Guest Post by Willis Eschenbach
There is a more global restatement of Murphy’s Law which says “Nature always sides with the hidden flaw”. Parasitic losses are an example of that law at work.
In any heat engine, either natural or manmade, there are what are called “parasitic losses”. These are losses that tend to reduce the temperature differentials in the heat engine, and thus reduce the overall efficiency of the engine. In general, as a percentage parasitic losses increase rapidly with ∆T, the temperature differences in the engine. In the climate system, two main parasitic losses are the losses from the surface to the atmosphere by way of conduction and convection (sensible heat), and the losses from surface to atmosphere by way of evaporation and transpiration (latent heat). Both of these parasitic losses act to reduce the surface temperature with respect to the overlying atmosphere, by simultaneously cooling the surface and warming the atmosphere … nature siding with the hidden flaw to reduce the overall system efficiency. So I decided to see what the CERES data says about parasitic losses. Figure 1 shows the parasitic losses (the sum of sensible and latent heat losses), as a percentage of the total surface input (downwelling longwave plus shortwave).
 Figure 1. Parasitic losses (latent and sensible heat loss) from the surface to the atmosphere. Percentage of parasitic loss is calculated as the sum of sensible and latent loss, divided by the total surface input (downwelling shortwave plus downwelling longwave).
Figure 1. Parasitic losses (latent and sensible heat loss) from the surface to the atmosphere. Percentage of parasitic loss is calculated as the sum of sensible and latent loss, divided by the total surface input (downwelling shortwave plus downwelling longwave).
I was most interested in how much the parasitic loss changes when the total surface input increases. Figures 2 to 4 shows that situation:


 Figures 2-4. Scatterplots, parasitic loss in watts per square metre (W/m2) versus total surface input (W/m2). Parasitic loss is loss as sensible and latent heat. Gold line shows the loess smooth of the data. Red dots show land gridcells, which are one degree square (1°x1°) in size. Blue dots show ocean gridcells.
Figures 2-4. Scatterplots, parasitic loss in watts per square metre (W/m2) versus total surface input (W/m2). Parasitic loss is loss as sensible and latent heat. Gold line shows the loess smooth of the data. Red dots show land gridcells, which are one degree square (1°x1°) in size. Blue dots show ocean gridcells.
I was very encouraged by finding this result. I’ve written before about how at the warm end of the spectrum, parasitic losses would increase to the point where most of each new additional watt striking the surface would be lost as sensible and latent heat, and that little of it would remain to warm the surface. These graphs bear that out entirely. Here’s why.
The slope of the gold line above is the rate of increase in parasitic loss for each additional degree of warming. As you can see, the slope of the line increases from left to right, although the rate of increase goes up and down.
In order to understand the changes, I took the slope (change in parasitic loss divided by the corresponding change in surface input) at each point along the length of the gold line for both the land and the ocean separately. Figure 5 shows that result.
 Figure 5. Change in parasitic loss (in W/m2) for each additional W/m2 of surface input. “Wobbles”, the looped parts in the two graphed lines reflect subtle changes in the loess smooth, and can be ignored.
Figure 5. Change in parasitic loss (in W/m2) for each additional W/m2 of surface input. “Wobbles”, the looped parts in the two graphed lines reflect subtle changes in the loess smooth, and can be ignored.
Now, what are we looking at here? Well, this is how the parasitic loss changes as more and more energy is input to the surface. Where there is little surface input, the loss is low. In fact, at the South Pole the situation is reversed, and the net flow of energy is from the atmosphere to the surface. This is the result of huge amounts of energy being imported from the tropics.
The key point, however, is that as we add more and more energy to a given gridcell the amount of parasitic losses rises, in perfect accordance with nature siding with the hidden flaw. And at the right hand end of the scale, the warmest end, for every additional watt that is added, you lose a watt …
Is this relationship shown in Figure 5 entirely accurate? Of course not, the vagaries of the smoothing process guarantee that it isn’t a precise measure.
But it clearly establishes what I’ve been saying for a while, which is that parasitic loss is a function of temperature, and that at the top end of the scale, the marginal losses are quite large, close to 100%.
Now, as you can see, nowhere is the parasitic loss more than about 30% … but the important finding is that the marginal loss, the loss due to each additional watt of energy gain, is around 100% at the warm end of the planet. Here is the parasitic loss for the planet as a whole versus total surface input as shown in Figure 2:
 Figure 6. Change in parasitic loss (in W/m2) for each additional W/m2 of surface input, as in Figure 5, but for the planet as a whole.Change in parasitic loss (in W/m2) for each additional W/m2 of surface input. “Wobbles”, the looped parts in the two graphed lines reflect subtle changes in the loess smooth, and can be ignored.
Figure 6. Change in parasitic loss (in W/m2) for each additional W/m2 of surface input, as in Figure 5, but for the planet as a whole.Change in parasitic loss (in W/m2) for each additional W/m2 of surface input. “Wobbles”, the looped parts in the two graphed lines reflect subtle changes in the loess smooth, and can be ignored.
Note also that across the main part of the range, which is to say in most of the planet except the tropics and poles, about half of each additional watt of energy increase doesn’t warm the surface … it simply goes into parasitic loss that cools the surface and warms the atmosphere.
Best to all,
w.
PS—If you disagree with what I’ve said please quote my words. That lets all of us know just exactly what you disagree with …
It is high time someone put Willis up for an Hon. PhD.
Might be a problem finding an honest enough University Department, though.
I have to admit that when I first laid eyes on this post, and I saw the word, ‘parasitic,’ in the title, the first thought that came to my mind concerned the intrinsic nature of our Global Warming Warriors.
Perhaps, being parasites themselves, they are incapable of recognizing a parasitic action such as that that insures no heat engine is 100% efficient. They better recognize it soon or they just might succeed in killing their host – modern, affluent society.
Many thanks, Willis. You have a gift for asking the right question and visualizing the answers. Two questions: (1) is Fig 3 supposed to be both land and ocean? It looks like land only (with ocean shown on Fig 4). (2) why does this “diminishing heat gain/increasing parasitic loss remind me of the Stefan-Boltzmann equation? I know that is for radiative losses but it has the same (general) negative feedback relationship. Funny how Nature might do that, notwithstanding the dire prognostications and hand-waving of the alarmists.
Is this Trenberth’s missing heat?
I have been using the CERES data myself for some research lately, so I am trying to get to know more about it. Clearly the below top of atmosphere (TOA) fluxes are all calculated since the satellite can only “see” what is at the top. Others may not be aware the data below TOA is calculated using a radiative model. I always get a bit nervous using model output data rather than actual observations. There are always lots of comments on this forum from people trashing any kind of model output. I asked the folks at CERES for a brief outline of how the data is calculated and here is their kind reply,
“Briefly, we compute “shortwave (solar)” and “longwave (emitted by the earth)” separately by radiative transfer models. The atmosphere is divided into ~50 vertical layers. Spatial and temporal resolutions for the computation depend on data product. Spectral region is separated into smaller spectral regions and absorptions by water vapor, ozone, CO2, and other trace gases are treated with approximations. Scattering by aerosols and clouds are treated by a two-stream approximation. Most inputs come from observations, including temperature, humidity profiles, cloud and aerosol properties.”
I am not saying the computed data is not good, just that people should be aware of what is actually observed data and what is model output.
A second point, what data set(s) did you use for the latent and sensible heat? I may have overlooked some but I could not find any of this direct data in any of the CERES data sets.
Thanks.
Willis E said this “These are losses that tend to reduce the temperature differentials in the heat engine, and thus reduce the overall efficiency of the engine. ”
dS = dQ/T ; ie the 2nd Law of Thermodynamics
In order to reduce Entropy, then one must have an large heat transfer surface and an infinitesimally small difference in temperature across the heat transfer surface, maximizing reversibility. You are only relying on Carnot Efficiency as related to heat engines.
Thermal power generating plants can’t afford large heat transfer surfaces so they accept the entropic inefficiencies (irreversibility) due to large temperature differentials.
I doubt the atmosphere can be solely evaluated using a Carnot Efficiency. It is an immense heat transfer system.
MaxLD says:
March 26, 2014 at 11:48 am
Thanks, Max. I’ve pointed that fact out in a number of posts, that the CERES TOA data is observations, and the surface data is calculated from those observations. I have compared the CERES surface data (where possible) to observations and other forms of estimates and calculations, and they are quite close. So I use them, with caveats, as the best that we have.
You are correct, there is no direct calculation of parasitic loss in the CERES dataset. The parasitic loss is computed as the input to the surface less the radiative loss from the surface. There is an uncertainty in the measurement due to the import/export of warm water from a gridcell, but that appears to be minor in the context of this particular analysis.
Regards,
w.
hi Willis,
An interesting post as always. “Parasitic losses” are, as you say, (“These are losses that tend to reduce the temperature differentials in the heat engine, and thus reduce the overall efficiency of the engine. “) mechanisms by which heat goes from hot to cold without doing any useful work. Could you please clarify what you mean by “useful work” and “efficiency of the engine” in this context?
Thanks
this graph look like the sperm whale. %3Bhttp%253A%252F%252Fwww.dailymail.co.uk%252Fnews%252Farticle-2370470%252FHeart-warming-moment-lost-baby-sperm-whale-jumps-joy-5ft-waves-reunited-family.html%3B634%3B417
%3Bhttp%253A%252F%252Fwww.dailymail.co.uk%252Fnews%252Farticle-2370470%252FHeart-warming-moment-lost-baby-sperm-whale-jumps-joy-5ft-waves-reunited-family.html%3B634%3B417
http://wattsupwiththat.files.wordpress.com/2014/03/scatterplot-parasitic-loss-vs-total-surface-input-global.jpg
Paul Westhaver says:
March 26, 2014 at 11:59 am
Thanks, Paul. I am far from the first person to discuss the Carnot efficiency of the climate seen as a heat engine. In Bejan’s work I find, for example:
and
Best regards,
w.
Willis:
I was reading on the CERES literature about the data and comparison to observation. They state that 26 stations were used to compare the global 1×1 grid cell data to observations. I could not find a list of where these stations are located Maybe I overlooked something. 26 stations around the globe is not a lot. I wonder if any of them are in the Arctic. It would be very hard (costly) to set up observing sites there just for CERES data usage.
Interesting. The total surface input equals the absorbed solar (by the surface). The downwelling longwave (atmospheric radiation) is no surface input. Net surface LW radiation is upwelling (cools the surface, warms the atmosphere). So, the “parasitic losses” percentage of the total surface input (absorbed solar) is much higher. Not parasitic at all. They’re the main surface cooling mechanism.
Michael D says:
March 26, 2014 at 12:11 pm
In terms of the greenhouse effect, the efficiency can be thought of as how hot the greenhouse effect can make the surface with respect to the atmosphere. This temperature difference ∆T is what ultimately drives the circulation of the atmosphere and the ocean.
However, there are a number of ways that heat escapes or otherwise interferes with the surface heating. In addition to parasitic losses, we have things like dust devils and a host of other emergent phenomena that spring up and cool the surface when it gets too hot.
My main point in this piece is to point out that these parasitic losses, like most parasitic losses, increase with temperature, with the corollary that at the high end of the scale almost all of any increase in forcing does NOT go into warming the surface.
Instead it is lost as either sensible or latent heat, which decreases the ∆T between the atmosphere and the surface.
w.
Re OldSeaDog’s comment and in the light of UWA’s behaviour regarding the Lewnatics fakery, “honest enough University Department” methinks might almost be considered an oxymoron.
Thanks, Edim, but I fear I don’t understand any of your claims. Comments follow.
Edim says:
March 26, 2014 at 12:25 pm
No, the total surface input is the sum of solar and longwave.
Say what?
True … so?
Edim, if you think that the only thing warming the surface is the absorbed solar, I fear you are beyond help. For example, we know (from measurements) that the ocean constantly loses about 400 watts per square metre (W/m2) of radiated energy.
We also know (from measurements and estimates) that the parasitic losses about about 100 W/m2. So the ocean is losing a total of about half a kilowatt per square metre.
However, we also know from measurements that the ocean only receives about 170 W/m2 of solar energy … so if your crazy theory were correct, what keeps the ocean from freezing? If your theory were correct it the ocean would have a NET loss of about 330 W/m2, so why isn’t it frozen solid?
I must confess, this idea that longwave radiation can’t warm the planet is bull goose looney but damn hard to kill. Everyone seems to agree that longwave radiation can warm them, common sense shows up that. And we all know that everyday objects far from a big fire are all heated by nothing but longwave radiation, sometimes to the point where they burst into flame.
But somehow, some folks think that although we have experience with LW warming all kinds of objects, it can’t warm the earth itself … and they often are totally impervious to logic of any sort.
So, let’s see what category Edim is in … Edim, what keeps the oceans from freezing if (as you claim) LW can warm you and me and all kinds of inanimate objects but it can’t warm the planet?
w.
“Others may not be aware the data below TOA is calculated using a radiative model.
glad you asked the CERES folks.
I’ve been trying to explain this to WUWT readers and folks who use CERES data or ANY satellite data for that matter
READ THE ATBD.
Here is what most people dont get.
1. When you use satellite “data” you are using a data PRODUCT, not observations
2. The observation is a voltage on sensor cell.
3. That observation is turned into a data product by applying PHYSICAL MODELS
4. IF you accept the data product as truth, you are epistemically committed to the
truth of the model.
Example: I shoot a laser at the moon. I measure the transit time. I accept a physical model
that dictates a speed for that signal. I also accept a model that says D=R*T. using
those two things I calculate the distance. When I use this distance I am commited to
two other truths: the speed of the signal and the model of D=R*T
So, what physical models are absolutely required to calculate data products?
Radiative Transfer Models:
Yup, the same physics that says doubling C02 will add 3.7 Watts to the energy balance
of the planet.
The same physics that says C02 warms the planet it does not cool the planet.
The same physics we use to design attenna for IR missiles, cell phones, radars.
Think of it this way
The satellite sensor records the signal AFTER it leaves the atmosphere.
Any inferences about what happens below this depends upon modelling.
how signals pass through the atmosphere. radiative transfer equations.
Everyone who uses and relies on satellite data is a closet AGW believer. They just dont know it.primarily because they dont read ATBDs. They just find data and use it without following it back to the original bits.
So, next: Is this an example of Prigogene’s self-organized structures emerging to increase the efficiency of thermal loss? You have documented an increase, and the increase appears to have some structure. If that structure can be connected to secondary measurements of dissipative quasi-particle structures (their frequency of occurrence, their strength) such as thunderstorms, large scale convective cells, tropical storms, cloud/albedo systems (which provide yet another highly nonlinear self-limiting mechanism) you might actually be able to provide a compelling argument that the climate is a self-organized system that more or less provides a “governor” that limits the temperature gain from any possible forcing that doesn’t move the entire system into an entirely novel dissipative regime. It is basically a strong argument for quasi-invariance of the primary attractors because if you add heat/surface radiative forcing, you simple increase the efficiency of surface loss mechanisms within the nonlinear structures that short-circuit the “simple” radiative loss mechanism. Even if there is additional e.g. water vapor feedback, it is simply canceled as it occurs and fails to actually heat the surface (by much).
Once again, one thing that puzzles me about the CERES data is, however, the lack of a substantial disparity between land surface and ocean, and between different kinds of land surface. In this case the deserts are clearly visible on the toplevel map — as one would expect, there is little latent heat transfer in a desert — and there is maximal transfer over the tropical oceans and a surprising large transfer over tropical rain forest, almost matching the ocean. However, this doesn’t seem to have much effect on the combined results — land, ocean and both together have almost the same general structure, although there is small bobble in the middle forcing region that might be exactly this, a limiting of the loss even for higher forcing in subtropical deserts that is then overwhelmed by the tropical rainforest returning to ocean-like behavior for the latitude (as the forcing is I’m guessing roughly monotonic in the latitude, so you could make the same diagram with latitude as the horizontal scale with only a small distortion of the scale).
If this slight subtropical/temperate flattening associated with middle latitudes/forcing does indeed reflect the lowered contribution of low-humidity deserts, it suggests that global warming from additional backradiation forcing should occur primarily (or most efficiently) in those climates, where there is reduced ability to transfer energy via latent heat. But those are precisely the climates that have the most efficient direct radiative loss, as there is little water vapor or cloud structure to feed back whatever additional radiation one develops due to CO_2 alone. Deserts seem to be the place where direct CO_2-linked warming should most easily be observed and where the effect should be maximal.
Since the tropics and subtropics represent the region of greatest annual insolation (by a pretty good margin) due to both the planetary Jacobean and because of the increasing angle of incidence as one moves towards the poles, the idea of self-organized structures merely linearly strengthening to provide an exponential attenuation of the warming effect of additional forcings and effectively limiting the probable temperature rise from any sort of additional forcing everywhere but in deserts or at the poles is an attractive one. Deserts might get a bit warmer, unless/until circulation patterns shift so that they are no longer deserts. The poles are one end of the heat transfer and dissipation cycle, and they could get a bit warmer. But all of the tropics and most of the temperate zone has (if your analysis is correct net negative feedback, reinforcing the idea that has been proposed in several places that the climate will warm strictly less than the amount predicted by analyzing CO_2 forcing alone. Instead of every additional watt/m^2 of CO_2 forcing being accompanied by a watt of additional water vapor forcing, the total effective forcing of an additional watt of CO_2 forcing is anywhere from 0.0 to 0.7 Watts of actual forcing, depending on where you are. This data suggests that the total warming from additional CO_2 that by itself might have warmed the planet by 1.5 C will only warm it by anywhere from 0.5 C to 1.C. Which actually does not badly correspond to the actual temperature data over the entire industrial era.
The really nice thing about this is that you (can) use data from one part of the globe effectively to make inferences about another part of the globe. You don’t have to assert a feedback to 1 degree of CO_2 linked warming globally as a guess — you can just look at what the feedbacks actually are someplace where the temperature is 1 degree warmer, as determined by the ratio between incoming and outgoing surface radiation. Not only do you get “water vapor” feedback, you get a very accurate picture of total feedback from all sources directly from the CERES data, ready to be plugged into even a very simple single layer model to get a very simple estimate of the plausible range of global warming. The data already knows everything you need to know to handle the global problem.
rgb
Paul Westhaver says:
March 26, 2014 at 11:59 am
While I am not totally comfortable with Willis’ used of the term “parasitic losses” here, as to me that implies an “intent” to the system as in an engineered design, this is just a semantic quibble on my part. I think his general point is well taken.
By analogy to an engineered system, let’s take the boiler in a steam-turbine generator. Any thermal losses from the boiler directly to ambient are considered parasitic losses, meaning energy put into the system that is unavailable to do what the system is designed to do: generate mechanical (and possibly then electrical) energy through the turbine.
If we had a poorly insulated boiler, significant energy would be convected away to the cooler atmosphere. If the outside were wet, as in the case where it was raining on the boiler, there would be significant evaporative energy transfer to the atmosphere as well. In such an engineered system, it would be worth some cost and effort to reduce these losses.
Note that neither of these losses have anything to do directly with the actual thermal-to-mechanical conversion process in the turbine. But what these losses do is to reduce the thermal energy available at the input to this process.
I view Willis’ use of the term “parasitic losses” as a bit of a metaphor, because it is not clear what they are losses “from” in a natural system. But still, these transfers do serve to reduce and limit the temperature at the high end of the natural system (compare our equatorial temperatures at midday to those of the moon…).
I must confess, this idea that longwave radiation can’t warm the planet is bull goose looney but damn hard to kill. Everyone seems to agree that longwave radiation can warm them, common sense shows up that. And we all know that everyday objects far from a big fire are all heated by nothing but longwave radiation, sometimes to the point where they burst into flame.
But somehow, some folks think that although we have experience with LW warming all kinds of objects, it can’t warm the earth itself … and they often are totally impervious to logic of any sort.
Damn skippy. You can show them pictures of it taken with IR cameras. You can show them broad spectrum spectrographs that directly measure not only the existence of the downwelling radiation but its actual spectrum, complete with CO_2 and water vapor bands and ozone/oxygen notches. You can show them arithmetic that indicates that without this downwelling radiation the sun alone might make it a lot hotter during part of the day in part of the globe, but it would get a lot lot colder everywhere else and all the rest of the time for a pronounced net cooling. And you’ll still have folks asserting that downwelling LWIR can’t have anything to do with surface temperatures.
They’ve obviously never heard of energy conservation or the laws of thermodynamics.
I feel your pain. I literally don’t know what to do with them (or for them, as they are to be pitied and helped as much as they can). I sometimes think that they are ringers who come to the site just to ensure that its science is never taken seriously, because there is often a sort of religious contempt for algebraic argument or even common sense that accompanies their assertions. But sometimes one does get such a comment from somebody who lacks the certainty that their own arguments, generally formulated in the absence of ever having taken a halfway decent physics class, are all that likely to be right and who are willing to learn.
So cross your fingers.
rgb
Willis, it’s not my crazy theory. The only surface input is the absorbed solar. This input is balanced by the surface outputs: evaporation, convection and net surface LW radiation.
http://science-edu.larc.nasa.gov/EDDOCS/images/Erb/components2.gif
The downwelling LW is only one ‘side’ of the LW radiative heat exchange at the surface – the net LW flux is upwelling and is a surface output.
Interesting. Willis gets 18.4% parasitic loss. A month or two ago I posted on WUWT a back of the envelope calcualtion for carnot efficiency for the global atmosphere of 20%, reducing to 18% due to global warming. Co-incidence, or perhaps the parasitic loss is the flip side of the carnot efficiency of the atmospheric heat engine?
Dear Edim,
That’s fine, but in that case one has to be very precise in what one is saying and make sure that you’re saying things in the same way that they are being said in what you are addressing.
Obviously you are interested in adding the word NET in front of each term in the energy flow. Equally obviously, Willis was not using that word. He was summing all of the downward terms into a total incoming/forcing (consistent with the usage of the term in much of climate science). He was then focussing on the DIFFERENCE between the measured downward forcing and the observed upward radiation, and (if I understand correctly) interprets this difference as the upward total heat loss in non-radiative channels, since CERES permits the direct observation of the heat loss in the radiative channels. His conclusion is that at some point, adding to the surface forcing does not increase the upward radiation at all — the surface reaches a constant temperature and additional forcing is eliminated by means of the alternative channels of conduction, convection, and latent heat, with the latter being the most important especially in the tropical ocean. So increasing the forcing (solar plus downwelling LWIR simply doesn’t chance the surface temperature (according to CERES) — the additional power goes directly into latent heat and vertical heat transport that short circuits the partially blocked radiative loss mechanism.
If all you are arguing about is the inclusion or lack of inclusion of the term “net”, then please adjust your usage to conform to Willis’s because right or wrong, it is clear enough and it is, after all, his post and thread.
rgb
Just found this post on climate change, and thought your readers would find it interesting and worth comment:
http://www.transitionnetwork.org/blogs/rob-hopkins/2014-03/prof-myles-allen-climate-change-flooding-and-carbon-capture-silver-bullet
Well done Willis, great addition RGB, it really is the only thing that explains how a crazy metric such as average annual global temperature could be as stable as it is.
v/r
David Riser
Curt says:
March 26, 2014 at 12:47 pm
Paul Westhaver says:
March 26, 2014 at 11:59 am
While I am not totally comfortable with Willis’ used of the term “parasitic losses” here, as to me that implies an “intent” to the system as in an engineered design, this is just a semantic quibble on my part. I think his general point is well taken…..
___________________________________________________________________________
Curt your point is well taken. I am elevating the notion of entropy production as a measure of efficiency to defeat so called “common- sense” assertions wrt heat transfer efficiency.
I recall be shocked as a young mechanical engineering student to discover that it is not in the interest of energy producers to seek max temps in a heat source and min temps in the low temp reservoir to yield max bang for your buck,
Yes you do increase heat transfer “rates” but at a huge cost.
The notion of reversible processes and entropy was introduced by Clausius and Kelvin around 1865 and it is an absolutely counter intuitive concept. All heat transfer systems are now evaluated based on minimizing entropy in the most cost effective manner possible. So Entropy is the true measure of efficiency. That makes the assertion that max delta T yield highest eff a fallacy limited to a categorical calculation, which is why I added the disclaimer of Carnot efficiency.
The most efficient processes are those which produce the least “disorder” from a second law perspective. That concept eludes most people and they are willing to buy into the popular phase repeated by Willis. If we speak in common parlance, we can get away with terms like “parasitic”. As scientists we rely on the 1st Law AND the Second Law of thermodynamics. The heat has to go somewhere, especially if a process is irreversible. Besides the atmosphere is an enormous heat transfer surface with relative low deta Ts from a heat engine perspective.
rgb, I think it’s misleading to count only one direction (downelling) of the LW radiation. The surface input is either the absorbed solar only (SW) or the net radiation (SW + LW), which is also acceptable. SW + LW(downwelling) as a surface input makes no sense and it’s misleading IMO.
Willis,
You make an excellent point about CERES data when you say,
So I use them, with caveats, as the best that we have.
My research for many years has dealt with boundary layer processes, and thunderstorms and tornadoes for which I have published a number of papers (yes, peer reviewed). There does not exist the complete physics of these processes, so we make approximations and parametrizations which often rely heavily on statistics. It is not ideal for sure, but, as with CERES data, it is the best we have right now. Hopefully our understanding will continue to improve. As such all weather forecasts should be used with caveats and all forecasts are probabilities…given the initial data what is the most likely outcome given our present knowledge. Many people (a lot on this forum) want deterministic forecasts and trash the models when a forecast does not work. The models (including climate models) are all approximations and the best we have.
I am not defending climate models, or more specifically the modelers. I have had many frustrating dealings with these modelers. But the models can have use if we know and abide by their weakness, something the modelers do not seem to want to do (too many perks involved as I well know.)
rgbatduke Mar 26 12:44pm – As always, yours is a very interesting and informative comment. Thank you. However, this time, your comment seems to go a bit further than usual, and indicates that the CERES data can be used to give a reasonably firm value (or range of values) for what the IPCC calls Climate Sensitivity:-
“ Not only do you get “water vapor” feedback, you get a very accurate picture of total feedback from all sources directly from the CERES data, ready to be plugged into even a very simple single layer model to get a very simple estimate of the plausible range of global warming. The data already knows everything you need to know to handle the global problem.”
If I am understanding you correctly, CERES data shows that Climate Sensitivity is “anywhere from 0.5 C to 1.C “), which is well below the IPCC estimates but in line with theoretical calculations that others have made using eg. the expected rate of increase of the hydrological cycle with temperature [see the table on page 2 of http://www.royalsocietyhighlands.org.au/WilliamKininmonthAnswersToAdvanceQuestions.pdf
“Rate of increase of evaporation : 6%/deg C; Surface temperature response : 0.7 deg C.“].
Is there any chance of getting any of this into the peer-reviewed literature, or is WUWT as far as it can get under the present journal gate-keepers?
PS. Regarding your 12:54pm comment re LWIR. There was a long and detailed discussion on this in WUWT some time ago, in which Leif Svalgaard explained the science in ever increasing detail and with amazing patience. I doubt that the other person budged their thinking one iota, but the real benefit was that there were large numbers of other people who did learn a lot. So as long as the resolutely dumb are few and open-minded observers are many then these arguments are still worth conducting. Rather like climate science, really.
@Steven Mosher
Some really good points about radiative transfer models. This strange phenomenon occurred to me as well, so many people completely trash every aspect of climate models but are, at the same time, totally accepting satellite data as gospel. And I thought, duh…they both use radiative transfer physics to obtain modeled data. Not defending climate models, as they have many many other assumptions, but an interesting observation.
Sister Michelle says:
March 26, 2014 at 1:24 pm
Just found this post on climate change, and thought your readers would find it interesting and worth comment:
_______________________
I did not find the article interesting. Besides being grossly off topic of this thread, I found your link to contain verbose pablum, with no hard data and many platitudes. I didn’t bother to read the whole thing, but skimmed it and re- read a few lines to make sure he’d really said (waffled around) a couple of stupid remarks, trying to make his inanities have merit and seem less false as he tried to maintain some sort of hip and cool status with the me- too climate alarmist crowd. I won’t point out the number of blatantly incorrect statements made by Myles Allen, but you can keep “believing in him”, if you wish.
What do YOU think of this current post by Mr. Eschenbach?
Ps to Sister Michelle (with all apologies to Willis et al for pursuing this off- topic subject)
Ma’am, Please stick around and become a regular WUWT reader. I have no doubt that you mean well and want to do the right thing. I visited your site and you have a good heart, but you have been led astray. Give yourself time to discover the truth of things, by becoming a regular reader of these threads. You will find your beliefs challenged and it may be painful, for a time, but you may find that the truth will be better for your purposes.
How did you do that Willis? Those first two scatter plots look just like dolphins. Ok, it’s late and maybe it’s my imagination, so let me get back to reading the rest of your post.
I’m intrigued already.
Regards
Willis, Have you a comment about the interesting green spot in your first image?
It is almost above Lake Eyre in South Australia, which is below sea level.
Good to see scatterplots. Not surprising to see the global view (first graphic) resembles NASA displays of global vapor pressure or temperature. Just overlay a plot of the vapor pressure of water from zero to 40 C. Then add clouds.
The land view is interesting. Likely deserves more study from the point of view of surface albedo due to photosynthesis.
Tropical ocean surfaces are the source of most global energy. Plot the same data against latitude, should be a higher slope in the tropics. Capped at around 30 C. by clouds.
Paul Westhaver says:
March 26, 2014 at 1:40 pm
“I am elevating the notion of entropy production as a measure of efficiency to defeat so called “common- sense” assertions wrt heat transfer efficiency.”
I am puzzled by this statement, and I don’t think you are distinguishing pure heat-transfer systems from systems that are producing thermodynamic work from thermal energy.
In a heat-transfer system that does not produce work, as in a heat exchanger, you always get the maximum entropy production. In the computationally simple case of a large hot reservoir at temperature Th and a cold reservoir at Tc, for a heat transfer of Q from hot to cold (“large” here means that these temperatures don’t change materially from this transfer), the entropy production is simply S = (Q/Tc) – (Q/Th), no matter how the transfer of this amount of energy is done. The math gets more complicated if the temperatures change during the process, but the principle is the same.
If you are producing any thermodynamic work (W) in the process of removing energy Q from the hot reservoir, there will be less entropy production, because the amount of thermal energy Qc (= Q – W) arriving at the cold reservoir is less, and so the entropy production is less: S = ([Q-W]/Tc) – (Q/Th). The more work W you produce for a given Q from the hot reservoir, the higher your thermodynamic efficiency (e = W/Q by definition), but also the lower your entropy production.
The 2nd Law says that the minimum entropy production is zero, and this would occur at the Carnot efficiency. If entropy production is zero, the process (and each sub-process) is reversible. Carnot worked out what this would mean in detail – the problem is the (theoretical) Carnot cycle would take infinite time. It should be thought of as no more than an idealized theoretical limit.
Now, there is a point that if you can get the details of the work production closer to the Carnot reversible limit, you can reduce entropy reduction and increase efficiency. This is why the gradual expansion of gases in a turbine gets higher efficiency than the explosive expansion in an internal combustion engine, for the same source temperatures.
But for a given type of process, if you can increase the temperature difference, you can increase the efficiency (and reduce the entropy production). This is why a diesel engine, with its higher compression ratio and therefore higher source temperature, has a higher efficiency than Otto-cycle gasoline engines.
Enough for now. I hope we haven’t veered too far off topic.
Interesting use of the term “parasitic losses”. More generally in thermodynamics and mechanical engineering these are referred to as “irreversibilities.”
Per figures 5 & 6: Why are losses between 250- 500 W/m2 decreasing as input increases? Why is the effect more pronounced over land than ocean?
Should Figure 3 be labeled “Land Only” not “Red = land, Blue = ocean”?
Curt says:
March 26, 2014 at 12:47 pm
While I am not totally comfortable with Willis’ used of the term “parasitic losses” here, as to me that implies an “intent” to the system as in an engineered design…
——-
Consider the “design” in the context of the constructal law http://en.wikipedia.org/wiki/Constructal_law as proposed by the same Bejan cited above.
Alan Robertson says:
March 26, 2014 at 3:04 pm
Per figures 5 & 6: Why are losses between 250- 500 W/m2 decreasing as input increases? Why is the effect more pronounced over land than ocean?
———
Melting sea ice/snow cover?
Thanks, Willis. You keep pointing at interesting aspects that are basic for climate science but seem to have been overlooked in the race to declare atmospheric CO2 content the key variable.
I’ve been telling you all for years that the thermal effect of GHGs in attempting to slow energy loss to space is negated by an increase in non-radiative energy transfer mechanisms which involve the entire global air circulation and not just local emergent phenomena such as thunderstorms and dust devils.
The correct issue to address is as to how much the global air circulation is affected by our emissions as compared to natural variations caused by sun and oceans.
I’d guess it would be too small to measure.
Having established that there is such a thermostatic mechanism one then needs to address the issue as to how it can work and at that point one just has to bring in the Gas Laws.
Nor should we ignore the adiabatic warming of descending air in helping to reduce radiative energy loss from surface to space.
It is no coincidence that most of the time the Antarctic is covered by a high pressure cell with descending air and that Willis points out that
“In fact, at the South Pole the situation is reversed, and the net flow of energy is from the atmosphere to the surface.”
That net flow from atmosphere to surface cannot all be coming from inflowing tropical air because the semi permanent high pressure cell blocks such inflows for much of the time.
It isn’t DWIR that reduces surface cooling around the globe. It is adiabatically warmed descending air which, at any given moment , is half the entire atmosphere.
“And we all know that everyday objects far from a big fire are all heated by nothing but longwave radiation, sometimes to the point where they burst into flame.”
Hmmm, that’s an interesting statement.
Willis said:
“Note also that across the main part of the range, which is to say in most of the planet except the tropics and poles, about half of each additional watt of energy increase doesn’t warm the surface … it simply goes into parasitic loss that cools the surface and warms the atmosphere”
In the tropics adiabatic uplift cools the surface and at the poles adiabatic descent warms the surface.. The net effect globally from adiabatic convection is therefore zero provided the scale and speed of the convective cycle changes to counter any radiative imbalances between poles and tropics.
The main part of the range (other than poles and tropics) is then warmed or cooled depending on the ever shifting balance between poles and tropics resulting in global air circulation changes which move the climate zones to and fro.
That movement of climate zones to and fro is the negative system response in action because it regulates radiative losses to space so that energy in always equals energy out over time.
