 1000 times that of the atmosphere. If we have lost, say 1 degree C of warming into the oceans, we can only expect the ocean to warm by 0.001 C. It seems to me that if the heat is getting lost in the ocean we do not have to worry about it, the global warming “problem” is solved.
I had thought that if some people are saying that all the missing heat is going into the oceans, what that means is that some people are running out of excuses.
I’d go for 4 times, Water at 1 Btu/lb-F opposed to air at 0.24 Btu/lb – F. The latent heat of evaporation/condensation is worth 950 to 1,000 Btu/lb depending on some details.
nickreality65 – we are talking about separate things. You are comparing heat capacity of a lb of air to a lb of water. I am comparing heat capacity of the ocean to heat capacity of the atmosphere.
Interesting model of Earth, Willis. And here I thought I was the one who developed it. However, there is a significant complication. The water at the equator is moving with the earth at just over a thousand miles per hour, while the water at the poles is just about stationary. The acceleration and deceleration going to and fro (coriolis anyone?) can significantly complicate the flow pattern. I can’t say any more about it, since I haven’t figured out how to put it in my model, yet.
The data from the BP Gulf of Mexico oil spill also messed me up. I was blindly assuming that the Gulf, with a relatively small percentage of it’s perimeter open only near the equator, would have a quite warm bottom. Yet it was barely a couple of degrees above freezing. Now, with a surface heated by the sun, and a bottom heated, just a bit, by heat flow up from the earth, where did the cold water come from? Obviously, the poles. The only question for a simple model, which now must include coriolis accelerations and a cold bottom in the Gulf of Mexico, is “how does this work?”
Tom, recall that the efforts to cap the Macondo well blowout were severely hampered by the cold temperatures, and the tendency for methane clathrates to form spontaneously. They plague deep water oil and gas operations, clogging pipelines and just making it very hard to work at times.
Many of the efforts to divert the escaping oils and gas into hoods and pipes was prevented from working due to the very cold water causing clathrates to form.
Buck Smith
“I am comparing heat capacity of the ocean to heat capacity of the atmosphere.”
Let’s try this approach.
According to IPCC AR5 between 1750 & 2011 (261 years) the 112.5 (278 to 390.5) ppm increase in CO2 added about 2 W/m^2 of radiative forcing, tilting the atmospheric energy balance, warming the atmosphere, oceans, etc.
A watt is a power unit, 3.412 Btu/h, 3.600 kJ/h. Use English hours w/ Btu and metric hours w/ kJ. ToA spherical surface area is 5.13E14 sq km. 8,760 hours per year. An additional annual heat load of 3.06E19 Btu/y.
This annual heat load absorbed by the 5.14E18 kg (1.13E19 lb) atmosphere, sensible heat capacity of 0.24 Btu/lb – °F, would raise the atmospheric temperature by 11.3 °F. Can’t say that such has actually happened.
This annual heat load absorbed by the 1.4E21 kg oceans (3.09E21 lb), sensible heat capacity of 1.0 Btu/lb – °F, would raise the ocean temperature by 0.010 °F. This would be hard to measure.
i.e. a factor of 1,136.
This annual heat load absorbed by the 1.4E21 kg oceans (3.09E21 lb), latent heat capacity of 970 Btu/lb, would require the evaporation of 10.24 ppm of the ocean’s mass. No change in temperature. A 13% increase in atmospheric water vapor.
More clouds, more albedo, less heat, lower temperatures. The ocean/atmospheric water vapor cycle as an organic thermostat w/ a bazillion years of proven success.
As many have stated , no one way valve is needed in the initial solar water heating diagram . Reverse flow does not occur unless the collector is above the storage tank , necessitating a pump and a non-return valve . Moreover the “advance” cold pipe can be teeed into the cold supply close to the tank and the “hot return” pipe can be teeed into the hot water draw-off pipe close to the tank and the system will still work . This enables a conventional tank to be employed that has no special “solar” connections .I have done this and it works fine .