Guest post by Bob Fernley-Jones by Bob Fernley-Jones AKA Bob_FJ
CAUTION: This is written in Anglo-Oz English.
Here is the diagram as extracted from their 2009 paper, it being an update of that in the IPCC report of 2007 (& also 2001):
The unusual aspect of this diagram is that instead of directly showing radiative Heat Transfer from the surface, it gives their depiction of the greenhouse effect in terms of radiation flux or Electro-Magnetic Radiation, (AKA; EMR and a number of other descriptions of conflict between applied scientists and physicists). EMR is a form of energy that is sometimes confused with HEAT. It will be explained later, that the 396 W/m^2 surface radiation depicted above has very different behaviour to HEAT. Furthermore, temperature change in matter can only take place when there is a HEAT transfer, regardless of how much EMR is whizzing around in the atmosphere.
A more popular schematic from various divisions around NASA and Wikipedia etc, is next, and it avoids the issue above:
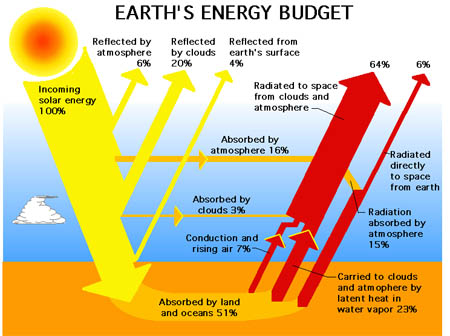
- Figure 2 NASA
Returning to the Trenberth et al paper, (link is in line 1 above), they give that the 396 W/m2 of EMR emitted from the surface in Fig.1 is calculated primarily by using the Stefan–Boltzmann law, and global year average conditions. Putting aside a few lesser but rather significant issues therein, it is useful to know that:
1) The Stefan-Boltzmann law (S-B) describes the total emission from a flat surface that is equally radiated in all directions, (is isotropic/hemispherical). Stefan found this via experimental measurement, and later his student Boltzmann derived it mathematically.
2) The validity of equally distributed hemispherical EMR is demonstrated quite well by observing the Sun. (with eye protection). It appears to be a flat disc of uniform brightness, but of course it is a sphere, and at its outer edge, the radiation towards Earth is tangential from its apparent surface, not vertical. It is not a perfect demonstration because of a phenomenon called limb darkening, due to the Sun not having a definable surface, but actually plasma with opacity effects. However, it is generally not apparent to the eye and the normally observed (shielded) eyeball observation is arguably adequate for purpose here.
3) Whilst reportedly the original Stefan lab test was for a small flat body radiating into a hemisphere, its conclusions can be extended to larger areas by simple addition of many small flat bodies of collectively flat configuration, because of the ability of EMR waves to pass through each other. This can be demonstrated by car driving at night, when approaching headlights do not change in brightness as a consequence of your own headlights opposing them. (not to be confused with any dazzling effects and fringe illumination)
4) My sketch below demonstrates how radiation is at its greatest concentration in the lateral directions. It applies to both the initial S-B hemispherical surface radiation and to subsequent spherical radiation from the atmosphere itself.
5) Expanding on the text in Figure 3: Air temperature decreases with altitude, (with lapse rate), but if we take any thin layer of air over a small region, and time interval, and with little turbulence, the temperature in the layer can be treated as constant. Yet, the most concentrated radiation within the layer is horizontal in all directions, but with a net heat transfer of zero. Where the radiation is not perfectly horizontal, adjacent layers will provide interception of it.
A more concise way of looking at it is with vectors, which put simply is a mathematical method for analysing parameters that 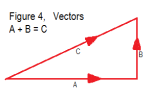 possess directional information. Figure 4, takes a random ray of EMR (C) at a modestly shallow angle, and analyses its vertical and horizontal vector components. The length of each vector is proportional to the power of the ray, in that direction, such that A + B = C. Of course this figure is only in 2D, and there are countless multi-directional rays in 3D, with the majority approaching the horizontal, through 360 planar degrees, where the vertical components also approach zero.
possess directional information. Figure 4, takes a random ray of EMR (C) at a modestly shallow angle, and analyses its vertical and horizontal vector components. The length of each vector is proportional to the power of the ray, in that direction, such that A + B = C. Of course this figure is only in 2D, and there are countless multi-directional rays in 3D, with the majority approaching the horizontal, through 360 planar degrees, where the vertical components also approach zero.
6) Trenberth’s figure 1 gives that 65% of the HEAT loss from the surface is via thermals and evapo-transpiration. What is not elaborated is that as a consequence of this upward HEAT transfer, additional infrared radiation takes place in the air column by virtue of it being warmed. This initially starts as spherical emission and absorption, but as the air progressively thins upwards, absorption slows, and that radiation ultimately escapes directly to space. Thus, the infrared radiation observable from space has complex sources from various altitudes, but has no labels to say where it came from, making some of the attributions “difficult”.
DISCUSSION; So what to make of this?
The initial isotropic S-B surface emission, (Trenberth’s global 396 W/m2), would largely be absorbed by the greenhouse gases instantaneously near the surface. (ignoring some escaping directly to space through the so-called “atmospheric window”). However, a large proportion of the initial S-B 396 surface emission would be continuously lateral, at the Trenberth imposed constant conditions, without any heat transfer, and its horizontal vectors CANNOT be part of the alleged 396 vertical flux, because they are outside of the vertical field of view.
After the initial atmospheric absorptions, the S-B law, which applied initially to the surface, no longer applies to the air above. (although some clouds are sometimes considered to be not far-off from a black body). Most of the air’s initial absorption/emission is close to the surface, but the vertical distribution range is large, because of considerable variation in the photon free path lengths. These vary with many factors, a big one being the regional and more powerful GHG water vapour level range which varies globally between around ~0 to ~4%. (compared with CO2 at a somewhat constant ~0.04%). The total complexities in attempting to model/calculate what may be happening are huge and beyond the scope of this here, but the point is that every layer of air at ascending altitudes continuously possesses a great deal of lateral radiation that is partly driven by the S-B hemispherical 396, but cannot therefore be part of the vertical 396 claimed in Figure 1.
CONCLUSIONS:
The vertical radiative flux portrayed by Trenberth et al of 396 W/m^2 ascending from the surface to a high cloud level is not supported by first principle considerations. The S-B 396 W/m^2 is by definition isotropic as also is its ascending progeny, with always prevailing horizontal vector components that are not in the field of view of the vertical. The remaining vertical components of EMR from that source are thus less than 396 W/m^2.
It is apparent that HEAT loss from the surface via convective/evaporative processes must add to the real vertical EMR loss from the surface, and as observed from space. It may be that there is a resultant of similar order to 396 W/m^2, but that is NOT the S-B radiative process described by Trenberth.
~~~~~~~~~~~~~~~~~~~~~~~~~~~~~~~~~~~~~~~~~~~~~~~~~~~~~~~~~~~~~
ADDENDUM FOR AFICIONADOS
I Seek your advice
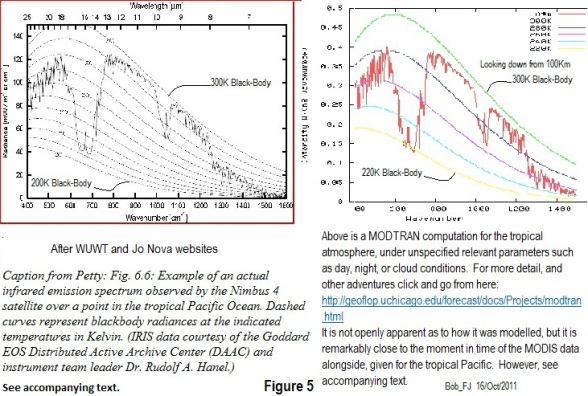
In figure 5 below, note that the NIMBUS 4 satellite data on the left must be for ALL sources of radiation as seen from space, in this case, at some point over the tropical Pacific. The total emissions, amount to the integrated area under the curve, which unfortunately is not given. However, for comparison purposes, a MODTRAN calculator, looking down from 100 Km gives some interesting information for the figure, which is further elaborated in the tables below. Unfortunately the calculator does not give global data or average cloud/sky conditions, so we have apples and pears to compare, not only with Nimbus, but also with Trenberth. However, they all seem to be of somewhat similar order, and see the additional tabulations.
| Compare MODTRAN & “Trenberth”, looking down from 2 altitudes, plus Surface Temperature | ||||
| Location | Kelvin | 10 metres | 100 Km. | (Centigrade) |
| Tropical Atmosphere | 300K | 419 W/m^2 | 288 W/m^2 | (27C) |
| Mid-latitude Summer | 294K | 391 W/m^2 | 280 W/m^2 | (21C) |
| Mid-latitude Winter | 272K | 291 W/m^2 | 228 W/m^2 | (-1C) |
| Sub-Arctic Winter | 257K | 235 W/m^2 | 196 W/m^2 | (-16C) |
| Trenberth Global | 288K ? | 396 W/m^2 | 239 W/m^2 | (15C ?) |
| Compare MODTRAN & “Trenberth”, looking UP from 4 altitudes: W/m^2 | ||||
| Location | From 10 m | From 2 Km | From 4Km | From 6Km |
| Tropical Atmosphere | 348 | 252 | 181 | 125 |
| Mid-latitude Summer | 310 | 232 | 168 | 118 |
| Mid-latitude Winter | 206 | 161 | 115 | 75 |
| Sub-Arctic Winter | 162 | 132 | 94 | 58 |
| Trenberth Global | 333 Shown as coming from high cloud area (= BS according to MODTRAN) | |||


“Leave a Reply”?….if only I could!
Unfortunately, an old draft of the article has been posted in errror. I’ve Emailed Anthony, asking for the intended version to be posted. It contains additional information.
There is lot’s to chew on here, but please clarify this statement:
“…temperature change in matter can only take place when there is a HEAT transfer..”
____
If by “temperature” you are referring the average translational kinetic energy, then of course your statement is erroneous, for the temperature of matter (as measured by average translational kinetic energy) can be changed by both heat transfer, AND/OR having work done on it. In the end, the results are indistinguishable. A perfect example is the compression of air when it flows between zones of different pressure…this is work done on the air molecules, their temperatures will rise as they are compressed and the average kinetic translational energy rises from the work done.
Well, in a word: YES!
In a transparent atmosphere and ignoring the curvature of the earth, the average 396 W/m^2 would be constant all the way up. The energy leaves the spherical surface of the earth. By conservation of energy, the energy MUST go somewhere. The energy does not get absorbed (by definition in a transparent atmosphere). Therefore it must leave the from any arbitrary spherical surface above the earth. In the approximation that the earth is not curved (or that we have not gone high compared to the radius of the earth) the same energy must leave upward thru every m^2 of the upper surface as left every m^2 of the surface. Unless you can find a good reason to disagree with euclidean geometry or conservation of energy, the average upward flux of thermal IR from the surface is constant in these circumstances.
R. Gates @ 5:46 pm,
Yes, you are correct, that work can result in heating, or cooling. An oversight on my part, where I was trying to show the difference between heat and EMR
If i followed your presentation correctly, then it seems to me that you left out an important fact. The radiation lost “sideways” above one point, should be exactly made up by the radiation “sideways” from some other point on the globe. I’m sure that, if you consider the earth as a sphere, these effects all average out and your concern is misplaced. I think that, to the extent that the earth is not a sphere, more surface radiation is absorbed in the atmosphere than in the spherical case.
Billy
“The length of each vector is proportional to the power of the ray, in that direction, such that A + B = C.”
Energy is not a vector! You cannot take components of energy. If a photon with energy 10^-19 J is heading north and at a 45 degree angle above the horizontal, it does not have 0.707 * 10^-19 J of “northward energy” and 0.707 * 10^-19 J of “upward energy”. It simply has10^-19 J of energy. If it passes upward through a surface, it carries 10^-19 J of energy upward thru the surface, not 0.707 * 10^-19 J.
An interesting observation about R. Gates. Has anyone else noticed that he pops up almost immediately in any thread where Trenberth’s work is being scrutinized? On other threads he is noticeably absent, but pick a hole in Trenberth’s work and POOF! there’s R. Gates defending it with one argument or another, usualy a tengential one intended to hijack the thread.
BTW R. Gates, you welched on your bet with me.
And I repeat my question from another thread…Do you have on official relationship with Trenberth, and if so, what is it?
Excellent article Bob Fernley-Jones.
Bob Fernley-Jones, I owe you a debt of gratitude. I have no idea if you just stumbled upon this though on your own or if it is that I have been harping on this on and off here since this spring when I personally realized it. But one thing I can say either way is you explained this hugely better than I have been able to explain it.
Stefan-Boltzmann is a three dimensional law, not one dimensional as so many of the climate related sciences is applying it. That is why the 396 Wm-2 is pure fantasy when speaking of energy net transfer as you have eloquently explained. This one fact in science is why global warming as we speak of it is now over, to me anyway. The maximum affect, or sensitivity, is 1/6th (numerically exact) of what so many of the calculation have been applied using S-B law. Pure radiation loss of energy from the surface is a minor, I’ll repeat, a minor player in Earth’s loss of the solar radiation gathered on the lit side every second of every day, not a major
Please help me to not let this thought go stale. It is one of the saddest states I could every imagine of how the physic branches of science have let this notion not only exist, but to become an accepted view of how energy transfers, always in one dimension, up and down, never accounting horizontally which is 4/6th of what actually occurs with symmetrical cancellation of effects. Any deviation from exactly 4/6th is due to the fact our world is a sphere, not a flat plane and the cooling and thinning of the atmosphere with altitude.
Excellent, excellent, article (paper). To me, the notion of global warming from CO2 died on this 3D thought. Radiation cannot do what they have led us to accept as truth… it isn’t.
I must say I don’t get any of this really, but what I have always found odd about this line of thinking is, what about kinetic and potential energy transfer of the heat. It would seem to me, that the sun shining and warmth associated converts into water moisture, which rises, forms clouds and falls. It creates wind from pressure variances. So when they start accumulating forcings and adding it all up, in fact that little extra just becomes one days little bit extra rain, just a little bit extra wind. It doesnt add up over the years, it just turns out to be a little more movement day to day.
When someone says climate engine, or weather engine, this is what I think of.
Tim Folkerts says:
October 26, 2011 at 6:07 pm
Well, in a word: YES!
In a transparent atmosphere and ignoring the curvature of the earth, the average 396 W/m^2 would be constant all the way up. […]
Wrong.
It decrease with temperature with an increase in altitude.
“…temperature change in matter can only take place when there is a HEAT transfer..”
Sorry , but no. Temperature is directly affected by absorption of radiation – so how and why are you distinguishing HEAT (in capitals!) from EMR.
Along with the paradox, the Trenberth et al. diagram also contains a statistical fantasy, which is the obscenely precise value of 0.9 W/m2 for the net absorbed. Considering the multiple W/m2 uncertainty in some of the other numbers (including cloud reflection), that 0.9 W/m2 could only have been extracted from some authorial orifice. A couple of years ago Vincent Gray and I had a short discussion of this statistical uncertainty, along with the unreality of the flat earth, on ICECAP
http://icecap.us/index.php/go/icing-the-hype/the_flat_earth
Thus, the “missing heat”, aka the “net absorbed” on the diagram, could be positive, negative, or zero.
Tim Folkerts says:
October 26, 2011 at 6:21 pm
“The length of each vector is proportional to the power of the ray, in that direction, such that A + B = C.”
Energy is not a vector! You cannot take components of energy. […]
Energy transfer is a vector and you must take it’s dimensional components when analyzing it in a real world. First study some real three dimensional physics.
I am no physicist or mathematician so let me see if I have this correct.
1. Energy from a point is radiated in all directions not just up or down. – no problem
2. the “apparent” vertical component of any line that is not straight up is represented by the side of a right triangle and is alway less than the hypotenuse.
However the energy from the point of view of an atom of gas can be thought of as a “packet” Upon absorption it kicks the atom to a higher energy level. when the atom falls back to the original energy level the exact same amount of energy “packet” is emitted.
I view it as sort of a pinball machine with the energy “packet” the ball getting whacked back and forth from atom to atom until it finally “escapes” The energy “packets” value does not change only its direction and the amount of time of travel.
If the energy “packet” is not the right “flavor” the atom will not “eat it” and therefore will not be absorbed and re-radiated. So it bounces off the atom. If it does not hit any atoms it just head out to space even if it is at a very long tangent with a very low angle to the ground.
Have I blown the physics somewhere?
(I really hate defending that model by the way)
One would assume that there would be some work term. The conversion of saline into airborne fresh water, then its transport onto land would be included, but no.
There is really no point in attempting to use such diagrams as this to calculate anything. The average temperature and average emission(s0 of a rotating planet that orbits a star every 365.25 days is a nonsense.
The difference between total radiative influx between the summer and winter solstices is 3.5%. If global is global then 3.5% of 341, 12 W/m2 should drive the difference between the global temperature around June 22nd and December 22nd. Sadly, this gives 12W/m2 = -0.17 degrees.
The problem is the heterogeneous distribution of different types of water and of land.
Trenberth ignores this complexity and presents box diagrams that were abandoned by everyone else some decades ago.
The Earths average temperature, see the BEST June+July vs Dec+Jan, is lower when it is closer to the sun than when it is further away.
“Energy is not a vector…etc”
But an energy flux is.
However once you start doing vector algebra, you have to integrate over all angles. So consider that little vector diagram in the article, and put next to it a similar diagram representing the neighbouring point on the surface, but think of the vector pointing the other way (ie same vertical component, opposite horizontal component). The horizontal fluxes will cancel. Once you integrate over all spherical angles, and over the surface area, all components except the vertical will cancel. This I think is why these diagrams only ever show a vertical component.
You could profitably read the discussion on the Trenberth diagram at ScienceOfDoom
http://scienceofdoom.com/2011/06/21/whats-the-palaver-kiehl-and-trenberth-1997/
SoD is not a warmist or an alarmist or even a “sceptic” site in the sense used here – it is that unusual thing in this field, a “pro-science” site (and if you doubt that, just consider how much they talk about fundamental physics, and how little about computer models…)
>>Tim Folkerts says:
>>In a transparent atmosphere and ignoring the curvature of the earth,
>>the average 396 W/m^2 [of upward thermal IR] would be constant
>>all the way up. […]
>wayne says:
>Wrong
>It decrease with temperature with an increase in altitude.
The temperature of the atmosphere certainly decreases — ie the energy of the molecules decreases as you get higher. But the energy of the PHOTONS does not change. If 396 J worth of photons leave the surface, those photons will still have 396 J of energy when they are 10 m or 10^9 m from earth.
(OK — there is actually a very small gravitational redshift of he photons, but that is immaterial here and almost certainly not what you were intending.)
The 333W/m2 back-radiation supposedly heating the Earth is nonense.
You CANNOT transfer heat from a colder body (the upper atmosphere) to a hotter body (the lower atmosphere and Earth surface) without doing work.
The 2nd Law of Thermodymanics avbsolutely forbids it.
Interesting post.
One of things that I’ve noticed over the years is that Climatologists don’t seem to differentiate between surface temperature and surface air temperature (SAT). GISS seems to understand this problem (see this for example). Trenberth’s diagrams treat both temperature types as one and the same.
There’s another problem. The Earth’s surface isn’t a perfect black body radiator. It does seem to radiate as a gray body, but then you have to use a modified S-B law that includes emissivity. Now emissivity is wavelength/frequency dependent, so an average emissivity is somewhat problematical. I’ve seen estimates anywhere from 0.90 to 0.99.
KT 97 assumes a surface temperature of 15 °C and an emissivity of 1.0. This gives us his surface radiation of 390 W/m². Trenberth’s 2009 update uses a surface temperature of 16 °C and an emissivity of 1.0 to give us a surface radiation of 396 W/m².
If we use a more realistic emissivity of say 0.95, then the KT 97 surface radiation drops to 370.6 W/m² and his 2009 surface radiation drops to 375.8 W/m². That’s almost a 20 W/m² difference. Trenberth needs add more reality into his diagrams instead of wringing his hands over a missing 0.9 W/m².
Jim
jimmi_the_dalek,
“Temperature is directly affected by absorption of radiation – so how and why are you distinguishing HEAT (in capitals!) from EMR.”
because EMR can be reflected with no net heat transfer among other issues. Then there is the issue of frequency. Not everything absorbs energy from every frequency whether it reflects or just scatters the frequency or simply ignores it.
This is the root of a lot of uncertainty in the so-called settled radiative transfer equations. The climate types are telling us that every bit of IR going down IS absorbed 100% by whatever composition of the earth is there and nothing else can happen to it. Wanna bet?!?!?!?!
Mr. Fernley-Jones;
With respect, I do understand that this particular post is an “initial draft” and perhaps additional details are forthcoming. I await these with interest.
In the meantime I would just like to point out a few observations relative to your (or more accurately NASA’s) Figure 2;
All of the following energy flows are travelling at the speed of light; “incoming solar energy = 100%”, “reflected by atmosphere = 6%”, “reflected by clouds = 20%”, “reflected by Earth’s surface = 4%”.
The following energy flows that are travelling at “close” to the speed of light after a slight delay (caused by absorptions and remissions by gases with thermal capacities) include; “absorbed by atmosphere = 16%”, “absorbed by clouds = 3%”.
Some of the remaining energy flows are made up of various flows that travel through the system at a combination of speeds; “radiated to space from clouds and atmosphere = 64%” which travels in part at close to the speed of light after absorptions and emissions but also contains energy flows that are “absorbed by land and Ocean’s = 51%” and “carried to clouds and atmosphere by latent heat in water vapor = 23%” which travel through the system at a close to the speed of heat.
One interesting thing to note is the ABSENCE of any text that indentifies the source / destination / and magnitude of the lower rightmost red arrow which starts as “absorbed by land and Oceans = 51%” (after removing 23% + 7% (or 30%) this would equal 21% and seems to leave the Earth as “radiated to space directly from Earth = 6%”. Somehow this 21% gets 15% “deleted” from to it and it becomes 6%. Seems to make sense, HOWEVER upon careful observation the arrow labeled “absorbed by atmosphere = 16%” MAGICALLY becomes “radiation absorbed by atmosphere = 15%” after passing behind the BIG 64% red arrow. Perhaps a typo, perhaps an accounting error?
In summary, while these nice little graphics do a reasonably good job of describing the first “order” understanding of the energy flows through the Sun / Atmosphere / Earth / Atmosphere / Universe system they have MANY faults. The largest of which is totally discarding any consideration of the speed at which each of these energy flows travel through the system.
In electrical engineering this is considered as a “DC” (direct current) analysis. Once the “speed” / “lag time” / “response time” / “delay time” is incorporated into the analysis a totally different prediction of the systems response usually results.
Hey, a percent here and a percent there, it probably cancels out and we are STILL WARMING FROM THE “GREENHOUSE” EFFECT RIGHT ???
Cheers, Kevin.
Wayne,
I agree that you can define a direction that energy is flowing, just like you can define a direction that the mass in a stream is flowing. But that does not make energy or mass a vector. If 10^19 photons of energy 10^-19 J pass thru a 1 m^2 surface oriented perpendicular to the earth’s surface, then 1 J passes thru the surface independent of the direction that the photons are moving. If the photons are all traveling at 45 degrees to the normal, the flux is still 1 W/m^2, not 0.707 W/m^2 “upward” and . 0.707 W/m^2 “sideways”
Figure 4 in the post seems to suggest that only the “upward component” of photon energy counts, which is NOT the case. My comments fit in with Billy’s comment October 26, 2011 at 6:17 pm. The total upward energy flux from the IR photons from the surface (in a transparent atmosphere) will remain constant. A proper integral over all directions will confirm this.
Now when the IR absorption of the atmosphere is added in, this will complicate the analysis a bit. But I have not been addressing that yet.
What about evaporation, condensation and changes of state? Are those really equal in time and balancing each other?
Let’s see an update. This doesn’t move me.
Mr. Watts,
The earth is a globe. Horizontal radiation will leave the atmosphere at an angle if it makes it that far. Shallower angles would leave at angles approaching vertical. Of course, with so much more atmosphere to traverse the likelihood of it leaving on that path is much smaller than straight up, the shortest path!!! As the emission point rises the angle that can irradiate the earth is increasingly small and the radiation has a larger area that points to space through increasing amounts of atmosphere (your cone).
The IR from the surface is most likely going to be absorbed by GHG’s close to the surface anyway. At the pressures and density there I am told collisions will transfer much of that energy to oxygen or nitrogen and be convected away as collisions happen oftener than the emission of IR.
As we go up in the atmosphere that gradually changes until the Strat. where the density apparently favors emission.
GHG’s therefore speed the heating and cooling of the bulk of the atmosphere which has a magnitudes lower absorption and emission rate in the far IR. This also brings into question just how much IR there is to actually get back to the surface if the energy is being preferentially transferred though collisions in the far IR opaque lower trop.
Another interesting bit is that GHG’s absorb in slightly wider frequencies near the ground due to the line broadening. It would seem if they emit at the same frequency the IR going up would have a better chance of escaping as the pressure/temp decreases narrowing the bands and not absorbing as much as the lower.
This would all seem to be especially apropros as to why Venus does NOT have a huge Greenhouse. The effect drops off so quickly only near the surface is it possible to be so massive, yet, the collisional transfer even though it is from CO2 to CO2 must be almost total under such temp/pressure/density!! It would almost seem to be more like water, conducting instead of radiating internally.
I must be wrong, but I don’t know enough to see why.
Tim Folkerts @ 6:07 pm,
I don’t have an issue with your transparent atmosphere discussion, if I understand it correctly. (there do seem to be a few typos). However, we do have in our atmosphere GHG’s, particulates, clouds, and precipitation. Note that the MODTRAN calculations in the tables in the addendum, suggest that up-down radiation diminishes with increasing altitude.
See also Wayne @ 6:44 pm below and that ol’ T^4 thingy
jimmi_the_dalek,
“The horizontal fluxes will cancel. ”
The surface is a sphere. The adjoining points would NOT cancel. Surface irregularities will confuse this so that the surface is irradiating itself just as the surface of the water does. One of the requirements for S-B to apply is that the surface geometry does NOT allow it to irradiate itself. OOOPS!!!!
Billy @ 6:17 pm,
I think my item 5) in the article should answer your concern, if not, please elaborate.
jimmi_the_dalek says:
“Sorry , but no. Temperature is directly affected by absorption of radiation – so how and why are you distinguishing HEAT (in capitals!) from EMR.”
Since long wave radiation is absorbed and then re transmitted, how can there be a NET absorbtion to affect temperature?
>>
KevinK says:
October 26, 2011 at 7:46 pm
In summary, while these nice little graphics do a reasonably good job of describing the first “order” understanding of the energy flows through the Sun / Atmosphere / Earth / Atmosphere / Universe system they have MANY faults. The largest of which is totally discarding any consideration of the speed at which each of these energy flows travel through the system.
<<
These diagrams are “steady-state.” That means the transients have had time to damp out or stabilize.
>>
In electrical engineering this is considered as a “DC” (direct current) analysis. Once the “speed” / “lag time” / “response time” / “delay time” is incorporated into the analysis a totally different prediction of the systems response usually results.
<<
That is unless you’re measuring flows that have had time to stabilize. These diagrams represent such measurements. I admit that these diagrams have a multitude of problems, but bad DC analysis isn’t high on the list of those problems.
Jim
Billy said:
I’m sure that, if you consider the earth as a sphere, these effects all average out and your concern is misplaced.”
I agree that in theory at any point in space all the scattered rays would cross, “averaging out” to be the total energy. However, don’t need to test the measuring device to see if it properly registers the energy from a beam scattered on a very shallow angle?
Its design could reflect those rays.
An interesting analogy is a PV cell on my roof. Calculating the angle of the sun and its cosine allows me to produce the expected electricity production from the cell. Effectively it is the ratio of the area exposed to the sun, compared to the area of the PV cell. But because of the design of the cell when the sun is low in the sky, most of the rays reflect off the surface of the protective cover of the PV cell. So whenI should see ~300W, I see ~30W.
I wonder if the measuring devices have been calibrated or designed to measure emissions at low angles of incidence. The height of satellite orbit would also affect this, the higher the better for reducing this problem.
Tim Folkerts @ 6:21 pm
I can understand that you find the vector consideration a tad obscure, but nevertheless, vectors are very useful for analysing parameters having directional information. In fact I wondered if someone might come up with your concern, and contemplated instead showing the classic inclined plane with a mass upon it, but decided it was too tedious and possibly thought to be off-topic.
If you place a mass upon an incline, where it overcomes the coefficient of friction, assuming it, and flatness, to be constant, and ignoring elasticity in the materials, then it will exert a perpendicular force upon the reacting surface according to the vertical component of its mass. If you complete the vector loop there is also an unseen horizontal component vector. This is pure vector maths, but nevertheless the mass can only slide down the direction of the incline
I’m not sure I understand this article but just to clear a few things up, radiation is not heat. Heat is the result of the absorption of radiation by a surface or molecule. 2nd Law of Thermodynamics does state that heat (without work) will only travel from higher to lower temperature, that is correct, however, radiation is not heat. Radiation can travel in any direction and because of this radiation reflected or re-emitted can also travel in any direction, up, down, sideways. Is the question here how much of the radiation is actually absorbed by the system, surface and atmosphere, and how much is lost (unused) out to space, leaves the system without being absorbed? I just don’t know how any of these figures are even calculated. If the earth reflects 4% that presumes that every day, the same amount of energy and direction of radiation and absorbing surfaces never change. This presumption is preposterous. Climate scientists and Physicists cannot do their calculations until they have an average figure but the real world is an every changing system that doesn’t know what an average is. I’m still trying to work out how long wave radiation can produce more heat than short wave radiation thereby causing global warming. I suspect it just ain’t so.
Wayne @ 6:34 pm,
Thanks for your comments. I’ve actually been ruminating over the Trenberth/IPCC cartoon for several years, and wondering why I’ve not seen anything in the literature critiquing it. (and I have not seen your comments on it). Oh well, I thought just recently; why not be brave and challenge the great authority.
Myself i am a layman, but i know that the sun is a flaming band and it ebbs and flows. So my question is who came up with the figure on the amount of radiation coming in and is that always constant. Can anyone tellme how it is measured and is it the same amount in the UK as in the USA or Australia, or is it what is measured at the Eqautor and considered the measurement for all of Earth? I have been told by warmist that a specific radiation amount sqaured is constant, on paper yes, but IMHO, the real world, i do not see it.
Sorry, Flaming BALL not Band
jimmi_the_dalek @ 6:49 pm
Jimmi, you are describing ONE of the ways of transporting HEAT. In the case of radiation this ONLY happens when there is a potential difference between two sources of radiation.
Check – out Wikipedia on heat transfer, for a start;
http://en.wikipedia.org/wiki/Heat_transfer
A large block of ice and a small candle flame both emit the same amount of EMR. However, only one is capable of warming a human being.
Trenberth’s analysis ignores this simple observation and assumes that since two object emit the same EMR, they contribute the same amount of warming.
I would love to see this in a 3d ray traced model animation. With the multiple freq bands it would have to be broken up into several animations to visualize it. I really enjoy these type threads even though I only understand about 2/6th of the content! Thanks for the puzzle.
I have several problems with these types of simplistic diagrams, and the first of which was mentioned by others that there is no way in hell that you can provide an average measurement with a granularity of 0.1 w/m2. Not possible.
I do a pretty good bit of engineering and space science. We have done a lot of work on the Nimbus II HRIR data sets and clouds are a lot more of an influence than what these simplistic diagrams indicate. In the Nimbus data the temperature of clouds were all the way down to below the 210 kelvin calibration of the sensor over large areas. This indicated a very high altitude for these clouds and a LOT of reflection.
I have measured a 90% decrease in insolation at the surface from cloud cover. This is a broad spectrum measurement with solar panels. Multiply this by the average global cloud cover and it is a hell of a lot more than is indicated.
I also hate using this funky 396 w/m2 average number. The vertical number is 1366 w/m2 and so the second graph does a better job of fixing this problem.
R. Gates says:
October 26, 2011 at 5:46 pm
A perfect example is the compression of air when it flows between zones of different pressure…this is work done on the air molecules, their temperatures will rise as they are compressed and the average kinetic translational energy rises from the work done.
Under that argument, air rising and falling also has work done on it. Sort of like the explanation that the brakes on a car stop the car by turning the motion of the car into heat.
Gail Combs @ 7:18 pm
What may not be commonly realized is that the GHG molecules are not the sole carriers of thermal energy as a consequence of their absorption of photons from EMR energy. There are countless molecular collisions between them and the N2 and O2 molecules etc that comprise the vastly greater bulk of the atmosphere. It is commonly called thermalization, of non-greenhouse gasses. These collisions result in a change of kinetic energy (heat) of individual molecules, and just because a GHG molecule absorbs a photon, it does not mean that it will re-emit it, because there is a lot of other stuff going on.
Bob Fernley-Jones says:
October 26, 2011 at 6:12 pm
R. Gates @ 5:46 pm,
Yes, you are correct, that work can result in heating, or cooling. An oversight on my part, where I was trying to show the difference between heat and EMR.
____
Thanks for your reply. I assumed it was an oversight on your part. But to be clear, in a pure sense, heat is a measurement of energy in transit, and should not be taken to be a measurement of the average translational kinetic energy (normally called temperature) nor the total internal energy of the object. Again, heat is a energy in transit flowing between objects. An excellent resource on this can be found at:
http://hyperphysics.phy-astr.gsu.edu/hbase/hframe.html
and on the whole issue of heat and thermodynamics, see:
http://hyperphysics.phy-astr.gsu.edu/hbase/hframe.html
To your point about the difference between heat and EMR, They are completely different things,as one is the measurement of the flow energy, and one is a form of energy.
How is it that solar radiation is partially reflected by the surface, but back radiation is 100% absorbed? How is it that the is 0% reflection of back radiation?
Well I have always been critical of the Trenberth “cartoon” earth energy budget, or whatever they currently call it in your new posting. And I should add, that I do not use the word”cartoon” in any derogatory sense; simply a descriptive label for a diagram that is not very scientific.
So tell me; why would anyone (including me) take any notice of anything written by a “scientist” apparently with a Doctorate Degree (Dr Laura has a PhD, and she doesn’t know beans about climate either) who uses “Watts” or “Watts per metre squared” as units of ENERGY.
When I went to school and studied Physics, Watts, was a unit of POWER, and “Watts per metre squared” would be a unit of AREAL POWER DENSITY.
And at the position of earth’s orbit (average) the arriving power density is 1362 Watts per metre squared; it certainly is NOT 341 W/m^2.
The response of real materials to an incident power density of 1362 W/m^2 (irradiance) will be quite different to the response to 341 W/m^2. For example the former might melt or vaporize a material whereas the latter may not. Such a change might be irreversible, in that the altered material may then become widely dispersed.
An example would be, a somewhat attenuated proportion of each of those purported radiances being incident on the surface of say an Arctic (of Greenland) ice mass.
The former larger radiance might result in warming the ice surface sufficiently for it to melt, whereas the latter could do no such thing.
Remember that WATTS is a rate of supply (or loss) of energy, and if the supply rate does not exceed the loss rate from other processes, such as simple thermal radiation; or conduction, then melting will NEVER occur, no matter how long that condition is maintained.
And since the earth rotates, under that incident power density (radiance) of 1362 W/m^2, if melting does not occur, during the irradiation portion of the daily cycle it never will melt, and it certainly won’t melt under 341 W/m^2 irradiance.
So Trenberth’s cartoon is unscientific gobbledegook.
ferd berple says:
October 26, 2011 at 9:34 pm
A large block of ice and a small candle flame both emit the same amount of EMR. However, only one is capable of warming a human being.
_____
Depends how cold that human being might be. Energy will flow from a block of ice to a human if that human is chilled below the temperature of the block of ice (by floating in interstellar space for a few minutes for example). Also, on what basis do you contend that a “large” block of ice and “small” candle flame emit the same “amount” of EMR? How can you know this?
I have just published a little monograph on this subject titled ‘The Dynamic Greenhouse Effect and the Climate Averaging Paradox’ . The links are:
Paperback:
http://www.amazon.com/Dynamic-Greenhouse-Climate-Averaging-Paradox/dp/1466359188/ref=sr_1_2?s=books&ie=UTF8&qid=1319675042&sr=1-2
Kindle:
http://www.amazon.com/Dynamic-Greenhouse-Climate-Averaging-ebook/dp/B005WLEN8W/ref=sr_1_1?s=books&ie=UTF8&qid=1319675042&sr=1-1
I put my dynamic or time dependent version of the K-T diagram on the front cover. Just copy it from Amazon. This may be challenge to Trenberth that you are looking for. Journals like Nature and Science wouldn’t dare touch this one. Once the dynamics are understood then the whole global warming problem disappears into the noise of the daily surface energy transfer. The ‘average’ solar heating and convective cycle is 12 hours not 24.
The funamental issue is that there is no such thing as a climate equilibrium state. The whole radiative forcing approach is just plain wrong. There are no ‘forcings’ or ‘feedbacks’. The climate models have been wrong at least since 1967 when Manabe and Wetherald started down the wrong path.
There is more info on my little web site at http://www.Venturaphotonics.com for those who are interested.
What he said:
George E. Smith; says
October 26, 2011 at 10:00 pm
Since delving in the mire of IPCC climate modeling I came to the opinion that some climate “scientists” think that physics is a la cart: pick and choose what you like, feel free to redefine what you like and create new terminology that sounds like physics. They have managed to dominate their field so even quite solid climate scientists use some of their terminology, making life very hard for physicists :). It feels like wading through hair.
There is a lot more examples of contention even in the second NASA version. While it is headed ENERGY budget, it diesn’t give units so that is ok.
BUT !! just let’s start with the INPUT at the left edge in yellow. Starting at 100%, 20% is reflected by clouds. Well average global cloud cover is supposed to be in excess of 60%, so if only 20% is reflected from 60% coverage, then the cloud reflectance must only be 33%. And the reflectance of clouds over the solar spectrum is well known to be much higher than 33%, so that 20% number doesn’t fly.
Now look there at the cloud ABSORPTION; only 3%
Now if the clouds REFLECT 20% and ABSORB 3%, what does that say about how much of the solar energy the clouds TRANSMIT ?
Well anyway, that is just an aside; the real point is that in CLEAR AIR there are NO clouds; so the clear air losses of solar energy are then 6% REFLECTED by the atmosphere; 16% ABSORBED by the atmosphere and 4% REFLECTED by the surface (oceans plus land). So assuming that is all true, then the ground level solar insolation (clear air) is 26% less than the TSI or 74% of 1362 which comes out to 1007 W/m^2, and that seems like a believable number since 1,000 W/m^2 is the usually accepted maximum power density for solar energy availability.
The 6% reflectance of the atmosphere is news to me; I’ve never been aware of it. Is it possible that they are using the term REFLECTANCE to describe processes like Raleigh scattering, that produces the blue sky color, and results in a like amount of solar energy being scattered to space.
The incoming solar rays are certainly not obeying the reflection laws of geometrical optics, so it is incorrect to describe Raleigh or Mie scattering as REFLECTANCE.
As to the 4% reflected from oceans and land, about 75% of the earth surface in the major solar input latitudes, is ocean, and the normal relfectance is 2%. That leaves another 2% to be reflected from the land, which comprises only 25% of that surface area. a simple calculation then shows that the land reflectance must only be 10%; and that would be a maximum, because, while 2% is the normal incidence reflection coefficient of water, over all angles, the total reflectance is more like 3%, since the 2% number holds relatively constant up to teh Brewster angle (57 deg incidence), but then increases rapidly beyond that.
So in that case, the land must only contribute 1% to the total reflectance and that reduces the land reflection coefficient down to 7%.
Whether 7% or 10%, both numbers are way too low for the reflectance of land areas.
But at least it looks as if they may be closer than Trenberth, and they avoid the incorrect units problem, and its consequences
“”””” R. Gates says:
October 26, 2011 at 10:04 pm
ferd berple says:
October 26, 2011 at 9:34 pm
A large block of ice and a small candle flame both emit the same amount of EMR. However, only one is capable of warming a human being.
………………………………………………..
Also, on what basis do you contend that a “large” block of ice and “small” candle flame emit the same “amount” of EMR? How can you know this? “””””
Well clearly ferd berple’s statement is correct, if we agree on say total WATTS of power output, and then agree on how large a block of ice is necessary to achieve equality, assuming we know the flame temperature or otherwise know its output power.
But R Gates’ objection is valid too, since the optics of illumination by a large block of ice, and a near point source candle, are quite different, and never could produce the same result; even if they were spectrally identical; which they are not.
So I agree with R on this and ferd’s example is not instructive.
I know this is going to sound really dumb to a lot of you out there,
but I’m just a humble individual whose job it is to control, temperature and humidity…so could someone explain to me please exactly how or more precisely where heat would accumulate in the Warmist model.
Given that the air temp at 300kPa is minus 30 degrees C over the equator and minus 50 over the poles…(and we all know from traveling in planes that air reguarly rises to this height then cools and falls) I’ve just never understood WHERE the warming is meant to BE and when it is there what stops it from rising up to the frigid void at the top of our atmosphere?
Be gentle with me!
“”””” CRISP says:
October 26, 2011 at 7:33 pm
The 333W/m2 back-radiation supposedly heating the Earth is nonense.
You CANNOT transfer heat from a colder body (the upper atmosphere) to a hotter body (the lower atmosphere and Earth surface) without doing work.
The 2nd Law of Thermodymanics avbsolutely forbids it. “””””
Well CRISP, nobody (but you) is transporting “heat” from a colder body to a hotter body, with or without doing work; so the second law of thermodynamics is not invoked. What IS being transported from the colder body to the hotter body, and can do so with impunity is ELECTROMAGNETIC RADIATION which is a form of energy, which is quite unrelated to “heat”; which is a different form of energy requiring physical matter to be present. EM radiation needs NOTHING in order to go where it chooses to.
EM radiation knows absolutely nothing about either “heat” or “Temperature.”, and it can go wherever it darn well pleases .
For me, thermals seemed always greatly underestimated.
Second, all atmospheric molecules radiate IR, not only so called “greenhouse gases”. Those fantasy diagrams being obsessed with wrong greenhouse analogy ignore 99% of the atmosphere and things like simple heat retention.
As I look at Trenberth’s work, I have to wonder if some of these climatologists have a fundamental grasp of how radiation (electromagnetic/photonic*) interacts with matter. Anyone who works with gamma, X-ray, IR, light, radio, and et cetera radiation quickly recognizes the 3D issues presented as photonic radiation interacts either by full or partial adsorption of the energy. Perhaps a primer on how radiation interacts with matter would be useful to readers of this article?
* Of course there is a large amount of energy transferred to the earth via particulates (muons, pions, et al) but much gets converted to photonic energy in the atmosphere.
kuhnkat says:
October 26, 2011 at 8:21 pm
Sorry, but it seems to me that that assertion was refuted by the replication of Al Gore’s high-school science experiment. CO2, at least, is a green-house gas that actually retards the speed of heat transfer through the atmosphere.
.. and they call us skeptics ‘Flat Earthers’ when their calculations are based exactly on that notion.
Walter H. Schneider….. During the ATTEMPTED replication of Al Gore’s high-school science experiment….. AKA, Science 101…..;-)
Anthony Watts showed quite clearly that Al’s experiment was busted….. But did prove to be informative of the fact that pure, or high concentrations of CO2 have a different thermal conductivity than that of air….
Very interesting discussion, which demonstrates clearly that the view that sceptics are politically rather than scientifically motivated is false.
Good to see R Gates removing more of his mask. He starts off posting in the arctic threads arguing with steve m, then reveals he is a buddy of Trenberth, and now demonstrates in this thread that his understanding of the subject goes way above a layman. R Gates is I believe someone far closer to the heart of the debate than he has let on until now. Genuinely sceptical mind turned, or trojan horse?
I think everyone is making this way too complicated. Physics is all about symmetries. For the purpose of explaining Figure 1 (which, after all, is a cartoon) imagine the processes shown are equal across the sphere of the Earth. Since there is no preferred direction in that case, there can be no net radiative or convective transport, except in the direction normal (up or down) to the surface. There is no net horizontal energy flow.
Obviously the real Earth breaks this symmetry and has a preferred direction – namely the axis of rotation. That is why calculations are done for energy transfer as a function of latitude. When you do that you get Hadley cell circulation north and south, which then produce the trade winds, etc., by the Coriolis effect.
Suffice it to say that Trenberth understands this, and that Figure 1 is a very useful and informative graphic.
Bob Fernley-Jones says:
October 26, 2011 at 9:51 pm
Gail Combs @ 7:18 pm
What may not be commonly realized is that the GHG molecules are not the sole carriers of thermal energy as a consequence of their absorption of photons from EMR energy. There are countless molecular collisions between them and the N2 and O2 molecules etc that comprise the vastly greater bulk of the atmosphere…..
_____________________________________________
Thanks,
That is sort of what I was getting at. (bouncing being collisions) however I did forget that during a collision energy can be split with some transfered as heat or added momentum without absorption or did I mix that up? (my physics training is over forty years old)
A collision unlike absorption and emission of a photon does not have to be in “discrete packets” that is at specific wavelengths.
So this means there are at least 4 separate types of activities happening to the radiation leaving the earth.
1. absorption of a photon
2. emission of a photon
3. Collision (bounce) with no transfer of energy (reflection)
4. Collision with some transfer of energy to the atom.
I also specified atom (or molecule to be more precise) because air is a mix of gases and absorption can take place with CO2, H2O, CO and possibly others. Collisions with all the different molecules.
Hopefully my fumbling around will help clarify this for others who are lurking. WUWT can proved to be one heck of an education in physics!
DocMartyn says:
October 26, 2011 at 7:19 pm
“Trenberth ignores this complexity and presents box diagrams that were abandoned by everyone else some decades ago.
The Earths average temperature, see the BEST June+July vs Dec+Jan, is lower when it is closer to the sun than when it is further away.”
Sunlight, falling on the Earth when it’s about 3,000,000 miles closer to the sun in January, is about 7% more intense than in July. Because the Northern Hemisphere has more land which heats easier then water most people state that the Earth’s average temperature is about 4 degrees F higher in July than January, when in fact they should be stating that the ATMOSPHERE is 4 degrees higher in July. In January this extra SW energy is being pumped into the oceans where the “residence time” within the Earth’s ocean land and atmosphere is the longest. There are also other factors, such as the Northern hemispheres winter increase in albedo exceeds the southern hemisphere’s winter albedo due to the far larger northern hemisphere land mass. So at perihelion we have a permanent loss to space of ? W/2m SWR due to increased albedo and a loss of SWR to the atmosphere as at perihelion the SWR is falling on far more ocean, where it is absorbed into the oceans for far longer then if that SWR fell on land. Do these balance (unlikely) or is the earth gaining or losing energy during perihelion??? The TOA flux should tell us and climate models should accurately predict the observation.
Leg says:
October 26, 2011 at 11:29 pm
As I look at Trenberth’s work, I have to wonder if some of these climatologists have a fundamental grasp of how radiation (electromagnetic/photonic*) interacts with matter. Anyone who works with gamma, X-ray, IR, light, radio, and et cetera radiation quickly recognizes the 3D issues presented as photonic radiation interacts either by full or partial adsorption of the energy. Perhaps a primer on how radiation interacts with matter would be useful to readers of this article?
* Of course there is a large amount of energy transferred to the earth via particulates (muons, pions, et al) but much gets converted to photonic energy in the atmosphere.
_________________________________________________
Go for it.
The uninformed like me would love to see the information and Anthony has asked the WUWT community to pinch hit for him for a while so this would be a really good time.
Just a thought! anti phase sine waves of the same frequency cancel out. Does this happen at infra red wavelengths?
R. Gates @ 9:56 pm
I was hoping this matter of semantics would not arise, and which I discussed in an earlier draft of this article which was not approved by Ric Werme, (For Anthony Watts)
In the classical meaning of HEAT, it can be calculated within any particular material phase from its specific heat (factor) and temperature per unit mass, but can also be described as sensible heat. If there is phase change involved then latent heat needs to be added or subtracted. BTW, doesn’t Trenberth/NASA mention sensible and latent heat?
Gail @ 12:47
You are getting at the basics of how radiation interacts with matter, which makes it a little easier to understand Trenberth’s graph and its refutation in this article. Adding to your list of potential radiation interactions: for incoming cosmic radiation, which may be >10E6 eV/photon, there is enough energy in the photon to disrupt the nucleus of an atom. This is how we get C-14 from nitrogen in our atmosphere as one example. Just looking at the atomic level, energy distribution from radiative forces in our atmsophere is clearly complex.
One thing I have not seen is how all the different forms of electromagnetic energies are measured for determining the relative amounts of energy transfer to earth and space (once measured and summed, conversion to potential energy such as watts/meter square is relatively easy). I know we have instruments that measure incoming radiation for a lot of spectrums (maybe the entire spectrum), but do we have instruments measuring outgoing radiation? Is outgoing measured or just calculated? I’d love to see some references that talk to how they measure outgoing radiation if someone has this handy.
@ Eric Rambert
The key word in your post is : IMAGINE. That seems to be what too many climate “scientists” are doing. The article of Bob Fernley-Jones raises important questions, as do many of the posts, those of George E. Smith being one example. And no, it is not true “that everyone is making this way too complicated.” The fact is, REALITY is much more complicated than the simplistic “models”, “cartoons”, “theories”, “assumptions” employed in climate “science” suggest.
This is really disappointing – both the article and the meandering, and largely confused, blog responses about 3-D geometry. It casts no light at all for me on the important central questions:
1. Does back radiation to the Earth’s surface occur at all or is it “unphysical”?
2. If it does, what proportion of that back radiation is due to CO2?
3. What proportion of the back radiation due to CO2 is due to man-made CO2?
Any answers to the above questions should then be tested for credibility against the real-world data which indicates that near-surface average temperatures between 1850 and 2010 increased at the decidedly unalarming long-term trend rate of about 0.4degC per century. (See: http://www.thetruthaboutclimatechange.org/tempsworld.html.)
Cmon guys – please let’s get focussed!
Few points:
– both diagrams, Trenberth and NASA, show the summary of simple energy balance models over an idealized 24h day and from poles to equator for the whole globe, assuming that any other parameters are in steady state. The diagrams are useful to grasp orders of magnitude but not to explain the underlying physical processes. Each flux is the results of many parameters that cannot be discussed at that coarse summary level.
o Trenberth diagram: to emit 396 W m-2 with an assumed emissivity of 1.0, the irradiating Earth surface (a mix of land, ice and sea) should have a temperature of 16°C (289K).
o NASA: if 70% (240 W m-2) of the incoming solar radiation is re-emitted to the outer space by Earth surface and atmosphere, then either the average surface temperature should be -18°C (255 K) or the average emissivity should be 0.604, or something in between.
But average temperatures or average emissivities don’t have any physical meaning. They are just calculation aids for these simplistic models.
– The NASA diagram is more understandable because it does not introduce the “back radiation” concept
– In the Trenberth diagram I could not understand why the 333 W m-2 back radiation is only going back and not also forth? Has anyone an idea?
– Ground and sea surfaces, as well as dust particles and droplets suspended in air emit radiation at their surface temperature following Planck’s law. At Earth temperature levels it is in the infrared range (IR) of roughly of 2 to 100 micrometers. The Stefan Boltzmann equation is the integral of Planck’s law over the whole spectrum. Gases do not irradiate (or only at very high temperature in the plasma state like in a flame or at the surface of the sun).
– IR absorption takes place at finite wavelengths. Each IR absorbing gas like CO2, H2O has an own absorption spectrum calculated line by line from the possible rotational and vibrational transitions that the given molecule allows. The absorption spectra can be downloaded from the HITRAN Database with a 0.1 cm-1 resolution from http://spectra.iao.ru/ .
– The radiated energy would not “largely be absorbed by the greenhouse gases instantaneously near the surface”, as writes Bob Fernley-Jones.
In a line by line calculation over a 2000 metre layer the absorbed energy (also called forcing) is approx. 10% of the total in dry air, and 28% in very wet one.
See graphs: http://dl.dropbox.com/u/6905434/Air-Full-Spectrum.png and http://dl.dropbox.com/u/6905434/Air-Full-Spectrum-tropic.png
– The CO2 forcing can be calculated: results are in line with the typical forcing of 3.7 W m-2 for any doubling of the CO2 concentration, as published by Myhre et al. in Geophysical Research Letters, vol. 25, no.14, p. 2715-2718, July 15, 1998.
That should no more be a debated issue, neither in its nature, nor in its extent.
– However, looking again at the numbers: 3.7 W m-2 are only approx. 1% of the Earth re-emitted IR energy, one order of magnitude less than what can be related to changes of humidity. The Trenberth and NASA diagrams are helping us grasping these sizes.
– This is why we should concentrate on the consequences of the increase of CO2 in atmosphere:
o Water displacement? Change of emissivity?
o Resulting in Warming? Cooling?
o and combined with other driving forces?
– And let’s not forget that neither weather nor climate have ever been in a steady state that would need to be preserved.
Trenberth’s ‘Back Radiation’ is the biggest scientific cock-up in History. He has confused it with ‘Prevost Exchange Energy’, exactly offset by IR from the ground plus a bit from lower, hotter gas. A Dutch PhD student recently shinned up an 800 foot radio mast and showed that the [up-down] signal decayed exponentially to zero, Beer’s Law.
Because the modellers include this imaginary energy in their CO2-AGW myth, they have to hide it by cloud cooling. The first thing they do is to overestimate low level cloud albedo by >2. The second is to claim the 1st AIE applies to thicker clouds. It doesn’t, so the sign is wrong.and it’s the real AGW which heated the World as Asian industrialisation spewed out aerosols which switched off a direct backscattering mechanism. It’s why Asian low level clouds look dull from the top compared with 30 years’ ago; they transmit more energy. It’s self-limiting hence the oceans stopped warming in 2003, and Trenberth’s ‘missing heat’.
Because they also overestimate present greenhouse warming by a factor >3, the IPCC ‘consensus’ CO2 climate sensitivity is a factor of at least 9 too high.
So long as Trenberth continues his elementary mistake [he clearly wasn’t taught the correct physics] , he is condemning himself to be considered by history as a failure. As for Hansen with his recent claim of over double 1st AIE to keep his 4.2K climate sensitivity despite no warming, he is an expert on aerosol optical physics so has no excuse for what is almost certainly fraud, the claim by NASA from 2004 of imaginary ‘surface reflection’ purporting that clouds with small droplets have high albedo,.
Look at any rain cloud – it’s dark underneath because albedo is high, and it’s a complex large droplet phenomenon. Yet workers in climate science believe the surface reflection myth.
The answer to the “paradox” is contained in the two emission curves depicted. The flux emitted through the “atmospheric window” (approx.750-1150 cm-1) can be seen to approximate to a black-body curve for about 298°K, or about 25°C, consistent with the “Tropical Pacific” source, and an emissivity close to 1. There is no paradox.
Secondly, a correction – K&T 2009 does not compute surface radiation for a “surface temperature of 16 °C and an emissivity of 1.0”. They did so in K&T 1997:
“For example, in KT97, we used a single column model constrained by observations, to represent the average fluxes in the atmosphere. We compared results at TOA with those from the NCAR CCM3 and found good agreement, so that the spatial structure was accounted for. At the surface, the outgoing radiation was computed for blackbody emission at 15°C using the Stefan–Boltzmann law”
In K&T 2009:
“To compute these effects more exactly, we have taken the surface skin temperature from the NRA at T62 resolution and sampling and computed the correct global mean surface radiation from (SB) as 396.4 W/m² . If we instead take the daily average values, thereby removing the diurnal cycle effects, the value drops to 396.1 W/m² , or a small negative bias. However, large changes occur if we first take the global mean temperature. In that case the answer is the same for 6-hourly, daily, climatological means at 389.2 W/m² . Hence, the lack of resolution of the spatial structure leads to a low bias of about 7.2 W/m² . Indeed, when we compare the surface upward radiation from reanalyses that resolve the full spatial structure the values range from 393.4 to 396.0 W/m² .
The surface emissivity is not unity, except perhaps in snow and ice regions, and it tends to be lowest in sand and desert regions, thereby slightly offsetting effects of the high temperatures on LW upwelling radiation. It also varies with spectral band (see Chédin et al. 2004, for discussion). Wilber et al. (1999) estimate the broadband water emissivity as 0.9907 and compute emissions for their best-estimated surface emissivity versus unity. Differences are up to 6 W/m² in deserts, and can exceed 1.5 W m² in barren areas and shrublands.”
I see plenty to argue about in the detail of K&T 2009, but let’s get the facts straight.
According to Trenberth’s figure the downward radiation from the GH gasses in the atmosphere. is 333 W/m2. But there must be an equal amount upward. Does’nt the atmosphere radiate infrared in all directions. If 333 W/m2 were radiated from the atmosphere to space we would have far more energy leaving the Earth then received! Surely this backradiation figure is a nonsense.
Can some one answer another question. All up 239 units of IR radiation are emitted to space, 40 directly from the Earths surface, 199 from the atmosphere (including clouds). How is this 199 units radiated to space? – It has to be by Greenhouse Gases in the atmosphere. If these gases were not present this energy could not be emitted. By increasing GH gas concentrations the ability for the atmosphere to radiate energy to space is increased. Where does warming come from?
FJ a most profound analysis of an old graph, all imputs to the discombubilation of the hockey team are a step forward to reality and common sense. Well done mate.
MODTRAN looking down will almost always be different than satellite measurements because a significant amount of the available heat is moved from the equator toward the poles via the wind. Thus, at the equator a satellite measures less heat than computed, and at the winter poles, much more.
Because the energy coming from clouds is sometimes much more than predicted by Stefan’s equation, it makes more sense to treat them as mirrors than as blackbody emitters. Yes, I have measured the energy using an IR thermometer. Very interesting.
Bob Fernley-Jones says: October 26, 2011 at 8:57 pm
“Tim Folkerts @ 6:21 pm
I can understand that you find the vector consideration a tad obscure, but nevertheless, vectors are very useful for analysing parameters having directional information.
Actually, I don’t find vectors particularly obscure, I work with vectors regularly, and I have done the vector surface integrals that show the upward flux will indeed remain constant (in the idea case: uniform temperature, transparent atmosphere, not TOO high above the surface). Furthermore, these integrals are indeed overkill, as Erik Ramberg said earlier: “I think everyone is making this way too complicated. Physics is all about symmetries.” The symmetry of the idea case makes it obvious that the net upward flux is indeed constant.
Fred Berple raises the old ‘ice cube and hot object fallacy’. He says at 9:38 PM,
“A large block of ice and a small candle flame both emit the same amount of EMR. However, only one is capable of warming a human being.”
Normally we think of an ice cube as being cold, because most things around us are normally warmer than that. Thus the analogy seems reasonable at first sight. How could an ice cube warm us? We all make the implicit assumption that if the ice cube wasn’t there, there would be something else, warmer, in its place – this is our normal experience.
But lets say you are in the cold vacuum of space, close to absolute zero. Now the ice cube is relatively warm, and you will receive much more radiation from it than you were previously receiving from ‘nothing’. If the energy received from it is the same as from the candle flame (which was the predicate) then It will provide the same heating effect as the candle flame. A black body absorbs all the radiation falling on it, whether from an ice cube or a candle.
.
wstannard says: October 27, 2011 at 3:31 am
According to Trenberth’s figure the downward radiation from the GH gasses in the atmosphere. is 333 W/m2. But there must be an equal amount upward. Does’nt the atmosphere radiate infrared in all directions. If 333 W/m2 were radiated from the atmosphere to space we would have far more energy leaving the Earth then received! Surely this backradiation figure is a nonsense.
The diagram is indeed “nonsense” to the extent that it is intended to be about the simplest possible diagram. One vast simplification is that it shows the atmosphere as one object, when in fact the calculations and observations that support it must look at the atmosphere as layered.
Willis Eschenbach created a great “slightly more realistic” diagram that splits the atmosphere into a lower layer and and upper layer. This helps explain why the atmospheric radiation is not the same up into space as down to the surface.
http://homepage.mac.com/williseschenbach/trenberth_mine_latest_big.jpg
A question inspired by this article though only slightly connected. If there is some warming from the increased CO2 in the atmosphere and so the air gets warmer, but because it is an open system the atmosphere will expand slightly and increase the mean free path of the photons leaving the surface. This would mean the window to space would get slightly larger and so act as a negative feedback. Has anyone, anywhere quantified the magnitude of this and is it significant?
“This is not science, it is an attempt to understand a climate system so complex that it can be discussed, modelled, hypothesised, even analysed, but can never confidently be understood and defined in scientific certainties. Not even attaching statistical degrees of uncertainty resolves this issue. The number and degree of uncertainties far outweigh our ability to make meaningful conclusions. In particular conclusions that require or support changes to our civilization such as are currently being FORCED upon us.”
I suggest the bigger picture is, by far, the more important aspect. The above comment is extracted from my blog http://tgrule.wordpress.com/2011/10/27/global-warming-more-questions-than-answers/, where this post is acknowledged.
Thanks to Anthony for the usual ‘on the ball’ posts.
For one heart-stopping moment I thought someone had really got what was wrong with ” EMR is a form of energy that is sometimes confused with HEAT.”
Ask why is it so? says:
October 26, 2011 at 9:06 pm
I’m not sure I understand this article but just to clear a few things up, radiation is not heat. Heat is the result of the absorption of radiation by a surface or molecule. 2nd Law of Thermodynamics does state that heat (without work) will only travel from higher to lower temperature, that is correct, however, radiation is not heat.
Thermal radiation, thermal infrared, is heat. It is the thermal energy of the Sun. It is heat on the move. Not all electromagnetic radiation is heat.
What is wrong with this energy budget is that LIGHT from the Sun, shortwave, non-thermal, has been given the properties of HEAT from the Sun, which is thermal energy on the move, thermal infrared from the Sun direct to us, thermal radiation.
For example, visible LIGHT cannot heat water because it doesn’t have the mechanism to do so, water is a transparent medium for visible LIGHT and so visible light is transmitted through without being absorbed, visible light cannot move the molecules of water into the vibrational state which is heat.
HEAT on the other hand, does have the mechanism to heat water, and does. This is the real energy which is heating the land and oceans directly from the Sun.
Not only has the KT taken out the world’s real energy budget the energy direct from the Sun to the surface which is the real source of heating the Earth, the Sun’s thermal energy, thermal infrared – it has given the properties of thermal infrared to visible, it has swapped HEAT to LIGHT around. It is saying that shortwave is thermal energy.
Not so much a paradox, more junk science fiction produced by the AGWSF department to sell its wares.
I have just had a long discussion on this and do not have the time to go through it all again here, but for any interested in telling the difference between this junk energy budget in order to understand what is really going on in our world, this has to begin with disentangling the science fiction memes about heat and light from the real physical properties of energy and matter.
You can start here with the difference between Heat and Light:
http://wattsupwiththat.com/2011/10/18/replicating-al-gores-climate-101-video-experiment-shows-that-his-high-school-physics-could-never-work-as-advertised/#comment-778960
=======================
Bob Fernley-Jones – your link to HEAT takes to a page of junk physics.
Here’s real world physics on HEAT.
The radiation budget graphic should be re-done in various scenarios:
– at noon when it is cloudy;
– at noon when it is clear;
– middle of the night when it is cloudy;
– middle of the night when it is clear.
– for the surface;
– for the tropopause
It will be a far less cloudy picture and be much more clear in that case.
Incoming solar irradiance is 1366 W/mw at noon and Zero at night. All the numbers are vastly different when it is cloudy versus clear. The tropopause energy levels barely changes at all from day to night, from clear to cloudy conditions, everything happens below that level.