It is the speed and scale of the non-radiative processes that changes to negate any radiative imbalances so as to achieve overall radiative equilibrium despite internal system variables such as differences in atmospheric composition.
That system deals with variations in atmospheric conductive AND radiative capability, both of which can vary with atmospheric composition.
As long as the categorical distinction between conservative HEAT TRANSFER and non-conservative RADIATIVE INTENSITY is ignored, confused depictions of the climate system and mistaken attributions of “forcing” will persist. What Willis here calls “parasitic” effects are, in fact, the principal mechanisms of thermal energy transfer between Earth’s surface and the atmosphere, as seen from the myopic viewpoint of a heat engine. That may satisfy low-level academic preconceptions, but it hardly represents a credible specification of the climate system.
Willis in this post, you showed the maximum temperatures that occur when the Ocean heats http://wattsupwiththat.com/2012/02/12/argo-and-the-ocean-temperature-maximum/ Is there any way that you can correlate the “parasitic” heat loss with the energy that appears to be missing based on the Argo float temperatures? When the Ocean does not heat beyond 30C, the heat is still there, but it is somewhere else.
Curt,
One piece at a time…
“In a heat-transfer system that does not produce work, as in a heat exchanger, you always get the maximum entropy production. In the computationally simple case of a large hot reservoir at temperature Th and a cold reservoir at Tc, for a heat transfer of Q from hot to cold ”
detaS = Q/(ThTc) x (Th-Tc), for heat transfer between 2 reservoirs.
deltaS –> 0 as (Th-Tc) –> 0.
I think MaxLD used to play with aerofoils.
Not that there’s anything wrong with that . . . .
Paul: You say:
“deltaS = Q/(ThTc) x (Th-Tc), for heat transfer between 2 reservoirs.”
That is what I said – just one algebraic manipulation away…
“deltaS –> 0 as (Th-Tc) –> 0.”
Of course.
Ok Curt we agree!
Cheers.
Convective/conductive losses are primary. Radiative loss from the surface itself lags far behind as soon as air motion begins.
This is a reversed-mechanisms blog post about something having to do with hot cold but it hasn’t got anything to do with global atmospheric energy handling.
Steven Mosher says:
March 26, 2014 at 12:39 pm
Well, I agreed with you until you switched from models to physics. They used radiative transfer MODELS … but you say that PHYSICS says doubling CO2 will warm the planet.
In fact the physics says nothing on the subject of how a resonant, driven, chaotic system will respond to a slight change in one of the inputs. This precise fallacy is one that drives AGW, the crazy idea that the laws of physics say that the climate MUST do X or Y … doesn’t work that way. We have nowhere near that level of understanding of the climate to say anything like that.
Agreed.
Not true in the slightest. You seem to forget that there are models, and models, and then there are models. The CERES folks used two different groups of models.
First, they used several models to convert the sensor data from the various sensors (which as you point out is a changing voltage or other analog signal) to the final result, e.g. the strength of the sun at that point in time and space.
Next, once the observations were converted to numbers, they used a second set of models (and a bunch of other data from both ground and satellite observations) to calculate the instantaneous surface radiation flows corresponding to the instantaneous TOA radiation flows.
But neither of those two sets of different models that they used were GCMs, global climate models. Nor were they attempting to calculate (as GCMs do) the century-long evolution of a chaotic system.
So your claim seems to rest on the idea that if I use one group of computer models (say modeling the numbers from the analog voltages from the sensors) I have to accept the results of totally different models which are being used for a totally different purpose … or as you put it,
Bad news for your theory, Mosh. Accepting the results of the CERES computer models converting instantaneous voltages to instantaneous numbers doesn’t mean that we have to accept the results of radically different computer models trying to say what will happen to a chaotic system in fifty or a hundred years, or even the next couple of decades … speaking of which, how is that “pause” thing going? You know, the one that can’t happen according to the computer models? Not the CERES models of course, they don’t do predictions/projections/forecasts/scenarios or whatever the weasel word is these days, but the GCMs. How are their predictions working out?
My thanks to you, when you are not drive-by commenting you usually raise interesting issues,
w.
Curt says:
March 26, 2014 at 12:47 pm
Thanks for that explanation, it’s an excellent one.
That’s a most interesting question, Curt. For me, they are losses from a perfect theoretical greenhouse, something like my “Steel Greenhouse”. With a perfect single-shell greenhouse system, you can double the watts/square metre (W/m2) at the surface. As you expect this would raise the temperature by the fourth root of two.
Now, for a perfect greenhouse to attain that 100% efficiency, it has to have a vacuum between the shell and the planet. Otherwise, in addition to losing energy via radiation the heat can “leak” from the surface to the atmosphere. Remember that the ∆T of interest in the game is the fact that anywhere you might go on this planet, there are freezing temperatures only a few miles from where you are standing … and it is exactly that temperature difference that allows the formation of the natural heat engines we call thunderstorms. Like any heat engine, they run off of the difference in temperature ∆T between the hot and cold ends of the engine. In the climate system, that is provided (inter alia) by the surface at one end and the atmosphere a couple of miles up at the other end.
So when you have sensible and latent heat loss, it reduces the ∆T, and thus the efficiency of the entire system.
I mentioned it above, but an excellent read about this is from Adrian Bejan, called “Thermodynamic optimization of global circulation and climate“.
w.
Oh I see Willis you’re using the term parasitic as in the original meaning of the word parasitic as in ”taking from one to give to another.”
Nevermind.
D.J. Hawkins says:
March 26, 2014 at 3:19 pm
Than\ks, D.J. You’re right, but by the time I noticed it, it was already up on the silver screen and I couldn’t be bothered … I figured folks would figure it out, so I consoled my self with some choice expletives and kept moving.
w.
Andres Valencia says:
March 26, 2014 at 3:39 pm
Thanks for that, Andres. The beauty of climate science from my perspective is that it is such a new science, and is so full of fascinating datasets, that finding novel oddities and curiosities and relationships and correlations is not that hard …
w.
James Rollins Jr says:
March 26, 2014 at 5:31 pm
Say what? Convective/conductive losses (sensible and latent) are only about one-fifth of total losses from the surface (about 100 W/m2 out of half a kilowatt/m2) … how is that “primary” on anyone’s planet?
w.
insightful and thought provoking, as always. Thanks Willis.
James Rollins Jr says:
March 26, 2014 at 6:06 pm
Not really. I’m using it in the sense it is used as regards engines and machinery. Here’s one of many examples from the web:
For example, when you drive your car, the energy it takes to overcome wind resistance is referred to as a “parasitic loss”. Here’s an example from the DOE:
That’s the sense in which I am using it. It is a loss of energy which reduces overall efficiency and generally increases with increasing speed/load/temperature/rpm/work performed.
Hope that clarifies it,
w.
Willis, it’s not out of half a kW/m2. It’s out of around 160 W/m2.
Willis concludes with-
“Note also that across the main part of the range, which is to say in most of the planet except the tropics and poles, about half of each additional watt of energy increase doesn’t warm the surface … it simply goes into parasitic loss that cools the surface and warms the atmosphere.”
Bill Gray stated the same thing on page 3 of his 2012 paper-
“The Physical Flaws of the Global Warming Theory and Deep Ocean Circulation Changes as the Primary Climate Driver”
http://hurricane.atmos.colostate.edu/Includes/Documents/Publications/gray2012.pdf
“Only half of the blockage of 3.7 Wm-2 at the surface should be expected to go into temperature rise. The other half (~1.85 Wm-2) of the blocked IR energy to space will be compensated by surface energy loss to support enhanced evaporation.”
You are in good company!
No, I read through it again fully and you’re claiming that more heat arriving makes things colder.
It’s rank warmist rubbish from beginning to end. I see you claimed the surface is predominated by radiation and that the parasitic losses are only 30%,
this is a well known falsehood for anyone who ever designed or studied heat removal in the atmosphere at normal operating air pressures.
Convection and conduction always predominate.
Always. It’s why every single air cooled object on earth is – air cooled – not radiation cooled.
Omg LoL.
Woah.
Willis I suggest you figure out what you tried to claim and why everywhere you go people laugh at you when you bring this with you because in the atmosphere things don’t cool, primarily,
radiatively.
You’re supposed to have sense enough to know that.
Woah in a big way. I saw you claim parasitic losses rise to 30% because you believe
looking at every set of motorcycle fins, heat cooling fins on light ballasts, volkswagon motors, computer chips, large fan cooled equipment –
you’re not aware things predominately convection cool in air on earth?
Mosher writes “The satellite sensor records the signal AFTER it leaves the atmosphere. Any inferences about what happens below this depends upon modelling. how signals pass through the atmosphere. radiative transfer equations.”
Absolutely true but you have to look even deeper to understand the satellite measurements. For example a satellite cant “see through clouds” so it cant know how thick they are and consequently cant know how to apply the radiative transfer model for an accurate picture of anything other than the top of the cloud and hence TOA. In that case any derived surface measurements have much more assumption built in than say a clear day over desert where there are no clouds and we can expect little water vapour.
IMO the best thing about satellite measurements is not their “absolute” precision but rather their reproducibility of results which means they can see trends quite well but even those can be artifacts of how the underlying model is applied.
rgbatduke says: “I sometimes think that they are ringers who come to the site just to ensure that its science is never taken seriously, because there is often a sort of religious contempt for algebraic argument or even common sense that accompanies their assertions. ”
I’m starting to suspect Mr Cotton is one of those fabrications.
typo there at the end of my own last post sorry.
Things primarily convectively cool in air.
If you think because CERES calculations don’t indicate it, or if you think for whatever reason radiant transfer predominates in air, all I can say is the only people I ever heard who believed in that were global warming alarmists.
[I figured that’s what you meant. Fixed. -w.]
Edim says:
March 26, 2014 at 6:37 pm
Edim, both rgb (Dr. Robert Brown at Duke) and I have tried to point out exactly where you are wrong. Undeterred, you just keep up the nonsense. Look, Edim, it’s not working. Nobody is buying what you want to sell.
Please, please, re-read what I said and what Dr. Brown said. If you can’t understand why scientists look at the individual energy flows and not just the NET energy flows that you keep harping on, then read it again until you can understand it. And if you still can’t understand how we are analyzing the individual flows, then go buy a text on radiative energy exchanges.
Because here, what we’re discussing are the individual flows, not the net flows, You seem to want to discuss net flows, which is fine, but I’m sorry, that’s not what this thread is about.
So if you can’t wrap your head around the mathematics of using individual flows instead of net flows, well, then please go somewhere that people are discussing net flows. At that point you can jump in and actually say something that makes sense.
Here … not so much …
w.
Your web assertions noted, I wasn’t asking you what you were using I was noting independently what usage you were using until I re-scanned and saw you specify losses parasitic as sensible heat losses for purpose of your post.
You then mistakenly asserted things at nominal atmospheric pressures lose most energy through radiant loss which is wrong, which is why the cooling fins on a volkswagon or a computer chip or any other heat dumping object in atmospheric air, aren’t arranged for best radiation dispersal but best solid-to-atmospheric-air transfer.
So I was correct: it was all a bunch of non-reality based pseudo science.
Willis says: “Not really. I’m using it in the sense it is used as regards engines and machinery. Here’s one of many examples from the web:”
Willis wrote;
“There is a more global restatement of Murphy’s Law which says “Nature always sides with the hidden flaw”. Parasitic losses are an example of that law at work.”
Well…. Highly trained engineers know all about “parasitic losses”. It’s why we insulate heat reservoirs to “trap” the heat (actually we just slow the velocity at which the heat escapes from the source, everybody knows that you can’t “trap” heat, well, except of course for climate scientists).
Well skilled electronic engineers know all about “parasitics” and we carefully consider them when selecting components to build an actual physical representation of a theoretical circuit design.
There are such things as “low inductance” resistors available (have been for decades), these are in some cases a wound up coil of wire that produces the desired resistance but by alternating the direction of the wires cancels out the “parasitic” inductance.
Any well practiced engineer understands the effects of parasitic losses within their design and has carefully selected components to mitigate those effects. They are hardly a “Murphy’s Law” effect, if you understand them correctly.
A nice analysis when applied to climate science, but it does not extend into well executed engineering designs.
Cheers, Kevin.
The individual throes of not having realized the earth is cooled via infrared gases linger on.
Willis I don’t suppose it means a thing to you that the entire world of cooling uses air as soon as any cooling need arises because it’s the predominating effect on the earth, does it.
Has it ever occurred to you that if the entire world of cooling repeatedly says ”put a fan on it, or build it so the air flows well across it because in most environments, convection predominates as soon as it starts competing with radiant loss”
it means the people who told you it’s actually reversed from that
whose work has also been found filled with willing mathematics fraud
are in need of assuring you somehow the
entire world of cooling
has their cooling model for objects in the atmosphere wrong?
James Rollins Jr says:
March 26, 2014 at 7:15 pm
James, as I recall you’ve made the claim before that for the planet the loss through conduction/convection far exceeds that from radiation.
Now, every authority on the subject that I know of disagrees with you, as do my own calculations using the CERES dataset, and my own calculations using the TAO buoy array.
So if you want to establish the opposite position, that conduction/convection is the predominate method of heat loss, you need to stop making claims and start producing actual data that supports your case. And given the breadth and depth of the evidence (not theory but evidence) that you are wrong, you need to produce a lot of data to support your unusual claim.
Looking at the fact that all of the things that you listed are man-made, constructed of metal, and specially designed to enhance the conduction/convection, I’m certainly aware that IF man-made metal objects need to lose more heat than they lose by radiation, we add fins to them.
To use your style and tone … you’re not aware that objects on the surface of the planet notably are not man-made, are not constructed of metal, and lack fins?
But like I said, this is just discussion. I cordially invite you to either come up with hard evidence for your theory and lots of it, or to stop making unsubstantiated claims.
Regards,
w.
KevinK says:
March 26, 2014 at 8:16 pm
Thanks, Kevin, your comments are interesting. Actually, when I parse what you said, it seems you are saying that they are indeed a Murphy’s Law effect. I mean, nature must be siding with the hidden flaw rather than with the engineers, or else the engineers wouldn’t need to take special measures and select special components in order to counteract the tendency implied by the Law … n’est pas?
I mean … you don’t hear much about “parasitic gain”, do you?
w.
The CERES outputs aren’t absolute flux, they’re layered shots with interpolation. The fact you try to tell people you thought 4/5ths or whatever of the loss of energy from objects at large is radiant is staggering to even try to imagine how you visualize energy loss.
If there was in fact predominate energy loss in most cases we would all understand the ins, and the outs, of the various ingenious ways, all these different mechanisms for exploiting your mythical majority of loss of energy through radiant transport.
Whereas in actual fact we all know of the many varied ways the atmosphere is manipulated so it’s predominative effects can be maximized throughout civilization.
LoL. wow Willis. Just woah. That’s a biggie there bud, you are going to have to just get mad or whatever you do when you don’t get told you’re right, but there are so many ways just off top of the head to check that claim it’s not even like one book is the best example: all books everywhere describe the maximization of convection where possible, to cool nearly everything under the metaphorically and literally correct, sun.
Go tell someone who builds things that generate heat, that – you don’t really see why they’re paying all that attention to making sure there’s enough clearance for air to get to it because
that’s only about one fifth, average, of most objects’ energy loss,
and try to not get laughed out of the place.
Not meaning to be insulting to you, you obviously seem to have put a lot of thought into what you are saying but woah – I don’t know what else to say LoL because – obviously you’re not working in the heating/cooling heat removal/containment fields.
Willis if everything cooled predominately radiatively there wouldn’t be all the attention paid to wind breaks and making sure air movement through a place is good, we’d all design our properties so the radiant losses were maximized.
I just am sorry but you just had someone call total b.s. Willis I thought about what, I thought you said the first time and I wasn’t wrong, I just didn’t realize I had it exactly the first time as I see I did.
This is errant thermodynamic analysis. End of story fella. Sorry to disagree so firmly but there’s a dozen ways to point out the absurdity of that call Willis.
I like your high seas stories; no I love em.
But you aren’t a trained thermodynamicist.
Than\ks, D.J. You’re right, but by the time I noticed it, it was already up on the silver screen and I couldn’t be bothered … I figured folks would figure it out…
Yep, five whole seconds shot to heck. If that. Good post.
Wilis your calculations from the CERES data are based on a layered interpolative model system not actual measurements, it’s why we don’t all talk about CERES as a surface temperature sensing device in the thermodynamics management fields, normally.
The fact you even mention having calculated such from CERES is all anyone in instrumentation would need to know about your claim Willis thanks for the discussion but I consider your words alone to be sufficient to convince anyone with any technical training at all or even not having any,
which of us is trained and experienced thermodynamicist and which of us isn’t.
Like I said your “I was a roving south seas guitar player” stories are very good.
Your thermodynamics, not.
You just really need to ask yourself how many times you’re going to argue with why every human inhabitant of the planet doesn’t know the ins, and outs, of a dozen ways to help with radiative heat loss from something,
rather than what we all over the earth actually learn about as we go through our individual lives,
how to maximize the predomination of convective thermal losses wherever and whenever possible.
I’ll just leave you to explain how you and your climate model friends all insist everything’s cooling by radiation
but the entire humanity on earth,
all discusses and buys products at the store to maximize convective losses
and the radiative heat loss products for all your stuff – they aren’t right there by the fans, or the air conditioners, or the misters.
LoL Willis I can not believe what I read but I see you continue to insist it’s so.
Just explain to us all where the radiative heat loss augmentation products are, over near the fans, the air conditioners, and the misters,
which all use conduction/convection, to cool things,
and we’ll compare the size of that section at all the stores, to your claims.
In other words Willis I was laughing at your claim so hard I was making light of it,
if what you are saying is true, then after years working on keeping heat on and off things using insulation I wouldn’t be asking myself how you can even conceive it being true, firstly,
and then,
when I tried to think of something that would reveal the hilarity of your claim
I thought of how everything reveals the hilarity of your claim about “radiant loss predominatiing”
and I thought ”It might be hard to express it not trying to sound like I’m joking.”
Well it did sound like I was laughing Willis but I’m not joking.
I’m trained in thermodynamics to work professionally in thermal management and know for a fact that your claim is false.
But how to prove that to Joe Sixpack reading your claim?
Simply ask everyone two things: if what you are saying is true, then why is it that firstly we know that all through history the management of air flow through an area determines it’s temperature much more powerfully than it’s radiative capability
and the “A” portion of it is ” why don’t we all know, of many myriad ways to manipulate and maximize the radiative heat loss of everything we have? ”
The answer of course is they’re so minimal in everyday applications
“B” is “why do we all instead know of many myread ways to manipulate and maximize the convective and conductive heat loss of things?”
The answer to which is because it’s what predominates since the beginning of time and today,
And if what you are claiming is real then where are all the products that show that mankind knows this and has been manipulating it as predominate method for heat removal for – everything ?
We should be able to go to any field and see the highly predominating radiative heat loss augmentation products,
and see a very minor section for the convective/conductive products.
But of course everybody reading your hilarious claim knows, it’s exactly the opposite of your claim.
All over the world in every field,
convection and conduction are the go-to heat removal methodologies exploited
where radiative heat removal is way down the list.
LoL Willis you need some training in foundations of thermodynamics.
Jones can ‘lose’ his original data and forget what all of the ‘adjustments’ were.
Manniacal can ignore requests for data and code
Others insist that the warmth is ‘hiding’ and tell everyone AGW is warming the earth but they just don’t know where the energy snuck off too.
Mostly they earn a lot of money using the classic shell game all singing “watch the pea”.
Willis: This type of research (your CERES articles) are where CAGW meets it’s demise. As long as the fakers hid their data, jumbled word definitions, pal’d their way to published papers and used non scientific descriptions they were able to keep clamor for real science at bay.
But science is advancing and has left the alarmists dancing as fools.
You may not be willing to actually publish papers, but you are seeding a lot of thoughts and ideas on fertile ground Willis! Remember to properly attribute Willis folks!
Say what?
Perhaps you meant to write the formula as (D=R*T)/2?
Or, after you shoot the laser at the moon you’re also waiting at the moon to observe it’s arrival? Not an unusual type of claim for many of the CAGW crowd; just not your normal stance.
Not a closet AGW believer. Just a believer, so long as the model has had all of it’s components certified and verified!
Yeah, that silly little requirement that model output is validated by observations! Validation that is replicated by any interested enough to try.
I don’t think this is issue for most of us. The issue is the dodgy application of models to CAGW chicanery. Many who mention why they’ve found WUWT, Bishop Hill, JoNova, Climate Audit is because of the shameful actions and claims of the alarmists and the inability of the models to show any accuracy or semblance to reality.
Virtually none of your flights of fancy make any sense, least of all what you think Willis posted.
Settle down, write exactly what CERES is showing, list all calculations and then highlight where you think you can help the discussion. Otherwise you’re just mouthing trash talk.
James, you are making Willis’ argument for him. The argument that you cannot augment radiative heat transfer to nearly the extent that you can for conductive/convective transfer is different from claiming that radiative transfer is not significant. Engineered heat transfer systems exploit the fact that you can augment conductive/convective transfers with pumps and fans, etc. to use these mechanisms primarily.
Also, most people underestimate how much radiative transfer is going on, even when temperature differences are relatively small. When temperature differences are large, the fact that radiative transfer is related to the difference in the 4th power of temperatures means that these transfers predominate.
When talking about the earth in space, the near-absolute-zero effective temperatures of space mean that radiative transfers would predominate even if there were stuff out there to conduct/convect to. There is a reason that astronauts’ space suits have seven layers of radiative insulation.
Curt you tried. So did AtheoK. A+ for willing to say anything for the cause, F for factual reality awareness. The reason the space programs of the world use reflective insulation is because the heat from the sun can’t escape till things overheat.
Good try but factually reversed so not so good at understanding who’s telling the truth here bud.
In other words Curt you thought it was cold in space and that’s why space programs insulate everything with that shiny insulation?
No, the reason they have those highly insulated surroundings on themselves and all the equipment is because in space where there is no atmosphere to cool the surfaces of objects,
the heat travels through the solid objects building up until the equipment overheats. Not because it’s cold out there. Where there’s an atmosphere the air washes off heat that causes equipment to fail in space for that reason.
James, you simply do not know what you are talking about. Inside an astronaut’s spacesuit, there are seven layers of aluminized mylar, each one providing a layer of radiative insulation, with the plastic mylar itself keeping the aluminum layers from conducting to each other.
The reason for this is that the human body produces about 100 watts through its metabolism. But the surface of the body radiates away close to 500 watts. Without the “back radiation” we are used to on the earth’s surface, without excellent radiative insulation, the astronaut would very quickly freeze to death when out of the direct line of the sun.
And I note that you did not address my larger point about your confusion between the ability to augment some types of heat transfer in an engineered system and their relative magnitudes in a natural system. I should point out that their is a reason (beside style) that many metal shells of electronic devices are black anodized – it does significantly enhance radiative transfer to ambient.
No Curt that isn’t correct actually about the space suits.
“Temperature
To cope with the extremes of temperature, most space suits are heavily insulated with layers of fabric (Neoprene, Gore-Tex, Dacron) and covered with reflective outer layers (Mylar or white fabric) to reflect sunlight. The astronaut produces heat from his/her body, especially when doing strenuous activities. If this heat is not removed, the sweat produced by the astronaut will fog up the helmet and cause the astronaut to become severely dehydrated; astronaut Eugene Cernan lost several pounds during his spacewalk on Gemini 9. To remove this excess heat, space suits have used either fans/heat exchangers to blow cool air, as in the Mercury and Gemini programs, or water-cooled garments, which have been used from the Apollo program to the present.”
From N.A.S.A. : How SpaceSuits Work:
Parts of a Spacesuit
NASA spacesuits have many pieces and parts. Learn about the parts and why each piece is important.
Primary Life Support Subsystem
The PLSS is worn like a backpack. It provides astronauts many of the things they need to survive on a spacewalk. Its tanks supply oxygen for the astronauts to breathe. It removes exhaled carbon dioxide. It contains a battery for electrical power.
The PLSS also holds water-cooling equipment, a fan to circulate oxygen and a two-way radio. A caution and warning system in this backpack lets spacewalkers know if something is wrong with the suit. The unit is covered with protective cloth layers.
Layers
The spacesuit arm has 14 layers of material to protect the spacewalker. The liquid cooling and ventilation garment makes up the first three layers. On top of this garment is the bladder layer. It creates the proper pressure for the body. It also holds in the oxygen for breathing. The next layer holds the bladder layer to the correct shape around the astronaut’s body and is made of the same material as camping tents. The ripstop liner is the tear-resistant layer. The next seven layers are Mylar insulation and make the suit act like a thermos. The layers keep the temperature from changing inside. They also protect the spacewalker from being harmed by small, high-speed objects flying through space. The outer layer is made of a blend of three fabrics. One fabric is waterproof. Another is the material used to make bullet-proof vests. The third fabric is fire-resistant.
As far as your other commentary Curt I’m happy to remind you everyone already understands you’d only be confused about the above matter if you were utterly unable to grasp what we’re talking about.
So thanks for the invitation to chat about your belief space suits have giant heaters inside.
I’ll pass.
James, I invited you to provide numbers, references, or citations to back up your claims. To date you have provided nothing.
Onwards to your main claim, which is that humans increase the heat loss of objects by building fins or installing fans. This is for a simple reason. It’s hard to increase heat loss from radiation. As you say you are in the business, perhaps you could list for us some possible ways to increase radiative cooling.
Now, of course the obvious one is to paint the radiator black. Because Kirchoff’s Law says that emissivity = absorptivity, that will make the object nearer to being a blackbody, so it will cool by radiation. That’s why automobile radiators are painted black, for example..
And make no mistake, engineers definitely use that method of cooling, changing the surface characteristics. It’s used e.g. in solar cells, to radiate away the heat from the back side. But once you’ve done that, how else can you increase the radiation? I know of no way to do that. According to Stefan-Boltzmann, radiation is the SB constant times emissivity times T^4 … which doesn’t leave a whole lot other than emissivity to mess with.
Once we move away from radiation, of course, we find that there are a host of ways to increase the physical removal of heat from a solid object through conduction/convection/evaporation. We can put fins on it. We can put a fan on it. We can spray water on it and let the water evaporate. We can use a heat pump, or a heat pipe, to remove the heat. The possibilities are endless … which is why as you point out almost all of the engineering work on cooling goes on in areas other than radiation.
Whether radiation or conduction/convection predominates in any given situation, however, is a function of the situation. For example, the earth loses 100% of its energy by way of radiation, and 0% by either conduction or convection … which shows that there are no general rules. You can’t depend on theory, you have to look at the actual measurements of the radiation and of the conduction/convection to see which one predominates in any given situation.
Now, all of the observations and measurements and scientific estimates I know of say that conduction/convection is about a fifth of the total surface heat loss. If you think different it is YOUR JOB to come up with the links to the OBSERVATIONS and MEASUREMENTS and SCIENTIFIC ESTIMATES to back up your case.
Because until then, you’re just flapping your gums, and nobody’s gonna believe your unadorned word in the face of lots of evidence. This is a scientific site, come up with evidence to support your claims or forget it.
w.
Willis says:
“And if you still can’t understand how we are analyzing the individual flows, then go buy a text on radiative energy exchanges.”
Thanks, but I got the highest grade in my Heat Transfer and the course was very comprehensive and the professor VERY demanding. We had to solve (and understand) all of the problems (some very difficult and tricky) from our textbook before even thinking of taking the exam.
Of course you can analyze the individual flows, my point that it can be confusing and misleading. It seems to me that it misled you to think that the atmospheric radiation is a surface input. The direction of radiative heat transfer at the surface is surface -> atmosphere, and it’s a surface output, together with the non-radiative surface cooling fluxes (convection and evaporation or sensible and latent heat). The input is the absorbed solar energy (by the surface).
Alternatively, the net radiation (Rn = SW + LW, where LW = LW↑ + LW↓) can also be viewed as a surface input (available energy) and I can accept that too (the way it is in climatology). It makes no sense to me to regard SW + LW↓, without the LW↑, as the surface input.
Willis you’re the first human being in my entire adult working life
dealing with sophisticated thermodynamic principles
who had the nerve to try to tell me
“metal is different from other things so the reality of convection predominating in nature is by that assertion evaded.”
You’re
Willis Eschenbach says:
March 27, 2014 at 12:59 am
“Flapping gums etc”
You’re free to stay here and talk about gum flapping Willis it’s your venue; but you are obviously not trained in any field related to thermodynamics, formally, or you’d realize the gross malfeasance exemplified in trying to claim refined man made materials lose and gain energy differently than those very same elements embedded raw in ore.
That’s what’s called absolute evidence of lack of formal thermodynamical comprehension Willis, it’s what people are tested for when they take beginner classes in the field, much less more sophisticated ones requiring algebraic calculation etc.
You’re just an obviously, not formally educated man, trying to bullshoot professionals. You’re coming across a bumbler because the errors you’re making are extreme in their nature.
I’ll leave the “Space is cold that’s why astronauts wear space suits” and “4/5ths of all energy emitted by natural and man made material is is through radiant transfer” crowd to your discussion and you can justify your assertions without even being bothered having me do it to you, all over again.
Good night and as I say I like your roving south seas guitar player stories. One thing I can recommend to get you into the real thermodynamic analysis field is take some courses and get yourself a degree of some kind in physical sciences and instrumentation if you’re going to talk about our fields. It’ll help you get along with us a lot better by being able to understand what the foundational principles are.
And Willis you and I both know, there’s no fundamental difference in the ways man made vs natural materials emit energy.
So you really need to recognize trying that kind of infantile insanity in a discussion about physical matter, is as low rent intellectually as it gets.
It’s outright, unrecoverable confession just how profoundly out of contact with the essentials of matter and energy, you are each time you make it.
If you want to you’re free to go ahead and claim yet again
you think “metal emits energy differently when it is ore, than when it is refined,”
and that “refined material uses different laws of physics to emit energy than natural”
it’s your right to state it : but any trained technical personnel in any field of matter and energy handling who sees you say it recognizes it instantly as revelation of profoundest ignorance.
Profoundest ignorance Willis I seriously can not imagine that you say that and don’t know what a torpedo to anyone believing anything you ever say afterward, to claim you think refining material makes it emit energy according to different laws of thermodynamics. Ouch Willis. That’s… that’s simply said Willis unforgivable error in physical understanding.
The simplest chemical engineering minor student in a Jr College Willis, knows this to be utterly ridiculous and yet you say it to me
like you expect to say it and not be somehow flagged as utterly remiss, in even the foundations, of physical science. It’s the kind of thing one would mark-off a 16 year old for putting on a test, on energy and matter.
“Matter that is refined and graded through handling by man emits energy according to
the same laws of physics in matter as unrefined ore.”
True
False
It’s a sixteen year old class question and you’re arguing with people who have actual careers managing energy in matter, about their not understanding how it can really be true.
No Willis. You really need to get some authentic education in thermodynamics so you can join the people who actually know what we’re talking about relative to atmospheric energy.
Sorry bro but the public needs to be protected from you if you can’t even be bothered to realize refined ore and unrefined ore, emit energy according to the same laws of thermodynamics.
That protection comes from having other people point out that the things you’re saying don’t even pass the judgement required in a test for a 16 year old Willis, because those of us who have some education and working functioning credentials, know far better and want others to know just how far what you’re saying has slipped into the realm of “and from that point on not a single word he said made much more sense than that statement.” The one about how refined ore emits energy according to different laws of thermodynamics than raw ore in the ground.
Willis just because you don’t have anyone call you to a halt with your misleading of laymen campaign doesn’t mean you aren’t harming the perception of people about atmospheric energy;
and if you can’t be bothered to say things that actually make functional sense, you need to stop arguing that the professionals who come by seeing you slaughter the laws of physics
deserve to go educate them or our selves.
You’re the one without the education and career in thermodynamics
insisting
refined man made material
emits energy
according to different laws of thermodynamics,
than raw ore
lying on the ground.
That’s not just intellectually incompetent it’s outright insult to expect people to come by and not object to it’s being touted as a scientific possibility:
much less a reality.
I’ll leave you and bid you good night – but you need to think about the claims you make to people Willis.
And no I don’t need to hear about how catching you claiming “unrefined ore, and refined ore emit energy according to different thermodynamic mandate”
means because of some chant or invective, I was the one who got caught making such bizarre claim.
Thing is Willis I don’t even dislike you: I just recognize you as an utterly untutored wannabe in so far over your head you can’t even begin to dream how swiftly the laughing starts.
You don’t deserve some free pass to publish bull*** even a sharp 15 or 16 year old would catch and you’re not going to get one from the very people whose fields you couldn’t pass into
if asked if natural and man made material emit energy
according to the same set of thermodynamic laws.
If you were trying to get a job as a high school dropout who wanted to install fire alarms you might well run into the test question “Refined ore and unrefined ore obey the same set of thermodynamic laws: true/false.”
You would be viewed as too scientifically illiterate to give the correct answer to that. Do you think you’re enhancing your legacy by suggesting to readers they get an education when they catch you peddling all this as reality based science?
For shame,
for real.
You should really ask yourself how many perfectly correct and honest well meaning people you’ve unleashed this torrent of fantasy physics onto Willis because I have no doubt if you’ll try it on me you’ve done it scores of times before.
It’s just a shame what utter lack of conscience about what one will publish will bring to a man.
For shame.
I’m not quite sure I follow you on the ‘parasitic loss analogy’ here, Willis. Aren’t the convective processes (conduction/convection/evaporation) simply the mechanism by which the heat moves through the system (the troposphere), from where the heat enters (surface) to where it exits (ToA)? And aren’t they simply getting stronger the more powerful the surface heating becomes and weaker the lesser the surface heating?
They’re not reducing the efficiency of the heat engine, they’re the result of the efficiency of the heat engine: surface heating, lapse rate -> temp difference between the two endpoints + atmospheric weight.
The true ‘hidden flaw’ here lies in treating the atmosphere as a second provider of energy to the surface, as if it adds an extra INput.
In the real world, the warm body (the surface) receives no energy from the cold body (the atmosphere) at all. It delivers energy to it.
Radiation (in the form of EM waves) is of course being emitted in all directions, but the FLOW OF ENERGY between two bodies at different temperatures (not to say the least, where the one body actually heats the other one), ALWAYS and ONLY goes one way, from hot to cold.
This seems to be a very hard concept for people to grasp. Much easier then to naively imagine two opposite and distinctly separated ‘highways’ set up between the two bodies where energy in the form of ‘light particles’ can be transported back and forth.
No, simply compare it with an electrical current or a flow of air (wind) between a high pressure and a low pressure. It’s all about gradients/differences in POTENTIAL.
Individual electrons naturally fly around in all directions, but the CURRENT moves only one way. Driven by the voltage across the circuit. Likewise, individual air molecules naturally fly around in all directions, but the FLOW of air, the wind, moves only one way. From high to low pressure.
The size of the difference in potential, the gradient between high and low, whether it’s represented by voltage or pressure … or temperature, determines the strength of the flow (resistance disregarded).
Follow the HEAT through the system. It’s all that matters. Everything else is just confusing the matter.
165 W/m^2 worth of HEAT comes IN to the surface (from the Sun) and 165 W/m^2 worth of HEAT goes OUT from the surface (to the atmosphere (and space)). 68% of the outgoing heat loss from the global surface comes through convective processes, only 32% through thermal radiation (of which only about 62% goes to the atmosphere whereas about 38% goes directly to space). But the real situation is much more lopsided than even this. Radiation is not transporting energy through the troposphere, up from BoA to ToA. Convection is. As soon as surface IR is absorbed by the air immediately above it, the air is warmed, grows less dense and buoys up. It happens instantaneously, naturally, automatically. The only thing radiation works towards within the atmosphere after this is progressively cooling it to space.
James Rollins Jr says, March 26, 2014 at 9:48 pm:
“Simply ask everyone two things: if what you are saying is true, then why is it that firstly we know that all through history the management of air flow through an area determines it’s temperature much more powerfully than it’s radiative capability
and the “A” portion of it is ” why don’t we all know, of many myriad ways to manipulate and maximize the radiative heat loss of everything we have? ”
The answer of course is they’re so minimal in everyday applications
“B” is “why do we all instead know of many myread ways to manipulate and maximize the convective and conductive heat loss of things?”
The answer to which is because it’s what predominates since the beginning of time and today,”
We could also ask people why humanity still tries its utmost to perfect the capture of solar energy but NOT the capture of ‘back radiation’ energy. Why do we waste our time and effort on building solar power stations and not on ‘atmospheric back radiation’ plants? The energy flow from the atmosphere to the surface after all allegedly provides it with 345 W/m^2 whereas the Sun can only muster 165 W/m^2, the former flux being more than twice as intense as the latter one.
James, do you really not understand that metals have near-zero emissivity in the long-wave infrared, but virtually all natural substances, including metal ores have near-unity emissivity in the LWIR? Seriously?
Edim says, March 26, 2014 at 12:57 pm:
“Willis, it’s not my crazy theory. The only surface input is the absorbed solar. This input is balanced by the surface outputs: evaporation, convection and net surface LW radiation.
http://science-edu.larc.nasa.gov/EDDOCS/images/Erb/components2.gif
The downwelling LW is only one ‘side’ of the LW radiative heat exchange at the surface – the net LW flux is upwelling and is a surface output.”
Somewhere along the way Climate ‘Science’ managed to corrupt basic physical principles to such an extent that people today walk around believing this utterly fundamental and childishly intuitive truth to be false, that the solar flux is NOT the only energy INput to the surface of the Earth.
I weep for humanity.
Hehe, what on earth is ‘childishly intuitive’? Skip the ‘childishly’ term from the above.
Willis Eschenbach says, March 26, 2014 at 12:35 pm:
“For example, we know (from measurements) that the ocean constantly loses about 400 watts per square metre (W/m2) of radiated energy.”
Again with this nonsense, Willis?! This has been shown so many times to be false and still you’re regurgitating it.
We haven’t MEASURED the 400 W/m^2. We have CALCULATED it by imagining that the earth’s surface emits into a vacuum at 0 K, by splitting the radiative heat transfer equation into two separate Stefan-Boltzmann equations. You can’t do that. That is, you can do it mathematically, but then you’re no longer describing reality. The only thing we MEASURE, Willis, the only REAL energy flow here is the P/A term (the ‘heat’) in the former equation.
I didn’t read all of the comments but it seems one would also need to measure any changes in reflected SW to determine all the gains/losses over time.
Willis, the Bejan et al. modeling has been extended since the first publication in 2005. The most recent version of which I am aware has appeared in at least three places as follows:
Clausse, M., Meunier F., Reis, A.H. and Bejan, A. (2012) ‘Climate change, in the framework of the constructal law’, Int. J. Global Warming, Vol. 4, Nos. 3/4, pp.242–260. https://dl.dropboxusercontent.com/u/26966144/Climate%20change%2C%20in%20the%20framework%20of%20the%20constructal%20law%20.pdf
http://www.earth-syst-dynam-discuss.net/2/241/2011/esdd-2-241-2011.pdf
Earth Syst. Dynam. Discuss., 2, 241–270, 2011 http://www.earth-syst-dynam-discuss.net/2/241/2011/ doi:10.5194/esdd-2-241-2011
http://www.earth-syst-dynam-discuss.net/2/C243/2011/esdd-2-C243-2011-supplement.pdf
M. Clausse, F. Meunier, A. H. Reis, and A. Bejan, Climate change, in the framework of the constructal law
https://dspace.uevora.pt/rdpc/bitstream/10174/6817/1/Using%20the%20Constructal%20Law%20to%20predict%20climate%20changes.pdf
F. Meunicr, M. Clausse, A. H. Reis and A. Bejan, Using the Constructal Law to Predict Climate Change versus Atmospheric Properties, Proceedings of the Global Conference on Global Warming 2011, 11-14 July, 2011, Libson Portugal.
It appears people are talking around each other. Willis defined his view of parasitic losses. There is nothing wrong with his calculations based on his definitions. I think most of the objections come from disagreements with his definitions. Nothing wrong with that either but that doesn’t mean his calculations are wrong. They are just different.
Willis gets a % number based on total downwelling LWIR + surface SW (18%). One could also compute a % number based only on surface SW. The number would be substantially different (~65%). To me neither number is right or wrong unless it is used inappropriately.
Edim says:
March 27, 2014 at 1:32 am
Two comments on that, Edim.
First, why do you think people call something “INPUT”? Because it is the total of what is going INTO something else, and so of course it ignores what is going OUT OF the something else in question.
For example, the INPUT to a car is gasoline, that is to say petrol. It is not petrol plus some of the exhaust. Exhaust is OUTPUT from a car, fuel is INPUT. Surely you don’t claim that the input to the car is petrol plus exhaust, do you?
As a result, the idea that something called “surface input” should measure anything but the input to the surface makes no sense.
My other point goes deeper, which is that if you want to engage in a discussion, you need to use the terminology of that discussion. If they are talking Russian, it’s useless to insist that the right way to go about it is to speak French.
Similarly, when we are talking about surface input (curiously defined here as what goes INTO the surface, it is useless to insist that the right way to do it is to say that “surface input” means all of the input plus some but not all of the output. All that happens when you insist on speaking French is that you get left out of the conversation …
Doesn’t mean you’re wrong, French may indeed be better … but not when everyone’s speaking Russian.
w.
James Rollins says:
March 27, 2014 at 2:40 am
james, I never said that. You made up that fake quote root and branch.
Putting that load of bollocks out and claiming it is a direct quotation of my words is an extremely dishonest and dishonorable tactic.
w.
James Rollins says:
March 27, 2014 at 3:18 am
Actually … there is a fundamental difference. It is in the very thing we are discussing, the ratio between radiation and conduction/convection.
Suppose we have a solid lump of aluminum ore. It loses little by convection/conduction, and mostly by radiation.
Suppose we replace it with a motorcycle head, same weight of aluminum, with all kinds of cooling fins.
The motorcycle head will have a different ratio of radiation and conduction/convection, since much more of its heat will be lost through conduction/convection due to two factors: physical shape and heat conduction.
The motorcycle head has fins, and is made of a metal which rapidly conducts heat.
The lump of ore has no fins, and is made of ore which is a much poorer conductor of heat.
If you think the two of those, the natural material and what we shape it into, have the same ratio of heat loss (radiation vs conduction/convection), I fear I can’t help you.
w.
rgb, I think it’s misleading to count only one direction (downelling) of the LW radiation. The surface input is either the absorbed solar only (SW) or the net radiation (SW + LW), which is also acceptable. SW + LW(downwelling) as a surface input makes no sense and it’s misleading IMO.
I have to keep this short — quizzes to write, class to teach — but it is a simple matter of fact that treating the surface as SW + LW downwelling DOES make PERFECT sense — in a single layer model such as the one Grant Petty walks you through in A First Course in Atmospheric Radiation. I promise — fact, not opinion, as proven by the fact that it is easy to turn the concept into a quantitative model. One cannot turn senseless ideas into quantitative models.
Nowhere did I or does the theory assert that only one direction of LW radiation is treated — this is your imagination, not the actual theories. Again I refer to Petty, where he treats absorption and emission as symmetric processes (as they must be according to Kirchoff’s Law!) with the same coefficient. In a single layer model, the atmospheric layer absorbs some fraction of upwelling surface radiation and reradiates it both up and down. The up component is part of the energy balance loss that keeps the atmospheric layer itself in detailed balance. The down component is pure gain for the surface, added to the SW (that makes it through the atmosphere) gain. This total gain has to be balanced by the LW upwelling loss in steady state (plus, as Willis points out, a much less well defined loss associated with conductivity and latent heat transfer — it isn’t only about the radiation).
None of the models are senseless (at least, not deliberately so as anybody can make an error) — at worst they are incomplete, oversimplified, and/or just lead to incorrect answers for the processes they attempt to model.
Also, I do not understand what the difference is between SW+LW as a surface input and SW+LW (downwelling) in your statement above. Surface inputs are by definition going to be downwelling (except for trivial amount of e.g. geothermal heat). Surfaces losses are going to be upwelling. Both of these might as well be written as SW(downwelling) + LW(downwelling) or “total radiation(downwelling)” — there is no difference between the four descriptions except in how you partition downwelling radiation by wavelength. So I cannot understand your objection quite aside from your mistake in calling any of these partitionings senseless.
rgb
Kristian says:
March 27, 2014 at 7:37 am
Glad you asked. The answer is twofold.
1. We can’t harvest thermal IR the way that we do solar energy (photocells). While it assuredly contains energy, the thermal IR simply doesn’t have enough energy to kick an electron out of its orbit … so no photoelectric harvesting is possible.
2. When photoelectric is ruled out, that only leaves a heat engine to harvest the energy. The problem with that is that a heat engine works off of ∆T, a temperature difference. As a result, we’d need a colder place to exhaust the waste heat to … and the incoming heat is only at about 0°C or so on average. The atmosphere straight up a few miles is generally the coldest place within a few miles, by a long ways … so we have no place to exhaust the waste heat.
So the answer to your question, why doesn’t humanity try to harvest the back radiation, is that we haven’t figured out a single way to do that, or even an approach that might possibly work.
Doesn’t mean it doesn’t exist, however, that’s nonsense … but like many kinds of energy, we haven’t figured out any way to utilize it.
Hogwash. Absolute hogwash.
The downwelling IR has indeed been MEASURED, to use your capital letters, thousands and thousands of times, all over the planet. We have special instruments to measure it. We have people whose job is to measure it. We have scientists who’ve spent their lifetimes measuring downwelling IR. Have you been asleep for the last century? You can measure the downwelling IR with a cheap IR thermometer, for heaven’s sake.
Your bizarre claim that we haven’t ever measured the downwelling IR is a perfect example of your terribly flawed understanding of the situation, Kristian … do a google search and you find hundreds of articles describing what you say doesn’t exist, their measurements of downwelling IR.
Of course you might not have noticed them, because often they have deceptive titles like Measurements of Downwelling Infrared Irradiance … read that, and come back and tell us what you found out.
w.
Dan Hughes says:
March 27, 2014 at 8:38 am
Dan, my thanks, and good to hear from you as always. I am aware of (although not fully conversant with) Bejan’s later work, but I usually use the earlier one because it’s more of an introduction to the subject.
All the best,
w.
Thank you Willis for a new installment in what looks to be developing into a comprehensive text on real climate science. Looking at the figs 5 and 6 blue traces and imagining a transition from night to day in a single grid cell suggests that losses at the low input end for the oceans has a dominant conduction component into the the atmosphere, the mid range a conduction-convection dominating transitional range followed at the higher input by a dominant latent heat range particularly aided by a jump in the convection component since higher water vapour reduces atmospheric density at the surface.
Willis, don’t you understand that when you read a mercury “thermometer”, you aren’t measuring temperature. You are simply measuring the height of a column of mercury. Then you are relying on a mathematical model of the column in which the mercury resides, and another mathematical model of the properties of mercury, to calculate the supposed temperature.
Need I say ?
The last line was supposed to be:
Need I say “/sarc”?
rgbatduke says, March 27, 2014 at 11:07 am:
The point here is that you let the postulated ‘downwelling LW’ component from the cooler atmosphere alone (not in collaboration with other fluxes) raise the temperature of the warmer surface (increase its internal energy) directly (not indirectly) and in absolute terms (not in relative terms). Such an energy flow with such a result is defined in physics as HEAT (or work). And heat in nature does not and cannot go from cold to hot. You seem completely impervious to this simple demonstration that there is clearly something fundamentally wrong (and/or misunderstood) with the traditional ‘Prevost energy exchange principle’.
Compare an earth with an atmosphere without so-called GHGs (but with albedo unchanged) with an earth with an atmosphere with so-called GHGs.
In the first situation the global surface absorbs an average solar flux of 239 W/m^2 and then, according to you, emits an equal flux directly to space to balance it. This according to you gives a mean surface temperature of 255K.
In the second situation the global surface absorbs an average solar flux of 165 W/m^2 which alone would’ve set the mean temperature to 232K. So how do we get to 288K? What’s changed? Is the solar increased? No. Is the resulting outgoing radiative flux from the surface reduced? No.
We ADD an extra energy flux, from the cooler atmosphere. That’s the (only) difference. And by that go from 232K to 288K. Your added 345 W/m^2 from the atmosphere to the surface in no way reduces the outgoing flux. It INCREASES it. Your 398 W/m^2 going out could not reach that level without the added flux from the atmosphere: 165+345 (510) = 112*+398 (510) (*convective losses).
So you let the 165+345 heat the surface FIRST (well, actually after strangely having subtracted the convective losses even before this: (165+345) – 112 = 398) and THEN only is the surface allowed to cool.
So in reality what you do is treat the 345 flux as a second HEAT flux to the surface, because it (and ONLY it) increases the internal energy of the surface (and thus the outgoing flux) and thereby its temperature … directly and in absolute terms. You will of course never admit to this, but that is what you DO (if not saying). Worse than that even, what you ultimately end up claiming is that any object can raise its OWN temperature purely by absorbing its OWN previously thermally emitted and then recycled radiative energy loss.
This is impossible in nature. In fact, it’s ridiculous. The laws of thermodynamics simply do not allow it.
The only reasonable (and reality oriented) way to set up the energy (heat) budget for the earth’s surface is like this:
ENERGY IN: 165 W/m^2 = ENERGY OUT: 53 [398-345] W/m^2 + 112 W/m^2
Edim is completely correct. Your postulated DWLWIR flux is a component of the surface’s energy OUTPUT, not INPUT.
Curt says, March 27, 2014 at 1:01 pm:
“Willis, don’t you understand that when you read a mercury “thermometer”, you aren’t measuring temperature. You are simply measuring the height of a column of mercury. Then you are relying on a mathematical model of the column in which the mercury resides, and another mathematical model of the properties of mercury, to calculate the supposed temperature.”
*Sigh*
You’re just displaying your utter lack of understanding, Curt.
What you describe above is equivalent to the change in the voltage in a pyrgeometer sensor detecting a ‘heat’ flux. The physical conversion is direct and firsthand: reading > conversion. Same with a speedometer.
The equivalent between stating that a 400 W/m^2 upward component is ‘measured’ and the thermometer would be if, AFTER you’ve observed the height of the mercury column, you applied a formula to claim that the temperature shown in reality is made up of a cold component and a hot component, as if the air in contact with the thermometer contained a volume at 280K and another one at 300K and therefore what you read off the column is actually a ‘net’ temperature of 290K.
I don’t expect you to understand the difference, Curt, but most likely other readers will.
Willis
in the link you provide claiming “proof of 400 wm sq”
the graph showing nightly readings
on site at your claimed proof it IS
measured at 400
it says clearly on Figure 3,
“Figure 3 Time series of downwelling infrared irradiance
for May 21, 2007. A clear sky was present from
midnight-midnight local time.
350 – 353 Watts/Sq. Meter.
I don’t know how important it is to the argument you’re having with the other man but the fact is
your link clearly disproves what you said at least in the instance of the study you provide
Willis Eschenbach says, March 27, 2014 at 11:20 am:
“Of course you might not have noticed them, because often they have deceptive titles like Measurements of Downwelling Infrared Irradiance … read that, and come back and tell us what you found out.”
Yes, and here is HOW it’s ‘measured’, Willis:
http://en.wikipedia.org/wiki/Pyrgeometer#Measurement_of_long_wave_downward_radiation
http://tallbloke.wordpress.com/2013/04/26/pyrgeometers-untangled/
You’ve been shown this several times before and you just continue to close your eyes. Your pigheadedness on this particular subject is what’s bizarre, Willis.
Wilis obviously it’s easy to check on your claim of 4/5ths loss via radiation from natural materials and it’s simply not coming up true. In spite of the fact that you claim natural material emits 4/5ths of it’s energy via radiation I checked the ratios for stone and everywhere the heating and cooling industry claim convection and conduction are primary removal means for energy when stone’s used.
Your claim is simply not within the realm of even the possible Willis
much less the probable.
If you expect to talk science with people Willis what you say had better meet with the ”that sounds like what I know to be true” across a wide swath of fields and one of the largest that your claim flies into the face of is everything mankind ever built or builds.
Everyone remembers being taught some thermodynamics.
Nobody remembers being taught radiant loss predominates in nature or we’d all agree it sounds right: if it were different your friends would be in here with every single link from every single building insulation site describing how all structures radiate, predominately;
but they don’t; because the fact is the vast majority of structures made by man cool primarily, convectively/conductively.
Everything you claim is propped up on tenuous arguments demanding everyone reading what you say utterly disregard the entire history of human endeavor keeping heat in and out of structures.
The entire industry of architectural thermal management is fighting against the predominating 4/5ths radiation as thermal loss then lying about it on tables online, around the world, claiming in structures actually are losing the majority of their heat via conduction and convection.
Because they’re part of big oil?
Because they’re against the science?
No what has happened is you’ve come into a field where every fake bulls ** story you tell is as easily checked
as simply comparing what you claim,
to what I, and hundreds of thousands, nay millions of others know, and have made a lot of our living displaying we know.
Your claim is that the millions and millions of people who have referred to architectural thermal management sites in being told most everything mankind can find to build with has a larger conductive/convective heat loss than radiative transfer
All those people are utterly clueless that right next to us, to them, all over the world, everything else has a 4/5ths radiant loss ratio and it’s only the wood, and stone, and paint that mankind has touched,
that has a predominating convective/conductive loss.
Do you understand the absolute maximum goofy associate with that claim Willis?
You should have but obviously you don’t. Well heres a hint:
that assertion pegs the ”absolute goofy” meter and keeps it pegged as you try to find anything to say, to keep from going and checking how everything loses heat, yourself.
Using something other than climate speculators’ assertions as your personal byword for scientific excellence because they’ve already got you claiming man made materials emit primarily conductively/convectively,
while forty feet away the very forest they were taken from
are busily radiating 4/5ths of theirs. No they are not. Not remotely.
No Willis which is why the only thing you can think of is “No it ain’t.”
There’s no such thing as a “man made vs natural materials” conduction/radiation ratio that everybody knows and remembers to refer to, when thinking about insulating, or heating, or cooling something.
There’s not a materials science on earth of any significance, which claims that material’s primary method of energy loss to the atmosphere is radiant.
Kristian says:
March 27, 2014 at 1:16 pm
“By his own Bootlaces” might one add dear and rare teller of sensible climate related science relative to the big Non-Warming issue that some seem to be letting slip away.
But maybe one or more will properly react to the fact that you wrote of 400Wm2 of (calculated) rising LW and not the DWIR substituted in insulting reply an then go on to tellus how it can, at 0degC he says, help to fry our eyeballs but not drive any realistic energy converter.
My nearest CH radiator to where I am sitting which can can belch out about 3kW when fed with 85degC watter can`t raise my little IR thermometer to 40degC even when placed one centimeter away from its metal surface nor warm my bare face against the -40 or so degC DWIR pouring in on me from the night sky through the bare glass of the window above it. NO?!
What you say Willis sounds like pseudo-science.
What this says, sounds much more like actual science:
How Much Water Is Evaporated Into the
Atmosphere Each Year?
On average, 1 meter of water is evaporated
from oceans to the atmosphere each year.
The global averaged precipitation is also
about 1 meter per year.ESS55
Prof. Jin-Yi Yu
How Much Heat Is Brought Upward By
Water Vapor?
Earth’s surface lost heat to the atmosphere when
water is evaporated from oceans to the atmosphere.
The evaporation of the meter of water causes Earth’s
surface to lose 83 watts per square meter, almost half
of the sunlight that reaches the surface.
Without the evaporation process, the global surface
temperature would be 67° C
instead of the actual 15° C.”
Evaporative cooling.
Convective cooling.
Conductive cooling.
Finally dead last, comes radiant transfer.
That’s what real science teaches about the real world.
Evaporative and Convective being spoken of as inter-related, the fact is, radiation comes dead last in the real world where people actually are right when they repeatedly assert something is science.
James Rollins Jr says:
March 27, 2014 at 5:29 pm
Still no observations facts, citations, or quotes to support your claims? I’m totally uninterested in your further claims until you come up with evidence.
w.
James Rollins Jr says:
March 27, 2014 at 5:40 pm
Dang, James, actual numbers. You surprise me in a good way.
Yes, I agree that somewhere around 80 W/m2 of energy is lost from the surface by evapotranspiration, and that this is around half of the solar absorbed by the surface. This agrees also with the KT energy diagram, as well as the CERES data.
Now, we know that the upwelling LW from the surface is about 400 W/m2. We derive these figures in a couple ways, both from ground measurements and from satellite measurements.
In addition, as we agree, about 80 W/m2 is lost from the surface by evapotranspiration. That makes 480 W/m2.
So … how much energy goes from the surface to the atmosphere by sensible heat.
I say it’s about 20 W/m2 or so. Estimates range from 15 to 20 W/m2. See Table 1b here for the estimates and their sources.
In order for your theory to be true, the total of sensible and latent would need to be well over the 400 W/m2 lost by radiation … which means sensible heat loss would have to be ~ 350 W/m2 for your idea to hold water.
Now, if you have evidence of that purported huge sensible heat loss of ~350 W/m2, bring it on … me, I’ve never seen it.
Regards,
w.
James: You could have saved yourself a huge amount of time and effort if you had simply said, “Willis, I think you should be talking about net radiative transfer from the surface, not gross radiative losses from the surface.”
That’s all your objections boil down to. Then we would have had something to discuss – when it is better to use gross flows and when to use net.
But even if we are talking about the net radiative transfer from the surface, it is about as big as the latent heat transfer, and significantly larger than the sensible heat transfer.
Willis: I had a fun problem today in my day job that is somewhat related to our discussions here.
One of our customers is using electronic power amplifiers whose heat sinks are black anodized to enhance the radiative transfer that helps cool it. In wiring up the system, they tried to ground the amplifiers through the heat sink surface, not realizing that the anodized layer is a very poor electrical conductor. The result was that they really were not grounded at all, which led to all sorts of spurious electrical problems.
Of course, you cannot enhance the radiative transfer to nearly the extent that you can enhance conductive/convective transfers (you cannot achieve emissivities higher than 1.0). I’ve recently been arbitrating arguments between our own power electronic designers, who want the most powerful possible fans on our heat sinks, and our marketing guys, who want the quitest possible fans. (With forced convection, you can get many times the transfer of free convection.) Always the tradeoffs…
In the climate system, two main parasitic losses are the losses from the surface to the atmosphere by way of conduction and convection (sensible heat), and the losses from surface to atmosphere by way of evaporation and transpiration (latent heat). Both of these parasitic losses act to reduce the surface temperature with respect to the overlying atmosphere, by simultaneously cooling the surface and warming the atmosphere
To me, what you are describing as parasitic loss, is actually the intended function of the system. It is the work that is needed to be done at the surface to transport the latent heat and sensible heat for release aloft. The “engine” doesn’t function until the temperature differentials develop via SWR, then the evaporation / lapse rate cause instability to develop and physically transport the heat away. The largest effect is transport of latent heat. The transport system emerges (as you have described before), which carries the energy aloft until water droplets condense, then the droplets act as black bodies and radiate the heat to space, nearly uninhibited by GHGs above at that point. The engine functions like any conventional air conditioner, using the condensing gas H2O, except that its primary heat rejection mode aloft is radiation. The process is a shunt that develops on demand to dump the excess heat basically directly to the drain (space), as you have also described as emergent phenomena. The more heat available, the more work is done by the condensing gas, until the energy is disposed of and the differential collapses. If this is a parasitic loss, what is the intended “work” of the system that is not a loss (its useful function)?
Kristian, you say:
“Your postulated DWLWIR flux is a component of the surface’s energy OUTPUT, not INPUT.”
So the downwelling flux is really the surface’s (upwelling) output. Down is up. Yikes! Orwell would be proud. That takes a level of confusion far more profound than I thought you had in you.
Heat transfer is a process, not a thing. Nobody (but you, apparently) believes in the 19th century caloric theory of heat transfer anymore. Please get into the 20th century, if not the 21st. Radiative heat transfer is simply the difference between two opposing electromagnetic radiation energy “flows”, as in upwelling vs downwelling. This has been well understood for a hundred years now.
I have a question that I need to clarify in my mind.
On a clear sky, no haze or visible clouds but with the air at say 60% humidity: is the water vapor still a greenhouse gas? It would would seem to be as the water is still there.
Willis, rgb,
The radiative (LW only) heat transfer at the Earth’s surface, which cools the surface, equals LW↑ + LW↓ = LW. It is about 60 W/m2 (~ 395 minus 335) in global and annual average, according to the various Earth’s surface energy budgets. You have to take the net radiative heat exchange, because that is what carries away the energy (or heat) from the surface. The evaporation is ~80 W/m2 and convection ~20 W/m2. These 3 are balanced by the solar input of ~160 W/m2. Of course, it makes no difference mathematically to view LW↓ as an input and LW↑ as an output, but physically it’s very confusing, misleading and also wrong, IMO. It makes the radiative surface cooling output look larger than it is (395 instead of 60 W/m2), as well as the surface input (495 instead of 160 W/m2).
EDim said:
The evaporation is ~80 W/m2 and convection ~20 W/m2.
Both those are proposed as net cooling effects at the surface but:
I) Water vapour that evaporates also has to condense and rising air cools at the moist adiabatic rate yet descending air warms at the dry adiabatic rate (twice as fast) so I have some doubt as to whether the net effect is of cooling at all.
ii) Adiabatic cooling on ascent is reversed by adiabatic warming on descent so I doubt a net cooling for adiabatic convection.
If surface cooling from those two processes has been overstated then where doe that leave DWIR ?
Curt says, March 27, 2014 at 9:02 pm:
“Heat transfer is a process, not a thing. Nobody (but you, apparently) believes in the 19th century caloric theory of heat transfer anymore. Please get into the 20th century, if not the 21st. Radiative heat transfer is simply the difference between two opposing electromagnetic radiation energy “flows”, as in upwelling vs downwelling. This has been well understood for a hundred years now.”
Then you absolutely don’t get what I’m saying, Curt. Or you do and you’re just deliberately trying to obfuscate it.
I’m not talking about ‘the caloric theory’. That’s simply you trying to degrade my argument (nice rhetorical tactic). I’m talking about HEAT. Heat remains the same phenomenon today. Here’s the modern definition of heat:
“If a block of hot copper is placed in a beaker of cold water, we know from experience that the block of copper cools down and the water warms up until the copper and water reach the same temperature. What causes this decrease in the temperature of the copper and the increase in the temperature of the water? We say that it is the result of the transfer of energy from the copper block to the water. It is from such a transfer of energy that we arrive at a definition of heat.
Heat is defined as the form of energy that is transferred across the boundary of a system at a given temperature to another system (or the surroundings) at a lower temperature by virtue of the temperature difference between the two systems. That is, heat is transferred from the system at the higher temperature to the system at the lower temperature, and the heat transfer occurs solely because of the temperature difference between the two systems.”
“Heat, like work, is a form of energy transfer to or from a system. Therefore, the units for heat, and for any other form of energy as well, are the same as the units for work, or at least are directly proportional to them. In the International System the unit for heat (energy) is the joule.”
You see, Curt, this definition holds also for radiative situations. According to the ‘energy exchange principle’ (which actually originated in the 18th century (Prevost), a very crude and ‘macroscopic’ concept, but which is still applied pretty much unchanged today, even long after it was discovered that the quantum world does not operate in such a ‘macroscopic’ manner – but hey, this appears to be totally fine with Curt) the heat is defined as the NET transfer. But the heat is still the heat. You can’t separate the two (postulated) opposing ‘streams’ of energy that make up the heat. They’re not separate entities. They’re part of one and the same process. One and the same energy (radiation) field. The transfer occurs instantaneously, automatically, spontaneously.
This is why it is COMPLETELY and UTTERLY un-physical to treat the smaller (least intense) of two such ‘streams’ as an independent ‘thing’ and, as a result, treat it like a separate energy INPUT to the object emitting the larger ‘stream’. As if it were a separate addition of energy to the hot system, that is HEAT.
So yes, Curt, heat transfer is a process, not a thing. You’re the ones treating it like a thing. That can allegedly be split up into two separate energy streams.
The spontaneous transfer of energy, the FLOW of energy, between two objects at different temperatures ALWAYS and ONLY pass from hot to cold. Just like wind from high to low pressure (even as individual air molecules fly around in all directions). Just like an electric current from high to low potential (even as individual electrons fly around in all directions). And this holds true even when radiation is involved. The thermodynamic laws apply to radiative heat transfer also, Curt. I know you would like to argue that somehow they don’t really, but I’m afraid they do.
For an atmosphere as a whole it must be the case (if the atmosphere is to be retained) that UWIR and DWIR nets out to zero for PROCESSES OCCURRING WITHIN THE ATMOSPHERE.
That leaves incoming solar energy and outgoing IR to space in balance. It just gets a free pass straight through whilst the atmosphere does its own thermodynamic thing by way of a mix of radiation and conduction and the balance between the two is mediated by convection.
It is atmospheric mass that allows conduction to occur.
Conduction being slower than radiation the surface temperature must rise above that required for the immediate in/out radiative energy exchange predicated by the S-B equation.
Circulation changes prevent differences in conductive capability of molecules destabilising the system Why should the same not apply to differences in radiative capability of molecules ?
eyesonu says:
March 27, 2014 at 9:06 pm
Assuredly.
w.
Edim says:
March 28, 2014 at 3:13 am
The cooling output IS 395, and the surface input IS 495 W/m2, Edim … that’s what “output” and “input” mean. Output means what is leaving the surface. Input means what is entering the surface.
Of course these are different from the net flow of energy … so? Those are in fact the inputs and the outputs, whether you like it or not.
Look, Edim. Is saying I paid $300, and I got a $100 rebate “very confusing, misleading and also wrong” to you?? Because that’s all I’m doing. If you think saying “I paid $300 and got a $100 rebate back” is very confusing and misleading to you, I fear I can’t help you.
Because that is EXACTLY THE SAME as saying that the surface radiated 300 W/m2 and received 100 W/m2 in back radiation.
Please note that either way is correct. You can either deal with net flows or individual flows. However, for a situation of any complexity, in order to do the math you pretty much have to deal with individual flows … and that’s how it’s been done for hundreds of years.
So I truly don’t understand your ceaseless objection to dealing with individual flows. That’s how people do these kinds of calculations, Edim, whether for money or for radiation, so you might as well get used to it or else you’ll be complaining about it for the rest of your life. The world ain’t gonna change how it does the math or discusses the flows just because you don’t happen to like it …
w.
Thanks Willis.
That leads me to the next question. Assume a column of air with a given amount/quantity of water. At the lower/warmer altitude the water vapor is visually transparent while at a little higher altitude pressure/temp decreases causing some of this water to condense to a lliquid or frozen state (clouds). Would the change of state change the overall GHG effect with regards to the entire column of a fixed quantity of water? I guess in more of a simple way of looking at this; would be would there be a difference in the GHG effect if the water (fixed amount) was in the form of a cloud or completely visually transparent?
There are more questions to come but I want to address a single aspect at a time. I’m breaking it down to specifics one step at a time.
My grammer in the above post leaves much to be desired.
Quote: I guess in more of a simple way of looking at this; would be would there be a difference in the GHG effect if the water (fixed amount) was in the form of a cloud or completely visually transparent?
Please disregard the first “would be” in that sentence. Multi-tasking on my end. 🙂
eyesonu asked:
“would be would there be a difference in the GHG effect if the water (fixed amount) was in the form of a cloud or completely visually transparent?”
The basic rule is that the more transparent the atmosphere the greater proportion of solar input reaches the surface.
The S-B equation relies on 100% transparency to a perfect blackbody (the Earth is not a perfect blackbody) and also relies on radiation arriving at the surface being radiated out immediately as can only occur with no atmosphere. Additionally,Earths oceans complicate the situation by introducing considerable variability between energy in and energy out, hence ocean cycles in general and ENSO in particular..
The more solar input reaches the surface the higher the surface temperature can get at any given level of insolation and the more conduction can occur to the mass of the air above. Deserts with dry, descending air above are a good example.
The less solar input reaches the surface the lower the surface temperature can become at any given level of insolation and the less conduction can occur to the mass of the air above. Tropical jungles with lots or water vapour taking energy upward are a good example. They do not attain the surface temperatures of deserts due to the abundant presence of that GHG water vapour. That is because water vapour is lighter than air and so more convection occurs in humid regions rather than high surface temperatures.
Deserts tend to be at higher latitudes than the equatorial jungles due to the Earth’s Hadley cells yet the surface temperatures are higher than at the equator where insolation is greater.
GHGs reduce atmospheric transparency so they must also reduce the amount of solar energy reaching the surface thereby causing a lower surface temperature than otherwise would have been the case and less conduction.
That is the precise opposite of AGW radiative theory. That theory suggests that DWIR from GHGs adds to the solar input to the surface over and above what would have reached the surface beneath an atmosphere as transparent as the one in which they float.
I don’t follow that reasoning.