Anglo-Oz “physics”.
It would be better, WUWT abstains from such “contributions”.
Sorry, but the Earth has no lid, and not having a lid impedes heat confining, as the so called “green-house effect” it is not other thing than confined heat:
http://www.scribd.com/doc/28018819/Greenhouse-Niels-Bohr
What would you choose for keeping your feet warm, a bottle filled with warm air or, instead, a bottle filled with hot water?….That´s simply because its Volumetric Heat Capacity is:
Air=0.001297 joules cm3/kg
Water=4.186 joules cm3/kg
This means that water holds heat 3227 times more than air.
Why is it that the 161 W/m^2 incoming from the sun can heat my sun tea, but the 333 W/m^2 down from the atmosphere (CO2) cannot?
wstannard says:
October 27, 2011 at 3:31 am
According to Trenberth’s figure the downward radiation from the GH gasses in the atmosphere. is 333 W/m2. But there must be an equal amount upward.
I have a similar problem with the first graphic. On the left hand side it has 161 W/m^2 being absorbed by the surface as the initial forcing but on the right hand side 396 W/m^2 as out going surface radiation.
If this figure is derived from near surface back radiation then the value in an open system can only be twice the constant input value of the black body (BB) radiator. This is because the near gas molecule can only accumulate enough energy to radiate (in this case) 161 Watts back too the BB and 161 Watts away. The BB and molecule will then be at radiative equilibrium of 322 Watts. I don’t understand where the 396 W/m^2 comes from.
jason says:
October 27, 2011 at 12:24 am
Very interesting discussion, which demonstrates clearly that the view that sceptics are politically rather than scientifically motivated is false.
Good to see R Gates removing more of his mask. He starts off posting in the arctic threads arguing with steve m, then reveals he is a buddy of Trenberth, and now demonstrates in this thread that his understanding of the subject goes way above a layman. R Gates is I believe someone far closer to the heart of the debate than he has let on until now. Genuinely sceptical mind turned, or trojan horse?
_____
Really, aspersions as to my intentions, relationships, etc. are quite unecessary and, more importantly, completely inaccurate. I comment here when I see something that sparks my interest, I have no relationship to Dr. Trenberth other than the fact that we live in the same state, and I think for many of those frequenting WUWT our knowledge of the subject at hand go well beyond the “layman”.
In terms of my own position of AGW. I remain more convinced than not that it is happening (I put the percentage at 75% just to show it is more than 51/50, but not 99%). Finally, I am currently not a believer in catastrophic AGW.
@R. Gates says:
October 27, 2011 at 7:11 am
“[…]
In terms of my own position of AGW. I remain more convinced than not that it is happening (I put the percentage at 75% just to show it is more than 51/50, but not 99%). Finally, I am currently not a believer in catastrophic AGW.”
I’ve known known your position stated in the first portion of that last paragraph, as anyone who’s read WUWT for a while has seen you post that a number of times phrased a number of different ways. I don’t recall you ever posting a statement as direct about CAGW as that last sentence, though you could have and I missed it. (P.S. Thanks for your comments. WUWT is not an echo chamber and it’s because you are one of several who helps keep it that way with some good points that you throw into the mix. Thanks again.)
R. Gates;
Really, aspersions as to my intentions, relationships, etc. are quite unecessary and, more importantly, completely inaccurate.>>>
REPLY: If you would provide straight forward answers to questions that wouldn’t be the case. But you don’t.
R. Gates;
I comment here when I see something that sparks my interest, I have no relationship to Dr. Trenberth other than the fact that we live in the same state,>>>
REPLY: You proudly proclaimed yourself as the “go between” who arranged for an invitation for 20 people from WUWT to meet with Kevin Trenberth. You accomplished this merely by living in the same state as Trenberth? I call BS.
R. Gates;
and I think for many of those frequenting WUWT our knowledge of the subject at hand go well beyond the “layman”.>>>
REPLY: Putting aside the royal “our”, excuse me, but you barely qualify as a layman. Consider the bet with me which you jumped into and then welched on. You agreed to wager that Al Gore’s experiment, if conducted as illustrated, would show the results as illustrated. You failed to notice that Al Gore’s experiment as illustrated made use of infrared heating lamps, making it IMPOSSIBLE for the experiment to demonstrate the greenhouse effect in the first place. Then, to compound your so called “well beyond” the knowledge of a layman, you suggested taking the globes OUT of the jars as they were superflous to the experiment. Really? Tell me please, with nothing in the jar to absorb SW and re-radiate it as LW, exactly how could the “greenhouse effect” be demonstrated? That thread alone demonstrated how limited your understanding of the physics is, and I could draw many more examples from many more threads. You constantly parrot the words of others, and when people with knowledge start asking pointed questions, you get backed into a corner and simply stop responding. Or welch on the bet. Whatever.
R. Gates;
In terms of my own position of AGW. I remain more convinced than not that it is happening (I put the percentage at 75% just to show it is more than 51/50, but not 99%). Finally, I am currently not a believer in catastrophic AGW.>>>
REPLY: Really? Can you point to a single skeptic comment about any part of the CAGW debate anywhere in the WUWT forum? Ever? Just one? As for not believing in “C”AGW, shall I cut and paste from all those comments of yours about chaos theory and sand piles collapsing under the weight of a single grain of sand?
The difference between solar radiation and back radiaiton is that the latter is hardly absorbed at all by a volume of water. ( 65 percent solar) Water in the energy buget diagram is completely ignored is this situation and despite the oceans being the most important with regulating global atmospheric temperatures. This is easily shown by comparing how a volume of water warms during one day in the sun and in the shade. Therefore the heat transfer is demonstrated to be huge by solar, but negligible with back radiation. I always new the energy buget diagram was wrong and didn’t reflect observed reality.
Myrrh says:
October 27, 2011 at 5:15 am
For example, visible LIGHT cannot heat water because it doesn’t have the mechanism to do so, water is a transparent medium for visible LIGHT and so visible light is transmitted through without being absorbed, visible light cannot move the molecules of water into the vibrational state which is heat.
Yet it does. 100 meters of water absorbs all the visible light falling on it, so heats it. Visible light move water molecules into overtones of symmetric and anti-symmetric vibrations.
R Gates says…In terms of my own position of AGW. I remain more convinced than not that it is happening (I put the percentage at 75% just to show it is more than 51/50, but not 99%). Finally, I am currently not a believer in catastrophic AGW.
Well there is wisdom in that, would you care to quantify the warming thus far between natural, UHI, and AGW?
One more question if you are in the mood. If a hypothetical GHG were to increase the LWR in the atmosphere, but decrease the SWR entering the oceans by an equal amount, which would have a greater effect on the earths energy budget and would that affect be warming or cooling?
Richard Keen says:
October 26, 2011 at 6:52 pm
Along with the paradox, the Trenberth et al. diagram also contains a statistical fantasy, which is the obscenely precise value of 0.9 W/m2 for the net absorbed.
http://icecap.us/index.php/go/icing-the-hype/the_flat_earth
Thanks. I’ve always had a hard time even looking at Trenberth’s representation, because it’s really too much like a Cartoon out of some pre-Kindergarten book.
R. Gates says:
October 27, 2011 at 7:11 am
“Finally, I am currently not a believer in catastrophic AGW.”
That’s quite a statement because the main sceptic argument has been this, not that a little AGW likely doesn’t have at least a little influence.
Matt G says:
October 27, 2011 at 8:23 am
The format doesn’t like “” close together with numbers.
Therefore the bracket in this previous highlighted post should read ” (absorbance less than 1 percent LWDR v greater than 65 percent solar)”
mkelly askes:
“Why is it that the 161 W/m^2 incoming from the sun can heat my sun tea, but the 333 W/m^2 down from the atmosphere (CO2) cannot?
Perhaps you are looking at it incorrectly. The “natural” temperature of the universe is 3K. Absent some input of energy, objects would radiate away energy until they reached this temperature. If you took your sun tea far from the earth and sun, the sun tea would cool to 3K. Bring that sun tea back to the earth in a transparent, insulated container that removed any heat transfer by conduction. Even if all sunlight is blocked, the IR radiation would heat the sun tea to ~ 300 K.
Conversely, if you kept the tea far from the earth’s IR, but exposed it to 161 W/m^2 of sunlight, it would warm up, but it would end up well below 300 K (around 230 K for a blackbody).
So it is easy to argue that the earth’s IR is BETTER at warming your sun tea than the sun. The sun can only effectively warm the tea if the IR is ALREADY present.
Myrrh,
Without going in to the rest what you wrote, let me simple state that the source you choose as “real world physics” gives two completely contradictory definitions of “heat”.
1) “Heat is thermal energy in transfer.”
2) “Heat is always the thermal energy of some system.”
“Heat” cannot be both always within a system and always between two different systems.
If you base your posts on a source that is clearly inconsistent, then there is a good chance that any of your conclusions will also be inconsistent.
This is a most welcome article. Many thanks to WUWT for having the cahones to showcase it.The comments are also a delight as they reveal that more and more skeptics are waking up to the ‘Slayers’ science. The paradigm shift is upon us: there is no greenhouse gas effect and the K-T energy budget is utterly bogus.
Astrophysicist, Joe Postma says it all here:
http://principia-scientific.org/publications/The_Model_Atmosphere.pdf
Tim Folkerts says:
October 27, 2011 at 8:44 am
mkelly askes:
“Why is it that the 161 W/m^2 incoming from the sun can heat my sun tea, but the 333 W/m^2 down from the atmosphere (CO2) cannot?
Mr. Folkerts says: “Even if all sunlight is blocked, the IR radiation would heat the sun tea to ~ 300 K.”
Balderdash. The IR given off by CO2 cannot do this. The 15 micro emission has an associated temperature via Wien’s Law of -73 C. The best that could happen is the tea would attain the air temperature via conduction.
Also, please let us talk of the earth and the atmosphere and not of space. I don’t live in space beyond the atmosphere. So what is important is what does or can happen where I live. I was looking at it correctly. The KT diagram says 161 W/m^2 at the earth’s surface that is where I put my sun tea and the sun heats it, CO2 cannot.
David Socrates asks:
1. Does back radiation to the Earth’s surface occur at all or is it “unphysical”?
Yes it does. It is easy to measure it with an IR thermometer. It can be seen in lapse rate plots. Back radiation is what causes the morning temperature inversion over land.
2. If it does, what proportion of that back radiation is due to CO2?
That is the important question. No one knows. My analysis indicates that it is close to zero at the surface and about 100% in the stratosphere.
To all, the fact that the amount of energy toward space is less than the amount toward the surface is proof that the atmosphere is IR opaque over a significant part of the spectrum.
R Gates
I have asked you this many times and perhaps you have answered but it got lost within the deluge of comments.
When do you believe the globe started warming? (we shall leave aside the one third of stations that are cooling that we pointed out in our article ‘in search of cooling trends’
I believe it started warming from 1607/8 albeit it has been in fits and starts with numerous serious reversals and astonishing advances (such as around 1700.)
Berkely appear to partially support my view with a rise from their start date of 1800.
Supplementary question; Who do you believe, Michael Mann or the Berkely study?
tonyb
We are just waiting for a very “cool” cooling….enjoy it!, it will be better than in the Maunder Minimum 🙂
You might like more writing on the paradox summarized quite nicely here:
http://www.tech-know.eu/uploads/Copernicus_Meets_the_Greenhouse_Effect.pdf
and a more lengthy version here:
http://www.tech-know.eu/uploads/The_Model_Atmosphere.pdf
Leif Svalgaard says:
October 27, 2011 at 8:24 am
Myrrh says:
October 27, 2011 at 5:15 am
For example, visible LIGHT cannot heat water because it doesn’t have the mechanism to do so, water is a transparent medium for visible LIGHT and so visible light is transmitted through without being absorbed, visible light cannot move the molecules of water into the vibrational state which is heat.
“Yet it does. 100 meters of water absorbs all the visible light falling on it, so heats it. Visible light move water molecules into overtones of symmetric and anti-symmetric vibrations.”
A great deal of the ocean has greater clarity and a small percentage, about 1% goes deeper. (sometinmes called the bad light zone) However this 1% may be in a spectrum that varies more then most over solar cycles. The intensity of visible light transmitted through water decreases rapidly with
increasing depth. Roughly 60 percent of incoming light is attenuated in the first meter (3.3 ft), 80
percent in the upper 10 meters (33 ft), 99 percent at a depth of 150 meters (500 ft) in
very clear water, and there is essentially no light penetration below 1000 meters.
http://www.google.com/url?sa=t&rct=j&q=solar%20radiation%20penetrates%20bad%20light%20zone%20oceans%20800%20meters&source=web&cd=3&ved=0CDQQFjAC&url=http%3A%2F%2Fwww.mhhe.com%2Fearthsci%2Fgeology%2Fduxfund4e%2Fstudy%2Fchap5.pdf&ei=dYapTuCMHoKhsQLUtonmDw&usg=AFQjCNGnVOSvWJbH6orsFOLUGmjjve5hMQ
Leif, a second reference for you…
http://www.google.com/url?sa=t&rct=j&q=bad%20light%20zone&source=web&cd=10&ved=0CHUQFjAJ&url=http%3A%2F%2Fwww.scienceclarified.com%2FMu-Oi%2FOcean-Zones.html&ei=CIepTrzlLsHnsQKqy52vDw&usg=AFQjCNHtNuybbpchX9S35Pp8IywjkMq8hQ
From 660 to 3,000 feet (200 to 900 meters), only about 1 percent of sunlight penetrates. This layer is known as the dysphotic zone (meaning “bad light”). Below this layer, down to the deepest parts of the ocean, it is perpetual night. This last layer is called the aphotic zone (meaning “without light”). At one time, scientists thought that very little life existed within the aphotic zone. However, they now know that a variety of interesting organisms can be found living on the deepest parts of the ocean floor.
Read more: Ocean Zones – body, used, water, process, Earth, life, plants, chemical, form, energy, animals, carbon, oxygen, parts, primary, plant, surface http://www.scienceclarified.com/Mu-Oi/Ocean-Zones.html#ixzz1c06eJJzu
Bob, thank you for the article! It is clear that you “ruminated” some time on it give us some time to digest the information. Here my two cents:
As you correctly showed – in divergence to T&All (F1 diagram) – the radiative exchange does not happen between the “floor” and a “ceiling”, but between “floor” and all levels of the atmosphere. The 333 W/m2 “back radiation” from a high ceiling is an erroneous number leading to misunderstandings – this is clearly showed in the tables – high cloud area is above 7 km – looking up from 6 km in the table we have between 58 and 125 W/m2 pointing down. There is no “back radiation” but energy transfer through radiation in all directions.
Figure 2 is a much more clear and without bias overview of the energy budget.
Furthermore the second point was that radiation results also in heat transfer between various strata of the atmosphere, especially with warm air raising up.
The third point I retained was that the outgoing radiation seen by Nimbus 4 is a result of radiation from several strata. The use of S-B formula is of limited help and should be used very carefully.
Finally I think there are bigger problems with the F1 diagram as pointed out by Mydogsgotnonose says: October 27, 2011 at 3:03 am, Myrrh says: October 27, 2011 at 5:15 am (is any radiation equally transferred into heat?), George E. Smith; says:October 26, 2011 at 10:00 pm (bogus flat world asumption etc) and others.
David says:
October 27, 2011 at 9:35 am
Leif, a second reference for you…
You are missing the point completely. The point is that the oceans eventually absorb all the visible light falling on them [minus that small part that is reflected] and hence is heated by that.
Mkelly, we are a bit off topic, but let me say a couple quick things
>>Mr. Folkerts says: “Even if all sunlight is blocked, the IR radiation
>>would heat the sun tea to ~ 300 K.”
>Balderdash. The IR given off by CO2 cannot do this.
The ~ 333 W/m^2 of IR we are talking about comes partly from CO2, but also from H2O (both gas and liquid). (In addition, the sides of the sun tea will be exposed to both the atmosphere and the ground, further raising the incoming IR). On a cloudy day, the IR spectrum will be nearly black body, and would warm the sun tea to ~ room temperature without any input from the sun or from conduction.
>The 15 micro emission has an associated temperature via
>Wien’s Law of -73 C.
No, Wien’s Law specifically applies to BB radiation. You can’t conclude that a non-BB like CO2 emits most strongly at 15 um has an effective temperature of (2.90 E-3 K/m) / (15 E-6 m) = ~ -73 C.
>Also, please let us talk of the earth and the atmosphere and not of space.
>I don’t live in space beyond the atmosphere.
The connection to space cannot be ignored. In fact, that is precisely the reason that CO2 by itself could not heat the sun tea to ~ 300 K as you correctly concluded above. CO2 by itself only emits in certain bands, leaving large “windows”. These “windows” connect space to everything on the ground, so we are all “connected” to space, just as we are connected to the sun. This is why the tops of cars are often frosted when the sides are not.
>The KT diagram says 161 W/m^2 at the earth’s surface that is where
>I put my sun tea and the sun heats it, CO2 cannot.
Actually, that is not what the diagram says. It says that during an average 24 hour period, the average solar energy is 161 W/m^2. During the night the average is 0 and during an average the day the average is 2 * 161 W/m^2 = 322 W/m^2. Assuming you are making tea not too early or late on a sunny day, the solar energy at that time could be up to about 1000 W/m^2. This is one other reason that sunlight is more effective.
The Sun does not surround the Earth, illuminating and heating it from the vertical all day long at all points on the surface. The Sun illuminates/heats the dayside of the Earth as if it were 1/2 a circle the size of the Earth….from the vertical. So, the Earth’s dayside gets, at most, 1/2 of the 1366 W/m^2. 1366/2 = 683.
rbateman says:
October 27, 2011 at 10:45 am
The Sun does not surround the Earth, illuminating and heating it from the vertical all day long at all points on the surface. The Sun illuminates/heats the dayside of the Earth as if it were 1/2 a circle the size of the Earth….from the vertical. So, the Earth’s dayside gets, at most, 1/2 of the 1366 W/m^2. 1366/2 = 683.
And because the Earth is round, you can cut that number in half as well, getting to the 342 W/m2.
Anyway, shouldn’t it be A^2 + B^2 = C^2 for that little vector diagram, not A + B = C?
A black body emits W/m2 AT A POWER according to it’s temperature.
A gray body emits AT A LOWER POWER than a black body (at the same temperature) for many well known reasons.
THAT, is NOT an energy flow, for a gray body IT IS the power the energy flow is emitted at…….
The volume of the energy flow IS NOT DESCRIBED.
So, as the gray bodies depicted in the K&T budgets are all different sizes and temperatures,
the diagram CAN NOT be correct (for power or volume),
UNLESS THEY ARE ALL (EQUAL) BLACK BODIES….
This also applies to all present explanations of GH “theory”.
Did you realise K&T and GH is ALL explained in “black body”???
It has taken me ages to realise the above, and it’s importance. It’s all imaginary.
http://www.globalwarmingskeptics.info/forums/thread-1071-post-10470.html#pid10470
(Particularly post 21 onwards.)
I doubt I am alone in that.
K&T depicts a black body world, in all it’s parts, AND, with no life, as does GH “theory”.
Makes me wonder how they “measure” the figures supposedly from (gray body) “reality”….
It’s a scam, plain and simple. A politically convenient, imaginary hobgoblin.
The world ain’t flat, and CO2 does not drive, nor even measurably influence, climate.
Wake up people.
There can not possibly be a “greenhouse effect” as presently hypothesized,
IN ANY OF IT’S PRESENTLY TOUTED “FORMS”.
Rich says:
Anyway, shouldn’t it be A^2 + B^2 = C^2 for that little vector diagram, not A + B = C?
If you assume he meant that as a vector equation (with the little arrows over the letters), then (vector A) + (vector B) = (vector C).
But if they represent the magnitudes, then you are right, the Pythagorean theorem would apply.
Leif Svalgaard says:
October 27, 2011 at 8:24 am
Myrrh says:
October 27, 2011 at 5:15 am
For example, visible LIGHT cannot heat water because it doesn’t have the mechanism to do so, water is a transparent medium for visible LIGHT and so visible light is transmitted through without being absorbed, visible light cannot move the molecules of water into the vibrational state which is heat.
Yet it does. 100 meters of water absorbs all the visible light falling on it, so heats it. Visible light move water molecules into overtones of symmetric and anti-symmetric vibrations.
Utter codswallop. Water is a transparent medium to visible light, visible light is transmitted through without being absorbed because that’s what what transparent means. Visible light isn’t capable of moving molecules of water into vibration, it ain’t big enough and doesn’t have the power to do so, it works on electron scale, electronic transitions, and doesn’t even get in to play with electrons in water (as it does in the atmosphere where the electrons of the molecules oxygen and nitrogen absorb it. It takes real power, the power of heat, thermal infrared, to move molecules of water. And this is what it does, direct from the Sun, the land and oceans are heated by thermal infrared.
The AGW/KT/NewScienceFiction is a nonsense. The Science Fiction department of AGWInc has given the properties of heat, the thermal energy from the Sun, to light, and, then says that the Sun’s real thermal energy thermal infrared, doesn’t get to the surface to heat it, and takes it out of the budget! To claim the actual heat from the Sun doesn’t heat the world is bad enough, but for anyone here calling calling himself scientist who thinks visible light is capable of doing this, needs to go back to primary school, in the real world, there are still some teachers left…
….
Tim Folkerts says:
October 27, 2011 at 9:04 am
Myrrh,
Without going in to the rest what you wrote, let me simple state that the source you choose as “real world physics” gives two completely contradictory definitions of “heat”.
1) “Heat is thermal energy in transfer.”
2) “Heat is always the thermal energy of some system.”
“Heat” cannot be both always within a system and always between two different systems.
If you base your posts on a source that is clearly inconsistent, then there is a good chance that any of your conclusions will also be inconsistent.
No contradiction – “Using the word heat helps physicists to make a distinction relative to the system they are talking about” It’s all of those things in context, because “thermal energy and heat are the same thing.”
What I am talking about here in context is the Sun’s heat, the Sun’s thermal energy on the move, thermal infrared which is this heat energy, this heat from the Sun travelling to us here and heating land and oceans and us. Or simply, heat from the Sun is thermal infrared, it is invisible, but we can feel it as heat because it warms us up from the inside. Because it is heat and that’s what heat does.
Visible light cannot heat us up.
You have excluded the primary means of heating of the Earth, it is missing from your energy budget, this is the real travesty of the missing heat.
Man Bearpigg says:
October 26, 2011 at 11:54 pm
.. and they call us skeptics ‘Flat Earthers’ when their calculations are based exactly on that notion.
A blast from the past:
“NASA covered up for forty years proof that the greenhouse gas theory was bogus. But even worse, did the U.S. space agency fudge its numbers on Earth’s energy budget to cover up the facts?”
http://sppiblog.org/tag/stefan-boltzmann-equations
“What ignited this latest Climategate-linked rumpus is a sensational new research paper, ‘A Greenhouse Effect on the Moon?’ otherwise called the ‘Moon Paper.’
Researchers for the paper scientifically proved that since at least 1997 climate scientists knew that guesswork was underpinning the whole greenhouse gas theory. In fact, so flaky are these numbers that they can be rendered to show a GHG effect on Earth’s moon, where no greenhouse gases exist! Thus, skeptics argue, the burning embers of political heat generated by the discredited theory should now finally and unequivocally be extinguished.
But more sinisterly, it turns out that NASA climate scientists, with access to better climate equations used for the Apollo Moon mission, forsook those in favor of dodgy Dr. Schmidt’s ‘back of an envelope’ numbers.”
http://usactionnews.com/2010/06/nasa-charged-in-new-climate-fakery-greenhouse-gas-data-bogus/
NASA knew how to calculate it properly, it had to junk Stefan-Bolzmann to get accurate figures for the moon landing.
Mr. folkerts says: “Assuming you are making tea not too early or late on a sunny day, the solar energy at that time could be up to about 1000 W/m^2. This is one other reason that sunlight is more effective.”
This I fully agree with the 1000 W/m^2 is why the diagram is not correct. However, you obfiscate on other issues, but on this we are in total agreement. You are coming along my little padawan.
climatereason says:
October 27, 2011 at 9:15 am
R Gates
I have asked you this many times and perhaps you have answered but it got lost within the deluge of comments.
When do you believe the globe started warming? (we shall leave aside the one third of stations that are cooling that we pointed out in our article ‘in search of cooling trends’
I believe it started warming from 1607/8 albeit it has been in fits and starts with numerous serious reversals and astonishing advances (such as around 1700.)
Berkely appear to partially support my view with a rise from their start date of 1800.
Supplementary question; Who do you believe, Michael Mann or the Berkely study?
tonyb
_____
Tony, by your question, I take it you mean when did human activity (specifically the burning of fossil fuels) begin to warm the climate? I think the issue would be one of measureability of the signal through any natural background fluctuations. Certainly, IMO, most of the decadal, century scale, and even 1500 year cycles in climate (i.e. non-Milankovitch) can be traced back to the sun. They may be reflected in ocean cycles, but the oceans themselves are not the cause of the cycles, but only a resultant effect. Then there are the shorter term cycles in climate linked to events like ENSO, volcanoes, etc. So in trying to find an anthropogenic signal amongst all this other natural background climate noise is a difficult proposition. For example, there can be times when the natural cycle is toward cooling (i.e. a Maunder or Dalton Minimum), but there could be human influences which make the cooling less severe or deep than it might have been. Identifying that type of effect (a less intense cooling) as in indication of warming is certainly quite a challenge. However, not to dodge your question, in my opinion the first really visible signs of human warming of the planet would be early in the 20th century, with the signal growing progressively stronger throughout (even though there were periods of natural cooling). I think for certain by the early 1980’s we could see temperatures begin to deviate from the natural solar influenced cycle, meaning that the influence of human GH forcing became greater than the solar influence. As to who I believe, Mann or Berkeley, I find that an odd choice. For example, I think the MWP was probably a bit warmer and bit more global than Mann might like to posit, but I also think that in general, the trend of global temperatures increases over the past century as reflected by Berkeley et. al, are pretty much on target, give or take some inconsequential adjustments. Based on natural cycles plus human forcings, we are due for continued warming (despite the AGW skeptics excitement over the flattening of the upward trend over the past decade). So we are due for warmer, not cooler times ahead…and the next glacial period by Milankovitch cycles is not due for more than 10,000 years.
One final note, of course human influences on the climate go both ways , with some leading to cooling and some leading to warming. So even these can be competing signals against the background of natural forcings.
Bob_FJ,
As to the last section where you claim “333 W/m^2 Shown as coming from high cloud area (= BS according to MODTRAN)”
First, the 333 W/m^2 is coming from the atmosphere as a whole, not “high clouds”. It includes CO2 and humidity and high clouds and low clouds.
And following up on that point, the calculations you have are all for clear skies. If you model a cumulus cloud base, the numbers are much higher. For example, the first line for looking up from 10 m in a clear tropical sky was 348 W/m^2 in your table, but 418 W/m^2 when cloudy. Arctic winter goes from 163 W/m^2 to 243 W/m^2. All the other numbers will also be higher for cloudy weather. As such, you would have to average not only the different zones, but also cloudy and clear for each zone.
Given that the downward radiation with cloudy conditions is so much higher than the clear conditions you quoted, it is now plausible that the average from MODTRAN (all areas, all cloud covers) would agree with trenberth’s 333 W/m^2.
Leif Svalgaard says:
October 27, 2011 at 11:11 am
rbateman says:
October 27, 2011 at 10:45 am
The Sun does not surround the Earth, illuminating and heating it from the vertical all day long at all points on the surface. The Sun illuminates/heats the dayside of the Earth as if it were 1/2 a circle the size of the Earth….from the vertical. So, the Earth’s dayside gets, at most, 1/2 of the 1366 W/m^2. 1366/2 = 683.
And because the Earth is round, you can cut that number in half as well, getting to the 342 W/m2.
And surely the same argument goes for backradiation, only 1/4 of it will reach the surface of the earth?
mkelly says:October 27, 2011 at 12:03 pm
“This I fully agree with the 1000 W/m^2 is why the diagram is not correct.
This seems like a non-sequitur. We seem to agree that sometimes sunlight is bright (1000 W/m^2 on a sunny noon); sometimes it is sort of bright (~200 W/m^2 later in the afternoon); sometimes it is gone (0 W/m^2 at night or under clouds). A weighted average that comes out to 161 W/m^2 would certainly be possible — how does this invalidate the Trenberth diagram?
Kelvin Vaughan says:
October 27, 2011 at 12:27 pm
And surely the same argument goes for backradiation, only 1/4 of it will reach the surface of the earth?
No. The IR comes from all directions, not just from one direction. There is no “night side” where IR from the atmosphere does not shine.
Don’t forget the Venus comparison: http://theendofthemystery.blogspot.com/2010/11/venus-no-greenhouse-effect.html
@Leif Svalgaard says:
October 27, 2011 at 8:24 am
Just to be clear, are you talking about pure water, or the soup that is sea water?
Tonyb;
Congrats on getting a response from R. Gates… well, sort of.
Me he continues to ignore entirely because the only answer he has left is to admit that he was totaly wrong and welched on his bet with me.
R gates
Thanks for your answer. So the observed 400 year warming trend only became a warming trend influneced by humans in the last 20 years or so? So for 380 years it was natural and then it switched to being man made. Is that correct?
tonyb
Myrrh;
Utter codswallop. Water is a transparent medium to visible light, visible light is transmitted through without being absorbed >>>
In various threads you demanded experimental proof, and when it was provided to you, declared it impossible. You demanded text books, and when reffered to same, declared them irrelevant. You demand that others read and understand your blather, but when confronted with facts, you scream “codswallop”.
Tell me please. Since it gets dark at about 100 meters in the ocean, where did the light go? If it wasn’t absorbed, where did it go? Did it make a U turn and head back to the surface? One would think the casual observer would notice light shining up from the bottom of the ocean? Did it come to a stop and just sit their in suspension in the water? Did it turn into flying pigs? Where does the light go if it wasn’t absorbed by the water? Maybe it just disappeared?
Tim Folkerts says:
October 26, 2011 at 7:49 pm
“If 10^19 photons of energy 10^-19 J pass thru a 1 m^2 surface oriented perpendicular to the earth’s surface, then 1 J passes thru the surface independent of the direction that the photons are moving.”
Unh-uh. If that were true, we would not need to steer solar arrays to point at the sun to get maximum power. You have to integrate over the projected area, which is proportional to the cosine of the angle between the (-) surface normal and the direction of propagation.
davidmhoffer says:
October 27, 2011 at 1:32 pm
Myrrh;
Utter codswallop. Water is a transparent medium to visible light, visible light is transmitted through without being absorbed >>>
_______________________________________
I don’t have any proof to the unhindered transmission of light threw pure water. However the sea is not pure. Particles and dissolved organics will decrease penetration of light in the sea. How these effect temperature I don’t have a clue.
Myrrh says:
October 27, 2011 at 11:30 am
Utter codswallop. Water is a transparent medium to visible light
You have been shown several times that this is not true. Are you not embarrassed by displaying such ignorance? and repeatedly.
Not only did you get a reference to such a textbook, but also a condensed explanation that should be understandable to the general reader: “A water molecule consists of an oxygen atom with two hydrogen atoms sitting on ‘hydrogen bonds’ [like little springs sticking out from the oxygen atom]. The two springs form an angle of some 105 degrees. There are three main modes of vibrations: mode 1, where the hydrogen atoms vibrate in and out in unison [‘symmetric vibration’]; mode 2 where the bonds bend back and forth, changing the angle;and mode 3 where the hydrogen atoms vibrate in opposite directions [when one goes in, the other goes out – called ‘asymmetric vibration’]. Because of the dense packing in liquid water, mode 2 does not happen [there is not enough room], so in liquid water only mode 1 and 3 occur. As with any vibration, there is a fundamental frequency [which is activated by far infrared], but here are also overtones [or harmonics – this is what makes a note, like ‘A’, sound differently on a violin and a trumpet]. The overtones that combine mode 1 and 3 [combined to 6th and 5th harmonics] correspond to the visual frequencies of light of 511 nm [green] and 606 nm [yellow], so visual light can and does excite vibrations. Overtones are normally of much less amplitude [strength] than the fundamental vibrations, so the visual light absorption is up to million times smaller than that of far IR. That infrared is absorbed [and thus heats] in the first few millimeters of the water column, while it takes 100 meters or more of water to absorb [and be heated by] visual light. But the ocean is deep enough for that, so even visual light gets absorbed [otherwise the ocean floor would be bathed in light – which it is not] and thus heats the oceans.”
Myrrh says:
October 27, 2011 at 11:30 am
Water is a transparent medium to visible light
Here you can learn more about absorption of visible light by water:
http://www.lsbu.ac.uk/water/vibrat.html
” Water is very slightly blue in color [131]c as overtone and combination vibrational absorption bands (albeit far less intense, see above [130]) extend through the red part of the visible spectrum with a small peak at 739 nm and shoulder at 836 nm, both varying somewhat with temperature [268] plus a smaller fourth overtone of the v1:v3 stretch at 606 nm, and very small fifth overtone (at 514 nm) and combined overtone (at 660 nm) bands. This absorption spectrum of water (red light absorbs 100 times more than blue light), together with the five-times greater scattering of blue light over red light, contributes to the blue color of lake, river and ocean waters.”
MostlyHarmless @ October 27, at 3:18 am
You seem to have misunderstood the article in two ways:
1) The problem is expressed in the absorptive spectra, which is why I wrote: (ignoring some [radiation] escaping directly to space)
2) The problem is concerning the S-B isotropic radiation when affected by an absorptive atmosphere. It is not chrystal clear to me what they did with their initial S-B calculation in 2009, but it doesn’t matter how the final number of 396 was derived, and the same argument would apply if they stayed with their 390 number of 1997. That is why I wrote: they give that the 396 W/m2 of EMR emitted from the surface in Fig.1 is calculated primarily by using the Stefan–Boltzmann law, and global year average conditions. And why I also put a question mark against the temperature in the tables
Nice Job Bob FJ,
A couple interesting points,
1. The 63 Wm-2 (396-333) is a good bit different than the NASA value of 83Wm-2 (21% at surface) and 59Wm-2 absorbed by the atmosphere versus only 23Wm-2 absorbed in the K&T.
2. The 396Wm-2 from the surface is via S-B with emissivity = 1, perfect black body. If the 333 is assume as a real energy source, via S-B emissivity = 1, The clouds where the DWLR would be originating are at approximately 277 degrees K, I have always found that interesting.
3. The assumption of only up or down radiant energyflux is perfectly fine, until the tropopause roughly. Then the horizontal window should be considered.
The cartoon needs a proper funeral so things can move on.
Michel @ October 27, at 2:58 am
What I wrote in full was: The initial isotropic S-B surface emission, (Trenberth’s global 396 W/m2), would largely be absorbed by the greenhouse gases instantaneously near the surface. (ignoring some escaping directly to space through the so-called “atmospheric window”).
The reason I claim this is that most of the radiation is in lateral directions, and even if the photon free path lengths are long, their initial absorption would be near the surface. Of course those streams of a more vertical aspect would mostly go much higher.
>>Tim Folkerts says: October 26, 2011 at 7:49 pm
>>“If 10^19 photons of energy 10^-19 J pass thru a 1 m^2 surface oriented
>>perpendicular to the earth’s surface, then 1 J passes thru the surface
>>independent of the direction that the photons are moving.”
>Bart says: October 27, 2011 at 1:43 pm “Unh-uh. If that were true,
>>we would not need to steer solar arrays to point at the sun to
>>get maximum power. You have to integrate over the projected area… ”
If you read carefully what I said, then we are not in disagreement. Whatever angle a photon hits a solar array, if that photon gets absorbed, it deposits all of its energy. It does not deposit Ecos(theta) or any such thing. This deposits E. Bob_FJ seems to be saying that only the ‘perpendicular component of the photon energy” gets deposited.
I fully agree that if you change the angle of the surface, then the number of photons hitting the surface will change. That is separate issue.
Well, It can be put this way:
According to Trenberth heat transfer by thermal radiation from surface to atmosphere is 23 W/m², while thermals + latent heat (related to phase transitions of water) carry off 97 W/m². As 40 W/m² is supposed to escape directly from surface to space through the atmospheric window by thermal radiation, it is only 14.4% of the entire heat flux leaving the surface that can be connected to the greenhouse effect.
More importantly in Table 2a of their paper they say global land area has an overall 15.6 W/m² heat flux deficit, that is, it radiates 5.57 PW more to space than it receives from the sun. This figure matches exactly the latent heat carried by the amount of water vapor carried by winds over land that is seen again as global river runoff to the ocean, glacier discharge added (slightly more than a million cubic meter per second).
Their value of 80 W/m² for the latent heat of evaporation also matches global average precipitation closely (as it should). However, water, once up in the atmosphere in the form of vapor, seldom returns to the surface upon first condensation. Most droplets and ice crystals, up to 90% of them evaporate again as they descend to lower layers, well before reaching the surface. That vapor is then raised again until it re-condenses, and so forth.
That is, even that 14% of heat flux that left the surface as thermal radiation and was re-absorbed by the atmosphere at some (pretty low) level, does not have to proceed painfully upwards as radiation, but is aided in its progress by the water cycle, until it makes up to a level where the air is clear & dry & cold enough to let radiation escape to space.
This level is at the cloud top, of course, and most of the radiation there comes from ice crystals. Emissivity of ice (and liquid water) in the thermal infrared is very close to one, that is, it behaves like a black body. This broad band radiation can hardly be intercepted any more by narrow absorption lines of dilute greenhouse gases at low pressure, which are not even subject to pressure broadening up there.
If the atmosphere gets a bit more opaque in the thermal infrared (due to GHGs), this intermediate water cycle simply gets more vigorous, making the overall average upward heat flux nearly constant.
Anyway, radiative transfer can’t give a serious contribution to heat flux between atmospheric layers as long as various forms of water are not involved, otherwise thermal conductivity of carbon dioxide could not be smaller than that of dry air (which, unlike carbon dioxide, neither emits nor absorbs em radiation in the thermal infrared band).
Myrrh @5:15 am
As noted earlier by Gail, there are a number of processes that can happen to a photon as it interacts with matter(adsorber). Partial interaction of the photon with an atom will lessen the energy of the photon thereby changing its frequency/wavelength and the photon goes on to interact the adsorber at some distant spot. With complete adsorbtion, the atom will gain all of the photon’s energy. Interaction with a particular atom depends on the frequency/wavelength of the incomng photon and the chances that the photon will interact with an electron or the nucleus of the adsorber. The chances of interacting depend on: how many adsorber atoms there are; how big is the nucleus of each atom; and how many electrons associated with the atom? If there is enough of the absorber (lots of atoms), all photons will be absorbed.
Water does not adsorb light photons very well, but it does adsorb some and it will adsorb all of it if there is enough water. Therefore all of the energy from the sun’s light spectrum (and the rest of the sun’s electromagnetic spectrum photons such as microwave and IR) will get transferred to water just because there is so much water in the oceans.
When a photon, such as a light photon, is partially or completely absorbed by an atom, the atom’s electrons gain extra energy. The atom is now excited and its vibrational state increases. This is not the state that the electrons want to be in, so eventually that extra energy is re-radiated away from the atom at a different wavelength or the energy may be transferred to another atom. For example in water, the excess energy gets shared among the three atoms, H-O-H, and the water molecule is now in an excited state, which we call a “heat” state. I’m using the term heat very loosely here. When we experience hot water, we are experiencing the release of excess energy from the water in the form of photons that transfer energy to our atoms or as a direct transfer of energy to our body’s atoms that are in direct contact with the water molecules.
Anything will act as a photon adsorber, but some things are better adsorbers for specific wavelengths/frequencies than other adsorbers. A black piece of paper will stop most light photons, but not all. This is easily demonstrated using low intensity (few photons) light and a high intensity light (lots of photons). Some light will go through the paper with the high intensity. However, if you put enough pieces of paper together, the high intensity light will get stopped because the black (carbon) molecules are quite good at adsorbing the wavelength/frequencies of the light spectrum. If it was an X-ray photon, at its different wavelength/frequency, the black paper is basically transparent. The same basics apply to water as an adsorber no matter what the frequency/wavelength/frequency.
What is happening to the energy being deposited in the paper? It is exciting the paper molecules and some of it will expressed as heat, much of which gets radiated/transferred to the surrounds. If the energy deposition is too great, the paper molecules may shift and break down (burning).
“”””” Kelvin Vaughan says:
October 27, 2011 at 1:16 am
Just a thought! anti phase sine waves of the same frequency cancel out. Does this happen at infra red wavelengths? “””””
Kevin, the question EXACTLY as you have phrased it has a very simple answer; YES.; well actually with one little detail you left out; those sine waves also have to be of the exact same amplitude, to completely cancel. And I bet you knew that too, if you had just thought about it.
So let’s just say that your sine waves are coming from some electronic oscillator circuit. You can of course switch the power to that circuit off and on; whenever you like.
Just what do you suppose is the likelihood, that if you switched the power off, and then on again, that the oscillator will come up in EXACTLY the same phase, that it might have had if you DIDN’T switch the power off; well not bloody likely as they say.
So ingeneral any oscillator goes on and off from time to time, either deliberately or accidently, so it may not remain PHASE CONTINUOUS all the time.
Well most light sources, and most Infra-red sources, are like that off and on sort of sources. They don’t remain phase continuous FOR VERY LONG; ACTUALLY THEY REMAIN CONTINUOUS FOR A VERY DAMN SHORT TIME.
The infra red radiation, may only travel two inches, and suddenly the phase jumps discontinuously to some other place in the cycle. We call it the COHERENCE LENGTH.
Ordinary light sources or IR sources, don’t normally cancel out, because they never remain exactly out of phase, because of theat coherence length.
The most obvious exception is the LASER. A laser, is not unlike an ordinary oscillator, with its phase remaining coherent for quite alarge distance (or time if you will).
As anybody knows, if you shine one of those little red laser pointers at a wall or something, you see this bright SPECKLE PATTERN apparently dancing in front of the wall, and as you move your eye the speckle patter seems to follow you around.
That speckle pattern is nothing more that the laser beam travelling towards the wall, INTERFERING with the beam reflected off the wall, in the space above the wall, where they overlap, and the speckle is a pattern of dark spots, where the two beams cancel, and the bright spots are where they add together to make an even brighter spot.
So the short answer to you r question is yes they can cancel, but only for the time duration when they really are out of phase.
Non laser ordinary light or infrared sources, usually do not cancel each other because the coherence length is too short..
Now Michelson, measured the length of the standard Metre bar against some lines in the spectrum of Cadmium, which happen to be very narrow bandwidth atomic spectral lines, and in that case they have a much longer coherence length than other sources. The Cadmium Red line as measured by Michelson came out at 6438.4696 Angstrom units, or aqbout 643.84696 nano metres. He had to have his coherence length at least 10 cm long to do that measurment, otherwise he would have seen no interference fringes.
There is a green line in the Mercury 198 spectrum, which is even sharper, than the Cadmium Red line, and makes even nicer interference patterns. Trouble is Mercury 198 is not a natural isotope, or is extrremely rare; so you have to make it in a nuclear reactor out of gold; so 198Hg is far more valuable than gold. There are some even better ones, before you have to go to lasers.
Ordinary sources; specially thermal ones, are very noisy so have low coherence lengths, maybe not even mm in extent.
Tim Folkerts @ October 27, at 12:05 pm
The problem with your argument is that Trenberth gives:
Sunlight absorbed by surface (161) = energy leaving surface + the controversial heat retained of 0.9.
161 = 17 + 80 + 63 + 1
The radiative heat transfer from the surface of 63 can also be obtained from the net of the alleged up-down radiation: 396 – 333 = 63
Thus the 396 & 333 is at the surface, but you argue that it is constant up to the high cloud level, including clear skies.
You earlier seem to admit that this can only be the case for an ideal transparent atmosphere, but clear skies are closer to this condition than are cloudy skies
davidmhoffer says:
October 27, 2011 at 1:26 pm
Tonyb;
Congrats on getting a response from R. Gates… well, sort of.
Me he continues to ignore entirely because the only answer he has left is to admit that he was totaly wrong and welched on his bet with me.
_____
Tony simply is far more polite than you and seems to have a better grasp of the the issues.
Tim Folkerts @ October 27, at 3:22 pm & Bart @ October 27 1:43 pm
Tim said: Bob_FJ seems to be saying that only the ‘perpendicular component of the photon energy” gets deposited.
Please see this simple analysis of Lambert’s law (or cosine law in optics, Lambertian or black body reflection)
http://escience.anu.edu.au/lecture/cg/Illumination/lambertCosineLaw.en.html
In the first figure, see the effect of incoming light angle variations. There are more detailed descriptions around, but you can see the effect of vectors in the simple diagrams.
In my previous post I neglected to mention the reflective property of water to light photons, which is relatively high compared to other matter. This is the result of partial interaction of the incoming photon without much energy deposition and is related to the unique structure of the water molecule, the frequency/wavelength of the incoming photon, and the incidence angle. Someone with a better theoretical physics background than I may be able to explain this phenomenon in laymen terms better than I. Obviously with reflected light photons, which do not enter into the water mass, this energy does not get adsorbed by the water. However, most of this light energy will be adsorbed by other matter, though a very small portion may escape into space.
Berényi Péter said “Anyway, radiative transfer can’t give a serious contribution to heat flux between atmospheric layers as long as various forms of water are not involved, otherwise thermal conductivity of carbon dioxide could not be smaller than that of dry air (which, unlike carbon dioxide, neither emits nor absorbs em radiation in the thermal infrared band).”
I don’t understand this paragraph, CO2’s thermal conductivity peaks at -20C and the Kinematic viscosity of air at -20 is very high relative 15C. Antarctica has a conductive/convective relationship that appears to negate most of the radiant impact of a change in CO2 forcing. The reason it is not warming as advertised.
Tim Folkerts says:
October 27, 2011 at 3:22 pm
“Whatever angle a photon hits a solar array, if that photon gets absorbed, it deposits all of its energy. “
Nobody is interested in individual photons. The question is, what percentage of photons in an incident plane wave are being absorbed. That is very much dependent on the angle of incidence.
climatereason says:
October 27, 2011 at 1:29 pm
R gates
Thanks for your answer. So the observed 400 year warming trend only became a warming trend influneced by humans in the last 20 years or so? So for 380 years it was natural and then it switched to being man made. Is that correct?
tonyb
____
There was some recovery from the lower temps of the LIA, but it was hardly as strong as the temperature increases we’ve seen since 1980. Temperatures were wavering in the 19th century…up a little then back down before starting a pretty constant rise in the 20th century, becoming really pronounced after about 1980. Of course warming (or cooling) doesn’t switch from one cause to another, and I know you’re being facetious here, but climate is always a combination of factors, some reinforcing and some competing with each other. Human forcings on climate are a mixed bag, with some causing cooling and others causing warming. Over the several centuries since the industrial revolution, humans have been putting increasingly large amounts of carbon into the atmosphere and oceans. As this contribution has grown, and as the accumulated effect begins to show, you would expect the human forcing signal to begin to dominate over the natural background forcings at some point. Those other forcings are still there, but the anthropogenic signal would begin to dominate, with the signal getting easier and easier to see, even though the other forcings are still there. The next 10-30 years or so will be very interesting to watch, as if we have a quiet sun and cooler oceans, we will get to test first hand the relative strength of the human contribution. If you think this was similar to the Maunder Minimum period, we certainly know what happened then, so we can compare today’s climate response directly.
R. Gates;
Tony simply is far more polite than you and seems to have a better grasp of the the issues.>>>
Then by all means, answer his follow up question, it was politely worded, was it not?
As for the notion that you don’t answer me because I’m not polite…in one of my comments I suggested you were a girl and you instantly jumped all over me. Seems to me when I’m totaly rude you respond right away. But to factual questions you either go silent or, as in your response above, come up with some inane excuse that is patently and obviously not true.
I’ve asked if you had some sort of relationship with Trenberth on many occassions. You finaly answered by claiming you had no relationship other than you lived in the same state. I repeat my follow up question:
Did you or did you not arrange for Anthony Watts and 20 guests from the WUWT forum to meet with Kevin Trenberth? You claimed very proudly in another thread that you did. How did you accomplish this without having any contact with Kevin Trenberth other than living in the same state as him?
As for our bet, you welched. You can fail to respond to me, or come up with excuses, but the record is there for all to see. You accepted the wager, and asked me how much I would like to bet. Then you tried to redefine the experiment. Then you tried to claim that Anthony’s results showed only that Anchor Hawking glass absorbs IR. That wasn’t the bet. The bet was that if the experiment were done as illustrated, it would not show the results illustrated. You said they would, and they didn’t.
Of course when it became totaly obvious that you’d lost the bet, you turned from defending Al Gore’s “illustration” to throwing the guy under the bus as if we’d forget what you said in his defense only a few weeks before.
Frankly R. Gates, I’m being perfectly factual, and while you might accuse me of being blunt, there is nothing impolite about anything I said or the questions I asked. If you are uncomfortable with answering the questions, might I suggest that your discomfort originates from another cause?
Ooops. I just did. How impolite of me.
I do agree that every photon incident on the Earth will get processed in some way. But, the total percentage is 1/2 of the area of incidence (pi*r^2, being the projected area, rather than 2*pi*r^2, being the total area facing the Sun).
If what I am saying is not germane to the argument, nevermind. I haven’t read through the article carefully yet, I was just commenting on what appeared to be an erroneous argument which caught my eye.
Modtran results looking up from the surface in the tropics (looking at the back-radiation, the red line) when it is Clear.
http://img171.imageshack.us/img171/4308/rad12081141.gif
Modtran results looking up from the surface in the tropics when there is low cloud cover. A perfect black-body radiating at 20C (and it is cloudy 65% of the time).
http://img171.imageshack.us/img171/7268/tropicalsurfacelookingu.gif
Where is this described in Trenberth’s diagram?
It is an illustration which gives everyone a false impression of how the real atmosphere works, and how it really works over a 24 hour period.
Another good example, in the middle of the day in clear conditions, the solar irradiance coming in can be as high as 1200.000 joules/m2/second. But the surface energy level in the same conditions is only increasing by 0.008 joules/m2/second. It is flying out as fast as it is coming in. Where is that shown is Trenberth’s diagram?
Jim Masterson wrote;
“These diagrams are “steady-state.” That means the transients have had time to damp out or stabilize.”
Thank you, I fully understand the concept of “steady-state”.
Unfortunately, the complex climate system of the Earth is NEVER in a “steady-state” condition.
My point is if you model/analyze a complex chaotic system as if it is a simple “steady-state” problem you are VERY LIKELY to get the wrong answer, no matter how well you analyze the “steady-state” condition.
For example, an airplane flying towards the surface of the Earth at 500 mph is in a “steady-state” condition. Once the plane and the Earth’s surface intersect the “steady-state” becomes chaotic very quickly.
Cheers, Kevin.
George E. Smith; says:
October 26, 2011 at 11:11 pm
“”””” CRISP says:
You CANNOT transfer heat from a colder body (the upper atmosphere) to a hotter body (the lower atmosphere and Earth surface) without doing work. The 2nd Law of Thermodymanics avbsolutely forbids it. “””””
EM radiation knows absolutely nothing about either “heat” or “Temperature.”, and it can go wherever it darn well pleases .
—————————————————–
George I hope you don’t want to imply that the 2nd Law doesn’t hold for radiation, Planck and Einstein surely build on it when they dug into the BB-radiation problem.
Anyways, I notice that many people think the entropy in k*ln*W has to do with the probability of jiggling atoms. It does not. It has to do with possible energy microstates, entropy is about the dispersal of energy itself. W stands for “Ways of energy distribution”, so in the case of atoms the energy distribution among all possible translation, rotation and vibrational states.
Why doesn’t the earth surface heat up infinitely by radiation? Because after absorption from a SWR photon the energy can leave dispersed as 20 diffuse LWR photons.
Backradiation may be real but it is clear that it cannot warm up the surface further because it can’t be dispersed any further than it was when it left the surface in the first place, it can only do this if it would be radiated away at an even LOWER surface temperature.
The ‘heat from hot to cold’ phrase that is often used, should be: Energy tends to flow from being concentrated in one place to becoming diffused and spread out and therefore less concentrated. This more concrete formulation with respect to the 2nd Law is well known in chemistry.
So EM radiation cannot transfer (heat)energy from a colder to a hotter body because the photon energy cannot be dispersed by the hotter body. Energy is still more concentrated here.
For an excellent website about the 2nd Law look here.
Bob_FJ says: “Thus the 396 & 333 is at the surface, but you argue that it is constant up to the high cloud level, including clear skies.”
That was not what I said (or at the very least, not what I meant). This seems to be a combination of two different ideas.
1) The 333 W/m^2 downward would be the net result (on average, of course) of all the atmosphere. The higher up you go, the less the you would see looking up. This is because 1) there is less atmosphere above you and 2) the atmosphere is getting cooler.
The value must depend on the GHGs and clouds, since in an IR transparent atmosphere, it would be 0.00 W/m^2 of IR from the 3 K background of space.
2) Conversely, the 396 W/m^2 upward IR from the earth’s surface would continue upward in a transparent atmosphere. THIS is the number I claim would be constant at any altitude. (The curvature of the earth would make a small correction. By the time you are 64 km high, you are 1% of the earth’s radius up, and there would be a 2% correction to the IR — it would decrease by ~ 8 W/m^2 to 388 W/m^2.)
In a real atmosphere, this value will decrease. Some of photons from the warm surface will get absorbed. They will partially be replaced by the photons from the cooler GHGs/clouds higher up. This is exactly the “bites” taken out of the observed and calculated spectra you posted.
Hans;
George I hope you don’t want to imply that the 2nd Law doesn’t hold for radiation, Planck and Einstein surely build on it when they dug into the BB-radiation problem.>>>
That’s not what George said. He said that all bodies radiate energy as per Stefan-Boltzmann and they do so in a spectrum as defined by Planck. The photons emitted by a body at any temperature don’t care WHAT the temperature of what they are aimed at is. They either get absorbed or they don’t depending on the properties of whatever they run into. SB, Planck and Einstein all require this to be the case. The 2nd Law holds up just fine if you define the NET exchange of radiation. So, a cold body does in fact send energy to a warm body, but not as much as it GETS from the warm body. That said, if you insert a cold body in between a warm body and a SUPER cold body (outer space for example) the warm body still cools, just not as fast as it would without the cold body in between.
Bob Fernley-Jones says:
“Please see this simple analysis of Lambert’s law …
I don’t think that really applies here. There is no “surface” that is diffusely scattering the light. Mei scattering or Rayleigh scattering would be more applicable.
Even more applicable, however, would be isotropic emission of IR from the various sources in the atmosphere. The molecules of CO2 and H2O, along with droplets of water, would emit IR uniformly in all directions.
I think we are still not communicating clearly the key issues to each other. Hopefully various outer comments will also get at these central issues.
Again, again, again, only crickets when the facts appear. Nobody wants to address the real issues. Just argue about photons…
The Atmospheric GHG theory has been proven to be toast in several ways, yet that is ignored and the silly debates about radiation go on, as if there were no elephant in the room at all.
Rather deserving of a comical play, no?
Very worrisome, I think.
But, even of more concern, the same problem applies to many other totally irrational things going on now, because of a stupid, lazy, uneducated, rich society. Like substituting bumper sticker slogans for policy. “Hope and Change,” as a “policy,” e.g. Like calling a war a “kinetic military action” and Muslim terrorism “man-caused disasters.” Like the climate scientists and Obamabots saying that “our ideas are really right, we just need to communicate them better, and the (stupid fools) will learn to appreciate us. Like the absolute stupidity of the concepts of “green energy,” “renewable energy, and “sustainability.”
1984 is coming true in 2011, unless more people wake up!
So enlighten us, JAE.
What do you consider to be “the Atmospheric GHG theory” and in what specific ways is it “proven to be toast” ?
How does that support or refute what Bob_FJ has written?
Tim Folkerts @ October 27, at 6:37 pm
My comment related to Bart’s comment pertaining to the surface alignment of solar panels relative to sunlight. They are fairly devoid of specular reflection or if you like are close to Lambertian reflectors/emitters. It is a tad off topic, but had relevance particularly with it giving another demonstration of vector analysis.
We could also get into semantics again, in that there are different terminologies again between optics, and your view of physics/elementary quantum theory.
Tim Folkerts @ October 27, at 6:18 pm
OK, maybe I misunderstood you. Are you now saying that although Trenberth shows the 396 & 333 arrowed up at the high cloud level, (and adjacent clear skies), that this is the net result at the surface from all of the above? In other words, the arrows are in the wrong place, and the columns depicting the EMR transmission should really taper-off towards the higher levels?
Not all that hard to draw it that way is it?
davidmhoffer says: (to R. Gates)
October 27, 2011 at 5:19 pm
“Did you or did you not arrange for Anthony Watts and 20 guests from the WUWT forum to meet with Kevin Trenberth? You claimed very proudly in another thread that you did. How did you accomplish this without having any contact with Kevin Trenberth other than living in the same state as him?
___
100% true. I emailed him, and told him what we wanted to do…including who it was that would like to do it (i.e. a small group that I would bring and Anthony Watts and a group he would invite). He politely emailed me back his schedule of when he’d be in Boulder, and told me his assistant would be in contact. Within a day or so she contacted me and we saw that Nov. 10th was open. I then communicated directly with Anthony to set up the details. Very simple, very straight forward. Though I have been to NCAR several times, I’ve never met Dr.Trenberth in person, and have only exchanged a few emails (no more than 2 or 3) over the past few years. He’s always been quite generous and responded within a few days.
So Gates is part of the conspiracy to backstab Anthony.
Figures.
Smokey says:
October 27, 2011 at 9:06 pm
So Gates is part of the conspiracy to backstab Anthony.
Figures.
_____
Smokey, I would expect an apology from a reasonable person, but that might be asking too much from you. I would also like to see Anthony make some remark here, as he knows the full truth of the situation, and there was never any attempt to back-stab Anthony or anyone else. Though Anthony might have become more hesitant to go to NCAR after the BEST issue happened, the two were never connected in any manner, and based on things I heard, I think Dr. Trenberth and Anthony probably would have found some common ground in being critical of the way the BEST results were handled. it was a missed opportunity, but I respect Anthony’s decision not to go, but to insinuate that their was a “conspiracy to backstab Anthony” is…well, beyond the normal level of paranoia even for you.
REPLY: You wanted a response, OK here it is. I made the decision not to go, because every time I’ve ever tried to outreach to climate science by invitation, I end up getting burned. It happened with NCDC after my visit in 2008, and it happened with BEST in the spring of this year. On both occasions, people that I confided in then went to great lengths to put me and the work I’ve done down in public and in very unprofessional ways. Karl et al used preliminary data against my objections, data that was put up briefly for the benefit of survey volunteers, and never part of the final product, and then Dr. Thomas Peterson had the gall to write a ghost authored “talking points memo” about it. He circulated it to every NOAA climate division and middle manager, naming me and my work and telling everyone how it was flawed, but didn’t have the integrity to put his own name to the internal paper. Then later, Karl, Menne, and Vose offer me an option to put my name on their upcoming paper…a week before it gets submitted, but I’m not allowed to offer any criticisms, revisions, or changes to it. Name only. I ask them for a letter of invitation, they refuse to even put it in writing. The idea was to get me to endorse their paper by putting my name on it. Sheesh.
I don’t know that Dr. Trenberth had any designs, he probably didn’t, but I do know this: I asked him personally not to address people as “deniers” at the AMS conference and he declined, only rewriting his speech to change the inaccuracies we called him out on. He didn’t even have the courtesy to respond. After my two previous experiences with “professional” climate scientists and the resulting outcome, I couldn’t see spending my own money, effort, and time to stand next to a man who has so little respect for me and others that he could not refrain from calling people like myself who question the issues on a scientific basis “deniers”.
That, and none of the other 8 scientists I invited wanted to attend. It seems they couldn’t justify making the effort for visiting Dr. Trenberth. So all in all it became simply R. Gates was the only one who really wanted this to happen. In better times, perhaps it would have worked out. But Dr. Muller has put the kiss of death on any further cooperation with his media blitzkrieg. And even now, he’s complaining that “I broke the embargo” because I published my essay on the BEST paper an hour before they got their website up…he seems to think that the Economist publishing a story hours before doesn’t count, and that I was bound by an embargo that somehow wasn’t broken by the Economist, but I did. I found myself being deluged with emails and requests for interviews even before BEST got their stuff out. Judith Curry published 30 minutes before BEST got their stuff on their website, but I’m the bad guy.
There’s no scruples it seems in climate science where the treatment of skeptics is involved. And after what I’ve been through, I won’t trust any of these people again.
Leaning from that, Dr. Trenberth can go fly a kite. – Anthony
>>
KevinK says:
October 27, 2011 at 6:12 pm
Thank you, I fully understand the concept of “steady-state”.
<<
There’s no insult intended. Some here may not know what steady-state means.
>>
Unfortunately, the complex climate system of the Earth is NEVER in a “steady-state” condition.
<<
True, but I never said the climate system was. I said these models were in a steady-state mode.
>>
My point is if you model/analyze a complex chaotic system as if it is a simple “steady-state” problem you are VERY LIKELY to get the wrong answer, no matter how well you analyze the “steady-state” condition.
For example, an airplane flying towards the surface of the Earth at 500 mph is in a “steady-state” condition. Once the plane and the Earth’s surface intersect the “steady-state” becomes chaotic very quickly.
<<
Neither can these models balance your bank statement, fly you around the moon, or simulate supernovae. They are simply, back-of-the-envelope, global average energy flow models. If you want to model the climate (or a supernova) you’ll need more horsepower.
I have modeled these simple, steady-state models with a spreadsheet, and they require a macro to get around the self-referencing problem. That macro just copies the contents of one cell to another cell. After I make a small change, the macro needs to cycle several times. I’ve programmed a loop and it usually needs about fifty iterations before that small change finally stabilizes.
Jim
Speaking as a former meteorologist who observed and reported innumerable surface and air temperatures with surface observations and rawindsonde probes during routine and special operations, Increasing altitude certainly does not result in a decrease in air temperature in all atmospheric layers or in all geographic locations and their time intervals. It is quite common for air temperatures to increase with increases in altitude in some atmospheric layers, in many geographic areas, at certain times, and in many common atmospheric conditions. There are a multitude of ways in which inversion layers develop as warm air masses become positioned above cooler air masses. The adiabatic lapse rates which assume decreasing temperatures with increasing altitude are based upon Tropospheric conditions and ideal assumptions about dry air conditions adjusted for dew point measurements in the upper air soundings. Variations in atmospheric temperature and density at different altitudes also change the quantities of electromagnetic energy being modulated by the air volumes. The presence of water vapor, water, and water ice changes the electromagnetic environment and thermal environment in complex ways. Models which fail to accurately account for these widespread and common exceptions are inherently lacking in reality.
R. Gates;
100% true. I emailed him, and told him what we wanted to do…including who it was that would like to do it (i.e. a small group that I would bring and Anthony Watts and a group he would invite).>>>
Was that so hard? Of course you are now on record with the answer which I shall accept at face value unless evidence to the contrary emerges. In the meantime, thankyou for your clarification.
BTW, who is “we”?
How about the answer to Tonyb’s follow up question?
As for the bet…you’re still avoiding it. If you think Tonyb has a better grasp of the issues than I do, well, I think you are probably correct. But your over confidence in your own grasp of the issues is what led you to accept a wager that was in fact a sucker bet. There was no possible way the results as illustrated could be derived from the experiment as illustrated. Further, the experiment as illustrated could not possibly demonstrate the greenhouse effect because it used LW as the external heating source rather than SW. Even funnier, you, who claim to have a grasp of the issues, suggested changing the experiment by removing the globes because they were superfluous. Might I ask how one can demonstrate the greenhouse effect when there is nothing to absorb the SW and re-radiate it as LW? And you question MY grasp of the issues?