If GHGs do introduce net DWIR towards the surface then surely that cannot be any greater than the opposite effect of their reduction in atmospheric transparency otherwise one breaches the Laws of Conservation of Energy ?
A cloud, being water vapour condensate (liquid rather than gas) greatly reduces atmospheric transparency and so must have a greater cooling effect on the surface than water vapour.
It all comes down to atmospheric transparency in the end.
AGW theory ignores the adiabatic warming of descending air and so to balance the books needs to propose net downward infra red radiation warming the surface.
That results in double counting.
In any atmosphere, that is retained long term, around a planet, UWIR and DWIR must cancel out to net zero within the atmosphere as a whole.
Any long term discrepancy between DWIR and UWIR within the structure of an atmosphere must result in the loss of that atmosphere.
Atmospheres always settle at a sustainable vertical thermal structure and that is revealed by the lapse rate (or collection of lapse rates) for that particular atmosphere.
GHGs cannot be permitted to permanently upset that vertical thermal structure otherwise the atmosphere will be lost.
Instead, the circulation of air within the atmosphere simply changes via changes in convective overturning to cancel out any potentially destabilising influences such as changes in the radiative or conductive capability of constituent gases.
eyesonu says:
March 28, 2014 at 10:07 am
Water vapor, like the other GHGs, absorbs only part of the upwelling radiation. On average it absorbs about 30% in clear-sky conditions.
Clouds, on the other hand, are basically black bodies w.r.t. longwave radiation. So there is definitely a difference. One absorbs 30%, and the other absorbs 100%.
w.
The point here is that you let the postulated ‘downwelling LW’ component from the cooler atmosphere alone (not in collaboration with other fluxes) raise the temperature of the warmer surface (increase its internal energy) directly (not indirectly) and in absolute terms (not in relative terms). Such an energy flow with such a result is defined in physics as HEAT (or work). And heat in nature does not and cannot go from cold to hot. You seem completely impervious to this simple demonstration that there is clearly something fundamentally wrong (and/or misunderstood) with the traditional ‘Prevost energy exchange principle’.
I have no idea what you are talking about with your directly/indirectly. Flux is flux. Nor do I understand what you are talking about with your introduction of absolute vs relative terms. I was quite clear in my definitions and what is being compared, and Grant Petty is even clearer when he writes out term by term a physics based single layer model and finds the fixed point. In actual fact, when I refer to a single layer perfect SW transmitter, perfect IR absorber atmosphere and the 1.19x surface warming it produces via downwelling radiation from the interpolated perfect absorber layer, I am talking about relative warming, relative precisely to the temperature the surface would have with identical incoming insolation but with no interpolated differentially absorptive layer. Under no circumstances can the energy flow involved be called work, at least not past the molecular level, and at that level there is no such thing as heat as all interactions are reversible.
However, it is your final remark that at last reveals you as a closet Dragonslayer. You somehow think a) heat cannot go from cold to hot; and b) that this observation has anything to do with the process I describe.
I believe that what you mean to say is the second law of thermodynamics has to be satisfied the processes involved — and fortunately, it is enormously simple to prove that it is, especially in the single layer model, which never has net heat transfer from cold to hot in the steady state of an open system between the Sun as one reservoir and outer space as the second reservoir. In fact, in the single layer “steel-greenhouse” limit, proving that the second law is happy is one line long, and one can even compute the net entropy increase of the Universe as the heat/energy flows from Mr. Sun, through Mr. Earth, and out into Ms. Space. Consequently you are simply mistaken in your claim of b) — the fact that net heat does not spontaneously flow from a cold reservoir to a hotter reservoir has nothing to do with whether or not the Greenhouse effect leads to a warmer surface with greenhouse gases in an atmosphere than without it because this never happens in the energy flow.
Now if you want to actually get substantive about this, I’m only going to say to you what I say to Postma when he spouts this sort of crap — show me the equations that prove that the second law is violated by a perfectly valid statement of the first law of thermodynamics that leads one to the 1.19T_0 conclusion.
rgb
Stephen Wilde says:
March 28, 2014 at 12:23 pm
Stephen, I’ve basically given up reading your comments, as they are almost invariably incorrect. Some things I’m unwilling to let slip by, however. This is one of them.
The S-B (Stefan-Boltzmann) equation has nothing to do with “100% transparency” of the atmosphere. It also has nothing to do with how long the radiation might remain in the object before being re-radiated. Here is the whole equation:
Radiation (w/m2) = S-B constant * emissivity * Temperature4
You see anything in there about the transparency of the atmosphere or how long the radiation stays absorbed? … didn’t think so …
Look, Stephen, if you can bring in a reference for either of those claims, we can discuss it. But to just throw out such radical statements without the slightest attempt at support or citation? That’s the very reason I mostly stopped even reading your comments. You just blurt out whatever crazy belief you have in your head at the moment, and you make no attempt to support it with quotes, citations, observations, logic, math or anything but your mouth.
It gets old … here’s another example:
That’s not true in the slightest. It also reveals a profound misunderstanding of the physics. GHGs have almost no effect at all on the transparency of the atmosphere to solar radiation. They simply don’t absorb energy at the wavelengths of visible light. Once again, you’re just running your mouth off, spouting something that is totally untrue without any attempt to back up your BS with anything factual.
It gets old …
w.
Willis said:
“Radiation (w/m2) = S-B constant * emissivity * Temperature4”
Emissivity relates to how long the radiation stays absorbed, doesn’t it ?
100% emissivity means it comes out immediately.
Zero emissivity means it doesn’t come out at all.
and Willis said:
“GHGs have almost no effect at all on the transparency of the atmosphere to solar radiation”
Transparency works two ways.
What matters is not just the transparency to incoming solar radiation. What matters is how much delay the atmosphere introduces compared to direct and immediate in / out radiation.
That delay is a consequence of conduction to the atmospheric mass.
The conductive absorption by mass is what delays the exit of radiation to space.
A purely radiative solution involves no delay since all radiation is at the speed of light.
To get the delay you have to introduce conduction because it is slower than radiation.
Then, to hold the balance between radiation and conduction you need variable convection.
The amount of conductive absorption that can occur is affected by transparency. It doesn’t matter whether the obstruction is on the way in or on the way out.
You can argue that GHGs change the balance of UWIR / DWIR which is fine by me but if that happens then the amount of conductive absorption by the mass of the atmosphere changes too and in an equal and opposite direction.
More DWIR and more conduction, less DWIR and less conduction, either way a net zero effect on surface temperature.
The surface temperature being stabilised by the change in convection as per your very own thermostat hypothesis. The phase changes of water merely assisting the process by making the necessary air circulation changes less violent than would otherwise be necessary.
If it were otherwise then the atmosphere could not be retained.
You need a mechanism like that for your thermostat to work.
If there is a thermostat, such as you propose, then how else do you see the physics working ?
Kristian:
Once again you manage to get things completely backwards. Let’s go through it step by step.
Bodies emit electromagnetic radiation as a function of their temperature and their emissivity at that temperature. (Their overall emissivity is the integral over all wavelengths of the product of the relative emissivity at each wavelength (between 0 and 1) and the blackbody intensity at that wavelength.) The overall emissivity can, and usually does, vary with temperature. Integration can give you the overall amount of power emitted.
It does not matter what the body is radiating towards, and in most cases it is radiating towards many different bodies. In the case of semi-transparent bodies like the atmosphere, each “slice” of the atmosphere is treated as a body.
This has been well understood even down to the level of statistical mechanics and quantum mechanics for about a century now. It is utterly non-controversial.
Next step: If Body A can radiate toward Body B, then Body B can radiate toward Body A, and will do so through the same geometric path. These streams of electromagnetic radiation are, contrary to your assertion, independent in many important aspects. The path between the two objects is not independent.
We also know in a completely uncontroversial sense that two (or more) streams of electromagnetic radiation pass through each other without impeding each other. For purposes of both analysis and measurement, we can look at individual opposing streams.
For example, the two of us are standing some distance apart on a very dark night. I shine a flashlight at you. Willis, standing between us, points a couple of photosensors at my flashlight. One uses thermal effects; the other uses photoelectric effects. (They agree.)
Now you shine a flashlight at me. Willis’ sensors are still pointing toward my flashlight. By your logic, their readings should change. They don’t. The beams of our flashlights pass through each other unimpeded so we can each see each other’s light using the frequency-sensitive radiation sensors in our retinas.
Next step: What about “heat transfer”? Here we need to take into account three important factors. First, as we have already discussed, the path from A to B is the same as from B to A. Second, the blackbody curve for a higher-temperature body is higher at all wavelengths that the blackbody curve for a lower-temperature body. Third, for any substance, the relative (0 to 1) absorptivity is exactly equal to the relative emissivity at every wavelength.
Given these well-known constraints, it is ALWAYS true that more radiant power from the higher-temperature body is absorbed by the lower-temperature body than radiant power from the lower-temperature body is absorbed by the higher-temperature body. The difference is the net power transfer, and if thermalized (as opposed to, say generating current in a photo-electric device), what we call heat transfer.
What we call the “heat transfer flux” (with flux just being the Latin term for flow) is simply a somewhat useful, but definitely limited, metaphor. Despite your protestations, you are definitely treating it as too real a thing, which is why I can say that you are acting too much like the 19th century believers in the caloric theory of heat. (They had no way of knowing better at the time.)
In a fluid flow between two pressure potentials, the bulk flow rate can be determined from the average velocity of the fluid molecules, with a somewhat normal distribution about that average. In radiant transfer between two bodies at different temperature potentials, there are two opposing radiation streams each at the speed of light. (There is an average only in the sense that the average human has one breast and one testicle…) This very different underlying nature permits us to perform different styles of analysis and measurement.
Perhaps a further comment will help Willis and Curt (and others):
If DWIR and UWIR become unbalanced for the global atmosphere as a whole (i.e, if changes in convection fail to eliminate the imbalance) then surface temperature will indeed change but that would be a self correcting event.
i) If DWIR exceeds UWLIR then the surface does indeed warm but then the system radiates more energy to space than the system is receiving and the system cools until surface temperature is back where it ‘should’ be.
ii) If UWIR exceeds DWIR then the surface cools but then the system radiates less energy to space than the system is receiving and the system warms until surface temperature is back where it ‘should’ be.
That is the true thermostat hypothesis.
A double indemnity.
If convection falls short of its job then radiation completes it.
QED
Willis said:
“GHGs have almost no effect at all on the transparency of the atmosphere to solar radiation. They simply don’t absorb energy at the wavelengths of visible light”
All energy passing through the system,including outgoing IR was initially solar radiation . Absorption of outgoing IR affects overall atmospheric transparency as much as absorption of incoming solar shortwave.
Both add to the delay in transmission of solar energy through the Earth system and thus both add to the time available for conduction to occur.
It is the time available for conduction of energy to atmospheric mass that determines surface temperature and then convection ensues to ensure that the radiation / conduction balance does not get out of alignment with radiation in and out.
GHGs may absorb outgoing IR but in doing so they radiate about half of that absorbed out to space.
In contrast, non GHGs have to absorb by conduction and since they cannot radiate out to space that energy must be passed back GHGs for direct radiation out or to the surface via adiabatic warming of descending air before it can be radiated out to space from the surface.
Thus GHGs do have a cooling effect on the surface because less energy needs to be returned to the surface by adiabatic warming on descent. They provide a radiative window to space from within the atmosphere that non GHGs cannot provide.
Convection with GHGs in an atmosphere needs to be less vigorous than convection without GHGs if balance is to be maintained. Add the phase changes of water and even less convective vigour is required due to upward radiation from the higher level condensate.
All that is consistent with, and indeed essential for, your thermostat hypothesis.
Stephen:
You say:
“Emissivity relates to how long the radiation stays absorbed, doesn’t it ?”
No, not at all.
“100% emissivity means it comes out immediately.”
No, it doesn’t. Not even close.
“What matters is how much delay the atmosphere introduces compared to direct and immediate in / out radiation.”
It’s not a question of delay at all.
Get yourself some good thermodynamics and heat transfer textbooks and study them closely, working through a lot of problems. You presently don’t even come close to having the conceptual background to discuss these issues intelligently.
MrEschenberger@ 12-50
ended a reply to Stephen Wilde by saying:-
That’s not true in the slightest. It also reveals a profound misunderstanding of the physics. GHGs have almost no effect at all on the transparency of the atmosphere to solar radiation. They simply don’t absorb energy at the wavelengths of visible light. Once again, you’re just running your mouth off, spouting something that is totally untrue without any attempt to back up your BS with anything factual.
It gets old …
w.
So the Magician slips “visible light” out of his sleeve and into his denial of there being notable atmospheric SOLAR HEAT absorption when he knows very well that around (14%) 200W/m2 of Solar IR HEAT is blocked according to reports on line.
The folly of treating backradiation (~335W/m^2) as a “forcing” on equal thermodynamic footing as insolation (~160W/m^2) at the surface is seen in the resulting BB temperature of ~306K. The fact of the matter is that Earth’s surface temperature is by no means the product of radiative processes alone. Nor can it be determined by a simplistic, mass-less radiation balance, wherein thermal energy stored in various reservoirs within the system is represented by oppositely directed LWIR fluxes. Clearly ANY such fluxes with a NET value of ~60W/m^2 will make the TOA power budget balance, but leave the surface temperature undetermined. That’s why advanced models depend upon empirical determinations..
rgbatduke says, March 28, 2014 at 12:48 pm:
”I have no idea what you are talking about with your directly/indirectly. Flux is flux.”
Good. Then we seem to agree: You let your flux down from the atmosphere to the surface heat it directly, that is, not by reducing the outgoing flux (decreased cooling), but by adding to the incoming flux (increased heating). Can’t do that.
”Nor do I understand what you are talking about with your introduction of absolute vs relative terms.”
Relative warming relates to flattening a cooling curve so that at a certain point in time the cooling object (now cooling slower than before) is at a higher temperature than what it would’ve been if the cooling went faster. Relative warming never makes the object hotter than originally.
Absolute warming is simply making an object hotter than what it was before. The surface of the earth would for instance according to you equilibrate at 255K with only solar input (239 W/m^2 IN, 239 W/m^2 OUT). At this point it doesn’t cool and it doesn’t warm. There is no cooling curve to flatten. But then you put your radiatively active atmosphere on top of it and apparently introduce an extra flux to the surface. This flux according to you physically raises the temperature of earth’s surface. It makes it warmer than before.
”In actual fact, when I refer to a single layer perfect SW transmitter, perfect IR absorber atmosphere and the 1.19x surface warming it produces via downwelling radiation from the interpolated perfect absorber layer, I am talking about relative warming, relative precisely to the temperature the surface would have with identical incoming insolation but with no interpolated differentially absorptive layer.”
That is not relative warming, Robert. That is absolute warming. You’re not letting your postulated ‘downwelling radiation’ reduce cooling. You’re letting it increase heating. You’re not subtracting. You’re adding. You say so yourself.
”You somehow think a) heat cannot go from cold to hot”
It can’t. In nature.
”b) that this observation has anything to do with the process I describe.”
It does. Because you expect your ‘downwelling radiation’ to produce a result exactly as if it were a heat flux. You don’t SAY it. But you DO it.
”I believe that what you mean to say is the second law of thermodynamics has to be satisfied the processes involved — and fortunately, it is enormously simple to prove that it is, especially in the single layer model, which never has net heat transfer from cold to hot in the steady state of an open system between the Sun as one reservoir and outer space as the second reservoir.”
You refuse to address what I’m pointing to. You refuse to see it. It’s not hard. In fact, it’s ‘enormously simple’. If the 2nd Law is to be satisfied then you will HAVE TO treat the alleged exchange of energy between the surface and the atmosphere as ONE process, the NET FLOW. You can’t just ignore the one part and treat the other is if it were a heat flux unto itself. That’s what you do.
You hope to circumvent what the 2nd Law clearly states by first expecting the one ‘stream’ of energy in a spontaneous NET energy exchange, the one going from cold to hot, to give a result AS IF IT itself were a net flow, that is, HEAT, meaning raising the temperature in absolute terms of the object receiving it.
Then, when this is pointed out to you, then all of a sudden you appeal to the NET concept. But this is simply the nonsensical ‘HEAT flows both ways, only more from hot to cold than from cold to hot’-argument.
The sun doesn’t help you, because the sun has no part in the EXTRA heating of the surface of the earth, the part going from 255 to 288K. The flux in from the sun is not increased. The resulting flux going out from the surface is not reduced, not obstructed in its free escape at all. There is ONLY the extra flux down from the atmosphere. IT does the extra heating. As if it were a second HEAT flux to the surface. Making the atmosphere a heat source to its own heat source, the surface. Or, in reality, making the surface, through recycling of emitted energy, its OWN heat source.
You’re breaking both the 1st and the 2nd Laws, Robert.
”Consequently you are simply mistaken in your claim of b) — the fact that net heat does not spontaneously flow from a cold reservoir to a hotter reservoir has nothing to do with whether or not the Greenhouse effect leads to a warmer surface with greenhouse gases in an atmosphere than without it because this never happens in the energy flow.”
I’m not mistaken in my ‘claim’ b). You don’t understand (or you don’t want to understand) what I’m getting at. You make energy from the cold reservoir of the surface make the surface, the hot reservoir of the atmosphere, even warmer. That doesn’t work in the real world. Because that is a transfer of HEAT from cold to hot.
What you have to do is reduce the energy flow actually leaving the hotter object. You’re not doing that. You increase it rather by adding more incoming. Reducing the outgoing will obey the 2nd Law. And this is done by decreasing the temperature difference (gradient) between the two objects at different temperatures. You know, like the radiative heat transfer equation shows us.
So an atmosphere on top of a solar-heated surface will certainly create a gentler temperature gradient away from the surface than in the no-atmo case. Because the atmosphere can be warmed. Space can’t.
The point is, the atmosphere would’ve been warmed even without radiative gases in it, through other heat transfer mechanisms than radiation and the temperature gradient away from the surface would surely not become any steeper than with an atmosphere with radiative gases in it. (Lapse rate, solar surface heating and convection determines that.)
The atmosphere, however, couldn’t be adequately cooled to space without radiative gases (your so-called GHGs). Because there are no other available heat transfer mechanisms than radiation in this case.
”Now if you want to actually get substantive about this, I’m only going to say to you what I say to Postma when he spouts this sort of crap — show me the equations that prove that the second law is violated by a perfectly valid statement of the first law of thermodynamics that leads one to the 1.19T_0 conclusion.”
You’re not this stupid, Robert. You’re only trying to redirect from your blatant violation of both the 1st and 2nd Laws in one go. You expect ‘back radiation’ from the cooler atmosphere to heat the warmer surface that already emitted the very same energy as a thermal loss, after it first warmed the atmosphere.
This is how this whole thing started. You can’t just split a heat flow into two separate entities and then pretend that they can be treated as individual heat fluxes. The quantum world doesn’t work in a ‘macroscopic’ fashion like that. The postulated ‘downwelling radiation’ from the atmosphere is not a separate INput to the surface. It is PART OF, a COMPONENT of the spontaneous and continuous radiative HEAT transfer going from the surface to the atmosphere, the OUTput.
I told you in front of all your readers: all someone has to do is check with any field on earth where mankind causes heat to leave things. Every major field of cooling on earth relates that convective/conductive losses supercede radiant ones.
You’re actually the one coming up with some work the type of which is renowned for being filled with false information trying to turn the entire world of thermal engineering on it’s back because you want radiation to predominate in most things in the natual world.
You’re just wrong as referring to any field on earth such as architecture, or any other field:
proves everyone uses convection/conduction models worldwide to a far, far greater extent than radiant heat loss.
You’re simply pretending because people don’t even have to be shown, and can find enough sciences to check for themselves, you somehow didn’t get shown erroneous.
Willis Eschenbach says:
March 27, 2014 at 6:40 pm
Still no observations facts, citations, or quotes to support your claims? I’m totally uninterested in your further claims until you come up with evidence.
w.
Which fields do you know of that cool things via primarily radiant heat loss Willis? I’m curious what field you personally ever worked in managing heat, where the objects you cooled were cooled by a ratio of 4/5ths radiant loss.
For instance if you’re right, – 4/5ths of energy lost in nature is about 80%
the entire world is going to know you’re right.
Will I go to say, Google, or Bing, to see anybody else beside you claiming “80% of total energy losses are radiant in nature” ?
I’ll see that all over the place people are relying on the well known fact that 80% of energy lost from objects in nature is lost through radiant loss, and people will be talking about it all
over
the world.
Won’t I Willis?
Let’s check: “80% of total energy losses are radiant” search return:
Shortened for ease of handling http://goo.gl/xS8OGZ
What about Bing maybe.
Maybe not a soul who knows thermodynamics worldwide predominate 80% radiant uses Google but they all do, over on Bing. http://goo.gl/BBjZaT
Nothing there.
There’s nothing there because you’re bluffing Willis, there is no wide and credible field which teaches most losses from objects in the atmosphere – 80% – are radiant.
The fact is you’re the one who’s in here trying to chide professionals in a field you’ve never worked in about falsehoods so easily disproven you don’t even bother trying to explain how you thought “about half the energy lost to the atmosphere is through evaporation.
In any responsible conversation where an adult human being got caught as swiftly and badly as you have, there would be a moment of reflection on the absurdity of your trying to claim 80% radiative loss to the atmosphere while you admit nearly 50% through evaporation.
You have got to be one of the poorest excuses for someone claiming to b thermodynamically literate I know around Willis.
Let’s see you indicate which fields of science agree with your bombastic 80% claim.
The one you make commenting on the post
where you got caught not knowing nearly half the losses are from evaporation.
While you claimed 80% losses from radiation.
You then provided a chart showing 353 watts from the surface claiming it proves your 400 watts claim.
You’re a disgrace to scientific discussion with this kind of intellectual dishonesty and if you tried what you do here professionally you wouldn’t last a week.
—————————-
“The evaporation of the meter of water causes Earth’s
surface to lose 83 watts per square meter, almost half
of the sunlight that reaches the surface.”
Dang, James, actual numbers. You surprise me in a good way.
Yes, I agree that somewhere around 80 W/m2 of energy is lost from the surface by evapotranspiration, and that this is around half of the solar absorbed by the surface. This agrees also with the KT energy diagram, as well as the CERES data.
Now, we know that the upwelling LW from the surface is about 400 W/m2. We derive these figures in a couple ways, both from ground measurements and from satellite measurements.
I can’t believe the things I have seen you say in series, like when you were caught bald faced, not knowing nearly half the energy losses from the earth’s surfaces are from evaporation,
insulting credentialed peoples’ knowledge of the fields they make their livings in, suggesting they learn what they’re talking about,
being caught repeatedly – by and in front of all of us – not having the slightest clue what you’re talking about.
I’m really disappointed and I don’t see how anyone so obviously out of touch with thermodynamic reality – your 80% claim is a perfect example, along with your showing a measurement that clearly indicates 353 watts,
claiming proof that 400 watts per sq. meter is measured regularly..
You really are very confused Willis. That’s obvious, as these posts make it perfectly clear you don’t possess the intellectual honesty to confess you’re wrong when the information’s put right there on the page in front of you by me.
Something very serious is wrong with you if you believe just because somebody will publish it, you have the right to mislead people en masse with your bombastic and error riddled claims.
Here’s something I think every Jr College or High School Student has had: CalTech talking about methods of heat loss.
When referring to the common convective heat loss example we might all reflect on – we see the atmosphere mentioned expressly.
CONVECTION:
In liquids and gases, convection is usually the most efficient way to transfer heat. Convection occurs when warmer areas of a liquid or gas rise to cooler areas in the liquid or gas. As this happens, cooler liquid or gas takes the place of the warmer areas which have risen higher. This cycle results in a continous circulation pattern and heat is transfered to cooler areas. You see convection when you boil water in a pan. The bubbles of water that rise are the hotter parts of the water rising to the cooler area of water at the top of the pan.
You have probably heard the expression “Hot air rises and cool air falls to take its place” – this is a description of convection in our atmosphere. Heat energy is transfered by the circulation of the air.
Let’s see right after that if they mention the atmosphere when they discuss radiation since it predominates 80% Willis. LoL. Think so?
http://coolcosmos.ipac.caltech.edu/cosmic_classroom/light_lessons/thermal/transfer.html
RADIATION:
Both conduction and convection require matter to transfer heat. Radiation is a method of heat transfer that does not rely upon any contact between the heat source and the heated object. For example, we feel heat from the sun even though we are not touching it. Heat can be transmitted though empty space by thermal radiation. Thermal radiation (often called infrared radiation) is a type electromagnetic radiation (or light). Radiation is a form of energy transport consisting of electromagnetic waves traveling at the speed of light. No mass is exchanged and no medium is required.
Objects emit radiation when high energy electrons in a higher atomic level fall down to lower energy levels. The energy lost is emitted as light or electromagnetic radiation. Energy that is absorbed by an atom causes its electrons to “jump” up to higher energy levels. All objects absorb and emit radiation.
Not mentioned.
Here’s another place Willis that says – once again: you’re so wrong it’s not even possible that you don’t know it. You’re posing.
Here’s what they’re teaching people from the 9th grade to the 12th Willis.
Introduction to The Atmosphere
http://www.ucar.edu/learn/1_1_2_7t.htm
“Alignment to National Standards
National Science Education Standards
Physical Science,
Earth and Space Science,
Grades 9 to 12, pg. 189,
Item #3: “Heating of earth’s surface and atmosphere by the sun drives convection within the
atmosphere and oceans, producing winds and ocean currents.”
Benchmarks for Science Literacy, Project 2061, AAAS
“Convection currents are found in many places and on many scales, from huge convection currents in the atmosphere, oceans, and even in the earth’s interior to smaller convection currents found in a cup of hot cocoa or a fish tank.
Meteorologists usually use “convection” to refer to up and down motions of air. Heat gained by the lowest layer of the atmosphere from radiation or conduction is most often transferred by convection.
“Convective motions in the atmosphere are responsible for the redistribution of heat from the warm equatorial regions to higher latitudes and from the surface upward.”
——-
They aren’t talking about your fantasy, false, mythical 4/5ths transfer by radiation.
Here’s a remedial college teaching the basics to children in some online University.
it turns out, they’re using lessons that say the same thing Cal Tech said.
And that Ucar said.
“Convection leads to the counterintuitive fact that good insulators (like air) can transfer heat efficiently — as long as the air is allowed to move freely. Trapped air, as between panes of a double window, cannot transfer heat well because it cannot mix with air of a different temperature.
Radiation
Radiation is the simplest means of heat transfer. Heat radiation is carried not by moving atoms (as in conduction or convection) but by electromagnetic waves. Radiation is the only way that heat can move through a vacuum, and is the reason that even a closed thermos bottle (which has a vacuum between the inner and outer parts) will eventually come to the same temperature as its surroundings.
Heat transfer is most efficient by convection, then by conduction; radiation is the least efficient and slowest means of heat transfer. Low efficiency of heat transfer means that vacuums make excellent insulation.”
https://www.bluffton.edu/~bergerd/NSC_111/thermo1.html
But you, some amateur in here admitting you see it printed before your face, “about half the energy of the sun” are telling us all,
that’s all wrong? No. You don’t have the first clue how transparent the level of goofy is here, Willis that’s what’s going on. And your pretense this is like an opinion poll is another big clue something is seriously wrong with your thinking process about what you’re claiming.
It’s really a shame to see this kind of stuff put up by you as your perception of reality, Willis, I didn’t know you actually said things like I’m seeing you say.
You are in serious need of some education somewhere, besides wherever you had someone convince you, 80% of natural heat removal in the atmosphere occurs via radiant transfer.
Willis here is yet another site describing the atmospheric/surface relationship and it’s predominating convection action without
one
single
word
of the insane claim you made to everyone here that 80% of losses to the atmosphere are radiant.
http://web.physics.ucsb.edu/~lgrace/chem123/troposphere.htm
“The uneven heating of the regions of the troposphere by the sun ( the sun warms the air at the equator more than the air at the poles )
causes convection currents, large-scale patterns of winds that move heat and moisture around the globe.
In the Northern and Southern hemispheres, air rises along the equator and subpolar ( latitude about 50 to about 70 north and south ) climatic regions and sinks in the polar and subtropical regions.
Air is deflected by the Earth’s rotation as it moves between the poles and equator, creating belts of surface winds moving from east to west ( easterly winds ) in tropical and polar regions, the winds moving from west to east ( westerly winds ) in the middle latitudes.
This global circulation is disrupted by the circular wind patterns of migrating high and low air pressure areas, plus locally abrupt changes in wind speed and direction known as turbulence.”
There are many, many, many more, Willis, all of them talking on and on – about the convective nature of surface/atmospheric interaction without a single word of your bombast and I mean that not simply as an insulting thing to say but just referring to it as exactly, what it is. Utter fable.
There isn’t a single place in reality based energy mechanics that teaches 80% of energy loss in the atmosphere is via radiation.
Here’s yet another place Willis, that says outright
convection predominates and barely mentions radiative losses:
They say:
“The uneven heating of the regions of the troposphere by the sun ( the sun warms the air at the equator more than the air at the poles )
causes convection currents,
large-scale patterns of winds that move heat and moisture around the globe.
In the Northern and Southern hemispheres, air rises along the equator and subpolar ( latitude about 50 to about 70 north and south ) climatic regions and sinks in the polar and subtropical regions.
Air is deflected by the Earth’s rotation as it moves between the poles and equator, creating belts of surface winds moving from east to west ( easterly winds ) in tropical and polar regions, the winds moving from west to east ( westerly winds ) in the middle latitudes.
This global circulation is disrupted by the circular wind patterns of migrating high and low air pressure areas, plus locally abrupt changes in wind speed and direction known as turbulence.”
http://web.physics.ucsb.edu/~lgrace/chem123/troposphere.htm
“Convection is the mechanism responsible for the vertical transport of heat in the troposphere while horizontal heat transfer is accomplished through advection.
The exchange and movement of water between the earth and atmosphere is called the water cycle.
The cycle, which occurs in the troposphere, begins as the sun evaporates large amounts of water from the earth’s surface and the moisture is transported to other regions by the wind.
As air rises, expands, and cools, water vapor condenses and clouds develop. Clouds cover large portions of the earth at any given time and vary from fair weather cirrus to towering cumulus clouds.
When liquid or solid water particles grow large enough in size, they fall toward the earth as precipitation. The type of precipitation that reaches the ground, be it rain, snow, sleet, or freezing rain, depends upon the temperature of the air through which it falls.”
—————————-
To which Willis your reply has been – for how long I wonder – that you have thumbed your nose at reality to publish lies you know very well are just that. Never in your entire life anywhere on this planet were you taught that
“80% of surface losses to the atmosphere are radiant.”
No you did not. And the proofs are able to get very, very long Willis, because every place we visit we aren’t going to see one single mention of your totally mythical bullshoot story about
“4/5ths radiant losses.”
Sorry moderator I meant the second of those two last two posts, I thought I lost one, Thanks
James R
Oldseadog says:
It is high time someone put Willis up for an Hon. PhD.
Agree wholeheartedly.
It won’t happen [good ol’ boy network won’t allow it], but I learn more from Willis than from just about any PhD.
Messrs JRjr & K have written:-
A whole load of Established Science sense in response to the frantic drivel and patent mischief issued by W.E. and his obvious acolytes in defense of the “Warmist” claim that is the hand of GHG`s which are on the Earth Oven thermostat control in order to wrongly elevate the role of Co2 to that of being some kind of inbuilt radiant element that could turn the pie to toast if tweaked above the abysmal level Industrial Man inherited, upon which specious argument AGW has been built, and that its REAL LIFE role in Climatology is to help emit to Space the likely self assisted too surplus IR that has managed to negotiate its way through our maelstrom of an atmosphere below and to slough off some of the Suns N/IR similar to the way Ozone does its UV to give us a habitable surface on which to LIVE.
(AND) “upon which specious……..” tutt tutt!
When you decide to stop beclowning yourself, Mr. James Rollins Jr. You might want to review the KT diagram that Willis mentioned above.
http://citeseerx.ist.psu.edu/viewdoc/download?rep=rep1&type=pdf&doi=10.1.1.210.2513
Here’s another widely known site in outright contradiction of your claim Willis.
Wikipedia:”Convective Heat Transfer: The very first lines in the definition:
“Convective heat transfer, often referred to simply as convection, is the transfer of heat from one place to another by the movement of fluids.”
“Convection is usually the dominant form of heat transfer in liquids and gases.”
Shortened – http://goo.gl/B8fqg
James Rollins Jr says, March 29, 2014 at 7:27 am:
““Convection is usually the dominant form of heat transfer in liquids and gases.””
Which everyone and anyone should darn well know!
The other heat transfer mechanisms would basically get nowhere without convection as a transporter of the energy provided through the fluid (from heating end to cooling end).* If the air directly above the solar-heated surface didn’t buoy up and away instantly and automatically upon absorption of the conductively and radiatively tranferred energy from the surface (and hence warming), to make way for new transferred energy, then the heat would pile up down there, at the surface, in huge amounts. Convection is the process by which the surface heat is brought out into the atmosphere. Convection is the process that maintains the tropospheric temperature profile, that prevents energy from building up near the surface, that gets it up to where it can finally radiate out of and away from the system, through the conductive (‘convective’) insulation layer that is the troposphere.
*Well, evaporation actually helps with the convective lift.
The best way to illustrate this relationship, how conduction and radiation provides the energy going from surface to atmosphere, but as soon as this energy is absorbed into the atmosphere, convection takes over completely, is with the burning candle. The radiation streams out from the hot flame in all directions in equal amounts, up, down and to the sides. But that’s not where the HEAT ends up. Hold your hand just a few inches away from it to the side (or below it) and you can practically no longer feel its warmth. Hold your hand at the same distance only over the flame and you’ll burn right away. Convection does the job. The energy (heat) comes from the candle flame combustion, but it is all almost immediately absorbed by the air, which instantly warms, expands and lifts up. So the flame cannot make its surroundings any warmer outside of the ‘zone of free radiation’. Convection does not allow it. That is, IF we do not find ourselves inside a closed room. THEN the candle (and our presence) will after a while noticeably warm the room. But why? Because convection is suppressed by the walls and ceiling. The heated air in the end has nowhere to escape. Open a window, though, and the warming will soon be erased. Or light your candle outside in the open air (even on a dead calm night). No warming of the general surroundings. You have to move inside the ‘zone of free radiation’.
This is such a simple everyday truth, but no one seems to connect it at all with the large-scale global surface/atmosphere interaction.