Since no one has really picked up on how wrong the K&T cartoon is, think about this. If CO2 doubled and that caused water vapor forcing to double twice the CO2, there would be a total of 3 times 3.7 or 11.1 Wm-2 increase in that 333Wm-2 down welling radiation. 11.1/333 = 0.033 or 3 percent increase in forcing. If that 333 is related to all of the 33C greenhouse effect, a 3 percent increase would be 1.1 degrees of warming due to a doubling of CO2 forcing and water vapor forcing. Not just a no feed back forcing, the whole enchilata. That is what Richard Lindzen has said, K. Kimoto has said, and Monckton has said. They are right, if you use that cartoon.
Isn’t it ironic how some people defend the indefensable even when it is shooting themselves in the Foot? 🙂
Unfortunately, it is not as simple as discrediting another flawed icon of global warming past. The reality is a little more complicated.
“Leaning from that, Dr. Trenberth can go fly a kite. – Anthony”
Wow. Thanks for the inside info. Things truly are much, much worse than I thought.
R Gates
Thank you very much for your reply in which you said;
“There was some recovery from the lower temps of the LIA, but it was hardly as strong as the temperature increases we’ve seen since 1980. Temperatures were wavering in the 19th century…up a little then back down before starting a pretty constant rise in the 20th century, becoming really pronounced after about 1980”
With respecf, that isn’t really so. I wrote an entire article on 19th Century temperatures viewed through the Life of Dickens
“Dickens life demonstrates the extraordinary variability of the British winters during that era, when the coldest and warmest winters in the CET records can be juxtaposed. Generally there are few examples of constant cold winters year after year-the LIA was becoming much more sporadic than it had been several centuries earlier, when bitter cold weather appears to have been the norm. To put this era into perspective mature English people might be surprised to learn they lived through a much colder winter than Dickens ever experienced. 1962/3 at -0.33C was the third coldest in the entire CET record compared to Dickens coldest year 1814 at 0.43c, the fourth coldest in the record. (1962/3 was a bit of a one off-Dickens experienced a greater number of relatively cold winters)
HH Lamb, (in ‘Climate, History and the Modern World’), says: “Indeed, the descriptions of ‘old-fashioned’ winters for which Charles Dickens became famous in his books may owe something to the fact – exceptional for London – that of the first nine Christmases of his life, between 1812 and 1820, six were white with either frost or snow.”
http://wattsupwiththat.com/2010/12/05/has-charles-dickens-shaped-our-perception-of-climate-change/
Within my article are also studies of Europe and the US.
The low point was the first decade of the 17th Century. The subsequent steady rise saw increases as great as the 1980’s. I am currently recostructing the temperature back to 1550 which is taking a great deal of research (and includes visits to the Met offive archives) . Good as part of Dr Manns experimental work was, it wasn’t as accurate as Hubert Lambs, although more recent evidence seems to suggest his adapted 1991 IPCC chart wasn’t wholly accurate. How Hubert Lamb would have loved the internet and the opportunities it gives someone like me to stand on the shoulders of giants
with best regards
tonyb
Dallas @ October 27, at 2:55 pm and more recently:
Sorry for a slow response, and I note that you have made a bunch of interesting comments, including, if I can paraphrase:
I think you inferred something like: Up or down radiant energy flux is OK until reaching the TOA, when radiation can then also escape freely to space sideways
Yes, indeed, and I was wondering at one stage if I should include an observation in the article that if the TOA could be treated as a surface, (which is a tad conceptual), then the idea of surface integration at “that level” might be applied. This is a concept that has been put forward by Tim Folkerts relative to the Earth’s surface, IF we had a transparent atmosphere. However, I thought it might get into a quagmire of semantics and whatnot, and I wanted to keep it simple and towards lay language.
Bob Fernley-Jones @ October 27, 2011 at 3:13 pm
My understanding of the 396 W m-2 is that it is an averaged net upwards oriented energy flux resulting from all phenomena taking place on a non-even emitting surface. The Trenberth and the NASA diagrams are just energy balance MODELS, not a fundamental explanation of all the physics going on on a lukewarm plate.
Even for lateral going radiation, a high enough concentration of IR absorbing gases (so called GHG) would be needed to absorb this energy. CO2 at 391 ppm and H2O at 0 – 40000 ppm cannot play that role on a very short distance (may be some methane at high concentration in cows’ flatulence could do the job). The major air components O2, N2, and Ar don’t absorb in the IR range.
To debate if [or not] a 15°C surface emits [or not] energy in its perpendicular [or not] direction is a “Nebenkriegschauplatz”, a battle not worth conducting.
What is more important is the unbalance shown in Trenberth, 333 W m-2 going only downward, and to understand what is wrong there (or if it’s right, to expain it).
And even more important is to know if a 3.7 W m-2 effect (GHG forcing for doubling CO2) can bring to a tipping point a system with huge shock absorbers like water evaporation and cloud albedo.
Re Smokey @ October 27, at 9:06 pm, and R.Gates.
ANTHONY, in your reply thereto; you have to be admired for having initially contemplated intercourse with Trenberth. But whilst you would like to make peace with such alarmists, I think it is a stretch to hope that such leopards could change their spots. For example, Trenberth’s and the IPCC’s treatment of Chris Landsea was disgusting. (and we don’t see any acknowledgement that Chris has been right so far!)
BTW everyone, may I suggest that regardless of what his relationship with Trenberth might be we should listen to R. Gates, because it seems to me that he does produce some arguments that we should consider as worthwhile thinking-on.
>>
MostlyHarmless says:
October 27, 2011 at 3:18 am
Secondly, a correction – K&T 2009 does not compute surface radiation for a “surface temperature of 16 °C and an emissivity of 1.0″.
In K&T 2009:
“To compute these effects more exactly, we have taken the surface skin temperature from the NRA at T62 resolution and sampling and computed the correct global mean surface radiation from (SB) as 396.4 W/m².
. . .”
I see plenty to argue about in the detail of K&T 2009, but let’s get the facts straight.
<<
In the first sentence (that you quote) they explicitly state that they use the S-B law to compute that 396.4 W/m². Using an emissivity of 1.0, the S-B constant, and absolute zero at -273.15 °C, we get exactly 16.00 °C to four significant digits. The rest is all hand waving.
Jim
“There have been decades, such as 2000 – 2009, when the observed globally averaged surface-temperature time series shows little increase or even a slightly negative trend (a hiatus period). However, the observed energy imbalance at the TOA for this recent decade indicates that the net heat flux into the climate system of about 1Wm^-2 should be producing warming somewhere in the system “. This quote from the warmist publication ‘nature climate change’ shows a measure of desperation in their cause along with ‘climategate’ when hockey predictions of CAGW do not materialise. They resort to bogus claims of global energy imbalances by Trenberth (‘where has all the heat gone’) and Hansen; B F-J’s post showing the paradox in trenberth’s claims is excellent.
George E. Smith; says:
October 27, 2011 at 4:04 pm
“”””” Kelvin Vaughan says:
October 27, 2011 at 1:16 am
Thanks for the lesson George, I appreciate it
Kelvin
Bob Fernley-Jones says:
“BTW everyone, may I suggest that regardless of what his relationship with Trenberth might be we should listen to R. Gates…”
Since R. Gates was actively trying to manipulate Anthony into a public meeting, I suspect a setup. Gates was doing what he was told, and I have no doubt that some kind of dog and pony show was planned to make Anthony look bad. Trenberth and Muller are two peas in a pod. Neither one can be trusted, and Gates is their water boy, even offering here to personally bus people from the airport to the meeting.
As I’ve often said, there should be a series of debates conducted on the subject of AGW. BUT they must be held in a neutral venue, with agreed-upon rules, and a debate moderator chosen by mutual agreement. After losing all debates the AGW crowd refuses to publicly debate any more, preferring instead to have proxies like Abraham take pot shots from the safety of his ivory tower. Alarmists fear debating with skeptics. So now Anthony has been invited to come to their home turf and be subject to their unstated rules. Based on their prior unethical treatment of a straight shooting, stand-up guy, IMHO Anthony was wise to decline their suspicious invitation.
Smokey @ October 28, at 3:14 am
Yep, I sort-of agree with your rant, but nevertheless, I think that R. Gates does come-up with some interesting thoughts. Call it playing devil’s advocate if you like, but worth considering, whereas for instance in comparison, the views of Myrrh are a waste of time and space when he cannot even accept that IR shielding glass allows high energy visible sunlight to pass through with observable consequences, but not IR which he thinks is the only source of heat.
Leif Svalgaard says:
October 27, 2011 at 8:24 am
Myrrh says:
October 27, 2011 at 5:15 am
For example, visible LIGHT cannot heat water because it doesn’t have the mechanism to do so, water is a transparent medium for visible LIGHT and so visible light is transmitted through without being absorbed, visible light cannot move the molecules of water into the vibrational state which is heat.
Yet it does.
No it bloody well doesn’t. Shine blue light on water and let me know when it’s hot enough for you to make a cup of coffee..
Bob Fernley-Jones says:
October 28, 2011 at 4:17 am
Smokey @ October 28, at 3:14 am
Yep, I sort-of agree with your rant, but nevertheless, I think that R. Gates does come-up with some interesting thoughts. Call it playing devil’s advocate if you like, but worth considering, whereas for instance in comparison, the views of Myrrh are a waste of time and space when he cannot even accept that IR shielding glass allows high energy visible sunlight to pass through with observable consequences, but not IR which he thinks is the only source of heat.
Yeah right, that’s why there’s a whole industry selling products to stop thermal infrared passing through window, but letting in as much light as possible. According to you, generic, that’s not stopping heat getting in because it’s visible that creates it…
You are of course fully entitled to take yourselves seriously.
Bob Fernley-Jones says: October 27, 2011 at 8:12 pm
” Are you now saying that although Trenberth shows the 396 & 333 arrowed up at the high cloud level, (and adjacent clear skies), that this is the net result at the surface from all of the above? In other words, the arrows are in the wrong place, and the columns depicting the EMR transmission should really taper-off towards the higher levels?
Not all that hard to draw it that way is it?”
Yes, that is pretty much what I am saying. This diagram is the simplest possible diagram to try to get across the points he is making. The atmosphere is being treated as a single object, and any of the arrows anywhere in or out of the atmosphere simply mean the energy is entering or leaving the atmosphere as a whole. It is not intended to mean that the back-radiation only occurs from the middle of the atmosphere or that it only occurs on the “right side” of the atmosphere.
But actually, it would be considerably more difficult to draw “correctly”. He could have drawn an arrow down from high (altitude) clouds and from low clouds and from middle clouds and from high O3 and low H2O and middle CO2. But soon the diagram would have 100+ arrows distinguishing flows to and from land and ocean and clouds and CO2 and sun and ….
The cartoon is what it is – a vast simplification to highlight major overall energy flows. People who want more detail need to actually read the papers in the field.
Myrrh says:
October 28, 2011 at 5:40 am
You are of course fully entitled to take yourselves seriously.
http://www.leif.org/EOS/unskilledandunaware.pdf
Michel says:
October 28, 2011 at 1:08 am
The major air components O2, N2, and Ar don’t absorb in the IR range.
———————–
Sure they do.
About 0.00000000015 seconds after a CO2, H2O, or CH4 molecule absorbs a photon in the IR range.
Myrrh says:
October 28, 2011 at 5:36 am
http://hyperphysics.phy-astr.gsu.edu/hbase/chemical/watabs.html
Your own link shows that the absorption of visible light by water is a million times weaker than that of infrared, but that only means that you need a layer of water a million times as thick, i.e 100 meters instead of a fraction of a millimeter. Since the oceans are more than 100 meters deep they will absorb all the visible light falling on them. The mechanism involves overtones of vibrational stretching as you have been shown many times.
Surely the real con in the diagram is that it treats clouds as static and not a variable and fails to show that the Latent heat rising causes cloud formation which then modulate the amount of solar radiation reaching earth and back radiation.
Or to translate from Sceptish: when the sun shines, the ground dries and this moisture rises up causing clouds. These clouds hide the sun during the day making it cooler and keep us warm at night.
Both the atmosphere and the oceans delay the incoming heat from being radiated out to space. We are living on the surface of the earth and probably this is why we think atmosphere first, but is it not in reality ruled by the oceans?
As Leif explained a couple of times the oceans are heated by visible light up to 200 m and more. The warming is distributed as water is almost 100% transparent to visible light.
As on the other side water is completely opaque to IR radiation, the deeper levels will not lose heat by radiation. Furthermore any IR from the atmosphere will hit the only the surface and not have any warming effect on the deeper levels.
As Stephen WIlde explains the oceans get themselves in equilibrium through evaporation & radiation at the surface.
“Importantly both the atmosphere AND the oceans delay the incoming solar heat from being radiated out to space.”
http://climaterealists.com/index.php?id=1487&linkbox=true&position=5
Myrrh, thank you for your reply however I think you seem confused. I was talking about radiation not whether it is visible or invisible. The 2nd law of thermodynamics is often brought up to prove that CO2 cannot cause warming because heat does not travel from cold to hot, and the surface of the earth being hotter would not receive the heat CO2 apparently sends back to the surface to cause global warming. If it were heat, this would be true, however, radiation unlike heat can travel in any direction including back down to the surface. I believe it is important for it to be understood that heat is controlled by the Laws of Thermodynamics, radiation isn’t. I’m sorry if my explanation was too simple. I often get lectured about that but I believe the KISS theory much more effective than the complicated gobbledygook scientists use.
I’m still trying to work out how long wave radiation can produce a higher temperature than short wave radiation.
Bob_FJ
I linked to this image earlier in the thread and it might be worth repeating now: http://homepage.mac.com/williseschenbach/trenberth_mine_latest_big.jpg
It is a slightly more detailed version of the Trenberth diagram. For people who understand the original diagram, this one helps show a bit more detail by splitting the atmosphere into an upper layer and a lower layer. For those who don’t understand the original, it only makes things more confusing.
Bob FJ
“Yes, indeed, and I was wondering at one stage if I should include an observation in the article that if the TOA could be treated as a surface, (which is a tad conceptual), then the idea of surface integration at “that level” might be applied.”
To quote the silly movie, Night Shift, “I am a Big Picture, kinda guy.”
I don’t do complicated though, so I keep it as simple as possible, which is a challenge in atmospheric physics.
This article, http://redneckphysics.blogspot.com/2011/10/330-watt-weirdness.html could use a little better grasp of the Queen’s English. You may find it humorous and enlightening once you go back to the basics then work toward the true relationships. Besides the dig that Lindzen and K.Kimoto seems to be playing on K&T, the Arrhenius relationship based on perfect blackbody is illuminating.
Feel free to edit as you wish.
I am working on some Tropopause posts that explain the impact of the inversion more clearly. Fun stuff.
“”””” Hans says:
October 27, 2011 at 6:12 pm
George E. Smith; says:
October 26, 2011 at 11:11 pm
“”””” CRISP says:
You CANNOT transfer heat from a colder body (the upper atmosphere) to a hotter body (the lower atmosphere and Earth surface) without doing work. The 2nd Law of Thermodymanics avbsolutely forbids it. “””””
EM radiation knows absolutely nothing about either “heat” or “Temperature.”, and it can go wherever it darn well pleases .
—————————————————–
George I hope you don’t want to imply that the 2nd Law doesn’t hold for radiation, Planck and Einstein surely build on it when they dug into the BB-radiation problem. “””””
Well Hans, I just had a lengthy answer blown away, by an inadvertent key push.
so a shorter response.
1/ “Heat” as a noun or “heat” or “heating” as a verb has NO MEANING in the absence of real physical materials; atoms/molecules, because it is the kinetic energies (mechanical energy) of those atoms/molecules that defines what “heat” (noun) is. Absent, materials, NO HEAT !
Likewise “Temperature” has NO MEANING in the absence of atoms/molecules, since it is the mean kinetic energy per particle which defines Temperature.
2/ Radiation requires NO MATERIAL; it exists, and propagates, in the complete absence of atoms/molecules; which is why it is able to get to earth from the sun. “Heat” on the other hand CANNOT travel from the sun to the earth, because there is no material (atoms/molecules) to transport it.
3/ Radiation understands NOTHING about Temperature, so radiation has no way of knowing whether some destination (sink) has a higher or lower Temperature, than where it came from (source). So I’ll leave it up to you to explain, just how “Radiation” could possibly comply with the second law of Thermodynamics.
I would recommed to you the second law as phrased by Gustav Clausius; and probably preferrably in the original German.
“Thermal radiation” has nothing much to do with “heat” or “heating” , EXCEPT that the ORIGIN of thermal radiation, is the acceleration of electric charge; AS A DIRECT CONSEQUENCE of the interparticle collisions of a collection of particles that have a Temperature higher than zero Kelvins. All materials above absolute zero emit “Thermal radiation” or “Planckian ” radiation if you like, with a limiting (envelope) (continuum) spectrum given by the Planck Radiation formula.
4/ Note that the second law says nothing about ENERGY; just “heat” and remember “words have meaning”.
Readersa need to stop confusing the resonance lines/bands of molecular spectra, which are a consequence of, and characteristic of the particular molecule structure; with the quite separate continuum spectrum that arises (at the atomic and molecular level) solely due to the Temperature of those materials.
The 15 micron bending mode emissions of the atmospheric CO2 molecule don’t have anything to do with the Temperature of the CO2, other than the molecular collisions that arise as a consequence of that Temperature will alter the width of those spectral lines, due to Doppler and other effects.
The reason that SOLIDS and LIQUIDS appear to emit “Thermal Radiation” and not GASES, is simply that the atomic/molecular density numbers are such, that many more molecules are emitting in a given volume.
The reason that solids can absorb strongly and appear “black” at least in some spectral range, is there simply are more molecules to do the absorbing. A big enough volume of gas, eventually would look quite black too. So we don’t usually talk about gases as being good sources of near black body radiation, but each and every single gas molecule is emitting, and absorbing its fair share, according to the Planck formula.
For a particle Physicist, the cause is acceleration of electric charge (over some non zero distance or time interval) whereas the Radio-Physicist would simply say you need a varying electric current in some non zero length antenna. Well the physical antenna is superfluous, so long as the current can flow somehow (stream of charged particles). For our molecular sources; we would simply say, we need a non-zero electric dipole moment; and then we can emit or absorb to our heart’s content.
We get ENERGY from the sun (via radiation); we get NO HEAT from the sun; and we don’t need to because we can make all the heat we need right here on earth by simply wasting most of the energy we get from the sun. And remember ; WORDS HAVE MEANING, so use the right words.
On SW absorption in water. With a ;long enough path, it is absorbed. The more interesting part is suspended particulates at varing depths greatly impact the depth and degree of absorption. Woods Hole has quite a few interesting links. As a fisherman, this is something nice to know, in case you wonder why fish stocks and climate seem to correlate.
Smokey says:
October 28, 2011 at 3:14 am
Bob Fernley-Jones says:
“BTW everyone, may I suggest that regardless of what his relationship with Trenberth might be we should listen to R. Gates…”
Since R. Gates was actively trying to manipulate Anthony into a public meeting, I suspect a setup. Gates was doing what he was told, and I have no doubt that some kind of dog and pony show was planned to make Anthony look bad. Trenberth and Muller are two peas in a pod. Neither one can be trusted, and Gates is their water boy, even offering here to personally bus people from the airport to the meeting.
_____
This is so laughable as to be hardly worthy of a rebuttal, suffice to say that it indicates why perhaps you should consider a career as fiction writer…perhaps novels involving complex conspiracies. Rather than ‘manipulate” Anthony, I was trying to accomodate and act as a mediator or go-between to try and get these two men together. As far a “dog and pony” show being planned, Dr. Trenberth was asking exactly what we wanted him to talk about. Really Smokey, there are honest people in the world…get rid of your paranoia.
REPLY: I’ll have to back up R. Gates here. I saw no evidence of anything like that in his dealings with me. – Anthony
Tim Folkerts:
“So enlighten us, JAE.
What do you consider to be “the Atmospheric GHG theory” and in what specific ways is it “proven to be toast” ?”
Multiple lines of evidence. I’ve linked to this twice in this thread alone:
http://theendofthemystery.blogspot.com/2010/11/venus-no-greenhouse-effect.html
If it’s wrong, why do you keep ignoring it?
tonyb,
Do you agree or disagree with the trends and uncertainty shown in this chart:
http://www.appinsys.com/GlobalWarming/GW_Part1_HistoricalRecord_files/image010.gif
And if you agree, then how can you dispute the accelerated warming of the 20th century versus what we saw from 1600 to 1800? Seems the curve over the past century is far more steep than the previous 300 year average. It seems that temperatures starting warming in general about 1690-1700 (end of the Maunder), and continued a gradual warm-up, with a pause of course for the Dalton Minimum dip in the early 1800’s, which of course was the reason for the cold winters that influenced Dickens so greatly. At least to my eye, the warming accelerated in the later half of the 20th century.
In general of course (with some notable exceptions) , global temps have pretty much followed this curve of solar activity:
http://en.wikipedia.org/wiki/File:Sunspot_Numbers.png
With exceptions of course being during periods of strong volcanic activity, and also since about 1980, when global temps and solar activity begin to diverge greatly. I would welcome any evidence that shows conclusively that the divergence of global temperatures from solar activity since 1980 as not been caused, at least in part, by anthropogenic greenhouse gases.
Gates,
I stand by what I wrote. No one else but you tried to push Anthony and his supporters into a meeting controlled by Trenberth, with no agenda or rules. If it was naive innocence on your part I understand. But those of us who are wise to the ways of the world and human nature would naturally have deep reservations when someone with reason to be hostile invites them onto their home territory with no pre-agreed ground rules. Anthony was smart to decline under the circumstances.
Sorry, but I disagree strongly with much of what has been said about heat lately.
In classical thermodynamics, “heat” refers to the net flow of energy from one object to another due to a temperature difference. The 2nd law states that the net flow of energy is always from a warmer object to a cooler object. This applies to conduction and convection and radiation.
It is valuable to recognize that the modern definition is stated in terms of statistics and probabilities. There is a chance during any given time interval that the net flow is actually from cool to warm. But the larger the objects, the longer the time and the greater the temperature differences are, then the lower the probability is of heat going from cool to warm. If you look at objects with only a few atoms, or look for nanoseconds, or look at objects within microkelvins of each other, you might actually have a good chance of seeing a net transfer of energy from the warmer object to the cooler object
For typical objects, the probabilities are so vanishingly small of net energy flow from cool to warm, that we can conclude it never happens = 2nd Law.
This does NOT require that zero energy moves from the the cooler object to the warmer object. For example, if a 350 K coffee cup is placed in a 300 K room, the net flow of energy is going to be from warm coffee to cool air. However, individual air molecules could be traveling much faster than average, and when they hit the coffee, they could easily transfer energy FROM the cool air TO the warm coffee. There will simply be many more collisions each second that transfer energy TO the cool air FROM the warm coffee.
Same for photons. Photons from a 270 K atmosphere can and do transfer energy to 300 K ground. But the 2nd Law requires that MORE energy be transferred from the ground to the atmosphere, so there are more photons with more average energy moving from the warm ground to the cool air than vise versa. Yes — the 2nd law DOES apply to heating via radiation.
Even though the net flow of energy must be from warm ground to cool air, the cool air can still help keep the ground warm. The reason for this is simple. The warm ground would still radiate its ~ 395 W/m^2. Without an atmosphere radiating ~ 330 W/m^2 toward he ground, the ground would instead be receiving ~ 0 W/m^2 from the 3 K background radiation of outer space. Even though there is a net loss of energy to the atmosphere, there is a much SMALLER net loss than there would be for an IR transparent atmosphere. If we magically made the atmosphere transparent to IR, the surface would suddenly start cooling dramatically because it would be receiving ~ 330 W/m^2 less energy!
Ask why is it so? asks:
October 28, 2011 at 8:05 am
I’m still trying to work out how long wave radiation can produce a higher temperature than short wave radiation.
Try this – the long wave energy is absorbed in a few millimeters of water, but the short wave energy is absorbed over 100 meters. Assuming that each group of frequencies carries the same amount of energy, the energy absorbed in the smaller volume will produce the greater temperature rise. If this case several million to one.
In the case of a solid surface – the absorptivity is frequency based. As a result, many more long wave photons are absorbed than shortwave. This is one of the main failings of the KT diagram – it does not indicate that the reflected radiation has a different spectrum than the absorbed radiation.
Tim Folkerts says:
October 28, 2011 at 9:03 am
[This] is a slightly more detailed version of the Trenberth diagram.
http://homepage.mac.com/williseschenbach/trenberth_mine_latest_big.jpg
Please provide a reference. Was this created by Willis? That images significantly disagrees with how I understand the atmosphere. In particular, what does “Lowest Stratosphere” mean. In some sources (including the IPCC documents), that actually means the tropopause, in the others, it is the layer above the tropopause.
At any rate, that image makes no sense at all.
Tim Folkerts says:
October 28, 2011 at 12:56 pm
Sorry, but I disagree strongly with much of what has been said about heat lately.
Same for photons. Photons from a 270 K atmosphere can and do transfer energy to 300 K ground.
Mr. Folkerts please show me where in the this formula I can do what you say can or does happen.
W/m^2 = sigma*SB*(T1^4-T2^4)
If T1=T2 then the W/m^2 equal ZERO. If you can show me where ZERO equals some other number or amount please do.
Robert Clemenzi @ October 28, at 1:09 pm, & Tim Folkerts @ 9:03 am
I recollect that Willis launched his version over at Steve McIntyre’s site several years ago, based on K&T97.
I’m a bit surprised that he has not joined in, in this here.
I’m not a scientist, just an old Air Force photo interpreter with 20+ years’ experience. I’ve worked with about every kind of photography imaginable, from B&W, Color, false-color IR, regular IR, Radar, and a few others I can’t talk about. I’ve physically SEEN that the atmosphere isn’t equally transparent to all wavelengths of EMR. LOTS of different things can degrade reflectivity (photography being the recording of reflected energy, regardless of the type). You can sometimes see quite well through very thin clouds, other times those same types of clouds are virtually opaque. The resolution of IR imagery is directly affected by the ambient-environment temperature in which it’s being recorded – the greater the difference in temperature, the better the resolution. RADAR has no-return zones, either caused by blocking the radar energy from a given area (building “shadow”, etc.), or in the case of relatively calm water, the majority of the reflected energy isn’t returned to the recording site (the angle of incidence is equal to the angle of reflection. Visible light has a greater variety of angles of incidence than other types of imagery). Dust and smog can also affect any imagery negatively.
The atmosphere works the same way for solar radiation. Some of the radiation is allowed through, some of it’s blocked, some of it is absorbed or reflected away in odd angles. Since the atmosphere is a constantly-changing soup of elements, chemicals, and aerosols, some of which exist in more than one phase, I can see no way to develop a direct measurement of its’ overall transparency for any given location, much less for the earth as a whole. The best we can do is come up with what we in the business call a SWAG – a ‘Scientific’ Wild-A$$ Guess”. Even that would require taking a constant, highly-accurate, 24/7/365 measurement of the transparency (both ways – receiving and emitting) from multiple points on the earth to develop that. Until then, all of this is just an exercise in futility, proving nothing.
Ask why it is so? says:
I’m still trying to work out how long wave radiation can produce a higher temperature than short wave radiation.
Well, the short-wave radiation isn’t really changing, and the long-wave is increasing. This is based on the fact that different types of radiation are more readily trapped than others (in systems like Earth’s), at least until the system equilibriates.
The sun is radiating in shortwave. A general recipe for creating a greenhouse effect in something (in a car, a planet, whatever) is as follows:
Find a material that’s transparent at the incoming radiation frequency, but opaque to the outgoing radiation (the outgoing radiation being the blackbody radiation). When you add a layer of this material to your system, the incoming short-wave radiation will still get through, but now the outgoing longwave radiation will be absorbed by the covering layer, and some of it will be back-scattered/back-emitted. The heat will take longer to escape, so the average temperature will increase. In essence, you’re not changing how your system absorbs heat, but you’re slowing down how quickly it loses heat.. so it will heat up.
For Earth, water vapor is also an excellent greenhouse gas, and in the lower atmopshere its concentration is so much higher than that of CO2 that adding a little CO2 doesn’t change the absorption of long-wave radiation much. But at higher altitudes, the water vapor condenses out, and other greenhouse gases dominate the back-emission of long-wave radiation. And by slowing down the loss of heat through the stratosphere, the troposphere will also become warmer.. and thus the overall global warming effect.
Of course, we’re not breaking any laws of thermodynamics.. just moving our average temperature a bit closer to that of our heat source (the sun) and a bit further from that of our heat sink (the rest of the universe).
Mkelly requests:
Mr. Folkerts please show me where in the this formula I can do what you say can or does happen.
W/m^2 = sigma*SB*(T1^4-T2^4)
I’m not sure how it could be more obvious in the equation.
(Net Power/Area) = (Rate of heat from 1 to 2) / Area
= sigma*SB*(T1^4-T2^4)
= sigma*SB*(T1^4) – sigma*SB*(T2^4)
= (power/Area leaving 1) – (power/ Area arriving from 2)
The first half of the equation is the “radiative flux” leaving object 1. The second half is the “radiative flux” arriving from object 2. These are measures of the photons heading in and out.
If T1 > T2, more energy leaves Object 1 than arrives from Object 2
** ie heat flows from Object 1 to Object 2.
**ie more photons with higher energy flow from 1 to 2 (but some still flow from 2 to 1)
If T1 = T2, the same energy leaves Object 1 as arrives from Object 2
** ie no heat flows
If T1 < T2, less energy leaves Object 1 than arrives from Object 2
** ie heat flows from Object 2 to Object 1.
(This version of the equation requires both objects to have emissivity = 1 (which you seem to call “SB”). Also, either Object 1 and Object 2 should be large and flat, or Object 2 should be surrounding Object 1 and “A” would be the area of Object 1. For a more general version, see http://en.wikipedia.org/wiki/Thermal_radiation)
kuhnkat @ October 26, at 8:30 pm, you wrote:
The surface is a sphere. The adjoining points would NOT cancel. Surface irregularities will confuse this so that the surface is irradiating itself just as the surface of the water does. One of the requirements for S-B to apply is that the surface geometry does NOT allow it to irradiate itself. OOOPS!!!!
I doubt if this is a significant issue; for instance water that is wave affected only has a fairly momentary oopsy, because what was concave alternates with being convex, at which time, it will radiate to below the horizontal. (= radiate more than S-B). Also, on land, (even on collectively flat ground), vegetation might at first seem to be an issue, but broadly speaking it’s all in equilibrium. (Well, according to Trenberth anyway). Thus that vegetation will simultaneously radiate to below the horizontal in more or less isotropic fashion.
Smokey says:
October 28, 2011 at 12:26 pm
Gates,
I stand by what I wrote. No one else but you tried to push Anthony and his supporters into a meeting controlled by Trenberth, with no agenda or rules.
_____
Smokey, read Anthony’s last reply when I said you’d make a good fiction writer. You are off base on this in a serious way, so let it go. It was a meeting that was completely above board and it simply didn’t work out. No one tried to push anyone into anything, and the Dr. Trenberth was willing to talk about any climate issue we wanted him to, and answer Q & A. After BEST, Anthony (understandably) became a bit gun-shy. Can’t blame him, but that’s all there was to it. End of story.
Robert Clemenzi,
“David Socrates asks:
1. Does back radiation to the Earth’s surface occur at all or is it “unphysical”?
Yes it does. It is easy to measure it with an IR thermometer. It can be seen in lapse rate plots. Back radiation is what causes the morning temperature inversion over land.
2. If it does, what proportion of that back radiation is due to CO2?
That is the important question. No one knows. My analysis indicates that it is close to zero at the surface and about 100% in the stratosphere.
To all, the fact that the amount of energy toward space is less than the amount toward the surface is proof that the atmosphere is IR opaque over a significant part of the spectrum
”
In case someone else hasn’t answered this,
You are measuring the BRIGHTNESS of the atmosphere with your IR thermometer. It is most likely calibrated for an emissivity of greater than .95 which is too large for the atmosphere as it was NOT designed for atmospheric work. IF it has adjustable emissivity you should do some research and set it correctly.
Next it reads this brightness from about 7-14 microns. If you will look at the numerous charts of the atmospheric emissions you will find that this is primarily WINDOW where only water vapor has a small absorptivity and emissivity.
Additionally S-B is BUILT INTO the instrument. That is, the emissivity is used along with the temperature to do an S-B relationship to give you a reading. There is absolutely no direct reading of the actual IR emitted by CO2. So, when you claim you can tell how much DLR there is, you should understand that it just may not be quite as accurate as you are assuming. It is based on assumptions that your conditions may not meet.
Tim Folkerts.
“And following up on that point, the calculations you have are all for clear skies. If you model a cumulus cloud base, the numbers are much higher. For example, the first line for looking up from 10 m in a clear tropical sky was 348 W/m^2 in your table, but 418 W/m^2 when cloudy. Arctic winter goes from 163 W/m^2 to 243 W/m^2.”
As most IR from the ground is absorbed within the first 10m, I would also think that any IR from above, if emitted from over 10m up would have little chance of getting to the ground!!! As this area is where much of the thermalization of the IR occurs I keep wondering what the real numbers are at sea level and the surface of the ocean. At higher altitudes we are cooler as there is more radiation directly to space due to less atmosphere.
Anyone got some actual ground measurements? The gradient at the surface is apparently a lot higher due to the density of the atmosphere and humidity!!!
When absorbable photons (with the right frequency or in the correct waveband) are absorbed to extinction in the lower or near ground atmosphere (relatively) by CO2 and H20 molecules what happens to them? Do they become more ‘energetic’ ie with higher velocities and therefore higher KE which can be measured as an increase in temperature? The answer logically must be yes. The temperature reached must be less than the ground emitter to obtain a net flow of photons into this system. An increase in CO2 and H2O would reduce the depth of the absorption layer but not the number of first generation photons absorbed ie Q in the equation Q=M*Cp*dT would remain unchanged. Any change in dT would depend on the depth and composition of the absorption layer (M & Cp); but whatever the change, any increase in CO2 would have virtually no effect because it is an insignificant absorber when compared with H20. There is !00 times more H2O present and H2O’s absorption bands are much wider.
Tim Folkerts @ October 28, at 6:05 am, you wrote:
But actually, it would be considerably more difficult [for Trenberth] to draw “correctly”. He could have drawn an arrow down from high (altitude) clouds and from low clouds and from middle clouds and from high O3 and low H2O and middle CO2. But soon the diagram would have 100+ arrows distinguishing flows to and from land and ocean and clouds and CO2 and sun and ….
Well yes, but very easy to draw it partly right, rather than really daft. See my quick sketch. (ignoring the values)
http://bobfjones.wordpress.com/2011/10/29/quick-sketch-for-trenberth-cartoon/
Tim Folkerts,
“No. The IR comes from all directions, not just from one direction. There is no “night side” where IR from the atmosphere does not shine.”
You use a common dodge. How much thermal storage does a GHG molecule have?? How long can it emit without absorbing more IR??
Without the earth emitting IR as it cools and the non-GHG’s to collide with to gain energy the GHG’s would stop emitting within a second after sundown. Trying to explain a portion of an energy flow is misleading.
Further to mine just above, does anyone think it a bit odd that Trenberth shows 40 escaping directly to space passing through a window in high clouds. It would be very easy to draw so as to avoid the depicted clouds.
jae,
for a treatment of Venus that covers much of the misapprehensions and the actual DATA from the space missions John Ackerman’s paper is probably the best. The explanation of how it got so hot in the first place I would skip till later as it is speculation as everyone else’s ides. The rest on what is happening NOW probably comes closest to the reality of the planet.
http://www.firmament-chaos.com/papers/fvenuspaper.pdf
>>
Bob Fernley-Jones says:
October 28, 2011 at 5:12 pm
Further to mine just above, does anyone think it a bit odd that Trenberth shows 40 escaping directly to space passing through a window in high clouds. It would be very easy to draw so as to avoid the depicted clouds.
<<
It’s drawn the same in KT 97. I question the computation of that 40 W/m² window. But I’m the only one who seems bothered by it.
Jim
kuhnkat says: October 28, 2011 at 4:56 pm
“As most IR from the ground is absorbed within the first 10m, I would also think that any IR from above, if emitted from over 10m up would have little chance of getting to the ground!!!
IR within bands that are emitted & absorbed by CO2 and H2O may have traveled only short distances, but IR from outside those bands (for example, from the nearly BB radiation from clouds) can travel quite far. So at least SOME IR from high above the ground can reach the ground.
kuhnkat also says:
“You use a common dodge. How much thermal storage does a GHG molecule have?? How long can it emit without absorbing more IR??”
I’m really not sure what your concern is here. As you say in the next paragraph, the GHG’s can ‘recharge’ by either absorbing IR from the ground or by colliding with the other gases in the atmosphere. We agree on that point. Based on this, the IR from GHGs can continue to radiate all night long, which is all I was claiming.
Bob FJ
Your diagram with sloping arrows has some good points.
My concern is that – by trying to indicate a depth to the atmosphere – you now open yourself to all sorts of questions about how quickly the arrows should taper of and how high the clouds are and how energy is transferred within the atmosphere and how high convection goes and ….
Trenberth apparently chose to avoid those details in his diagram. You are welcome to include more details (like Willis did), but I don’t think that necessarily makes one “better” than the other — just different.
Bob Fj
“Further to mine just above, does anyone think it a bit odd that Trenberth shows 40 escaping directly to space passing through a window in high clouds. It would be very easy to draw so as to avoid the depicted clouds.”
I was reading a thesis on minimum local emissivity variances by David Taylor of UWM. There was 1RU ~ 20K unaccounted for in the Arctic that appeared to be absorbed by the atmosphere and not a calibration issue, i.e. missing in action. The Antacrtic flux readings are even more bizzare, +/- *0 Wm-2 in some areas. I believe the 40 is a don’t know indication. NASA shows the absorption and indicates atmospheric window radiation from the clouds as it should be, water and ice have a different spectrum.
About the only thing that can be learned from K&T is that they aren’t very sure what’s going on other than models are much more accurate determining 0.8Wm-2 net warming +/-0.18 CI 🙂
Windchaser,
“And by slowing down the loss of heat through the stratosphere, the troposphere will also become warmer.. and thus the overall global warming effect.”
And just how much slowing do you think extra CO2 causes, 1 second 2???? If it isn’t at least 24 hours…
Bob FJ,
“for instance water that is wave affected only has a fairly momentary oopsy, because what was concave alternates with being convex, at which time, it will radiate to below the horizontal. (= radiate more than S-B). Also, on land, (even on collectively flat ground), vegetation might at first seem to be an issue, but broadly speaking it’s all in equilibrium. (Well, according to Trenberth anyway). Thus that vegetation will simultaneously radiate to below the horizontal in more or less isotropic fashion”
For water there are always waves from small to large. The SB would be at worst there. Very small irregularities like cracks would cause small irregularities in SB. I haven’t seen any work on how much would cause a significant problem, but, my wag would be that Mountainous areas, water that isn’t very calm, and vegetation would not provide surface areas that would allow reasonable SB computations. The crude experiments where Solar Cookers are used to freeze water at night shows why this could be an issue. When surface areas radiate themselves there is a much larger energy flux than when just the atmosphere is at work. The water won’t freeze. When surface irregularities are blocked from irradiating the cookers view of a clear sky, water can be frozen at an air temp of 40F. Clouds also can interfere, but, not as much as trees, buildings…
We are told that DLR slows cooling of the surface. These crude experiments show that surface roughness can slow the cooling of the surface!!!!! As you point out there would appear to be more radiation in a horizontal mode. This would be an important issue here.
Do you know if there are computations where the Planck Equations have been used to estimate the actual emissions from the atmosphere or their absorption?? SB simply doesn’t apply and the Spectra simply doesn’t provide data as to where radiation is actually coming an going. Only that there are “holes” at the top and the bottom. It doesn’t tell us if that radiation has been frequency shifted by collision and emission.
The horizon issues are in reference to your geometry picture mostly. As anything but straight up is almost certainly to be reabsorbed the IR has a second (or 3,4,5,6…) chance to be transferred thru collision or emitted to the ground. I have issues as to whether much ever gets thru the dense surface layer to hit the ground, but, that is for another day.
Tim Folkerts,
“IR within bands that are emitted & absorbed by CO2 and H2O may have traveled only short distances, but IR from outside those bands (for example, from the nearly BB radiation from clouds) can travel quite far. So at least SOME IR from high above the ground can reach the ground.”
SOME IR is NOT ~320w/m2.
“As you say in the next paragraph, the GHG’s can ‘recharge’ by either absorbing IR from the ground or by colliding with the other gases in the atmosphere. We agree on that point. Based on this, the IR from GHGs can continue to radiate all night long, which is all I was claiming.”
Thank you. Many people seem to think that GHG’s somehow BLOCK IR and RETAIN it in the atmosphere or HEAT the earth with it. I simply wish to make sure the flow is understood and that it never stops. A continuous COOLING except when an energy supply is present.
I apologize for the snarky comment.
kuhnkat says:
October 28, 2011 at 4:39 pm
You are measuring the BRIGHTNESS
That is a good point. I interpret the readings as “number of photons”, more or less.That way I don’t really care about the emissivity. On a clear day, it might read -20F, but on a cloudy day, about 40F. This clearly shows that more photons come from clouds than from a clear sky. When pointed at the Earth, the “temperature” can also be interpreted as a number of photons.
The point is that a simple $10 tool can demonstrate the reality of back radiation. But kuhnkat is correct – it is wrong to interpret the reading as a “true temperature”, but I think is is still a good representation of an “effective temperature” – the temperature of a blackbody emitting the same number of photons that the atmosphere is currently emitting.
it reads this brightness from about 7-14 microns
Unfortunately, I have no way to determine the tool’s frequency range. I agree that this is one area that could (should) cause major problems. Thanks for pointing it out.
In my October 28, at 2:44 pm, I wrote:
Robert Clemenzi @ October 28, at 1:09 pm, & Tim Folkerts @ 9:03 am
I recollect that Willis launched his version over at Steve McIntyre’s site several years ago, based on K&T97.
I’m a bit surprised that he has not joined in, in this here.
Well here it is; the Willis-wisdom from January 2008: Energy Balance at the Tropopause, at CA:
http://climateaudit.org/2008/01/10/energy-balance-at-the-tropopause/
If you have the energy to go through all the comments, you may recognise some commenters that have also appeared here.
In my previous life, under a “nom de blog” of Black Wallaby, (indicating Oz heritage), I also appeared there, but not as Bob_FJ.
Back in those days Willis Eschenbach, when he constructed his “improved” Trenberth thingy, he was obviously still learning on some of the physics. For instance, how about this absolute gem of his:
Honest; me not joking; that is what he actually wrote…… (and with some repetition), read through the 2008 thread comments if you don’t believe me.
kuhnkat @ October 28, at 8:32 pm
Thank you for your comments, but I need time to think on some of them, and will get back to you later
RE: Bill Illis October 28, 2011 at 7:10 am
Michel says:
October 28, 2011 at 1:08 am
The major air components O2, N2, and Ar don’t absorb in the IR range.
———————–
Sure they do.
About 0.00000000015 seconds after a CO2, H2O, or CH4 molecule absorbs a photon in the IR range.
_______________________________
Bill,
Sorry, you can’t be right, or you would have to show an absorption spectrum in the IR range for those molecules (O2, N2, Ar).
But you are also right: the consequence of absorbing IR radiations is a temperature increase, (energy absorbed divided by the heat capacity = delta T).
This heat is dissipated within the whole air mass by conduction (and later convection).
This is the mechanism explaining “forcing”.
And this is approx. 3.7 W m-2 for each doubling of CO2 concentration (it’s a logarithmic scale):
– from 280 to 560 ppm: 3.7 W m-2
– we are now at 391 ppm: therefore the forcing is at about 1.8 W m-2 as compared to the alleged historic value of 280 ppm
– from 391 to 782 ppm: another 3.7 W m-2
So, let’s not panic for these small and slow, nonetheless existing, effects.
Tim Faulkner says:
“Trenberth apparently chose to avoid those details in his diagram. You are welcome to include more details (like Willis did), but I don’t think that necessarily makes one “better” than the other — just different.”
I have to disagree. It is not just another way to portray the energy budget. Since radiation in a gas can emit in any direction in a full spherical manner, Trenberth, you I assume and others like to view it as ½ traveling upward, 396 Wm-2 from a 16 °C average surface and then there’s the 333 Wm-2 radiation raining down from the sky blue. But, under those conditions there must be 666 Wm-2 “up there” radiating in all directions ½, or 333 Wm-2, returning to keep the surface warm at 16 °C as people who believe in back-radiation see it.
Didn’t know before tonight that you have a PhD in physics so maybe you can clarify for us here just where this 666 Wm-2 (kind of prophetic) is located vertically in the sky and why there is not the other half 333 Wm-2 radiating upward to space plus the 396 Wm-2 from the surface. (seems it is you that said radiation “knows nothing of it’s surrounding matter”).
You talk like you know physics, so, don’t you see why so many rightfully have problems with Trenberth’s presentation of the IR portion? And that is just one aspect. Bob hit another one on the head in his article.
kuhnkat says:
October 28, 2011 at 4:39 pm
“2. If it does, what proportion of that back radiation is due to CO2?
That is the important question. No one knows. My analysis indicates that it is close to zero at the surface and about 100% in the stratosphere.”
kuhnkat if I correctly understand gases do radiate only in the same bands that they receive, only if the temperature is high enough. This would mean the CO2 from the stratosphere will radiate in the lower CO2 bandwidths, not the higher ones, and the radiation will be intercepted by CO2 about 10 meters below and above it. All this radiation in both directions is still net heat transfer from warm to cold.
Michel says:
October 29, 2011 at 1:43 am
N2, O2 etc do absorb a little infrared, if you have ever used infrared spectroscopy before, the background absorbance reading is around 10 percent when none of the main greenhouse gases cover this band. (H2O, CO2, CH4, or O3 etc) This is down to the main gases in the air, nitrogen and oxygen. (only small yes, but still there.
Bob Fernley-Jones says:
October 28, 2011 at 11:13 pm
Well here it is; the Willis-wisdom from January 2008: Energy Balance at the Tropopause, at CA:
http://climateaudit.org/2008/01/10/energy-balance-at-the-tropopause/
Thanks, but I don’t see it there. Perhaps you are referring to greenhouse.bmp, which is not found. The earliest example I have found is
http://wattsupwiththat.com/2010/03/16/another-look-at-climate-sensitivity/
At any rate, it appears that Willis invented that image himself. In my opinion, it is wrong at many levels. First, the water in the troposphere emits a significant amount of energy directly to space. The proof is the increasing emissions from 400 to 600 cm-1 in figure 5 above. Next, the peak in the CO2 emissions to space (also in figure 5) comes from above the stratopause. In addition, there are no significant atmosphere to atmosphere photons that cross the tropopause. In addition, there is a significant energy transfer from the stratosphere it the tropopause.
I don’t see any allowance in the charts for the nuclear furnace beneath our feet. Something other than the sun is heating mines of 1,000 feet to 120F degrees and more. Magma isn’t being kept fluid by the sun or gravity. Seems it should be at least a few percent, and should show up in measurements and models if we think they are accurate to 10ths of a percent.
There seem to be lots of flaws in Trenberth’s diagram. The most obvious to me is designating all the downward LW radiation received at the surface as ‘back radiation’. This is highly misleading, as downward LW has three potential sources and only a fraction of it is ‘back radiation’ (that which last originated surface emitted LW). The diagram makes it look like of the 396 W/m^2 emitted from the surface, 333 W/m^2 is coming back from the atmosphere, which is incorrect.
He also designates 78 W/m^2 of the post albedo is being ‘absorbed by the atmosphere’ and then brings this to the surface as part of the 333 W/m^2 designated as ‘back radiation’. This portion isn’t not ‘back radiation’ but ‘forward radiation’ from the Sun yet to reach the surface (key distinction).
Also, has anyone else noticed that he returns all the non-radiative flux from the surface to the atmosphere (latent heat and thermals) in the form of downward LW lumped in as part of the 333 W/m^2 designated as ‘back radiation’? What then is the source of the energy in the temperature component of precipitation? It’s not there.
Robert Clemenzi @ October 29, at 8:47 am
Sorry, I did not check the links, but I can assure you that the Willis version was there, back in 2008. He remains proud of it, because he cited it again last August in his article here; “Radiating the Oceans”:
http://wattsupwiththat.com/2011/08/15/radiating-the-ocean/#more-45114
Somewhere in the over 900 comments. He sure does generate debate!
HEAT: Semantic misconceptions
Here is an extract from Wikipedia under that heading:
http://en.wikipedia.org/wiki/Heat
My recollection is that physicists, not long ago, were quite happy to define HEAT at the quantum level as the state of kinetic energy within matter. Nowadays it seems that they prefer to consider it as a transfer of thermal energy from A to B.
The latter terminology is in conflict with engineering, where there is a whole field on “Heat Transfer”. In engineering, the heat content of matter can be calculated for any constant temperature; it is not necessarily a transient condition.
I think the sensible solution to the semantics issue is to consider the context of the use of the word.
Ari Tai says: October 29, 2011 at 12:24 pm
I don’t see any allowance in the charts for the nuclear furnace beneath our feet.
Estimates I have seen are considerably less than 1 W/m^2 for geothermal energy flow. This is pretty small compared to most other energy flows.
kuhnkat @ October 28, at 8:32 pm
I can’t really answer your questions, but would think that it is a case of “swings and roundabouts” and that the real world has an equivalence that is near enough to a flat surface for it to not matter within the scale of other uncertainties. Whatever, Trenberth also seems to assume that, and I would say it is the least of the problems in his diagram. (e.g. his emissivity = 1)
One of the things that have for a long time irritated me is the CAGW talk of significant albedo positive feedback when sea-ice melts and exposes “highly absorbent” water. (NO, I’m not going off-topic). Yet, it is well known that when the sun is low in the sky, as in the polar-regions, that specular reflection from water is high, and about the same as old snow, atop sea-ice. This led me, in connection with your issue, to wonder if there is specular reflection of IR at shallow angles on water. However, it seems to be a hard question, and I suspect that water would give close to Lambertian/ black body reflection/emission. Whatever the answer is, a lot of the self-absorbing issue that you raise would be minimised by the generally shallow angles involved.
Here is a simple description of Lambertian surfaces etc, from the perspective of optics. You can see the effect of shallow angles (and vectors) at the far right of figure 1. Caution: if you search around on the topic, you’ll find that some physicists seem to get over-excited by it, if you know what I mean.
http://escience.anu.edu.au/lecture/cg/Illumination/lambertCosineLaw.en.html
Robert Clemenzi,
your simple $10 tool is not measuring what you think it is. It is not measuring the potential energy to be transferred. It isn’t even measuring the number of photons as you claim. The magnitude it measures is adjusted based on preset assumptions of emissivity of the object which you apparently haven’t checked.
I am glad that you have at least been awakened to the issue of the frequencies. There are a number of good manuals by manufacturers and other info on the net that explains how the instruments work and their limitations.
As mentioned above, NOTHING is a perfect Black Body with an emissivity of 1. The oceans and ice can be the closest with, I think, about .99 depending on their condition. The surface ranges from about .91-98. The atmosphere will also depend on the actual composition, but, I believe it is closer to .8 than .9. That may be something for you to research. I think I remember CO2 as about .16 in our atmosphere, but, like the surface, all the gases and aerosols contribute.
Basically, if you have the $10 unit it is designed to gather IR from up to about 1o ft from surfaces with an emissivity of about .98. I haven’t read what issues the distance would cause so can’t suggest anything there. The incorrect emmissivity will cause a reading higher than it should be.
The expensive instruments used by the big boys have adjustable emissivity. I would hope they have correctly computed the emissivity when they take their readings, except, the emissivity will depend on the humidity, clouds and aerosols and who has all that data?? They would need a massive instrumentation, satellite msu and/or sondes to even get in the ballpark.
Yeah, I don’t trust anybody!!!! 8>)
Robert Stevenson,
Pekka Parilla over at Climate Etc. tells us that the time to collision is shorter than time to emission for GHG’s in the lower atmosphere. That and the multidirection emission when it does happen seems to be telling us that less IR would be going to the ground than the energy chart indicates. A smaller portion will get to the ground based on geometry. More energy is transferred by collision than gets emitted. The collisional energy will typically be convected. I don’t see how they can come up with those numbers. Emissions from higher up have a low probability of getting through the dense surface layer just like ground emissions are unlikely to get directly to the upper trop or TOA, yet, somehow we are to believe that there is almost as much radiation being absorbed by the ground as the atmosphere even though it would appear to be substantially biased up.
Bob F-J,
thank you for the link.
I tend to think you may be right with the reflection issue in water unless the swells are large, which happens reasonably often. I had forgotten to consider the low angles and reflectivity. With the ground there is less reflectivity with most surfaces. We also have a lot more vertical roughness.
Of course, with the ocean, it would seem that pretty much all the DLR goes back up with evaporation anyway.
Tim Folkerts @ October 28, at 6:19 pm, you wrote:
Bob FJ, Your diagram with sloping arrows has some good points.
My concern is that – by trying to indicate a depth to the atmosphere – you now open yourself to all sorts of questions about how quickly the arrows should taper [off] and how high the clouds are and how energy is transferred within the atmosphere and how high convection goes and ….
NO, definitely NO, it is not my problem, but a problem for Trenberth et al and his IPCC collusionists.
R. Gates @ October 26, at 5:46 pm, you early-on wrote in part, concerning my article:
”There is lot’s to chew on here…”
I appreciate that since then you have probably been distracted by some rather hostile insinuations against your character/personality, but I would like you to put that aside and offer your views on my article.
Please.
Bob says:
“One of the things that have for a long time irritated me is the CAGW talk of significant albedo positive feedback when sea-ice melts and exposes “highly absorbent” water. (NO, I’m not going off-topic). Yet, it is well known that when the sun is low in the sky, as in the polar-regions, that specular reflection from water is high, and about the same as old snow, atop sea-ice. “
I don;t think this is as much of a problem as you might think for two reasons.
1) Snow typically reflects at least 60 % of light (http://en.wikipedia.org/wiki/File:Albedo-e_hg.svg). To get that sort of value for water, the light would have to hit at 85+ degrees from the normal (5 degrees from the horizontal) (http://en.wikipedia.org/wiki/File:Water_reflectivity.jpg). Much of the arctic ocean for much of the summer has the sun considerably higher than 5 degrees.
2) If the water is not perfectly smooth, the average angle if incidence will decrease (ie the water will hit more directly) leading to a lower reflection. The “upslope” of the wave facing the sun will block the light from the “downslope” on the other side of the wave. If the sun is 5 degrees above the horizon, but the wave slopes up at 10 degrees, the light hits at a 15 degree angle on that part of the wave. This will lower the reflectivity for that sunlight from 70% to 20%.
kuhnkat says: October 29, 2011 at 10:02 pm
Of course, with the ocean, it would seem that pretty much all the DLR goes back up with evaporation anyway.
I disagree. For starters, there is ~ 330 W/m^2 average DLR, but only ~ 80 W/m^2 goes up as evaporation. So no more than ~ 25 % of the DLR could go up as evaporation. The vast majority of the DLR must go up as “ULR”.
And of course, at least SOME of the evaporation is driven directly by sunlight, so less than 80 W/m^2 of the evaporation is due to DLR.
Bob says:
“… Yet, it is well known that when the sun is low in the sky, as in the polar-regions, that specular reflection from water is high, and about the same as old snow, atop sea-ice. “
Bob, see of MODIS and CERES. more precise than Tim’s logic:
NASA: Sea Ice and Snow Change, but Reflection Remains the Same
http://earthobservatory.nasa.gov/Features/ArcticReflector/arctic_reflector4.php
If anyone is worried of decreases in albedo with sea-ice melting at the poles, they need not, so far this effect cannot be found by the satellites of any significant amount, clouds compensate.
Tim Folkerts @ October 30, at 10:47 am
I did not want to go off topic, so I’ll be brief.
1) If you look around, you should find a variety of albedo’s and incidence angles.
2) If the wave directions are “pointing at the sun”, part of them will absorb more, and part less. (another case of swings and roundabouts)
Wayne @ October 30, at 11:59 am
Thanks for the link Wayne…… very interesting; must study.
Dear Moderator,
I’m puzzled and disappointed as to why Willis Eschenbach, has had nothing to say on this thread, since it touches something close to his heart; his own version of the Trenberth diagram. It might be something to do with this:
http://wattsupwiththat.com/2011/08/15/radiating-the-ocean/#comment-727406
But whatever, please contact him and make sure that he is aware of this thread. I would appreciate his input.
BTW, a gerbil, as he described me, I discovered is a mouse-like creature not native to my homeland of Oz.
Tim Folkerts,
No problem Tim, just my being very imprecise. I was trying to refer to the issue that little if any warming is from top down as it all goes back up one way or another.
My main thrust was that the objects on the surface irradiate themselves. Our host was pointing out that the reflection would prevent most of that in the case of the ocean and waves!!
What is your opinion of the effects of a rough surface causing self irradiation as another source of reduction of cooling?? Isn’t this why SB isn’t as accurate with a self irradiating geometry?? It would seem to be with solar cookers freezing water. When exposed to the sky only they can freeze water at higher air temps. When there is a wall, tree, or other object in their “view” it doesn’t work as well indicating significant radiation from solid objects!!
So, how is this included in the energy balance cartoons?? It isn’t. Only the DLR from GHG’s are which makes me think the effect is at least a little overestimated!!
Leif Svalgaard says:
October 28, 2011 at 7:12 am
Myrrh says:
October 28, 2011 at 5:36 am
http://hyperphysics.phy-astr.gsu.edu/hbase/chemical/watabs.html
Your own link shows that the absorption of visible light by water is a million times weaker than that of infrared, but that only means that you need a layer of water a million times as thick, i.e 100 meters instead of a fraction of a millimeter.
?! And I thought my maths was bad, but the logic fail here.. 🙂 Water is a transparent medium for visible light, that is bog standard physics, well known knowledge used in countless industries, that chart shows it. It doesn’t absorb visible, not when there’s a millimetre of water or when there’s ten thousand feet of it. If there’s something happening at some points of zilch significance in the different visible wavelengths it doesn’t alter the basic mechanism which makes water a transparent medium for visible light, transparent means it is not absorbed, it cannot therefore be all absorbed the greater depth of water. Not absorbed means that is can’t heat water.
The AGWSF claim is that visible light, shortwaves, ‘is the energy direct from the Sun which is heating the Earth’s land and oceans’. This graphic shows just how utterly stupid the claim. And I’m supposed to be impressed by your reading of the information and the reading of other scientists like you priding themselves on their great scientific credentials? When you don’t even know what transparent means?? Transparent means the energy doesn’t have the mechanism to heat the medium, it is transmitted through without being absorbed.
From the link:
“Transparency of Water in the Visible Range
Water is strongly absorbing at most of the wavelengths in the electromagnetic spectrum, but it has a narrow window of transparency which includes the visible spectrum. The span of the absorption spectrum shown is from wavelengths on the order of a kilometer down to about the size of a proton, about 10-15 meters. It doesn’t absorb in the wavelength range of visible light, roughly 400-700 nm, because there is no physical mechanism which produces transitions in that region – it is too energetic for the vibrations of the water molecule and below the energies needed to cause electronic transitions.”
The water molecule keeps the tiddly visible out. Which words in the analysis of the information given by Georgia uni are you having a problem understanding in those I’ve bolded?
but? it? has? a? narrow? window? of? transparency? which? includes? the? visible? spectrum?
Since the oceans are more than 100 meters deep they will absorb all the visible light falling on them. The mechanism involves overtones of vibrational stretching as you have been shown many times.
So what changes the water to being not transparent the deeper it is..?
It? doesn’t? absorb? in? the? wavelength? range? of? visible? light?, roughly 400-700 nm?, because? there? is? no? physical? mechanism? which? produces? transitions? in? that? region?
What I’ve shown you many times is that vibrational stretching is not something that electronic transmissions can effect since visible works on the electronic transition levels of electrons.
Reflection/scattering in the atmosphere is the molecules of nitrogen and oxygen getting rid of piddling visible energy.
It takes the oomph of real heat energy, the invisible thermal infrared energy direct from the Sun, to heat land and oceans, to vibrate the whole molecules. Water is greatly absorbing in the thermal infrared because thermal can move whole atoms and molecules into vibration which is what it takes to heat matter.
Thermal infrared, heat, moves molecules into vibration, this is heat.
Visible can move electrons but doesn’t have the power to move whole molecules into vibration.
Visible is absorbed by the electrons of the molecules of oxygen and nitrogen in our atmosphere, and gets reflected back out by this, blue is more easily scattered so we have a blue sky.
Visible is being bounced all over the place like a ball in a pin ball machine, it doesn’t have the oomph to move the whole molecule, instead the molecule kicks it around all over the sky just with its electrons.
The AGWSF claim that the atmosphere is transparent to visible is another false, science fiction, meme introduced to confuse real physics as if real fact. How much is it heating the fluid gaseous air atmosphere above us since you claim that all absorption is directly creating heat and reflection/scattering is by nitrogen and oxygen electrons absorbing it?? Where is this in your ‘energy budget’? Logic fail. Science incompetance.
Water is really transparent to visible, (not like your fake greenhouse cartoon claims for visible in the atmosphere). Water is really transparent to visible Because there is no physical mechanism which produces transitions in that region.
Whatever you think you’re saying about vibrational stretching, it’s gobbledegook. Vibrational stretching is the movement of whole molecules which infrared can effect, visible’s limitations to electronic transitions can’t do this.
Either you have misunderstood it or you’re deliberately muddying the waters here. I don’t much care which, either way, you show yourself promoting a science fiction world where visible light has been given the properties of thermal infrared which is the only real heat energy direct from the Sun, the Sun’s real invisible thermal energy direct to us which heat we feel; we cannot feel visible light, it is not thermal. I have tended to give you credit for your mangling of the real facts as being deliberate because a great scientist and all that, but perhaps it’s just because you have no idea what you’re talking about..
… perhaps you’re simply out of your depth…
Again, the different mechanisms between Visible and Thermal Infrared, visible doesn’t do molecular vibration.
http://en.wikipedia.org/wiki/Transparency_and_translucency
“Mechanisms of selective light wave absorption include:
Electronic: Transitions in electron energy levels within the atom (e.g., pigments). These transitions are typically in the ultraviolet (UV) and/or visible portions of the spectrum.
Vibrational: Resonance in atomic/molecular vibrational modes. These transitions are typically in the infrared portion of the spectrum.”
Ask why is it so? says:
October 28, 2011 at 8:05 am
Myrrh, thank you for your reply however I think you seem confused. I was talking about radiation not whether it is visible or invisible. The 2nd law of thermodynamics is often brought up to prove that CO2 cannot cause warming because heat does not travel from cold to hot, and the surface of the earth being hotter would not receive the heat CO2 apparently sends back to the surface to cause global warming. If it were heat, this would be true, however, radiation unlike heat can travel in any direction including back down to the surface. I believe it is important for it to be understood that heat is controlled by the Laws of Thermodynamics, radiation isn’t. I’m sorry if my explanation was too simple. I often get lectured about that but I believe the KISS theory much more effective than the complicated gobbledygook scientists use.
I’m still trying to work out how long wave radiation can produce a higher temperature than short wave radiation.
I don’t have the time to go into this further with you here, sorry, I’ll just take your last sentence.
Longwave radiation is thermal, it is heat on the move, it is invisible. It is the heat you feel from the Sun. You cannot feel visible light, it is not hot. It is the heat in the Sun, the thermal energy of the Sun, that creates the visible. The visible light from the Sun is the product of the Sun’s great thermal energy, visible is not that energy. That great thermal energy of the Sun is what travels to us at the speed of light and reaches us direct on the surface of the Earth; we feel its heat, it is invisible. It heats us from the inside because water is the very great absorber of thermal energy, and we are mostly water, (and around 20% carbon). Visible light cannot heat water, water is really transparent to visible light, which means that the water molecule does not absorb visible ligh; visible light passes through water without being absorbed, this is called transmission.
See the link in my last post (to Leif), there are basic differences in how visible and thermal infrared, which is heat, work on meeting matter. Visible’s electronic transitions is interaction with the electrons, the visible light is too small to do even much here, as I explained, in the atmosphere the molecules of oxygen and nitrogen absorb visible in their electrons and bounce it back out again. This is the second possible way that electrons affect and are affected by matter in electronic transitions as described in this section on that page, the third possible is transmission through a transparent medium, when the energy is not absorbed.
I have extracted the information about these differences in another discussion here in this post: http://wattsupwiththat.com/2011/10/18/replicating-al-gores-climate-101-video-experiment-shows-that-his-high-school-physics-could-never-work-as-advertised/#comment-778960
Visible light cannot heat land and oceans, the invisible thermal energy of the Sun we receive directly here, heat from the Sun, can and does heat matter. Not only does water greatly absorb this heat direct from the Sun, but it has a very great capacity to store it, which is its heat capacity. Land also absorbs heat direct from the Sun, thermal infrared, but has a lower heat capacity than water, so it warms up more quicky but releases it quickly. This is how our weather comes to be on Earth, from the different temperature gradients created by this, and so we have winds, which is volumes of the fluid gas atmosphere on the move, as a heated volume of air rises and is displaced by the volume of cold air above it. The smaller energy of visible light is used in many other ways; we see the world by it, all the colours and so forms and it creates sugars, not heat, in photosynthesis, and so on.
Confusing by deliberately taking out the scale and property differences between these energies by saying they are all the same energy is giving the false impression that ‘highly energetic’ means greater power, it just means that the more highly energetic the smaller, gamma billions of times smaller than radio..
All these wavelengths are distinctly different from each other, they have their own properties distinct from each other, affect matter in different ways. There are also category distinctions, Heat and Light are the basic ones in the differences between ‘shortwave visible and invisible from the Sun around the Visible wavelength’, i.e. Solar, which includes near infrared which is not thermal, and the category Heat, which is the thermal energy from the Sun on the move, the invisible longer waves of thermal infrared. Light is not Heat, Light is not thermal, we do not feel it as heat.
It is utterly disgraceful that this deliberate change of giving visible light the properties of the thermal invisible infrared has been introduced into education to support the fake AGW money grab scheme. That all these discussions and arguments between those believing in AGW and those not are using this faked property in their posts is sure indication that we are seeing those who have walked through the looking glass with Alice, where you can believe all kinds of impossible things, create all kinds of fictional worlds.
Visible light heating land and oceans is a science fiction world, it is not reality here, where I am, where real world traditional physics contradicts it..
The equation for the interchange of radiant heat between air at temp Tg containing CO2 at partial pressure Pc and the ground surface at temperature Ts through a distance L, per unit of surface, is:
Q = sigma x (aTs^4 – eTg^4)
where ‘e’ denotes gas or air emissivity and ‘a’ the gas or air absorptivity for black body radiation
from the surface at Ts.
Although the absorptivity equals its emissivity when Ts = Tg, a correction is made when ‘a’ for CO2 is evaluated as emissivity at Ts by multiplying the result by (Tg/Ts)^0.65.
As well as temperature ‘e’ for CO2 depends on product term PcL and the total pressure Pt (for the lower atmosphere this can be taken as 1atm).
Equating the absorption by CO2 of land IR ( Sigma x aTs^4) to the energy absorbable by the CO2 wavebands, gives a value of L (for Pc 0.0004 atm) of not less than 2000 m.
Repeating the calc for atmospheric water vapour glves a value for L of 120 m.
Myrrh, if water is transparent, then why can’t we see through clouds? Why is the sky clearer on a cold night than on a warm night?
I assume that you realize that sea water has many salts dissolved in it. Those salts provide the additional absorption. As a result, as every diver knows, the oceans do absorb visible light. Whether it is the water, or the stuff dissolved in it, does not matter – the light is still absorbed.
On the other hand, you claim that “Visible light cannot heat land and oceans” has some merit because most of the surface albedo is in the visible part of the spectrum. Thus, the reason we do not feel visible light as heat is simply because our bodies reflect it. This is why most of the heat we feel is from the infrared. However, when a pigment (or the ocean) absorbs a visible photon, that energy is eventually converted into heat. In the case of leaves, the light is first converted into sugar, but even that will eventually oxidize and produce heat at sometime in the future.
In the case of UV in the stratosphere, the photons are not directly converted to heat. Instead, they break molecular bonds and create free radicals. When the free radicals take part in various reactions, the stored energy is converted to heat. As a result, the temperature of the stratosphere increases with height.
Robert Stevenson says:
October 31, 2011 at 8:20 am
Nice post Robert. Emissivity of an H2O-CO2 combination is always an important question. Hottel from memory had CO2 as very low on emissivity and always lowered emissivity of H2O when in combination.
Where is you get this, (Tg/Ts)^0.65, from?
Robert Stevenson,
please excuse my ignorance, but, wouldn’t you need to combine these two calculations in some way to give a net height for absorption as water vapor and CO2 absorptive wave lengths overlap?? Or am I completely misunderstanding what you are stating??
Robert Clemenzi says:
October 31, 2011 at 9:14 am
Myrrh, if water is transparent, then why can’t we see through clouds? Why is the sky clearer on a cold night than on a warm night? etc.
Good grief. I’m arguing about the stupid fictional science of the ‘energy budget’ you’re all working to, where shortwave is falsely credited the heating mechanism for all land and oceans, having been given the properties of thermal infrared which is the real heat from the Sun and which we can all feel as the thermal energy of the Sun, which real heat energy you have excluded from your ‘energy budget’ saying it doesn’t even reach us even though we can feel it.., and, make the effort to give you real world physics on the difference in scale between these two energies of the sun, which shows that on visible scale which works to electronic transitions it is not possible to heat the gazzillions of gallons of water on our planet, while thermal infrared which is the real thermal energy from the Sun can and does, because electronic transitions cannot move even one molecule of water into vibration and is not absorbed which is what makes water transparent, while thermal infrared heats exactly by this doing this, its absorbed energy moving the molecule into vibration, and you widdle off about why you can’t see through clouds so how is it transparent..?
I’m talking here about the fact that the very basic physics premises have been changed in support of AGW, that non thermal shortwave Solar energies have been given the properties of heat, which is actually the invisible thermal infrared. This is a complete, and obviously, efficient scrambling of basic physics, leaving all you and your arguments in a totally ludicrous fictional world created by this sleight of hand. You’ve been had. Light is not at thermal energy, it is not Heat, it cannot and does not heat matter. And your claim is that it heats all land and oceans!
Water is transparent to visible light because it is transparent, transparent in physics, real physics here, means that visible energy cannot, I repeat, cannot be absorbed, the volumes of the molecules of water keep visible light out. Water does not allow visible light to play with its electrons and the tiddly visible isn’t big and strong enough to move a molecule into vibration, so it is passed through. This is called transmission.
It’s what you generic claim in your fictional energy budget that the atmosphere is for visible light, but, in the real physics in the real world the volume of the fluid gaseous atmosphere is not transparent to visible, because visible on the electronic transition level appropriate to its size is absorbed by the electrons of the molecules of the nitrogen and oxygen, before being kicked out, this is what creates the reflection/scattering of visible light. Since you claim that all ‘absorption creates heat’ in your one dimensional reality, how much is that blue in the sky heating your atmosphere? I don’t see it mentioned on your energy budget cartoons.
Either take it out of your budget and put back thermal infrared direct from the Sun to Earth, or admit you’re all garbling nonsense arguments at each other about a science fictional world because you’ve changed the basic properties and processes of Heat and Light energies from the Sun.
Kuhnkat asks: “What is your opinion of the effects of a rough surface causing self irradiation as another source of reduction of cooling?? “
I can imagine several affects of surface roughness.
First of all, the area that should be used would be the area as seen from a point of interest. Or perhaps more precisely, it is related to the solid angle subtended by the various surfaces involved. So within a valley (or between large waves) the energy from the sky would be less and the energy from the ground (or water) would be larger. Hence frost not forming on the sides of cars facing a heated wall. However, once you are high enough that the horizon looks flat, then the surface topology should make less difference.
On another front, rough surfaces generally make the emissivity larger. See http://www.ib.cnea.gov.ar/~experim2/Cosas/omega/emisivity.htm for example. The extreme case would be a deep pits, which becomes an extremely effective black body (http://www.imagesfromhere.com/archives/Grate%20Work%206794.jpg). This might suggest that rough water should have a higher emissivity than smooth water. So it would be better at both emitting and absorbing light. There could a slight complication that water will form “whitecaps” when it gets rough. This could affect the emissivity.
“My main thrust was that the objects on the surface irradiate themselves.
True, but that also means that there is more surface area. If there are waves irradiating themselves, that means the waves have more surface area than the flat ocean.
Overall, I suspect the surface topography would have little affect on overall absorption or emission from he ocean.
Ask why is it so? says:
October 28, 2011 at 8:05 am
….The 2nd law of thermodynamics is often brought up to prove that CO2 cannot cause warming because heat does not travel from cold to hot, and the surface of the earth being hotter would not receive the heat CO2 apparently sends back to the surface to cause global warming. If it were heat, this would be true, however, radiation unlike heat can travel in any direction including back down to the surface. I believe it is important for it to be understood that heat is controlled by the Laws of Thermodynamics, radiation isn’t. I’m sorry if my explanation was too simple. I often get lectured about that but I believe the KISS theory much more effective than the complicated gobbledygook scientists use.
I’m still trying to work out how long wave radiation can produce a higher temperature than short wave radiation.
_____________________________________________________
The temperature depends on selective absorption. For example I have a livestock trailer, It is painted midnight blue (almost black) and white. When inside (painted uniform gray) I can tell by the difference in heat whether the paint on the outside is white or blue. At high noon the dark area is very warm to the touch while the white area is not.
That is one part (absorbed or reflected)
The second is the actual amount of energy in the photon. Depending on the wavelengths it might take two or three LW photons to equal the energy of an EUV photon.
Myrrh.. it hurts my brain to read your posts.
Visible can move electrons but doesn’t have the power to move whole molecules into vibration.
Visible has higher energy than infrared, so it most certainly does have “the power” to vibrate atoms.. visible light has too high of a frequency (and thus, the sign of the electric field switches too fast) for most atomic dipoles to react quickly enough, and this is what makes water more transparent to visible light. Electrons move at higher frequencies, so they can match with higher-frequency light than dipoles (atoms) can.
Water is really transparent to visible, (not like your fake greenhouse cartoon claims for visible in the atmosphere). Water is really transparent to visible Because there is no physical mechanism which produces transitions in that region.
Not true – 1 cm of water is about 99% transparent to deep-red visible light, and about 99.99% transparent to deep-blue visible light. Not purely transparent in either case. Absorption of visible light is mostly at the overtone frequencies of the stretch and/or bend frequencies… so the absorption is weaker, but definitely there.
Will you take wikipedia as a source? If not, I can provide papers from physicists.
http://en.wikipedia.org/wiki/Electromagnetic_absorption_by_water
Observe, the absorption in the visible spectrum is not zero.
You cannot feel visible light, it is not hot.
Want to bet? Humans are reasonably opaque to visible light, which means it gets absorbed, which means, yes, you can feel it if it’s strong enough. Try it! Go stand in front of a 10,000-Watt set of concert LED lights, with a few panes of glass in between to block the infrared and any convection, and you’ll see for yourself.
We tend to feel more infrared because there’s more of it around (from low-efficiency lighting and background radiation), not because it’s more energetic or more absorbed.
As Robert mentioned, there are other ways for high-energy light to interact with matter, even “transparent” matter, via ionization, scattering, conversion to chemical energy, etc.
Wayne says: “But, under those conditions there must be 666 Wm-2 “up there” radiating in all directions ½, or 333 Wm-2, returning to keep the surface warm at 16 °C as people who believe in back-radiation see it. ”
That is not quite how I would say it. The ~ 330 W/m^2 should be though of as the integrated affect of the atmosphere acting on a given square meter of the surface. It does not come from any specific cubic meter of space, or from some specific square meter of surface in the atmosphere somewhere.
I could set some sort of 1 m^2 empty picture frame on the ground. Presumably ~ 390 W/m^2 of IR would be heading up thru the frame from the ground. The downward radiation through the frame would be a combination of radiation from many parts of the atmosphere heading many directions. In the bands that CO2 or H2O gas emit IR, much of the IR comes from quite close by — within a few meters of the ground. Along the edges of the bands, the distance could be 100’s of meters. In the bands where these don’t emit, then the IR come be coming from several km away from clouds that emit a continuous spectrum of IR. The net value should on average be ~ 330 W/m^2
If you take that same frame to the “top of atmosphere” (perhaps 50 km up), you get a completely different situation. The IR in CO2 bands should come from near/just below the tropopause. Since there is little H2O that high, the IR from H2O gas would come from a little lower in the atmosphere (as I understand it). The IR “in the gaps” would come from the tops of the clouds far below.
There is no reason to expect this value to be ~ 330 W/m^2 since it is coming from very different places. You cannot simply double the DLR arriving at the surface and expect to get the ULR at any particular location.
Tim Folkerts,
good job side stepping the issue of the surface irradiating itself SLOWING THE COOLING and invalidating the correctness of SB!! 8>)
So, since it irradiates itself similar to the GHG’s, except it is a wider range of frequencies and higher amplitude I think the simplified models have more problems. Are the Big Boys dealing with this any better?
Tim Folkerts @ October 31, at 5:16 pm and Wayne@ October 29, at 3:49 am
Tim, I don’t think you have adequately addressed the fact that radiation is isotropic, not just up and down. A very important aspect is that the horizontally opposed stuff amounts to nothing in terms of HEAT transfer or temperature, yet it is PART of the S-B vertical calculation, yet out of view of the vertical (=normal). If you think that it is too hard for Trenberth to draw multi directional arrows, what about a simple notation that the radiation is isotropic?
Meanwhile, please examine this following sketch, and advise if you see any conflicts with drawing capability and/or reality:
http://bobfjones.wordpress.com/2011/10/29/quick-sketch-for-trenberth-cartoon/
Windchaser says:
October 31, 2011 at 5:07 pm
Not true – 1 cm of water is about 99% transparent to deep-red visible light, and about 99.99% transparent to deep-blue visible light. Not purely transparent in either case. Absorption of visible light is mostly at the overtone frequencies of the stretch and/or bend frequencies… so the absorption is weaker, but definitely there.
Oh just brilliant, what a great sense of scale. So this 1% and .001% is heating the oceans of the Earth as the primary mechanism of this junk energy budget’s only shorwaves heat the land and oceans and thermal infrared, real effin heat energy, which we can really feel from the Sun direct to us, doesn’t have any role to play in heating land and oceans?!
What are those numbers saying anyway? That towards the longer wavelengths water sometimes accepts them? How does the electron transitions of visible blue sometimes get through the molecule of water’s standard rejection of it? Whatever the reason, this is so effin insignificant that for all practical bog standard physics water is 100% transparent to visible, it does not in any significant way change that because some exception may or may not be proved. Transparent means that water does not absorb visible. Transparent means that water is not being heated by visible. Transparent means that the junk energy budget you all think is so real physics, is based on junk science fiction. Real science understands this:
Get your head around that. It’s your generic idiotic twisting of physics properties and processes which not only gives those with real interest in physics a headache, but higher taxes and loss of our freedoms. Those we can feel, visible and UV and near IR we cannot feel, they are not hot, they are not thermal energies, they are not heat. They have for all practical physical purposes and sense no ability to raise the molecules of the matter of our bodies into the vibrational states which is heat, which thermal infrared, heat, from the Sun, does every day. They work on the electronic transitional level, appropriate to their puny, tiddly nature..
That shortwave only heats land and oceans is not proved. It is already falsified because in real physics the properties of these are already well known and used in countless applications because they are well known, the building blocks are not imaginary ‘shortwave heats water’ or ‘water is not transparent to visible it is absorbed’.
Show and tell.
Tell how much visible light is heating the atmosphere in all the reflection and scattering since that is done by electronic transitions of the electrons of the molecules of oxygen and nitrogen absorbing it. Go on, tell us, how much hotter are these getting? Why isn’t this in the your junk ‘energy budget’?
Until you can show some real physics logic in these base premises of that energy budget, you don’t have real world physics energy budget.
You have a science fiction world depicted, that’s all you have.
You’re all arguing about an imaginary world as if it is real.
How can so many people calling themselves Scientists not see that when it is pointed out to them?
This is your basic claim: http://wattsupwiththat.files.wordpress.com/2011/10/greenhouseeffects1.jpg
Prove your base premises, prove that these shortwaves heat land and oceans and that thermal infrared the direct heat from the Sun doesn’t play any part in it. This is your generic claim. Prove it. It goes against everything that is known in real physics.
Damn well prove it.
Bob,
I am really not quite sure what your objection is. There certainly is energy moving horizontally back and forth thru the atmosphere. But the diagram is showing energy flows between 4 broad regions: the sun, the atmosphere (as a whole), the solid/liquid earth (as a whole), and outer space. It is not trying to describe the flows within the various parts. It would be like having a diagram showing foreign trade for a country, then complaining that trade between state/provinces within the country are not included.
The 330 W/m^s IS the total IR from the atmosphere as a whole to the surface (appropriately averaged over time and space). Whatever other IR is flying around being emitted and absorbed within the atmosphere is not important (for this diagram anyway). This 330 W/m^2 does not need to be further divided into up and down and left and right components.
mkelly says:
October 31, 2011 at 9:16 am
The term (Tg/Ts)^0.65 can be found in McAdams – ‘Heat Transmission’ (Radiation from Nonluminous Gases). For water vapour it is (Tg/Ts)^0.45
The terms are also found in Perry – ‘Chemical Engineer’s Handbook’ (Heat Transmission by Radiation) 5th Ed.
kuhnkat says:
October 31, 2011 at 11:13 am
I calculated CO2/H2O separately ie for dry air and air without CO2 to show the huge difference between the two absorbers. Pc for CO2 is so low that it takes 30 times the traverse compared with H2O which has a much larger Pw. In furnace calculations when dealing with the CO2/H2O system I calculate a combined emissivity; this could be done for atmospheric calculations in which water vapour would dominate etc. In furnace calcs the two are roughly equal.
Robert, thanks for adding some great real-world experience to the discussion.
Myrrh expounds: “Prove your base premises, prove that these shortwaves heat land and oceans and that thermal infrared the direct heat from the Sun doesn’t play any part in it. ”
Of course no one can prove that, because no one believes it! Anyone with an ounce of understanding knows that BOTH help heat the earth.
* “Shortwave EMR” [Which I will define as “Near IR” (0.7 um – 4 um) + Visible (0.4 um – 0.7 um) + ultraviolet (less than 0.4 um)] from the sun DOES plays a role in heating the earth.
* “Longwave EMR” [which I will define as greater than 4 um] from the sun DOES play a part in heating the earth.
“Thermal IR” is ill-defined, so you would have to give your definition. One standard definition is any IR beyond 3 um. This definition basically equates “thermal IR” with “Longwave EMR”. This definition means that only a few % of the sun’s EMR is “thermal IR” so only a few % of the energy warming the earth is due to “thermal IR”
I suppose you could make your own definition of “thermal IR” like “any IR generated from thermal energy” in which case all of the sun’s IR would be “thermal IR” and would account for ~ 50% of the sun’s EMR. This definition certainly increases the importance of “thermal IR”, but still leaves ~ 50% of the absorbed energy coming from visible and UV.
————————————————
“Tell how much visible light is heating the atmosphere in all the reflection and scattering … Why isn’t this in the your junk ‘energy budget’?”
[sigh] If you look, it IS there. The incoming 342 W/m^2 includes 67 W/m^2 “Absorbed by Atmosphere”. Now, this includes all wavelengths, so I can’t tell you off-hand how much of that is attributed to absorption of IR vs visible vs UV. It is all wrapped up into the one factor — 67 W/m^2 of absorbed energy from sunlight heating the atmosphere.
————————————————
And that is enough interaction with Myrrh for me for the next few months. The rest of you are free to keep up the discussion if you wish.
Myrrh continues:
What are those numbers saying anyway?
Well, that 1 cm of pure liquid water will absorb about 1% of the deep-red and 0.01% of the deep-blue visible light that passes through it (i.e., 99% and 99.99% transmitted). The effect is multiplicative, i.e., 2 cm of water would absorb about 1 – 0.99*0.99 of the deep-red visible, and 1 meter of water would absorb about [1 – (0.99)^100]. It would certainly be transparent enough for many real applications.
Using these numbers, it takes about 70 meters of water to absorb 50% of the deep-blue visible, or about 70 cm for deep-red. That’s hardly insignificant when you’re talking about how oceans absorb light.. and the oceans aren’t pure water anyway.
How does the electron transitions of visible blue sometimes get through the molecule of water’s standard rejection of it?
Water absorbs at overtone frequencies of its IR stretching/bending frequencies. As is normal, it absorbs much more weakly at overtone frequencies than at the prime frequencies.
Transparent means that the junk energy budget you all think is so real physics, is based on junk science fiction. Real science understands this:
http://www.esa.int/esaMI/Herschel/SEMNGHFTFQG_0.html
…
That’s a press release; it’s not meant to be a rigorous description of the physics of water (just like the cartoon is not meant to be a rigorous/exhaustive description of how IR transmits in the atmosphere). The point of that release was the story – that they’d missed the jets of water until now, because this telescope is much more sensitive to the IR (where water absorbs strongly) than other telescopes, which see in visible (where water absorbs weakly). You’ll need much more sensitivity to see water vapor in the visible range.
We can see visible light with our eyes, but astronomers have found many stars that we can’t pick out with our eyes .. not because the stars don’t transmit in the visible range, but because they transmit much more *weakly* in the visible range than in, say, radio waves.
Or, putting it another way – can you pick out Jupiter’s moons with your bare eyes? No? Does that mean that they give off / reflect any visible light, or just that your eyes aren’t sensitive enough to see them?
Prove your base premises, prove that these shortwaves heat land and oceans and that thermal infrared the direct heat from the Sun doesn’t play any part in it. This is your generic claim. Prove it. It goes against everything that is known in real physics.
Nope.. it’s not our claim. Like Tim said, they both play a part.
Robert,
thank you. Yes, at much higher temps CO2 actually does a bit.
Bullshit chaps, the ‘greenhouse claim’ is on the basic premise that shortwaves (UV/Visible/NearIR aka Solar) directly heat the land and oceans converting to heat which radiates out the said thermal infrared, shorwave in, longwave out. The claim is the the real heat from the Sun, the Sun’s thermal energy, doesn’t reach us and plays no part in heating land and oceans. The claim is that visible light is thermal, because you have subsituted the properties of Light with the properties of thermal infrared.
You can fiddle with yourselves as much as your like around this, but since that is the claim that is what you have to deal with. That’s what the AGW claim is based on, that is what is now being taught in schools. That visible light heats matter. It’s pathetic nonsense, as are your arguments about ‘the energy budget’ here and elsewhere because that is the basic premise you work to, garbage in garbage out.
kuhnkat says: October 31, 2011 at 8:54 pm
“good job side stepping the issue of the surface irradiating itself SLOWING THE COOLING and invalidating the correctness of SB!! 8>) “
Every surface is rough at some level. The heating element of an electric stove would look rough under an electron microscope — does this invalidate the SB law for the heating element? Does the “surface irradiating itself” slow the cooling of the heating element when you shut off the power? The answer is “no” — in fact the rough surface will radiate MORE efficiently and cool MORE quickly (see the link earlier for emissivity of rough vs smooth metals).
If you are looking at distances on the same scale as the surface roughness, then yes, it will have local effects on EMR levels. Once you are on scales significantly larger than the roughness of the surface, then the roughness effects will become less and less noticeable. So if I am standing in a ditch, I will get extra IR from the ground. If I am standing on a pillar, I will get less IR from the ground. If I am 100 meters above the ditch or the pillar, I can pretty safely ignore the topography and treat the ground as “flat”.
The key is finding the solid angle subtended by the various surfaces. Once you are even slightly above the level of the roughness, you will have very close to 2 pi steradians of sky above you and 2 pi steradians of ground below you and the exact roughness of the ground will have little affect.
Myrrh says: November 1, 2011 at 11:56 am
Bullshit chaps, the ‘greenhouse claim’ is on the basic premise that shortwaves (UV/Visible/NearIR aka Solar) directly heat the land and oceans converting to heat which radiates out the said thermal infrared, shorwave in, longwave out. The claim is the the real heat from the Sun, the Sun’s thermal energy, doesn’t reach us and plays no part in heating land and oceans.
I shouldn’t bother …
Myrrh, what you call ” shortwaves (UV/Visible/NearIR aka Solar) ” is “the Sun’s thermal energy” reaching earth. Tells us, please, what percent of the energy in the sun’s EMR is not these shortwaves? How does the sun’s thermal energy reach us if not thru shortwave EMR — what other mechanism is there?
So the claim is actually the opposite of what you understand — the claim is that the much of the “real heat from the sun” (most of the Visible/NearIR; some of the UV/Mid IR) does reach us and does play a major part in heating the land and ocean.
Tim Folkerts,
Saying that a geometry where the surface being evaluated irradiates itself “invalidates the correctness” of the SB equations is probably poorly stated . I believe it makes the calculation less accurate just as not being in equilibrium would give a less accurate computation.
So, considering the actual earth isn’t particularly smooth and that plenty of roughness causes it to be irradiating itself and it is never in equilibrium, has any work been done to determine the amount of variance that this would cause? Any papers you could link?
Correction when I said in furnace calcs CO2/H2O are roughly equal, I should have said they both make strong contributions as opposed to the atmospheric case where CO2 is very weak when compared with water vapour.
Natural gas combustion typically gives 13.4% H2O and 6.7% CO2 and consequently H2O makes the greater contribution to their combined emissivity and absorptivity.
Tim Folkerts says:
November 1, 2011 at 12:32 pm
Myrrh says: November 1, 2011 at 11:56 am
Bullshit chaps, the ‘greenhouse claim’ is on the basic premise that shortwaves (UV/Visible/NearIR aka Solar) directly heat the land and oceans converting to heat which radiates out the said thermal infrared, shorwave in, longwave out. The claim is the the real heat from the Sun, the Sun’s thermal energy, doesn’t reach us and plays no part in heating land and oceans.
I shouldn’t bother …
Myrrh, what you call ” shortwaves (UV/Visible/NearIR aka Solar) ” is “the Sun’s thermal energy” reaching earth. Tells us, please, what percent of the energy in the sun’s EMR is not these shortwaves? How does the sun’s thermal energy reach us if not thru shortwave EMR — what other mechanism is there?
So the claim is actually the opposite of what you understand — the claim is that the much of the “real heat from the sun” (most of the Visible/NearIR; some of the UV/Mid IR) does reach us and does play a major part in heating the land and ocean.
🙂 These are Light energies, they’re not thermal. They’re not Heat from the Sun, they are incapable of heating the Earth’s land and oceans.
Why is that so difficult to understand?
They are not the heat we feel from the Sun, this is thermal infrared, it is actually capable of heating the Earth’s land and oceans, it is actually capable of heating water.
Visible light, a Light energy in trad physics, doesn’t heat water.
These shortwaves are Light energies, not Heat energies. They are not thermal. They don’t do heat..
The ‘energy budget’ you are all arguing about is based on fictional physics. You are not arguing about the real physics of this world.
Is that clearer?
Okay.. let’s back up.
To a physicist/materials scientist, no type of EMR is “thermal energy”. Thermal energy is the kinetic energy of a system’s atoms and molecules.. and since light has no kinetic energy or atoms, it has no thermal energy.
In other words, all light has energy, but no light has/is thermal energy. Sometimes thermal energy is *converted* to light, and this is called the “thermal radiation” or the blackbody radiation, but it’s not thermal energy after it’s converted, any more than coal’s chemical energy is the same thing as electricity.
So, thermal energy can be converted to light via blackbody radiation (amongst other ways), and vice versa – materials have distinct frequencies at which they can absorb EMR directly as kinetic energy. These are frequencies correlating to the natural resonances of parts of the atoms/molecules/material. For instance, in water, we have frequencies for twisting, bending, and stretching the O-H and H-O-H bonds, as well as other frequencies for intramolecular interactions, like the hydrogen bond (the bond between one water’s O and another water’s H).. and so on.
Water will also absorb at linear combinations of these frequencies, at the “overtones”, which are similar to harmonics on a guitar. Higher multiples of the native frequencies.
Now, very nearly 100% of the Sun’s light is from its “thermal energy”, i.e., the Sun’s light is just its blackbody radiation. Because the Sun is much hotter than the Earth, it emits blackbody radiation much more strongly in the visible range than the Earth.
Okay, you say
“[Visible/UV light] are Light energies, they’re not thermal. They’re not Heat from the Sun, they are incapable of heating the Earth’s land and oceans.”
Oh dear.
Okay, first – if I give you a bit of light at a specific frequency, you have no way of telling whether it’s “thermal radiation”, coming from blackbody radiation, or whether it was emitted via some other process. There’s no such thing as this “thermal” and “light” energies for EMR, as all light has “light energy”.
Visible light carries energy, as does every other kind of light. When visible light goes into the ocean, and doesn’t come back out – which we know it doesn’t, as you can see that the oceans look darker than deserts from orbit – what happens to the energy in the visible light? It is absorbed, and converted to heat (well, some small portion of it is converted to chemical energy via photosynthesis, but mostly heat).
Definitions:
– Thermal energy: the kinetic energy of the constituent parts of a system. Vibrational, translational, phonon, etc.
– Heat: the transfer of thermal energy between two systems, whether directly or indirectly.
– EMR: electromagnetic radiation, or light.
– Thermal radiation: blackbody radiation, a type of EMR.
Myrrh says “”These shortwaves are Light energies, not Heat energies. They are not thermal.””
Just answer two simple questions:
1) What percent of the energy in the sun’s EMR is NOT these shortwaves (UV/visible/near IR = wavelengths shorter than 4 um)?
2) How do you define “heat energies” — preferably with a specific mathematical expression.
Tim Folkerts @ November 1, at 5:52 am and Wayne@ October 29, at 3:49 am
Tim, well at least this is on-topic! You wrote in part:
”…The 330 W/m^s IS the total IR from the atmosphere as a whole to the surface (appropriately averaged over time and space). Whatever other IR is flying around being emitted and absorbed within the atmosphere is not important (for this diagram anyway). This 330 W/m^2 does not need to be further divided into up and down and left and right components”.
Starting at the basics:
1) The 396 is derived from an S-B calculation and is isotropic into a non transparent atmosphere (equal in all directions hemispherically)
2) The horizontal components are always there, and are not additional EMR energy, and therefore are not part of the vertical flux as claimed by Trenberth
3) The backradiation is claimed to be 333, presumably as a progeny of the 396, and of course, it too must be isotropic, but this time it must originate from the atmosphere so is spherical. It too must have continuous horizontal components which are not part of the vertical flux. There is nominally the same flux upward as there is down. Which is the puzzle raised by Wayne….
4) I guess the solution to the puzzle in 3) is that the 333 is there only for schematic purposes; that it is really part of the 395 and its progeny.
5) There must be significant additional backradiation that is not shown, which arises from the warming of the atmosphere from the 65% of total heat loss from the surface in thermals and evapotranspiration. However, I guess this may be rather high-up to be significant at a few metres from the surface
Repeating; the problem is that Trenberth assumes the isotropic radiation is pure vertical flux, when it is not. Please look again at my crude sketch, and explain if you see anything faulty in it.
http://bobfjones.wordpress.com/2011/10/29/quick-sketch-for-trenberth-cartoon/
Bob says:
The horizontal components are always there, and are not additional EMR energy, and therefore are not part of the vertical flux as claimed by Trenberth
You are thinking about this incorrectly. A decent analogy is sprinklers on the roof of a large warehouse. If the sprinklers spray out 390 kg/s/m^2, then 390 kg/s/m^2 will land on the floor each second. It matters not one bit that the water drops may have some horizontal component when they are sprayed out. The horizontal component of the motion of the drop does not reduce the mass of water landing on the ground by some sort of cos(theta) factor.
The same goes for photons. The 390 W/m^2 of photons leaving the surface with any upward component will carry 390 W/m^2 upward.
(this analogy would be “upside down” and the atmosphere would be transparent. Also, we need to ignore the effects of gravity on the water drop. The analogy could be made better, but that would come at the expense of simplicity.)
“The backradiation is claimed to be 333, presumably as a progeny of the 396”
No it is the progeny of ALL the energy into the atmosphere — the 396 from the ground and the 80 from evaporation and the 20 from thermals and the 70 directly from the sun. Once the atmosphere absorbs the energy, it is all just energy, with no tags saying where it came from.
The IR sideways that you talk about does help spread the energy around within the atmosphere, equilibrating the temperature to some extent.
“Please look again at my crude sketch, and explain if you see anything faulty in it.”
I don’t find any fault per se. But as I said before, it is adding more details than the image is intended to portray.
Using the import/export analogy again, this is sort of like drawing arrows from Australia to other countries to show the amount of exports — then continuing the arrows within the countries, letting them taper off to show how far into the countries those goods go. It could be done; it could be interesting; but it doesn’t directly relate to the amount of exports.
Windchaser says:
November 1, 2011 at 2:29 pm
Okay.. let’s back up.
To a physicist/materials scientist, no type of EMR is “thermal energy”. Thermal energy is the kinetic energy of a system’s atoms and molecules.. and since light has no kinetic energy or atoms, it has no thermal energy.
? Maybe not those physicists/materials scientist, do you mean applied scientist here?, who have been AGWSFmeme educated..
Thermal energy is thermal infrared. That’s what we know is heat on the move because we can feel it, from the Sun, from a stove producing no visible light and so on, it is heat, thermal energy radiated out, the thermal energy of the Sun radiating out to us is the invisible thermal infrared.
In other words, all light has energy, but no light has/is thermal energy.
Agreed, light has not/is not thermal energy, that’s why it is called Light. Longwave infrared however is thermal energy. that is why it is called Heat.
Sometimes thermal energy is *converted* to light, and this is called the “thermal radiation” or the blackbody radiation, but it’s not thermal energy after it’s converted, any more than coal’s chemical energy is the same thing as electricity.
Thermal energy in the Sun creates visible. Quite, it’s not thermal energy after it’s been converted to light, thus light isn’t thermal energy. However, the thermal energy in the Sun which has created the light, the heat of the Sun, is also being radiated out. That is thermal infrared.
I really don’t know know what you mean by equating ‘blackbody’ with ‘thermal energy’, but I have found it extremely tedius to keep seeing the meme repeated that ‘everything radiates out thermal energy above zero K’ which does make it appear that thermal energy is being downplayed, I’m thinking of the contexts I see this. So, let’s just forgo delving into blackbodies and concentrate on the problem here, that you generic call light from the Sun thermal which is not its property. You generic have downplayed the real thermal energy from the Sun to us which really is the Sun’s thermal energy we feel to the extent that you claim it doesn’t even reach us. This is not a description of the physics of Light and Heat in this world. Light in this world, is not thermal. We can feel heat from the Sun in this world.
You’re living in a science fiction world.
This has been so brainwashed into what appears the majority, that they now think light is heat, that light is thermal. Other brainwashing memes are as you’ve given before, that ‘light is higher energy therefore they are powerful and can move atoms’ when in my world they work on an electronic transition scale and don’t move atoms. You equate ‘higher energy’ with ‘more power’, but in your world you have no sense of scale, these a piddly little energies, they can just about manage to interact with some electrons, but not visible light in water because the water molecules don’t let them in, for all practical real world physics, 100% of the time.., while the molecules of oxygen and nitrogen do, visible light excites their electrons they absorb it, and then it gets spat right back out, irritating little git that it is.., this is known as reflection/scattering. Blue visible is more highly strung than than the longer colours and so smaller, this highly energetic tiny thing gets bounced around all over the sky just by the electrons of the molecules of oxygen and nitrogen like a ball in a pinball machine.. It doesn’t have any ‘power’. It’s a wimp.
This AGWSF meme that ‘all electromagnetic energy is the same’, has confused you. You fail to appreciate the differences in properties between these, the differences such as size, and size matters. A highly energetic teeny little energy that is flicked all over the sky by electrons is not powerful. To call it powerful is ludicrous. Thermal infrared is around the size of a pinhead, now that’s powerful when it bumps into molecules. And that’s exactly why the more powerful heat energy moves the whole atom and molecule into vibration, which is what heats matter up.
So, thermal energy can be converted to light via blackbody radiation (amongst other ways),
Via? You said blackbody was thermal. So you’re still saying what I’m saying, let’s leave out this blackbody silliness, is that thermal energy creates light. There are other ways of creating it, as we now have begun exploring and utilising, LED’s and such, but we’re talking about the Sun, the thermonuclear creator of it.
and vice versa – materials have distinct frequencies at which they can absorb EMR directly as kinetic energy.
No not quite, they do not so much absorb EMR directly as kinetic energy, they absorb kinetic energy which is part of the EMR range of energies. Matter doesn’t create kinetic energy out of ‘passing EMR’ just because it can absorb heat. EMR is divided into many distinct groups, kinetic energy, heat, can be absorbed, a radio wave could be passing straight through at the same time without stopping. Because how matter absorbs energies also depends on the kind of energy it is.
These are frequencies correlating to the natural resonances of parts of the atoms/molecules/material. For instance, in water, we have frequencies for twisting, bending, and stretching the O-H and H-O-H bonds, as well as other frequencies for intramolecular interactions, like the hydrogen bond (the bond between one water’s O and another water’s H).. and so on.
Right.., and water and thermal infrared like to party. Visible is transmitted through without being absorbed.
Water will also absorb at linear combinations of these frequencies, at the “overtones”, which are similar to harmonics on a guitar. Higher multiples of the native frequencies.
Interesting as that is, water is transparent to visible light, visible light is transmitted through without being absorbed. Deal with it. The claim in this science fiction world’s energy budget is that visible light is absorbed by the oceans and heats it up. Visible light does not heat me up. I don’t care if that’s your world, imagined or even real, some alien world intersecting mine, I do care that you are pretending that it is this world around me, where the physics is well known and understood. That water is transparent to visible light is bog standard traditional science in my world. I gave an example from my real world science:
Will you kindly spend a little time taking that in?
Water is transparent to visible light in my world.
It is a well known physical fact, because it is a physical fact that is well known to real scientists in my world, who use this information successfully in their work because they know the differences between the different properties of EMR. EMR is not ‘all the same’.
Stop claiming you speak for my world.
Now, very nearly 100% of the Sun’s light is from its “thermal energy”, i.e., the Sun’s light is just its blackbody radiation. Because the Sun is much hotter than the Earth, it emits blackbody radiation much more strongly in the visible range than the Earth.
Visible light created by the Sun’s thermal energy I would have said 100%, is there something else creating visible light in the Sun? Not all the radiation from the Sun is thermal, visible light is not thermal, but being created by the Sun’s thermal energy.
Sure, the Sun is hotter than the Earth and emitting more visible…
Oh dear.
Okay, first – if I give you a bit of light at a specific frequency, you have no way of telling whether it’s “thermal radiation”, coming from blackbody radiation, or whether it was emitted via some other process. There’s no such thing as this “thermal” and “light” energies for EMR, as all light has “light energy”.
Oh dear, it’s that old all EMR is the same. We can measure the amount of thermal infrared coming from a body, we can tell if it’s thermal or light, because we know the difference between the two, that’s irrelevant to the process which produced it. Just because you can’t tell the process which produced this “bit of light at a specific frequency” doesn’t mean we can’t tell what that is produced, by it’s frequency we know what it is. Your conclusion does not having any logical connection to the spiel which came before it. We know there are such things as thermal and light energies for EMR, but “no such thing .., as all light has light energy”? What’s that supposed to mean when it’s at home?
Visible light carries energy, as does every other kind of light.
Hmm, I’d have put it, that visible light is energy in the form of visible light. It’s the electromagnetic waves which are carrying the energy. There are three ways of transferring energy, conduction, convection and radiation.
Heat travels from the Sun, the Sun’s thermal energy radiating out to us, is heat energy carried by radiation. It’s radiation that transports it. So what have you, generic, done here? Light is a form of electromagnetic radiation, such as radio waves are a form of electromagnetic radiation. So, you’ve just called all of these ‘light’?
When visible light goes into the ocean, and doesn’t come back out – which we know it doesn’t, as you can see that the oceans look darker than deserts from orbit – what happens to the energy in the visible light? It is absorbed, and converted to heat (well, some small portion of it is converted to chemical energy via photosynthesis, but mostly heat).
Well actually, light can come back out, it can be refracted and reflected all ways, including back up, depends what it reflects back from. But what happens to the energy is that it is not absorbed by the water. Visible light energy does not heat water. Water is transparent to visible light. If the visible is being absorbed it is in photosynthesis, pigmentation, fluroescence and so on, converting to heat is just not what visible does directly because it works on a minute scale in electronic transitions. You can claim it does, just as you claim that visible is thermal, just as you claim that ‘because it disappears in water must mean that water absorbs it and since absorption means creating heat therefore visible heats water’, but they are simply statements that bear not the slightest semblance to reality. Because: Water is a transparent medium for visible light. That is simply a physical fact here, at whatever depth. Go down in a submarine into the deepest trench and shine a light, you’ll be able to see through the water, it doesn’t absorb your light. That’s why they didn’t spot this huge mass of water around Saturn before, because the water was transparent to visible.
This is far too much like the memes about CO2 and temp rises, ‘because we can’t think of anything else it could be so it must be’ – ?? Think harder.
It is a fact that water is transparent to visible light. This means that water does not absorb it. This means that visible light cannot be heating it. Adjust the rest of what you think to that, to that physical fact.
Definitions:
– Thermal energy: the kinetic energy of the constituent parts of a system. Vibrational, translational, phonon, etc.
– Heat: the transfer of thermal energy between two systems, whether directly or indirectly.
– EMR: electromagnetic radiation, or light.
– Thermal radiation: blackbody radiation, a type of EMR.
And Tim’s post which touches on this, I think what you really need to get to grips with is what heat is. Heat is thermal energy. Heat is used by some to mean specifically that thermal energy on the move, but they do so knowing that it is the same thermal energy. You can’t just pick and choose what you want from the way traditional science uses these terms, and then claim that any other use of it is wrong.. No wonder you’re all so confused.
===================================
Tim Folkerts says:
November 1, 2011 at 2:31 pm
Myrrh says “”These shortwaves are Light energies, not Heat energies. They are not thermal.””
Just answer two simple questions:
1) What percent of the energy in the sun’s EMR is NOT these shortwaves (UV/visible/near IR = wavelengths shorter than 4 um)?
2) How do you define “heat energies” — preferably with a specific mathematical expression.
1. Right now I don’t bloody well care. And, I wouldn’t take any figures for them as fact because of the amount of corruption of basic science has taken place by those promoting AGW. What I am interesting in, is in getting a proper energy budget for this world, the one I’m in, where shortwaves are not the heating mechanism of all the land and oceans of Earth. To that end, I’m interested in getting these crook premises sorted out which are the basic building blocks of the science fiction energy budget you’re touting.
2. If you can’t make sense of it in English then you have no effin idea what you’re talking about in the maths language, Tim. So far, you make not one iota of sense in English. Your basic premises are junk, just what do you think your maths is worth when you don’t even understand the difference between light and heat or that water doesn’t absorb visible light? Stop playing your how educated you are card, it really doesn’t impress me..
…what has happened to the 95% thermal infrared being radiated out from an incandescent bulb if the 5% visible is the thermal felt as heat..? Gets trapped behind the glass does it? Runaway greenhouse in every bulb?
I posted a wodge on heat here: http://wattsupwiththat.com/2011/10/18/replicating-al-gores-climate-101-video-experiment-shows-that-his-high-school-physics-could-never-work-as-advertised/#comment-784290
I think you could both do with going back to basics as traditionally still taught in these descriptions, heat is thermal energy, thermal energy from the Sun on the move is heat, this is the invisible thermal infrared package of the electromagnetic spectrum, i.e. thermal infrared is heat. It’s heat that has the power to move atoms and molecules into vibration, which is heat, kinetic energy.
Bob Fernley-Jones says:
“1) The 396 is derived from an S-B calculation and is isotropic into a non transparent atmosphere (equal in all directions hemispherically)
2) The horizontal components are always there, and are not additional EMR energy, and therefore are not part of the vertical flux as claimed by Trenberth
3) The backradiation is claimed to be 333, presumably as a progeny of the 396, and of course, it too must be isotropic, but this time it must originate from the atmosphere so is spherical. It too must have continuous horizontal components which are not part of the vertical flux. There is nominally the same flux upward as there is down. Which is the puzzle raised by Wayne….
4) I guess the solution to the puzzle in 3) is that the 333 is there only for schematic purposes; that it is really part of the 395 and its progeny.
5) There must be significant additional backradiation that is not shown, which arises from the warming of the atmosphere from the 65% of total heat loss from the surface in thermals and evapotranspiration. However, I guess this may be rather high-up to be significant at a few metres from the surface”
The fundamental confusion lies with Trenberth’s designation of the 333 W/m^2 as all ‘back radiation’ when it is really the total downward LW flux received at the surface. If you read the tables in paper from which this diagram comes, it states this quite clearly.
Downward LW received at the surface has three potential sources, and only a portion of it is ‘back radiation’ as defined as that which last originated from the surface LW flux of 396 W/m^2. Another source is post albedo solar energy absorbed by the atmosphere and emitted down to the surface in the form of LW. This however is not ‘back radiation’, but ‘forward radiation’ from the Sun yet to reach the surface (key distinction). The other source is the non-radiative or kinetic energy fluxes from the surface (latent heat of water in particular) which also radiates in the LW infrared, some of which back in the direction of the surface.
If you add up the numbers, what Trenberth does is take 78 W/m^2 designated as ‘absorbed by the atmosphere’ from the Sun and the 97 W/m^2 of non-radiative flux (latent heat and thermals) and lumps them in the return path of 333 W/m^2. By deduction, only 157 W/m^2 is ‘back radiation’ as defined as that which last originated from surface emitted of 396 W/m^2. He has the atmosphere emitting 169 to space and 70 W/m^2 is passing straight from the surface to space (40 W/m^2 through the clear sky and 30 W/m^2 through the clouds). 169 + 70 = 239 leaving at the TOA. 161 directly from the Sun + 78 indirectly from the Sun + 157 from ‘back radiation’ = 396 W/m^2, which is the net energy flux entering the surface (396 – 169 – 70 = 157).
Because he returns all of the entire non-radiative flux from the surface of 97 W/m^2 back to the surface as part of the 333 W/m^2, it’s a net zero flux (78 + 97 + 157 = 332). Trenberth has an extra watt in there to make it look there is an energy imbalance causing global warming.
Via? You said blackbody was thermal. So you’re still saying what I’m saying, let’s leave out this blackbody silliness, is that thermal energy creates light. There are other ways of creating it, as we now have begun exploring and utilising, LED’s and such, but we’re talking about the Sun, the thermonuclear creator of it.
Okay.. one comment, and then I’m done.
You’re confusing thermal energy (i.e., kinetic energy of atoms and molecules) with thermal radiation (light that is given off as blackbody radiation, i.e., light that is produced from thermal energy). Thermal radiation for a body like the Sun (at, what, 7000 Kelvin?) is a mix of UV, visible, and IR light. Thermal radiation for the Earth is only IR, since the Earth is cooler.
“Thermal radiation” is just a tag we put on some light, to say how this light was created. It doesn’t say anything about the frequency of the light or about its spectra. “Thermal radiation” could be radio waves, IR, visible, UV, whatever, depending on how hot or cold the body emitting it is.
I showed you links (and there are plenty more to find, from respectable journals) showing that water does absorb visible light, although not so strongly that you’d see it from looking at a glass of water. These frequencies exist, and can be found on any chart of the absorbance of water.
You keep saying that water doesn’t absorb visible light. Would you mind providing a link to a scientific article showing zero absorbance in the visible range? I betcha you can’t. =p
Even if you have a light+material combo that don’t interact thermally (in the range where, for that material, light can be directly converted to kinetic energy), light may still interact with the electrons of the material, and then be converted to heat. Look at phosphors, for example – the reason we have trouble getting high efficiencies using these as light sources is because the excited electrons (which are supposed to be giving off light) keep dissipating their energy as thermal energy instead. They relax in the wrong way. It’s caused by phonon scattering, mostly.
Light is a form of electromagnetic radiation, such as radio waves are a form of electromagnetic radiation. So, you’ve just called all of these ‘light’?
Yep. That’s it. “Light” is just a shorthand way of saying “electromagnetic radiation”.
Tim Folkerts @ November 1, at 6:35 pm & RW @ November 1, at 7:26 pm
Tim, you wrote in part:
Sorry Tim, but that is a VERY poor analogy because the water sprays would interfere with each other thus disrupting their initial kinetic energy and converting the lateral spray to chaotic cumulative downward deflections, aided by the entirely foreign force of gravity such that it all ends up on the floor. It is an entirely different situation to isotropic IR radiation because light waves pass through each other. (well, until their quanta are absorbed). (see point 3) in the article).
AND, with my bold added:
Tim Folkerts @ November 1, at 6:35 pm & RW @ November 1, at 7:26 pm
Tim, you wrote in part:
Sorry Tim, but that is a VERY poor analogy because the water sprays would interfere with each other thus disrupting their initial kinetic energy and converting the lateral spray to chaotic cumulative downward deflections, aided by the entirely foreign force of gravity such that it all ends up on the floor. It is an entirely different situation to isotropic IR radiation because light waves pass through each other. (until their quanta are absorbed). (see point 3) in the article).
AND, with my bold added:
ERH; the atmosphere is NOT transparent. I don’t know why you repeatedly obfuscate as to how it could be in a transparent atmosphere!
AND:
Yes OK, I was fooled by Trenberth’s term; Backradiation, and its apparent diagrammatic linking with the 396. I see that RW has clarified this very well, and please see my separate response to RW.
Meanwhile, you also wrote:
QUE? It is a consequence of the temperature and emissivity within the layers. Dost though have thine cart before thine horse?
And, I might get back to you later on the sketch.
Tim Folkerts,
love the way you and Fred over at Judith’s lapse into near incomprehensibel jargon that only vaguely touches on the issue brough up when you don;t want to constructively engage.
Have a good day!!
Bob Fernley-Jones,
I should also add that of the 396 W/m^2 LW flux emitted from the surface, 70 W/m^2 goes straight space, which means 326 W/m^2 is the amount absorbed by the atmosphere (396 – 70 = 326). Of this, 169 W/m^2 is ultimately emitted up out to space and 157 W/m^2 is emitted down back to the surface. This is fairly close to a 50/50 split (52% to space and 48% back to the surface). If you read the paper, Trenberth’s 70 W/m^2 direct surface to space transmittance is not really referenced in the paper and only appears to be a rough estimate or guess. The fact that he has more than 50% going to space, suggests it’s low. Most estimates I’ve seen put this number more like 90 W/m^2. It should be virtually an exact 50/50 split because all the radiative emission in the atmosphere – be it directly from GHGs or from the heated gases of the atmosphere itself, is always isotropic. This means that half of what’s absorbed by the atmosphere from the surface LW flux ultimately goes to space and half goes back to the surface.
The non-radiative flux from the surface to the atmosphere, from the atmosphere to other parts of the atmosphere, and from the atmosphere back to the surface doesn’t affect or change this, as all of these fluxes are in between the surface and the TOA. The non-radiative fluxes are just moving energy around so the planet’s energy balance is what it is – about a 390 W/m^2 net energy flux into the surface.
Windchaser,
nice clear explanation. Hopefully others see it because it is wasted on Myrrh. He has a mind like a steel trap, with no hinge.
Bob Fernley-Jones,
You’re definitely correct that the diagram is highly misleading in many ways and really not an accurate depiction.
One of the most glaring examples is returning all the “Evapo-transpiration” back to the surface in the form of downward LW. What then is the source of the energy in the temperature component of precipitation? It’s no where to be found.
RW @ November 1, at 7:26 pm
Thanks very much for that clarification RW, in which you wrote in part after Trenberth:
”…Downward LW received at the surface has three potential sources, and only a portion of it is ‘back radiation’ as defined as that which last originated from the surface LW flux of 396 W/m^2…”
My word, you are a brave fellow! I confess that I did not examine the body text of Trenberth et al 2009, with much thoroughness, partly because I was fearful of breaking out in hives. If you don’t know; let me explain that hives is a most fearful allergic/reactive rash, in which I’ve been prone in past years; and I’ve had times when I’ve contemplated chopping-off an arm having such angry welts.
The problem I have is that many sources claim that the Trenberth/IPCC diagram gives that there is massive HEAT loss from the surface greater than the energy received from the sun. I feel that this needs some clarification.
RW:
Bob Fernley-Jones:
If your still here:
Finally you guys are talking some real physics to me anyway! I have kept raising these points out there (not exactly the same but close, I tend to talk in NET transfers only) hoping someone would eventually get it and you two are on it. If you ever re-read my view, keep in mind those figures I stated are strictly net transfers and all of the figures on Kiehl-Trenberth’s graphic are also net but the two 333, the 356 and the 396. Miskolczi figures are a bit different as Trenberth’s 97 paper, all trying to nail it down. Those large IR figures are only true right at the surface, not ever 100 meters up on an averaged world, (and in my room, matter of fact, if you would pop the roof off and make it 16°C, not 23°C. so the upward component could escape (1/6th)). That is also why you see divide by 3 or divide by 6 in so many astrophysics papers when radiation is part of the equations, after all, this is a 3d universe. Way to go! You give me hope others might soon “get it”.
If you look at Mars this is not so. The atmosphere being about 1/100th of Earth’s even though nearly pure CO2 does not impede even horizontal radiation from escaping directly to space, not up but sideways. That is why the surface is 210K same as the theoretical black-body temperature of 210K (see NASA’s fact sheet on Mars). Venus’s is so incredibly thick there is no upward ‘window’ at all so the 17000 Wm-2 at the surface stays there by a tiny fraction and that moves upward strictly by SB layer by layer. IR radiation cannot move a millimeter without recapture in any direction. Try that, all figure tend to coalesce for all three bodies in that light.
Bob,
I admit that there are many shortcomings with the model, but we seem to be talking past each other a bit. I could embellish the analogy and shoot drops one at a time so they never hit each other. I could set up my warehouse in space where there is no gravity, I could set up little sponges that delay some of the water (or even shoot some of it back toward the ceiling).
But that is all secondary to the core of the analogy. The core point is that despite any of these shortcomings the angle that the water travels does affect the net flux. 1.0 grams of water heading at a 45 degree angle does not carry 0.707 grams of water left and 0.707 grams of water down. There are no vector components of the mass the way there are with momentum or force. Similarly, 1.0 J of photons heading at a 45 degree angle does not carry 0.707 J of energy left and 0.707 J down.
We would have to agree on this before moving deeper. I conclude that if 390 W/m^2 of photons are emitted at random angles from the surface of a large spherical planet with a transparent atmosphere, then we will still have 390 W/m^2 of photons heading up thru any concentric sphere slightly higher up (subject to corrections due the area of the upper sphere being slightly larger). Do you agree with this conclusion for this idealize though experiment?
Once was can agree on that, then we could try coming to agreement on the to decide what effect the atmosphere has when it absorbs and emits IR radiation itself.
———————————————-
As for the term “back radiation”, I can agree with RW’s semantics (and even more with his physics). I guess after a brief study of the figure, I simply thought about the physics and interpreted “back radiation” as meaning “in the general direction of the surface” or “back toward us from the sky”. I never equated “back radiation” specifically with “radiant energy that was heading one direction and came back” (which would exclude the downward radiation that might have originally gotten its energy from convection or evaporation or direct absorption of sunlight).
(As with the discussion of “heat” — the physics is can be crystal-clear even when the semantics is befuddled.)
Perhaps a better term would be “Surface-bound radiation”. (Although now I can imagine people equating “bound” with “tied to”. I guess you just can win.)
If you take a cubic meter of atmosphere, it will emit some small amount of total radiation. For the sake of argument, lets call it 10 W (but obviously it will depend on the temperature, the humidity, and the presence of water droplets). This should be isotropic.
* I could divide the total radiation into two sets of photons — those heading generally upward and those heading generally downward. That would be 5 W upward and 5 W downward.
* I could divide the total radiation into two sets of photons — those heading generally east and those heading generally west. That would be 5 W west and 5 W east.
* I could divide the total radiation into two sets of photons — those heading generally north and those heading generally south. That would be 5 W north and 5 W south.
You would divide the total radiation by TWO, not by SIX to get the amount going in any general direction.
Bob (or anyone else),
FYI, here is an excellent paper that illustrates the atmosphere is fundamentally limited to 50% opacity – meaning half of what’s absorbed from the surface LW flux goes to space as part of the total LW flux leaving at at the TOA and half is returned to the surface as part of the total energy flux entering the surface from the atmosphere:
http://www.palisad.com/co2/div2/div2.html
The model presented by the author is much more straightforward and is derived from the physics of black body/grey body emission and basic top level constraints dictated by Conservation of Energy, rather than the mostly assumptive methods and heuristics employed by Trenberth. It’s important to note though that the author’s model is not a model of the actual behavior but a model of the net result equivalent of the actual behavior at the boundaries of the surface and the TOA, where only radiation enters and leaves. It’s purpose is to show net energy flux in and out of the system, which is what really matters in the end, and as you can see, there is some doubling counting going on in regards to net effect of the 3.7 W/m^2 of ‘forcing’ from 2xCO2.
Tim Folkerts @ November 2, at 3:23 am
Tim,
You cannot salvage your warehouse sprinkler analogy by making the changes you suggested. If you remove gravity, make the spray distribution isotropic, (hemispherically), magically arrange that the droplets do not collide with each other, remove the warehouse walls, and eliminate air drag and turbulence, then Newtonian mechanics would mean that the droplets would fly-off into space in all directions. The greatest number would be those approaching the horizontal; see item 4) in the article.
If you reinstate air drag, it gets a tad closer to an analogy of EMR in our absorptive atmosphere because the droplets would be quickly slowed to a virtual standstill, remaining on-course. (with no turbulence).
S-B surface emissions in our absorbent atmosphere are rather different in that the photons have a wide range of energy levels and frequency distribution a la Planck. A significant number are absorbed, (extinguished), by GHG molecules at various altitudes. They may or may not quickly emit a new photon but not necessarily of the same energy level, because meanwhile they have to thermalize a vastly greater number of O2 & N2 molecules etc, by transferring KE in collisions. (and of course that is two-way, and ignores some EMR that escapes directly to space)
wayne @ November 1, at 10:49 pm
Wayne,
you wrote in part:
”…That is also why you see divide by 3 or divide by 6 in so many astrophysics papers when radiation is part of the equations, after all, this is a 3d universe…
That’s interesting; do you have any links handy?
If I eyeball, item 4) or Fig 3 in the article, it seems to me at a guess that the “conical” portion of the sphere described as crudely UP, might be about a sixth of the volume of the upper hemisphere. I went looking for it under “spherical geometry”, but couldn’t find a formula.
I’m not sure that I fully understood all that you wrote, but interesting.
Bob,
I think we are perhaps addressing slightly different issues, so maybe it is best to skip the analogy and go back directly to what you concluded
“The vertical radiative flux portrayed by Trenberth et al of 396 W/m^2 ascending from the surface to a high cloud level is not supported by first principle considerations.”
I agree that 396 W/m^2 does not reach high clouds. I disagree that this is what Trenberth portrayed. The diagram is intended only to show the radiation ascending to the atmosphere as a whole. Some goes to high clouds, some goes to low clouds, some goes to GHGs. (And some goes clean thru the atmosphere to outer space)
“The S-B 396 W/m^2 is by definition isotropic”
I agree with this.
“…as also is its ascending progeny, ”
I’m not quite sure what you mean here, so I am not quite sure what I think.
“with always prevailing horizontal vector components that are not in the field of view of the vertical.”
This is problematic IMHO. Any photon with any upward component will be in the “field of view” as I understand you to use it. Any photon with any downward component will NOT be in the “field of view”. So half will be visible, not some small amount as suggested by Figure 3.
“The remaining vertical components of EMR from that source are thus less than 396 W/m^2. ”
The vertical components do decrease, but I would attribute it to the lapse rate, not to “prevailing horizontal vector components.”
If the temperature was constant, then the upward radiation should be constant. Suppose a given layer of air emits 10 W/m^2 upward and 10 W/m^2 in a particular IR bad of the GHGs. The layer above and below would be the same (Again, assuming a constant temperature). So every layer absorbs and emits a total of 20 W/m^2 in the band.
Of course, the lapse rate will lower the temperature as you go up. The layer that absorbs and emits 20 W/m^2 would receive something like 10.1 from below and 9.9 from above (in that IR band). So as you go higher, the energy upward will taper off, much like you tried to draw.
If you play with MODTRAN looking down from varying heights up to ~ 1 km, this is confirmed. The upward energy flow is nearly constant. The higher you go, the more you can see that the energy up from the top layer is less than the energy up from the surface directly.
Again, this is NOT due to the layer absorbing 10 W/m^2 from below, then emitting only 1/6 upward = 1.67 W/m^2 so that the upward component is reduced by sending the radiation mostly to the sides instead.
Bob also says: “it seems to me at a guess that the “conical” portion of the sphere described as crudely UP, might be about a sixth of the volume of the upper hemisphere.”
What you were looking for is the AREA of that top surface relative to the entire surface (although as I think about it, the two answers will be proportional, so either will be the same). That fraction of the area would be
1/2 [ 1 – cos(phi) ]
where phi is the angle down from the top. So the surface of the “top” of the sphere is
1/2 [ 1 – cos(45) ] = 14.6%. Your intuition about 1/6 was only a couple percent off !
(But as I argued before, what you REALLY want is the photons with any upward component
= 1/2 [ 1 – cos(90) ] = 50%)
Bob Fernley-Jones says:
… do you have a link?
No Bob, that is merely from studying physics, especially astrophysics, for decades and thousands of papers read. Are you wanting to see the third and sixth factors? When I get some time I’ll dig. I’ll just start by going back to texts like Planck, Boltzmann, and Stefan’s, maybe Fourier’s on heat transfer written before modern ‘science’ has gotten a hold and re-written the equations into more modern symbolic mathematics with the dimensionality buried deep within. This is not necessarily bad but once spherical coordinates and solid angles (steradians) are used the dimensionality is not so apparent in the terms, but the equations are perfectly equivalent and the three dimensions are always there. It’s just an ‘old science’ – ‘new science’ thing. Both are actually identical.
I’ll dig but may be few days. (this kind of speak is a bit of pure poison to those studying modern climate science so you might have to not get sidetracked)
But you figure 3 is just partly completed. You drew red for top and bottom but you could have also painted red the right and left. The forward and backward would be rather hard to portray but they are there too. These four portions of 1/6 of the area of the sphere all point horizontally to a degree until they are pointing in one of the other 5 directions.