You can’t just put so-called GHGs in the atmosphere and expect the lower part of the atmosphere to warm more than if they weren’t there. Because any excessive energy absorption (heating) would just be negated by convection right away. The tropospheric temperature profile is what it is. Established and maintained by the interaction between solar surface heating and convective response to set it up globally fluctuating around in generally close proximity to the adiabatic lapse rate. You don’t make it less steep by introducing ‘GHGs’ into the atmosphere. If anything, they would work toward steepening it, making heat transport (by convection) through the troposphere MORE efficient. And as we all know, those radiatively active gases are what enables the atmosphere to cool approprately to space. They are however NOT what enables the atmosphere to be heated by the surface.
James: You read but you don’t understand. It’s been repeatedly pointed out that Willis has been talking about GROSS radiative transfers in this post (because that’s what you can measure, and this post has been about radiative measurements and their variation), and you are talking about the NET radiative exchange.
Willis is correct about the general magnitude of the gross radiative losses from the surface. For a high-emissivity (say 0.95) body at 15C (=288K), any textbook will tell you that:
Q = e * sigma * T^4 = 0.95 * 5.67×10^-8 * 288^4 = 370 W/m^2
At 20C, it is 424 W/m^2.
These numbers are right in the range of what Willis was talking about.
Now, in the case of an atmosphere with greenhouse gases, as ours is, the NET radiative losses from the surface (which is what you are talking about) are much lower because of the downwelling radiation from the greenhouse gases. If the atmosphere did not have these gases, the gross from the surface would be the same as the net.
Kristian: In introductory calculus, you learn that when you perform the definite integral of a function, you need a constant of integration. So when you integrate the dT/dz lapse rate to try to obtain T(z), you need a constant of integration. With an atmosphere that is transparent to infrared, that constant is derived from the fact that the surface must be in radiative balance between sun and space.
With greenhouse gases, the constant is derived from the fact that this balance occurs at an elevation range higher up in the atmosphere. Given the lapse rate, this yields a higher surface temperature.
Curt says, March 29, 2014 at 9:56 am:
“It’s been repeatedly pointed out that Willis has been talking about GROSS radiative transfers in this post (because that’s what you can measure, and this post has been about radiative measurements and their variation), and you are talking about the NET radiative exchange.”
It’s also been repeatedly pointed out in this post that there are no GROSS radiative transfers. There is only what you would call a NET transfer. The transfer of energy between two objects at different temperatures is spontaneous and indivisible and goes only from hot to cold. You can’t apply the ‘macroscopic’ concept of splitting a radiation field between two such objects into two separate ‘streams’ of energy (as if they were two opposing highways) and then treat each of them independently from the other, as if they both were individual heat fluxes (they both heat the receiving system in your model!).
It’s not like the surface first heats a bit from the alleged incoming atmospheric flux and then cools a bit (only a bit more) by its resulting outgoing. There is ONLY cooling. The radiative transfer is from surface to atmosphere. And that’s it. Any ‘exchange’ would occur continuously and simultaneously as the radiation field between the two objects constantly adapts to their temperature difference (the potential gradient through the field), changing instantaneously with it. The spontaneous FLOW of energy (the only one that can be detected, meaning, the only ‘real’ one) between the two objects goes only ONE way, from hot to cold. Like an electric current. Like wind. This is the HEAT. It’s all there is. Everything beyond this is purely theoretical speculation and extrapolation.
What you can MEASURE, Curt, is the ‘heat’ (the NET). What you CALCULATE from this and any sensor’s temperature are the assumed individual (‘gross’) fluxes.
Curt says:
March 29, 2014 at 9:56 am
James: you read but you don’t understand.
—————————-
Your grasp of thermodynamics is best described as thinking space suits are insulated because it’s freezing cold in space Curt.
I’ll remind everyone who we’re dealing with in you:
“Curt says:
March 26, 2014 at 11:32 pm
James, you simply do not know what you are talking about. Inside an astronaut’s spacesuit, there are seven layers of aluminized mylar, each one providing a layer of radiative insulation, with the plastic mylar itself keeping the aluminum layers from conducting to each other.
The reason for this is that the human body produces about 100 watts through its metabolism. But the surface of the body radiates away close to 500 watts. Without the “back radiation” we are used to on the earth’s surface, without excellent radiative insulation, the astronaut would very quickly freeze to death when out of the direct line of the sun.
—————————-
That childish lack of grasp on reality on your part has had to be straightened out but you’re sure you’re all about thermodynamic understanding :
James Rollins says:
March 27, 2014 at 12:26 am
No Curt that isn’t correct actually about the space suits.
“Temperature
To cope with the extremes of temperature, most space suits are heavily insulated with layers of fabric (Neoprene, Gore-Tex, Dacron) and covered with reflective outer layers (Mylar or white fabric)
to reflect sunlight.
The astronaut produces heat from his/her body, especially when doing strenuous activities.
If this heat is not removed, the sweat produced by the astronaut will fog up the helmet and cause the astronaut to become severely dehydrated;
astronaut Eugene Cernan lost several pounds during his spacewalk on Gemini 9.
To remove this excess heat,
space suits have used either fans/heat exchangers to blow cool air,
as in the Mercury and Gemini programs,
or water-cooled garments, which have been used from the Apollo program to the present.”
From N.A.S.A. : How SpaceSuits Work:
Parts of a Spacesuit
NASA spacesuits have many pieces and parts. Learn about the parts and why each piece is important.
Primary Life Support Subsystem
The PLSS is worn like a backpack. It provides astronauts many of the things they need to survive on a spacewalk. Its tanks supply oxygen for the astronauts to breathe. It removes exhaled carbon dioxide. It contains a battery for electrical power.
The PLSS also holds water-cooling equipment,
a fan to circulate oxygen and a two-way radio. A caution and warning system in this backpack lets spacewalkers know if something is wrong with the suit. The unit is covered with protective cloth layers.
Layers
The spacesuit arm has 14 layers of material to protect the spacewalker. The liquid cooling and ventilation garment makes up the first three layers.
—————————-
Whatever you do for a living you don’t have formal thermodynamics handling training or you’d have understood the insulation isn’t on equipment in orbit “because they will freeze to death!”
Your grasp of the entire thing is well summed up by your charging in here trying to preach the above class of amateurish wishing.
It’s not even like you can even say you were wrong through some typo. You vomited out your amateur answers in front of professionals who caught you trying to fake it.
Kristian: You say,
“It’s not like the surface first heats a bit from the alleged incoming atmospheric flux and then cools a bit (only a bit more) by its resulting outgoing.”
That’s exactly what is happening (although simultaneously, not sequentially). Every photon absorbed adds to the surface’s internal energy, every photon emitted reduces its internal energy. This has been well understood for a hundred years now.
You also say, “You can’t apply the ‘macroscopic’ concept of splitting a radiation field between two such objects into two separate ‘streams’ of energy (as if they were two opposing highways) and then treat each of them independently from the other, as if they both were individual heat fluxes (they both heat the receiving system in your model!).”
Sure you can split them, because that is exactly what is happening in the underlying physics. (And I don’t know why you would call this the “macroscopic” concept – you can use the “macroscopic” metaphor of heat flow to talk about it simply; I’m talking about the “microscopic” mechanics.) The opposing “streams” both carry energy, reducing the energy of the body emitting them and adding to the energy of the body absorbing them.
You continue to treat heat flow as something real, rather than a convenient metaphor. This is why it is so easy to mock you as being stuck in the 19th century, when they had no way of knowing better. What is the underlying physical entity transferring your supposed one-way real heat flow — the “heaton”?
Have you ever studied statistical mechanics, radiative physics, or even engineering heat transfer? All of this is treated early in any of those courses.
James: You seriously miss some very important points here. Let’s break them down into baby steps.
First, the human body’s rest metabolism produces about 100 watts. With exertion, you can produce more for intermittent periods.
Just considering an adult’s torso alone, the skin is about at 35C with an emissivity of 0.97. There is about a square meter of surface area on the torso. So the radiative power output from the torso alone is:
P = e * sigma * T^4 * A = 0.97 * 5.67×10^8 * (273+35)^4 * 1.0 = 495 watts
Of course, your limbs will be radiating as well, probably from a slightly lower temperature, but we’ll ignore that for now, and just say that the body is radiating away 500 watts on a continuous basis. So far we are at a 400 watt deficit that has to be made up somehow.
Now, let’s consider the point of the thermal design of a space suit. It has to make sure that the astronaut neither overheats nor freezes under any of the foreseeable conditions. The astronaut can be in sunlight or shade, can be exerting heavily or at rest.
Let’s consider the similar problem of going outside on a cold winter’s day. You may be in sunlight or shade; still air or wind; and you may be working hard or at rest. Do you take enough insulating clothing (here we are talking primarily about conductive/convective insulation, but radiative can help) to handle the worst-case “cold” conditions (lowest expected temperature, shade, wind, rest), being able to open them up (e.g. unzipping the parka) or remove them in warmer conditions, or do you only bring enough for warmer conditions and count on some separate energy source to help when the conditions get colder?
Obviously, it’s the first case. Many people have died of hypothermia attempting the second strategy.
So it is also with space suit design. The suit must be insulated with the worst-case “cold” conditions in mind – rest metabolism and shade conditions. In this case, there are no conductive/convective losses to ambient in space, so we are concerned primarily with radiative insulation. We must make sure that the body’s losses are only about 100 watts to match the metabolic gain. This is achieved primarily by using multiple layers of reflective radiative insulation, so the inner layer can reflect back about 400 watts.
Now, what happens if things are not worst case – the astronaut is exerting himself and/or in the sunlight?j Well, the radiative insulation does work both ways, so that helps, and there is a reason all spacesuits are white, to reflect as much sunlight as possible. But with just a passive system, the astronaut could overheat.
Now, if you start working hard when you are outside on a winter day on the earth’s surface, can open up your jacket to permit more losses to ambient. In space, of course, it is not possible to do this in the same way.
But you can get the same effect by running coolant over the surface of the body and passing it to the outside of the insulation (through very small gaps in the insulation). Once outside, it is very easy to radiate the energy out to deep space. The only power that is needed is a few watts to drive the pump motor.
So the thermal strategy of spacesuit design is to overinsulate radiatively, then add an active cooling system to regulate the interior temperature. This requires a far smaller energy supply (battery) to be carried along, because a few watts to the pump motor can dissipate hundreds of watts of thermal power. If the suit were underinsulated, the battery would have to supply every watt of added thermal power.
It’s not a good idea to rely on stuff written by NASA PR flacks as being authoritative technical sources. They seldom get the subtleties right, and often miss the basics as well. I have seen some truly awful stuff out of them, far worse than Wikipedia.
Thanks Curt. Keep up the good fight. Love reading your analysis.
Willis,
I am concerned about the impact of one possible oversimplification in your analysis, you assume a simple surface that either gets rid of input energy via upward IR or other losses to the atmosphere. Here are you relevant quotes:
“The parasitic loss is computed as the input to the surface less the radiative loss from the surface.”
“In terms of the greenhouse effect, the efficiency can be thought of as how hot the greenhouse effect can make the surface with respect to the atmosphere.”
“Otherwise, in addition to losing energy via radiation the heat can “leak” from the surface to the atmosphere.”
“The parasitic loss is computed as the input to the surface less the radiative loss from the surface. There is an uncertainty in the measurement due to the import/export of warm water from a gridcell, but that appears to be minor in the context of this particular analysis.”
It seems to me what the analysis is missing is the depth of that gridcell and the heat capacity of the ocean water. Most of the solar SW input will probably be deposited in the top few meters, but as someone who lives in the tropics, you know that some SW radiation penetrates 10s of meters, and there are even kelp forests over 100 meters deep. It appears to me that heat stored in the ocean is a “parasitic loss” in your analysis, not a loss to the atmosphere on the time scales being considered. This might be a figure so small as to be in the noise in your analysis. We heard frequently, especially in response to the “pause”, that 90% of AGW global warming heat is stored in the ocean. This however, is 90% of the net energy imbalance, variously quoted as from 0.6W/m^2 to 0.8 or 0.9W/^2 globally and annually averaged. This gridcell depth may be much less of an issue for LW/IR radiation since it penetrates mere microns into the ocean and it thus closer to the idealized surface or skin effect. Models that couple LW radiation to the whole mixing layer just like SW, would miss the enhanced surface coupling of the LW radiation. I wonder if this enhanced LW coupling to the complex ocean surface (foam, waves, etc) might result in the enhanced feedback you are seeing with increased input.
Hmmmmn.
Gonna have to disagree with you here, Willis, on a couple of points.
Granted, you are working with averages from CERES – and THAT may be the fundamental problem! – but, for example, the earth receives just a little under 1000 watts/m^2 between the tropics of Cancer and Capricorn all year, (at noon) and each square Mkm^2 gets that radiation only around noon each day.
The initial graphs, stopping at 600 watts/m^2, would be misleading then, right?
So, should we not begin instead with the plots for the parasitic losses over 24 hours for each latitude; then attempt to improve on that approximation by incorporating the percent of land/snow/ice/water being hit by the that calculated radiation for each 5 degree latitude band from pole to pole?
Instantaneous losses from
Radiation.
Convection.
Conduction.
Evaporation.
vs the (somewhat simple to calculate) instantaneous heat input for that minute from the sun.
All parasitic heat losses are instantaneous: Most vary directly by delta T (from surface to local air temperature or from the surface to the ultimate radiation absorber in the stratosphere) and a constant that varies instantaneously with pressure, contact conditions (conduction), geometry of the two surface (convection changes with wind velocity, humidity, heat surface locations, fluid conditions (Prandlt number, Nusault number, Raleigh number, relative turbulence, length of constant area, etc.), humidity or surface condition (emissivity of both surfaces for example, percent of pressure between surfaces, type of roughness of surfaces, etc).
All heat losses occur ONLY due the immediate local conditions, and cannot be “recalled” or changed once losses occur. No one should make global assumptions from global averages over the year, because that is NOT how heat transfer occurs. Heat transfer – fro a hot surface to ta cold surface depends immediately and ONLY on the immediate conditions of those two environments at that particular second in time.
So, the net impact can ONLY be judged looking at EACH particular instantaneous situation at EACH particular latitude and assumed weather condition (temperature of surface, wind speed at 2 meters, air temperature, sky condition, pressure, relative humidity, T wet bulb, T dry bulb.) What IS important at the equator at noon is irrelevant at the Antarctic in the winter, but the “delta T” difference between each of the two surfaces, might even be the same. Or be very different! Radiation losses, for example, in the arctic will change because the surface (ice or open water) are very different temperatures in degrees K.
But, air temperature could be the same all day, but 3 hours of evaporation losses alone will be larger than the entire days’ heat gain from the sun. Parasitic heat losses from an open water at 2 degrees C into an air temperature of “only” -15 C will vastly different from a ice-covered surface also -15 degrees C into that same arctic air at -15.
When the Arctic is getting well under 200 watts/m^2, but parasitic losses are 50 or 100 or 150 watts per m^2, then much more precise definitions and calculations of those exact parasitic losses are critical to the problem.
For example, if air temperature is 35 C, surface water temperature = 15 C with 2 m/sec wind at 2 meters and relative humidity = .85%, what are the evaporation and convection losses and radiation losses into a sky covered with clouds in the the stratosphere at -30C?
Input SW radiation at noon = 1030 watts/m^2. Do small parasitic losses matter?
Input solar (SW) radiation at midnight = 0, input long wave (LW, proportional to Tsky^4) radiation at midnight = ???? watts/meter^2.) Do parasitic heat losses matter now? 8<)
Now, change that problem to the Arctic or Antarctic: If air = -15 C, water = 2 C, wind = 4 M/sec, and the the relative humidity is 45% (for -15 C !!!) what are the parasitic losses?
Yes I did see it, and so did everyone else. You’re another amateur faking it online.
Curt says:
March 29, 2014 at 4:08 pm
You didn’t see me show up claiming outer space is freezing cold removing heat faster than convection or conduction. You didn’t see that.
You also didn’t see me try to crawfish out of it through desperately talking in circles, then claim that N.A.S.A. and other educational organizations don’t know as much as me.
Please say you didn’t see that and you can forget I revealed how little I actually understand.
Come on James, this is trivial introductory undergraduate stuff. Do the numbers for yourself!
Repeating from above, the skin of the human torso is about 1 m^2 at 35C with 0.97 emissivity. This gives a radiative power output of:
P = e * sigma * T^4 * A = 0.97 * 5.67×10^8 * (273+35)^4 * 1.0 = 495 watts
Conservatively, I’ll add the outer surface of the limbs at another 0.5 m^2 at 30C, again with 0.97 emissivity, for additional radiative power output of:
P2 = e * sigma * T^4 * A = 0.97 * 5.67×10^-8 * (273+30)^4 * 0.5 = 232 watts
So that’s a total of about 725 watts, far higher than the body’s rest metabolism of 100 watts generated internally.
Now in normal earth surface conditions, you are surrounded by surfaces that are not much colder. If you are inside, you are surrounded by high-emissivity walls at about 23C. These radiate at a power density of
Q = e * sigma * T^4 = 0.97 * 5.67×10^-8 * (273+23)^4 = 422 W/m^2
So your 1.5m^2 of body surface is receiving 422 * 1.5 = 633 watts of radiant power, for a net outward radiative power flow of about 90 watts. You would also lose similar power by conduction/convection, which is why you would want some clothes on you at rest at an ambient of 23C.
Now, in space and out of the sun, you would have no conductive/convective losses, but virtually no ambient radiation toward you (the cosmic microwave background radiation is less than a microwatt per square meter). So without radiative insulation, you would have a power imbalance of over 600 watts. That could not be maintained for long.
When astronauts expel urine from the space ship, it immediately evaporates from the lack of pressure, but then almost immediately freezes into crystals. It is not losing heat from conduction or convection – it is from a huge radiation imbalance.
Curt there’s a way a man talks when he’s a professional whose work is always right.
We don’t swear we can do all the calculations – then spray the crowd like a pimple, with the insanity that comes out of the self taught amateur:
“That’s why they have insulation on those space suits – cause it’s so cold out there!”
The kind of unforgivable thermodynamic errors you’ve made don’t vanish because you gin up and try to Manic-Magpie yourself into some respectability.
Posers – such as yourself all swear you can do all the calculations,
and yet are notorious for doing what you and Willis have both done: make statements the entire educated world knows are the height of folly.
For those wondering if you, Curt actually know what you’re saying, when you claims you “calculates” your, and Willis’ folliy – a purely radiative surface –
here’s the difference between a professional thermodynamicist and some amateurs who washed up on the internet insulting the professionals, willing to give away their analyses free.
That’s because no one will pay money for it.
For those of you who aren’t trained math heads,
I’m gonna explain to you the kind of “work”
being done by Willis
and by Curt.
Being a professional who’s had to explain the ins and outs of thermodynamic flows to clients and various people I don’t need a long time to do it.
I’ll just do it the same way I would, If I had showed up and someone said “I had some guys over here and they claimed they did all the calculations and everything but they can’t come up with a right answer to save their lives: can you help? If you can, my company’s contract is effectively yours, because something’s obviously wrong with what these guys are doing.”
So the client takes the “work” done by Willis
the amateur
and the client takes the “check” of his work by Curt
the amatuer,
and we see they both insist none of the rest of us – the actual professionals – know what we’re talking about because
“They Did The Calculations” and “They Checked Each Other.”
If you are a reader trying to see who, is checking on whose work, finding it in error, watch –
– this is gonna take about 15 seconds for you, the non math-trained reader,
to understand why trained, experienced, knowledgeable certified thermodynamics professionals,
stay in business.
And you’re going to see why amateurs, are always getting caught: busted: outright: so wrong it’s literally something you take back to the office and show the other
certified professionals, what’s trying to pass itself off as “analysis.”
Ya know how Curt and Willis have gone on, and on, with their “I calculated,” – ?
Let’s do a real quick flyby on these two and see how “calculated”
their so-called “calculations”
really are.
—————————-
Willis Eschenbach says:
March 28, 2014 at 12:50 pm
“Here is the whole equation:
Radiation (w/m2) = S-B constant * emissivity * Temperature ^4”
—————————-
Ok.
Now here we have Curt: the other amateur: proudly claiming he’s checked, and he sees it just that way, too:
“Curt says:
March 29, 2014 at 9:56 am
“Willis is correct about the general magnitude of the gross radiative losses from the surface. For a high-emissivity (say 0.95) body at 15C (=288K), any textbook will tell you that:
Q = e * sigma * T^4 = 0.95 * 5.67×10^-8 * 288^4 = 370 W/m^2
At 20C, it is 424 W/m^2.”
—————————-
What these two amateurs are trying to do, is calculate the amount of energy, leaving the surface of the earth.
What they’ve done, is calculate it
using the wrong calculation.
Look for yourself. Here’s the page of something called “The Engineers’ Tool Box.”
Here is the page, at the Engineer’s Tool Box, that describes the calculations for radiative loss.
Just look at the page and specifically, look for something called “Hc” or the
“hc= convective heat transfer coefficient of the process”.
Now what the Hc or convective heat transfer coeeficient does, is include the amount of heat removed by the air.
Do you see the proper accounting for the convective removal by the atmosphere itself, these amateurs are advising you all, about?
No you don’t. The two amateurs who have been telling the professionals we need to learn to count,
forgot the air when they calculated losses from the earth’s surface.
http://www.engineeringtoolbox.com/radiation-heat-transfer-d_431.html
Right there above is the page with the radiation transfer calculation.
Notice it is identical to the one the two amateurs are using to calculate losses from earth’s surface.
Notice there’s nothing there even acknowledging the air removes anything at all?
That’s because the two amateurs don’t even have the correct calculations for removal of energy from the surface of the earth.
The Atmosphere removes part of the heat from the surface of the earth: the amount that is taken off by convection is a function of the difference between the temperature, in the solid and the fluid washing it which is in this case, atmospheric air.
http://www.engineeringtoolbox.com/convective-heat-transfer-d_430.ht
Notice the real calculations for determining the surface of the earth, take into account the temperature and thermal transfer of air – just like you’d expect.
Instead, these two amateurs have spent a week in here, arguing with us, insulting the people correcting their horrifically mauled assertions of a physical world that simply can not be:
You’ve seen now yourself: we all know that regardless of what some wannabes on the internet say, the air takes a lot of energy directly from the surface of the earth through non radiative means.
These two have taken the calculations for an object without any atmosphere at all, and tried to claim they’re the calculations for a surface for an atmosphere –
yet I just showed you the calculations for a surface with an atmosphere touching it: a gaseous ‘fluid’ environment that removes heat conductively, and convectively.
You can see that even a casual pass by these peoples’ backward, error riddled fantasies, shows they don’t even grasp, they’ve tried to calculate the temperature of the surface of the earth,
having forgotten the atmosphere even is there. Thats what the “Hc” portion is, in the “Convective Heat Transfer: page: the effect of the atmosphere.
Look above at Curt – smart mouthing me, smart mouthing others – in here telling us all that the reason space ships have insulation is because it would be so cold in space without it.
Then when he’s shown to be utterly, utterly reversed, he goes on the long, amateur hour, Manic-Magpie “You didn’t catch me because I’m still typing” expedition.
From there he’s on to “I calculated the temperature of the surface of the earth. I forgot to include the atmosphere like real mathematics requires.”
And he’s still fervently typing to try to save something he perceives as a reputation.
Curt doesn’t have a reputation to damage. Neither does Willis. They’re amateurs, arguing with professional thermodynamicists who have
while you watched –
simply let they themselves express to you, their grasp of atmospheric energy and thermodynamic fundamentals.
They both forgot to include the atmosphere at all.
Look for the atmosphere in their calculations. It’s not there, is it.
No it is not.
That’s why these people despise the real, thermodynamically trained professionals, coming around checking their bullshoot stories.
Because everything they do and say is so easily debunked and not only is it easy, there’s no place for them to hide, because their own words
are what they’re defending here. It’s not like they’re defending someone else’s words, they came here and volunteered that
when calculating the amount of energy radiantly leaving the surface, you don’t even need to acknowledge the atmosphere is there in your calculations. Just calculate it like there’s no atmosphere.
That’s who is in here trying to act snide to their betters. The amateurs caught by the professionals not even knowing they have to include the atmosphere in their calculations for earth.
With regards to your Figure 1 the North African and Arabian deserts are glaring at me. It’s a big area and it would seem that there should be a large loss in upwelling IR to space if the deserts were to be considered to have a dry atmosphere. That doesn’t seem to be the case there. Could there be a stratification in the upper atmosphere containing high level water vapor near 100% RH effectively creating a GHG effect even though the low altitude is very dry?
I have returned to this post numerous times. It is interesting. I think the plots may offer more than what has been discussed here. A future post expanding on the data presented here would be most welcome.
And further, readers, check this:
when I was typing my last post revealing to you how transparently and just brainslessly –
they forget to even include the atmosphere at all,
when calculating the temperature of the earth,
I didn’t know Curt was up there wagging away trying desperately to bury his
“Its freezing in outer space which is why they to insulate space suits” debacle of revealed ignorance
(there are several places listed above including NASA revealing space suits aren’t insulated because it’s cold – heat can’t leave an object in vacuum, except through radiant loss – so the inside of a space suit gets hot as blazes – because there’s nothing to remove the heat)
by running his way through yet another set of error riddled “calculations”
where – once again –
he forgot to include the atmosphere.
At all. There’s nothing there about the atmosphere, at all.
No convective heat transfer coefficient: no calculation for air being present. At all.
You’re speaking with people who believe in calculating the temperature of the earth,
using the calculations for “no atmosphere present.”
You’ve been watching, you saw, and you see it, yourself.
Look at my last post.
Look at where I showed you the calculations for “radiant loss” without any atmosphere at all –
and look at the places I showed you
– Willis using it –
– then Curt using it.
– and then Curt doing it again
– while I was actually typing to you, that it’s the kind of stuff
we, the trained certified thermodynamic professionals, are always having to check.
Everyone here – everyone reading this thread – has seen what the caliber of the “math” Curt thought he was “calculating” the surface temperature of the earth with, same for Willis who showed him how to do it.
They don’t even know the atmosphere must be counted in their calculation of the surface temperature of an object with an atmosphere.
They both admit and brag about it – their idea of calculating the surface temperature of the earth, involves doing the calculations for an object with no atmosphere.
Curt @ 9-56am29/3 said
Q = e * sigma * T^4 = 0.95 * 5.67×10^-8 * 288^4 = 370 W/m^2
At 20C, it is 424 W/m^2.
These numbers are right in the range of what Willis was talking about.
————————————————————————————————-
…………….but as they have to compete at ringing the water vapor bells with `first boy on the block`down-coming Solar N/IR etc.@ hundreds of times more vigorous the actuality
is that those WV molecules are already down-loading at a higher frequency and so resist the colder radiation much as the gas in your double glazing so does and which would be near as good as a vacuum were it not for the inevitable mini air circulation system within.
But, like many, he has got his head stuck too far into his subject, so to speak, that he cannon now, like the proverbial woodcutter, see the woods anymore.
Curt says, March 29, 2014 at 3:27 pm:
“Kristian: You say,
“It’s not like the surface first heats a bit from the alleged incoming atmospheric flux and then cools a bit (only a bit more) by its resulting outgoing.”
That’s exactly what is happening (although simultaneously, not sequentially). Every photon absorbed adds to the surface’s internal energy, every photon emitted reduces its internal energy. This has been well understood for a hundred years now.”
No, Curt. Heat does not go both ways. Simple as that. There is no exception made for radiation even though I know that’s what you people would like to think.
If everything happens ‘simultaneously’ as you seem to agree, then there is no instance of heating of hot. We never see the surface temperature rise in absolute terms. There is only cooling.
And then there is no sense in separating the two postulated ‘opposite streams of energy’ as if they were independent from one another, both being individual heat fluxes, only of different sizes.
What you end up doing then is creating direct heating of hot by ‘energy from cold’. Because it’s not the sun doing the extra warming. The solar IN doesn’t increase. And the resulting terrestrial OUT isn’t reduced/obstructed in its escape. The energy raising the surface temperature in your explanation is the ADDED flux from the cooler atmosphere.
This isn’t how nature works. This is a violation of both the 1st and the 2nd Laws of Thermodynamics.
Everyone sees this if they just think it through a tiny little bit …
Curt @ 9-56am29/3 said……………….
…………and relative to the view that the human body is frail to the natural elements of hot and cold conditions it does of course have those metabolic rate elevating processes we call perspiration to increase its riddance of excess heat upon extreme exercise and/or being in exceptionally warm ambient one one hand along with the ability to reduce skin temperature decisively to help combat both low temperature and “Wind Chill” on the other and which, in particular, does not exist in the Boffin Capsule as it would massacre as it can turn the torrid into bearable, the chilly to #n` cold and the freezing into fatal!
Willis,
I’m burning my tabs up following your most recent posts on the CERES data. Now looking for the post prior to “Water vapor feedback” http://wattsupwiththat.com/2014/03/24/water-vapor-feedback/
All this is very interesting analysis. I think you have opened a door that no one else has.
It would be nice to have a post with a summary/list of your previous posts broken apart into “subjects” with regards to the most recent analysis of the CERES data and another category for your thermostat hypothesis.
Your research is too important to be read only once (in my case multiple times).
Maybe Anthony could possibly organize a summary in the reference pages.
continuatio……..
as said by a healthy enough (he looked) and robust Greenlander boy to Ban Ki Moon + large entourage and Media presence when he visited Uummannaq just the other day on an AGW jauntand questioned as to what he thought of Climate Change –
“I dunno about Climate Change (sir) – but I`m Freezin!”
Kristian, “there is no instance of heating of hot. We never see the surface temperature rise in absolute terms.” Would your understanding be satisfied if the incident radiation from the cooler body, merely slowed the heat loss and caused a temperature differential in the warmer body, assuming other surounding incident radiation is even cooler? That law of thermodynamics is ultimately statistical. You task is to explain what happens to the photons emitted by the cooler body, when they incident upon the warmer body. If you propose there is no effect, then either the warmer body is transparent to incident radiation from cooler bodies (perhaps also refracting it), or it is 100% reflective of it. In the reflective case, someone monitoring radiation from the surface of the warmer body would see that side nearest the cooler body as warmer, in the transparent case, someone would see the side of the body opposite the cooler body as warm, due to the extra photons.
Stephen Wilde says:
March 28, 2014 at 1:39 pm
No, Stephen. Emissivity has absolutely nothing to do with what you call “how long the radiation stays absorbed”. In fact, how long the radiation stays absorbed is generally not even capable of being measured … once radiation is absorbed, it is totally indistinguishable from any other energy, so there is no way to tell when it is released.
But in any case, even if we could measure it, it still has nothing to do with emissivity. Emissivity is equal to absorptivity, which is the percentage of the impinging radiation that is being absorbed … and that is totally independent of how long (even in theory) that radiation might hang around.
w.
Curt said:
“It’s not a question of delay at all.”
Willis said:
“Emissivity has absolutely nothing to do with what you call “how long the radiation stays absorbed”. In fact, how long the radiation stays absorbed is generally not even capable of being measured … once radiation is absorbed, it is totally indistinguishable from any other energy, so there is no way to tell when it is released.”
Thanks to Willis for clarification of the definition of emissivity and thanks to Curt for confirming that the S-B equation does not consider delay in transmission of energy through an atmosphere.
I humbly submit that convection and convection do cause a delay, that such delay is not taken into account in the S-B equation nor in radiative theory generally, that it is the delay that causes the increased surface temperature (255k to 288k), that the delay is caused by mass and not radiative capability and that therefore radiative capability only affects the rate of convection and not surface temperature for a planetary surface beneath an atmosphere.
An inconvenient fact for some but there it is. No need to shoot the messenger.
Martin Lewitt says, March 30, 2014 at 8:53 am:
“Kristian, “there is no instance of heating of hot. We never see the surface temperature rise in absolute terms.” Would your understanding be satisfied if the incident radiation from the cooler body, merely slowed the heat loss and caused a temperature differential in the warmer body, assuming other surounding incident radiation is even cooler? That law of thermodynamics is ultimately statistical. You task is to explain what happens to the photons emitted by the cooler body, when they incident upon the warmer body. If you propose there is no effect, then either the warmer body is transparent to incident radiation from cooler bodies (perhaps also refracting it), or it is 100% reflective of it. In the reflective case, someone monitoring radiation from the surface of the warmer body would see that side nearest the cooler body as warmer, in the transparent case, someone would see the side of the body opposite the cooler body as warm, due to the extra photons.”
Martin, I can’t but laugh. You also DO NOT GET what I’m saying.
Yes, of course, this is what I’ve been pointing out all along. The cooling of the warmer object is reduced when the temperature of the opposing cooler object closes up on the temperature of the warmer object. This is what the radiative heat transfer equation is telling us.
But you do not reduce the COOLING of something by adding MORE energy to it. Then you increase its HEATING. And that is a very different thing.
Still, this is exactly what Willis, Robert B. and Curt claim is going on. They state it loudly and clearly. The ‘back radiation’ does not subtract from the surface’s OUTput. No, in their world it adds to the surface’s INput. AS IF IT WERE AN EXTRA HEAT FLUX. The solar flux raises T_sfc to 255K, then the extra atmospheric flux raises it further to 288K. Same result, same definition: HEAT fluxes. (If it looks like a duck, quacks like a duck and so forth …)
But in nature, heat does not pass from cold to hot.
You can’t have the cake and eat it at the same time. But that’s what they expect. They want us to believe them when they say that the ‘back radiation’ is NOT heat, but at the same time they want the ‘back radiation’ to directly give a result as if it were. It can’t not be heat but still heat the receiving system (unless they want to argue that it’s in fact ‘work’).
It is the whole ‘heating by back radiation’ concept that is so absurdly un-physical. That is what I’m objecting to. Not the effect of a warm atmosphere on the surface temperature.
The flawed physics appear as soon as you split the actual radiative heat going from sfc to atm into two assumed hemifluxes, separate them, discard the bigger hemiflux going up and in this way allow yourself to pretend the smaller hemiflux going down creates a direct surface heating (as if it were HEAT from cold to hot).
You can’t do this! You can’t make an energy flux from cold to hot directly raise the temperature of hot. It’s not a ‘thing’ that happens as a distinct occurrence in nature. It’s all just hypothetical ramblings. Do I have to say it again? I guess I do: In nature, HEAT DOES NOT PASS FROM COLD TO HOT.
A transfer of heat is ONE integrated, spontaneous physical process that moves ONE way – down the potential ladder!
There is only ONE real energy flow between the warmer sfc and the cooler atm, and that is the HEAT going up. Heat does not go both ways. There can’t be simultaneous heating in both ends.