Put your sphere somewhere above the surface a couple of 100 meters high. Call this point A. Place another identical sphere 100 meters away horizontally, any direction, and call this B. A will radiate in all directions but a small portion of the four steradians of the sphere will pass right through sphere B. When A radiates it cools, and, a portion passing through B will be absorbed warming B. BUT, B is also radiating toward and through A and the same amount of it’s radiation is being absorbed by A. This cools B and warms A. On an averaged Earth, these would always be at the same temperature so these amounts are identical statistically.
What of the portion passing through B but not absorbed by B? There is some sphere C further or nearer that is in symmetry with A the same as B is, C and A passing radiation back and forth per SB and emissivity. Taking all possibilities and you get the point. This horizontal radiation that is constantly being passed back and forth horizontally does absolutely nothing.
Of course this example is exactly horizontally but even if A and B are not at the same altitude therefore different temperatures they have a portion per the cooler of the two that is in symmetry and the small variance becomes part of what we generally call up and down radiation energy transfer. Integrate this over all directions and you get the 1/6th up and the 1/6th down figures.
But one big questions is if this horizontal radiation is counted in any Stefan-Boltzmann calculation as the 396 or 333. Of course it is even though this portion is not cooling OR warming anything. The same thing is happening on the walls of your room. If you stick a pyrometer in the path of this radiation it is going to read it depending on the temperature and emissivity but you cannot claim this is warming or cooling anything for the temperatures are identical in such cases.
Is that clear enough for you to see my point. This 3d aspect is being totally ignored (or hidden, maybe just because of ignorance and bad training).
wayne @ November 2, at 9:39 pm
Wayne,
Thank you for another interesting comment in which I have no disagreements! YES, it is the origin and logic of the third and sixth factors that have particularly caught my interest!
Hopefully, see later, a comment that I intend in part to make to Tim, concerning the “size” of an elemental parcel of air, and as to whether its surface area and/or its volume should be considered in relation to emissions from it, depending on its “size”, or if you like, its molecule count. (and absorptions into)
I doubt if I have time to do that today
Wayne,
Oh, but just one quick thing; you wrote in part, with my bold added:
“…But one big question is if this horizontal radiation is counted in any Stefan-Boltzmann calculation as the 396 or 333…”
I don’t understand how S-B can be applied to the general atmosphere because of widely varying photon free path lengths and the absence of any definable surface. The underside of substantial clouds, well, yes; I recognise them as likely being almost equivalent to black bodies.
Windchaser says:
November 1, 2011 at 8:21 pm
Myrrh: Via? You said blackbody was thermal. So you’re still saying what I’m saying, let’s leave out this blackbody silliness, is that thermal energy creates light. There are other ways of creating it, as we now have begun exploring and utilising, LED’s and such, but we’re talking about the Sun, the thermonuclear creator of it.
Okay.. one comment, and then I’m done.
You’re confusing thermal energy (i.e., kinetic energy of atoms and molecules) with thermal radiation (light that is given off as blackbody radiation, i.e., light that is produced from thermal energy). Thermal radiation for a body like the Sun (at, what, 7000 Kelvin?) is a mix of UV, visible, and IR light. Thermal radiation for the Earth is only IR, since the Earth is cooler.
I’m not confusing it, you are. I have given you a post, my link above, to some very good explanations about this. Until you read it and take on board what it is saying you are dismissing this world’s physics as it is traditionally taught, and which makes a coherent whole from the parts, i.e. is not full of illogical connections which come about from extrapolating from your fictional physics. Thermal radiation for a body like the sun is thermal infrared, it is not uv, visible or near ir which are not thermal.
Just because you have chosen to call them thermal, does not make them thermal. UV is not heat in the Sun, it is not the Sun’s thermal energy. It cannot therefore be the Sun’s thermal energy on the move to us. Ditto visible and near infrared. These are not hot, they are products of the Sun’s thermal energy. They do not heat matter. They are puny in size and work on an electronic transition level. The difference in size between near infrared which is not hot, we do not feel it any more than we can feel visible or uv, is that near infrared is microscopic compared with thermal infrared which is the size of a pinhead. It is this thermal infrared, heat, the thermal energy we receive from the Sun, which does have the power to move atoms and molecules into vibration which creates heat. Heat creates heat in matter because it does have the power to move atoms and molecules into vibration. Just as friction creates heat, rub your hands together, you are moving the atoms and molecules in your skin into vibration – visible light doesn’t do this, it can move electrons, but it doesn’t have the power necessary to move the whole atom or molecule into vibration. In water, visible light doesn’t even move the electrons, which it does of oxygen and nitrogen molecules in the atmosphere, it is transmitted through without being absorbed, that’s why water is transparent. That’s why they missed this massive amount of water vapour around Saturn, because water vapour is transparent to visible light.
“Thermal radiation” is just a tag we put on some light, to say how this light was created. It doesn’t say anything about the frequency of the light or about its spectra. “Thermal radiation” could be radio waves, IR, visible, UV, whatever, depending on how hot or cold the body emitting it is.
? All the different wavelengths of the electromagentic spectrum have unique properties, thermal radiation is not radio waves, you have absolutely so destroyed the known science which has already established that there are these differences, that you have utterly lost all sense of basic science perspective here. That’s why you generic now erroneously teach that ‘visible light is thermal; that the visible light we feel from the Sun is thermal, that the heat we feel from an incandescent lightbulb is visible light’. You’ve lost the plot completely. You’re no longer scientists in the line of scientists who’ve worked hard over the centuries to understand the differences between things. An incandescent lightbulb radiates 95% of it energy in heat, which is thermal infrared, and only 5% in visible light, which is not thermal, which is not heat. But because you have so twisted real world physics out of shape with this idiocy of calling all energy thermal, you can no longer tell the difference between heat and light.
But because you have so twisted real world physics out of shape with this fictional physics idiocy of calling all energy thermal, you can no longer tell the difference between heat and light.
I suggest that you get rid of the ‘blackbody meme’ and come back to basics.., if you want to understand how the world works on a physical level. All electromagnetic radiation is not the same, visible light is not thermal, it is not thermal infrared. And then you’ll have a better grasp of the use of words in context.
I showed you links (and there are plenty more to find, from respectable journals) showing that water does absorb visible light, although not so strongly that you’d see it from looking at a glass of water. These frequencies exist, and can be found on any chart of the absorbance of water.
You keep saying that water doesn’t absorb visible light. Would you mind providing a link to a scientific article showing zero absorbance in the visible range? I betcha you can’t. =p
Can you provide figures for the amount of heating of the oxygen and nitrogen in our atmosphere that visible light is creating by the electrons of these molecules absorbing visible light in reflection/scattering?
Your reference to exotic studies is again pure physics nonsense, irrelevant, in real world physics water is treated as 100% transparent to visible light, because its actual properties make it so to the actual properties of visible light.
You claim that Visible, shortwave, light is the primary heating mechanism of the world’s lands and oceans.
What you should be doing is giving me proof of this, instead of avoiding it by this over-emphasis on an insignificant number in the scale of things which is not well understood and irrelevant to the scale of what you are claiming – that visible light heats all the waters of the oceans, of the lakes and rivers, and all the land.
Water is a transparent medium to visible light is a basic physical fact about water, visible is therefore not absorbed, it’s energies are not absorbed, it cannot be heating it.
Discussion about what is happening in this insignificant ‘absorption’ does not alter the basic physical fact that water is transparent to visible because water’s molecules are ‘resistant’ to its wiles, visible is transmitted through water without being absorbed is basic standard physics. Water’s molecules do not let visible in to play with its electrons, visible isn’t powerful enough to move a molecule of water into vibration which is what creates heat. Do I have to put that in bold or even caps before you’ll think about it?
Enough of this bs avoidance to providing proof of your claim that shortwaves heat land and oceans. Damn well prove it, prove visible is capable of heating water and land. This is your energy budget, your claim, I don’t have to prove anything, you have to prove it is fact. I have already given you traditional physics on this which falsifies your claim. Do you understand what I’m saying here?
Even if you have a light+material combo that don’t interact thermally (in the range where, for that material, light can be directly converted to kinetic energy), light may still interact with the electrons of the material, and then be converted to heat. Look at phosphors, for example – the reason we have trouble getting high efficiencies using these as light sources is because the excited electrons (which are supposed to be giving off light) keep dissipating their energy as thermal energy instead. They relax in the wrong way. It’s caused by phonon scattering, mostly.
Who the f can tell what you’re saying here when you use the words heat and thermal energy.. 🙂
Do concentrate. I want you to prove that visible light heats water. Show and tell. This is the basic claim of the junk energy budget you keep promoting. Stop avoiding it.
Myrrh: Light is a form of electromagnetic radiation, such as radio waves are a form of electromagnetic radiation. So, you’ve just called all of these ‘light’?
Yep. That’s it. “Light” is just a shorthand way of saying “electromagnetic radiation”.
Well, I suggest if you really want to get a grip on the difference between heat and light, you stop saying that. A radio wave is not a gamma ray is not light is not heat, it’s a radio wave.
==================================
kuhnkat says:
November 1, 2011 at 9:36 pm
Windchaser,
nice clear explanation. Hopefully others see it because it is wasted on Myrrh. He has a mind like a steel trap, with no hinge.
As long as you think shortwaves can heat land and oceans and that you can feel heat from visible light you don’t know what you’re talking about.
That you agree with someone else saying this is irrelevant. Prove it, show and tell how.
Electronic transitions level do not create heat, because they don’t move whole atoms and molecules into vibration which is what creates heat. And until you get your mind out of the AGWSF meme trap and make at least some bloody effort to understand what I am saying you will continue to confuse yourselves and others by believing such utter stupid claims that visible light is thermal and you can feel it as heat.
In the real world, not your imaginary science fictional one, an incandescent light bulb radiates around 95% heat and around 5% light. Got that? There is a difference between these. 95% is heat, it is not light. 5% is light, it is not heat. The 5% visible light does not have the power to heat you, to move your molecules into vibrational states which is heat. The 95% which is heat, thermal infrared, does have the power to heat you, to move your molecules into vibrational states. That is the difference between light and heat. This is the traditional physics of my world, your physics is garbled nonsense in my world. In my world it is garbled nonsense.
The incandescent light bulb is proof that your world is fictional, because you can’t explain it with your claims that visible light from the Sun is thermal, your fictional science meme doesn’t make sense of the physical world we see around us. The parts don’t fit because you don’t understand words in context. You don’t understand words in context because you are regurgitating memes which have given the properties of one thing to another. Visible light is not thermal. If you can grasp that, the real physical difference between heat and light, you’ll know how you’ve been manipulated to the level of confusion you, generic, have achieved here, when in extrapolating from your science fiction memes you think ‘visible is thermal therefore the visible light from the incandescent lightbulb is what you feel as heat’. When you can see how ridiculous that is, you’re back in the real world.
Thermal infrared is heat. Visible light is not heat. Visible light is light it is not heat, it is not thermal infrared. Until you can tell the difference between visible light and thermal infrared you are not describing my world.
Do you understand what I’m saying? Your physics doesn’t work in my world.
Your physics is full of logical disjuncts, such as shown by your explanations of the incandescent light bulb and the claim that water is not transparent to visible light.
You are so much in your meme trap that you can’t understand what water transparent to visible light means because the word ‘absorb’ ties you in physical knots which produce garbled illogical claims as do the meme that get you confused about thermal and light because they use these out of context.
In my world that water is transparent to visible light is basic bog standard knowledge, as I gave example of the discovery of a huge mass of water around Saturn, which they couldn’t see before because water is transparent to visible light. They were looking straight through it. Something is transparent when the molecules do not absorb it, visible light is neither absorped on an electronic transition scale, as it is in the fluid gaseous atmosphere by the electrons of oxygen and nitrogen, nor is it powerful enough to move the whole molecule of water into vibration.
That’s in my world. Your world is fiction because it claims that water is heated by visible light. Go on, try heating water with visible light.
REPLY: Please consider making your comment longer next time /sarc – Anthony
Bob Fernley-Jones says:
November 3, 2011 at 12:01 am
I don’t understand how S-B can be applied to the general atmosphere because of widely varying photon free path lengths and the absence of any definable surface. The underside of substantial clouds, well, yes; I recognize them as likely being almost equivalent to black bodies.
Bob, my advice is DO NOT try to make the IR figures in Trenberth’s graphics real and make sense. You will find you cannot do that. That is what first queued me that this energy budget they laid out is so wrong, totally wrong. SB equation does not give you the amount of radiation always occurring. It gives you the absolute maximum of radiation POWER that could be spontaneously transferred from A to B, if and only if B is absolute zero and the emissivity of A is one. That is what SB gives you, a maximum.
First, this hypothetical world in the graphic has not latitudes, it doesn’t have a sense of spinning, it is evenly lit by average solar radiation at all points evenly and there is no day and night, you know this. So ask yourself, if part of the energy moving from the surface is due to evaporation (80 Wm-2) and part is carried upward from thermals (17 Wm-2) then can the surface ALSO radiate at 396 Wm-2 upward by radiation even if the emissivity is one? This is the first clue I had that this is all pseudo-science on the IR side of this graphic.
Many of the figures are real NET figures by good papers and are satellite analysis to our best ability. But those figures on the left 2/3rd are NET energy transfer figures and they give a NET amount of energy moving from the surface to space or from sun to our surface, averaged, not into the atmosphere to return to the surface. NET energy moves ONLY from warmer to cooler and because our atmosphere via lapse rate is layered warm to cool as you move each 100 meters higher (~0.65 K ) this NET energy can NEVER return to the surface. See the NETs highlighted above. Don’t mix NET figures with relative figures that are used on the far right side.
So back to your question. The 396 and 333, toss them. Change the 333 to zero for NET energy, never flows downward on an averaged Earth. The 396 is actually about 63 as you said in your article above. (We are converting these to NET transfer now). Of the ~63, ~40 Wm-2 is window frequencies that actually zip at the speed of light directly to space. So, that leaves us with a NET of ~23 Wm-2 instead of 356 that is attributed to IR radiation warming the atmosphere for this is NET absorbed from IR radiation from the surface above what it would be if it was never absorbed within the atmosphere.
That is my take after two years of deciphering this subject.
So, 161 Wm-2 absorbed = 80 + 17 + 63 + 1 (NET). Check!
And 239 Wm-2 at TOA = 78 + 80 + 17 + 63 + 1 (NET). Check!
Other papers show variances in these figures but all are closely clustered. It is 23 Wm-2 (63-40) we are talking about in relation to warming by GHGs, not 396 Wm-2.
On a more comical side, one thing a pyrometer or radiation thermometer never clues you into is there is also radiation hitting the back side that is hidden from you, never accounted for. You really need to tape two radiation thermometers together facing opposite directions and then let that instrument cast a shadow in the local radiative field… convert this temperature of both back by SB with emissivities accounted for and subtract the two which are actually cancelling each other as I showed above. Then and only then will you get the real story of what is occurring with radiation.
You are so close to seeing my thoughts clearly. No one else has ever commented back to me showing their insight. Keep digging, thinking, keep it in real physics.
Tim Folkerts @ November 2, at 8:59 pm
Tim, you wrote in part:
As I’ve shown previously, the arrows and numbers are in the wrong place, and there is ZERO difficulty in drawing it to better show what may be happening. What makes things worse is that this misleading diagram is referred too without critique by others including its illustration by the IPCC
As a consequence of the initiating S-B 396 and its subsequent absorption and emission, there is an ascending progeny of absorption and emission which is isotropic. (spherical). “Its progeny” is a label used to distinguish it from additional EMR driven by thermals and evapotranspiration etc.
The surface integration that you have previously proposed, if that is what you are on about, has no relevance in an opaque atmosphere. Maybe at TOA, but that is not where we are at.
I’m referring to the fact that radiation is not straight-up-and-down. If you don’t want to believe in vectors, then just consider the significant portion of the purely horizontal components. By your own words, these are not up OR down, and because they are not, but are nevertheless part of the S-B 396, and its progeny, then the vertical stuff must be less than 396.
Anthony – Unless and until everyone using this junk energy budget as gospel in its basic premise that ‘shortwaves directly heat land and oceans and thermal infrared plays no part in this’ can show how this is physically possible then it remains science fiction. It is actually junk science fiction because real physics falsifies this claim, as I have shown traditional physics teaching on it.
Avoiding the import of this is not a substitute for producing the show and tell I have asked for. I only ask for it in the rapidly diminishing hope that some here in actually looking to provide me with real physical show and tell supporting their claim will come to see for themselves that it is junk science fiction and as such does not bear any relationship to the real world.
To simplify this request, I’m asking show and tell for only a part of that claim. I’m asking for proof that Visible light is actually capable of heating water, because if it can’t heat water then it can’t be heating the oceans. This then has to be taken out of the ‘energy budget’ to get nearer to what should be the energy budget of this real physical world around us.
Do have a go.
Tim,
Further to my November 3, at 5:06 pm
Concerning your belief that a photon stream cannot be analysed with component vectors, please see this brief summary of Lambert’s cosine law. It is written from the perspective of optics, and you may not like the semantics. However, if you look at the far right of figure 1, see how when the incoming light is at a shallow angle it is “reflected” less but still isotropically. This is because the vertical component is small, whereas the horizontal, which cannot be absorbed or reflected, is a large proportion of the light stream.
http://escience.anu.edu.au/lecture/cg/Illumination/lambertCosineLaw.en.html
CRX: 4π steradians, not 4 steradians. Just noticed that somewhere between MSWord to WordPress and back a couple of times the pi got lost. Hope that doesn’t negate the content.
Bob, Tom, and anyone else still here,
just to throw something else into the mix, photons have momentum. It is transferred when the photon is absorbed. This is not taken into account anywhere that I have seen this talked about. What would be the net effect of the surface irradiating the atmosphere knocking all those particles away from the point of emission? Wouldn’t it be a net upward force?
wayne @ November 3, at 4:11 pm
Wayne,
You wrote in part:
Yes, I know what you mean, and this had me puzzled too for a long time. I think that the answer is that the surface temperature is a consequence of ALL of the various heat transfers. Thus it may be valid to do an S-B calculation from that resultant surface temperature. (even though we do not have good T data for the actual surface! BTW).
To elaborate; without an atmosphere, it seems perhaps non-intuitively, that without the alleged 65% “convective” cooling, the surface would be significantly hotter, with thus a different S-B result. Thus whilst at first sight there seems to be additional energy over the top of S-B, I don’t think that this is actually the case.
I’m not sure if I explained this well, so please ask if ye non comprendo.
“…photons have momentum”
kuhnkat, yes, your right but very, very tiny once you stop to think of the scale.
Think of this… energy increases with the velocity squared as momentum increase with just velocity. So with a velocity of the speed of light the scale of energy so dwarfs any momentum transfer by about 150 million times (1/2 c) using SI units. Does that simple explanation make some sense?
An order of magnitude estimate of the momentum of a typical IR photon:
p = h/(lambda) ~ 6E-34 J*s / 6E-6 m = ~ 1E-28 kg*m/s
Momentum of a typical molecule in the atmosphere:
p = mv = ~ 3E-26 kg * 300 m/s = ~ 1E-23 kg*m/s
So photons have a momentum ~ 100,000 times smaller than molecules. I suspect that for most purposes, this can be ignored. (You all are welcome to double check the numbers)
There are places where the photon momenta ARE important. These typically involve higher energy photons (eg x-rays and “Compton Scattering”) and/or brighter sources (eg “radiation pressure” from the sun).
Bob, I comprehend. It does seem you are still hanging on S-B calculations, mixing relative figures with net figures, that can so easily mess you up if all factors are not applied correct. It’s a well respected equation that with two temperatures and emissivities can hand you the maximum net transfer power across vacuum between but this ignores such factors as the absorption coefficients of any matter components lying between the two surfaces, like part of an atmosphere, and you get stuck again for the S-B’s answer is not correct unless the distance is so small that such factors are irrelevant. And then there is the geometric aspect. I still say net is correct as your article stated.
If you never got the chance to read Hans (Hans says: October 27, 2011 at 6:12 pm) comment and the small blue link “here” in his last sentence, you should. Somewhat deep depending on your level in physics but if you can just skim off the essence it will help you answer such questions and really understand why and why not. It’s on the spontaneous transfer of any energy, microstates, and it has everything to do with radiation and entropy. See if that might help fill in some of the holes.
Wayne,
I agree, very very very tiny. Then again, a .22 with a cyclical rate of fire of 1200rpm can shoot through steel, concrete, bullet proof vests… That extremely tiny amount has a rather large multiplier.
Wayne,
Hans post does not appear to be there. Do you have the link?
Wayne,
ignore that last post, found it.
Bob,
I agree that there are many places where the vector nature of photons is important. The direction that light is scattered is one of those cases.
For radiation emitted by a chunk of atmosphere:
* 1/2 of the energy goes up and 1/2 goes down
* 1/2 of the energy goes east and 1/2 goes west
* 1/2 of the energy goes north and 1/2 goes south
NOT 1/6 goes in each direction. In that sense I feel Figure 3 is misleading. It seem to be saying that (combined with Fig 4 showing vector components) a photon heading 10 degrees above horizontal toward the left should only be considered as carrying sin(10) = 17.4% of its energy upward and cos(10) = 98.5% of its energy leftward. Or maybe it is being treated as simply additive — if 10% is upward, then 90% is sideways. I’m really not sure what you intended there. In fact, it is carrying 100% of its energy up and 100 % of its energy to the left.
On the other hand, momentum IS a vector. The component of the photon’s momentum upward would be 17.45 % of the total momentum.
(And to be just a little more precise about energy, on average 1/2 + delta goes down and 1/2 – delta goes up, because of the average temperature gradient in the atmosphere. )
Bob Fernley-Jones says:
“…So ask yourself, if part of the energy moving from the surface is due to evaporation (80 Wm-2) and part is carried upward from thermals (17 Wm-2) then can the surface ALSO radiate at 396 Wm-2 upward by radiation even if the emissivity is one? …”
Yes, I know what you mean, and this had me puzzled too for a long time. I think that the answer is that the surface temperature is a consequence of ALL of the various heat transfers. Thus it may be valid to do an S-B calculation from that resultant surface temperature. (even though we do not have good T data for the actual surface! BTW).
To elaborate; without an atmosphere, it seems perhaps non-intuitively, that without the alleged 65% “convective” cooling, the surface would be significantly hotter, with thus a different S-B result. Thus whilst at first sight there seems to be additional energy over the top of S-B, I don’t think that this is actually the case.
Bob (and Wayne),
Remember, to the extent that non-radiative flux (latent heat and thermals) is leaving the surface, it’s largely being returned somewhere else in equal and opposite amounts (as the temperature component of precipitation, wind, weather, etc). Remember also, the surface upward radiative flux of 396 W/m^2 is also just the net energy flux entering the surface from the atmosphere. So what happens if more non-radiative flux leaves the surface than is returned to the surface on average (i.e. a net convective energy loss from the surface to the atmosphere)? The surface will lose energy and subsequently cool down and radiate less, right? Since energy cannot be convected to space – only radiated, in this particular example, non-radiative flux at the surface is just being traded off for radiative flux at the surface, requiring the surface to emit less to achieve equilibrium output power at the TOA.
Wayne,
“So back to your question. The 396 and 333, toss them. Change the 333 to zero for NET energy, never flows downward on an averaged Earth. The 396 is actually about 63 as you said in your article above. (We are converting these to NET transfer now). Of the ~63, ~40 Wm-2 is window frequencies that actually zip at the speed of light directly to space. So, that leaves us with a NET of ~23 Wm-2 instead of 356 that is attributed to IR radiation warming the atmosphere for this is NET absorbed from IR radiation from the surface above what it would be if it was never absorbed within the atmosphere.
That is my take after two years of deciphering this subject.
So, 161 Wm-2 absorbed = 80 + 17 + 63 + 1 (NET). Check!
And 239 Wm-2 at TOA = 78 + 80 + 17 + 63 + 1 (NET). Check!
Other papers show variances in these figures but all are closely clustered. It is 23 Wm-2 (63-40) we are talking about in relation to warming by GHGs, not 396 Wm-2.”
First of all, the diagram is depicting a direct surface to space transmittance or ‘window’ transmittance of 70 W/m^2 (40 W/m^2 through the clear sky and 30 W/m^2 through the clouds or cloudy sky).
The surface emitted LW flux is solely due to the surface temperature and nothing else, because temperature is slaved to emitted radiative power via the Stefan-Boltzman law. Now I agree the emissivity of the surface is not 1, but it’s very close to 1 – probably 0.98-0.99+ on average. Taking this into account would only reduce the surface LW flux by about 4-8 W/m^2, which is negligible.
If the surface is emitting 396 W/m^2 in the LW infrared and 70 W/m^2 of this goes straight to space, that leaves 326 W/m^2 of it being absorbed or captured by GHGs and clouds in the atmosphere. Of this, 169 W/m^2 is emitted to space as part of the 239 W/m^2 flux leaving, and by deduction, 157 W/m^2 is returned or re-circulated back to the surface. So the net warming from GHGs is +157 W/m^2 into the surface (not 23 W/m^2). 157 W/m^2 from GHG re-direction + 239 W/m^2 from the Sun = 396 W/m^2 (the net energy flux entering the surface from the atmosphere).
I think you may not be realizing that the non-radiative fluxes from the surface to the atmosphere, from the atmosphere to other parts of the atmosphere, and from the atmosphere back to the surface are just moving energy around within the thermal mass so as to maintain the planet’s energy balance of about a net 396 W/m^2 flux into the surface and about 326 W/m^2 of atmospheric absorption from GHGs and clouds.
RW,
unless you are saying Trenberth is loony the flux entering the surface is simply 161 SW and 333 DLR for a total of 494 absorbed. Convection and evaporation is NOT returned as the heat is lost in the mid to upper trop and mostly radiated directly to space from there.
“First of all, the diagram is depicting a direct surface to space transmittance or ‘window’ transmittance of 70 W/m^2 (40 W/m^2 through the clear sky and 30 W/m^2 through the clouds or cloudy sky).”
No, that is 40 direct from ground + 169 from all atmosphere + 30 from clouds = 239. There is no 70 direct from ground to space.
The 169 would include the portions of the evapotranspiration and thermals as would the 30 from the clouds.
The emissivity of water is .98-.99. The ground ranges from about .93-.98 which includes plants. This is a mistake by Trenberth of a significant amount.
“Taking this into account would only reduce the surface LW flux by about 4-8 W/m^2, which is negligible.”
Trenberth’s missing heat is only .9 w/m2.
“If the surface is emitting 396 W/m^2 in the LW infrared and 70 W/m^2 of this goes straight to space, that leaves 326 W/m^2 of it being absorbed or captured by GHGs and clouds in the atmosphere.”
Again, there is only 40 going from ground to space. The other 30 is being emitted by the clouds. The diagram says what it says. You are overanalyzing something relatively straight forward.
17 thermal + 80 evapot + 396 + 1 = 161 + 333 = 494 (don’t ask me what the 1 is about!!)
The only involved issue is that it takes a number of cycles before the ground is emitting the full amount including the DLR coming back to it. That is, 396 and 333 include amounts that have been counted one or more times already.
A simplified way of seeing it is that when the OLR goes through the troposphere, tropopause, stratosphere… it is not counted when it goes through each layer, it is only counted once for the whole transit. In this diagram, because the flow reverses they count the same energy multiple times instead of taking a net figure. While a meter would “SEE” a flow there is no work being done or there wouldn’t be that large of a flux. Basically the atmosphere has EPICYCLES OF ENERGY!!! HAHAHAHAHAHAHAHAHAHAHA
Tim Folkerts @ November 4, at 3:09 pm
Tim,
You wrote in part (a repeat from a few days ago):
So, you are claiming that ½ + ½ + ½ + ½ + ½ + ½ = 1 ?
The rest of what you wrote seems to be claiming that the vectorial treatment of a photon stream as discussed in the Lambertian optics article I cited, is not applicable to a photon stream originating from the Earth’s surface or within the atmosphere. You don’t adequately explain why, but just reject it. Would you please be kind enough to elaborate why you think this?
Bob F-J,
“So, you are claiming that ½ + ½ + ½ + ½ + ½ + ½ = 1 ?”
And that doesn’t include the energy transferred through collision to non-GHGs!!!
kuhnkat,
I’m pretty sure it’s actually 30 W/m^2 from the surface directly through the clouds to space. The actual ‘window’ transmittance from the cloud tops directly space would be far greater than 30 W/m^2, as there is very little water vapor in between the cloud tops and space (and no clouds either).
“The 169 would include the portions of the evapotranspiration and thermals”
Yes it likely would, but those non-radiative fluxes originated from the surface and were in addition to the surface LW flux of 396 W/m^2. So if more non-radiative flux leaves the surface than returns to the surface on average, the surface will lose energy, cool down and subsequently radiate less. Because the diagram is assuming a steady-state condition, any non-radiative for radiative trade offs effects like this already are already embodied in the surface LW flux of 396 W/m^2.
BTW, I’m not defending the diagram. I think it’s a mess and really not an accurate depiction at all. I’m also aware of the counting of energy more than once, which make it look like there is more energy than there really is.
The bottom line is (ignoring Trenberth’s phony extra watt) there is 239 W/m^2 entering and leaving at the TOA and the surface is receiving a net flux of 396 W/m^2. If you really doubt if my depiction of the numbers is accurate, and you agree the atmosphere cannot create any energy of its own, what then is the origin of the +157 W/m^2 into the surface if not from GHG absorption and re-direction back towards the surface?
RW,
the lower atmosphere is very dense. Only 40 makes it past the first 10 meters (on average of course!!)
If you agree there is double counting why bother with:
“the surface is receiving a net flux of 396 W/m^2. ” The net surface flux is very small as it consists of the energy that is still in the surface each morning when the sun comes up.
According to Trenberth the surface receives 161 + 333 = 494 on average.
17 thermals + 80 evapo + 396 OLR + 1 = 494
Remember that .9 down at the bottom is the NET surface flux that is absorbed in the oceans and the ground and is why the outgoing needs the 1 added.
The 30 confusing you is direct from the surface into the clouds and then radiated by the clouds. The 30 is from the 356 that is going into the atmosphere.
The only values that are measured are the TOA in and out. The rest are estimated (made up).
Moderators,
I forgot to close the italics on the quote ““The 169 would include the portions of the evapotranspiration and thermals” in my last post. Can you fix? Thank you.
RW @ November 1, at 9:30 pm & 9:44 pm, and November 4, at 5:29 pm
Sorry to be so tardy RW; but just a couple of quickies:
• I don’t see 70 leaving the surface direct to space, but only 40. The additional 30 you mention is shown as coming from the high clouds.
• The latent heat is released in clouds when the water vapour condenses into water particles, thus resulting in increased temperature related radiation. It is not returned to the surface via precipitation, whilst there is a complication that some precipitation does not even reach the surface because of intervening evaporation
Hopefully I can find more time to study your comments in more detail soon. Thanks for your big interest
Bob,
“The latent heat is released in clouds when the water vapour condenses into water particles, thus resulting in increased temperature related radiation. It is not returned to the surface via precipitation, whilst there is a complication that some precipitation does not even reach the surface because of intervening evaporation.”
While yes, some of the latent heat that condenses to form clouds ends up radiated into the atmosphere and ultimately radiated out to space. But a lot of it stays with the water and returns to the surface as the temperature component of precipitation. Some of it is also ends up radiated back down to the surface. Some of it can also be replenished by post albedo energy absorbed in the atmosphere from the Sun.
If you doubt this, might I ask you then what is the primary source of energy in the temperature component of precipitation, if not from the latent heat of evaporation?
“I don’t see 70 leaving the surface direct to space, but only 40. The additional 30 you mention is shown as coming from the high clouds.”
You’d have to double check with Trenberth himself on this, but I’m pretty sure the diagram is depicting the 30 W/m^2 flux from the surface directly through the clouds to space. As I said earlier, there is no way there is only a 30 W/m^2 direct transmittance from the cloud tops to space. That is just way too low. But again, neither the 30 W/m^2 or 40 W/m^2 values are really referenced in the paper and appear to be arbitrary estimates or just guesses.
There are really so many things wrong with this diagram, it’s hard to discuss it without their being a great deal of confusion and misinterpretation.
Kuhnkat, you wrote in part:
Trenberth’s missing heat is only .9 w/m2.
Hey look; I have it from a confidential source that Trenberth has calculated the “missing heat” as greater at 0.931459265358979323846 W/m^2, but decided to round-it-off to avoid controversy. Apparently it has something to do with ye old ‘Pi’, a convenient mathematical constant.
Bob,
“I have it from a confidential source that Trenberth has calculated the “missing heat” as greater at 0.931459265358979323846 W/m^2, but decided to round-it-off to avoid controversy.”
You sure they carried the “1” correctly??
I calculated that the emissive power of the entire spectrum @ 15 C is 392 W/m^2.
I calculated also that CO2 at 380ppm absorbs 79.8 W/m^2 of these ‘first generation’ photons to extinction, and H2O at 23000ppm absorbs 248.2 W/m^2 in relatively short distances.
I may be too high with the H2O calc, does anyone else have any figures?
kuhnkat,
“According to Trenberth the surface receives 161 + 333 = 494 on average.
17 thermals + 80 evapo + 396 OLR + 1 = 494”
Yes I know, but 97 W/m^2 (from latent heat and thermals) of the 494 W/m^2 originated from the surface in addition to the radiative flux, so the net flux received at the surface is 396 W/m^2 (494 – 97 – Trenberth’s phony extra watt = 396). Why is this so hard to see? Do you not understand the fundamental T^4 relationship between temperature and emitted power? Whatever temperature a body is radiating at (in this case the surface of the Earth), the amount of energy it is radiating has to be continually replaced or else the body will gain or loss energy and subsequently warm up or cool down. If the surface is emitting 396 W/m^2 in the steady-state, it has to be receiving a net of 396 W/m^2 from the atmosphere in order to sustain it.
“The 30 confusing you is direct from the surface into the clouds and then radiated by the clouds. The 30 is from the 356 that is going into the atmosphere.
Not really. The cloud tops radiate according to their temperature an emissivity. If the direct surface to space transmittance were actually 40 W/m^2 as you claim, the 356 W/m^2 is the amount absorbed by GHGs and clouds in the atmosphere, some of which (half) ends up radiated up out to space and some radiated down back to the surface.
RW,
“there is no way that the clear sky atmosphere is radiating 169 W/m^2 to space.”
You continue to confuse a very simple and straightfoward, although wrong, diagram. The 169 is an AVERAGE and comes from clear sky AND cloud areas, night/day/poles. The 30 from clouds is only the extra from clouds AVERAGED over the whole earth.. That is, remove the clouds and that 66% of the atmosphere would still be radiating depending on the ground temp pumping it. The atmosphere above the clouds would still be radiating unhindered by the clouds whether they are there under it or not. Some cloud areas emit little above average clear sky and others emit a huge amount more. Some clouds actually reflect IR which means they do not allow the atmosphere under them to radiate out. This reduces the net cloud output as the radiation is from the upper surface instead of a 3d opaque atmosphere.
These are GLOBAL AVERAGES that include clear sky and clouds and monsoons and hurricanes…
All the AVERAGING has already been done on these figures. There is no percent taking to be done unless you wish a more detailed analysis.
You are worried about the 169 number Trenberth made up. Try this,
incoming SW absorbed by atmosphere 78
incoming SW absorbed by earth 161
ignoring how it gets back into the atmosphere that is 239 available to make its way out on average 24 hours a day seven days a week… That is about what DOES make its way out. I think we can agree that Trenberth has a problem of distorting the details of reality. Quibbling over which pieces doesn’t get us too far unless we have hard data to show it is different. Notice that 239 doesn’t leave .9 to be absorbed anywhere!!! Trenberth can’t even add!! Of course he wouldn’t want to be too clear because he might have to admit some of that absorbed is in the ground instead of the ocean or photosynthesis or any number of other chemical processes driven by temp that doesn’t spontaneously reverse!!!
Yes, his figures may be off, but, it is a cartoon. If anyone tries to use this to create a GCM they deserve what they get. Willis or anyone else depending on these numbers are wasting everyone’s time. Depending on the imaginary 396 up and 333 down (or whatever it is today) are REALLY wasting our time.
RW,
maybe y’all can answer a question I have. What size meter do all these figures fit. That is, does the 239 w/m2 out cover a meter of the sphere’s surface at orbit, a meter of the sphere’s surface at average radiation altitude, or a meter of the surface of the earth, which varies due to roughness and altitude!! It DOES make a substantial difference. Since they claim it is measured great. Is there a conversion factor or what??
kuhnkat,
Moreover, there is no way that the clear sky atmosphere is radiating 169 W/m^2 to space. According to the ISCCP data, clouds cover an average of about 2/3rds (66%) of the Earth’s surface, which means 1/3 (33%) is clear sky. 396 W/m^2 x 0.33 = 131 W/m^2 emitted to the clear sky, which is less than 169 W/m^2. And this is before even subtracting the ‘window’ of 40 W/m^2. The clear sky cannot emit more to space than is emitted to it from the surface.
One of the biggest problems with Trenberth is he does not clearly separate or distinguish the clear from the cloudy sky atmosphere, which only adds further to the confusion and ambiguity.
kuhnkat,
“You continue to confuse a very simple and straightfoward, although wrong, diagram. The 169 is an AVERAGE and comes from clear sky AND cloud areas, night/day/poles. The 30 from clouds is only the extra from clouds AVERAGED over the whole earth.. That is, remove the clouds and that 66% of the atmosphere would still be radiating depending on the ground temp pumping it. The atmosphere above the clouds would still be radiating unhindered by the clouds whether they are there under it or not. Some cloud areas emit little above average clear sky and others emit a huge amount more. Some clouds actually reflect IR which means they do not allow the atmosphere under them to radiate out. This reduces the net cloud output as the radiation is from the upper surface instead of a 3d opaque atmosphere.”
I’m having trouble understanding exactly what you’re referring to here. Yes I know the 169 W/m^2 depicted is from the whole atmosphere (cloudy and clear sky). I thought you were saying the 30 W/m^2 was the total amount emitted up from the cloud tops. If you’re saying that the 30 W/m^2 depicted is the ‘window’ transmittance from the cloud tops directly to space, I can understand this, although as I said, I dispute the number. I’m also well aware that the whole atmosphere radiates, both below and above the clouds, so I’m not sure what you are implying by this.
RW,
I am not claiming any of the numbers are right either, only that the TOA in and out are probably close.
What I am trying to get across is that Trenberth tried to simplify too much. I THINK he gives the 30 as the difference from what the average clear air and the average cloud is. There would be 33% of the area with 0 extra as there is no cloud. Maybe another 33% with a small extra contribution. So only about 33% of the area has a significant contribution to this 30 that is an average of the total cloudiness spread over 100% of the atmosphere.
Here is a newer cartoon from NASA:
http://mynasadata.larc.nasa.gov/Radiation_Explanation.html
kuhnkat,
“maybe y’all can answer a question I have. What size meter do all these figures fit. That is, does the 239 w/m2 out cover a meter of the sphere’s surface at orbit, a meter of the sphere’s surface at average radiation altitude, or a meter of the surface of the earth, which varies due to roughness and altitude!!”
Interesting question. I believe it means a path straight from the surface to the TOA, globally averaged. Or the shortest distance from the surface to the TOA, globally averaged. So it’s relative to a m^2 from the surface, I think is the answer to your question.
RW @ November 4, at 11:31 pm
You raise an interesting question RW, with:
If you [Bob] doubt this, might I ask you then what is the primary source of energy in the temperature component of precipitation, if not from the latent heat of evaporation?
If I remember correctly, the evapotranspiration of 80 was derived from estimates of rainfall, which was rather simplistic, but I think possibly a little better than a wild guess, and in the too-hard category.
To your issue, I don’t think the precipitation would warm the surface, because it comes from a colder region. Thus, if anything, it might cause further cooling? There would be some warming from braking of gravity driven KE but I guess it would be slight. (friction from air drag and impact with surface)
So I reckon it is a small issue compared with other stuff in the diagram.
~~~~~~~~~~~~~~~~~~~~~~~~~~~~~~~~~~~~~~~~~~~~~~~~~~~~~~
Incidentally, if the 80 is real, it might be that a small change in it would be more significant than the effect of a small change in the net surface radiative effects. I’ve had exchanges with Roy Spencer on this, and it seems that he and everyone are too busy competing on the radiative stuff to look at it. Strange; it could be a bigger negative feedback than in the area they are looking.
Bob,
“To your issue, I don’t think the precipitation would warm the surface, because it comes from a colder region. Thus, if anything, it might cause further cooling? There would be some warming from braking of gravity driven KE but I guess it would be slight. (friction from air drag and impact with surface)
So I reckon it is a small issue compared with other stuff in the diagram.”
I’m not claiming it warms the surface. Just that it’s a significant portion of the energy entering the surface from the atmosphere that is not depicted or accounted for in the diagram. If not primarily from the left over latent heat of evaporation that doesn’t get radiated out to space or back to the surface, then what is the source? Don’t forget too that some of the energy from the latent heat of evaporation condensed in clouds that is radiated into the atmosphere is also replenished from energy from the atmosphere radiated into the clouds, effectively sustaining much of the energy lost from the clouds via radiation.
I also agree that quite often precipitation is colder than the surface, but globally averaged at least, I wouldn’t think this in and of itself has a cooling effect the surface, especially if the energy primarily originated from the surface to begin with. Of course, since precipitation emanates from clouds and clouds reflect sunlight, there is a cooling connection associated with precipitation – just not a direct one.
Bob,
“Incidentally, if the 80 is real, it might be that a small change in it would be more significant than the effect of a small change in the net surface radiative effects. I’ve had exchanges with Roy Spencer on this, and it seems that he and everyone are too busy competing on the radiative stuff to look at it. Strange; it could be a bigger negative feedback than in the area they are looking.”
Agreed. I know Roy’s main focus is on the cloud feedback (i.e. changes in radiative flux at the TOA), but all of these components — the latent heat of evaporation, water vapor and clouds — are all interconnected to one another and constitute the whole of atmospheric the water cycle (ground state water -> evaporation -> water vapor -> clouds -> precipitation -> ground state water), which is the primary thing controlling the planet’s energy balance and ultimately the globally averaged surface temperature.
It’s true that warmer temperatures are associated with increased water vapor from evaporation, but increased water vapor from warming also increases the amount of energy removed from the surface as latent heat of evaporation, which has a cooling effect on the surface. And of course water vapor from evaporation is ultimately what drives cloud formation, and as the clouds form they reflect more and more sunlight, which further cools the surface. While the water vapor feedback by itself is likely positive, it’s ultimately the net combined feedback of all three of these things being positive that is nonsense and cannot be supported by any real science or data.
kuhnkat,
“What I am trying to get across is that Trenberth tried to simplify too much. I THINK he gives the 30 as the difference from what the average clear air and the average cloud is. There would be 33% of the area with 0 extra as there is no cloud. Maybe another 33% with a small extra contribution. So only about 33% of the area has a significant contribution to this 30 that is an average of the total cloudiness spread over 100% of the atmosphere.”
Forgive me, but I just cannot understand what you’re talking about here. Sorry.
The bottom line is the neither the value of 30 W/m^2 or 40 W/m^2 is referenced in the paper, and neither value is even in the right ball park. They are useless, as is most of the diagram.
As I mentioned before, if you really want an accurate depiction (and proof) of the net energy flux in and out of the system, I recommend this one here:
http://www.palisad.com/co2/div2/div2.html
RW @ November 5, at 6:23 pm
Hi RW, I don’t have a significant issue with your thoughts. I agree that precipitation will be initially warmed by the release of latent heat to its ambience. However, there is no doubt that precipitation typically comes from much colder regions than the surface, and that includes ice-hail and snow. You correctly argue that there is less cooling of the surface than if there were not evapotranspiration. It then boils down to whether it is a significant consideration; a matter of scale.
Furthermore I think if Trenberth tried to include every detail in his cartoon, it would be too complicated to follow. Nevertheless, some aspects of his team’s draftsmanship and analysis are very sloppy.
For instance, they show 80 leaving the surface and being released as latent heat, (the same value), in the clouds. However, this cannot be correct. The heat loss from the surface has an un-shown element known as evaporative cooling. In the process of evaporation, the higher energy water molecules are more readily able to escape and become gas, with the consequence that a higher proportion of lower energy molecules are left behind, resulting in cooling of the skin of water etc.
I don’t know the relative scales of this, and probably no one does, but I’d rather drive a nail into the coffin with the S-B consideration, than with these lesser and controversial considerations.
Bob,
“Furthermore I think if Trenberth tried to include every detail in his cartoon, it would be too complicated to follow.”
I agree. This is also the reason I really like the much more straightforward thermodynamic model derived from top level constraints dictated by Conservation of Energy in the paper I referenced. The author is stripping it down the net energy flux, which in the end is what really matters in the system (any system).
Bob,
“For instance, they show 80 leaving the surface and being released as latent heat, (the same value), in the clouds. However, this cannot be correct. The heat loss from the surface has an un-shown element known as evaporative cooling. In the process of evaporation, the higher energy water molecules are more readily able to escape and become gas, with the consequence that a higher proportion of lower energy molecules are left behind, resulting in cooling of the skin of water etc. I don’t know the relative scales of this, and probably no one does, but I’d rather drive a nail into the coffin with the S-B consideration, than with these lesser and controversial considerations.”
I’m not quite sure I understand what you’re saying here. Yes, I agree that the process of evaporation has a cooling effect on the surface or ‘skin’ of the water, but this would not be related to the S-B consideration, which is solely the result of the surface temperature and nothing else (assuming an emissivity of 1 or very close to 1). Any cooling effects like this are already embodied in the surface temperature and the resulting surface emitted LW flux derived from S-B (396 W/m^2 in the case of Trenberth’s diagram). Or am I misunderstanding you?
I am surprised that this paper:
http://www.biocab.org/Mean_Free_Path.pdf
hasn’t yet been thrown into the discussion. Could we be in the wrong building? GK
RW: (and Bob)
I’ve followed the last thirty or forty comments you and Bob have been tossing around and I still think you will never get the correct answers, for you keep mixing apples and oranges together, slicing and dicing them and then having trouble coming back with pure apples upward at the TOA. Don’t add (or subtract, or divide) relative numbers with NET numbers, otherwise you really need a proper, rather complex, program to track all changes IN THE ORANGES that are mixed into the apples (net) occurring simultaneously at all moments. Best to keep all numbers as net transfer of energy.
But, if you insist on keeping the “back-radiation” view, and you can do that, just remember those are relative figures, you need to listen to Bob about energy leaving the surface.
A moist averaged surface (see 80 Wm-2 evapo-transpiration) and a surface in contact with a gas that can conduct, warm, expand, and rise (17 Wm-2 thermals) cannot also radiate at 396 Wm-2 calculated from a 16°C perfectly dry and isolated (no air) surface by S-B and be correct in reality. The net radiation will be 97 Wm-2 less in reality. So the effective temperature radiating from that surface is ((396-97)/5.67)^0.25*100 or 296.5K (-3.6°C). So at least correct Kiehl-Trenberth’s IR figures to 396-97=299 and 356-97=259 and 333-97=236. If you do this you will notices that Bob’s figures at the top in the article’s text are still the same, the NET IR flux leaving the surface is still 63 Wm-2 but now you are one big step closer to reality. You will find this “shift-of-base” really affects nothing else in the graphic, it just corrects one wrong aspect.
BTW, that reduction of radiation happens at the molecular level in the top micro-meter of that averaged surface.
You will also notice that by reducing the 333 Wm-2 to 236 Wm-2 (-17 & -80) and getting the effective “back-radiation” happening right at the surface, √√(236/5.67×10^-8) = 254K or -19°C which is what most people get, on the average, when they point a radiative thermometer upward toward the empty sky. This is empirical evidence, and it’s real, that shows everything I’ve been trying to get you guys to understand is exactly correct. Re-read my comments above.
I can’t believe the peer reviewers let such a gapping mistake through. They were all, everyone of them, wrong. And to add insult the IPCC holds this up as high quality peer reviewed “science”, phooey!
RW @ November 6, at 9:15 am
I meant that the evapotranspiration heat loss at the surface is shown as 80 at the surface, AND also 80 in the clouds, presumably in the form of latent heat loss in condensation. However, there is also evaporative cooling at the surface. What the relative proportions of these two processes are, I don’t know, but I was just pointing out another “simplification”.
The S-B consideration I mentioned is what my article discusses, that it is isotropic, and there are continuous horizontal components which cannot be part of the vertical 396 portrayed by Trenberth. (they are prevented from escaping directly to space because of the opacity of the atmosphere).
The surface temperature, and hence the S-B calculation is a consequence of ALL the processes
wayne,
“A moist averaged surface (see 80 Wm-2 evapo-transpiration) and a surface in contact with a gas that can conduct, warm, expand, and rise (17 Wm-2 thermals) cannot also radiate at 396 Wm-2 calculated from a 16°C perfectly dry and isolated (no air) surface by S-B and be correct in reality.”
I don’t see why not. The 80 W/m^2 of evapo-transpiration and 17 W/m^2 of thermals are in addition to the radiative flux at the surface. These values might be better expressed in equivalent joules rather than W/m^2.
G. Karst,
if you have actual data and science that disagrees with the IPCC meme you are ALWAYS in the wrong building!! 8>)
Thanks for the link.
wayne,
It might be easier to think of the atmosphere as acting as a ‘filter’ between the surface and space, where each ‘pass’ through the filter about 62% of the surface LW flux escapes to space and about 38% is ‘blocked’ and returned or recirculated back to the surface. Of the roughly 390 W/m^2 surface LW flux, only about 240 W/m^2 is allowed to leave at the TOA (240/390 = 0.62) and about 150 W/m^2 is returned to the surface (150/390 = 0.38) in addition to 240 W/m^2 arriving at the surface directly or indirectly from the Sun (240 + 150 = 390).
The physical mechanism for the 150 W/m^2 returned back to the surface is the downward re-emitted half of the surface LW flux absorbed by the atmosphere (either from the heated atmosphere itself or directly from GHGs). Of the 390 W/m^2 LW flux emitted at the surface, about 300 W/m^2 is absorbed or captured by the atmosphere and about 90 W/m^2 passes directly through to space as if the atmosphere wasn’t even there. Of the roughly 300 W/m^2 absorbed by the atmosphere, about 1/3rd is from the fixed or homogeneous GHG components like CO2 and about 2/3rds is from the highly dynamic components of water vapor and clouds, which are driven by evapo-transpiration moved non-radiatively from the surface into the atmosphere. The water is returned to the surface as precipitation and the non-radiative energy (latent heat) that isn’t returned as the temperature component of precipitation contributes the forces driving weather (storms, precipitation systems. wind, lightning, etc).
It’s important to realize also that at the TOA only radiation enters and leaves (i.e. all photons), so all the non-radiative flux from the surface to the atmosphere and from the atmosphere back to the surface all takes place in between the surface and the TOA. To the extent that non-radiative flux leaves the surface, non-radiative flux is also being returned to the surface somewhere else. While it’s entirely possible for some of the non-radiative flux moved from the surface into the atmosphere to radiate into the atmosphere and find its way radiated out to space as part of the 240 W/m^2 flux leaving, all that is happening in this case is non-radiative flux at the surface is being traded off for radiative flux at the surface, requiring the surface to emit less radiative flux to achieve equilibrium output power (240 W/m^2) at the TOA. Any trade off effects like this are already embodied in net energy flux entering the surface from the atmosphere, which also determines the LW flux from the surface into the atmosphere as well as the surface temperature (from S-B).
Bob,
“The S-B consideration I mentioned is what my article discusses, that it is isotropic, and there are continuous horizontal components which cannot be part of the vertical 396 portrayed by Trenberth. (they are prevented from escaping directly to space because of the opacity of the atmosphere).”
You mean that once the 396 W/m^2 surface LW flux is absorbed in the atmosphere it then gets re-emitted isotropically, much of which happens close to the surface and is not depicted in Trenberth’s picture?
“The surface temperature, and hence the S-B calculation is a consequence of ALL the processes.”
Agreed.
G. Karst @ November 6, at 11:52 am, thank you for an interesting citation:
I’ve only read the body text excluding the maths, because I was hung-up on the following extract, and consequently did not want to grind through the mathematical model and so many references:
• Effective path length = 7000 m? Que?
• How can you have a path length of ANY dimension in a vacuum, unless there are annihilators of the photons?
• See also: Robert Stevenson @ November 5, at 3:47 am and October 31, at 8:20 am above giving his calculation that appears to show the free path length, as not less than 2000m for CO2, and 120m for H2O.
• Other blog sources suggest annihilation of surface radiation mostly within 10 metres altitude.
• It seems more than counter-intuitive that a combination of CO2 & H20 could possibly reduce the number of photon intercepts.
OK, let’s argue that I’ve misunderstood, but now I come to some realities of the real world as I see them. Given that CO2 is known to have IR absorptive properties on its own, it seems counter-intuitive to suggest that it has a cooling effect when combined with other absorptive gases. I’m NOT claiming that this is untrue, but the point is that it seems extremely unlikely that this paper would be accepted by any of the iconic journals or the IPCC, or by rampant journalists and politicians.
Thus whilst it may be a matter of great interest, I doubt if it can further the cause of our scepticism. (sadly)
What is more, it is a tad off-topic, and so big that it really deserves a separate thread. Perhaps you could suggest it to Anthony in a few weeks after he has finished his new paper and the BEST circus!
kuhnkat @ November 5, at 6:33 pm, you wrote in part:
“Here is a newer cartoon from NASA: http://mynasadata.larc.nasa.gov/Radiation_Explanation.html
Thanks K, I’ve added it to my library folder called “Funny Arrows”. I don’t know if I’m getting too senile but I couldn’t read all of the text, with the same result when I found two other sources and zoomed and whatnot. Ho hum!
Bob F-J,
I had to zoom to read it also. Fortunately I have a 17″ on the notebook and a 22″ on the desktop. Can’t imagine using a netbook for anything except possibly a phone!!
“G. Karst says:
November 6, 2011 at 11:52 am
I am surprised that this paper:
http://www.biocab.org/Mean_Free_Path.pdf
hasn’t yet been thrown into the discussion. Could we be in the wrong building? GK”
Could be/ 😉
Know if Nasif still frequences Jennifer Marohasy’s site? Haven’t had the time myself in the last few months to check.
>>
Bill Illis says:
October 27, 2011 at 5:47 pm
Modtran results looking up from the surface in the tropics (looking at the back-radiation, the red line) when it is Clear.
http://img171.imageshack.us/img171/4308/rad12081141.gif
Modtran results looking up from the surface in the tropics when there is low cloud cover. A perfect black-body radiating at 20C (and it is cloudy 65% of the time).
http://img171.imageshack.us/img171/7268/tropicalsurfacelookingu.gif
Where is this described in Trenberth’s diagram?
<<
and
>>
RW says:
November 5, 2011 at 10:22 am
One of the biggest problems with Trenberth is he does not clearly separate or distinguish the clear from the cloudy sky atmosphere, which only adds further to the confusion and ambiguity.
<<
In Kiehl and Trenberth 1997, the authors spend several paragraphs on cloud cover. They discuss a model of cloud cover where they take the percent of cloudiness from three levels: 49% low, 6% midlevel, and 20% high. They then use something called “random overlap” to calculate the total cloud cover of 62%. I have no idea what random overlap means, but I tried computing the same 62% using the Inclusion-Exclusion principle.
Now the Inclusion-Exclusion principle requires a specific order of terms and is rather error-prone to account for them properly. I prefer the mathematically equivalent technique of simply taking the complement of the product of the complements: 1 – (1-0.49)*(1-0.06)*(1-0.20) = 0.61648. This gives a similar answer to their 62%.
This supposedly, is the average Earth cloud cover. Their figure 7 should probably read “62% cloudiness assumed,” but it doesn’t. From this point on in their paper, the term “cloudy” is ambiguous. When KT 97 uses the term “cloudy,” do they mean 100% cloudy, 62% cloudy, or something else?
It appears that KT 97’s figure 7 represents 62% cloudiness, and most of the cloudy values that appear in figure 7 are referring to 62% cloudy. Then we have this little statement for computing the atmospheric window:
“The estimate of the amount leaving via the atmospheric window is somewhat ad hoc. In the clear sky case, the radiation in the window amounts to 99 W m−2, while in the cloudy case the amount decreases to 80 W m−2, showing that there is considerable absorption and re-emission at wavelengths in the so-called window by clouds. The value assigned in Fig. 7 of 40 W m−2 is simply 38% of the clear sky case, corresponding to the observed cloudiness of about 62%.”
That “cloudy case” 80 W/m² is a problem. If the 80 W/m² is the value at 62% cloudiness, then the window should be 80 W/m². If the 80 W/m² is the value at 100% cloudiness, then they should interpolate between 99 W/m² and 80 W/m² which gives us a window of 87 W/m². Instead they interpolate between 99 W/m² and 0 W/m², so that 80 W/m² must be a detractor and some other percentage of cloud cover (or it’s just wrong). The computation, 38% of 99 W/m², gives us 37.62 W/m². Why did they round to 40 W/m² instead of 38 W/m²?
There’s no mention of the 40 W/m² window value in their update paper. It just appears in their updated figure. It’s a puzzle.
Jim
Yes, here is another of Nasif’s posts, directly on topic:
http://jennifermarohasy.com/2011/03/recycling-of-heat-in-the-atmosphere-is-impossible/
I am not sure, how I am going to swing on these issues, as my paradigm has been undergoing subtle shifts lately. A lot of that, is directly due to reading WUWT and discussions like this. Old farts, like me, can experience difficulties with such shifting, as it must be downloaded throughout a life time of models and experience.
All I can say, is that, the present state of climate science is mostly a field of RED HERRING data. Fascinating, but we haven’t yet learned to frame the right question! Until we do, none of the “answers” will be very satisfying. GK
G. Karst @ November 7, at 9:18 am and Wayne
I had a quick look at Nasif’s article, “Recycling of Heat in the atmosphere”, which included in part:
This kind of analyses shows a strange “multiplication” of the heat transferred from the surface to the atmosphere and from the atmosphere to the surface which is unexplainable from a scientific viewpoint. The authors of those diagrams adduce that such increase of energy in the atmosphere obeys to a “recycling” of the heat coming from the surface by the atmosphere …
As an old fart engineer myself, I found his reference to HEAT a bit inapproriate. Trenberth shows up-and-down EMR, which is a different form of energy to HEAT, in the classical sense, with quite different behaviour. Certain physicists and climatologists seem to have defined a new meaning for heat in recent times; that it is ONLY thermal energy IN TRANSIT. Not so in engineering, and even the IPCC, NOAA and others don’t seem to have caught-up with it. For example, see these links discussing heat content:
http://www.ipcc.ch/publications_and_data/ar4/wg1/en/ch5s5-2-2.html
http://www.sciencemag.org/content/284/5421/1735.1.full.pdf
http://www.nodc.noaa.gov/OC5/3M_HEAT_CONTENT/
http://journals.ametsoc.org/doi/abs/10.1175/2010MWR3189.1
http://www2.ucdsb.on.ca/tiss/stretton/database/Specific_Heat_Capacity_Table.html
Does anyone know how, why, or when, this wobbly new definition arose?
http://en.wikipedia.org/wiki/Heat
But see the section on semantics
Jim Masterson,
““The estimate of the amount leaving via the atmospheric window is somewhat ad hoc. In the clear sky case, the radiation in the window amounts to 99 W m−2, while in the cloudy case the amount decreases to 80 W m−2, showing that there is considerable absorption and re-emission at wavelengths in the so-called window by clouds. The value assigned in Fig. 7 of 40 W m−2 is simply 38% of the clear sky case, corresponding to the observed cloudiness of about 62%.”
That “cloudy case” 80 W/m² is a problem. If the 80 W/m² is the value at 62% cloudiness, then the window should be 80 W/m². If the 80 W/m² is the value at 100% cloudiness, then they should interpolate between 99 W/m² and 80 W/m² which gives us a window of 87 W/m². Instead they interpolate between 99 W/m² and 0 W/m², so that 80 W/m² must be a detractor and some other percentage of cloud cover (or it’s just wrong). The computation, 38% of 99 W/m², gives us 37.62 W/m². Why did they round to 40 W/m² instead of 38 W/m²?”
That’s interesting. What they should do is use the weighted cloud and clear sky averages. So 80*0.62 + 99*(1-0.62) = 88 W/m^2 (38 W/m^2 through the clear sky and 50 W/m^2 through the cloudy sky), which is much closer to the figures I’ve seen from other sources. It’s generally in the ball park of about 90 W/m^2, give or take a few watts.
The entire edifice of the atmospheric greenhouse effect paradigm, and these flat-earth models which try to depict it, are based on a paradox in the first place.
First, you have the fact that the “atmospheric greenhouse effect” functions nothing like a real, actual greenhouse.
Second, you have the admitted fact (admitted on Climate Etc., and in personal email exchanges with GHE supporters, which I can produce) that the models used to mathematically explain the atmospheric greenhouse effect, and to teach the effect to physics students, are meaningless pieces of fiction which have no basis in reality. This has been admitted and conceded. When asked to present the mathematics which explains the ACTUAL atmospheric greenhouse effect, nothing can be produced.
Third: This would be the first time, that I am aware of after having spent 12 years in learning and teaching astrophysics, that physicists used a piece of fiction, instead of the real thing, to teach what it claimed to be real. We do A LOT of advanced mathematics and physics in astrophysics, including deriving: the equations of General Relativity; Maxwell’s Equations; fundamental theorems of Quantum Mechanics; ALL of Classical Physics; Radiative Transfer in Stellar Atmospheres; Lifetimes of Stars, and much more. In none of these do we use a paradoxical fiction which no basis in reality to teach a concept – the concepts learned are all concepts which exist in real world. That we teach the atmospheric greenhouse effect with a model which has no basis in reality, which is admitted by the proponents of the atmospheric GHE, is a first for science.
We also undergo several years worth of laboratory training and learning exercises: there exists no laboratory demonstration in the world supporting the mathematical theory of the basic function of the atmospheric greenhouse effect, as it is depicted in these (fundamentally tautologous) flat-earth models. It would be one of the most insightful and fundamental physics labs to have ever existed, and it would give EVERY thread of support to the cause of catastrophic AGW. Yet it isn’t performed. The premise is simple: passively trap OLR in a box and make the box hotter than the insolation heating it. Given the insolation on a clear day is about 90C, we should be able to make the box WELL over 100C and boil water inside it by trapping its OLR inside.
Fourth: The flat Earth models depict the Earth as flat. The Earth is not flat.
Fifth: Water melts at 0C. Insolation of -18C could therefore never melt ice.
The physics of heat flow is simple: the Sun heats the Earth surface (and sunlight is HOT, over 120C, and +30C on average as it impinges), the earth surface heats the atmosphere. QED. The atmosphere is NOT a source of additional energy or heat.
For more info:
http://www.tech-know.eu/uploads/Copernicus_Meets_the_Greenhouse_Effect.pdf
http://www.tech-know.eu/uploads/The_Model_Atmosphere.pdf
http://www.tech-know.eu/uploads/Understanding_the_Atmosphere_Effect.pdf
The actually physically-realistic model presented in the “Model” paper has been converted into a proper 1-st order differential heat-flow equation. Such as equation creates no additional heat, but simply describes the flow of heat from hot to cold, in real time, for any location on a rotating, spherical Earth. There is no need for additional heat creation by the atmosphere, and the surface temperature is explained with ease.
The entire edifice of the atmospheric greenhouse effect paradigm, and these flat-earth models which try to depict it, are based on a paradox in the first place.
First, you have the fact that the “atmospheric greenhouse effect” functions nothing like a real, actual greenhouse.
Second, you have the admitted fact (admitted on Climate Etc., and in personal email exchanges with GHE supporters, which I can produce) that the models used to mathematically explain the atmospheric greenhouse effect, and to teach the effect to physics students, are meaningless pieces of fiction which have no basis in reality. This has been admitted and conceded. When asked to present the mathematics which explains the ACTUAL atmospheric greenhouse effect, nothing can be produced.
Third: This would be the first time, that I am aware of after having spent 12 years in learning and teaching astrophysics, that physicists used a piece of fiction, instead of the real thing, to teach what it claimed to be real. We do A LOT of advanced mathematics and physics in astrophysics, including deriving: the equations of General Relativity; Maxwell’s Equations; fundamental theorems of Quantum Mechanics; ALL of Classical Physics; Radiative Transfer in Stellar Atmospheres; Lifetimes of Stars, and much more. In none of these do we use a paradoxical fiction which no basis in reality to teach a concept – the concepts learned are all concepts which exist in real world. That we teach the atmospheric greenhouse effect with a model which has no basis in reality, which is admitted by the proponents of the atmospheric GHE, is a first for science.
We also undergo several years worth of laboratory training and learning exercises: there exists no laboratory demonstration in the world supporting the mathematical theory of the basic function of the atmospheric greenhouse effect, as it is depicted in these (fundamentally tautologous) flat-earth models. It would be one of the most insightful and fundamental physics labs to have ever existed, and it would give EVERY thread of support to the cause of catastrophic AGW. Yet it isn’t performed. The premise is simple: passively trap OLR in a box and make the box hotter than the insolation heating it. Given the insolation on a clear day is about 90C, we should be able to make the box WELL over 100C and boil water inside it by trapping its OLR inside.
Fourth: The flat Earth models depict the Earth as flat. The Earth is not flat.
Fifth: Water melts at 0C. Insolation of -18C could therefore never melt ice.
The physics of heat flow is simple: the Sun heats the Earth surface (and sunlight is HOT, over 120C, and +30C on average as it impinges), the earth surface heats the atmosphere. QED. The atmosphere is NOT a source of additional energy or heat.
For more info:
http://www.tech-know.eu/uploads/Copernicus_Meets_the_Greenhouse_Effect.pdf
http://www.tech-know.eu/uploads/The_Model_Atmosphere.pdf
http://www.tech-know.eu/uploads/Understanding_the_Atmosphere_Effect.pdf
The actually physically-realistic model presented in the “Model” paper has been converted into a proper 1-st order differential heat-flow equation. Such as equation creates no additional heat, but simply describes the flow of heat from hot to cold, in real time, for any location on a rotating, spherical Earth. There is no need for additional heat creation by the atmosphere, and the surface temperature is explained with ease.
Sure it is a caution flag… do you know of a climate paper without any?
I am not advocating Nasif’s hypothesis nor Nasif himself. Investigation i/p. GK
G. Karst @ November 7, at 9:44 pm
I may have been a bit harsh on Nasif for him labelling EMR as HEAT. For instance he might just be going-along with the recent definition a la Wikipedia and various supporters of it such as Tim Folkerts and R. Gates on this thread. Trouble is, I get too grumpy sometimes when physicists and climatologists re-write the rule books.
But hey, I’ve just had one of those eureka moments. Remember how Trenberth wrote that infamous Email describing inability to find his missing heat as a travesty?
Well, since the elite definition is that heat is a transient thing, and not capable of residence in matter, then obviously, his heat is not missing, but still in transit!
“Bob Fernley-Jones says:
… then obviously, his heat is not missing, but still in transit!”
HAHAHAHA! There you go. Someone has left the trap door open to space wide open!
Off the top, one thing I see questionable in Dr. Nahle figures, and this does not invalidate his work, is simply where he converts the flux to ‘per steradian’. One I wonder why he is doing this for those units are generally not used in climate science and he is dividing by pi, not 4π if the four is already encapsulated in the previous terms. That I question. If that conversion is incorrect those figures listed as W/m^2/sr are really one fourth of his results but such an innocent slip is easily corrected.
This is playing right into my comment above of the total ‘real’ and net upward flux after removing the window portion is a mere 23-24 Wm-2 (63-40) per Trenberth et al’s figures, a fraction of what most people seem to think is happening as energy leaves the surface and is transferred to space, net-wise that is.
That is why it takes twelve hours for the air at night to cool a mere 8-12°C each night without further energy input. About the same energy as a small 24 watt CFL per meter squared.
RW, I might clarify, for you were speaking as if I was unaware that the thermal and evapo-transpiration figures listed in the graphic converts to IR. That’s not true. The problem is these figures are net figures which means there is 17 and 80 Wm-2 of energy per second that leaves the Earth system by these processes and that transfer ALREADY includes the conversion to IR outward, so, don’t remix this energy back into the atmosphere and then try to further move it around (“back-radiation”). Look in the papers listed as references at the bottom of Kiehl-Trenberth’s papers or Miskolczi’s papers where these figures were derived from other papers. For you are right, all energy lose from the Earth system is by IR in the end. Keep the net figures separated from the relative figures.
That incorrect mixing of net and relative figures IS the paradox in the cartoon. It will drive you mad!
Anyway, that’s the way I understand it.
I might add to:
“That is why it takes twelve hours for the air at night to cool a mere 8-12°C each night without further energy input. About the same energy as a small 24 watt CFL per meter squared.”
that this decrease in temperture also includes the window flux of course. Someone could read that dirrerently as if I didn’t understand the physics. I just assume people also realize that and I should not assume that, sorry.
wayne,
“RW, I might clarify, for you were speaking as if I was unaware that the thermal and evapo-transpiration figures listed in the graphic converts to IR. That’s not true. The problem is these figures are net figures which means there is 17 and 80 Wm-2 of energy per second that leaves the Earth system by these processes and that transfer ALREADY includes the conversion to IR outward, so, don’t remix this energy back into the atmosphere and then try to further move it around (“back-radiation”). Look in the papers listed as references at the bottom of Kiehl-Trenberth’s papers or Miskolczi’s papers where these figures were derived from other papers. For you are right, all energy lose from the Earth system is by IR in the end. Keep the net figures separated from the relative figures.
That incorrect mixing of net and relative figures IS the paradox in the cartoon. It will drive you mad!
Anyway, that’s the way I understand it.”
I’m not sure I understand exactly what you’re saying here, but perhaps you can clarify for me – do you know that the non-radiative flux at the surface is in addition to the radiative flux at the surface, and that the radiative flux at the surface is solely due to to the surface temperature and net energy flux entering the surface from the atmosphere?
RW @ November 6, at 8:21 pm
Whoops RW, I overlooked this that you wrote:
You [Bob_FJ] mean that once the 396 W/m^2 surface LW flux is absorbed in the atmosphere it then gets re-emitted isotropically, much of which happens close to the surface and is not depicted in Trenberth’s picture?
Well yes, sort of, but the real point was that Trenberth shows the vertical flux as 396, but it can’t be when a significant part of the 396 is horizontal and therefore not vertical. (the horizontal stuff is constant under Trenberth’s uniform conditions)
Bob Fernley-Jones says:
November 7, 2011 at 5:36 pm
G. Karst @ November 7, at 9:18 am and Wayne
I had a quick look at Nasif’s article, “Recycling of Heat in the atmosphere”, which included in part:
This kind of analyses shows a strange “multiplication” of the heat transferred from the surface to the atmosphere and from the atmosphere to the surface which is unexplainable from a scientific viewpoint. The authors of those diagrams adduce that such increase of energy in the atmosphere obeys to a “recycling” of the heat coming from the surface by the atmosphere …
As an old fart engineer myself, I found his reference to HEAT a bit inapproriate. Trenberth shows up-and-down EMR, which is a different form of energy to HEAT, in the classical sense, with quite different behaviour. Certain physicists and climatologists seem to have defined a new meaning for heat in recent times; that it is ONLY thermal energy IN TRANSIT. Not so in engineering, and even the IPCC, NOAA and others don’t seem to have caught-up with it. For example, see these links discussing heat content:
Also, they now define heat as the net exchange of photons, i.e., they have given every photon thermal properties so that heat also flows from colder to hotter. This they say still obeys the 2nd law, that it is the net exchange of these which gives the flow always hotter to colder. They have no explanation of the mechanism in place which results in this obeying the 2nd Law. Having no such mechanism, there is nothing to stop the heat flow from colder to hotter overwhelming in the exchange and thus breaking the 2nd Law.
This “net” is their justification for ‘atmospheric background upwelling from Earth back-radiating and heating up the Earth further.’ Some no longer defend that, saying instead that it is the ‘blanket qualities of CO2 trapping heat slowing the heat loss from that upwelling from Earth, the more CO2 the thicker the blanket the more heat gets trapped in the atmosphere’.
In other words, to them all EMR is the same, claiming all photon energies create heat on being absorbed. They have jettisoned the classic differences between Light and Heat which is how they get to claim Light, Solar (UV/Visible/Nr Infrared) the actual electromagnetic energies which physically converts to heat land and oceans. They claim that Thermal Infrared direct from the Sun doesn’t even reach the Earth’s surface and so plays no part in physically heating land and oceans, the only thermal infrared in their models is that which is upwelling from Earth after being heated up by Light, and this back-radiating/being trapped.
They have no idea what they are talking about.
Paradoxes have some logic, there is no logic in their claims because they swap properties around and take laws out of context.
You’re arguing against these physically impossible basic premises as if they are real and then wonder why their arguments don’t make sense..
Bob,
“Well yes, sort of, but the real point was that Trenberth shows the vertical flux as 396, but it can’t be when a significant part of the 396 is horizontal and therefore not vertical. (the horizontal stuff is constant under Trenberth’s uniform conditions)”,
Forgive me, but I’m still not quite I understand what you mean here. The radiative flux emitted by the surface is an upward flux in the direction of space (i.e the TOA), is it not? Now, I do realize that some of the surface is not perfectly perpendicular to space, so not all the radiative flux from the surface is emitted perfectly straight up, but this would be negligible relative to the total upward flux, especially given over 2/3rds of the surface is water.
Or am I still misunderstanding you?
In other words, they have actually excluded the real heat from the Sun from the direct downwelling from Sun to Earth and they have given the property of this heat, the Sun’s real thermal energy, to the Shortwave Solar energies (UV/Visible/Near Infrared) direct from the Sun to Earth, the mnemonic meme: Shortwave in Longwave out. They have not only missed out the real heat from the Sun, whatever amount of energy that actually is, but by giving this property to Shortwave which it doesn’t have, they are short of the real thermal energies direct to Earth from the Sun, THIS is the only missing heat worth looking for..
If, the energy budget is ‘energy from the Sun to Earth which should equal the energy from Earth to Sun’, then their energy budget is junk because they’re missing the actual heat from the Sun from their calculations, and so are your calculations all who are arguing as if their energy budget figures from the Sun are real physics.
What we really have – there is energy from the Sun direct to Earth which is Heat, that is, the actual thermal energy of the Sun radiating to Earth and heating up directly, land, oceans, and us critters and cabbages which is thermal infrared, longwave, and, there is Solar energies from the Sun, Light, shortwave, which is taken in by the land and oceans and us critters and cabbages which does not directly heat these, but, which in being utilised within the processes of life on Earth can also produce heat. For example in photosynthesis, the Light energies are chemical not heat creation, the creation of sugars, but once the sugars are burned by the plant for energy in growth this produces heat, which the plant transpires in the process. When the confusion creation by their science fiction is sorted, when Light is given back its own properties which does not include Heat, these energies can also be seen to produce more non-thermal lights in their electronic transition absorptions, in reflecting/scattering in the atmosphere for example. So:
There are two main distinct processes at work in any real energy budget. Downwelling direct from the Sun to Earth must include all energies and the direct upwelling from the Earth must then balance with the all downwelling direct from the Sun, is the premise being worked to. How this direct upwelling of Heat from the Earth is apportioned to the real Light and Heat energies direct from the Sun to Earth is what the real question is. Since not all photons are thermal, Light is not Heat, not all the upwelling direct from the Earth is Heat, some of it is Light, that reflected from the surface or from light produced on Earth, fires, cities aglow at night with electric light… And being Light and not Heat, it will not heat the atmosphere in upwelling from Earth, any more than it heats the atmosphere on the way down from the Sun; the Visible Light energies absorbed by the electrons of the molecules of oxygen and nitrogen in the atmosphere are reflected/scattered which does not create heat, because visible is not hot, it is not heat, it is not thermal, it is not capable of moving molecules to vibrational states which creates heat, but, it is still in the budget of the energies from the Sun upwelling if a straight energy balance is established.
Their more than wobbly definition of Heat arose to better promoted their agenda, which was to provide a scientific sounding base to push the AGW agenda. No internal consistency is required, the object is to confuse not enlighten.
Myrrh,
“Also, they now define heat as the net exchange of photons, i.e., they have given every photon thermal properties so that heat also flows from colder to hotter. This they say still obeys the 2nd law, that it is the net exchange of these which gives the flow always hotter to colder. They have no explanation of the mechanism in place which results in this obeying the 2nd Law. Having no such mechanism, there is nothing to stop the heat flow from colder to hotter overwhelming in the exchange and thus breaking the 2nd Law.”
There’s no violation of the 2nd law with the greenhouse effect or additional CO2 induced warming because its not about energy going from cold to hot through a conduction process. Do you actually think that a re-emitted photon cannot travel from the colder atmosphere toward the warmer surface? If yes, how do the photons from the Sun travel through the colder space and then through the colder atmosphere to the surface of the Earth? EM radiation emitted in the atmosphere does not have a temperature or thermal energy. It’s only when EM is absorbed by matter that the energy gets transferred into heat or thermal energy (i.e. absorbed by a GHG molecule and transferred to the other more abundant gas molecules in the atmosphere via collisions, which heats the atmosphere).
Don’t forget that the Sun is constantly adding energy to the system. The colder atmosphere is not heating the surface – it’s reducing the rate the incoming energy from the Sun, which is mostly transparent through the atmosphere, can leave the system.
The GHE in principle is not much different than the interior of a car heating up inside on cold day from sitting out in the Sun. The Sun’s energy is mostly transparent through the windows. It’s then absorbed and re-radiated by the interior car components. The rate at which the energy is entering the interior is faster than the rate the re-radiated energy can leave the interior; therefore, the interior has to heat up. The colder exterior components of the car are not heating the interior – they are slowing heat loss.
“This “net” is their justification for ‘atmospheric background upwelling from Earth back-radiating and heating up the Earth further.’ Some no longer defend that, saying instead that it is the ‘blanket qualities of CO2 trapping heat slowing the heat loss from that upwelling from Earth, the more CO2 the thicker the blanket the more heat gets trapped in the atmosphere’.
In other words, to them all EMR is the same, claiming all photon energies create heat on being absorbed. They have jettisoned the classic differences between Light and Heat which is how they get to claim Light, Solar (UV/Visible/Nr Infrared) the actual electromagnetic energies which physically converts to heat land and oceans. They claim that Thermal Infrared direct from the Sun doesn’t even reach the Earth’s surface and so plays no part in physically heating land and oceans, the only thermal infrared in their models is that which is upwelling from Earth after being heated up by Light, and this back-radiating/being trapped.”
The use of the word ‘trapped’ is an all too common misnomer. No energy is ‘trapped’ – its exit from the system is just delayed (and not delayed very long). In effect, the re-emitted downward half of the isotropic radiative emission in the atmosphere keeps the surface warmer by re-directing some of it back in the direction of the surface, slowing the rate at which the incoming energy from the Sun can leave the system.
For example, having reduced everything used in their climate base premises to gobbledegook they can make claims such as the following:
No it doesn’t, because they have excluded the thermal infrared direct from the Sun to Earth which really heats the Earth and this is what is, logically, actually being measured, at least majority. The only sensible measurements of ‘background radiating back to Earth from the atmosphere’ have to be minus the direct from the Sun thermal infrared they’ve excluded. They could try night time..
My conclusion is, that y’all arguing against their energy budget figures are going to continue to go around in circles until you get to grips with how they have mangled the basic physics premises in their energy budget.
They are giving the properties of Heat to Light, this is AGWScience Fiction. Saying that: Light, sunlight, shortwave, is now thermal. That this Light passes through the atmosphere unabsorbed. That this Light heats oceans. That this Light directly heats the Earth. That Heat, thermal infrared, direct from the Sun does not heat the Earth.
In the real world of physics: Light is not Heat. Light is not thermal. Light does not pass through the atmosphere unabsorbed. Light does pass through water unabsorbed. Light does not directly heat the Earth. The Sun’s Heat, thermal infrared which is the thermal energy of the Sun on the move to Earth, does directly heat the Earth.
Without directly dealing with their fictional base premises, arguing about what the ‘backradiating’ infrared is doing and what they haven’t included and so on, is a complete and utter waste of your time.
RW says:
November 8, 2011 at 6:50 pm
Myrrh,
“Also, they now define heat as the net exchange of photons, i.e., they have given every photon thermal properties so that heat also flows from colder to hotter. This they say still obeys the 2nd law, that it is the net exchange of these which gives the flow always hotter to colder. They have no explanation of the mechanism in place which results in this obeying the 2nd Law. Having no such mechanism, there is nothing to stop the heat flow from colder to hotter overwhelming in the exchange and thus breaking the 2nd Law.”
There’s no violation of the 2nd law with the greenhouse effect or additional CO2 induced warming because its not about energy going from cold to hot through a conduction process. Do you actually think that a re-emitted photon cannot travel from the colder atmosphere toward the warmer surface? If yes, how do the photons from the Sun travel through the colder space and then through the colder atmosphere to the surface of the Earth? EM radiation emitted in the atmosphere does not have a temperature or thermal energy. It’s only when EM is absorbed by matter that the energy gets transferred into heat or thermal energy (i.e. absorbed by a GHG molecule and transferred to the other more abundant gas molecules in the atmosphere via collisions, which heats the atmosphere).
Heat is transferred by radiation, you have excluded that just as you have excluded the thermal energy from the Sun directly heating Earth. You don’t understand the difference between Heat and Light because you are repeating science fiction memes, not real physics.
There are three ways heat is transferred, by conduction, convection and by radiation. It is actual thermal energy travelling to us from the Sun, not indistinguished ‘photons of electromagnetism’ which then somehow create heat when absorbed.
The Visible light from the Sun is absorbed by the atmosphere. It is absorbed by the electrons of the molecules of oxygen and nitrogen, this is what reflection/scattering is, this is why the sky is blue (blue light being smaller and even more nervy than red visible, gets scattered and bounced around the sky more easily), this is categorised as electronic transitions. Electronic transitions work on the smaller electron level of molecules, they are not powerful enough to move the whole atom or molecule into vibrational states which is how matter is heated
But, since you claim that visible photons absorbed create heat, how much heat does this create in the molecules of oxygen and nitrogen in our atmosphere? Where is this in your energy budget?
You are repeating science fiction memes, that’s why there is no logical consistency in your physics. Your memes do not describe the real physical world around us.
The thermal energy of the Sun is Heat. That thermal energy of the Sun is transferred direct to the Earth by radiation, one of the three methods of Heat transfer. That thermal energy from the Sun in radiation is thermal infrared. It is the actual heat from the Sun on the move to us. It is invisible. We can feel this heat radiating out from hot pavements, stoves and so on, which do not have any visible light. It is a powerful energy. It is greatly absorbed by water. Heat, thermal infrared radiation, penetrates your body and heats the molecules of water in your body, our bodies are mostly water (and around 20% carbon). Heat warms us up from the inside.
Light, does not do this.
“The GHE in principle is not much different than the interior of a car heating up inside on cold day from sitting out in the Sun. The Sun’s energy is mostly transparent through the windows. It’s then absorbed and re-radiated by the interior car components. The rate at which the energy is entering the interior is faster than the rate the re-radiated energy can leave the interior; therefore, the interior has to heat up. The colder exterior components of the car are not heating the interior – they are slowing heat loss. ”
This NOT what causes the heating inside a car. The heat generated inside the car is induced by the original insolation upon the surfaces inside the car. The windows serve to trap the hot air which is then generated by conduction with the surfaces. This is how a real greenhouse works, and it is NOT “in principle” the same way the atmospheric greenhouse is claimed to work. The rate at which energy leaves the interior will eventually equilibrate with the rate of energy coming into the interior – at this point there is radiative thermal equilibrium. This is just like the radiative thermal equilibrium with the Sun which keeps the Earth at -18C on average. The car will NOT become hotter inside than the insolation inducing the temperature upon the surfaces, just like the Earth is NOT hotter than -18C on average. The radiative temperature of the Earth IS -18C as measured by satellites in outer space. The surface temperature specifically – a very small component of the entire ensemble – is supposed to be warmer than the average due to the natural sorting of the temperature of a gas in a gravitational field.
So you see here in the quotation above the prototypical example of illogic and paradox in the GHE paradigm. Note the wording: “The ghe is [IN PRINCIPLE NOT MUCH DIFFERENT]…” Alright, “in principle”. But in reality, a real greenhouse does NOT work by trapping radiation, but by trapping hot air and preventing its convection. A real greenhouse is warmed by the direct and original insolation into it. So then the argument becomes a description of something that ISN’T. We describe a greenhouse to work a way that it DOESN’T, in order to say this establishes the desired GHE in the atmosphere. We argue like this nowhere else in physics.
The goal here, obviously then, is to invent some radiative scheme by which CO2 can be blamed. That’s the entire purpose of this paradigm. CO2 does not prevent convection. If it could prevent convection, THAT would be away in which it behaved similar to a greenhouse. But CO2 is a negligible component of the atmosphere…it does not prevent convection.
The vast majority of heat in the atmosphere is gained by direct conduction with the heated ground surface; the second majority is achieved by direct heating from insolation due to the extinction of sunlight. And CO2 has one more degree of freedom that the entire rest of the 9(% of the atmosphere doesn’t have: it can shed heat by radiating it away via a molecular vibration. All the other gases in the atmosphere just get heated and then hang on to it, unable to shed it away by radiation. CO2, as a radiating gas, has a much easier time shedding the energy it collects. So neither does CO2 prevent convection, it also doesn’t prevent radiation! It’s able to radiate (cool) when the rest of the atmosphere CAN’T.
Illogic and paradox IS the paradigm of climate science and the GHE. One best example is the question as to lag of CO2 to temperature in the ice-core records – the answer being, from Gavin Schmidt: just because CO2 didn’t cause the start of the warming, doesn’t mean it didn’t cause the rest of it.
It was that statement that turned me into a skeptic – read, proper scientist – and I have never found a solid line of logic or reasoning to welcome me back to the AGW cause.
RW November 8, at 6:01 pm
Forgive me, but I’m still not quite I understand what you mean here. The radiative flux emitted by the surface is an upward flux in the direction of space (i.e the TOA), is it not? Now, I do realize that some of the surface is not perfectly perpendicular to space, so not all the radiative flux from the surface is emitted perfectly straight up, but this would be negligible relative to the total upward flux, especially given over 2/3rds of the surface is water.
Or am I still misunderstanding you?
Well; Trenberth’s 396 is primarily based on an S-B calculation of a daft assumed global-year average surface temperature and stuff. By S-B definition, this is not a vertical flux, but isotropic. If the atmosphere were transparent then it would all radiate directly to space, in which case by integration it could effectively be described as a vertical flux. However, much of the radiation tends towards the horizontal or tangential to the surface, but is annihilated by absorptive gasses and stuff. Thus the horizontal vector components do not make it to space, but are still part of the 396, but not in the field of view of the vertical. (normal view). Thus these horizontal vector components should be vectorially deducted from the hypotenuse of the standard vector diagram to give the vertical component.
See item 5) in the article, and also next, a quote from a comment I made to Tim Folkerts on 3/Nov:
All this ignores whatever radiation that may be escaping directly to space, including that from “convective” stuff.
Bob,
“Well; Trenberth’s 396 is primarily based on an S-B calculation of a daft assumed global-year average surface temperature and stuff. By S-B definition, this is not a vertical flux, but isotropic.”
This is what I still don’t understand. Yes, the body of the Earth as a whole emits radiation from its surface in all directions out to space because it’s a sphere, but the emission from the localized surface itself is not isotropic but more or less straight up to space.
Now, it’s true that the spherical curvature of the Earth would have a slight effect on the isotropic emission from the atmosphere, as tiny fraction (1% or less) of the more sideways downward half would go over the horizon, but again the amount is negligible. I don’t think this is what you’re referring to though, is it?
Bob,
“See item 5) in the article, and also next, a quote from a comment I made to Tim Folkerts on 3/Nov:
Concerning your belief that a photon stream cannot be analysed with component vectors, please see this brief summary of Lambert’s cosine law. It is written from the perspective of optics, and you may not like the semantics. However, if you look at the far right of figure 1, see how when the incoming light is at a shallow angle it is “reflected” less but still isotropically. This is because the vertical component is small, whereas the horizontal, which cannot be absorbed or reflected, is a large proportion of the light stream.
http://escience.anu.edu.au/lecture/cg/Illumination/lambertCosineLaw.en.html
All this ignores whatever radiation that may be escaping directly to space, including that from “convective” stuff.”
I’m not following this. The radiation entering Earth’s surface, either from the SW directly from the Sun or from the LW from the atmosphere, is all absorbed and re-radiated — not reflected. Are you trying to say that radiation from one side of a flat surface is multi-directional over a 180 degree plane?
Bob!! I’m jumping up and down with glee! Someone finally sees that aspect clearly, for your words are now as I would speak them myself. I feel like taking a break and passing the baton to you, you seem to explain it better than I to others, no one has undestood me in nine months.
After reading what you just said to RW, there is one point you might be sure to include in your description… that near horizontal radiation from the surface, that would have to pass through some 50 km of atmosphere to make it to space, of course, will be absorbed at some point, being IR and passing through such a great amount of mass. This cools the spot where the radiation was released and warms where it is absorbed, BUT, the opposite is always occurring from that far point back to the point of initial radiation but a tiny residual exists for 89 degrees from vertical is not exactly horizontal, but close. Since all atmospheres have a lapse rate that far point will be some amount ABOVE the surface where it is cooler and that far point will never pass the same amount back to the initial point by symmetry. That tiny difference when integrated across all directions in the hemisphere (degrees from vertical) will end up being the 63 Wm-2 (actually exactly 1/6) you began this post with (396-333).
And, we are not just speaking of 89 degrees from vertical, that was just an exaggerated example, but the same occurs for any degree you pick but the parameters will flex of course. If at 60 degrees it would have to pass through twice the mass to escape. The rest is geometric adjustments.
What I’m saying is don’t forget the symmetrical passing back and forth at all points and in all directions or someone will pick on that very point to create a meaninglessly argument. It is this back and forth, cooling when emitted, warming when absorbed that causes the cancellation of the energy transfer by symmetry, not of the energy itself of course!
I always like to equate that to what is happening in any room you happen to be siting in, the back and forth between the walls at the same temperature. Some see gazzilion photons flying back and forth from all points at all times in all directions even if temperatures are identical, so let them, for that view does not really change anything we have been discussing, just a different way to look at the same thing. (Actually I personally don’t even “believe” that of photons, if real at all, for it is more of a standing wave between any two points if at identical temperatures, there is no physical reason, even at the microstate level, for the spontaneous emission to even occur at all (again, see Hans physics link on that subject, and I thank him for that, and at such a perfect time)). But discussion on that get to deep for here.
RW @ November 8, at 8:23 pm AND 8:36 pm.
It was experimentally determined by Stefan that a small flat surface radiates isotropically, (hemispherically), and Boltzmann later derived the formula mathematically. (via integration of Planck distribution and solid angles). As far as I’m aware, this is well accepted by the scientific community, although it should probably only apply on a small scale when in an absorbent atmosphere including the Earth’s surface; a single body situation. When there is an interaction between two bodies it becomes complicated by relative geometry and field-of-view considerations. When there is interaction between a body or bodies and an absorptive gas, it appears to be a bit of a mystery in the literature. (and certainly to me). In general engineering, the field of view between two or more bodies is sensibly all that is considered in the earthbound scale of things.
See also items 1), 2), & 3) in the article
One thing that should be noted from the diagram at the top of this article is that 396 W/m² is leaving the Earth’s surface in the form of LWIR and there is 333 W/m² coming right back from the atmosphere. As indicated by the author, this says that only 63 W/m² is actually escaping from the surface by LWIR and the 333 W/m² represents a trapped local energy flowing up and down. The 40 W/m² figure would seem to be bogus or unexplained.
Assume that the 333 W/m² number represented the energy flowing downward as observed one micrometer above the surface. In that case, there is no consideration of energy flowing sideways. 63 W/m² must be leaving the surface. Once that energy gets into the atmosphere, it can go anywhere it wants as long as it does not change the observed energy readings flowing up or down.
The figure also says that 239 W/m² is actually escaping from the Earth. This would suggest that the additional 176 W/m² must be radiated by the atmosphere. The atmosphere cannot be creating this flow except as it is warmed by the Sun and the Earth. The diagram also says that thermals and evaporation result in a energy flow of 97 W/m² from the surface into the atmosphere. Thus, solar heating must be adding 79 W/m² to the energy in the atmosphere. The diagram says this is 78 W/m², so there is only a 1 W/m² missing energy flow. The net total energy flow from the surface would thus be 160 W/m² escaping as LWIR to outer space. The diagram says 161 W/m² is arriving as non-reflected solar energy. Again, there is a 1 W/m² discrepancy. Perhaps this is covered by the unexplained “Net absorbed 0.9 W/m².”
Note that one can use the University of Chicago hosted MODTRAN utility to get a picture of Air Force model *predicted* radiative heat flow in the atmosphere by changing the sensor altitude settings and subtracting the down flowing energy (sensor looking up) from the upward flowing energy (sensor looking down) to measure the ‘outward energy flow’ at levels in the atmosphere up to 99 km. MODTRAN results nominally apply to energy flows over the wavenumber range of 100 to 1500 kayzers (cycles per centimeter, CM-1).
wayne @ November 8, at 10:23 pm
Wayne,
Concerning your doubts about wave-particle duality theory, if you do a Google search on:
photon two-slit
There is some interesting stuff, such as:
http://en.wikipedia.org/wiki/Double-slit_experiment
Bob, I’m well aware of the double-slit experiment and the duality. Don’t take me wrong, it’s just that I have also read some hundred papers, books, discussions in physics over the years with some very good physicists knowing worlds more than myself on the subject, both sides. I’ll talk photons, use them, and they are great way to have others visualize the quantization of energy, but, firm “belief” they concretely exist only as photons and no waves… I have seen no conclusive experiment results that such particles actually exist, as some physicists have pointed out, possibly just mathematical and statistical in nature, maybe not. In that subject lie some other very interesting paradoxes. But I didn’t mean anything negative, use them, I do.
You know, if someone were to design an experiment that proved light was actually just E/M waves and why they appear to be particles, it wouldn’t surprise me. Same on the flop side. As far as I know the duality still exists, really. Or… maybe I just missed the true answer somewhere along the way. ☺
Bob asks “So, you are claiming that ½ + ½ + ½ + ½ + ½ + ½ = 1 ?”
Suppose 100,000 people leave a stadium after a big game, driving out across the state to their homes. After tracking the people to their homes, i discover that
* 50,000 end up north of the stadium; 50,000 end up south of the stadium.
* 40,000 end up east of the stadium; 60,000 end up west of the stadium.
* 70,000 end up at a higher elevation than the stadium; 30,000 at a lower elevation
I’m sure neither of us thinks 300,000 people left the big game.
People are not vectors; photons are not vectors. A single photon can head east, north and up, being counted in each group. Even if it is heading more east than up, it is still heading up (as opposed to down).
Now, you could divide the photons into those heading more vertical (either up or down) than horizontal, in which case the first group has 2/6 of the photons and the other group has 4/6 of the photons. But the 4/6 that are “mostly sideways” still are divided in half as either generally upward or generally downward. No matter how you slice it, 1/2 of the isotropic radiation from a chunk of atmosphere is upward and 1/2 is downward. Not matter how you slice it, ALL of the photons from a flat part of the earth’s surface have an upward component and carry energy upward (at least until some get absorbed by the atmosphere.
Bob,
“It was experimentally determined by Stefan that a small flat surface radiates isotropically, (hemispherically), and Boltzmann later derived the formula mathematically. (via integration of Planck distribution and solid angles). As far as I’m aware, this is well accepted by the scientific community, although it should probably only apply on a small scale when in an absorbent atmosphere including the Earth’s surface; a single body situation. When there is an interaction between two bodies it becomes complicated by relative geometry and field-of-view considerations. When there is interaction between a body or bodies and an absorptive gas, it appears to be a bit of a mystery in the literature. (and certainly to me). In general engineering, the field of view between two or more bodies is sensibly all that is considered in the earthbound scale of things.
See also items 1), 2), & 3) in the article”
Very interesting. I have to admit I’ve never really given this much thought, but I think Tim Folkerts is likely correct when he says “Not matter how you slice it, ALL of the photons from a flat part of the earth’s surface have an upward component and carry energy upward (at least until some get absorbed by the atmosphere).”
I think the same could be said of the upward half of isotropic emission from the atmosphere itself that passes into space. It’s not all going straight out to space perpendicular to the surface, but it’s going in a direction that goes out to space, which is what really matters in the end.
Tim, no one said a photon is a vector. No one said you can split a single photon. However, it was said that a photon’s movement, or e/m wave, is a vector, you know, energy transfer. It seems you are having trouble following what has been said above. Perhaps a detailed re-read might help.
Wayne says:
>However, it was said that a photon’s movement, or e/m wave, is a vector, …
Yes. Fine.
>… you know, energy transfer.
Nope. You slipped up. A transfer of energy is NOT a vector and cannot be compared with things like the direction of the photon. If 1 liter of gasoline enters my gas tank at any angle, then 35 MJ of energy enters. The movement of the gasoline is certainly a vector, but the energy transferred is not. If 396 J of photons pass thru a surface at 45 degrees, 396 J of energy is transferred. Energy transfer is NOT a vector.
That said, I agree there are many aspects of vectors that are indeed important in the analysis here. The directions of the photons must be accounted for when determining how many photons actually end up reaching a particular surface.
Bob says: “For instance, they show 80 leaving the surface and being released as latent heat, (the same value), in the clouds. However, this cannot be correct. The heat loss from the surface has an un-shown element known as evaporative cooling. ”
I am pretty sure the 80 W/m^2 _is_ the evaporative cooling you speak of. The surface is losing those 80 W/m^2, cooling the surface. When the water re-condenses (in clouds), the air is warmed by that amount. I’m not sure what additional cooling of the surface you are thinking of, or where that energy comes from/goes to.
Bob,
So I think I now finally understand what you’ve been saying. I think you are correct that the emission from the surface is equally multi-directional, but I think Tim F. is correct in saying it doesn’t matter because it’s still in an upward direction away from the surface toward space. For calculations and all practical net energy flux depictions from the surface to the atmosphere, it doesn’t matter, or it’s still accurate to depict it as all being emitted more or less perpendicular to the surface toward space.
Think about it from this perspective. The upward half of isotropic emission from the atmosphere itself that ultimately passes into space. It’s not all going straight out to space in a direction perpendicular to the surface, but it’s none the less going in a direction that goes out to space, which is what really matters in the end. Is it incorrect to depict this flux from the atmosphere to space as perpendicularly upward from the atmosphere?
I don’t think so.
Tim Folkerts @ November 9, at 4:20 pm
You wrote in part:
What branch of mathematics is this, where people can travel simultaneously in several different directions up to 90 degrees apart, three dimensionally? Does it have a name? Maybe it has something to do with string-theory and multiple dimensions. Give me pause to think on this and do some research, and I’ll try and get back to you before Christmas.
Meanwhile, let me quote parts of two of mine to you that are connected:
November 3, at 5:59 pm
AND: November 4, at 10:05 pm
I can’t find a response, and would appreciate such before Christmas.
Bob says: “If the atmosphere were transparent then it would all radiate directly to space, in which case by integration it could effectively be described as a vertical flux. However, much of the radiation tends towards the horizontal or tangential to the surface, but is annihilated by absorptive gasses and stuff.”
You just agreed that in a transparent atmosphere, if 396 W/m^2 left the surface, then 396 W/m^2 would pass up thru any layer.
Let’s consider the opposite extreme — an atmosphere where each cubic meter is a perfect black body, absorbing all radiation hitting it.
So the ground emits 396 W/m^2 into a cubic meter. It all gets absorbed. The cube will emit 1/6 in each direction: 66 W/m^2 up, 66 down, and 4*66 sideways. The cube must be the right temperature to emit 66 W/m^2. And most of the energy _is_ going sideways as you deduced.
But wait, the cubes to the sides are also that temperature, so they emit 66 into the four sides. And the next cube up was getting 66, so it will return 66/6 = 11 . That is 396 + 66*4 + 11 = 671 W/m^2 inward, so there is 671/6 =112 W available to head out each 1mx1m side. Which is nearly twice as much from the second iteration as we got from the first iteration.
But wait, the cubes to the sides are returning 112, and the cube above is returning more than 11 W. There will be diminishing returns, but each iteration will get is closer to the “correct” value. It is clear that the final answer must be that each cube is hot enough to emit 396 W/m^2.
The cube at the bottom of the stack gets only 396 W/m^2 from the ground below. But it also gets 396 W/m^2 from the cubes on the other 5 sides. It emits 396 W/m^2 in every direction — INCLUDING UPWARD. So while it emits much more from the 4 sides than it does up or down (which I think was your original point), it also GETS much more from the sides that it gets from below, leaving it with sufficient power to radiate a full 396 W/m^2 upward, even though only 66 W/m^2 “of the energy attributable to the ground below” is actually still heading upward.
(I’m trying to decide if the previous discussion is too obvious, or to obscure!)
So in a perfectly transparent atmosphere, 396 W/m^2 is left to exit from the layer of atmosphere 1 m high around the surface of the earth.
And in a perfectly opaque atmosphere, 396 W/m^2 is left to exit from the layer of atmosphere 1 m high around the surface of the earth.
It ought to be intuitive that for any level of opacity in between, 396 W/m^2 is left to exit from the layer of atmosphere 1 m high around the surface of the earth.
[NOTE: this assumes no temperature gradient. In fact, the observed gradient would require that the top of he cube be cooler than the bottom. So the “true” radiation from the bottom cube would be more like 396 down, 395 up, and 395.5 to each side.
But to repeat, this decrease is due to lapse rate, not because “most of the radiation is going sideways rather then up or down”.]
Tim:
… Energy transfer is NOT a vector. …
Ok, we are clearly talking on two different planes of thought. I see the way you are thinking, will you stop see what Bob and I have speaking of. You look at energy transfers as a lump quantity (energy, Joules) the is added to a bucket or point irregardless of where that energy came from or even the direction. I can see that and in certain discussions that would be proper and what I would speak of in that case too.
But addressing what has been said above, using your example (using water, not gasoline), if you are tracking the droplets of water from various directions the transfer of a drop of water from point A to point B (the bucket) is vectors of quantum of energy being transferred and I know you know enough to see that (I hope). That is the transfer we have been speaking of. Most still here seems well enough versed in basic physics to understand those two different aspects of “energy transfer”.
Does that clarify it?
Bob asks “What branch of mathematics is this, where people can travel simultaneously in several different directions up to 90 degrees apart, three dimensionally? ”
Are you really confused by this???
Suppose my house is 40 miles from the stadium at a heading of 10 degrees east of north, and 200 ft higher in elevation. Then I would be counted as living north of the stadium. I would be counted as living east of the stadium. I would be counted as living higher than the stadium. The vector describing the location of my home has components in each of these directions.
If a stream of photons is heading 10 degrees east of north, and 5 degrees above the horizontal, then these photons are counted as heading north. These photons are counted as heading east. Most importantly, they are counted as heading upward. If this stream has 1 W of photons leaving the surface, it will have 1 W heading up thru any layer higher up. I don’t need to know ANYTHING other than the initial direction is more upward than downward (assuming again a transparent atmosphere).
~~~~~~~~~~~
As for the Lambertian reflection/emission, I have no problem with it. And I have no problem describing the direction or the momentum of a photon with vectors.
I just don’t see how it is important here. Lambertian reflection is an idealization. If a beam of IR photons or sunlight hit such a surface, they would reflect uniformly in all directions. But we are not hypothesizing any such surfaces for EMR to reflect from.
It turns out that black bodies are perfect lambertian emitters, but we are already assuming that IR from the surface is isotropic, so saying that the surface can ALOS be described as a Lambertian emitter seems superfluous.
Tim Folkerts @ November 9, at 6:52 pm
Tim,
You wrote:
I am pretty sure the 80 W/m^2 _is_ the evaporative cooling you speak of. The surface is losing those 80 W/m^2, cooling the surface. When the water re-condenses (in clouds), the air is warmed by that amount. I’m not sure what additional cooling of the surface you are thinking of, or where that energy comes from/goes to.
Tim, I’m shocked! Try this experiment: put on an old t-shirt or something and douse yourself with water. Notice that you will feel colder than if you were dry. It’s called evaporative cooling. In the same way, evapotranspiration cools the surface of the earth, (removes surface heat), and there is a convective warming of the atmosphere. Then, as the water vapour ultimately meets the ambient conditions that promote it, it condenses in clouds, thus releasing latent heat. These are two different things.
Evaporative cooling is exquisitely explained in quantum theory, as I explained above somewhere, and in fact I’m happy to call this example: Quantum mechanics. (rather than theory).
RW and Tim,
Straight up is the shortest distance to space. Any vector other than straight up will encounter more atmosphere. Out of 390 w/m^2 only 40 manages to make it all the way out of the atmosphere and most of that will be going straight up. Why?? Because the densest part of the atmosphere is next to the ground. Any almost horizontal vectors will extunguish fairly quickly due to the density. As the angle from horizontal increases the density decreases as does the distance to space.
There are actually 2 computations for SB:
http://www.spectralcalc.com/blackbody_calculator/blackbody.php
On this page for 288k with 1 for emissivity it gives both radiant emittance at 390 and radiance at 124. RE is for the full hemisphere. R is for a specific amount of angle.
http://en.wikipedia.org/wiki/Irradiance
Notice a more realistic .99 emissivity is only 386, not 390!! How much was Trenberth missing again?? What a sad little man. Then there is the issue of the atmosphere’s emissivity. Probably down around .7 which would only give 270 instead of 333 coming back down. As the atmosphere ISN’T as warm as the surface usually, even that number is inflated!!!!
Tim, less than half will be going in the direction of earth as soon as you clear any obstructions on the horizon. Remember the earth is round and finite, not an infinite flat surface to intersect the radiation. The higher up the less is going in the direction of earth in the cone.
RW @ November 9, at 7:04 pm
Hi RW,
The issue I’ve tried to explain is that in an absorptive atmosphere, only part of the initial S-B surface emission and its progeny escapes directly to space. Well, other than those recalcitrant naughty photons escaping through the so-called window by virtue of their particular wavelengths. The stuck-at-home guys are repetitively annihilated by absorption in the GHG’s, at various layers of altitude. A very large proportion of them are annihilated in the lateral directions, at all levels until approaching the TOA, and by proper vector analysis there are both vertical AND horizontal components. The greater horizontal components of the 396 W/m^2 and its progeny are constantly there, and cannot be part of the vertical flux, yet they ALL evolve as PART OF the S-B initial surface emission in the context of radiative emissions from the surface.
To that must be added the radiative effects of evapotranspiration and thermals. (per Trenberth terminology)
Bob is shocked: “Try this experiment: put on an old t-shirt or something and douse yourself with water. Notice that you will feel colder than if you were dry. It’s called evaporative cooling. ”
Yes, I know about evaporative cooling. I did exactly what you suggest one summer while driving through the desert in a car with no air conditioning.
I am simply saying that “evaporative cooling” is synonymous with the 80 W/m^2 of energy leaving the surface. The oceans are the ‘wet t-shirt around the earth’, cooling the surface of the earth by 80 W/m^2 (well, you do need to also include transpiration from plants). You seem to be saying that there is some OTHER evaporative cooling beyond the evaporative cooling already in the diagram.
“In the same way, evapotranspiration cools the surface of the earth, (removes surface heat), and there is a convective warming of the atmosphere. “
These are two different things, each of which is counted in the Trenberth diagram. Evapotranspiration removes ~ 80W/m^2 from the surface and deposits 80 W/m^2 into the atmosphere. Convection removes ~ 20 W/m^2 from the surface and deposits ~ 20 W/m^2 into the atmosphere.
Then, as the water vapour ultimately meets the ambient conditions that promote it, it condenses in clouds, thus releasing latent heat. These are two different things.
I’m not exactly sure which two things you are calling different.
Latent heat and convection are certainly two different things. Evaporation and condensation are two aspects of the same thing. If evaporation removes 80 J of energy, then conservation of energy requires that condensation deposits 80 J of energy somewhere else, namely the atmosphere.
Kuhnkat exclaims: “Probably down around .7 which would only give 270 instead of 333 coming back down. As the atmosphere ISN’T as warm as the surface usually, even that number is inflated!!!!
Once you calm down from your 4 exclamation points, you might want to switch to contemplating a few scientific points. In particular, the emissivity of clouds is very close to 1 and clouds cover ~ 2/3 of the earth. If the cloud base was 1 km up, the average temperature would be about 6 K cooler than the surface. Such clouds would radiate on the order of 5.67e-8 * (288K- 6K)^4 = 358 W/m^2.
Clouds come in all sorts of types and altitudes, but some of them clearly radiate well above 333 W/m^2, while areas of clear sky radiate less than 333 W/m^2. It sure SEEMS the average of numbers above 333 and below 333 could average to 333. Do you have clear support for your contention that the global average is indeed below 333 and that the number is inflated as you claim?
Tim Folkerts @ November 9, at 7:39 pm
Tim,
So 66 goes up and 330 goes sideways?
You can only sum two different sources of EMR when they have the same sign. If you add -66 to + 66 you have no heat transfer.
BTW, the 396 from the surface is isotropic, so a single square metre of ground will not radiate into just the cube above it.
Oh and your analogy was for a black body anyway Tim
Bob,
“The issue I’ve tried to explain is that in an absorptive atmosphere, only part of the initial S-B surface emission and its progeny escapes directly to space. Well, other than those recalcitrant naughty photons escaping through the so-called window by virtue of their particular wavelengths. The stuck-at-home guys are repetitively annihilated by absorption in the GHG’s, at various layers of altitude. A very large proportion of them are annihilated in the lateral directions, at all levels until approaching the TOA, and by proper vector analysis there are both vertical AND horizontal components. The greater horizontal components of the 396 W/m^2 and its progeny are constantly there, and cannot be part of the vertical flux, yet they ALL evolve as PART OF the S-B initial surface emission in the context of radiative emissions from the surface.”
I know what you’re saying, but I think you’re overlooking that the average flux is still really perpendicular to the surface even though on a photon by photon basis it’s going all over – much of it in a more horizontal upward direction as you describe. Remember, the surface to space direct transmittance or ‘window’ is the amount of surface radiative flux that passes straight through the atmosphere to space as if the atmosphere wasn’t even there. There is no distinction or stipulation that it is only from perpendicular or directly upward emitted photons. It is true that a more horizontally upward emitted photon has longer path to the TOA, resulting in a greater chance of being captured by the atmosphere, but this in and of itself does not affect what percentage of the flux ends up going straight to space.
Let me ask you this. The upward emitted half of isotropic emission from the atmosphere that ultimately escapes to space – is it incorrect to show or calculate this as an all upward flux from the atmosphere to space? Some of the more sideways emitted upward photons have to travel through more atmosphere in order to get to the TOA, right? But they ultimately still get there, right? How is this really any different from the emission from the surface?
I think what you’re arguing would have some validity if the Earth’s surface was a flat plane, but the fact that it’s a sphere will no ‘ends’ in any direction makes it moot.
Bob,
I wrote:
“It is true that a more horizontally upward emitted photon has longer path to the TOA, resulting in a greater chance of being captured by the atmosphere, but this in and of itself does not affect what percentage of the flux ends up going straight to space.”
What I meant was the so-called ‘window’ transmittance accounts for the equally multi-directional emission from the surface.
Bob,
You do know that the so-called ‘window’ transmittance or direct surface to space transmittance does not just include the parts of the emitted spectrum that are mostly transparent to the atmosphere (i.e. between about 8-13u), but also includes the non-transparent components, a portion of which still passes directly to space in the non-saturated bands, right?
RW @ November 10, at 3:39 pm
RW, you wrote in part:
Let me ask you this. The upward emitted half of isotropic emission from the atmosphere that ultimately escapes to space – is it incorrect to show or calculate this as an all upward flux from the atmosphere to space? Some of the more sideways emitted upward photons have to travel through more atmosphere in order to get to the TOA, right? But they ultimately still get there, right? How is this really any different from the emission from the surface?
No, they do not ultimately still get there. In fact, most of them are annihilated quite close to the surface. If you study Trenberth’s diagram you will see that 161 W/m^2 of descendants of surface heat loss is claimed to leave the TOA. (239 less 78 solar absorbed by atmosphere = solar absorbed by surface).
So, whatever happened to the 396 + 80 + 18 leaving the surface?
Have a play with the MODTRAN calculator looking down from various heights.
http://geoflop.uchicago.edu/forecast/docs/Projects/modtran.html
Bob,
“No, they do not ultimately still get there. In fact, most of them are annihilated quite close to the surface.”
I’m aware that most of the absorption happens close to the surface, but are you trying to say it’s not possible for a more sideways emitted photon from the surface to travel directly to space without being capture by the atmosphere? I realize of course the chances a photon of the same wavelength getting captured increases the longer the distance to the TOA, but that’s separate issue relative to the global average.
“If you study Trenberth’s diagram you will see that 161 W/m^2 of descendants of surface heat loss is claimed to leave the TOA. (239 less 78 solar absorbed by atmosphere = solar absorbed by surface).”
All of the post albedo of 239 W/m^2 gets to the surface, albeit not all directly. The 78 W/m^2 designated as ‘absorbed by the atmosphere’ does not mean that of the 239 W/m^2 flux leaving, 78 W/m^2 are from that designated as being ‘absorbed by the atmosphere’. This is the fundamental problem with his diagram – it doesn’t show net energy flux from the TOA to the surface and from the surface to the TOA, which is what really matters. All the energy that enters the atmosphere, from the Sun or the surface, is either on a path back to the surface or out to space.
While I certainly agree that not all the post albedo gets to the surface as SW radiation, and some of it is absorbed in the atmosphere (mostly by clouds). While it’s entirely possible for some of these absorbed photons to exit at the TOA without ever reaching the surface, all that is happening in this case is post albedo energy absorbed in the atmosphere from the Sun is being traded off for surface LW flux absorbed in the atmosphere that would otherwise have to be leaving the planet and subsequently getting to surface indirectly in this way as a result. The bottom line is by definition all the post albedo absorbed in the atmosphere enters the thermal mass of the planet, which is mostly surface but part atmosphere as well.
“So, whatever happened to the 396 + 80 + 18 leaving the surface?”
It’s all accounted for when the full post albedo is getting to the surface. 396 + 80 + 17 = 493 and 161 + 333 (78 + 80 + 17 + 157) = 494. Subtract out Trenberth’s phony extra watt and you have 493 W/m^2 entering the surface from the atmosphere and 493 W/m^2 leaving the surface into the atmosphere.
Tim,
you accept that only 40 w/m^2 makes it past the dense low atmosphere and eventually straight to space. The clouds do a really good job of irradiating mostly that same dense low band of atmosphere. Remember, over half of everything absorbed goes back up before getting to the ground!! Your point should have been that I should have taken into account that it would be somewhere in between 270 and 358. Of course, 1k or below clouds do not necessarily WARM as anyone who is around water, fog, and low overcast will tell you, they COOL!!
So, my 270 is only appropriate where there is no cloud and your 358 is only appropriate when the cloud temps are really as high as 288!! The averages are really poor. Of course, my 270 is based on the same temp as surface so is an overestimate and probably not as far from reality as you are trying to make it!! Or NOT!! The .7 or so is an AVERAGE so includes clouds!!
http://www.xylenepower.com/Emissivity.htm
Tim Folkerts @ November 10, at 8:56 am
Tim, you wrote in part:
* Evaporative cooling from the surface results in warming of the air at the interface and consequent additional convective activity.
* The process involved is that higher energy water molecules escape, leaving a greater proportion of lower energy molecules behind. (BTW there is a mass transfer to the atmosphere)
* The water molecules eventually end their convective travel upwards when the ambient conditions result in their condensation in clouds. This results in further warming.
* The latter process involved is latent heat loss, due to material phase change.
These are two entirely different processes, and there are also at least two other associated processes which are probably relatively trivial.
* Cooling of the surface by precipitation from a colder region.
* Warming of the air and surface by air friction and impact of precipitation.
Also, as a bit of a “David Karoly”, shortly after the climategate scandal, over at RealClimate, Gavin Schmidt ran a period of allowing many sceptical comments to be posted. (long-since abandoned). I raised the topic of evaporative cooling and latent heat of condensation there, and my recollection is that no one, zilch, disputed the two different processes. However, the message I got, very strongly was that latent heat loss in the clouds is the big deal, and that evaporative cooling was small by comparison. Of course those assertions were without any data or relative numerical foundation. (and I have no idea of their relative scales).
But actually, this is all wandering away from the paradox that I raised.
RW @ November 10, at 6:57 pm
A quickie:
Thankyou RW for your long post, but right at the moment I don’t want to grind through the detail. Please try to understand that the Trenberth alleged 396 W/m^2 surface emissions a la S-B are isotropic. As I understand you, you intuitively think that this is somehow converted to a normal (vertical) presentation. But, IF the atmosphere were indeed transparent, then such an integration that you visualize would be arguable, what with the horizontal stuff escaping directly to space. (if viewed from space with an encompassing wide-angle instrument). However we have an absorptive atmosphere, and a lot of stuff don’t make it to space.
Furthermore, my purpose in asking “what happens to the 396 from the surface” was to get you thinking that indeed it substantially dies, and does not head-out vertically to space as you seemingly wish to say it does.
Starting from a one layer model, going to 2, 3..and finally a multilayer model of chicken wire.
Applying it to the atmosphere gave coherent results.
Applying it to K&T and the like gave different results.
http://www.ilovemycarbondioxide.com/pdf/IRabsW27102011.pdf
Let’s go back to the top …
Bob, you claim “It may be that there is a resultant of similar order to 396 W/m^2, but that is NOT the S-B radiative process described by Trenberth.”
Could you provide a summary of what Trenberth actually claims from some paper? As far as I know, he only claims the surface radiates 396 W/m^2, which is (as far as I know) exactly in accord with SB calculations. I doubt he claims that the radiation higher up is exactly SB in nature (since it involves radiation from many places that that are many different emissivities, rather than from a single opaque surface). I suspect this is a bit of a strawman, attributing to Trenberth conclusions that he never specifically made.
Bob, thank you much for the link the paper by Joseph Reynen in France. Seems others have come to the same conclusion as being discussed above, and, in much more detail. That will take a while to absorb. Great reference, will use it. Also noticed his immediate jump to net transfers. Bravo!
BTW, this was one great post. I rank it at the very top being the real science.
Tim Folkerts @November 11, at 12:54 pm
Tim,
You are welcome to read the 2009 paper a link for which is in the first paragraph of the article. The diagram shows the surface emission as a vertical arrow into the high clouds other than the 40 directly to space. The earlier 1997 version is similar, and is used in the IPCC reports of 2001 & 2007
You are also welcome to look at the following sketch again:
http://bobfjones.wordpress.com/2011/10/29/quick-sketch-for-trenberth-cartoon/
Bob, love this statement in the paper:
“Back radiation is just an algebraic manipulation of the SB law which
happens to give the same result as for the present model when the
atmosphere is taken as one single slab.”
Could not agree more. That is a great way to put it.
Just a note: also found in multiple astronomy sites is the figure of how much more atmosphere mass radiation passes through when near horizontal in direction. It is about 38 times the zenith mass. This is closely related to “Mass Attenuation Coefficient” or “Mass Extinction Coefficient”, logarithmic in nature. Large telescopes greatly rely on such figures to calculate the dimming of radiation at various frequencies and vectors through the telescopes. This has a huge effect in most IR frequencies compared to visible frequencies.
Also took the time to write a numeric integration program over a sphere’s surface of random radiation from a point as seen in three dimensions. Just wanted to make sure some of my statements above might not be mathematically correct. Horror! As I suspected it is a bit harder than you would think at first. You cannot take random directions in +/- x, y, & z and you cannot do the same evenly distributed in theta and phi if in spherical coordinates for the integration will report more radiation going up and down than tangent. The trouble is these distributions both ignore the area weighting on a sphere being much smaller at the poles. Thanks to Wolfram Research for the proper corrections and it is as stated above, 1/6 exactly in all of the six possible half-axes when normalized to one. Just had to check that. Even though it seems so logical, I was saying it above with no real proof.
JWR @ November 11, at 12:00 am
wayne @ November 11, at 2:32 pm
Wayne, we have JWR to thank, for the link not me
JWR, thanks for the link. I’ve not had time to properly read it, but it looks interesting
Bob,
“Thankyou RW for your long post, but right at the moment I don’t want to grind through the detail. Please try to understand that the Trenberth alleged 396 W/m^2 surface emissions a la S-B are isotropic. As I understand you, you intuitively think that this is somehow converted to a normal (vertical) presentation. But, IF the atmosphere were indeed transparent, then such an integration that you visualize would be arguable, what with the horizontal stuff escaping directly to space. (if viewed from space with an encompassing wide-angle instrument). However we have an absorptive atmosphere, and a lot of stuff don’t make it to space.
Furthermore, my purpose in asking “what happens to the 396 from the surface” was to get you thinking that indeed it substantially dies, and does not head-out vertically to space as you seemingly wish to say it does.”
I’m a bit confused, as I’m not quite sure where it is we are disagreeing.
For the record, I do not think that the atmosphere is transparent to the more horizontally emitted photons from the surface. I agree that most of them would be captured by the atmosphere relatively close to the surface and relatively few would pass directly to space. I also agree that of the more horizontally emitted photons, a higher percentage of them would be captured by the atmosphere than those emitted more perpendicular to the surface, as a result of the longer distance between the surface and the TOA.
I also agree that of the 396 W/m^2 emitted radiatively at the surface according to Trenberth, not all of this makes it to the clouds before much of it is absorbed by the atmosphere and subsequently re-emitted by the atmosphere. No doubt that there are multiple absorptions and re-emissions along the way (either out to space or back to the surface).
So tell me, where is it we are disagreeing?
Ditto the thanks JWR. ( seems a maximized window would help ☺)
To follow-up on my previous post, I am thinking that much of the discussion has been focused on a side-track.
Bob says: “Furthermore, my purpose in asking “what happens to the 396 from the surface” was to get you thinking that indeed it substantially dies, and does not head-out vertically to space as you seemingly wish to say it does.”
I think we can all agree that (give or take a few W/m^2 in all the following numbers) ~ 396 W/m^2 of IR photons get radiated by the surface of the earth, and that these specific photons head out pretty nearly isotropically from the surface.
Beyond that, I think we agree that 356 W/m^2 of that 396 W/m^2 gets absorbed before escaping to space (either by GHGs or by clouds), while 40 W/m^2 gets emitted to space.
To a large extend, that is all that really matters — energy leaves the surface and energy enters the atmosphere. The claim (made by Bob in his post)
is, in my opinion, a red herring. There is no claim of 396 W/m^2 continuing up through the atmosphere. There is no “alleged 396 W/m^2 of upward flux” anywhere but at the surface. Any details withinthe atmosphere are glossed over in the Trenberth diagram. This is a “black box” approach (not “black body”) that ignores the details of the atmosphere.
Specifically, the diagram makes absolutely NO attempt to describe the energy flows by specific means at specific altitudes (other than the very generic claim that the NET flow at the TOA is ~ 0.9 W/m^2). It is easy to hypothesize/assume/conclude that the higher you go, the fewer LWIR photons from the surface you will find, but many of them will be replaced by LWIR upward from the atmosphere. To the extent that the air is the same temperature as the ground, the net change in upward LWIR will be rather small.
If I had to make a stab at the flow 100 m above the ground, I would say something like
The GHGs absorb LWIR isotropically; the GHGs emit LWIR isotropically. To the approximation that the atmosphere and the ground are the same temperature, the absorption of upward LWIR by GHGs and the emission of upward LWIR by GHGs will very nearly cancel out.
RW @ November 11, at 6:10 pm
Sorry RW if I misunderstood, but I thought you inferred somewhere that the isotropic radiation from the surface was vertical.
Tim Folkerts @ November 11, at 9:06 pm
Tim, briefly, you wrote in part:
Most of the 356 does not make it to space
Despite that the 1997 and 2009 diagrams show the vertical arrow into the high clouds, (which might be a mistake), the main concern is not that issue. The 396 has horizontal vectors which are not vertical, and which are extinguished. If you want to consider that these constantly prevailing horizontal vectors are vertical, don’t expect me to agree. This is getting a bit like sawing sawdust.
Can’t be bothered with the rest of it.
“Despite that the 1997 and 2009 diagrams show the vertical arrow into the high clouds, (which might be a mistake), …
I strongly disagree that the “the vertical arrow into the high clouds” might even partially be a “mistake”. The diagram specifically says it is “schematic”. The paper itself is clearly discussing only broad energy flows among the sun, the surface as a whole (land and water), the atmosphere as a whole (clouds and GHGs and non-GHGs). The arrow in no way represents energy flowing to any specific part of the atmosphere. Drawing the picture to look vaguely like the earth is only a visual aid to make it easier to remember, but it could just as well had 4 distinct boxes at the four corners of the diagram labeled “sun”, “surface” “atmosphere” and “outer space”.
“…the main concern is not that issue. The 396 has horizontal vectors which are not vertical, and which are extinguished.
Define “extinguished”. I would take that to mean “absorbed by the atmosphere, thereby transferring their energy to the atmosphere”. Obviously you do not mean the IR energy was destroyed.
Which of the following do you specifically disagree with?
1) If 396 W/m^2 of IR photons from the ground continuously enters some region of atmosphere and some of those photons get “extinguished”, then that region would be continuously gaining energy.
2) Since the temperature is not continuously rising, that region must be continuously losing the same amount of energy.
3) We know the region is not losing that energy via “horizontal vectors” since the horizontal vectors would merely exchange like amounts of photons with neighboring regions, for no net transfer horizontally.
4) The ONLY direction for net transfer is either down or up.
If you don’t like logic, then try an experiment. A completely analogous situation would be visible light in a high temperature furnace (say 1200 C). Put anything in the furnace – clear glass, colored glass, shiny metal, white ceramics, black ceramics. All of these “extinguish” the visible light coming from the hot wall of the furnace behind them in various ways. But all will look nearly indistinguishable inside the furnace once they reach the temperature of the surrounding walls. Whatever photons they are “extinguishing” from behind, they are emitting/reflecting from the other side. As long as it is all the same temperature, the color is the same.
See Bob, I told you that would be raised:
Tim says: “1) If 396 W/m^2 of IR photons from the ground continuously enters some region of atmosphere and some of those photons get “extinguished”, then that region would be continuously gaining energy.”
I told you above to watch out. Tim is so lost in physics, credentials or not, he is still simply wrong.