No, the atmosphere has to become warmer relative to the surface if it is to make the surface warmer. And it does so by making the energy flowing OUT OF the surface less, not by increasing the INCOMING. That is the difference between cooling and heating – OUT vs. IN. Just look at the radiative heat transfer equation and you should understand. P/A is the only real flow of energy, the only answer to the equation, the actual spontaneous transfer of energy from hot to cold. You make this flow smaller simply by increasing T_2 (or reducing T_1, or both).
The only thing we need to decide upon, then, is: Does adding ‘GHGs’ to the atmosphere make the atmosphere warmer relative to the surface? Will the temp gradient away from the surface become smaller?
James Rollins Jnr said:
“They don’t even know the atmosphere must be counted in their calculation of the surface temperature of an object with an atmosphere.
They both admit and brag about it – their idea of calculating the surface temperature of the earth, involves doing the calculations for an object with no atmosphere.”
That is how it looks to me too.
The mass of an atmosphere absorbs energy by conduction and convects it up and down.
That results in a delay in solar energy passing through the system and the surface warms up from the S-B expectation of 255K to 288K. That is the mass induced greenhouse effect.
Willis and Curt and many others remain oblivious.
The tragedy is that Willis sees that there is a thermostatic mechanism and that it involves convection but he fails to join the dots.
Conduction and convection through the mass of an atmosphere causes the delay in energy transmission that leads to the higher surface temperature than the S-B equation predicts.
Altering conduction and convection to negate changing radiative characteristics provides the thermostatic mechanism that Willis accepts to be in place.
Why can he not see it ?
Kristian said:
“It is the whole ‘heating by back radiation’ concept that is so absurdly un-physical. That is what I’m objecting to. Not the effect of a warm atmosphere on the surface temperature”
I agree.
DWIR does not warm the surface to a level any higher than it otherwise would be.
The atmosphere above the surface is kept warm by adiabatic heating on descent which offsets adiabatic cooling on ascent so that convection is thermally neutral for the surface and not a net cooling effect as proposed by radiative theory.
Since convection does not have a net cooling effect at the surface the concept of surface heating from DWIR becomes unnecessary. In fact it constitutes double counting.
In the end it all boils down to a very simple proposition.
The same parcel of energy cannot perform two separate functions simultaneously.
Willis admits:
“once radiation is absorbed, it is totally indistinguishable from any other energy, so there is no way to tell when it is released.”
Energy that is radiated to space cannot be simultaneously be used to hold the weight of the atmosphere off the surface via conduction and convection.
255K at the surface enables radiation to space to match radiation from the sun.
The extra 33K at the surface is constantly recycled through adiabatic uplift and descent to maintain atmospheric height.
It is all about atmospheric mass absorbing energy by conduction and nothing to do with the radiative characteristics of that mass.
It is the mass of the entire atmosphere working via conduction and convection that produces the greenhouse effect of 33K.
Stephen 11:57am: “That is the mass induced greenhouse effect.”
Translating Stephen “mass” word in contextual equivalent “radiation”: That is the radiation induced greenhouse effect because:
“All matter – gaseous, liquid, or solid – at all temperatures emits radiation of all frequencies at all times…there is no hedging here: all means all. No exceptions. Never. Even at absolute zero?…absolute zero is unattainable…” – Bohren 2006 p. 4.
“Altering conduction and convection to negate changing radiative characteristics..Why can he not see it ?”
Because conduction and convection are ~adiabatic process as you so often write; as sun increases Tmean from 255K to 288K due increasing atm. optical opacity above transparent atm., these adiabatic processes increase in lockstep; adiabatic processes can not negate diabatic sun radiation.
david(swuk) says:
March 28, 2014 at 4:51 pm
First, “Magician”? “Denial”?? “he knows very well”???
Dang, dude, you are most impolite, and you don’t have a clue what I “know very well”, so assuming you do just makes you look less than professional … does your momma know you talk ugly like that?
Second, you’ve made a very large claim without providing even a scrap of evidence. This is a science site, David, and so your unsupported flights of fancy won’t get any traction here at all.
You also say:
Sounds like you have either a busted IR thermometer, or a busted radiator.
More to the point, the downwelling IR (global average) is about 340 W/m2, which is about 5°C, not anywhere near -40°C, you’re just making that up. And as to whether it can “warm my bare face”, you’ve asked the wrong question.
The right question is “can it warm my bare face compared to what”?
When compared to what the warming would would be without the GHGs, which is IR radiation at about -270°C, yes, radiation from about 5°C will definitely warm your face compared to that.
Come back with some evidence for whatever it is you claim is happening, and we can discuss it. Let me suggest that when you do so that you lose the attitude and become a bit more congenial.
Until then? You’re just providing useless anecdotes, and doing so in an insulting and unpleasant manner … sorry, no takers.
w.
PS—Here’s a protip to improve your communication. If you write to someone and you want them to think that you are actually paying attention, you should learn to spell the person’s name you are writing to correctly. Actually, you don’t even need to learn to spell it, a simple cut and paste suffices … just sayin’ …
James Rollins Jr says:
March 28, 2014 at 10:00 pm
And I told you in front of all of your readers: your endless voluble ranting without making the slightest attempt to provide citations, observations, facts, or mathematics to support your manifold claims is pathetic, unpleasant, distractive, and totally meaningless. NOBODY CARES WHAT YOU THINK, JAMES. This is a science site. Look up the meaning of “Nullius In Verba”.
We care about science here, not your ceaseless raving about your incorrect thought processes.
w.
Trick said:
“adiabatic processes can not negate diabatic sun radiation.”
They don’t need to negate it and I never said they did.
They CAN keep the surface thermal effect of diabatic solar radiation fixed at the figure determined by atmospheric mass.
Conduction and convection work via mass and not radioactive capability. Conduction and convection with the decline in temperature with height would still be present even without radiative gases.
I know that that is a sticking point with some who say that without radiative gases the atmosphere would become isothermal i.e. the same temperature at every height.
I say that cannot be right because the highest molecules would then carry the same load of kinetic energy as molecules at the surface PLUS another load of gravitational potential energy.
In that situation the total energy content of individual molecules would INCREASE with height and that is physically impossible.
It is well established that the total energy content of molecules is constant right up the vertical column (on average).
radioactive capability.
Typo alert, should read radiative capability.
James Rollins Jr.
I think you are on the verge of making the same mistake as Willis and Curt.
The AGW theory does recognise convection as cooling the surface.
That is why their numbers don’t work and they have to introduce DWIR to make the numbers balance in the purely radiative world.
What they and you have missed is that descending air warms adiabatically so as to return the energy originally adiabatically removed upward, back to the air above the surface.
Adiabatic convection is thermally neutral for the surface of the globe as a whole.
So you don’t need DWIR to balance the figures. Indeed, the concept of net DWIR is double counting.
Curt said:
“With greenhouse gases, the constant is derived from the fact that this balance occurs at an elevation range higher up in the atmosphere. Given the lapse rate, this yields a higher surface temperature.”
I’m not sure that that is right.
GHGs change the slope of the lapse rate and so you don’t necessarily need a higher surface temperature to accompany the higher elevation.
In so far as the change in slope causes a divergence from the pure adiabatic lapse rate then convective changes will occur to rebalance the system.
Stephen 1:18pm: “They (adiabatic processes conduction and convection) don’t need to negate it and I never said they did.”
Yes. You did. Which is exactly what I responded to:
Stephen 11:57am: “Altering conduction and convection to negate changing radiative characteristics provides the thermostatic mechanism….Why can he not see it ?”
Trick.
I said that adiabatic processes negate the thermal effect of radiative characteristics within the atmosphere.
They do not negate the solar diabatic effect which is what you said I was saying.
You said:
“adiabatic processes can not negate diabatic sun radiation.”
The solar diabatic effect heats the surface to 255K as per S-B.
The delay in energy transmission caused by atmospheric mass via conduction and convection raises the surface temperature to 288K
If radiative or conductive variability within the atmosphere as a result of composition changes (other than mass) tries to change that 288K then convective changes occur to keep it at 288K.
Stephen 1:47pm: “It is well established that the total energy content of molecules is constant right up the vertical column (on average).”
Up until 1866. This was the solid/liquid/gas kinetic theory up until JC Maxwell’s 1866 paper when he reported the non-negligible pV term in a gas parcel total energy (enthalpy) found from experiments in applying Kinetic Theory to gases in balloons et. al. and noticing pV term was needed to reasonably agree with nature.
I’ve constantly reminded Stephen of this without effect. While U = KE+PE being constant is good for a single molecule, U = KE+PE sum over all molecules is not the total energy in a parcel of gas molecules where constant total energy (enthalpy) ever since Maxwell 1866 is H = U + pV. Changes in enthalpy then need dp*V and P*dV terms which give rise to consideration of constant pressure and constant volume processes (meaning dp=0 and dV=0 outside Stephen’s no-math world).
Stephen 2:01pm: ”The delay in energy transmission caused by atmospheric mass via conduction and convection raises the surface temperature to 288K.”
This is gibberish. Only the sun using up its fuel can raise surface temperature mean from 255K to 288K enabled by increased atm. optical depth (opacity). Any sort “delay” in energy transmission uses up no other fuel adding 33K energy to earth system which can only come from radiative energy transfer of a nearby star – at least in the basic analogue used by many modern authors as introduction to understand some basic radiative, conductive, convective energy transfer physics useful in meteorology.
James Rollins Jr says:
March 28, 2014 at 10:11 pm
Climate science, duh … and your lack of citations is still showing.
But heck, James, how do satellites cool? 100% by radiant losses last I looked. How do motorcycle heads cool? About 5% by radiant losses, at a guess, maybe less.
So why do you not believe that there are not a host of situations in between those two?
As to things that lose heat primarily by radiant cooling, we could start with Wikipedia’s article on radiant cooling:
So, in response to your question,
Um … the field of radiant cooling?
Are you truly unaware of this stuff? I thought you said you worked in this field.
Here’s another one for you, from long ago. In the Southwest, back in the day people made ice even though air temperatures were above freezing. How? By radiant cooling. They painted a shallow flat tray black, filling it with water, and putting up on the roof. On a clear night in dry air, the radiant loss is quite large, and the warming (not cooling but warming!) by conduction/convection from the air is not very large. As a result, the temperature of the water ends up BELOW THE AIR TEMPERATURE, and if you think that is happening by conduction/convection you’ve lost the plot entirely … which actually seems possible, given the rest of your claims.
What else. There is a test of a passive cooling system with some data here.
Let’s see. From the US Naval Academy Engineering Department we have the following:
See the last one in that list? While I’m sure that (as you stated) you never have and never would consider radiation when you are designing fins … there are assuredly engineers who do so.
It strikes me that a worked example would be useful, let me look into that
w.
Kristian, “In nature, HEAT DOES NOT PASS FROM COLD TO HOT.”
But photons can pass from cold to hot, if we were to shine a flashlight towards the Sun, the photons with their momentum and energy would reach it.
Consider a warm object in the vacuum of intergalactic space. It will gradually cool as by radiating its heat way in photons. Put that same object at the same starting temperature say 273K drifting in a dyson sphere vacuum bottle of a black body material, with that bottles walls held actively, or just having lots of thermal mass at a temperature of 200K. Even though the only coupling to the cooler bottle is radiative, that object will cool slower. Nothing is preventing it from emitting photons at the same rate determined by its temperature. It will cool slower because of the energy of the photons delivered to it. It’s temperature will never cool to lower than that of the surrounding walls.
The same principles apply in greenhouse warming. The surface cools more slowly even if the GHGs above are cooler than the surface. The surface however can get cooler that the GHGs because they are not a complete black body and photons escape to the vaccum of space, the surface “sees” the vacuum of space because all the frequencies it radiates at are not saturated. The surface is not warmed by the cooler GHGs, but if it started out warmer, perhaps because of the Sun it will be warmer than it otherwise would have been, due to the greenhouse gases, due to the slower cooling rate from the GHG photons it absorbs.
Here’s a recap :
Checking your “80% Radiant loss BS with Google search for it: Nothing.
http://goo.gl/xS8OGZ
That’s one
checking your “80% Radient loss Bs on Bing.
http://goo.gl/BBjZaT‘
Nothing from the world’s two largest search engines about your fantasy.
That’s two
More proof nobody teaches what you claim – no mention of “80% energy loss surface by radiation here.
http://coolcosmos.ipac.caltech.edu/cosmic_classroom/light_lessons/thermal/transfer.html
“You have probably heard the expression “Hot air rises and cool air falls to take its place” – this is a description of convection in our atmosphere. Heat energy is transfered by the circulation of the air.”
That’s three
UCAR.EDU contradicting your baloney 80% radiant loss from earth surface
http://www.ucar.edu/learn/1_1_2_7t.htm
““Convective motions in the atmosphere are responsible for the redistribution of heat from the warm equatorial regions to higher latitudes and from the surface upward.”
That’s four
More reality based scientific site contradicting your bombastic –
“80% earth surface losses are radiant claim:
https://www.bluffton.edu/~bergerd/NSC_111/thermo1.html
“Heat transfer is most efficient by convection, then by conduction; radiation is the least efficient and slowest means of heat transfer. Low efficiency of heat transfer means that vacuums make excellent insulation.”
That’ five
This was up there:
http://web.physics.ucsb.edu/~lgrace/chem123/troposphere.htm
“The uneven heating of the regions of the troposphere by the sun (the sun warms the air at the equator more than the air at the poles)
causes convection currents, large-scale patterns of winds that move heat and moisture around the globe.
That’s six,
http://web.physics.ucsb.edu/~lgrace/chem123/troposphere.htm
“Convection is the mechanism responsible for the vertical transport of heat in the troposphere while horizontal heat transfer is accomplished through advection.
Wikipedia:”Convective Heat Transfer:
“Convection is usually the dominant form of heat transfer in liquids and gases.”
Shortened – http://goo.gl/B8fqg
That’s seven
Me showing people you trying to calculate radiant loss to the atmosphere with a calculation that doesn’t include the atmosphere:
http://www.engineeringtoolbox.com/convective-heat-transfer-d_430.ht
That’s eight
Me showing people the calculation for temperature with an atmosphere,
http://www.engineeringtoolbox.com/convective-heat-transfer-d_430.ht
That’s nine.
Plus, there’s what everbody else reading knows, that you’ll never see posted here.
And the other ones I didn’t count.
You’re just owned so easily because your claims are so bombastic and preposterous Willis.
You can’t tell people the kind of stuff you say and not have the actual professionals show everyone you’re noobing the whole thing so bad it’s a train wreck.
Willis Eschenbach says:
March 30, 2014 at 12:55 pm
And I told you in front of all of your readers: your endless voluble ranting without making the slightest attempt to provide citations, observations, facts, or mathematics to support your manifold claims is pathetic, unpleasant, distractive, and totally meaningless.
Yes that field, the radiant cooling field. With 4/5ths of all energy leaving structures you’d need radiant cooling wouldn’t you?
If radiation was even important, somebody would make an industry of it even if it was niche or specialty clients or production.
Why doesn’t radiant cooling find itself ingeniously integrated into every one of man’s sophisticated,computer designed objects?
Because as soon as any thermally conductive atmosphere is present in the temperatures seen on earth, convection outstrips radiant loss.
But then those open roof radiators cool because when pointed to the sky they are receiving only minute amounts of energy down. Certainly not enough to warm them. In fact they cool to the point where they freeze sometimes,
these kinds of open radiant refrigerators are well known world wide. Open insulated container, fact sky at night away from direction of sun preferably, evaporation and radiation emit a lot of heat and things freeze even when the temp is above freezing.
That doesn’t help you though, the very core of my argument is the entire world uses something much, much more natural and easy to augment: convective cooling.
Willis Eschenbach says:
March 30, 2014 at 2:33 pm
Um … the field of radiant cooling?
Your lie filled rant doesn’t help your cause any either, where you simply melted down and started publishing bald-faced lies everyone on the thread knows aren’t true. I put like a dozen different references on this thread.
You’re just doing the “I’m not really in thermodynamics so I don’t have a reputation to blow: so I’ll just throw a low class invective filled meltdown into the thread.
I have one that went into moderation listing all my contributons Willis. It’s less than disingenuous to simply start ranting like someone off their meds just to tantrum and lie, when the evidence that you’re just tantruming is on the very page you’re doing it on.
Martin Lewitt says, March 30, 2014 at 3:26 pm:
You are trying to make the case that heat CAN pass from cold to hot. When it comes to radiation. Give it up. It can’t.
We agree on the result of reduced cooling. We do however NOT agree that you reduce a hot object’s cooling by letting the opposing cold object increase its heating. ‘Back radiation’ does not raise the temperature of the surface of the earth, Martin. Because that would make it an extra source of HEAT for the surface. A heat source heats (raises the temperature), a heat sink cools (lowers the temperature). And ‘back radiation’ to the surface comes from its heat sink. So you’re left in a check mate position. Because it can’t NOT be heat and then still heat the surface.
This is what I’m trying to tell you. You have to move away from the whole idea of the cooler atmosphere as a provider of energy (in effect a second heat source) to the warmer surface. It is ONLY a receiver of energy. A heat sink for the surface.
Reduced cooling comes from actually reducing the energy flow going OUT FROM the hot object to the cold object. By making the temp difference smaller (referring once again to the radiative heat transfer equation).
You see the difference? When you physically make the OUTGOING flux from the hot object smaller, THEN it is the energy supplied by a constant flux IN from the sun that piles up and make it even hotter. This is a situation that perfectly satisfies the laws of thermodynamics. You on the other hand are NOT making the outgoing flux smaller. You make it bigger by adding more INCOMING. And then it is NOT the energy from the sun that piles up and causes the extra warming. Then it is your extra addition of energy from the cooler atmosphere that does the trick.
And this is a direct violation of the laws of thermodynamics.
James (Rollins), I think I see the reason for the difficulty. We’re comparing net sensible and latent heat flows with individual radiation flows. Work with me here.

Let me take as an example the Kiehl-Trenberth global energy budget:
Now, set aside all of your considerations of whether it is right or wrong and look at what is being measured. The radiation flows are all the INDIVIDUAL flows, as is typical in multi-body radiation analyses.
But the sensible and latent heat flows are NET FLOWS. Let’s look at the sensible heat for example. It’s about 24 W/m2 as a global net average.
Now, during the day, the surface heats the atmosphere from the bottom, and the direction of the heat flow is from the surface to the atmosphere.
But during the night, the situation is reversed. The surface cools the atmosphere from the bottom, leading to thermal stratification, and the heat flow is from the atmosphere to the surface.
Note that the net heat flow, which is what is gained in the day minus what is lost in the night, is as we would expect, from the surface to the atmosphere. That’s the 24 W/m2 shown in the K/T diagram above as “Thermals”, a poorly chosen term meaning sensible heat loss.
And as a result, while as you point out the heat loss to the atmosphere during the day is quite significant, so is the nocturnal heat gain. it is the difference of these two, the net heat loss, that is shown above and that I have been discussing and referencing.
w.
Willis Eschenbach says: (to)
March 30, 2014 at 12:35 pm
david(swuk):
March 28, 2014 at 4:51 pm
So, ok bud, but your Bach is just a sharp as that Berger Bite I saw in your response to (Stephen was it – can`t reel back easy to check) so why the huff? Making you look “All Warmist” it is!
As for the term “magician” I of course was referring to the disappearing up your sleeve act you put on Solar N/IR Radiation when slipping from “Solar Radiation” to “Wavelenghts of Visible Light” in your claim that the Atmosphere does not absorb SR. There is no refuge for that switch and use of the words “denial” and “he knows….” are well justified in coming from a Scientist of your distinction on such fundamental fact.
But you don`t even stop there Sunshine in dishing me 5degC night sky temperature when it`s more like -40degF (pardon please my slip in writing deg,C) and the related and totally absurd 340W/M2 which is about all we are getting (minus the Real World wind chill effect) in full Sun here @ ~50degN just now.
But tempus fugit and I must wish you an early to you bon nuit if not goodbye on this subject just yet.
James Rollins Jr says:
March 30, 2014 at 4:12 pm
James, this is a perfect example of why I insist, not ask but insist, that people quote what they are objecting to.
To start with, who are you addressing? Me? Kristian? Curt? Someone else?
Second, what are you claiming to be a “bald-faced lie”? Accusing a man of lying without quoting the lie is a sneaky underhanded tactic. IF it is me that you are accusing of lying, either quote me or leave me alone.
Third, don’t put quotes around words that are your own. Those are YOUR WORDS, not mine, and I said nothing remotely similar.
You are a piece of work, James. I’m trying to play nice with you, but your rage-filled invective makes it hard.
w.
Kristian says:
March 30, 2014 at 4:43 pm
No, no, that’s not what he said. Read it again. He did not say that HEAT can pass from cold to hot.
He said that ENERGY can pass from cold to hot. If you shine a flashlight at the sun, the sun gets warmer … but not as much as the flashlight warms. In other words, there is a real physical flow of ENERGY from the flashlight to the sun. And there is a real physical flow of ENERGY from the sun to the flashlight. The energy is transferred in the form of photons of light.
But the HEAT, which is the NET ENERGY FLOW, only goes one direction—from hot to cold.
If you don’t understand what I just said, or if you disagree … buy a thermo textbook, I can’t help you.
w.
Kristian: Do you really not understand that electromagnetic radiation carries energy with it? Seriously?
Do you not understand that streams of electromagnetic radiation pass through each other unimpeded, like the example of two flashlight beams I recently brought up? And that this means that the energy these carry with them pass through each other as well?
Do you not understand that if a body absorbs electromagnetic radiation, it absorbs the energy carried by that radiation, and that this increases the internal energy of the object?
Do you not understand that if a body emits electromagnetic radiation, it emits the energy carried by that radiation, and that this reduces the internal energy of the object?
Do you not understand that we have known how this works down to the photon level since Einstein’s and Planck’s day, a hundred years ago? (And I will side with Einstein and Planck any day over Kristian!)
Do you not understand that a photon does not carry any information about its source, whether it was from thermal emission or not, and if from thermal emission, what the temperature of the emitting body was?
Do you not understand that for even for a thermally emitted photon of a given wavelength hitting a body of temperature T, the receiving body has no way of knowing whether the photon was from the “colder” (longer wavelength) part of the spectrum of a higher temperature body, or from the “hotter” (shorter wavelength) part of the spectrum of a lower temperature body?
Do you not understand that that the body’s probability of absorbing this photon and the energy it carries (= h * v) depends only on the body’s absorptivity at this wavelength, and not on any property of the emitting body?
Do you not understand that the metaphorical “heat flow” you keep talking about is simply a convenient way of talking about the difference between the countervailing gross energy flows carried in the electromagnetic radiation in the opposing directions – that it is not a physical entity at all?
Do you not understand that in any system but the absolute simplest of systems – those with more than two bodies, those with bodies of differing absorptivities/emissivities, bodies whose absorptivities/emissivities change with temperature, etc. – must be analyzed in terms of gross radiative flows, calculating heat transfer at the end?
Once you understand all of these things thoroughly, you can start providing a meaningful contribution to the discussion.
Curt says, March 30, 2014 at 5:23 pm:
I do understand one thing, Curt, and that is that you’re stuck in a naïvely simplistic 18th century concept world where the transfer of energy between two objects at different temperatures, where the hot object heats the cold object, is pictured as happening through distinct instances of heating and cooling in both ends by alternating absorption and emission of streams of ping pong ball-like particles flying along mutually independent and oppositely directed highway lanes that once accidentally put together (in what we today would call a ‘radiation field’) would still be readily distinguishable from one another and could also on a whim just as easily be disentangled again to stand as individual and working entities, take away the one and the other would still be there just as before, completely unaffected.
You STILL don’t understand even the slightest of what I’ve been saying on this thread, Curt. Still missing completely the EXCEEDINGLY simple point I’m making. You just don’t see it, do you?
The vigour of convection from a heated surface depends on the rate at which temperature declines with height as well as the absolute temperature of the surface.
The average temperature of a heated surface depends on both the amount of incoming radiation and the length of time that the radiation remains absorbed by the surface.
The rate of decline in temperature with height (the lapse rate) is set by the rate at which atmospheric density declines with height because the greater the density the more of any incoming radiation can be absorbed.
That all applies equally to atmospheres with and without radiative gases.
The slope of the lapse rate can potentially be affected by the presence of radiative gases in an atmosphere because the kinetic energy carried by such gases tends to spread the kinetic energy at the surface up through the vertical column.
That potentially changes the effective emission height for the atmosphere.
Note that changing the effective emission height need not involve a change in surface temperature if the lapse rate slope changes at the same time.
However, a reduction in the rate of decline in temperature with height also reduces the vigour of convection and less vigorous convection pushes less high up into such an atmosphere so the effective emission height drops back again.
Once more, the decline in temperature with height is determined by the decline in density with height and the original lapse rate is restored.
The reason for that being possible is the ability of the radiative gases to radiate directly out to space which means that the system can lose the same amount of energy to space as before radiative gases were introduced but without such a vigorous convective circulation.
The convective circulation in a non -radiative atmosphere must be more vigorous because it is then necessary to return all the energy flowing into convection back to the surface before it can be radiated out to space.
The thermal effect of radiative gases is, therefore, offset by an equal and opposite convective response.
The rules for condensing GHGs such as water vapour are different and need not concern us here.
Despite radiative capability within an atmosphere, the surface temperature, the effective emission height and the slope of the lapse rate are all kept stable on average over time.
If it were correct that radiative gases could contribute to a warmer surface than would otherwise be the case then the warmer surface would result in a faster decline in temperature with height and more vigorous convection.
The problem with that is that adiabatic convection returns as much energy to the surface on the descent as it takes away from the surface on the ascent so the more vigorous convective overturning would create a positive feedback loop which would result in a warmer surface and faster convective circulation with each successive convective cycle.
The atmosphere would soon be blown off into space by the heat building up at the surface but that hasn’t happened for the past 4 billion years.
Trick said:
“Only the sun using up its fuel can raise surface temperature mean from 255K to 288K enabled by increased atm. optical depth (opacity). Any sort “delay” in energy transmission uses up no other fuel adding 33K energy to earth system which can only come from radiative energy transfer of a nearby star – ”
Mass absorbs solar energy via conduction and any gases then engage in convection which is a slower method of energy transmission than radiation and so must heat the system if the rate of incoming energy remains the same.
No other ‘fuel’ is needed to raise the surface temperature beneath an atmosphere. All the necessary fuel was consumed in the sun.
To clarify, I should have said:
” the greater the density the more of any incoming radiation can be absorbed either directly or from the heated surface.”
It is important to realise that the bulk gases which have negligible radiative absorption capability are well able to absorb conductively from the surface especially when recycled by convection.
James:
Since you tried to pull rank on me by calling me an “amateur”, I will mention my background. I have worked professionally as an engineer for over thirty years now, dealing with thermal issues on an almost daily basis in my work. I have graduate and undergraduate degrees in mechanical engineering, with extensive coursework in thermodynamics, heat transfer, and fluid dynamics.
Some of the interesting thermal issues I’ve dealt with lately in my professional capacity – I mentioned the first two of these above:
A customer of ours is using a power electronic amplifier with a metal heat sink that has been black anodized to enhance its radiative output for cooling. The electrician wiring up the cabinet did not realize that the black anodized surface is not a good electrical conductor and tried to ground the amplifiers through the surface of the heat sink. All sorts of flaky electrical issues resulted.
On a new power electronic design, the electrical and thermal designers want very high-flow fans on the heat sink to keep the electronics as cool as possible, which would extend their lifetime. The marketing people want lower-flow fans so they will be quieter. We are in the midst of testing to validate the thermal model predictions. I am mediating this.
A customer of ours who was using motors in a vacuum chamber at a synchrotron did not adequately account for the added temperature that would result from the loss of conductive/convective cooling, leaving only radiative cooling. I had to work with them to reduce electrical current in the motor to the absolute minimum to keep the motor within temperature specs.
A lot of the world’s large telescopes use our electronics. Most of them are at such high elevations (e.g. 4500m) that they are beyond the standard range of conditions for listed cooling requirements due to the less dense atmosphere. I have had to work with them to qualify specialized cooling systems in these environments.
In one of these big telescopes, the scientists wanted to minimize the power dissipated by our electronics because the convection currents inside the dome were distorting their optics (like the air over a fire). We came up with a scheme of selective “underclocking” of the key digital circuits, which significantly reduced their dissipation at times when high performance was not required.
In each of our heat sink designs, we have to decide whether it is worth the cost of black anodizing the outer surface to enhance the radiative cooling. When there is forced convection as well, we usually conclude that going to a higher-flow fan is more cost effective.
In a new heat sink design, we have to decide whether we should go with an extruded design, where all of the fins must be parallel, or with a cast design, which provides for more flexibility in managing the convective flow, but has more up-front tooling costs.
Since I used to design opto-electronic components, I stay active on those engineering forums. I recently helped an LED lighting designer understand how light from an LED generated in the semiconductor “junction” at ~35C could melt his lens coatings, which meant they were getting close to 100C.
So no, I am not an amateur in this field in any sense of the word.
Now, on to the more substantive points. I said earlier that you read, but you don’t understand. With each post you make, you just confirm my assessment. In your post of March 29, 2014 at 11:43 pm, you object to Willis’ and my use of the basic SB radiative output equation to calculate the radiative output. You say we are ignoring other heat transfer modes.
This is a very odd claim, for several reasons. First, the whole point of Willis’ post was to try to calculate the magnitude of these other modes of heat transfer by subtracting out the radiative transfer. How can anybody claim he is ignoring these modes in an attempt to calculate them? (I also talked about other heat transfer modes in my posts, so I was not ignoring them either.)
Then you link to an Engineering Toolbox page that completely backs up our use of the SB equation. It even emphasizes the difference between gross radiative output and net radiative exchange that we keep talking about, and you still don’t seem to understand. But to repeat one more time: Willis is talking about GROSS output, you are talking about NET exchange.
By the way, the page YOU cite has a very nice link to a table of radiative emissivities of different materials that completely backs up Willis’ claim that metals have very low emissivities and most natural materials have very high emissivities. You claimed that this would require different laws of physics — do you really think Engineering Toolbox believes in different laws of physics for metals?
Now, you seem to be upset that we are not explicitly calculating convective losses in the same style that we are calculating radiative losses. (Any claim that we have ignored them is just factually false.) In Willis’ case – to repeat – he is using a database of radiative measurements to try to calculate the other losses by subtraction. Because in the natural world, it is very hard to compute or measure convective transfer directly given all of the variables in play.) I was able to show that even just gross radiative output from the human body led to a large power imbalance over the metabolic production; computing convective losses would just have made the imbalance worse.
I carefully explained how in typical earth environments, most of this imbalance is compensated for by the ambient radiation. This makes the net radiative balance small, such that the resulting radiative loss, plus convective loss and evaporative loss, often with slight insulation (i.e. clothing), balance the metabolic power gain of the body nicely.
I’m sure that in many of the thermal systems you deal with, convective transfer dominates over NET radiative transfer. In my professional experience, when forced convection is used (i.e. pumps or fans over heat sinks), the convective transfer so dominates over the NET radiative transfer that you can safely get away with ignoring the radiative transfer. I suspect that you often do this.
You keep talking about systems “on earth” having convective transfer as the primary transfer means. But you don’t understand the deeper reason why that is true. Willis and I keep talking about GROSS radiative outputs. Why? Because focusing on this underlying mechanism allows us to look at a much wider set of systems. The reason NET radiative transfer is small for a lot of earthly systems is that we are engulfed in a significant sea of background ambient thermal radiation.
By the way, an incandescent light bulb operating at its rated voltage transfers well over 80% of its power to ambient through NET radiative transfer. Only a small minority of the power is transferred through conductive/convective means. Many high temperature furnaces have multiple layers of reflective radiative insulation to minimize the input power required and to permit higher temperatures to be reached.
But if you don’t understand that NET radiative transfer on earthly systems is relatively minor because the temperature differences are relatively small, so the received radiation is almost as great as the emitted radiation, you aren’t prepared to analyze systems where this is not true.
Just as an incandescent bulb filament at 3000K transfers the large majority of its power to a 300K ambient through NET radiative transfer, a body at 300K can have dominant radiative transfer as well. That’s what the T^4 dependency dictates.
Getting “off earth” now, let’s look at space. We all agree that the mass density is incredibly low, so when we are considering space, conductive and convective transfers are completely negligible. When it comes to radiative transfer, how do we consider it? Well, it’s obvious that space is virtually transparent to electromagnetic radiation – we can detect it from billions of light years away.
Do we treat it as a very cold (~3K) radiating body, or as “nothing”? In any practical thermal analysis, it really doesn’t matter. If you account for the cosmic microwave background radiation that was discovered 50 years ago, you use an ambient radiation level of a few microwatts per square meter. Ignoring this, you change the results by about one part in a hundred million.
If you are in low earth orbit, you can get a few hundred W/m^2 of reflected solar and/or thermal emitted power on one side of you. If you are in direct sunlight, you get ~1360 W/m^2 on one side of you. But there are cases where you get neither of these, and you must be able to survive without any significant GROSS radiative flow coming to you, even as your GROSS radiative output remains high.
Just as with high-temperature furnaces on earth, one of the solutions is multiple layers of reflective insulation. This has the benefit of also working to keep out high levels of GROSS radiative flow coming at you, as with direct sunlight on you.
I brought up the well-known phenomenon of the virtually instantaneous flash freezing of waste water expelled from space ships into space. Since everyone agrees that there is no conductive/convective heat transfer in the virtual vacuum of space, how would you explain it?
No those are your
“frustrated, resentment fueled invective rant streams we all read Willis.
That was you who melted down and went into a paragraphs long invective filled rant that had absolutely zero scientific relevancy about any part of the arguments at hand.
————-
Willis Eschenbach says:
March 30, 2014 at 12:55 pm
your endless
voluble
ranting without making the slightest attempt to provide
citations,
observations,
facts, or
mathematics to support your
manifold claims
is pathetic,
unpleasant,
distractive, and
totally meaningless.
NOBODY CARES WHAT YOU THINK, JAMES.
This is a science site. Look up the meaning of “Nullius In Verba”.
We care about science here, not your ceaseless raving about your incorrect thought processes.
James Rollins Jr says:
March 30, 2014 at 9:11 pm
Great, James, I got it. You’re in a serious snit, plus you have a rogue quotation mark that has inserted itself into your text. I hate it when that happens.
Look, I’m sorry to hear that you have your knickers in such a twist, but so what? Given your past behavior, why should any of us care if you are happy or angry?