Dr. Richard Feynman explained all of this some forty years ago. All transfer of energy and photon-electron-matter interactions via those three (or four) processes of conduction, convection, radiation, and evaporation/condensation are all identical at the atomic/quantum level. Those processes are manifestations of this one principle. That is why the 396 is a figment, an imagination of someone who does not understand all of physics. The surface cannot manifest this difference in temperature, therefore difference in energy levels, between various points in the Earth climate system in a strictly radiation manner, ignoring the other three, except at the edge of the void of space. At that point there is no convection and conduction, no evaporation, to be manifested so all is IR radiation at this special “surface”.
The transfer by all non-radiative processes take energy away (see microstates) from the possible S-B hypothetic power (396 Wm-2) due to temperature differences and emissivity totally dry and against a void at zero K.
BUT, at the surface the energy held by the top few centimeters where the atmosphere touches the surface is divided between these four paths, convection, conduction, evaporation/condensation and radiation. It is evident Tim, Trenberth, Kiehl and others have never learned that aspect of quantum electro-dynamics and that is why you are so correct Bob, the 17 and 80 Wm-2 must firstly be subtracted from all four IR radiation terms listed on Trenberth’s diagram, 396, 356, and the two 333 Wm-2, for those 17 & 80 are net transfers of energy from the surface to space ignoring any and all inter-atmosphere processes and those IR radiation terms are all overstated in the diagram by approximately 97 Wm-2.
Tim is also ignoring energy moving opposite as you keep trying to explain it to him. But once again you are correct Bob, there is cancellation, it happens in the very room you are sitting in just like it happens as you step outside into the open atmosphere. A to B cools A and warms B, BUT, at all times, energy is also going from B to A cooling B and warming A and both are in the power Wm-2 terms. Yes Tim, there is cancellation in energy transfers depending on the vectors. There is no automatic “accumulation”. You are simply wrong on those aspects so stop trying to defend the mistakes of Kiehl-Trenberth et al. For starters at least subtract what is not in contention.
Or that is what my forty years of studying physics has left me with.
I still say it is very curious to me that if you take the (333-97)Wm-2 correction as temperature you get approx. minus 20°C which is what most people have reported their radiative thermometers read as they point it toward a cloudless sky. That is curious to me. It also tends to say those corrections are very real and correct.
Suppose you have a traffic monitor on a mountain pass. You measure an average of 333 vehicles per hour going east and 396 vehicles per hour going west. Suppose that road goes down into a valley with multiple intersections with other roads and then up into a second pass that is the only way through those mountains. Suppose, your traffic measured there is 239 cars per hour going west and 341 cars per hour going east. Is there a contradiction?
It is also curious that an analysis of readings from Mars Global Surveyor Thermal Emission Spectrometer pointed backwards toward Earth during it’s trip to Mars in 1976 gave a good whole-Earth emissivity of 0.75xx with readings across all frequencies responses averaged. Sorry, can’t remember the last two digits.
If you take the 396 Wm-2 * 0.75 emissivity you get guess what? … 297 Wm-2 in very good agreement with the same 396-97 = 299 Wm-2 according to Trenberth’s graphic after thermals and evapo-transpiration are removed. One more reason to properly assume that the 396 and related numbers are totally fictional.
Hang in there Bob, seems you are really on to something.
I see I overlooked one very important point, the reflected energy that reduces the net in-coming short wave radiation power to 239 W/m². (Cars going east = 239.) The net solar energy flow arriving at ground level is 161 W/m². The net energy flow leaving the ground is 63+17+80=160 W/m². The additional unexplained 0.9 W/m² “Net absorbed” might make up the inconsequential difference.
Tim Folkerts @ November 12, at 6:48 am
Tim, your following words are worth a comment:
Tim, you have nicely put your finger right on the issue, but should have added another point to complete the picture.
5) Since the EMR in 3) is continuous and greater than in 4), and both are part of the continuous 396 S-B surface emission, the vertical flux component must be less than 396.
I believe that the most important point made by the Trenberth diagram is that most of the energy being radiated from the top of the atmosphere actually originates in the atmosphere. I am not all that concerned about the actual accuracy of the diagram itself.
I have generally restricted my use of MODTRAN to clear Tropical air in its default setting. So far, I have not been interested in the modeled effects of clouds because I do not want to introduce too many uncontrolled variables.
It has been my understanding that the energy observed with the sensor looking down (energy flowing up) minus the energy observed with the sensor looking up (energy flowing down, i.e. back-radiation) should be the net energy moving out. Here is one example of results from a sensor altitude sweep I obtained with MODTRAN:
Note that ‘back-radiation’ virtually disappears above the Troposphere in clear Tropical air.
Spector @ November 12, at 4:45 pm
An interesting comment Spector.
In your MODTRAN table for the tropics, I see that there is a significant increase in net radiative loss between about 1.0 and 10.0 kilometres, and then a levelling off up to 99Km. I guess this is related to the effects of thermals, evapotranspiration and solar absorbed. It is only a model of course and I have no idea how they would have determined and distributed such data over the altitude and spectral range. Do you?
Bob,
“Sorry RW if I misunderstood, but I thought you inferred somewhere that the isotropic radiation from the surface was vertical.”
I think the average LW flux from the surface is vertical, but on a photon by photon basis it going all over as you say.
I don’t understand the thing about lateral angles at a cross section. To look at a simple limiting case, if you take a cross section far away from the earth, you find that almost every photon through it originating from the earth is parallel to the next (ie, not lateral but “up” almost perfectly if the cross section is slanted as I think you intended.. and, no matter the slant of the hypothetical cross section, the photons would be radial from the point-like earth). However, within the atmosphere, we have photons being generated all over, so we get a much nicer mix.. if perhaps still with a bias to be in the earth radial direction.
So if I understand the “lateral” claim, I think the truth tends to be rather the opposite. Maybe the author is confusing that some photons come from points where they left laterally, but they tend to go through a cross-section more and more parallel as you move further from the originating source (note that the same number of photons spread out over a growing volume of the universe so the density decreases as R increases).
And as for angle of origination (vs angle of arrival), the odds of lateral appear to be no greater than leaving at any other angle (I think this is a quantum mechanics/wave equation assumption.. although assumed probably no matter the standard theory used). Lateral origination might even be at a lower probability since it might imply greater energy needed to escape the surface as opposed to being normal to it (I haven’t done the math or experiments, but this might be my guess).
Also, a line normal to a surface is a standard way to represent something coming from a surface, as it is a simple diagram form to convey a particular piece of information that can be understood to apply evenly to the entire surface.
RE: Bob Fernley-Jones: (November 12, 2011 at 6:54 pm)
“I have no idea how they would have determined and distributed such data over the altitude and spectral range. Do you?”
No, I have no inside information on this program. I understand that it was generated by the Air Force for quality control purposes. I assume that these calculations are based on a typical static atmosphere.
If you select the “[save text for later retrieval]” save option, you can use “View the whole output file” to capture the calculation printout details. Note that they appear to be using cgs units for the radiance, i.e. watts per square centimeter. There is also a solid angle and bandwidth correction required. The values are calculated over the wave number range from 100 to 1500 kayzers (cycles per centimeter, CM-1) in 2 kayzer steps. I suspect the results would have been a little more accurate if the upper range had been extended to 2500 kayzers.
Nasif Nahle’s paper – ‘Emissivity of a mixture of gases – 5% water vapour and o.o39% CO2’ states that CO2 emissivity is almost zero below 33 C for PcL of 0.6096 atm cm. From Hottel’s graphs I found that for PcL 0.6096 atm cm (0.019988 ft atm) CO2 emissivity is 0.045 at 33 C (551 R) and for water vapour it is about 0.4. Emissivity of CO2 is small but not zero; it is 10% of water vapour emissivity .Allowing for spectral overlaps the combined emissivity would be 0.436
Bob, Since you were wondering about at what point the two dimensional approximation of the tropopause might be come significant in error, I thought you might find this method of approximating surface emissivity/transmittance amusing.
http://redneckphysics.blogspot.com/2011/11/building-better-model.html
Jose_X @ November 12, at 11:08 pm
Jose,
The issue applies to opaque parts of the atmosphere, particularly with the Stefan-Boltzmann radiation from the surface. (where the Trenberth alleged vertical 396 W/m^2 is in dispute). This is isotropic, hemispherically, the largest portion of which is lateral. Much the same applies at all emitting parts of the atmosphere, except that it is spherical. Perhaps my following exchange with Tim Folkerts above, (a physicist), may help.
Also, if you scan up a short way there is a table submitted by Spector that you may find interesting
>> This is isotropic, hemispherically, the largest portion of which is lateral.
I am not sure what you are trying to get at with this, but can you explain what derivation, logic, assumption, etc, leads you to claim that the “largest portion” is lateral? It’s a vague comment to say “largest portion is lateral”, so maybe you can give some sort of bounds by degree rise from horizontal (the x-axis). And if you talking about energy flux from point of origination being greatest in the lateral directions, are you also claiming this is the case when we look at a cross section higher up as a destination of photons? Note that if you move away from the earth, the near “lateral” angles trace back to points not on the planet. In contrast, the up direction traces back radially to the planet’s surface. Far enough away, the earth is like a point and only the photons coming exactly from that point hit the cross section (and at a 90 degree angle).
The other point is that, as has been mentioned by others, the lateral radiation is on average met by radiation of the same quantity in the opposite direction. The up radiation is the only one that generally dominates as we look at nearby horizontal slices one just above the other because the lower slice has more radiation than the higher up slice (on average) keeping in mind that some fraction of the originating radiation from the lower areas will not be absorbed by the higher areas (and note that extra radiation moving up that is not caught eventually makes its way into space while extra radiation downward eventually hits the ground leading to a steady state temperature and flux that are higher at the earth boundary than if there were no back radiation at all). Anyway, this is a hand waving (non-mathematical) argument, but the Spector table you mentioned shows experimental evidence of this effect for up/down, where the higher slices have a greater and greater upward component (and, again, I’m assuming the side-side versions of this would give about the same numbers with no net lateral).
Note, for comparison’s sake, the electric field lines direction for conductive surfaces in the presence of nearby charges (as taught in text): the field lines are normal at these conductive surfaces, as any other direction would imply a net current along that same electrical potential.. something that statistically is calculated (based on natural assumptions) to cancel out (net 0) and which appear to agree with experiments (and hence why it is taught in text books that field lines are normal to the conductive surfaces). For radiation distribution among large objects or molecules at large atomic separations within at the earth’s solid/gas boundary, we may not see the same extreme case as with electrons on a conductive surface, but we probably get close (at a different time scale). The “lateral” component of various gradients would generally be near zero at any point. [haven’t attacked this problem or looked it up, so I am willing to see if you have calculations or a more precise/rigorous discussion.]
Jose_X, as to ““largest portion” is lateral” Bob mentioned I always view it like this. A 1-cm2 spot on the surface of the Earth is covered by a glass hemisphere (imaginary will do) with a surface area of 1-m^2. That one cm^2 spot radiates randomly and evenly distributed through any and all points on that hemisphere. I mentally take a sharpie and draw 1 cm^2 squares to cover all of that bowl, 10,000 of them, then the same amount of radiation from that spot is going to pass through every square in equal quantity.
But, there are many more squares near the surface where the radius of the bowl is large that near the top of the bowl where the radius is small. In fact there is only one cm^2 square that is strictly zenith.
Now expand that example to be one meter^2 but the principle is the same, much more radiation travels horizontally (or laterally) than mainly pointed upwards and if integrated it is 2/3 mainly pointing horizontal and 1/3 mainly pointing upward with a hemisphere. If a complete sphere it is 1/6 down, 1/6 up, and 4/6 lateral. I think that is what Bob was saying, for that is exactly how I also view it and it explains Mars and why we see the net upward is approx 1/6th of 396 (if the 396 Wm-2 is even accurate which I think is not, due to the sharing of energy transfer modes leaving the 16°C surface, being convection, water state-change, and radiation, I think more like 396-80-17 or 299Wm-2 giving 59.3 though 63.114 is given by MODTRAN, but, we are on a sphere and not a flat plane).
You seemed to mentioned understanding the existence of lateral cancellation in your posts above so I probably need not go further on that.
Hope that helps and if you feel any of that is wrong please say so and why. I am, and I’m sure Bob, searching for confirmation on some of these thoughts with actual physics to back it up.
wayne, thanks for that. I never got back to the golf ball example or I may have realized what Bob meant.
A few notes:
One, that notation is acceptable when this is seen as a block diagram. This point was brought up (I looked back and could not find the comment).
Two, this simplification at ground level is justified (as several have mentioned) because all of these values originating at the “flat” ground eventually make it into some cross section or other at any given height from the ground (in the limit case far away from the earth’s curved surface and deep into space, the photons are actually normal to the earth). If you try to measure with a sensor at some point in the atmosphere, the sensor will get those photons coming at 90 degrees as well as some coming near 0 degrees (as a 2d projection angle) in all such directions in space (360 deg rotation) unless we go too far up in the atmosphere. The sensor shows how the average pick up is that figure, even if some photons came from miles away.
So the diagram is indeed a simple model, with the arrows representing net average rates measured near that level. [Note, I don’t know enough to say if the numbers are accurate or in what range. Also, a computer model would use, I assume, data from many many points (corresponding to actual measurements all around the planet) and not just at a few points. The model is useful at some level, but I would not use it to make too many predictions about the future.]
Jose, glad that gave you a clearer view, but I don’t know if you should elevate it to a full ‘model’ status, for if I was interpreting Bob correctly, he seemed to just to be highlighting some incredibly simple physics principles that conflict with what the Kiehl-Trenberth diagram tends to leave you with. And your right, holds no predictions of the future! ☺
http://greenhouse.geologist-1011.net/
Source of their AGW fictional world without convection – Arrhenius misunderstands Fourier.
wayne, the diagram is informative to the degree it follows measurements and would be consistent with an increasing effect on ghg. As concerns modelling, it is useful perhaps only for very rough modelling.
While I might be understanding Bob, I don’t see too much use in that presentation (except conceptually in order to move forward) or see it as inconsistent with the diagram; each is of marginal use by itself. Bob’s point seems consistent with what the Trenberth diagrams say, that there is an *average net* flux upwards. Bob appears not to like the idea that the diagram only shows average net. That it correct IMO that to model adequately (eg, for predictions) such a simplified diagram is too simplified to unleash on time-dependent differential equations and get much out of it (my inexpert opinion).
One paper-in-progress that tried to use the Trenberth diagrams to recalculate equilibrium climate sensitivity (to a lower value in that case) appears to have assumed those values would represent steady state in the future at 2xCO2. To calculate equilibrium climate sensitivity (as per IPCC definition), you need something much more complex than anything you could derive from those diagrams.
Myrrh, AGW doesn’t deny the cooling effect of convection. (a) “greenhouse effect” in atmosphere (re-rediation) and (b) the fact real greenhouses keep an elevated temperature that would otherwise drop if convection could take place freely with outside cooler environment are two separate things and consistent with each other.
Woods did not do much of an experiment (almost zero detail write-up). A professor, Nahle, tried to support Woods and failed to support anything against greenhouse effect despite concluding otherwise in the paper (I commented on that here http://climateclash.com/2011/07/19/wood-is-correct-there-is-no-greenhouse-effect/comment-page-1/#comment-2122 ). Pratt did a good experiment that clearly shows a greenhouse effect.
wayne, I should have clarified that I agree (but not 100%): The way the Trenberth’s diagram is drawn can give a bad oversimplified/misleading view of reality. Bob calls him up on it although I think via a presentation that itself might be a bit misleading to the extent it might suggest there is no (to first approximation, I believe) average net flux in the up direction at an arbitrary point on the earth surface.
>> average net flux in the up direction at an arbitrary point on the earth surface.
I don’t mean to say that the arrow represents the average at such an arbitrarily chosen point. Rather, the arrow represents (I think) an average for every point together.
Bob, the more I think of what you and I have discussed on the horizontal aspects of radiation from the surface and low troposphere the more this seems to be of great importance. Really, it has opened up my eyes.
See these before I say more and think radiation going out instead of starlight coming in (these mainly speak of astronomy in relation to visible light frequencies, not IR to which the atmosphere is much more opaque):
Air mass (astronomy)
http://en.wikipedia.org/wiki/Air_mass_(astronomy)
Mass attenuation coefficient
http://en.wikipedia.org/wiki/Mass_attenuation_coefficient
You seemed to grasp my comment on Mars horizontal radiation being more or less unencumbered by the extremely thin co2 atmosphere (1/100th of Earths’) except exactly in co2’s absorption frequencies. Therefore all of the 5/6th can travel unabsorbed no matter the angle components (+x,-x,+y,-y&+z). Not so on Earth and the main factor is the water bands and water’s continuum across the IR spectrum.
Since a much greater portion of radiation does tend to be more horizontal than vertical then an atmosphere is more controlled by the simple mass that the atmosphere contains than its specific chemical components vertically, the thickness of the atmosphere. How could I have not seen this for a year and half! Well, I know, they had me thinking 1-D, up and down, and tracing photons like they seem to have trained most people to do. And, every absorption starts the same process all over again with a re-emission going all directions, more horizontal and through much more mass of the atmosphere than up or down. Woo. Others here need to get that view. Have you considered another post on this?
One more ah-ha. Saw a plot about a year ago of Venus, Earth, Mars, Jupiter and Titan that shows that the turn of the lapse rate curves (where main-line absorption of energy ends and at that given altitude) always all but Mars occurs at close to the same density even though Venus is near pure co2, Titan methane, Jupiter hydrogen and helium and Earth of course water vapor with Mars so thin there is little absorption at all occurring and Mars is basically off the chart. The chemical components seem to have so little relative influence and the mass seems to set the surface temperature. Very curious.
I guess you might see where I am leading, that possibly climate science has been looking at this whole physics problem somewhat backwards, placing all emphasis on what is entering and leaving when it is what is held strictly by the mass, not pressure, not density, not co2’s 15 micron lines but mass by the mass attenuation coefficient of IR (hard to find references) and the mass. To me what is possible to go exit will exit no matter what, energy flows, in an open end system you cannot ‘trap’ it, but, if the horizontal is thick, that component cancels by symmetry if absorptions occur. Does that make sense to you? Not worded very good but you might get the drift.
I would offer some collaboration if you should need any. No strings. Heck, have spent two years now trying to untangle ‘atmospheric energy’ and this is the closest approach to a clear view, thank you much for this post! Boy, I jumped on this topic a bit hard the minute the first few paragraphs were read, hope I didn’t over-fill the thread. Just hoping there is not some overlooked aspect that negates this concept but can’t see any so far.
And to give credit where it belongs, the talk in my comments above of NET energy never returning to the surface once it has entered and been absorbed by the atmosphere, both solar SW in and surface IR out… that is from Dr. Miskolczi’s papers, not me. Being an astrophysicist I think he does know what he is talking about. He’s right, that keeps it strictly on thermodynamics, no back-radiation in that particular frame of reference, strictly net.
Bob says (in reply to my comments about flow of IR photons)
“5) Since the EMR in 3) is continuous and greater than in 4), and both are part of the continuous 396 S-B surface emission, the vertical flux component must be less than 396.
We are indeed getting closer to agreeing. In some ways I might even get more extreme than you, but I would be very wary of a phrase like “part of the 396 S-B surface emission”.
At an altitude of 0 km (ie at the surface), there will be an average S-B surface emission of 396.
At some altitude like 2 km, I would suggest that very close to ALL of the SB surface emission in certain bands has been absorbed. In particular, the band that CO2 absorbs around 13.5 – 16.5 um (wave number ~ 600 – 750 cm^-1) there would be effectively NONE of the “396 S-B surface emission” still present. Not because it has be “emitted horizontally” but because it has been absorbed in to the GHG molecules and ceases to exist as IR photons. Conversely, outside the bands where GHGs absorb, very nearly 100% of the surface IR photons will still be heading generally upward. The spectrum of IR photons would have huge gaps in in.
For the sake of argument, let’s suppose that GHG’s absorb 1/3 of the possible wavelengths (the exact number is not critical). Then 264 W/m^2 out of 264 W/m^2 of surface IR photons OUTSIDE the GHG absorption bands will pass upward thru the surface 2 km up (because the atmosphere has little or no effect on those photons). But 0 W/m^2 out of 132 W/m^2 of surface IR photons INSIDE the GHG absorption bands will pass upward thru the surface 2 km up. So in this case, only 264 W/m^2 of surface IR photons will pass up thru a 1 m x 1 m square 2 km up. (The cut off at the edges of the band is not perfectly sharp, of course. The bands tend to have ragged edges, but that can just factor into the net fraction of IR photons absorbed.) )
On the other hand, if you look at MODTRAN output looking down from 2 km, you do not see 100% missing from any bands. Why is that? Simply because those are also the bands where GHGs emit IR photons. To the extent that the GHGs are the same temperature as the surface, the spectrum would still look the same — ie like a S-B blackbody curve. By the time you are 2 km up, the gas is getting cooler, and the power is a little less in the GHG absorption/emission bands. If you go back to 0.1 km in MODTRAN looking down, the spectrum is indistinguishable from the BB curve, even though the gases from 0 – 100 m must be absorbing a big chunk of the IR surface photons.
(there are several other interesting comments lately, but I don;t have time to reply to them all right now.)
wayne, why would you think absorption features of the gases is not important? Sun energy reaching, number of particles (which, if we are talking about similar gases, correlates to first order with mass), and ability to absorb are all key ingredients. [Even the curvature of the planet is important.] Do you have specific evidence to suggest absorption is not an issue?
Mass is one tool we can use within the context of a particular system (where a particular “attenuation coefficient” might have a specific value), but generally mass in one atmosphere would not directly match mass in another without some scale factor to account for these other variables.
Keep in mind that near the ground, with lots of hills and mountains, there will be a lot more chances for “re-radiation” to hit the surface.. and there is no need to abandon re-radiation (which includes a downward slope).
A mostly flatter (temp) lapse rate curve results from the homogeneity of Mars atmosphere. Do you have samples of those curves that lead you to think absorption characteristics are not important?
>> the talk in my comments above of NET energy never returning to the surface once it has entered and been absorbed by the atmosphere
Saying “net” and “not returning” just means that we get a (net) distribution profile that is most intense at the excitation boundary (the planet) and trails off towards the other end.
Just think of putting your hand against a heating coil. The closer you are, the hotter it feels. This is true regardless of the contents of the atmosphere; however, this doesn’t negate the concept of greenhouse “trapping” (absorption and emission in all directions).. ie, raising the overall profile higher the more greenhouse effect there is.
Also, because of the curvature of the planet, the higher you get, the lower is the percentage of your potential re-emission direction that intersects the planet. Combine this effect with the lower density higher up (meaning that the photon is less likely to be intercepted). These two things (combined with absorption/radiation) are consistent with the table Spector provided a little higher up. [Note that if the planet was flat and infinite, a particle would always have half of its potential emission directions intercepting the planet no matter how high the particle was.]
Concerning climate models, I suspect they model particle interactions (or wave equations) at a fairly low level. Solving physics time-dependent differential (or short time step difference) equations at many subdivisions of the planet’s atmosphere (with the power of supercomputing) takes the need for intuition or for coarse approximations out of the picture. That “lateral” effect (and absorption, density, and everything else) would be factored in automatically.
I want to point out a few things
>> At some altitude like 2 km, I would suggest that very close to ALL of the SB surface emission in certain bands has been absorbed.
Whether 2 km is accurate or not would depend on the density of ghg in the atmosphere that could absorb in those bands. I mention this since Mars was mentioned. On some planets (maybe within our solar system or not), there could be no such value where one could say “close to ALL” because of the thinness of the ghg. Put differently, on one group of planets, we might be able to say “close to ALL” even as high up as several kilometers, but on other planets we might not be able to say “close to ALL” even when on ground level. This effect may play a role in the temp distribution in the atmosphere and help explain increasingly strong “skewing” of the profile beyond (or some bending at) some height. We have some form of “saturation” and then a trailing off much more quickly.
Additionally, as stated in an earlier comment, the curvature of the planet (aided by decreasing gas density with height) suggests that being higher in the atmosphere increases the chances significantly that the next emission will direct a photon essentially away from the planet even if the net direction is partially “downward” (the photon straight line path may initially approach the planet a little but then hit minimum nearness and diverge and keep diverging towards infinity).
>> Conversely, outside the bands where GHGs absorb, very nearly 100% of the surface IR photons will still be heading generally upward.
Note on “generally upward”: In the limit towards infinite distance, all such out-of-band surface emitted photons that don’t run into dust, mountains, etc, will essentially follow a path that approximates a radial path from the planet. They will all eventually be seen as mostly moving “up” from the center of the planet. [Eg, Bob’s discussion of some sun rays leaving from the edge of the sun laterally is approximated by saying that the ray is moving up from the center of the sun.]
Note: The chances of emitting in such a band is a probability calculated within the quantum mechanics framework (I have some but not too much experience here). The narrower are the absorption bands (and taking into account LTE), the greater the likelihood the next emission from a molecule will be “upwards”. To relate to the Mars example, having enough total ghg band coverage (aka, distinct ghg) greatly improves the odds of an emission _from the surface or other solid body_ being absorbed and not just passing through into space unimpeded.
>> For the sake of argument, .. GHG’s absorb 1/3 .. 264 W/m^2 out of 264 of surface .. photons .. will pass upward
Note: 264 is 2/3 of 396. This just says that at the initial “hemisphere” emission on the surface (assuming all such angles have equal probability) a full 2/3 will simply leave the planet unable to be impeded (ie, absorbed).
Note: Of the other 1/3 absorbed in the near atmosphere, a fair number of photons will then later be in a “re-emission” mode where we look at the full spherical direction, of which approximately half will be right back at the planet and will be absorbed essentially no matter the frequency.
Note: To improve this simple model, we would have to factor in the distance and direction the gas molecule would travel (including, on average, losing some of that energy with LTE) before the next photon release. So a “second” round of releases, on average, would be some distance from the planet that may not coincide with the average distance of (first) absorption of planet surface photons.
Note: FWIW, the “lateral” effect plays a role to the extent it lowers the average distance from the earth of a further collision. If we could somehow create a field that skews emissions to be mostly “up” then the average distance to next absorption would be higher.
>> On the other hand, if you look at MODTRAN output looking down from 2 km, you do not see 100% missing from any bands. Why is that? Simply because those are also the bands where GHGs emit IR photons.
Note: So even though certain bands get almost entirely caught in the lower 2 km. They eventually get re-emitted _always_ at that same band range, but, being higher up, now have an improved change to make it out of the 2 km (enabling it to be measured ..so to “fill up” that would-be missing band).
>> By the time you are 2 km up, the gas is getting cooler, and the power is a little less in the GHG absorption/emission bands.
Note: Although these re-emissions just mentioned are in the “same band”, because of average lower energy higher up, they will be redistributed within that band towards the lower energy ranges of that band as compared to the distribution we’d see nearer to the ground.
>> Note: 264 is 2/3 of 396. This just says that at the initial “hemisphere” emission on the surface (assuming all such angles have equal probability)
The angle probability part at the end is irrelevant.
>> To relate to the Mars example, having enough total ghg band coverage (aka, distinct ghg) greatly improves the odds of an emission _from the surface or other solid body_ being absorbed and not just passing through into space unimpeded.
What I mean here is that if a planet mostly has just one type of ghg, as Tim Folkerts’ explanation implies, a larger fraction of all surface photons would simply leave unimpeded into space in comparison to a planet that had more energy band coverage because the atmosphere had a wider array of ghg in significant numbers. This may address a complaint wayne mentioned recently about Mars’ mostly CO2 atmosphere. So for Mars, losing half their CO2 but doubling up on some other gases (even though the new number of total gas particles would still be much less) might lead to a greater greenhouse effect. [Maybe we would need to more than just double up on some of these trace gases, but we might still remain with many fewer total gas particles for the same gh effect.] And if we reverse the argument with methane playing the role of dominant gas, the same logic would apply (even though CO2 has a weaker gh effect). This invokes the principle of “diminishing returns”.
Jose_X November 13, at 11:51 pm
Jose, you wrote in part:
[1] Actually, both Trenberth and the MODTRAN model calculator do not show that, and if the 396 W/m^2 S_B surface emission did make it to high altitude/space, then there would be more energy leaving the Earth than was received from the Sun.
[2] All parts of the atmosphere emit photons isotropically. In successive layers approaching the TOA, some photons can escape more readily to the normal because of shorter path lengths, whereas the more horizontal are absorbed. When closer to the TOA, even those approaching the horizontal will also escape to space. Thus an observing instrument needs a wide angle field of view to pick-up all the radiation. (it is not all normal)
Regarding the MODTRAN calculations presented above, they seem perfectly logical and understandable.
I) The final column should clearly be labeled “Net IR Energy Flow Out”
II) The only 2 numbers on this chart that Trenberth is dealing are the first row (428.610 up – 365.496 down = 63.114 up at the surface) and the last row (292.334 up at the TOA). These numbers don’t quite match his numbers, but his numbers global averages and these are clear sky tropics, so there is no reason to expect them to match exactly.
III) The true Net Flow should be 0 W/m^2 (or ~ 0.9 W/m^2 if you want to trust Trenberth’s estimate of net warming) at any level. At TOA, the ONLY way to send energy up is IR radiation, so this should (approximately) match the net inflow. The global average inflow is 341 -102 = 239. This is less than the MODTRAN results, but the inflow at the tropics would be larger, so 292 vs 239 seem reasonable.
At the surface, there are many other ways to send energy up, so the net incoming solar (maybe 200 – 250 in the tropics), would be matched by the net outflow (the 63 IR outflow + evaporative outflow + convection outflow).
The higher you go, the less able the other energy flows are (evaporation & convection), so IR must take up the slack.
Wayne says “Dr. Richard Feynman explained all of this some forty years ago…quantum electro-dynamics…”
We seem to be having considerable difficulty with real photons; I don’t think getting into virtual photons is going to make things clearer. 🙂
Jose_X, I think I agree with pretty much all of your additional notes on my comments. You seem to be preemptively addressing a variety of issues that others might have. It is always tough to know how much detail to put in, and how much to wait until people have additional questions. And of course, things that are obvious to me (like 2/3 x 396 = 264) may not have been as obvious as I thought.
Jose_X, I read your tips and don’t even want to consider such complexity at this point. Simplicity first. But thanks anyway. After all, if I don’t already know to consider what you mentioned plus some, there’s probably no hope. ☺
Bob,
[1] You are right that saying “all” didn’t make sense there. “All” might only apply if we were just releasing photons from surface straight into space.
[2] The instruments cover approx a full hemisphere, yup.
Tim Folkerts, yes, I wanted to anticipate questions and take advantage of connecting various ideas/points with each other. Also, I too am shaping and refining my understanding, so the comments were part of Jose_X answering Jose_X’ [I have multiple internal identities. The debates can get really heated sometimes.]
wayne, some of those analysis “tips” were not intended for mere humans. I don’t blame you, but don’t blame me for trying to indulge a moment in the heavens.
Wayne,
Thanks for your suggestions and your offer to collaborate on a new article, but things have become complicated for me in the near term.
I had planned to re-write the article to include some pre-emptive stuff WRT various queries and criticisms in this thread of over 400 comments. I would then offer it to JoNova and/or WUWT. Oddly, if I tie-up all the loose ends it may result in not much traffic. On the other hand, if Anthony agreed to run it, it would be good if R. Gates and Willis Eschenbach among others would comment. (I’m disappointed they have not joined-in). Then if Joanne Nova ran it, it would hopefully stimulate a different audience, and it might provoke the interest of David Evans, her husband.
I was also thinking along the lines of a part 2, concerning some other issues with the Trenberth cartoon, the biggest of which seems to be the alleged 40 W/m^2 radiated directly to space from the surface, which is somewhat more than the remaining 23 of the 63 net leaving the surface absorbed by the atmosphere, and….
However, currently I’m hopping mad that the ACMA (Australian Communications & Media Authority) have sent me a 29 page report rejecting my appeal on complaint originally rejected by the National Radio, the ABC, concerning their so-called “Science Show”. They produced a whitewash that did not actually address the issues I raised. This process has taken over one year. I suspect that it may be political because it covertly criticises junk science from the AAS. (Australian Academy of Science)….. and boy it took them about 6 months to come-up with their 29 page report which they say they wil publish on their website.
I need to do something about this, and to raise a new complaint to ACMA showing how else the “Science Show” is knowingly biased.
I’m also planning to have a break in Tasmania of about a month, and where I’m going, radio contact may not be available.
It’s a bit off-topic, but there is more on my complaint here if you are interested:
http://bobfjones.wordpress.com/2011/03/01/abc-radio-australia%e2%80%a6-misleading-%e2%80%9cscience%e2%80%9d-%e2%80%a6-no-3/
Click on Complaint No.3 for the original complaint but there is also much correspondence.
Any comments I make from here-on for a while are likely to be brief and to the point!
Bob_FJ: “from here-on for a while are likely to be brief”
Hey, no problems. I mainly study science, scientists, and scientist’s logic and methods. That simple. And there is a pot-lot of work left to do in the realm of planetary atmospheric science. Addressing Earth, it is suck in a rut.
Thank goodness there are individuals like yourself willing to keep open eyes, such as admit there is even a 3-D aspect, mostly unaddressed, and bravely analyze it, and for goodness sake not merely follow “the script”.
Later Bob. Hope you endeavors to better that science show are successful. ( You mention Tasmania, enjoy! If I ever got that far south it would include a stop in Adelaide Yacht Club to give young Jessica Watson a big pat on the back. (youngest girl to solo circum-naviagate last year) )
Jose_X @ November 14, at 7:20 pm
Very quickly:
So you now agree that radiation from earth is not all normal to the surface?
And earlier:
Erh from memory the average diametr of the Earth is about 12,500 Km. Tim Folkerts suggested that the curvature effect amounted to about 1%, which is trivial compared with the other stuff. MODTRAN suggests that most of the radiative action in the atmosphere is almost over at an altitude of around 10 Km in the tropics. (presumably lower towards the poles). So how about you do a calc for 12,510 squared / 12,500 squared?
Tim Folkerts @ November 14, at 1:43 pm
Quickly;
You wrote in part:
Me no understand; the surface emission is isotropic!
Bob, the spectral gap I was referring to is with respect to the wavelengths, not locations or angles. So yes, I agree that the surface emission is indeed isotropic.
Refer to Figure 5 in your post. The envelop for the data seems to be a BB curve with a temperature ~ 295 K. So at ground level, the curve would (very nearly) follow the 295 BB curve. In particular, the scale would be ~ 140 (in the units of the graph) at 15 um.
As the photons travel thru the atmosphere, some will get absorbed by CO2. The deep “bite” around 15 um is (I am pretty sure) due to CO2. In the first km, a big fraction of the 15 um photons would get absorbed. For the sake of argument, lets assume that 99.99 % get absorbed.
If I understand your argument correctly, you would say that only ~ 1/6 of those 15 um photons (about 25 units on the graph) would be heading mostly upward, so the observed result should be ~ 25 for the “brightness” of 15 um photons at 1 km according to your model.
According to my model, about 140 of the 140 units of 15 um photons should be observed heading upward. Even though ~ 0% of the 15 um surface IR photons survive, I say the missing 15 um surface photons are replaced by an approximately equal amount of 15 um photons emitted by the atmosphere.
In other words, I predict approximately no “gap” in the IR spectrum at 15 um when looking down from 1 km. And (I believe) you predict a deep “gap” at 15 um. (Feel free to correct me if I am misinterpreting your model).
>> So you now agree that radiation from earth is not all normal to the surface?
Bob, I’m not sure where you think I said every emission is normal. I said (a) normal appears to be an accurate way to represent (what I anticipate is) the net average effect at ground level; (b) normal is accurate representation if we are doing simple diagrams (engineers use simple box models all the time); and (c) far from earth, as the earth can be approximated by a point, the photons we do find that originated there will be very near a radial line from the center of the planet.
I also told wayne I understood his explanation of your graph and agreed (in different words that) the majority of area on the surface of a sphere is not directly up or even mostly up.
I can understand you misinterpreted Trenberth’s diagram (as I don’t think Trenberth was trying to suggest every emission from the ground was exactly up at 90 degrees or only in the general “up” direction). Yes, that diagram appears to be an oversimplification and not great for learning about the process.
I haven’t changed my mind on any of this.
>> So how about you do a calc for 12,510 squared / 12,500 squared?
When I mentioned the curvature issue, I was not referring to anyone else’s context. It was partly to state that high up it becomes extra unlikely (assuming random direction emissions) a photon will head on a course to hit the planet. The times I mentioned that I was not necessarily thinking up of a figure for altitude, especially since the conversation has been general and even included talk of other planets; however, to demonstrate the point, we would not look at the 10km section since there it wouldn’t be much of an issue. Looking at wikipedia, the exosphere goes up till about 10,000 km. Whatever calculations you wanted me to do for 10 km would not be necessary now. 🙂
Point one:
“Up arrow” can certainly signify the _entire_ upper hemisphere rather than what you (Bob) are calling simply the upper portion of the upper hemisphere. That is a reasonable interpretation for an up arrow, especially in the context of an audience that knows obviously not all emissions are 90 degrees up.
Further, this interpretation makes even more sense as explained in the next point.
Point two (and clarification):
>> (a) normal appears to be an accurate way to represent (what I anticipate is) the net average effect at ground level;
A sensor would likely accept approximately anything from the full half space (think hemisphere) towards which it is oriented. This means that a photon coming quasi-laterally from far away would on average (at a given height, say, very close to the ground) make up for a photon directly under the sensor which was inclined laterally as well.
So by “net” I was looking at this from the perspective of a measuring device, where what matters is the number of photons hitting in a certain (eg) square meter and not the angle any particular photon might have.
I understand you were bothered by this, Bob, but think of the context around this paper and consider no longer thinking there were evil intentions behind it. I am sure many laypeople would go bonkers thinking you are trying to deceive them if they see some of your diagrams intended for other (mechanical) engineers.
Tim Folkerts @ November 16, at 4:08 am
So I guess you did not mean to say the following in your previous post; my bold added:
Tim Folkerts @ November 14, at 1:43 pm
Quickly,
You wrote in part:
Bob says “So I guess you did not mean to say the following .. ”
Actually, I think I do mean what I said. Or I mean what I thought I said.
Near some wavelengths — for example near 11 um (near 900 cm-1) — the atmosphere is quite transparent. Nearly 100 % of the 11 um photons emitted from ground level (“surface IR photons”) will reach a surface 1 km up.
Near some other wavelengths — for example ~15 um (~ 700 cm-1) — the atmosphere is quite opaque. Nearly 0 % of the 15 um photons emitted from ground level will reach a surface 1 km up.
So the spectrum observed (a la Figure 5) due to photons emitted directly from the surface would be “bright” near 11 um and “dark” near 15 um. The “dark bands” are the “gaps” I was talking about in the spectrum of the IR photons emitted from the ground.
Does that clarify my position?
~~~~~~
And I would really enjoy hearing your reply to my post:
“In other words, I [Tim] predict approximately no “gap” in the IR spectrum at 15 um when looking down from 1 km. And (I believe) you [Bob] predict a deep “gap” at 15 um. (Feel free to correct me if I am misinterpreting your model)”
Ie. i think the spectrum looking down from 1 km up will be ~ identical to the black body spectrum looking down at ground level even if the GHGs in between absorb a lot of IR photons and send them out “mostly sideways”.
Jose_X says:
November 16, 2011 at 11:20 am AND 11:38 am
You wrote in part:
I feel you should either retract that statement or clarify what you meant to say.
I don’t have time to respond to the rest of it.
Tim Folkerts, it seems you are saying at the top half of your last comment (“[a]ctually, I think I do mean what I said”) that if we could hypothetically track the photons to filter out all except the surface photons, then we would get gaps. Then at the bottom, it seems you are saying that actually measuring quasi-near the ground would reveal little distortion away from blackbody of ground.
Here is what I am thinking now, and it offers a way to explain figure 5.
You did say earlier (according to my interpretation) that the varying temperatures across the atmosphere would distort the observed in band spectrum from high above. I would agree with this statement but would add that we also get distortion from the following effect. [Here, “in band” and “out-of band” refer to energy ranges relative to ghg.]
Assume, to get a first order result that we can ignore night and day differences, clouds, etc (I’d have to think more to see what effects these might have on average).
The earth surface receives in band and out-of band radiation X from the sun and essentially only in-band backradiation Y. [For the record (but not used much in the analysis): the X is from immediate sunlight shine while the Y includes leftover atmospheric effects going back into the distant past (negative infinity in time).] This X+Y input gets output as the in band and the out-of band of blackbody spectrum at surface temp.
A) The out-of band makes it to satellite measurement.
B) However, only some of the in-band energy leaving the surface at time t=0 will eventually make it upward as in-band. A part of this surface radiated in band energy at t=0 will make it back to earth at various future times via the Y stream. This means that some of the photon energy released in band at t=0 never make it to space in band because it detours by the earth and gets redistributed to _full_ in surface temp blackbody spectrum. If we were keeping track of in-band energy released at t=0, we would find that some of it makes it to satellite as in band energy at numerous future times, but a large part of it becomes, in small chunks, part of out-of band radiation (at surface temp), each chunk at some future point in time.
So, (A), as out-of band energy radiated from the surface at time t, fairly evenly fills out the out-of band blackbody curve at surface temp as measured by the satellite at about time t. (B), as in band energy radiated from the surface at time t, helps build the out-of band (A) of many future points in time, with the leftover helping to fill out the in-band satellite measurements taken at many future points in time.
Alternatively, we can look at the picture from the point of view of the satellite reception: Out-of band satellite received photons at time t were radiated from the surface at about time t and accounts for energy from sun and that had previously in time been generated as in band from the surface but returned to the surface. In band satellite received photons at time t were radiated as in band in the atmosphere and/or the surface of the earth with “pre-cursor” photons having been generated at a distribution of times in the past (also from the surface and/or atmosphere). [“pre-cursor” is an approximate term that might make sense if we were accounting carefully for energy. Of course there is LTE, etc, to muddy the picture.]
The out-of band at satellite is fairly even and corresponds to surface temp. The in band at satellite is varied (due to radiating at varying altitudes/temps) and falls significantly short of the out-of band curve. This description qualitatively matches the satellite curves shown in fig 5.
I’ll have to think a bit more about these details. What do you think?
@Bob: sorry if that comment upset you. After posting I realized I was carrying over from your comment at your blog site (to Jose Testx), where you are upset at the IPCC. So what you quoted of my reply here doesn’t apply here. Sorry. [I may not get a chance any time soon to read any of the books you mentioned on that other thread at your site. I may reply there later on after I try to read some of what you mentioned.] I thought about clarifying but got distracted with my prior comment to Tim Folkerts.
@Bob: OK, this prior comment only applied to the “there were evil intentions behind it” part.
The part about your engineering diagrams was my way of saying that Trenberth’s diagrams were probably understood by much of his intended audience. A person not an expert in climate science might very reasonably misinterpret the meaning of the arrows in that diagram. If you read the entirety of that comment I wrote, you’ll read that I now think the up arrow more likely than not refers to upper half plane rather than to a region around the surface normal direction (as I think was your interpretation in this article).
>> The earth surface receives in band and out-of band radiation X from the sun and essentially only in-band backradiation Y.
Ooops, X is mostly just out-of band (since in-band would largely be blocked on way down).
And I am talking about _directly_ receiving the radiation from the sun in the sense that the photons were generated in outer space (likely at the sun).
So in my hypothetical energy accounting, I start off by partitioning the earth received radiation into what comes immediately from the sun and what comes from elsewhere (ie, from the atmosphere).
Tim Folkerts @ November 16, at 2:55 pm
Tim, look, I’m sorry but you are asking for discussion on something which is a tad complicated and speculative that I don’t have time for, as I explained above.
For instance, the IR radiation seen from space by Nimbus must include that originating from the surface a la S-B, together with thermals, evapotranspiration, atmosphere, and clouds. These things all have difficult gradation issues with altitude. They don’t have labels attached, and there are other bothersome things like lapse rate causing lengthening of wavelength with altitude, and varying H2O vapour level and, and, is that enough?
Bob says: “Tim, look, I’m sorry but you are asking for discussion on something which is a tad complicated … ”
I agree they are complicated. But remember you are the one who started this by questioning the Trenberth diagram and the physics of IR radiation. It is more complicated than drawing a colored circle with an “X” and guesstimating how much IR should go different directions.
A quote from Lord Kelviin comes to mind:
Until we are discussing actual numbers and actual 3D integrals, any discussions we have are destined to be “meagre and unsatisfactory”.
Tim,
Thank you for cherry-picking part of a line of mine that was couched in irony.
If you check back on my article, the prime topic related to isotropic radiation in an absorptive atmosphere. I have little interest in the actual numbers, but just to show that the vertical components of the 396 must be less than 396. The addendum showing absorption spectra etc, was just that: an addendum as a puzzling matter of interest, which I doubt can be solved within current knowledge. (See also Spector’s posts above)
WRT your philosophy on science:
There was a great Bishop a few centuries ago, (Upshott?), famous for precisely calculating the age of the Earth at that time as being 6004 years. It was based on sound data in the Holy Bible, a forerunner of the IPCC report. Then along came Kelvin, and shock-horror whilst personally facing ridicule, he said nah, Earth is much older, based on calculations on rate of heat loss through the Earth’s crust. (even though he knew not the origins of that heat…. Erh sorry, but for you Tim, read; thermal energy ILO heat). But, they were both wrong by some billions of years; so it seems.
Take Alfred Wegener; not all that long ago he had a fairly sound hypothesis of “continental drift”, (tectonics), but I understand he never got it past peer review, and got a good deal of cheek from his church. Yeah, that’s right, he didn’t have any numbers!
As for your concluding:
I can kind of visualize an integration somewhere around the TOA, wherever that is, isotropically to space, ignoring transitions. However at the surface and above in an opaque atmosphere? Eh? If you really think that, why not submit an article to Anthony and see how it goes?
Incidentally, my laptop is starting to run seriously slow at over 450 comments, including those Myrhh monsters.
Tis time for a new updated post methinks, if Anthony might agree, or bye-byes from me here.
Bob, I don’t think the key is mere calculations. A theory that has a quantitative component makes it easier to check against experiments and provides more useful predictive capabilities (from which we can also further test the theory).
I haven’t looked up Wegener, but I am sure the current theories are superior to whatever he presented, even if they adopted the essence of his theory.
It’s only a matter of time before good theories gain acceptance, but it does take time, partly because there are many many more “great” theories that fail in various ways under the microscope. Science isn’t about faith, it’s about weeding out to find the most useful models of reality (eg, repeatability/verifiability, comprehensive yet as simple as possible, computational components/utility, etc).
One of the biggest problems I have with challenges to current climate science is that there is no theory being presented that beats what exists. Most likely (“if I were a gambling man”) I’d say that nearby future changes will not be revolutionary but instead adjust the current theories.
The computer models can do a very good job of solving many differential equations (including doing all the necessary “integrating”) to improved degrees of precision as computational power increases. Most people challenging the status quo are not aggressively looking at these software programs, and so are really hurting their chances of providing a competitive computational framework. There are some open source versions of complex climate models that can be used for free, analyzed, documented, and forked if necessary. [giss/nasa has at least their model E as open source.. google.]
Jose_X @ November 18, at 3:55 pm
you wrote in part:
• Do you know of any empirical evidence that shows a connection between global warming and CO2?
• Please explain why more powerful computers will give better results on GIGO models? Won’t they just pump-out Garbage more quickly?
• Did you see that recent article at WUWT, where the IPCC has allegedly reported a change in heart about AGW over the next 20 or 30 years?
• I responded to your latest comment on my website, (which is not intended as a blog), concerning Donna Laframboise’s book. Don’t be a meanie; spend $4.99, it won’t kill you.
Jose_X
Please ignore/do not respond to my last post to you. I guess I was thinking you needed some help, but it is really going too far off topic
Bob,
I’ll have to just agree to disagree with your understanding of “the vertical component of the 396 W/m^2”.
~~~~~~~~~~~~~~~~~~~~~~~~~~~~~~~~~~~~~~~~
I *do* disagree with your characterization of science (or more specifically, your characterization of my characterization of science).
“It was based on sound data in the Holy Bible”
The Bible is an authoritative source of religious information for Christians, but it is not an authoritative scientific source of “sound data”.
” … based on calculations on rate of heat loss … ”
Now you are agreeing with me! He had *numbers* that allowed a scientific understanding of a situation. He didn’t have all the data (no one ever does!) but he could *calculate* a number for the age based on the assumptions he made. His knowledge was no longer “meager and unsatisfactory”. We wasn’t just guessing an age, or accepting an authority. He could be quite sure that the earth was not just 6000 years old.
“Take Alfred Wegener … “
OK. He had a fascinating hypothesis. According to wikipedia “He supposed the cause might be the centrifugal force of the Earth’s rotation (“Polflucht”) or the astronomical precession.”
From the 20’s to the 60’s, scientists made measurements and performed calculations to support or rule out various hypotheses. For example, both precession nor centrifugal force were ruled out by calculating the magnitude of such effects. As better data and better calculations come forward, the theory took shape and became accepted.
~~~~~~~~~~~~~~~~~~~~~~~~~~~~~~~~~~~~~~~~~
IMHO, your theory is similar to continental drift in the 1920’s — an interesting hypothesis in need of calculations to give it a firmer foundation. On the other hand, I see MODTRAN more like continental drift in the the 1960’s. MODTRAN uses detailed calculations based on absorption data of real gases and comes up with spectra that match data quite well (your Figure 5).
If you want to convince me, you will have to show why MODTRAN is wrong (even though it matches real spectra well) and/or show that your approach gives BETTER results.
Tim Folkerts @ November 21, at 5:59 pm
[1] Well actually old chap, I’m NOT putting forward a theory or hypothesis, but a simple first principles statement of fact for you, in which I assume that you agree with elementary quantum theory and the greenhouse effect. The initial S-B surface emission is indisputably isotropic, (hemispherically on average), and BECAUSE the atmosphere is absorbent, a good deal of it is absorbed. (apart from in those unaffected wavelengths escaping directly to space through “the window”). As a consequence, there are subsequent emissions of various values from the GHG’s at successive ascending levels in the atmosphere. (arguable from Kirchhoff’s law for a surface). These emissions are isotropic spherically, and would not exist in a transparent atmosphere, by definition. Because these emissions are isotropic, there are undeniable horizontal components in which the photons are annihilated in absorption, but it is a CONSTANT process of renewal by virtue of the gas temperature; consequent from a global constant surface T average specified by Trenberth. Thus, as I think you agreed somewhere, there is all this horizontal EMR whizzing around, in which there is no heat transfer, yet this EMR must have come from somewhere, and it ain’t part of the vertical stuff. To quantify the components at ascending levels would be phenomenally difficult, (see [2]), and frankly that is not my purpose. It is exquisitely adequate to say that the vertical components are less than the isotropic total….. ZERO need for a calculation!
[2] Concerning the DIFFERENT topic of MODTRAN modelling, Spector was also keen on it, but when I asked if he/she knew if and how the modelling included Evapotranspiration, Thermals, Solar absorbed, (and I forgot to mention incoming solar IR), and how that untagged stuff might be graded by altitude, he responded to the effect: Dunno.
OK Tim: What do YOU know?
Is your confidence based on real knowledge, or respect for “authority”
Do you like comparing bananas with apples?
Bob,
I can agree with everything you said up through “…yet this EMR must have come from somewhere”
Where we diverge is where the energy comes from and goes to. Consider a band of wavelengths where the IR gets absorbed by a small bit of the atmosphere right above the ground — suppose this band has 6 W/m^2 of photons being emitted from the ground. You could say that (in this band) ~ 1/6 of the energy absorbed from IR photons coming from the ground goes in each of the “six directions” N, S, E, W, Up, & Down — ie 1 W/m^2 in each direction. If that was the whole story, then the upward component of IR would indeed get dramatically attenuated — ie only 1 W/m^2 continuing upward instead of 6 W/m^2.
However, because the atmosphere to the south is about the same temperature as the ground (and because it is an efficient emitter of IR in that same band), it will ALSO be sending 6 W/m^2 to our bit of atmosphere. And again 1 W/m^2 will go generally in each of the six directions. The same holds true for the other directions.
NET RESULT: 6 W/m^2 is coming from each of the “six directions” and 6 W/m^2 will be heading out in each of the “six directions”.
NOTE: This assumes a constant temperature for the atmosphere. Now in reality, there is a lapse rate, and this will mean less coming in and less going out in the IR bands as you go higher. But I argue that this is a result of a change in temperature, not due to energy ‘getting scattered sideways’.
As for MODTRAN, I am not an expert, but I do know it reproduces the actual spectra pretty well (as shown in your figure above). This is the single most important feature of a calculation.
Looking at the output from MODTRAN, it appears that it assumes a temperature and pressure profile based on typical values. This would be a bit of a simplification, since all the other factors you mentioned are simply assumed to have an effect that produces the profile they use. In effect they are taking the standard lapse rate and calculating from there. For minor adjustments of the parameters, this is probably fairly safe. In more extreme cases, this would be a problem. For example, removing O3 from the stratosphere should remove much of the temperature rise in the stratosphere, but it has no effect on the assumed profile in MODTRAN. I suspect that the temperature profiles could adjusted, but not easily in the interface I have seen.
I don’t thinks this is really a apples/bananas comparison. You are saying that the energy upward must decrease significantly as you go up. MODTRAN says the energy upward does not show much change thru several km of altitude, even though nearly all of the IR photons from the ground (in IR absorption bands) must have been absorbed and “re-emitted mostly sideways”. To that extent, you are directly competing with MODTRAN to describe how the photons flow.
In 1954, Hoyt C Hottel conducted an experiment to determine the total emissivity/absorptivity of carbon dioxide and water vapour. From his experiments, he found that the total emissivity of carbon dioxide is almost xero below 33 deg C (551 deg R) in combination with a partial pressure of 0.00039 atm.
17 years later B Leckner verified Hottel’s results finding that the emissivity of carbon dioxide was insignificant below 33 deg C and a pp of 0.00039 atm.
Hottel and Leckner’s graphs show a total emissivity for carbon dioxide of zero under those conditions.
The same investigators found by experiment that the emissivity/absorptivity for 5% atmospheric water vapour at 33 deg C was 0.4.
The absorption/emission spectral bands of CO2/H2O overlap and for atmospheric concentrations of 5% H2O and 0.039% CO2 it was found that CO2 attenuates the total absorptivity/emissivity of water vapour and acts to cool rather than warm the atmosphere.
@Robert S, I could not find the paper by Hottel (didn’t check for others). Can you provide a link?
Let me ask, at what wavelength does the quoted emissivity apply?
Over what depth of atmosphere does the emissivity apply?
How was this measured?
Doesn’t such an allegedly negligible value disagree with satellite measurements?
I don’t have data for the above, but I seriously get the feeling anyone alleging CO2 has negligible effect in our atmosphere hasn’t looked at the relevant data and/or is confused about the meaning of various measurements. I think the accepted theories agree to first order with satellite measurements. I imagine the depth of the atmosphere is not being factored in properly by those saying CO2 has negligible effect. Additionally, emissivity is useful for seeing how much energy leaves from the body at equilibrium but not at how the internal temperatures of the components of the body vary or what temp value they reach.
Jose_X, and Robert S
Your comments are a tad off-topic, but interesting. I think that Robert may be referring to Prof Nasif Nahle. It seems that Hottel and Leckner are not the only authors to write on this. See:
Determining the Total Emissivity of a Mixture of Gases Containing Overlapping Absorption Bands: A Note from Nasif S. Nahle (My bold)
http://jennifermarohasy.com/2011/04/determining-the-total-emissivity-of-a-mixture-of-gases-containing-overlapping-absorption-bands/
Check-out the 15 references
I’ve not researched it, but it looks interesting at a quick look.
BTW Jose, not all the literature is in papers in journals; (possibly behind a pay-wall); some is in books, some of which cost even more than the massive US$ 4:99, you imply elsewhere that you can’t afford!
Funny how Hottel and Leckner are unknown to the IPCC and climategate I & II Email personnel according to boolean search.
Jose,
I share your concerns about CO2 emissivity. This is a good link to a relevant summary of the topic: http://dspace.mit.edu/handle/1721.1/42950.
1) 5% H2O is very high. Typical numbers are 1-4% near the ground. Higher up where the air is colder, this number drops to ~ 0. Since the higher altitudes are where the radiation to space mostly occurs, water becomes mostly irrelevant.
2) From one of the graphs, the emissivity of CO2 @ ~ 300 K is ~0.04 for 1 cm-ATM. For air with a 400 ppm CO2, the partial pressure would be 1/2500 ATM. So the situation quoted would be for 2500 cm, or 25 m of the atmosphere. I suppose they can say that 4% absorption is “almost zero” for lengths of a few tens of meters, of that less than 1% would be “almost zero” for lengths of less than a few meters.
For 2500 m, this would increase to an emissivity of ~ 0.18 according to the table. Given that much for much of the spectrum, CO2 is transparent to IR, the bands where it DOES absorb must be close to 100% absorbed over the first couple km.
In the upper atmosphere, absorption/emission would be nearly complete over lengths of maybe a few 10’s of km. This implies the “TOA” is on the order of 25 km thick.
Tim Folkerts @ November 24, at 1:31 pm
Sorry Tim, I don’t get it, but it doesn’t matter anyway. Regardless of how much EMR you think there is whizzing around in the horizontal, (which is part of the total isotropic EMR), there is no net energy transfer. The only portion that gives net energy transfer is vertical.
Yes, the only part that gives a net transfer is vertical. But I would say that near the surface, this net transfer upward is due (almost entirely) to the “gaps” in the IR spectrum, not in the absorption bands of the GHGs. Near 15 um, where CO2 absorbs well, the net flow will be very close to 0 in the vertical direction, because ~ as many IR photons will be emitted downward from the CO2 above you as are emitted upward from the ground below you. And the CO2 will emit that same amount upward (the “6 W/m^2) in my previous example).
[There will be a very small net upward flow because the lapse rate pretty much guarantees the CO2 above you is cooler than the ground below you. But with an inversion layer in the atmosphere, I would conclude there would be MORE 15 um IR heading down to the earth than there is going up from the ground. More interestingly, there would also be MORE IR heading up from the top of the inversion layer than there is heading up from the surface. The CO2 will ENHANCE the upward flow of IR photons, not diminish it as. In other words, the arrow in the trenberth diagram for upward IR would be INCREASING from 396 W/m^2 as it went up from the surface until it hit the top of the inversion layer, before it started decreasing again.]
In the “gaps” (say around 11 um) , the earth is still emitting IR upward, but the GHGs are emitting nothing downward. This is what is responsible for the net vertical flux. It is not the absorption (and scattering in various directions) that reduces the upward flux, it is the transmission upwards (with no compensating downward flux) that is responsible for the net flow.
Let me do a gedanken experiment, and add gases to the atmosphere that absorbed IR at ALL frequencies. Supppose the gases were dense enough that 99.99% of the IR photons from the ground were absorbed in the first 100 m. Suppose the ground emits 360 W/m^2 at whatever temperature it happens to be. How much IR flux would you expect to see if you looked down from 100 m? I would expect to see 360 W/m^2. I would also expect to see 360 W/m^2 if I looked up.
Tim Folkerts @ November 24, at 1:48 pm
Tim, if you look at fig 5a, it describes the data as being …over a point in the tropical Pacific Ocean… Oh OK; but it makes no mention of day, night, season, or cloud cover, and the tropical Pacific is quite a big place, with some islands and other stuff, such as substantially varying regional weather patterns.
Whilst fig 5b is remarkably similar, (by my selection, which is why I queried it as a matter of interest in the addendum), it too is lacking ID as far as certain essential parameters required for comparison with 5a are concerned.
BTW, I’ve just spotted a mistake in my text for 5b. Where I wrote NIMBUS, I meant to write MODIS…… Sorry.
Oh, and you admit that you don’t know how the MODTRAN modelling is done. Have you considered the possibility that they may have fed-in some data from NIMBUS for looking down?
I think that this truncated quote is pure speculation and of no value.
If you try to compare 5a with 5b and Trenberth’s 396 W/m^2, then you are not comparing apples with apples.
Note that the Trenberth 396 does not include the multiple other inputs that you speculate MODTRAN has somehow incorporated.
Jose_X says:
November 25, 2011 at 10:44 pm
@Robert S, I could not find the paper by Hottel (didn’t check for others). Can you provide a link?
Yes, the paper referred to is in the following:
http://www.biocab.org/Mean_Free_Path.pdf
This is the first time I have seen a reference to any experiments by Hottel on atmospheric CO2 and water vapour at 33 deg C with the very special conclusion that the emissivity of CO2 is almost zero and insignificant.
My own calculations are close to Tim Folkerts perhaps we were using the same charts for CO2 and H2O from Hottel’s 1935 papers which are reproduced in McAdams ‘Heat Transmission’ chapter 4.
Using a partial pressure of 0.0231atm for water vapour I calculated that the absorbable LWIR is absorbed to extinction in 120 m giving an emissivity of 0.57.
These comments may be a tad off comment but as global warming is all about CO2 and its absorption of LWIR I think it is important to point out that,(1) there is no correlation betwween CO2 and global Warming and, and (2) that Hottel ond others found that the emissivity of CO2 was either close to zero or insignificant in the atmospheric context.
Robert S, you aren’t making sense to me. Tim Folkerts’ note, if I understood, was that atmosphere CO2 has significant absorption, the opposite of what I think you are saying. And the charts he pointed to show that CO2 emissivity is negligible if you consider a small enclosing of CO2 but are very significant if you look at large enclosures (including anything resembling a mile of travel distance in a dimension). Additionally, I have no idea what you mean by CO2 and global warming having no correlation? Where are you getting that from? One of the favorite arguments by many “skeptics” includes that historically CO2 clearly lags temperature. Climatologists agree that historically CO2 lags temperature. Clearly lagging something else is a strong correlation. Are you saying you don’t agree with that data?
Jose_X, I am saying that after the absorbable LIR wavelengths have been absorbed by water vapour and CO2, an increase in atmospheric CO2 will not absorb any more heat, it will merely change the absorption distance slightly but there will no additional warming. If CO2 was leading temperature, which it does not, you would get a feedback and a runaway (hockey stick) temperature increase which is clearly not happening. In the last decade there has been a reduction if anything.
@Robert S, I will assume from “I am saying that ..[something Robert S had not said]..” that you are Robert Stevenson.
What physics are you invoking in saying that adding more CO2 won’t lead to higher temperature? More CO2 molecules means more opportunities to absorb and re-emit ever closer to the ground and in higher densities as we move away from the ground. Why do you think this increase in absorption instances won’t lead to greater average kinetic energy (temp)? The average time a CO2 molecule would be exited would be going up (and with LTE, we know that all other gas molecules will also be increasing).
Note that I am not asking for an equation or claiming the relationship will be linear, but why do you think temp would essentially stop increasing (all else remaining the same)?
Also, CO2 is not a constant multiple of temperature. There are other variables. When people talk about “leading”, they likely mean when looked at over long period averages (steady state and with assumptions on how other variables behave).
Robert S,
I agree that near the surface, a little more CO2 will have little affect on the the absorption of IR because of the significant overlap with water absorption. However, high in the troposphere there is little H2O, and there CO2 is the major GHG.
Look at Bob’s figure 5. There is a “bite” out of the spectrum near 15 um due to CO2 radiating from the cold upper troposphere. With more CO2 in the troposphere, the “TOA” for IR radiation to space from CO2 would be a bit higher, and hence a bit cooler. This would make the “bite” deeper, lowering the IR radiation to space. With the same sunlight in but less IR out, the earth must warm a bit. Eventually the ground will become slightly warmer and can radiate a little extra to make up for the reduced amount of IR energy that CO2 is radiating to space.