Now, how about you respond to this scientific comment of mine above, which is more to the point than your internal state of upset?
w.
You’ve got a cartoon drawn by a clown who thought CERES graphs spelled unstoppable hellfire in the sky, which you apparently believe in. You’re the one with the graph showing more energy leaving an object than hitting it, you respond to how you explain it.
People in the real scientific fields don’t make up charts with more thermal energy leaving objects than arriving. It breaks the laws of physics.
Willis Eschenbach says:
March 30, 2014 at 9:52 pm
Now, how about you respond to this scientific comment of mine above?
Also your graph shows the earth absorbing 324 watts of it’s own previously expended energy; coming down from the atmosphere as backwelling; however the very link you gave,
shows clearly: in figure 3:
“Figure 3 Time series of downwelling infrared irradiance
for May 21, 2007. A clear sky was present from
midnight-midnight local time.
353 watts – not the 400 you claimed it represented,
occurs, in daylight.
That’s
In the presence of the sun. That’s infrared downwelling, total, with the sun.
The amount of atmospheric infrared is much,smaller at night, and there’s most certainly no 400 w/sq/meter infrared back radiation seen.
http://vortex.nsstc.uah.edu/atmchem/docs/DEPSCOR_progreport_9_13_07.pdf
Everybody go look at Willis’ Figure 3. Look at the “353 watts downwelling infrared.”
Look at what time of day it is.
That’s not “back radiation” that’s infrared radiation primarily, from direct solar downwelling.
If you understood what you are talking about you’d have realized space suits aren’t made with heaters.
Pretense you could even calculate the temperature of an object washed with fluid
utterly ignoring the fluid, is exactly what you both did, in front of everyone here.
Repeatedly. You were doing it
while I was pointing out to everyone you were repeatedly doing it.
Nobody told you calculate the surface temperature utterly ignoring the portion removed by convection and conduction. Ever. In your lives.
When radiant transfer from an object is calculated, energy removed by conduction and convection are accounted for first.
Not second.
Then the radiant fraction is calculated.
Don’t pretend the people catching you in these fundamental errors need to have it explained to us.
Don’t pretend that if you are just allowed to say you didn’t do what you just did, it would be like you didn’t do it.
Don’t pretend because you gave up and used wrong calculations
because you can’t begin to dream how to do it with the right ones,
or because no one can, it makes it
like I’m the one who did it wrong, not you.
You did it wrong, multiple times. I caught you doing it wrong.
Regarding water changing phase in space, It’s the change of pressure that makes it turn to ice.
just as the change of pressure that makes it turn to ice in the vapor > ice phase change process in storm cells.
Water doesn’t work in refrigeration systems because when it goes from high pressure to a lower one, it turns to ice.
Moisture In A Refrigerant System
“A single drop of water may look harmless, but to a refrigerant system, it is a monster,
First, it creates “freeze-ups.” Moisture will be picked up by the refrigerant and be transported through refrigerant lines in a fine mist which forms ice crystals at the point of expansion (expansion valve).” Ice crystals retard or stop the flow of the refrigerant, causing loss of cooling.
http://www.robinair.com/acsolutions/acvacuum/acvacuum.php
There’s as much, or more sun-side infrared stream where the space shuttle orbits. If you were in the realm of understanding much of this you’d know why water turns to ice when it’s suddenly depressurized.
You’d also realize the amount of infrared flux where the shuttle orbits is quite high and the concept of there being less infrared where the sunlight arrives full strength is more evidence of you truly, amateurish stature regarding this entire subject Curt.
Curt says:
March 30, 2014 at 8:26 pm
You’re right I’m not formally trained in thermodynamics. I’ve worked around it a lot though.
I’m a mechanical engineer.
I answered you Curt but when I pushed Post twice the thread didn’t appear.
It’s the change in pressure that makes the water outside the shuttle change phase.
It’s what makes it change phase identically in the troposphere in storm cells when it rises as vapor and contracts into ice.
It’s what makes it change phase similarly when water gets into a refrigeration unit, and it’s the reason water can’t be used in a modern compressor type refrigeration unit.
For someone telling everyone we need to study you sure don’t know the answers to a lot of extremely basic questions, Curt.
Good. Then we seem to agree: You let your flux down from the atmosphere to the surface heat it directly, that is, not by reducing the outgoing flux (decreased cooling), but by adding to the incoming flux (increased heating). Can’t do that.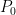 , the system will be in dynamic equilibrium at a constant temperature when the outgoing flux equals this. If we assume that the reservoir is a perfect blackbody that can only radiate energy out through the single transparent surface with surface area
, the system will be in dynamic equilibrium at a constant temperature when the outgoing flux equals this. If we assume that the reservoir is a perfect blackbody that can only radiate energy out through the single transparent surface with surface area 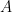 , we get:
, we get:
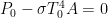


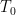 is the dynamic equilibrium temperature of the system in this case.
is the dynamic equilibrium temperature of the system in this case.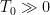 , its entropy change is zero. The entropy gain of the ~0 K “reservoir” of space (per unit time) is thus enormous, the entropy change of the reservoir is zero, and it is left as an exercise for the audience to realize that the entropy decrease of the star is much, much less than the entropy gain of anything else in the system or the surroundings at their much lower temperatures (per unit time). The second law is very happy, and of course the equations above are the first law for this system.
, its entropy change is zero. The entropy gain of the ~0 K “reservoir” of space (per unit time) is thus enormous, the entropy change of the reservoir is zero, and it is left as an exercise for the audience to realize that the entropy decrease of the star is much, much less than the entropy gain of anything else in the system or the surroundings at their much lower temperatures (per unit time). The second law is very happy, and of course the equations above are the first law for this system.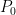 above. Because I am lazy and I only am proving a point, we will make this material perfectly transparent to the incoming SW radiation
above. Because I am lazy and I only am proving a point, we will make this material perfectly transparent to the incoming SW radiation 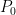 and perfectly absorbing at the LW radiation wavelengths emitted by the reservoir at the range of temperatures it might reasonable reach. This change is not necessary — Grant Petty, for example, provides a more detailed single layer model (still easily solvable) where one can make the absorptivity of the SW and LW radiation independent parameters from 0 to 1. One can make the absorptivity of the layer a full function of the wavelength instead of splitting it into only two pieces. The cost of making these changes is one has to work harder and integrate over the spectra in the end, but it changes nothing of the qualitative demonstration that is my purpose.
and perfectly absorbing at the LW radiation wavelengths emitted by the reservoir at the range of temperatures it might reasonable reach. This change is not necessary — Grant Petty, for example, provides a more detailed single layer model (still easily solvable) where one can make the absorptivity of the SW and LW radiation independent parameters from 0 to 1. One can make the absorptivity of the layer a full function of the wavelength instead of splitting it into only two pieces. The cost of making these changes is one has to work harder and integrate over the spectra in the end, but it changes nothing of the qualitative demonstration that is my purpose.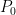 source and emits blackbody radiation as before, but the radiation it emits is all absorbed by the perfectly absorptive intermediate layer. This layer increases in temperature until it emits away all of the radiation it receives as blackbody radiation. The system still has to be in detailed balance — it has to lose energy to space at the rate it receives it,
source and emits blackbody radiation as before, but the radiation it emits is all absorbed by the perfectly absorptive intermediate layer. This layer increases in temperature until it emits away all of the radiation it receives as blackbody radiation. The system still has to be in detailed balance — it has to lose energy to space at the rate it receives it, 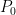 . The side of the thin surface layer of area
. The side of the thin surface layer of area 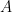 just outside of the transparent surface bounding the reservoir that is facing space therefore has to radiate at temperature
just outside of the transparent surface bounding the reservoir that is facing space therefore has to radiate at temperature 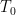 . Outer space is the only actual sink for the incoming power
. Outer space is the only actual sink for the incoming power 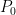 .
. —
— 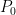 as SW radiation from the external energy source (which could be a laser, a star, anything hot enough to radiate with a peak in the SW/visible range where the thin layer is approximately transparent), and
as SW radiation from the external energy source (which could be a laser, a star, anything hot enough to radiate with a peak in the SW/visible range where the thin layer is approximately transparent), and 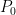 as LW back radiation from the thin layer interpolated between the reservoir and outer space at
as LW back radiation from the thin layer interpolated between the reservoir and outer space at  K.
K.


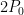 of long wavelength light from the reservoir and absorbs it all. It is at temperature
of long wavelength light from the reservoir and absorbs it all. It is at temperature 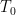 and radiates it symmetrically from its two (inner and outer) surfaces —
and radiates it symmetrically from its two (inner and outer) surfaces — 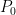 back to the reservoir and
back to the reservoir and 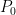 out to space. Space is cold, dark, and far away and acts as a perfect
out to space. Space is cold, dark, and far away and acts as a perfect  K reservoir. The first law is explicitly satisfied for both the reservoir and the interpolated absorber layer.
K reservoir. The first law is explicitly satisfied for both the reservoir and the interpolated absorber layer.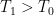 in detailed balance. Its net entropy change as it absorbs energy from the external source
in detailed balance. Its net entropy change as it absorbs energy from the external source 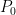 and as back radiation from the shell and and re-emits it all, neither heating nor cooling, back out through the transparent surface that bounds it as blackbody radiation is zero. The net entropy change of the thin absorber layer at the constant temperature
and as back radiation from the shell and and re-emits it all, neither heating nor cooling, back out through the transparent surface that bounds it as blackbody radiation is zero. The net entropy change of the thin absorber layer at the constant temperature 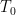 as it absorbs energy emitted from the reservoir at rate
as it absorbs energy emitted from the reservoir at rate 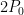 and re-emits it all, neither heating nor cooling, is zero. The entropy change of space is strongly positive as it temperature is very, very low. The entropy loss of the original source of the incoming SW power
and re-emits it all, neither heating nor cooling, is zero. The entropy change of space is strongly positive as it temperature is very, very low. The entropy loss of the original source of the incoming SW power 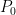 is much smaller than this if it is, as might reasonably be assumed, at either a much higher temperature or the result of operating a laser that overall produces entropy.
is much smaller than this if it is, as might reasonably be assumed, at either a much higher temperature or the result of operating a laser that overall produces entropy.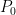 being SW radiation being produced by a very hot star with a temperature vastly greater than either
being SW radiation being produced by a very hot star with a temperature vastly greater than either 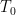 or
or 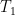 associated with its SW blackbody peak, for example). The only actual source of incoming power is the star. The only final sink in the system is outer space. Net energy transfer occurs from the star to the reservoir, from the reservoir to the thin layer, and from the thin layer to outer space but in dynamically steady state the entropy change of both reservoir and layer are zero anyway. Net heat never spontaneously flows from colder things to hotter things or whatever related claptrap you might wish to assert if you are a disciple of Postma.
associated with its SW blackbody peak, for example). The only actual source of incoming power is the star. The only final sink in the system is outer space. Net energy transfer occurs from the star to the reservoir, from the reservoir to the thin layer, and from the thin layer to outer space but in dynamically steady state the entropy change of both reservoir and layer are zero anyway. Net heat never spontaneously flows from colder things to hotter things or whatever related claptrap you might wish to assert if you are a disciple of Postma. , the reservoir with the LW absorptive layer is strictly warmer with the layer present (heated by SW that bypasses the layer altogether) than it would be without it. No laws of thermodynamics are harmed by this demonstration — the equations are the first law, and if one determines the entropy change of the constant temperature objects in the two systems, the overall entropy change of the Universe is strictly greater than zero. There is nothing any more surprising in this than there is when a block of passive perfect absorber material inside a shell maintained at temperature
, the reservoir with the LW absorptive layer is strictly warmer with the layer present (heated by SW that bypasses the layer altogether) than it would be without it. No laws of thermodynamics are harmed by this demonstration — the equations are the first law, and if one determines the entropy change of the constant temperature objects in the two systems, the overall entropy change of the Universe is strictly greater than zero. There is nothing any more surprising in this than there is when a block of passive perfect absorber material inside a shell maintained at temperature 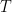 is itself, in dynamic equilibrium where the radiation it receives from the shell is balanced by the radiation it loses to the shell, at the same temperature
is itself, in dynamic equilibrium where the radiation it receives from the shell is balanced by the radiation it loses to the shell, at the same temperature  .
.
Of course I can. For one thing, I don’t tell the flux what to do. It doesn’t “heat” or “cool” the surface. The surface itself can be at a fixed temperature and emit radiative flux continuously as long as there is net energy delivery to the surface through all channels to balance it. Is it “heating” flux? No. “Cooling” flux? No. The temperature isn’t necessarily changing. It is outgoing flux. It carries energy away from the surface. It is, in fact, the flux of the Poynting vector through a surface with an outward directed normal, period.
Flux coming down from the atmosphere is nothing special. It has no memory of how it was created. It is electromagnetic radiation, the flux of the Poynting vector through a surface with a downward directed normal, period.
When I put that surface at the boundary of some (otherwise adiabatically isolated) heat reservoir with fixed volume and no internal source of free energy (so it does no work), we have three possibilities. If incoming flux exceeds outgoing, the reservoir will increase its temperature. If outgoing flux exceeds the incoming flux, it will decrease its temperature. If they balance, the temperature will remain constant. If one knows the heat capacity of the reservoir, one can make this entirely quantitative.
Nothing in this system knows about the temperature of anything else. The radiation incident on the surface of the reservoir does not have to come from a single source, be “black body” radiation (that is, have any particular spectral character) or come from a body or fluid that has any particular temperature or a single temperature at all.
The simplest description of a single layer greenhouse effect comes from just such a reservoir. Consider two systems. In the first case, incoming short wavelength flux enters the otherwise adiabatic reservoir through its one transparent surface, where it is completely absorbed by the unit emissivity medium within. It heats until the outgoing long wavelength flux from the reservoir through the transparent surface precisely balances the incoming radiation. If we assign the incident power (the integrated flux through the surface in the incoming direction) the symbol
or
or
Note well that the only source of incoming flux is the SW source indicated — otherwise we are assuming that the temperature of the surroundings of the system is 0 K, so there is no incoming thermal LW radiation. This works find as a model of a planet in a vacuum, as space surrounding the planet in all directions except the SW source (the star) is basically at 3 K.
We can easily compute the entropy changes associated with the energy movement through this system, because the system absorbs and radiates equal amounts of energy at a constant temperature
We now change the system in only one way. We add a thin layer of material that is a very good absorber in the LWIR band emitted by the reservoir, but is nearly transparent to the SW radiation delivered as
The reservoir absorbs energy from the
This surface radiates symmetrically on both sides, of course, as it is a thin layer of material that is a good conductor and its LW emissivity equals its absorptivity (Kirchoff’s Law). This means that it also radiates power $P_0$ back at the reservoir. The reservoir receives total power
The temperature of the reservoir in steady state is thus determined by:
or
The thin layer is in perfect balance. It receives
How about the second law? Well, as before the reservoir is at a constant temperature
In other words, the second law is entirely happy. Net energy always flows from hotter things to colder things (if we imagine
But in the end,
rgb
rgbatduke says:
March 31, 2014 at 12:34 pm
Dang, Dr. Robert, I do so enjoy your comments. They are detailed and solid, without getting sidetracked in the endless minutiae.
That is the clearest mathematical physics-based explanation I’ve read of why the Second Law is not harmed in building a greenhouse.
Live long and prosper, and continue to comment while you do, would be my wish …
w.
PS—As Dr. Robert commented on another thread, my own descriptions of how the “greenhouse effect” works break no new ground … but they are my own explanations that are a result of how I ended up understanding the poorly named “greenhouse effect”. Remember that I am totally self-taught, so I had to figure out for myself exactly what was going on.
I can’t express my surprise when I realized that the greenhouse effect works because a shell has two sides while a planet only has one side, and that the effect itself has nothing to do with gases, CO2, or even an atmosphere, you can build one out of steel … anyhow, my take on the same subject:
The Steel Greenhouse
People Living In Glass Planets
The R. W. Wood Experiment
So rgbatduke above proposes a perfect Black Body with a fixed window that is transparent to Solar Spectrum plus IR & LW EMR and which contains a reservoir of material that can reach a temperature at which it achieves radiative equilibrium with a Star by use of that fixed perfect window ALONE – AND – then go on to achieve an even higher temperature of equilibrium by the addition of a veil that is opaque only to the wavelength of its initially outward LWR.
Have I got that right rgb?
rgbatduke says, March 31, 2014 at 12:34 pm:
I’m sorry, Robert, that you had to spend all that time and effort to write such an extended post to try and distract from the real issue at hand here with words on top of words on top of piles of red herrings. For you see, all the words and red herrings in the world won’t help you cover up your central flaw, the blatant and embarrassingly obvious violation of physical laws that you commit.
Your base premise is wrong, Robert. And this renders your entire exercise thereafter in trying to drown my argument and displaying to the world your proficiency in physics utterly hamstrung.
I have to ask you the same thing I asked Curt: You simply don’t understand even the slightest of what I’ve been saying on this thread? Still missing completely the EXCEEDINGLY simple point I’m making? You just don’t see it?
I’ll try once more, then, to make it as clear as possible to everyone here what you’re doing.
Robert, what we’re trying to analyse here is a ‘heat transfer situation’. And trying to analyse such a situation, that is, interpret and describe what we think physically happens in a specific process giving a specific result, the first thing we need to make sure is that our explanation satisfies the Laws of Thermodynamics. If it doesn’t, our explanation, our interpretation of what’s going on, no matter how plausible and scientifically sound it appears to be, is WRONG. Start over. Try again. Then we have evidently misunderstood something essential.
I have explained to you and everyone else reading this thread now maybe ten times what exactly it is that you do that violates the Laws of Thermodynamics. But I guess I have to explain it one more time.
Energy comes in from the sun. It is absorbed by the earth’s surface, a cold reservoir (a heat sink) for the sun, warming it to a certain temperature, though far below the temperature of its hot reservoir, the sun. We will now follow this energy and see what happens to it according to Robert Brown.
First it is, after having warmed the surface, freely emitted back out as a loss, cooling the surface, making way for the next round of energy in from the sun. The energy ejected goes to the next cold reservoir (heat sink) in line, this time for the surface: the atmosphere.
The ejected energy is now absorbed by the atmosphere, warming it to a certain temperature, though one below the temperature of its hot reservoir, the surface.
So, here’s what Robert Brown does next. He takes the very same energy (well, not all of it, but some of it; quite a lot of it, in fact), originally come in from the sun, and throws it back to the surface, back into the hot reservoir, warming it one more time (the first time was when coming in from the sun, remember?), raising its temperature ABOVE where it ended up after the first time.
To sum up, Robert Brown want us to follow him in letting the ‘back radiation’ heat again some more its hot reservoir, where it just before came from as thermally emitted loss, warming the cooler atmosphere.
Remember, the energy coming in from the sun is all the time free to escape the surface again as loss. The extra surface warming is allegedly accomplished only upon the return of the ejected energy. The very same energy warming it on two separate occasions, raising its temperature first one time and then some more the next time.
This postulated back-radiated flux is apparently completely undiminished in power and ability to heat, no matter what it strikes, even after having been absorbed by the atmosphere, warming it. It is treated by Robert Brown just as an independent HEAT flux which is able to go and work as just that, heat, anywhere, even towards hotter.
I will end by once again repeating myself:
“(…) in nature, heat does not pass from cold to hot.
You can’t have the cake and eat it at the same time. But that’s what they expect. They want us to believe them when they say that the ‘back radiation’ is NOT heat, but at the same time they want the ‘back radiation’ to directly give a result as if it were. It can’t not be heat but still heat the receiving system (unless they want to argue that it’s in fact ‘work’).”
You do not reduce the cooling of something by supplying it with MORE energy, by ADDING energy to it. Then what you do is increase its heating.
All Balls and no Chain – in short (eh Willi Boy). Mental Masturbationism most unbeComing.
And Trendie`s (well he is isn`t he) peer reviewed by like-inclined individuals “Trick of the Light Fantastic” illustration is the same sort of stuff !
I think the point that is being missed is that the so called effective emission height (EEH) varies with density.
It isn’t a height above a surface that we should be looking for because the radiative fluxes within an atmosphere work in three dimensions, not two.
The greater the density of a radiatively active atmosphere (or any atmosphere, since no gases are completely inert radiatively) the closer to the surface will be the EEH.
So, how to envisage an EEH that varies with density ?
The adiabatic lapse rate slope.
As long as a molecule is at the correct height for its temperature along the adiabatic lapse rate slope then it will have DWIR and UWIR in balance and that is the EEH for that particular molecule.
Any GHG finding itself out of position, whether too high or too low, will be moved by convection back towards the correct height for its temperature.
Too cold for its height will result in it falling back towards the right position and it will warm adiabatically as it falls.
Too warm for its height will result in it rising up towards the right position and it will cool adiabatically as it rises.
The mechanism will be the temperature induced density variations that cause convection.
That is the global thermostat.
At any given moment there are the same number of molecules too warm as too cold so their net thermal effect is zero and convection always works to negate any imbalances that may arise.
Kristian said:
“It (DWIR) is treated by Robert Brown just as an independent HEAT flux which is able to go and work as just that, heat, anywhere, even towards hotter.
In reality it doesn’t heat anything. Instead it causes the GHG molecules to do additional work against gravity which cancels the HEAT by converting it to gravitational potential energy (NOT HEAT) at a greater height.
Having been pushed upwards by the temperature induced density change via convection the initially warmed GHG molecule finds itself above the adiabatic lapse rate slope and so cooler than it ‘should’ be but it continues radiating out to space which makes it even colder than it should be relative to its height and so it starts to sink again until it is below the adiabatic lapse rate slope when it picks up more IR from the ground, becomes too warm and rises above the adiabatic lapse rate slope again.
So, in rising and falling above and below the lapse rate slope a GHG first absorbs IR from the ground, carries it upward, offloads to space and falls back again for another load and the cycle continues.
GHG’s may absorb IR from the ground but they then act as a form of pumping mechanism which ensures that their thermal effect at the surface is zero.
Their absorption capability primes the pump. Density differentials then work adiabatically against gravity. Their radiating capability then discharges the energy used for priming the process to space. The molecule falls and is again primed and so it goes.
James Rollins Jr says:
March 31, 2014 at 12:34 am
How many errors can you pack into a single post?
“That’s not “back radiation” that’s infrared radiation primarily, from direct solar downwelling.”
“Downwelling” radiation, by definition, does not include incoming from the sun. How can we tell the difference? Because the spectra are totally different – there is virtually no overlap. Solar insolation includes a lot of shortwave infrared, but virtually no longwave (past, say 3um). Downwelling from the atmosphere is in the longwave infrared, with virtually no shortwave infrared. Measurements of this “downwelling” flux specifically block out the shorter wavelengths to isolate the longwave.
What Willis and others are talking about, and what was measured in the link you gave, was the longwave infrared, specifically toexclude the solar input.
The 400 W/m^2 Willis has been talking about is the typical upwelling flux density from the earth’s surface. The 324 W/m^2 typical downwelling longwave yields a difference of 76 (these are only very rough averages, of course), which is the NET radiative HEAT transfer between the surface and the atmosphere. Of course, if the atmosphere were transparent to longwave infrared, there would be no downwelling longwave flux, and the earth’s energy balance would be entirely different.
The link you provide shows the downwelling longwave infrared flux density varying between a daytime peak of about 350 W/m^2 – right where cited – and a nighttime low just below 300. I’d say an average of 324 W/m^2 is perfectly plausible given this data, wouldn’t you?
So rgbatduke above proposes a perfect Black Body with a fixed window that is transparent to Solar Spectrum plus IR & LW EMR and which contains a reservoir of material that can reach a temperature at which it achieves radiative equilibrium with a Star by use of that fixed perfect window ALONE – AND – then go on to achieve an even higher temperature of equilibrium by the addition of a veil that is opaque only to the wavelength of its initially outward LWR.
No, I don’t propose this. Read the text. This is a simplified version of a more detailed single layer model that has parameters for easily measurable differential absorption. Which is itself an approximation of the actual spectroscopy of the atmosphere, which (of you are interested in doing something other than picking nits) you can look at measured graphs of in Grant Petty’s book or many other places, which show that the atmosphere is, in fact, significantly transparent in the bulk of the approximately blackbody spectrum associated with the 6000K sun, and is, in fact, significantly opaque in the absorptive bands of CO_2 water vapor, and less opaque but still strongly absorptive in ozone, with little visible signature from other GHGs. Petty has spectrographs of that, as well, as measured both at the bottom of the atmosphere looking up and the top of the atmosphere looking down. But data, I’m sure, doesn’t matter to you.
In the end, though, yes, if there is differential absorption of the atmosphere between SW and LW radiation, there is a significant GHE. This model is without a doubt an oversimplified one, but it suffices to disprove the most often complaints of people that don’t understand thermodynamics — that the effect described in no way violates the first or second laws of thermodynamics, and results in a warmer surface with GHGs than without them. To determine accurately how much warmer is nontrivial, and cannot be done with a single layer model and ignoring other aspects of heat transport.
rgb
Duke said……………………
I`m nit picking,
I say,
I`m spoiled for choice.
it doesn`t matter how many times I might pour back on forth part of the contents of a pint glass into a smaller one it still ends up being just a pint (if I`m lucky)!!
[No, the final liquid will end up being entirely contained in the volume of the smaller jar. Or on the floor. Mod]
I want to take this time to encourage you, the individual reader, to go back and examine the claims made by Curt.
Curt and Willis are both saying things that even on cursory inspection,
are wildly inaccurate; Willis with his “80% of natural emissions from earth’s surface are radiant”
Literally so easily checked it makes one wonder what is going through his head – how could he believe a planet known to lose nearly fifty percent of it’s energy through evaporation alone,
is actually losing so much energy purely to radiation that all other losses, combined, are minor?
Reminded of the glaring conflict with reality, of this unforgivable thermodynamic error, Curt pipes up with his declaration of support for it,
and along the way has of course made a list of his own, hilarious claims.
We’ve watched Curt declare he’s very well educated, and able to understand the issues..
Curt is now saying he thought Sunlight and Earth emitted infrared light are so completely different in spectra
that the term “Downwelling Radiation” can’t mean from the sun because the sun doesn’t produce any medium or long wave radiation infrared radiation.
Look at his assertions; then look at this illustrated graph of solar spectrum.
“the Sun emits nearly zero energy to earth, in earth’s own frequency.
“The sun emits nearly no long wave infrared” Curt hilariously shouts as proof he’s right.
“The very type infrared being sampled for rules out any sunlight being in that at all!”
Look at the top of the atmosphere and the sunlight arriving.
Look at the bottom of the atmosphere and the sunlight arriving.
Compare this to Curt’s claim: “The sun emits almost no energy in the same frequency of earth.
Curt doesn’t even know generally what spectra the sun emits.
The things Curt is saying aren’t remotely possible because of
grammatical error, or inability to explain himself.
He told us all: stating it as a proof he’s thought about this, a long,
long time: The sun emits nearly zero earth spectra infrared.
Curt says:
April 1, 2014 at 7:59 am
“Downwelling” radiation, by definition, does not include incoming from the sun.
Because the spectra are totally different – there is virtually no overlap.
Solar insolation includes a lot of shortwave infrared, but virtually no longwave (past, say 3um). Downwelling from the atmosphere is in the longwave infrared, with virtually no shortwave infrared. Measurements of this “downwelling” flux specifically block out the shorter wavelengths to isolate the longwave.
I think everyone needs another opportunity to read what Curt wrote, as his proof, he thinks he understands what he’s saying so here it is.
Note that he’s referring to “the link you provided” meaning me.
That is the link Willis provided, as proof 400 w/sq/meter is upwelling from the earth. When what it actually is, is a link to an experiment showing 353 watts downwelling, from the atmosphere overall, including earth based “back” radiation and full sunlight infrared downwelling.
What I’m encouraging the typical scientifically oriented reader to do is ask themselves how it’s possible for so many kooky claims to come from people who have even a loose grasp of the realities of earth/atmospheric energy.
Curt says:
April 1, 2014 at 7:59 am
“Downwelling” radiation, by definition, does not include incoming from the sun. How can we tell the difference? Because the spectra are totally different – there is virtually no overlap. Solar insolation includes a lot of shortwave infrared, but virtually no longwave (past, say 3um). Downwelling from the atmosphere is in the longwave infrared, with virtually no shortwave infrared. Measurements of this “downwelling” flux specifically block out the shorter wavelengths to isolate the longwave.
What Willis and others are talking about, and what was measured in the link you gave, was the longwave infrared, specifically toexclude the solar input.
The 400 W/m^2 Willis has been talking about is the typical upwelling flux density from the earth’s surface. The 324 W/m^2 typical downwelling longwave yields a difference of 76 (these are only very rough averages, of course), which is the NET radiative HEAT transfer between the surface and the atmosphere. Of course, if the atmosphere were transparent to longwave infrared, there would be no downwelling longwave flux, and the earth’s energy balance would be entirely different.
The link you provide shows the downwelling longwave infrared flux density varying between a daytime peak of about 350 W/m^2 – right where cited – and a nighttime low just below 300. I’d say an average of 324 W/m^2 is perfectly plausible given this data, wouldn’t you?
Sorry James, I’m that individual reader, that thinks you’re a pompous wanker. Have a nice day. By the way Curt rules!
Curt has said to James somewhere (apparently).
“The link you provide shows the downwelling longwave infrared flux density varying between a daytime peak of about 350 W/m^2 – right where cited – and a nighttime low just below 300. I’d say an average of 324 W/m^2 is perfectly plausible given this data, wouldn’t you?”
Trendies Trip of the Light Fantastic Cartoon shows 168 (x~4 in all cases below as well) of Solar heat being absorbed by the surface from which 102 goes to thermal losses and so leaving only 66 available for surface radiation be it from cold ice through to hot Tropical surfaces and which can only heat the atmosphere by the amount provided. So to that 66 we can at best add say half of the Solar heat lost to the atmosphere at 34 (34 up and 34 down of course) and so arrive at a total flux of ~100 plus (perhaps) half (again) of the thermal loss to give at the most 150W/m2 available for DWIR back radiation – this however will be at much lower than ambient temperature than most of the surface upon which it will arrive and so then act as a cooling flux and not heating – Neither Water Vapour nor Co2 absorb nor radiate in the “Comfort Zone” below body heat and above freezing – I don`t think!
So unless you “Believe” that photons from a cooler source can accumulate to heat a warmer one upon which they fall and could be in trouble were you a financial accountant of such practice I think the IPCC and its accolytes look wrongly upon the net effect of GHG`s.
rgbatduke says, April 1, 2014 at 8:15 am:
“In the end, though, yes, if there is differential absorption of the atmosphere between SW and LW radiation, there is a significant GHE.”
The glaring problem with this statement is that the earth’s atmosphere (by the presence of the so-called ‘GHG’) actually absorbs a lot more radiative heat coming in from the sun than going out from the surface. You need only look at those famous (infamous?) global energy budget diagrams to see that. According to the numbers from Stephens et al. 2012 for instance, the atmosphere absorbs 75 W/m^2 of the incoming radiative heat, but only [398-345.6-20=] 32.4 W/m^2 of the outgoing radiative heat. (And as we all should know, HEAT (& work) is what ‘heats’ something. It is that ‘net’ flux that actually transfers HEAT (from sfc to atm), not those postulated individual radiative fluxes going up and down. They would simply spontaneously make up the heat.)
http://curryja.files.wordpress.com/2012/11/stephens2.gif
Well, 75 is a number a little bit more than 2.3 times larger than 32.4.
On top of this, the presence of ‘GHGs’ in our atmosphere, primarily through H2O, raises earth’s global albedo (0.3) far above the moon’s (0.13), causing between 55 and 60 of the W/m^2 worth of radiative heat coming in from the sun that would otherwise have been absorbed by the earth system (mostly by the surface), to be instead reflected directly back out to space.
How is this atmosphere warming the surface of the earth compared to a ‘non-GHG’ situation?
An atmosphere on top of a solar-heated surface would warm (have energy (heat) transferred to it) with or with without the presence of ‘GHGs’, through other heat transfer mechanisms than the radiative one. It could however not cool (get rid of this transferred energy (heat)) properly to space without the presence of ‘GHGs’. Because in this case, radiation is the only way. So what atmospheric gases really ‘trap’ heat from the surface? It is of course those that make up 99.5% of it: N2, O2 and Ar, that are able to absorb heat from the surface (conductively/convectively), but not adequately able to shed it to space (radiatively).
Putting ‘GHGs’ into an atmosphere does not enable it to warm. It enables it to cool.
=== === ===
Finally, think about this:
Why will a solar-heated surface naturally warm some extra once you put a massive atmosphere on top of it?
It’s all to do with the weight of the atmosphere on the surface. As soon as you insert the atmosphere between the solar-heated surface and space, there will be conductive/convective losses in addition to the radiative ones. But these losses work only by the effective movement of heated air away from the surface. So it is an inherently slow process. The atmosphere ends up functioning as a ‘conductive’ (in reality a ‘convective’) insulation layer on the surface, which can no longer release its absorbed energy as readily as before.
So it has to warm from the S-B temperature (that is, it will accumulate energy) to attain an ultimate balance between incoming and outgoing.
Ask yourself: Will an earth surface with an equally heavy atmosphere resting on top of it and with an equal input from the sun, but with a mean global temperature of only -18C (255K), manage to run an equally efficient convective ‘engine’ as the one we have at +15C (288K)?
And if it can’t, what will happen in between?
What if you made the atmosphere much lighter, same input? What would happen then?
rgbatduke says, April 1, 2014 at 8:15 am:
This model is without a doubt an oversimplified one, but it suffices to disprove the most often complaints of people that don’t understand thermodynamics (…)”
We know the Laws of Thermodynamics perfectly well, Robert. That’s the very reason why we can point out to you exactly how you violate them.
“(…) — that the effect described in no way violates the first or second laws of thermodynamics (…)”
The effect doesn’t violate them, Robert. Listen to me, it is your description of how this effect comes to be that violates (both the 1st and the 2nd) Laws of Thermodynamics.
It does not come about by adding more energy to the hot system (increasing the input), heating it from cold. It works by making less energy go out from the hot system, reducing its cooling (decreasing the output). To accomplish this all you need to do is make the temp gradient away from it smaller than before (T_2 larger relative to T_1).
It is your flawed interpretation of the radiative heat transfer equation that makes you violate the Laws of Thermodynamics. You see it as two individual Stefan-Boltzmann equations in one. It’s not. Well, mathematically it is. Physically, it isn’t. It describes ONE integrated, indivisible process. P/A (the heat) is the only answer, the only real flow of energy we find.
Recognise that the transfer of heat is ONE spontaneous and continuous process that cannot be divided into two separate heat fluxes going in opposite directions, and your problem is fixed.
Nope! David swuk goofed………………..
From the 150 DWIR deduct half of the uprising IR at 66 to revise the DWIR to 117W/m2 pl.
I too invite readers to compare James’ and my claims in the above threads. It will reveal that James has absolutely no idea what he is talking about when it comes to radiative heat transfer.
On the most basic level, no matter how many times it is pointed out to him, he does not (cannot?) understand the difference between GROSS radiative power output and NET radiative power transfer. Willis has been talking about GROSS outputs, as has been emphasized multiple times, and James thinks he is talking about NET. The difference is covered on the second or third page of the first chapter on radiative heat transfer in any text.
On to the latest point of contention: the difference between the sun’s radiative output spectrum and the earth’s. I said, “the spectra are totally different – there is virtually no overlap. Solar insolation includes a lot of shortwave infrared, but virtually no longwave (past, say 3um).”
James misquotes me multiple times (James – when you claim somebody said something they didn’t say, and put it in quotes, that’s called LYING.) and talks about the sun’s emissions. “Insolation”, the term I used, is defined as the received radiation. What’s the difference? Only about 150 million kilometers! And due to this little phenomenon called the inverse square law, the radiative flux density is about a factor of 50,000 lower at the earth than at the sun’s surface. But what’s a factor of 50,000 between friends, right?
Only about 2% of the sun’s spectrum is at wavelength’s longer than 3um, and the large majority of that is between 3um and 4um. Typically, measurements of the downwelling longwave infrared start at 4.5um, where less than 0.5% of the sun’s spectrum lies. So with a total radiative flux density from the sun of 1365 W/m^2 at earth, only about 6 W/m^2 is in the range measured for DWLWIR.
So when James says of these measurements, “That’s not ‘back radiation’ that’s infrared radiation primarily, from direct solar downwelling”, talking about measurements on earth that show over 300 W/m^2 DWLWIR (over 4.5um wavelength), he is completely and utterly wrong.
Here is a good plot of the difference between the sun’s spectrum (at earth’s distance) and earth’s own spectrum:
http://labspace.open.ac.uk/file.php/5177/moddata/resource/62834/Items/S250_3_004i.jpg
(By the way, James, I have never gotten such stringent security warnings – from multiple browsers – as on the link you provided.)
Maybe I can help James with an simple explanation using net amounts of energy. Energy in minus energy out equals zero. Using the KT figures we have 168 w/m2 input. We have 3 ways to lose this energy. Thermals (24 w/m2) Latent heat (78 w/m2) Radiation (66 w/m2).
Therefore we have 168-24-78-66= 0
Go Whitecaps!! said:
“We have 3 ways to lose this energy. Thermals (24 w/m2) Latent heat (78 w/m2) Radiation (66 w/m2).
Therefore we have 168-24-78-66= 0”
That’s not right because only radiation gets out to space.
Thermals only release radiation to space from GHGs and aerosols lifted up within them. The rest of their energy gets returned to the surface in adiabatic descent for radiation out from there.
Latent heat is contained within thermals when water vapour is present but it only releases radiation to space from condensate such as clouds and raindrops. The rest of the latent heat energy gets back to the surface on the subsequent descent of air after the condensate has been removed because the dry adiabatic lapse rate on the descent is twice the wet adiabatic lapse rate on the ascent.
In effect ALL radiation to space is either from the surface or from GHGs, aerosols, clouds and raindrops.
Anything that radiates out to space from within the atmosphere causes less convection within the atmosphere which leaves surface temperature unaffected.
The reverse is also true which is why the only effect from GHGs is a change in convection rates whether the net effect be warming or cooling.
CURT – said at 7-44am2Apr. %3Bhttp%253A%252F%252Fscienceofdoom.com%252F2010%252F01%252F20%252Fco2-%2525E2%252580%252593-an-insignificant-trace-gas-part-two%252F%3B480%3B303
%3Bhttp%253A%252F%252Fscienceofdoom.com%252F2010%252F01%252F20%252Fco2-%2525E2%252580%252593-an-insignificant-trace-gas-part-two%252F%3B480%3B303
“Believe” me???
No bud because if you were playing straight you would not have fished out from all the others the graph that presents the BB emissions of Sun and Earth side by side without reference to relative size which I think is 10 the power of six, Sun over Earth, nor the one that shows the absorption lines that verify the ~200W/m2 Solar IR taken out by the cold atmosphere and so then beamed down among the relatively paltry amount of rebounded Up-rising IR/LW as one can easily infer from another graph detailing Solar intensity rating of 15,000 units max.against the Earths 0.05max. These graphs are referenced “Marble in Space” – “The Habitable Planet”- “Co2, an Insignificant Gas?”
You can find `em all @
………..but you know that already don`t you: Curt(ains!).
David, you are making the same mistake I explained to James that he made, which means that you don’t understand the argument at all. So let’s break it down.
The sun’s spectrum is very close to that of a black body at 5770K, which means that the radiative power flux density is sigma * T^4 = 62.8 million W/m^2 AT THE SUN’S SURFACE. But this radiation spreads out according to the inverse square law, so that each doubling of distance reduces the density by a factor of 4.
The sun’s own radius is 700,000 km. The earth’s orbital radius is 150,000,000 km. So the radiative flux density from the sun AT EARTH is about:
Qearth = 62,800,000 * (700,000)^2 / (150,000,000)^2 = 1368 W/m^2
This figure for the power flux density of the solar radiation at the top of earth’s atmosphere is utterly uncontroversial. It has absolutely nothing to do with any real or imagined greenhouse effect.
In all of the plots you referenced, as well as the one that I referenced, the fraction of the solar output at wavelengths longer than 4.5um is minuscule. Referencing the tables in one of my heat transfer textbooks, it is less than 0.5%, or 1 part in 200.
At the sun’s surface, this is still a high number. But at the earth, it is only 1368/200, or about 6 W/m^2. If the atmosphere filters any of this out, it is an even lower number So James’ claim that daytime measurements of downwelling longwave infrared radiation of 300 W/m^2 or more, which measure at wavelengths longer than 4.5um, are primarily from the solar radiation, is completely false.
Curt you’re exactly right about that and as soon as I started talking about the various spectra relative to the pyrgeometer Willis showed, I was making error after error.
Curt says:
April 2, 2014 at 5:45 pm
David, you are making the same mistake I explained to James that he made, which means that you don’t understand the argument at all. So let’s break it down.
The sun’s spectrum is very close to that of a black body at 5770K, which means that the radiative power flux density is sigma * T^4 = 62.8 million W/m^2 AT THE SUN’S SURFACE. But this radiation spreads out according to the inverse square law, so that each doubling of distance reduces the density by a factor of 4.
The sun’s own radius is 700,000 km. The earth’s orbital radius is 150,000,000 km. So the radiative flux density from the sun AT EARTH is about:
Qearth = 62,800,000 * (700,000)^2 / (150,000,000)^2 = 1368 W/m^2
This figure for the power flux density of the solar radiation at the top of earth’s atmosphere is utterly uncontroversial. It has absolutely nothing to do with any real or imagined greenhouse effect.
In all of the plots you referenced, as well as the one that I referenced, the fraction of the solar output at wavelengths longer than 4.5um is minuscule. Referencing the tables in one of my heat transfer textbooks, it is less than 0.5%, or 1 part in 200.
At the sun’s surface, this is still a high number. But at the earth, it is only 1368/200, or about 6 W/m^2. If the atmosphere filters any of this out, it is an even lower number So James’ claim that daytime measurements of downwelling longwave infrared radiation of 300 W/m^2 or more, which measure at wavelengths longer than 4.5um, are primarily from the solar radiation, is completely false.
I had misinterpreted what I thought you said by not reading it Curt
This comes from trying to mix pyrgeometer discussion with the over-arching principles of whether it’s possible for the surface of the planet to emit 80% of it’s energy through radiant transfer which is of course impossible.
Adiabatically warmed descending air needs neither to warm the surface nor inhibit surface cooling.
All it needs to do is return the temperature of the air at the surface to the temperature of the surface itself.
It achieves that on completion of the first cycle of convection.
The point being that once the temperatures of the surface and of the air above it are the same then no further conduction to the air can occur because conduction only occurs when there are temperature differences.
That is important because only by diverting radiation to conduction can one increase surface temperature because conduction is slower than radiation so if conduction stops increasing then the surface temperature stops rising.
The ability to conduct is a property of mass and is independent of the radiative properties of that mass.
Thus the greenhouse effect is caused by conduction alone which increases surface temperature for the Earth from 255K (or whatever) by 33K.
Only an energy transfer mechanism that is slower than radiation can increase surface temperature above the S-B prediction.
Whilst the atmosphere continues to recycle a fixed amount conducted energy up and down via convection no further surface temperature increase can arise.
DWIR does not increase the amount of conduction that can occur and thus cannot slow down the rate of energy transmission through the Earth system and so cannot raise surface temperature more than the 33K already caused by conduction.
Instead there are changes in convection which negate any thermal effects from GHGs, aerosols, clouds, ice crystals or raindrops.
“This comes from trying to mix pyrgeometer discussion with the over-arching principles of whether it’s possible for the surface of the planet to emit 80% of it’s energy through radiant transfer which is of course impossible.”
Pyrgeometers do not measure DWIR.
They measure the temperature of the air at the height at which they are designed to treat optical depth as a solid surface.
That is how one gets them to focus on the surface of a solid object which it is intended to measure remotely.
Pointed at a fully transparent atmosphere they record the temperature of space.
Pointed at a fully opaque atmosphere they record the temperature of the air at the instrument.
Pointed at a cloud they record the air temperature at the height of the cloud.
In every case the temperature recorded will be set by the lapse rate slope and not DWIR.
Stephan says: “That’s not right because only radiation gets out to space”
If I was discussing radiation to outer space you would be correct. However, I was discussing surface energy transfer into the atmosphere as per the KT diagram. Radiative energy balance into outer space would be 235 w/m2 as per the KT diagram versus 168+67 w/m2 input.
Of course you do not believe in GHG acting as a absorber/emitter of energy so this explanation is moot.
James Rollins Jr says:
April 1, 2014 at 7:06 pm
James, I explained this to you above. You completely ignored it. I asked you to respond to it. You have not done so. Here is the link again.
Your comments on my explanation would be interesting. Your endless, mindless attacks? Not so much …
w.
An oft overlooked feature of the adiabatic lapse rate (never mind local or ambient lapse rates for the moment) is that it represents the heights at which molecules are at their ‘correct’ heights for their temperatures. Those correct heights being determined by density, and therefore weight, differentials.
At any point where a molecule is at its correct temperature along the adiabatic lapse rate slope all energy transfer mechanisms net out to zero. UWIR equals DWIR and conduction up equals conduction down.
We can ignore the rest of the atmosphere for present purposes because there is equal atmospheric mass too warm for its height and too cold for its height so that nets out to zero too.
The thing is that the adiabatic lapse rate intersects with the surface.
Think about that.
A molecule at its correct temperature at the surface experiences no net radiative flux.
Therefore no net DWIR at the surface when a molecule is at 288K.
That 288K must include the 33K surface temperature enhancement caused by the diversion of radiative energy transfer (fast) to conductive energy transfer (slow).
Combine the S-B equation for the surface (255K) with the Gas Laws for the atmosphere (33K) and the correct surface temperature for Earth is 288K.
It cannot be otherwise because energy flow through the Earth system can only be slowed by conduction to mass which causes a build up of heat at the surface if the solar energy flow through the system is held constant.
There has been confusion between optical depth and atmospheric transparency. They are not the same.
Transparency of an atmosphere does not distinguish between absorption on the way in or absorption on the way out. Either way, the speed of transmission of solar energy through the atmosphere is slowed down.
It is that slowing down via the involvement of conduction that causes the surface temperature rise. It makes no difference whether the slowing down is on the way in, on the way out or a mix of the two.
An observer from space will only note the S-B temperature whilst an observer within the atmosphere will note the surface temperature enhancement as well.
“Of course you do not believe in GHG acting as a absorber/emitter of energy.”
Yes I do.
I just aver that they result in changes in convection and not surface temperature.
That involves climate change, but insignificant compared to natural climate changes.
The real greenhouse effect lurking in the background is mass induced.
Curt at 5-45pm
replied….
No. Sun is ~50K. times smaller in effect -so ok-work from there even – where do you account for the ~200W/m2 of Solar IR that is absorbed as is depicted on the “Marble….” graph shown among the others above because I don`t see it in Trendies Trick o` the Light cartoon depicting that state of `never ever anywhere` climate affairs here on Earth.
continuation……
I`d love to see his midnight and noon shots at them!
Willis I already told you, that chart is messed up. And for any scientific endeavor of any importance, for there to be one major, unforgivable error on it, means it’s trash.
The first thing I see is the 324 w/sq/meter down. Okay.
Fine.
Where’s the identical 324 w/sq/meter that must by definition be emitting up due to the very effect showing 324 emitting down?
There’s unforgivable error 1, rendering the diagram trash. Unless you show me where the other 324 emitting up, is accouted.
Willis Eschenbach says:
April 3, 2014 at 12:56 am
Ignore whether it’s right or wrong just analyze Kiehl-Trenberth
James Rollins Jr says:
April 3, 2014 at 2:02 am
James, you are not listening to me. Here’s what I said again
I implore you again to let go of your considerations of whether it is right or wrong and LOOK AT WHAT IS BEING MEASURED!!! In the case of radiation, in the diagram we are looking at INDIVIDUAL FLOWS. In the case of sensible and latent heat, we are looking at NET FLOWS.
If you wish to use all NET FLOWS, that’s fine. In that case we see that the NET LOSS by radiation is 66 W/m2 (390 up minus 324 down), the NET LOSS by sensible heat is 24 W/m2 (unknown amounts up and down), and the NET LOSS by latent heat is 78 W/m2 (again unknown amounts up and down).
You have insisted that sensible and latent heat losses are larger than radiation losses. And this is true … but only for NET losses. Net losses are 66 W/m2 by radiation, and about 100 W/m2 by sensible+latent. Does that sound about right to you?
Regards,
w.
You need to show me the other half of that 324 accounted for properly in this document or it’s trash as is your reputation as an analyst for not having realized it isn’t accounted for and that something very important is wrong with it, Willis.
I apologize for being snappy Willis I just think you’re going on and on, but… you’re really buying this Kiehl-Trenberth style analysis where you don’t have to be right, and account accurately for your own claim, you just have to be adamant nobody else could be right.
James Rollins Jr says:
April 3, 2014 at 2:22 am
I’m sorry, James, but I don’t understand that. What do you mean by “the other half of that 324 accounted for properly”? Here’s the diagram again:


If you mean that the Kiehl/Trenberth energy budget omits important processes, I can only agree. One of the things that originally drew me to climate science was figuring out the simplest possible model of the climate in order to fix K/T budget diagram. He shows 324 W/m2 downwards and only 195 W/m2 upwards. So, here is a more detailed view …
Note that all layers (surface and two atmospheric levels) are balanced, with as much entering each layer as is leaving it.
To recap. About a decade ago, I realized that there was something very wrong with the Trenberth global energy budget. The atmospheric level is shown as radiating different amounts up and down. Unlike you, however, I didn’t just go Oh, it’s wrong, it’s wrong … I figured out where it was wrong and I fixed it.
I guess that’s why I have the deserved reputation as an analyst … and you?
w.
Willis Eschenbach says:
April 3, 2014 at 12:56 am
“Ignore whether it’s right or wrong just analyze Kiehl-Trenberth”
OK Mr. `Bach and Bite – how about ~400W/m2 of Reflect Solar Frequency Radiation for starters, diffused as it would be?
That`s near about what we are getting on the ground at 50degN UK around now.(minus all that net uprising IRLW stuff of course) and which, at anywhere near Trendies intenisity inferences, would have me still wrapped in my thermals instead of testing our 10degC Briney for conformity with SST anomally blurb and actual tolerability of the ambient!
But whilst TOA must be about right on this liquidised 4 course meal all in one pot potion of his regarding energy dissipation it answers not the real ??? about how the HEAT from the Sun is managed by the atmosphere through all Seasons of the Solar and indeed Milankovitch cycles.
With the atmosphere being a thermal mass ALL of it must be radiating some energy into Space surely and not just the active gases at their responsive frequencies to prevent disasterous bottling but which active gases will have greater effect the more the temperature rises and so cause turbulence that will send them to higher levels of liberation and all of which confirms long history of Mother Earth being capable of dealing with varying levels of insolation and active gases all by Herself, thank you muchly. (but ta for letting out some of the excess gas in mi tanks to feed mi trees but not for then taking `em down nor digging big holes all over the place, choking and covering me in soo and crunchin` under, over and around mi cooler private parts, if you don`t mind).
david(swuk) says:
April 3, 2014 at 3:34 am
I said no such thing. That was James.
I fear that that is totally unintelligible, David. Let me suggest that you leave out all of the flowery stuff. Lose all of the cutesy circumlocution, it doesn’t help. Quit going round the block, and simply say what you mean. Your words often hold no meaning at all to me.
For example. What is “actual tolerability of the ambient”? Why are you talking about a “liquidized four course meal”, and what is that when it’s at home? And what on earth does “TOA must be about right” mean? TOA what?
I’m not kidding, David. I don’t have a clue what your point is, and I’m totally unwilling to guess.
I suggest that you first clarify what it is that you want to say. What is your point? If you are not clear on it, no one else will be either.
Then start with clear sentences. Limit your sentence length to about 12 words or so. Avoid most adjectives and adverbs. Explain unfamiliar concepts. Because here’s the thing.
If you want to get an answer to a scientific question, or make a scientific point, someone out here needs to be able to understand you.
And I can’t. Not when you write like that. It’s not funny. It’s not appealing. It doesn’t make me want to read more, it makes my head hurt. And most importantly, rather than forwarding communication, all that style of writing does is obfuscate and efface and conceal whatever it is you are unsuccessfully trying to get across.
In friendship,
w.
PS—Making fun of someone’s name is never funny. It just irritates people. Just sayin’ …
And there’s no question there’s 159 w/sq/m missing in the “emitted by atmosphere” portion.
There’s no question if the people who tried to call that an ”analysis” tried it in real science
where tenure doesn’t surpass sense, they’d be fired; and you’d be fired for admitting you couldn’t understand, why they were fired, because the people taking money for being able to balance simple thermal in/out problems
are supposed to be able to do just that.
The people who gave you that diagram can’t. As is obvious. We have their very best effort at it.
When I say ‘there’s no question’ I mean is there’s no question there’s the initial obvious difference.
Then there’s the ”absorbed” notation, the cloud functions being ridiculously, grossly over simplified, etc. I’m not looking for a word count I’m pointing it out like I would if I were talking to someone in a real scientific discussion where real scientific realities predominate.
The other half of the 324 that you see clearly marked going down. For someone who thinks of yourself as a smart guy you too, miss a lot of simple, simple things, that are intuitive to people who work in knowing what’s happening to energy.
Willis Eschenbach says:
April 3, 2014 at 2:53 am
I’m sorry, James, but I don’t understand that. What do you mean by “the other half of that 324 accounted for properly”? Here’s the diagram again:
[This message stops abruptly in mid sentence. Do you want to delete it and then try again? Mod]
Willis you have another cartoon, that’s an accomplishment, but what’s that got to do with me having to show you nobody else on earth but you, and maybe a very small number of people,believes 80% of earth surface energy leaves via radiant transport?
Willis Eschenbach says:
April 3, 2014 at 2:53 am
Note that all layers (surface and two atmospheric levels) are balanced, with as much entering each layer as is leaving it.
To recap. About a decade ago, I realized that there was something very wrong with the Trenberth global energy budget. The atmospheric level is shown as radiating different amounts up and down. Unlike you, however, I didn’t just go Oh, it’s wrong, it’s wrong … I figured out where it was wrong and I fixed it.
I guess that’s why I have the deserved reputation as an analyst … and you?
david(swuk) says:
April 3, 2014 at 3:34 am
Willis Eschenbach says:
April 3, 2014 at 12:56 am
“Ignore whether it’s right or wrong just analyze Kiehl-Trenberth”
and
I said no such thing. That was James.(to James?)
(I just copied and pasted like you said I should previously.)
anyway app`s if I intruded:-
Otherwise, in answer to your response nevertheless, is seems that joined-up English doesn`t cut across The Pond as doesn`t a more conversatiopnal approach between amateurs either (and) so I`ll try to adopt staccato style henceforth but only in writing and not thinking (because) I believe that is where part of the problem lies. But don`t feel obliged to reply please if I do get too wordy agenagen).
The gist of my dismay is at the way the Climate Cartel & Co. via the likes of K-T are getting away with justifying their specious claims of (forthcoming as it is now) AGW by presentation of such fallacious documents never mind cultivated data etc. to a Scientific community that prefers looking peevishly compliant than looking down the Sit`s Vac, columns and a general public who can`t gets their heads around anything bigger than a Double Whopper with Cheese – please.
(phew, only 5 lines)
So when I see relatively educated in the Science persons burying themselves in such baloney and correcting/refining it or at least hoping to I, like others, do like to remind them that all those Watts of upwelling radiation well below say the 15degC ambient temperature as in LIR/LW which do not escape the claws of GHGs at either first or second pass go to cooling the planet and not reinforcing (adding to) the ground temperature which first dispatched them. Period.
continuation….
and no, your name is fine by me, just live up to its better connotations maybe.
d.
Willis I worked out the several ways, “it’s wrong, it’s wrong”, years ago, then moved on.
Basically you’re admitting that with hundreds or thousands of hours of study, you came to the conclusion the earth’s convective transport is a fable and 80% of surface losses are radiant.
You’re forgetting Willis studying things longer and longer doesn’t necessarily mean you know more and more: particularly when the professional experts you claim to know more than, using the identical instrumentation you are, are exclaiming among themselves, openly, in stunned humiliation, they realize how completely inadequate their combined data acquisition and observation mechanisms are, for any and/or all of them together, to have any understanding of what they’re really doing, at all.
James Rollins Jr says:
April 3, 2014 at 7:00 am
“Willis I worked out the several ways, “it’s wrong, it’s wrong”, years ago, then moved on.
Basically you’re admitting that with hundreds or thousands of hours of study, you came to the conclusion the earth’s convective transport is a fable and 80% of surface losses are radiant.
You’re forgetting Willis studying things longer and longer doesn’t necessarily mean you know more and more: particularly when the professional experts you claim to know more than, using the identical instrumentation you are, are exclaiming among themselves, openly, in stunned humiliation, they realize how completely inadequate their combined data acquisition and observation mechanisms are, for any and/or all of them together, to have any understanding of what they’re really doing, at all.”
Indeed James, if I may latch on. My reply to Willis above was far too generous in saying ALL THOSE WATTS of upwelling IR/LW as I firmly believe most of the heat is carried up thermally.
In temperate and tropical zones that is along with water vapour particularly.
Wind Chill, which is rated to reduce the ground level by <5degC and ever common in some parts like the usually draughty UK, must so totally overwhelm IRLW that it has only marginal effect at best.
And so the task is that of countering the K-T travesty with a diagram of (likely) Real World
situations showing HEAT FLUX & TEMPERATURES, not WilliWatts, and not tinkering with it.
James, Dr. Spencer, in a 2010 article I was just reading, stated that based on the KT diagram the energy transfered from the surface to the atmosphere by conduction & convection is 4 times greater than by radiation. That is sensible & latent heat equals 102 watts/m2 whereas radiation emits 26 watts/m2 (350-324 watt/m2.) into the atmosphere.
Why do you disagree with this? It seems to me that this would be agreement with your ideas yet you state that the KT diagram is rubbish.
This is a rhetorical question. No need to answer.
Go Whitecaps!! says:
April 3, 2014 at 2:37 pm
James, Dr. Spencer, in a 2010 article I was just reading, stated that based on the KT diagram the energy transfered from the surface to the atmosphere by conduction & convection is 4 times greater than by radiation. That is sensible & latent heat equals 102 watts/m2 whereas radiation emits 26 watts/m2 (350-324 watt/m2.) into the atmosphere.
Why do you disagree with this? It seems to me that this would be agreement with your ideas yet you state that the KT diagram is rubbish.
This is a rhetorical question. No need to answer
——————-
joining your tete a tete if I may I point out that that is 390 by the way and that there surely isn`t sufficient heat capacity/radiative power in scarce GHGs to dispose of 235W/m2 (x~4 on the Sunny side I have read) Solar Heat (~50:50 Vis & IR) reaching and warming the atmosphere and surface.
Sorry Dave, I don’t understand what you’re saying. However, my figure of 350 is correct as that is the radiation into the atmosphere as I stated. 40 watts radiates directly into outer space. Therefore 350 + 40 = 390. (See the KT diagram)
Sorry GW:-
It`s irrelevant as to whether the theoretical 40 is absorbed or not as it only shuffles the numbers around on the quacky no-where, never-ever land depicted in the Warmist K-T diagram.
How, by the way, does it dispose of all that heatgenerated by the natural and industrial worlds – fossil fuel heat for instance – that already “perfect balance” presented by Trendie et al first came out of The Eagle I reckon!
“And we all know that everyday objects far from a big fire are all heated by nothing but longwave radiation, sometimes to the point where they burst into flame.”
So said Willis to another doubter much earlier on.
But campfire IR is well inside the Solar Spectrum (not visible of course eh) whilst the LW which he is seeking to attribute REAL HEAT is not even of body heat intensity.
Also said (to Edim):-
(The downwelling longwave (atmospheric radiation) is no surface input).=Edim
“Say what?”=Wills
and………..
(Net surface LW radiation is upwelling (cools the surface, warms the atmosphere).(=E)
True … so?(=W)
(So, the “parasitic losses” percentage of the total surface input (absorbed solar) is much higher. Not parasitic at all. They’re the main surface cooling mechanism).(=E)
Edim, if you think that the only thing warming the surface is the absorbed solar, I fear you are beyond help. For example, we know (from measurements) that the ocean constantly loses about 400 watts per square metre (W/m2) of radiated energy.
We also know (from measurements and estimates) that the parasitic losses about about 100 W/m2. So the ocean is losing a total of about half a kilowatt per square metre.
View – mine that is:-
DWLW (from top of Troph) delivers near Twice the heat of direct Solar Rad to the surface??
Even tho` radiating in the ~40degC band
“UWLW (from the surface) cools it (in the process and) warms the atmosphere” (Hallelujah)
“The ocean is losing a total of (400+100W) about half a kilowatt of heat per M2 (and would freeze without DWLW – in a further reply)”
Polar Oceans would and do and Tropical ones could not and do not!
So- work in progress at the very best methinks.
Another point – ~40% of Sunlight reflected back into Space?
kind regards to all.
The KT diagram is not warmist. It was first constructed by Dines in 1917.
Whatever it is/has become a Warmist tool to wonderous effect……
One example then I have to dash – you or me laying out uncovered on a Tropical beach will lose little thermal heat to the air as neither will the ocean at similar temperature – transfer though ourselves to a Northern UK beach enjoying 10degC and air and we would be losing about 2KW of body heat (1KW/m2) but the ocean NOT.
The KT “process” simply mixes water and air temperatures globally and ends up with a non-existent differentia,l save in a few areas perhaps, of 10deg C say that could drive stillthat illusory cold IR.
The energy budget is a consequence of Solar heating of Earth and not a cause and Willis`s wild claim that the Oceans are belching out 500W/m2 of heat is absolute “Warmist Gold”!
In the KT diagram we see the classic energy budget analysis supporting the hypothesis that the surface of the Earth is warmer than the S-B equation would predict due to 324 Wm2 of ‘Back Radiation’ from the atmosphere to the surface.
It is proposed that it is Back Radiation that lifts the surface temperature from 255K, as predicted by S-B, to the 288K actually observed because the 324 Back Radiation exceeds the surface radiation to the air of 222 Wm2 ( 390 Wm2 less 168 Wm2) by 102 Wm2. It is suggested that there is a net radiative flow from atmosphere to surface of 102 Wm2.
I now discuss an alternative possibility.
The portions I wish to focus on are:
i) 390 Wm2 Surface Radiation to atmosphere
ii) 78 Wm2 Evapo-transpiration surface to atmosphere
iii) 24 Thermals surface to atmosphere
iv) 324 Back Radiation atmosphere to surface
The budget needs to be amended as follows:
The 78 Wm2 needs to be corrected to zero because the moist adiabatic lapse rate during ascent is less than the dry lapse rate on adiabatic descent which ensures that after the first convective cycle there is as much energy back at the surface as before Evapo-transpiration began.
The 24 Wm2 for thermals needs to be corrected to zero because dry air that rises in thermals then warms back up to the original temperature on descent.
Therefore neither ii) nor iii) should be included in the radiative budget at all. They involve purely non radiative means of energy transfer and have no place in the radiative budget since, being net zero, they do not cool the surface. AGW theory and the Trenberth diagram incorrectly include them as a net surface cooling influence.
Furthermore, they cannot reduce Earth’s surface temperature below 255K because both conduction and convection are slower methods of energy transmission than radiation. To reduce the surface temperature below 255K they would have to work faster than radiation which is obviously not so.
They can only raise a surface temperature above the S-B expectation and for Earth that is 33K.
Once the first convective overturning cycle has been completed neither Thermals nor Evapo-transpiration can have any additional warming effect at the surface provided mass, gravity and insolation remain constant.
As regards iv) the correct figure for the radiative flux from atmosphere to surface should be 222 Wm2 because items ii) and iii) should not have been included.
That also leaves the surface to atmosphere radiative flux at 222 Wm2 which taken with the 168 Wm2 absorbed directly by the surface comes to the 390 Wm2 required for radiation from the surface.
The rest of the energy budget diagram appears to be correct.
So, how to decide whether my interpretation is accurate?
I think it is generally accepted that the lapse rate slope marks the points in the atmosphere where there is energy balance within molecules that are at the correct height for their temperature.
Since the lapse rate slope intersects with the surface it follows that DWIR equals UWIR for a zero net radiative balance if a molecule at the surface is at the correct temperature for its height. If it is not at the correct surface temperature it will simply move towards the correct height by virtue of density variations in the horizontal plane (convection).
Thus, 222 UWIR at the surface should equal 222 DWIR at the surface AND 222 plus 168 should add up to 390 and, of course, it does.
AGW theory erroneously assumes that Thermals and Evapo-transpiration have a net cooling effect on the surface and so they have to uplift the radiative exchange at the surface from 222 Wm2 to 324 Wm2 and additionally they assume that the extra 102 Wm2 is attributable to a net radiative flux towards the surface from the atmosphere.
The truth is that there is no net flow of radiation in any direction at the surface once the air at the surface is at its correct temperature for its height, which is 288K and not 255K. The lapse rate intersecting at the surface tells us that there can be no net radiative flux at the surface when surface temperature is at 288K.
A rise in surface temperature above the S-B prediction is inevitable for an atmosphere capable of conducting and convection because those two processes introduce a delay in the transmission of radiative energy through the system. Conduction and convection are a function of mass held within a gravity field.
Energy being used to hold up the weight of an atmosphere via conduction and convection is no longer available for radiation to space since energy cannot be in two places at once.
The greenhouse effect is therefore a product of atmospheric mass rather than radiative characteristics of constituent molecules as is clearly seen when the Trenberth diagram is corrected and the lapse rate considered.
Since one can never have more than 390 Wm2 at the surface without increasing conduction and convection via changes in mass, gravity or insolation a change in the quantity of GHGs cannot make any difference. All they can do is redistribute energy within the atmosphere.
There is a climate effect from the air circulation changes but, due to the tiny proportion of Earth’s atmospheric mass comprised of GHGs, too small to measure compared to natural variability.
What Happens When Radiative Gases Increase Or Decrease?
Applying the above correction to the Trenberth figures we can now see that 222 Wm2 radiation from the surface to the atmosphere is simply balanced by 222 Wm2 radiation from the atmosphere to the surface. That is the energy being constantly expended by the surface via conduction and convection to keep the weight of the atmosphere off the surface. We must ignore it for the purpose of energy transmission to space since the same energy cannot be in two places at once.
We then have 168 Wm2 left over at the surface which represents energy absorbed by the surface after 30 Wm2 has been reflected from the surface , 77 Wm2 has been reflected by the atmosphere and 67 Wm2 has been absorbed by the atmosphere before it reaches the surface.
That 168 Wm2 then warms the atmosphere by conduction and convection during the first convective cycle leaving a total of 235 Wm2 in the atmosphere (168 plus 67).
It is that 235 Wm2 that must escape to space if radiative balance is to be maintained.
Now, remember that the lapse rate slope represents the positions in the atmosphere where molecules are at their correct temperature for their height.
At any given moment convection arranges that half the mass of the atmosphere is too warm for its height and half the mass is too cold for its height.
The reason for that is that the convective process runs out of energy to lift the atmosphere any higher against gravity when the two halves equalise.
It must follow that at any given time half of the GHGs must be too warm for their height and the other half too cold for their height.
That results in density differentials that cause the warm molecules to rise and the cold molecules to fall.
If a GHG molecule is too warm for its height then DWIR back to the surface dominates but the molecule rises away from the surface and cools until DWIR again equals UWIR.
If a GHG molecule is too cold for its height then UWIR to space dominates but the molecule then falls until DWIR again equals UWIR.
The net effect is that any potential for GHGs to warm or cool the surface is negated by the height changes relative to the slope of the adiabatic lapse rate.
Let’s now look at how that outgoing 235 Wm2 is dealt with if radiative gas concentrations change.
It is recognised that radiative gases tend to reduce the size of the Atmospheric Window (40 Wm2) so we will assume a reduction from 40 Wm2 to 35 Wm2 by way of example.
If that happens then DWIR for molecules that are too warm for their height will increase but the subsequent rise in height will cause the molecule to rise above its correct position along the lapse rate slope with UWIR to space increasing at the expense of DWIR back to the surface and rising will only stop when DWIR again equals UWIR.
Since UWIR to space increases to compensate for the shrinking of the atmospheric window (from 40 Wm2 to 35 Wm2) the figure for radiative emission from the atmosphere will increase from 165 to 170 which keeps the system in balance with 235 Wm2 still outgoing.
If the atmosphere had no radiative capability at all then radiative emission from the atmosphere would be zero but the Atmospheric Window would release 235 Wm2 from the surface.
If the atmosphere were 100% radiative then the Atmospheric Window from the surface would be zero and the atmosphere would radiate the entire 235 Wm2.