That is the basic theory. Of course, then you can start talking about other feedbacks that could enhance or diminish the warming (eg changes in cloud cover).
Bob,
The hypothesis that MODTRAN assumes a temperature profile was a little more than speculation on my part. I had tried some rather drastic changes in he atmosphere (eg removing all the CO2) and the temperature profile remained exactly the same.
>If you try to compare 5a with 5b and Trenberth’s 396 W/m^2,
>then you are not comparing apples with apples.
Quite right, Those figures are connected to the 239 W/m^2 emitted to space.
>Note that the Trenberth 396 does not include the multiple other
>inputs that you speculate MODTRAN has somehow incorporated.
What I speculated was that MODTRAN has incorporated the net energy flows by assuming a temperature profile consistent with the observed profile, which is a result of all the energies.
However, MODTRAN’s energy and spectrum calculations are for IR only, and hence should relate to trenberth’s IR numbers (396, 333, 239) when looking at appropriate outputs from appropriate locations/directions.
@Robert S, I forgot to finish the reply..
Could you explain what you interpret as “leading” and then also explain what you meant by, not leading or else “you would get a feedback and a runaway (hockey stick) temperature”?
Reading Tim Folkerts reply, I should qualify that I was addressing the behavior of raising ghg gases, generally.
Specifically for CO2 at low heights (where a much greater concentration of water vapor exists) and in overlapping bands with water vapor, the water vapor effect overshadows the CO2 effect as long as the CO2 level is rather small. Put differently, a 20% increase in CO2 in a volume where that increase constitutes say a 1% increase in ghg effect (for a given absorption band) will be as if the increase was of around 1% and not 20%.
Regardless, at any given band where the earth is radiating, adding more of any gas that absorbs in that band should continue to increase the temp (under this simple model.. ie, ignoring other potentially counterbalancing effects), I think, for the reason I mentioned earlier: essentially that there is more traffic to intercept the photons, so their energies will remain in the atmosphere for a longer period of time, implying the atmosphere will remain at a higher temperature.
Tim Folkerts @ November 27, at 5:42 am
Tim, if there were 6 W/m^2 going up, and the same amount going down, the upward emission in that layer would be 6 W/m^2. The downward flux from either within or above the referenced layer would not impede the upward emissions in any layer.
If you are describing an S-B calculation, the total emission from the surface would be in all hemispherical directions, (isotropic). Thus, if you look down from 100m, you would not see the horizontal components, and would thus see less than 360 in total.
Oh BTW, the atmospheric widow is a separate issue, where I’ve repeatedly said like; ignoring stuff escaping directly to space.
Bob,
“Oh BTW, the atmospheric widow is a separate issue, where I’ve repeatedly said like; ignoring stuff escaping directly to space.”
Do you agree that not all of which passes directly from the surface into space is from that emitted perpendicular?
Bob says “If you are describing an S-B calculation … ”
I’m not describing any calculation; I am describing what you would measure with an actual IR detector (eg a pyrgeometer or simple IR thermometer). I would be willing to make a friendly wager that the spectrum and/or total IR power observed when the detector is 100 meters over a uniform, level surface would be indistinguishable from the spectrum 10 m above the surface or 1 m above the surface. (The only differences would be from 1) the *slight* affect of curvature of the earth and 2) a *slight* drop in temperature of the GHGs as you go up (~ 0.6 K for typical lapse) ).
And even with a S-B calculation, the IR intensity would not show any (noticeable) drop at 100 m. The surface will STILL cover 2 pi steradians of solid angle, and the incoming radiation will still be the same.
For example, we could buy one of these cheap IR thermometers http://www.thermoworks.com/products/ir/ir_mini.html#Specifications. It has a relatively wide field of view. I would argue that if the ground is a uniform temperature & emissivity, the thermometer will read the same value, regardless of how high you are above the ground (until the lapse rate and/or curve of the earth become important).
~~~~~~~~~~~~~~~~~~~~~~~~~~~~~~~~~~~~~~~~~~~~~
At this point, I think it is time to call it good for this discussion. I don’t think I am getting my point across, and without actually talking and drawing pictures on sheets of paper in front of us I don’t think we are going to make further progress.
I wish you well in your quest for understanding.
Tim Folkerts @ November 26, at 7:23 am to Jose
Tim, have you considered the possibility that above the clouds, not only is water mostly irrelevant, but so too is CO2? I think this is so, because not only is the air colder, resulting in lengthening of EMR wavelengths, but also the air is getting thinner, resulting in greatly increased photon free path lengths. It’s all over up there denken sie?
AND, concerning atmospheric water, it is also interesting to follow the war between that elitist alarmist atmospheric physicist Andrew Dessler, (with whom I’ve had some interesting intercourse a few years ago), and Roy Spencer et al. Back in January 2009 Dessler wrote this great wisdom at Grist; (my bold):
Negative climate feedback is as real as the Easter Bunny. http://www.grist.org/article/Negative-climate-feedback-is-as-real-as-the-Easter-Bunny
That was before some more recent research. Well Tim, don’t be timorous; how about you research the literature on this, and not just water vapour, but clouds
No mention of the much greater evapotranspiration cooling and stuff according to Trenberth of course.
Jose_X, the physics I am invoking is the concentration (partial pressure of CO2, Pc), distance mean free path of photon beam, L) relationship (PcL). Reducing atmospheric CO2 to 100ppm would not rduce the number of first generation photons absorbed in the relevant bands, but the distance over which they were absorbed would increase. Conversely increasing CO2 beyond 380ppm would reduce the absorption to extinction distance but would not absorb any more first generation photons, The subsequent retention time of these photons as they are re-emitted may be slightjy altered but not to affect the overall heat balance.
Bob, Tim, Jose, might I reenter this conversation for a moment, but first, I have come across a great link you might find helpful, pure correct physics and I finally found it here on the web, halleluiah!
http://astronomyonline.org/Science/RadioAstronomy.asp
Nothing like good ‘ol radio astronomy text which deal with radiation in these thermal/microwave/radio frequencies to give us a reference we can refer to ( had a bit of a problem physically getting my physics books onto the web, but this will now do ☺) and talk apples to apples.
See Tim and Jose, I agree with what that short book explains basically in toto. It’s a little skimpy in details but didn’t detect any scientific flaws. That is the science I have learned over the decades pre-AGW “climate science”. All matter radiates, EVEN GASES in a blackbody manner. All electrons ALWAYS radiate when externally accelerated, EVEN BUMPING O2 and N2, across the entire spectrum per T. Radio astronomers know this well. Our entire atmosphere radiates. Yes, GHG’s have strong absorption/emission lines and continuum but look at any spectrum of the atmosphere and this BB radiation underlies all of them. I know, would be called blasphemy to many today. The actual science has been re-written for a cause.
Bob, after reading all comments since I left, I have to bring up one point where you seem to keep stopping one step short of saying what is happening horizontally in thermal resonance. You end you statement with extinction. But that is just normal energy transfer and Tim is right then taking your one-step short statement and saying that is warming, it is. Extinction warms the destination and cools the source. That one step short of my A to B and B to A description above which is a null, or, oh, what word… resonance, or two-directional transfer with no NET transfer so no change in the temperature at either point. Do you see that subtle difference there? The identical and opposite side has to be included for cancellation to occur, and this happens even if you separate vectors across axes and are addressing those components.
All of that I said in comments above was an attempt to explain this, it is merely another way to view the energy transfers in contrast to Trenberth’s graphic. He has 396-333=63 of which 40 shuttles directly to space leaving 23 Wm-2. A pure geometrical 3d view gives you 396/6=66 of which 40 would also go directly to space leaving 26 Wm-2. The 4 horizontal components always completely cancel in our rather thick troposphere in the infrared/microwave. One question I have not answered yet for myself is what then exactly causes this 3 Wm-2 difference between those two views, one 63 and the other 66. In a geometric view, is that concerning GHG’s absorption or purely the atmosphere’s mass attenuation?
To me the geometric view is much cleaner, for that very aspect mention way back in these comments. It so very simply explains Mars atmosphere completely and the radiation transfers within. Nearly zero is absorbed so that any direction radiation goes it is to space, on all five axes. Mars temperature IS its BB temperature. Both 210K.
It also seems to explain Venus’s also. At the surface the radiative density is ~17000 W/m2 (740K), not 390 as here on earth and the DWLR is, let’s guess, about 16985 W/m2 at the surface with a NET upward of 15 W/m2. But 65 W/m2 is measured at TOA (compared to ~239 here) so means some 45 W/m2 in this example is absorbed by Venus’s atmosphere (and those are very rough guesses for example only). At the surface, in a near liquid co2 state, all 6 axis direction axes are in resonance but upward z+ still has a very tiny tendency (that’s why I brought in the ‘mass attenuation coefficient’, there are 94 equivalent earth atmospheres for the surface radiation to pass through when exiting, 17000/6 is attenuated down to a mere 15 Wm-2). Just skim off the gist of that explanation.
That is the good I found shining from your approach laid out by your post. Much more realistic on first principles, and getting closer to the approach that I think that should be applied to further analyze our atmosphere’s radiation.
Still have a hunch that 3 W/m2 difference is from our atmosphere’s shear mass that surface radiation must pass through, therefore the non-window radiation is attenuated, and not having anything to do with GHG’s absorption, water vapor or co2.
Of course, this view could also be very wrong, but I don’t seem to see it, but tear away all.
Tim and Jose, I know that is a very unconventional way to view the atmosphere’s radiation but that is what science is, questioning every aspect, from every direction possible. That is always where the hidden answers lie (though they sure don’t happen very often!).
Robert S, if first generation photons get absorbed closer to the ground, this has a cascading effect. Second generation would also get absorbed closer. The effect is that photon energy that would otherwise have left the atmosphere will still be in transit. Up and down the atmosphere, there will be a larger amount of total energy from earlier generations (going back to negative infinity). This implies a higher average kinetic energy per molecule, aka, higher temperature.
Where you say “negligible”, I say, yeah if a few degrees hotter is negligible. In any case, either of us would have to provide computations to give a more definite answer in the context of our current atmosphere. However, without performing more calculations, I certainly find it reasonable that there would be a rise of say 1 degree C (or something near that) solely on account of this higher CO2 doubling. In any case, we seem to both agree that higher CO2 concentration promotes higher temperatures (although you claim “negligible”), at least if we ignore more complex phenomenon (clouds and what have you), which we’d have to analyze additionally.
@wayne
>> All matter radiates, EVEN GASES in a blackbody manner.
For easier reference and comparisons, could you point out where on that page this is stated or implied.
>> All electrons ALWAYS radiate when externally accelerated, EVEN BUMPING O2 and N2, across the entire spectrum per T.
Then an electron moving around a nucleus should be radiating nonstop, right, even if the atom is alone in space, right? Wouldn’t then a static isolated neutral atom seemingly radiate an infinite amount of energy in the limit? Or what do you mean by “externally accelerated”?
And remember that we are generally talking about neutral atoms. What exactly is happening there with that positive and negative charges so close to each other and “moving” around (aka “accelerating”) so much?
The link you gave includes this:
“Molecules, as well as atoms, in their gas phase also absorb characteristic narrow frequency bands of radiation passed through them. In the microwave and long wavelength infrared portions of the spectrum, these lines are due to quantized rotational motion of the molecule. The precise frequencies of these absorption lines can be used to determine molecular species. This method is valuable for detecting molecules in our atmosphere, in the atmospheres of other planets, and in the interstellar medium. Organic molecules (that is, those containing carbon) have been detected in space in great abundance using molecular spectroscopy. Molecular spectroscopy has become an extremely important area of investigation in radio astronomy.”
So, if everything radiated as blackbody, we would not be able to identify anything by its spectra (that concept would not even exist) because the blackbody frequency distributions would vary only with temperature no matter the material.
This obviously is not what that article is saying. It specifically is saying that gases can be identified by unique fingerprints.
Gas molecules bumping require momentum and energy to be conserved as we observe with large bodies (or so go many theories); however, I think quantum mechanics probability wave functions stipulate that we might also get radiation (or not) and this would affect the final energies and momentums of the 2 molecules (and possible photon(s)). At these low scales, whether a photon is absorbed or released and when is a probabilistic phenomenon, which in theory would be described by the molecule’s QM wavefunction. In aggregates of many atoms, such probabilities may lead to a “smooth” range of observed values or at a “smooth” range of times so that we may approximately say that acceleration of charged particles always leads to “EMR”, but, at an individual level, any given photon may not release any photons.
People who work with antennas (engineers and scientists) likely use classical theories. The antennas are massive items loaded with molecules. When you have a huge number of such molecules interacting with each other closely (as in a solid), you get “thick” statistics and so can end up with blackbody. From a QM view, you have such complex wave functions that essentially have as potential measurable energies a continuum of energy values. With gases, you probably don’t get ready access to anything resembling a continuum of values. [I am not sure (don’t remember). We obviously have both kinetic energy of the molecule’s motion and the electron energy levels to trade off, but I think experiments suggest more quantized behavior when you have photon and molecule interacting vs say two molecules’ nucleus coming into the vicinity of each other and the resultant changes in kinetic energies. I need to get back to the books at some point… and check this out: http://www.colorado.edu/physics/2000/lasers/lasers2.html ]
>> A pure geometrical 3d view gives you 396/6=66
What is so fundamental about “6”? Might you be favoring “6” specifically because you noticed the relationship above? Why isn’t “top” defined by the “top hemisphere” (aka 1/2)? or why not top be the top 1% of surface area only? Why should “6” somehow define what is or isn’t “lateral”?
There really is no reason for “6”. We have a continuum, and approximating it as 6 is an arbitrary approximation.
>> The 4 horizontal components always completely cancel
No cancellation, I don’t think (ie, in the quantum wave function interference sense).
What we have is that for every lateral photon deviating to the side at some point, we have others from nearby (or faraway) points that also release lateral photons towards the original point. A sensor above any area gets photons from directly below and from the side. So while it doesn’t capture the photons originating below and moving sideways, it compensates (on average) from photons originating far away and moving laterally to the sensor.
If we hypothetically cover the whole earth in sensors quickly at a point in time, we’d likely observe that all “lateral” photons from below, would have to hit some sensor or other. Every photon would hit some sensor and the average would be the same as if all photons went straight up — we could not tell either way as we’d have no way to identify the origin.. the totals would add up just the same.
wayne, I just found this link http://www.cv.nrao.edu/course/astr534/LarmorRad.html
Skip to the bottom few paragraphs (right after they finish deriving “Larmor’s equation”).
At first, it appears to say what you were saying:
“It states that any charged particle radiates when accelerated and that the total radiated power is proportional to the square of the acceleration. Since the greatest astrophysical accelerations are usually electromagnetic, the acceleration is usually proportional to the charge/mass ratio of the particle. Thus radiation from electrons is typically 4106 stronger than radiation from protons, which are 2103 times more massive.”
Notice that it even uses the example of a single electron or proton.
However, as we read further we get the disclaimers:
1 — “Beware that Larmor’s formula is nonrelativistic”
and then the one relevant here:
2 — “Also, Larmor’s formula does not incorporate the constraints of quantum mechanics, so it should be applied with great caution to microscopic systems such as atoms. For example, Larmor’s equation incorrectly predicts that the electron in a hydrogen atom will quickly radiate away all of its kinetic energy and fall into the nucleus.”
So my point is that classical mechanics uses a language that appears to imply it applies to subatomic particles, atoms, and small molecules by themselves, but it only does so as a vehicle to help explain how to use the formulas and theories on macroscopic systems. [Hypothetical example: “If we have 1 billion atoms, take this equation that applies to any number of atoms and put in a value of 1 billion for n.” However, in reality, we likely could not apply this formula to a small number n of atoms.]
@ Jose_X
Larmor’s formula? Sounds interesting, I’ll look at it later.
Whoa.. I’m not here to teach you or argue. All of the objections you site happen to also be answered in the book of which I gave you the link. And yes Jose, I was not speaking of electrons falling into the nucleus but if you had any insight in physics at all you would have known that yourself, I even specified external and you still can;t seem to grasp it.
Spend an hour or two and read it yourself, maybe twice. If you still object, maybe send those perceived mistakes concerning radiation to:
http://www2.jpl.nasa.gov/radioastronomy/
That is the mirror site offering that same book in PDF form.
With your help, maybe they can correct their mistakes. Of course, most is also cross-reference to Kaufmann, W.J., III, Universe and that book might also need your corrections.
Sorry, my time is short and can’t chitchat things you seem not understand or disagree with. All I wrote is for others to consider.
Jose, one of your complains is clearly my mistake, in:
“Yes, GHG’s have strong absorption/emission lines and continuum but look at any spectrum of the atmosphere and this BB radiation underlies all of them”,
you are correct, should have read:
“Yes, GHG’s have strong absorption/emission lines and continuum but look at any spectrum of the atmosphere and this low emissivity radiation that follow Planck curve underlies all of them”.
I was lying trying to get some sleep mulling your comments and then it hit me, did I really type that… I think I did. Knew exactly where one of your points was aiming. Thanks for the catch.
wayne, I did not make a single complaint about that website. [not sure if you realized that on your follow up comment or not] And I quoted the other website (Larmor) to show that classical theories are not intended to be applied at all levels, even though the descriptions might sound as if they apply to atoms and what not. I didn’t find a problem (skimming a bit here and there) with the radioastronomy site. For many forms of EMR analysis, they can get away with classical analysis (although I quoted that same page talking about the value of quantum effects).
Now, I am not sure what you mean by “low emissivity radiation that follow Planck curve underlies all of them”.
Anyway, *one* reason I come back to this thread is (to learn, share, and) to defend attacks against climatology to the degree I understand the arguments and think the status quo is defensible. The burden (and likelihood of failure) is always on those trying to undermine years of work and agreement from a whole field of educated scientists focused on solving a particular problem and generally coming to agreement. Where they don’t agree, there is greater hope. Where they agree, the odds are not in Spencer’s or anyone else’s favor. That shouldn’t come as a surprise to anyone. To take over the castle, you have to get past the established and proven defenses. If you think it will be easy, you are probably underestimating something. Expect to be wrong more (perhaps many more) times than not. In fact, generally, people consider themselves students *until* they have a good grasp of the essence of the literature that was built up over time. .. but keep firing away because that will make this and future castles stronger.
RW @ November 28, at 7:12 pm
Welcome back RW
Yes, I agree. If the atmosphere were totally transparent at those wavelengths, it would all escape directly to space. (including horizontally). Why do you ask?
Bob,
“Yes, I agree. If the atmosphere were totally transparent at those wavelengths, it would all escape directly to space. (including horizontally). Why do you ask?”
Do you also think then that it is incorrect to show the direct surface to space transmittance or ‘window’ as going all vertically from the surface to space?
RW @ November 29, at 4:53 pm
Intriguing question RW:
Well technically it is indeed imprecise, but since the 40 W/m^2 is claimed to escape directly to space, then effectively, and for diagramatic simplicity, I think it is OK to show it that way. (I don’t know about the actual value though). Similarly, the heat transfer from thermals and evapotranspiration will have ascending isotropic emission effects, but from the cartoon arithmetic, their 17 + 80 are also claimed to escape to space, in a somewhat understated manner; that I can also live with. (putting aside Travesty’s incredibly accurate 0.9 missing heat)
However, on the other hand, the 396 S-B surface emission is in itself isotropic, and is substantially annihilated by absorption quite close to the surface. (within ~10m altitude is a popular assessment, but I’ve not seen verification, and BTW, Tim Folkerts did not dispute this number when stated severally above)
Clearly, the 396, by definition, comes from the surface, but does not make-it to space. (or even to the high clouds as shown in the cartoon). The 396 and its progeny of new-generation emissions contain horizontal vector components which cannot be seen in the normal view. Thus, the vertical components must be less than the total S-B emission.
wayne @ November 29, at 6:58 am
Thanks Wayne, but I thought item 5) in my article explained the basics adequately. For the purpose of the article, I felt that it was enough to explain that because there is no change in temperature within a typical small regional layer of air, there cannot be any heat transfer. (heat transfer can only occur when there is a potential difference). Any more detailed explanations were in my view likely to make it more difficult for lay readers.
It’s relatively trivial, but you might be interested in an earlier draft of the article, where I did go into some finer detail, but redacted stuff after discussion with WUWT personnel:
http://bobfjones.wordpress.com/2011/09/22/is-the-trenberth-et-al-%e2%80%9cearth%e2%80%99s-energy-budget-diagram%e2%80%9d-a-travesty-a-cartoon-or-both/
Tom Folkerts says:
Look at Bob’s figure 5. There is a “bite” out of the spectrum near 15 um due to CO2 radiating from the cold upper troposphere. With more CO2 in the troposphere, the “TOA” for IR radiation to space from CO2 would be a bit higher, and hence a bit cooler.
Unfortunately fig 5 is indistinct and pretty much unreadable on my screen so can’t draw anything from it. Concerning the spectrum near 15μm due to CO2 doesn’t Wien’s law show that maximal emission λmax for that wavelength occurs at minus 80 deg C; is that the temperature of the cold upper troposphere?
At 15 deg C the temperature lower down or at ground level, maximal emission λmax occurs at wavelength 10μm.
CO2 absorbs infrared emissions from the Earth’s surface only minimally in the range 7 to 13μm and it is within this range where the greatest proportion of radiation emitted by the Earth is found. This range is called ‘open radiation window’, because it is here that the least amount of absorption by water vapour and CO2 takes place. This window allows 70 to 90% of the radiation from Earth to escape into space. It includes the peak emission.
The biggest problem with the KT graph is that you cannot add and subtract W/m^2 when talking about temperature. 10 W/m^2 of sunshine plus 10 W/m^2 of IR from atmosphere does not equal 20 W/m^2 of radiation to heat an object. It will only be heated to the temperature of the highest radiator.
>> The 396 and its progeny of new-generation emissions contain horizontal vector components which cannot be seen in the normal view. Thus, the vertical components must be less than the total S-B emission.
When photons hit a sensor or a molecule, it doesn’t matter if they come 90 degrees from the bottom or at some other angle. The point remains that the lateral photons may not hit the molecules or sensors directly above, but they hit those over on the side. The net result is the same. This is why I think the 396 doesn’t represent 90 degrees vertical but instead the entire upper half of the horizontal plane.. ie, 0<angle<180 is "up" and 180<angle<360 would be "down". The horizontal cross sections still get 396. When you say Watts/meter squared, you don't care from which angle the photons arrive.
>>
Robert Stevenson says:
November 30, 2011 at 6:43 am
Concerning the spectrum near 15μm due to CO2 doesn’t Wien’s law show that maximal emission λmax for that wavelength occurs at minus 80 deg C; is that the temperature of the cold upper troposphere?
At 15 deg C the temperature lower down or at ground level, maximal emission λmax occurs at wavelength 10μm.
<<
I tried this argument on another thread and was thoroughly chastised (by alarmists on the thread) for not knowing that Planck’s equation gives different results in the frequency domain vice the wavelength domain. That means that Wien’s law derived in the frequency domain is different than the same law derived in the wavelength domain.
Here I was taught that wavelength and frequency are related by wave velocity and were interchangeable–silly me. Ahhh, the various magical mysteries of radiation physics continually haunt us.
Jim
mkelly, that doesn’t make sense. If I have a heater on the left and another on my right and another in front and behind and above and below, I get 6 times the amount of roasting (and some temp rise based on the 4th root of total power being received from all sources in my environment.. SB) if each oven is at the same heating/power output.
A photon is a photon. If the sun keeps sending more photons and these keep bouncing around in increasing numbers because they dissipate into space slowly, then the atmosphere is getting extra power and will rise in temp accordingly.
A photon is a photon, and 6 photons are 6 photons. It doesn’t matter that they all come from different directions and originated in different places.
Jose_X, the majority of photons from the surface rollock off to deep space without even seeing a CO2 molecule. As I mentioned in an earlier post to Mr Folkerts, CO2 absorbs infrared emissions from the Earth’s surface only minimally in the range 7 to 13μm and it is within this range where the greatest proportion of radiation emitted by the Earth is found. This range is called ‘open radiation window’, because it is here that the least amount of absorption by water vapour and CO2 takes place. This window allows 70 to 90% of the radiation from Earth to escape into space. It includes the peak emission.
[Robert Stevenson]>> This range is called ‘open radiation window’, because it is here that the least amount of absorption by water vapour and CO2 takes place. This window allows 70 to 90% of the radiation from Earth to escape into space. It includes the peak emission.
Looking at this rough picture at the top section http://en.wikipedia.org/wiki/File:Atmospheric_Transmission.png, it looks like the area that is blue is much closer to 40% than to 70-90% range.
This is not a big deal, but might as well take the opportunity to link to that pic.
Jim Masterson, all the Planck’s law charts I use, plot Iλ (intensity of monochromatic emission) versus λ, wavelength in microns; I find it easier to understand and integrate on a spreadsheet. I get told off for being rooted in the 1950’s and not being upto date.
[Robert Stevenson]>> Concerning the spectrum near 15μm due to CO2 doesn’t Wien’s law show that maximal emission λmax for that wavelength occurs at minus 80 deg C; is that the temperature of the cold upper troposphere?
At 15 deg C the temperature lower down or at ground level, maximal emission λmax occurs at wavelength 10μm.
I’ll assume you are correct although I’m not sure why you mention that. Tom Folkerts specified 15, I think, in reference to the range where CO2 has an effect.
[Jim Masterson]>> I tried this argument on another thread
I don’t see what argument that was.
[Jim Masterson]>> thoroughly chastised (by alarmists on the thread) for not knowing that Planck’s equation gives different results in the frequency domain vice the wavelength domain.
Without seeing the argument I have no idea what those people attempted to say. Yes, where the speed is essentially speed of light, labeling the graph using frequency or wavelength can be done consistently and is really immaterial. It sounds to me that it would have been an odd argument.
Robert S, what are you trying to say by saying most photons don’t see CO2? Are you disagreeing with my observation that we each agree more CO2 will lead to higher temperatures of some amount and that to be precise we would have to perform calculations?
Jose_X , Yes I am disagreeing with your observation that we each agree more CO2 will lead to higher temperatures of some amount and that to be precise we would have to perform calculations?
Firstly what do you mean by ‘some amount’?
>>
Jose_X says:
November 30, 2011 at 9:43 am
[Jim Masterson]>> I tried this argument on another thread
I don’t see what argument that was.
<<
The argument that per Wien’s law 15 microns is -80 °C. The surface has to be pretty cold for CO2 to absorb in this range.
>>
[Jim Masterson]>> thoroughly chastised (by alarmists on the thread) for not knowing that Planck’s equation gives different results in the frequency domain vice the wavelength domain.
Without seeing the argument I have no idea what those people attempted to say. Yes, where the speed is essentially speed of light, labeling the graph using frequency or wavelength can be done consistently and is really immaterial. It sounds to me that it would have been an odd argument.
<<
See the May 7 post by Ira (http://wattsupwiththat.com/2011/05/07/visualizing-the-greenhouse-effect-light-and-heat/). Specifically see Phil’s May 19, 2011, 8:19 pm response to me. That should be enough context to see if the argument was odd.
Be careful. This is where jae called me a “kook.”
Jim
Jim Masterson, I don’t know why he was talking about wanting to focus on 340K. I didn’t notice anything wrong with your reply (1-1 correspondence), but his response didn’t make much sense to me. I might have to read more, but I am not very interested :-).
Robert Stevenson, OK. You seemed to have acknowledged that the photons might find an absorber quicker and said that any gain (in temp) would likely be negligible. I pointed out that if you find CO2 quicker generally, that you’d get a larger number of photons bouncing around before leaving. Since the Sun is “constant”, this means we have a greater amount of energy in the atmosphere, which means higher temp somewhere. I figured you would agree and took your “negligible” comment to mean you’d accept that there might be some gain in temp.. say north of 0.00001 C. 1 C gain (purely due to CO2) for a doubling, to me, might be called negligible by some people.
Anyway, so you don’t think there would be anything resembling 1 deg C gain for CO2 doubling? I don’t think anyone can argue that one way or other without calculations.
And saying most photons from earth miss CO2 is not specific enough to say whether temperature may rise a lot or hardly at all.
Although the blanket example is not a broad analogy for the atmosphere “greenhouse effect”, it suggests that slowing down the escape of radiation leads to increased temperature over what we’d otherwise have.
An oven with the door closed is the same thing. We have very little heat leaving relative to the energy in the oven and the high temp.
Remember that the sun keeps firing photons at us nonstop. If the earth keeps radiating away and those photons take a long time to escape the atmosphere, that is a lot of kinetic energy among the molecules (think of the oven example). So slowing down the radiation escape means a greater concentration of energy.. higher temp.
Now, if you have no prob with greenhouse effect yet specifically think CO2 will have negligible effect, why would you say that without calculations?
btw, I know convection is what helps even the temps inside and outside quickly, but the analogy is useful to show that temp rises when we slow down the rate of loss of heat. This slowdown is what I am referring to.
>>
Jose_X says:
November 30, 2011 at 12:07 pm
I might have to read more, but I am not very interested.
<<
I don’t blame you. I wasn’t either.
Jim
With a little care, the issue of looking at graphs of [intensity vs wavelength] or [intensity vs wavenumber] doesn’t matter. It is not a question of “where the peak is” but rather “the total energy in the band”. Presumably calculations for the total energy absorbed by CO2 will be the same whether you deal with wavelength or wavenumber.
In one representation, CO2 might be near the peak, but have a narrow band. In the other representation, CO2 might be far from the peak, but have a broader band. If you want real numbers, you will have to integrate areas under curves. Presumably, if CO2 absorbs 20% of photons in when discussing wavelength, it will still absorb 20% when discussing wavenumber. Just looking at peak wavelength (or peak wavenumber) is insufficient.
The simplest analogy that springs to mind would be something like this. Suppose I have a whole bunch of speakers set to 20 Hz, 20.1 Hz, 20.2 Hz ….. 100 Hz. If I plotted (speakers per Hz) as a function of frequency, there would be a flat graph with (10 speakers per Hz) across the entire graph.
But If I plotted (speakers per meter) as a function of wavelength, I would find there are many more speakers with a wavelength between 4m and 5 m (~ 170) than between 16 m and 17 m (~ 12).
[Jim Masterson]>> The argument that per Wien’s law 15 microns is -80 °C. The surface has to be pretty cold for CO2 to absorb in this range.
I think what you are saying is that you have to have a fairly cold blackbody radiation if you want the “15 micrometer CO2 band” to end up centered at the max wavelength. However, the key point is area under curve and not whether a particular band is centered or not.
mkelly, I agree that “It will only be heated to the temperature of the highest radiator.” I disagree with the rest.
If I completely surround an object with 270 K ground/atmosphere/walls/clouds that emit ~ 300 W/m^2, the object will never get warmer than 270 K (absorbing ~ 300 W/m^2 if it is a black body).
And if I completely surround an object with 5780 K plasma (like the sun) that emits ~ 63,000,000 W/m^2, the object will never get warmer than 5780 K (absorbing ~ 63,000,000 W/m^2 if it is a black body).
If I partly surround the object with hot plasma @ 5780 K and partly surround the object with 270 K ground/atmosphere/walls/clouds, the object will absorb somewhere between 300 W/m^s and 63,000,000 W/m^2, reaching a temperature somewhere between 270 K and 5780 K.
The energy from the cooler surroundings is still absorbed by the object, even though the object is warmer than the surroundings. The object does not magically know to absorb 4 um photons from the sun but reject 4 um photons from the surroundings.
Jose_X @ November 29, at 12:22 pm
Of course the defences are not just made-up of the bowmen, but also politicians, green organisations, and the media, some of which are truly fearsome warriors.
The main Oz national broadcaster, the ABC, consistently breaches its editorial policies, and charter by act of parliament when it comes to AGW. For instance, I am not alone in noticing that they have been silent on the so-called Climategate II Emails. (as is also reportedly the BBC). Given that a significant majority of Australians are polled showing that they would have a strong interest in them, they have clearly breached their published editorial policy. It is particularly damming because of the timing of the release to coincide with that latest jamboree in Durban.
I recommend that you carefully read, as it contains some awful naughties between your heroes.
http://wattsupwiththat.com/2011/11/22/climategate-2-0/
The exchange between Severinghaus and Mann is rather telling, particularly since the infamous Hockey-stick, was so brilliantly applied to deceive the policy makers and media into thinking that recent warming, (prior to about the last decade), is unprecedented.
Oh BTW, unlike the Laframboise book at $4.99, it’s FREE
@Jose_X…
Two questions for you:
1. What is a photon?
2. How long a molecule of carbon dioxide retains the absorbed energy from a photon?
Thanks for answering.
Jose_X @ November 30, at 8:51 am
In the context of elementary quantum theory:
You may be lacking in spatial perception when considering an absorbent atmosphere. For argument’s sake, let’s contemplate that the mean photon free path length from the surface is 10m. Let’s say your implied multiple sensors are uniformly distributed over a wide area at some height of less than 10m. Your suggested “sideways intercepts” of horizontal or near horizontal photons would need to be a great distance away from their source, but such photons cease to exist long before they could get there at only around 10m away from source. Therefore they cannot be detected in the direction that you suggest. Multiply these numbers by a factor of 10 if you like, but you get much the same story. Much the same situation exists in succeeding progenies of isotropic radiation into absorbent air at higher altitudes. (except of course in “transparent wavelengths”)
Jose_X,
Did you properly answer Robert’s November 29, at 4:53 am, particularly the bit I’ve bolded below?
I, and I guess so too Nasif, would be interested to hear your wisdom on this!
(although as I understand it, photons are rarely reemitted: it is a misnomer. Photons are typically annihilated upon absorption according to QT, and subsequent emissions depend on mutual transfer of KE in collisions, mostly with N2 and O2, and temperature and stuff).
Hi Nasif. Now I’m sure this is going to be quite interesting. I’ll just listen.
[wayne]>> Now I’m sure this is going to be quite interesting.
Well, I’m finding it to be interesting as well. I won’t know if I’m right without a fair amount of thinking and discussion. Whatever the outcome, it’s a free physics tutorial/lesson.
To revisit mkelly’s point, my reply considered that the multiple heaters were above ambient. No matter the case, a photon lost is energy lost. A photon gained is energy gained. Radiating is a cooling effect. I suppose I was also assuming the heaters were being driven by a source of energy that allowed them to remain at the elevated temperatures. For the planet case, we have the sun as a “constant” source of energy. So I think a better reply to:
[mkelly] >> The biggest problem with the KT graph is that you cannot add and subtract W/m^2 when talking about temperature. 10 W/m^2 of sunshine plus 10 W/m^2 of IR from atmosphere does not equal 20 W/m^2 of radiation to heat an object. It will only be heated to the temperature of the highest radiator.
would be:
(a) We don’t have enough information in general to make a determination about the last sentence based on arbitrary radiating bodies. We’d need to know the energy sources for the radiators and also the environment of the radiators. On earth, most radiators would wind down in energy if not constantly heated by the sun. The environment plays a major role. Meanwhile, we aren’t much affecting the sun, so that radiator can be assumed to be driven by an infinite reservoir of energy and radiate independently from the affects on earth.
(b) 10 W/m^2 from the atmosphere will lead to cooling of the atmosphere unless replenished. The earth and almost all bodies on it (to first approx, for the purposes of the earth system analysis) can also be assumed to be like the atmosphere in that, as they radiate and contribute energy to other bodies, they lose it and must have it replenished to preserve their temp (eg, by being radiated back).
(c) 10 W/m^2 from the sun is additional energy (and infinite stream essentially). If we on earth keep getting more of it and not radiating out into space (quickly enough), we will keep cooking — meaning on average getting hotter.
[Bob Fernley-Jones] >> The main Oz national broadcaster, the ABC .. For instance, I am not alone in noticing that they have been silent on the so-called Climategate II Emails.
From my perspective, calling climate science Oz is at this point in my understanding an assumption. I don’t see it that way. I will note that as pointed out by Jon Stewart (assuming he was correct), the BEST preliminary results — and generally we can say this about all the literature adding up monthly which supports climate science — were not covered by most media outlets. So I can easily call it a bias that the media is widely ignoring all the evidence and studies supporting current climate science.
I think a fair representation of climategate II is that the media jumped the gun before (CG I) and got burned to some degree. The media goes after what will attract eyeballs to sell ads. You can call conspiracy, but the fact remains that formal inquiries found nothing improper about climategate I (at least not beyond normal bureaucratic failures and politics that exists in all human organizations), and at least there is enough doubt about that doing any significant damage to climate science. Of course, that had an impact of sorts, but the science was not refuted because of it (although it obviously has attracted extra scrutiny).
http://mediamatters.org/blog/201111220013
>> The previous release of hacked emails triggered a storm of ill-informed media coverage in December 2009, with news outlets rushing to quote the documents without taking the time to research the context or ask experts to translate the scientific language
[Bob Fernley-Jones] >> between your heroes
I show respect to the growth of the science. I am certainly open to improvements. I really don’t know enough to take a strong position for or against any particular accepted climate specific theory, but the majority of attacks I see fall short IMO (so far of what I have experienced).
Can current results be hacked? Well, I know little enough that from that limited perspective I would say of course I think that would be a possibility. This is one reason I have taken interest in hearing the objections.
[Bob Fernley-Jones] >> The exchange between Severinghaus and Mann is rather telling, particularly since the infamous Hockey-stick, was so brilliantly applied to deceive the policy makers and media into thinking that recent warming, (prior to about the last decade), is unprecedented.
Can you point to a precise discussion? The link you provided had a load of stuff.
Also, when you say “telling”, what do you think is the conclusion? That result x or y are wrong? What specifically?
[Bob Fernley-Jones] >> unlike the Laframboise book at $4.99, it’s FREE
I likely wouldn’t be looking at it if it wasn’t free. I really wasn’t trying to deceive you. I have not bought reading material in a long time (I think the last time I bought reading material was $20 for an electronic ansi standard years ago). I also don’t make a lot of money.
[Nasif Nahle] >> 1. What is a photon?
What point are you trying to get at?
I am not an expert in photons. I have taken some quantum mechanics courses. I can research. I am more than willing to consider your definition.
In short, if you want a response form me in order to get a clue about my background and biases and lapses, a photon is a massless entity with a fixed amount of energy. All “entities” I think, potentially exhibit particle and wave behavior to some degree (even if almost immeasurable for large complex entities).
[Judging from a different comment I reply to further down, let me add that a photon effectively is created when it is emitted and then destroyed when “absorbed”. I think theory considers these particles to exist at a given quanta of energy and not to exist any longer once they are “absorbed”. The theory isn’t that a photon gets re-emitted later after being absorbed, no; however, I am not sure that a model where photons do live forever would necessarily be incorrect, but it appears it would simply lead to a more complex theory and potentially less intuitive. Tomorrow, however, we may find a new model if we appear to discover that there are 3 centillion photons in the universe, not one more or less, and they hide within other particles and change their energy levels when they leave the other particles, etc.]
[Nasif Nahle] >> 2. How long a molecule of carbon dioxide retains the absorbed energy from a photon?
I don’t know the specifics (haven’t set up the model or done the math or read it). The duration in time falls out of the wave function in some way. The actual time for any molecule is expected to vary, but we can calculate expectation values if we know enough about the system. Whether that event is likely to happen before the molecule reacts with another molecule, I am not sure.
On a related note: I think I heard that the time between absorbing photons (not sure in what part of the atmosphere this applies) is perhaps 2 orders of magnitude longer than between encountering and interacting with another molecule (ie, having it’s kinetic energy and momentum changed is likely to happen before another photon is absorbed).
[Bob Fernley-Jones] >> but such photons cease to exist long before they could get [to the sensor]
I don’t see your point.
If the photon hits something less than 10m (which is where you placed the hypothetical sensors), then it imparts energy. That energy didn’t disappear. It arose from the planet at some point X and was absorbed at some other point at least slightly above it.. regardless that it went laterally or straight up. So the point is that (the energy associated with) all photons created within some surface m^2 eventually make it higher up. Diagramming that as 396 “up” sounds perfectly reasonable (assuming the values are correct of course).
[Bob Fernley-Jones/Robert S]>> The subsequent retention time of these photons as they are re-emitted may be slightjy altered but not to affect the overall heat balance.
I addressed this before by saying that the concluding part of this statement is qualitative opinion. It isn’t quantitative. I am sure many people have many opinions here. It’s even ambiguous what “slightly” would mean. Yes, I addressed this. If you want more, we need numbers and/or calculations (or, if you think you can measure it, measurements).
You also mentioned contact of these molecules with other molecules (exchange of KE). I am not sure what point you want to communicate.
Also, I know that photons don’t bounce around. It is energy transfer essentially. My point is that if the odds of absorption of a photon are greater because of more molecules, then this leads to a longer time period before the energy radiated at ground level dissipates. The energy contribution of a given photon produced at ground level has to go through more intermediaries (via energy exchanges, whether absorption and regeneration later on of photons or by molecules coming into contact with other molecules.. leading to the eventual photon radiation that simply leaves the atmosphere). A hypothetical measurement of this extra time would be seen by measuring the time constants of the earth temp dropping towards 0 K if we turn off the sun when the earth is at current temps.
Remember that the sun provides a constant addition of energy. If it takes longer to radiate into space any given addition of energy, then we have on average now a greater amount of energy on the earth’s atmosphere at any new point in time. By definition, I think, temperature of gas represents the average kinetic energy of each particle. More energy in the atmosphere with the same number of particles, volume, etc means a higher energy. — Sure, the volume might increase… that is something to consider. — And with higher temp, we get a more rapid rate of radiation, which may eventually lead to greater or equal radiation outward as we receive from the sun (but at that higher temperature). When the rate out is equal, we should have stable global average temps (assuming no other chemical or other reaction disturbs this balance). When rate out is greater, we will now start to cool as more energy will be leaving then entering.
We can look at temp as a measurement of the amount of energy (eg, kinetic energy) in place in the (eg) atmosphere at any given point in time. An analogy would be with water level (temp) in a tub where we have a source of water into the tub and a drain.
To clarify on the comment I made recently:
1: “On earth, most radiators would wind down in energy if not constantly heated by the sun.”
In other words: Most objects on earth would lose “all” (aka, “most of”) their energy in a noticeable time period if it weren’t for the continuous energy the planet receives from the sun. Technically, many objects on earth pretty much “get their energy from” other objects directly (but ultimately from the sun if we follow the chain of energy acquisitions).
This might be a silly clarification to make, normally, but I’ll do it now in the context of the current discussion of, “what is a photon?”
I may have misspoke any number of times (and will in the future) because I frequently wear the hat of a layperson and enough times don’t use technically correct jargon. Ask away if anything I say sounds odd. We are all on this same boat anyway.
To sum up that that first part (to mkelly’s comment), basically I’m saying that power does add up, in the sense that energy adds up, but, to know what happens to temperature, we’d have to know enough details to know the power/energy values to add AND to subtract, before then applying S-B or equivalent to get the temperature. So I think mkelly’s comment was too vague to conclude that the final temperature is simply the temp of “the highest radiator” (not sure what that means). Also, I can see how we might achieve here on earth a temp higher than the sun’s temp even though ultimately the energy probably came from the sun at some point in the past.
2: “More energy in the atmosphere with the same number of particles, volume, etc means a higher energy.”
The last word should be “temperature” and not “energy”.
The main point in that section is that temperature of the earth system can have many internally different temperatures (eg, average temp at TOA vs. close to the ground vs. in the oceans vs. in volcanoes, etc). Think of power going into an oven and power radiating out. A well insulated oven might have an internal high temperature for a given measured amount of power in and out (in/out in equilibrium), while a less quality oven might have a lower internal temperature for the same given amount of power in/out. The earth might receive some power and radiate some power yet have built up furnaces of high temp deeper below the TOA.
So I think its rather natural to accept the possibility of “greenhouse effect” within our atmosphere. We may disagree over the details of which gas (if any) has how much greenhouse effect, but the mere idea that energy would be retained to enable a higher temperature (and power radiation) in one part of the earth system internally vs. at its boundary with space should not be too hard to accept. We see many related examples all around us. If conduction and convection (which are what specifically?) can be kept at gradients via judicious use of barriers in the context of given “energy sources”, why can’t radiation also be a part of that equation and possibly apply within the atmosphere?
@Jose_X…
“In short, if you want a response form me in order to get a clue about my background and biases and lapses,”
Forget about it… Professional background, and biases and lapses do not matter in science when they are not related with the information that is being examined.
“…a photon is a massless entity with a fixed amount of energy.”
In physics and biology, entity is an abstraction of the real world. If we add the word “analytical”, for example “physics analytical entity, we are referring to an abstraction created with the purpose of describing and analyzing physical processes. If we classify photons as physical analytical entities, we would be creating an abstraction which would permit us to describe photons and processes related to photons. Therefore, the label “entity” does not fit with the definition of photon; at least, it is not the concept of photon handled in quantum physics.
Photon is a perturbation of the electromagnetic field which manifests itself as a particle with inertial mass and a wave with wavelength and frequency according to the energy that it has available which is usable on other fields.
Given that wavelengths and frequencies of quantum/waves depend on the amount of energy that they transport, it is evident that the energy of quantum/waves is not fixed.
On the other hand, matter particles also exhibit wave’s nature.
The great question is about the definition of photons as massless particles; we cannot say that photons are massless particles because they have inertial mass, and in a given moment it can be interpreted as gravitational mass. The latter was evidenced when researchers measured the redshift of quantum/waves due to gravity. Gravity makes a photon to shorten its wavelength and to increase its frequency:
Δf = f ((gH)/c^2)
Consequently, a quantum/wave originated in the Sun carries a higher amount of energy which diminishes as the condensation goes farther from the source and increases as the quantum/wave goes approaching to any interfering system with gravitational mass which is capable of absorbing it.
C = Cp (δ) (r)
For atmospheric carbon dioxide in current Earth’s atmosphere, the capacity of carbon dioxide to store energy during 120 picoseconds (1.2 × 10 ^ (-10) seconds) is 609.7 J/m^2 K.
t =√(ΔE*(1/((m^2 kg) )) )= √(1.345 x 10^(-20) (kg m^2/s^2) * (1/((m^2 kg) )) ) = 1.16 x 10^(-10) s
As way of comparison, the whole atmosphere retains 2000 times more energy than carbon dioxide during 138 picoseconds.
It is preferable to think about a distribution of energy more than about “heat retained”.
The major mistake that I have read from some articles published in the Internet involves the amount of quantum energy absorbed by a system. No system in the known universe absorbs the whole load of energy transported by a quantum/wave. It is erroneous to suppose that the load of energy carried by absorbed photons is transformed into stationary energy. There is a retrograde movement of the absorbent molecules when a quantum/wave hits upon them. Additionally, there is an amount of dynamic energy that is transformed into unusable potential energy; consequently, the absorbed energy by a molecule of carbon dioxide is not the same as the energy carried by the absorbed photon.
Regarding the question about the annihilation of a quantum/wave as soon as it is absorbed by a system, it is true; the quantum/wave emitted is not the same quantum/wave that was absorbed. Emitted photons have longer wavelengths and lower frequencies, so emitted photons are less energetic than absorbed photons. This characteristic avoids that molecules of same species absorb photons emitted by molecules of their own species.
There is not physical basis in support of global warming caused by carbon dioxide.
Nasif
@ Nasif
Very clear as usual. I do have a question for you along the lines of the atmosphere’s shear mass in relation to what Miskolczi’s work tends to imply on tau, but I will defer that for later, not to jostle this thread here.
@ Jose_X
I’ll deem this as a free lesson/tutorial the minute either you or I actually do learn something new to our respective understanding of these topics.
The x/6’ths seemed to throw you above. I could have placed that in spherical coordinates with equal ease but as I told you before, I like to say things here on WUWT as simply as possible for the many different backgrounds of those taking the time to read these comment, many of them never comment at all.
You see Jose_X, if you are so intelligent in these topics you should be able to mentally transform my simple x,y,z Cartesian example into the more complex proper physics, principle by principle, and agreedwith most of what I was explaining. IR photographs do not show that low-level whole-atmosphere IR radiation because that portion a CCD registers is zeroed out to give you a good photograph. Those ‘events’ do occu. It does not mean the quantum/waves are not present (at an event level).
Read it again later. (and yes, those energy transfers are ‘after-the-fact’ absorptions of the quantum/waves in an explicit direction and at an exact time since it was separated into vectors and accumulated to give a rate.) Personally, I will continue to take the time to word things here in a manner that any person should understand with a little thought, regardless of their background or nomenclature.
If I were you I would not assume people here are as clueless as you apparently assume. Your smart, translate, fill in, assume correctness, read between the lines. You keep saying your willing to learn but if you continue to splice out partial thoughts just to tear them into pieces, I for one will stop feeding you. You say you want to learn, climb off that horse.
Nasif Nahle, OK, what you wrote did not land smoothly upon my eyes. Some of my objection might be reasonable. But surely this is an opportunity for me to learn and review some physics.
>> massless
Note, I did not clarify that by “massless” I meant zero rest mass only. E=mc^2 implies all energized particles have mass.
>> If we classify photons as physical analytical entities, we would be creating an abstraction which would permit us to describe photons and processes related to photons. Therefore, the label “entity” does not fit with the definition of photon; at least, it is not the concept of photon handled in quantum physics.
I did not understand what distinction you were trying to make with my use of the word “entity”. I chose what I thought was a simple dictionary word that is rather general in ability to describe something we believe exists. I did not realize there was a specific special meaning for that word in physics and biology. Can you provide a reference where I may be able to read up on the use of that word in these fields of study?
I am not a philosophy major by a long shot, but, at least in science, the only meaning that really exists is that which forms a part of some model or other. I don’t see what distinction you would create between an actual object and a model of that object.
>> Photon is a perturbation of the electromagnetic field..
I will note that electromagnetic field (and everything else in physics) is just a model.
Anyway, this appears to be a philosophy diversion. Feel free not to clarify this point (I’m not sure why you brought it up), and I will just move on regardless. I generally don’t want to get hung up with words.
And like I said, I will try to work with whatever definition you find suitable (which will depend on what tools/models you use to analyze the problem).
>> ..which manifests itself as a particle with inertial mass and a wave with wavelength and frequency according to the energy that it has available which is usable on other fields.
To the extent I cut short my education and likely will have to read up on this, are you referring to classical E&M field theory?
>> Given that wavelengths and frequencies of quantum/waves depend on the amount of energy that they transport, it is evident that the energy of quantum/waves is not fixed.
I don’t follow. Do you mean anything beyond the Heisenberg Uncertainty Principle?
>> we cannot say that photons are massless particles because they have inertial mass
Agreed. I meant zero rest mass.
>> Consequently, a quantum/wave originated in the Sun carries a higher amount of energy which diminishes as the condensation goes farther from the source and increases as the quantum/wave goes approaching to any interfering system with gravitational mass which is capable of absorbing it.
Can you specify the mathematical form of this wave or at least specify if you are referring to a single photon or a group of photons (what is the quantum system you refer to)?
I have very little experience with wave equations, but I will try to follow. Or you can use some other description (eg, dump the wave function approach for the sake of this discussion/analysis).
>> For atmospheric carbon dioxide in current Earth’s atmosphere, the capacity of carbon dioxide to store energy during 120 picoseconds (1.2 × 10 ^ (-10) seconds) is 609.7 J/m^2 K.
>> t =√(ΔE*(1/((m^2 kg) )) )= √(1.345 x 10^(-20) (kg m^2/s^2) * (1/((m^2 kg) )) ) = 1.16 x 10^(-10) s
Do you mean the capacity to store energy for a time interval of at least 120 picoseconds or is 120 the average?
Can you specify from what you derived this (or name of this formula). Is this a calculation of some sort of time expectation on a wavefunction? Can you give details. I cannot follow your logic without more clues.
I’ll repeat I am not practiced here, so any derivations I may have to perform may take time, but you can help by pointing to the name of the equations you started with or something.
>> C = Cp (δ) (r)
This may be obvious to you, but can you specify the name of these quantities or the formula name?
I am willing to do research, but since I don’t use these formulas and may never have seen them, it helps to at least specify what these variables represent.
>> No system in the known universe absorbs the whole load of energy transported by a quantum/wave. It is erroneous to suppose that the load of energy carried by absorbed photons is transformed into stationary energy.
Wave functions refer to probabilities, so I am not sure what you mean here by total energy.
[I find this very interesting because these are calculations I have not done.]
..If the wave is of many photons, then of course no molecule is going to absorb all the energy. I am not sure why you would look at the wavefunction of many photons yet then talk about a single molecule, but if that is what you are doing, I’m all ears.
>> Additionally, there is an amount of dynamic energy that is transformed into unusable potential energy; consequently, the absorbed energy by a molecule of carbon dioxide is not the same as the energy carried by the absorbed photon.
..If you are talking of a single photon, can you reference which experiments support the view that only a fraction of the energy of a photon is absorbed by a molecule?
You can likely get away with many models that in the end come to the same conclusion (we would have to know the details of the theory); however, I have not delved deeply into this area and the simple models I remember have each photon energy be imparted entirely or not at all, iirc. The classical view works differently, but that is because it describes many photons and a reduction of that set gives the appearance that a fraction of the energy of the whole was imparted.
Again, if you could be clear about your wavefunction or the theories/experiments you are invoking, I could better follow your argument.
I do recognize that a “collision” between any given particles may produce some of the same particles (with different energy levels, etc) as we started out with, so perhaps this is what you mean. [Can you clarify and provide experiment or math?]
>> Emitted photons have longer wavelengths and lower frequencies, so emitted photons are less energetic than absorbed photons.
Well, this is an extension of your point.. which I don’t agree with, generally, from what I remember of basic quantum mechanics (and I simply may not remember my physics from many years back). However, I can certainly change my mind if I have some basis (eg, experimental basis or a mathematical analysis that requires the same type of particle exist afterward but with different values).
>> This characteristic avoids that molecules of same species absorb photons emitted by molecules of their own species.
This is a conclusion I cannot accept since I don’t currently buy your earlier points.
I do get the feeling that this is not standard accepted physics.
Also, it doesn’t readily follow from you claims earlier since molecules of the same specie can absorb different amounts of energy (within some “band” range of values), so it would be quite possible to have one CO2 molecule emit a less energized photon (on this argument you provide) that would still be acceptable to a different CO2 molecule (ie, would still fall within the overall absorption band range).
I suppose it’s also possible (and I noted this confusion on my part in an earlier comment.. I think to wayne, iirc) that the photon energy can be used to affect the electrons in the molecules and also the whole molecule’s average kinetic energy. I have to believe this is very possible, but I have not thought about this very much or remember explicitly reading this (which may be a very basic common fact). In any case, this would not support your conclusion that CO2 cannot absorb photons emitted from other CO2 since we’d have a clear mechanism for the same molecule to accept a very wide range of values .. In fact, I really think this is true; however, a very high energy photon probably is more likely than not (QM wavefunction probability analysis) to simply pass over a CO2 molecule rather than to impart its energy and ionize the molecule.
>> There is not physical basis in support of global warming caused by carbon dioxide.
I disagree and suppose you deduce this from the view you present that CO2 cannot pass photons to other CO2.
Honestly, this sounds rather strange to me: that no molecule can ever pass energy to another of the same type via a photon seems rather absurd.
wayne, why are you going back to that x/6? There is no fundamental rule or definition that creates that and says “up” is associated with it (and if you believe their is, please reference that definition). The coordinate system is immaterial. Your “6” is an arbitrary division of the space you decided to create because you wanted to define “up” in that context.
You mentioned Nasif was “very clear as usual”, in which case, every question I posed Nasif I pose to you as well because I found a number of things that weren’t very clear to me about that comment.
Also, based on your earlier replies, I find it suspect you would claim Nasif’s comment was clear. [BTW, you never addressed what part of that radio astronomy webpage you referenced stated something that was inconsistent with what I have been saying. Supposedly much on that page was, so it would be useful to me if you could be specific.]
>> Personally, I will continue to take the time to word things here in a manner that any person should understand with a little thought, regardless of their background or nomenclature.
I agree that is generally a good idea for everyone to follow, but many conversations cannot procede except with math (it depends on the nature of the particular sub-discussion).
>> If I were you I would not assume people here are as clueless as you apparently assume. Your smart, translate, fill in, assume correctness, read between the lines.
I am all ears for healthy discussion. There is little need for assumption of what others know or don’t. Simply put, let’s argue/discuss. I’ll try to follow as much as I can and state my views, of course.
>> You keep saying your willing to learn but if you continue to splice out partial thoughts just to tear them into pieces
I don’t think I have been closed minded. In fact, I just asked Nasif (and you) a load of questions so I can better understand what models he(?) is using.
Jose_X @ December 1, at 7:22 am
[1] It shouldn’t have taken you long to find it. See item 38: http://wattsupwiththat.com/2011/11/28/a-response-from-jeff-severinghaus-on-why-the-trees-dont-make-good-thermometers-after-1950-i-did-indeed-feel-at-the-time-that-mike-mann-had-not-given-me-a-straight-answer/
WUWT item 52 is also relevant, and takes you to Steve McIntyre’s new article:
Hide-the-Decline Plus: http://climateaudit.org/2011/12/01/hide-the-decline-plus/
Remember “Hide the Decline” in one of Phil Jones’ emails in climategate I?
Somewhere above in all that waffle, you say that you are willing to study and learn. You should apply this to what I’ve recommended that you read.
[2] It is yet more proof that Mann and other hockey-stick creators, e.g. Jones, Briffa, etc, omitted tree ring data from the mid 1900’s without explanation, because it showed an inconvenient downturn against the published instrumental data. Esper 2002 seems to be the only Dendro’ to openly discuss it, but he was Swiss, not part of the US/UK “team”, and was unpopular with the IPCC. He subsequently removed that paper from his publication list
If you study this you will see that the team were far from honest in their dealings… see Climategate I & II emails
“Hide the decline” is also known as “The Divergence Problem”
Oh, BTW; Oz =Australia(n) in this context.
Jose says: “Note, I did not clarify that by “massless” I meant zero rest mass only. E=mc^2 implies all energized particles have mass.”
Actually, you were closer to correct originally. Photons have no mass! I challenge anyone to find any authoritative reference that says photons have mass!
The full equation is not E = mc^2, but rather E^2 = (m^2 c^4) + (p^2 c^2). For particles with mass at rest, it becomes E = mc^2. For particles with no mass moving at the speed of light, it becomes E = pc.
So photons have energy. Photons have momentum. Photons are even affected by gravity. But they have no mass.
Jose says: “Anyway, this [the definition of “entity”] appears to be a philosophy diversion. ”
I agree. I have never seen “entity” used as a specifically defined term in physics. I think your use was understandable and reasonable.
Bob,
“Clearly, the 396, by definition, comes from the surface, but does not make-it to space. (or even to the high clouds as shown in the cartoon). The 396 and its progeny of new-generation emissions contain horizontal vector components which cannot be seen in the normal view. Thus, the vertical components must be less than the total S-B emission.”
Do you agree that the required emitted flux from the atmosphere that eventually passes into space at the TOA is not all from vertical emission from the atmosphere? By ‘vertical’ I mean emission perpedicular to the surface?
@ Nasif
I am reading a bit between the lines but when you said:
—> This characteristic avoids that molecules of same species absorb photons emitted by molecules of their own species.
didn’t you mean “This characteristic avoids that molecules of same species absorb photons emitted by molecules of their own species at the same frequency of the quantum/wave that was originally absorbed?
To me, and you from what I have gathered from you, that the re-emitted radiation will always be of lower frequency, lower energy, multiple photons, due to both the momentum transfer/loss and that all thermal absorption is of ro-vibrational in nature, never strictly vibrational. (that is unless a path existed where some other event could separately add more energy to that same molecule.) Close?
Or are you saying due to the above characteristics that the frequency is basically always shifted so much lower and the energy split to prevent basically any like-species cross radiative transfer.
I can see both sides, but which is closer to reality? Would love a clue.
RW @ December 1, at 5:01 pm
Yes, of course RW. Is it a trick question?
Nasif says:
Did you mean …
“Consequently, a photon originated in the Sun carries a higher amount of energy which diminishes as the photon goes farther from the source and increases as the photon goes approaching to any .” ?
I would agree with that — it sounds like a standard gravitational redshift.
What is this equation? What do the symbols stand for?
Where does this come from? Why is 120 picoseconds significant for energy storage? What is the square meter factor?
1.345 x 10^(-20) (kg m^2/s^2) seems to be the energy of a ~15 um photon. What is the significance of 1/(m^2 kg)? What is this equation?
I can’t tell quite what your objection is. Yes, there will be some energy that goes into KE of the CO2 molecule as it recoils. So, yes, the energy is not transformed entirely into “stationary energy”.
But what sort of “unusable potential energy” does the atom gain?
Molecules are generally quite good at absorbing the same photons that the same species of molecule emits. Between Doppler broadening and the natural line width related to the Heisenberg Uncertainty Principle, there are a variety of energies of photons that can be emitted and absorbed by a particular molecule.
But all this is rather off-topic from the original topic….
Bob,
“Yes, of course RW. Is it a trick question?”
No, it’s not a trick question. Truthfully, I’m still not quite sure I understand your fundamental objection here, so I’m attempting to get clarification.
Yes, I agree you are correct that the 396 W/m^2 LW flux emitted at the surface is not all emitted perpendicular to the surface.
Yes, I agree you are correct that most of the absorption occurs close the surface and subsequent LW emission in the atmosphere from that absorbed would be isotropic.
What am I missing?
Bob says: “For argument’s sake, let’s contemplate that the mean photon free path length from the surface is 10m.”
For the sake of argument, let’s also assume the atmosphere in those first 10 m is the same temperature as the ground. Ie the two are in thermodynamic equilibrium. And to be specific, we are considering a 1m x 1m x 10m tall rectangular volume of air right above the ground.
Bob says: “such photons cease to exist long before they could get there at only around 10m away from source. ”
Yes I agree. But we had assumed that the ground and air were the same temperature. As photons flow from the surface to the first 10 m of air, there is a flow of energy. But for two objects at the same temperature, there can be no net flow of energy (because that would contradict the thermodynamic equilibrium). Therefore, there must be exactly as much energy flowing down from the first 10 m of air to the ground in the form of IR photons as there is flowing up into the first 10 m of air.
But we also know the radiation from the air is isotropic. So the energy flowing up out of the top 1m x 1m is the same as that flowing down from the bottom 1m x 1m.
So the ground emits photons. Some of them are absorbed by the column of air. The column of air emits its own photons up thru the of the column that exactly matches the photons absorbed in the column of air. The spectrum up thru the top will be indistinguishable from the spectrum coming into the bottom of the column.
It is quite true that it is not the same photons 10 m up, but the energy and spectrum will be indistinguishable from the spectrum at the surface.
To disagree with this conclusion, you pretty much have to disagree either with radiation from the air being isotropic, or disagree with the definition of thermodynamic equilibrium.
Bob Fernley-Jones, I asked what you thought of those emails, if they disproved something from climate science and you didn’t state anything, so I assume you think they make certain scientists look bad/dishonest and perhaps invoke extra scrutiny over their other work. If you want to answer affirmative that more is at stake, go ahead.
Now, you may want to read this interpretation of those emails about hiding the decline and using the Nature trick: http://bbickmore.wordpress.com/2010/05/27/series-climate-conspiracy-theories-in-utah-part-1/ On first reading, you may want to skip to around the middle (where it first starts talking about “trick”).
Some of the main arguments there are (plus some other points I want to note):
(a) Current decades tree ring data appears not to represent temperature, and this is corroborated by satellite data. Experts have taken note of this as well.
(b) Further into the past tree ring data appears to do a good job and this is supported by other forms of temperature readings and proxy analysis. You may not want to accept tree ring data at all, but there is support for accepting it. The main divergence problems also appear to be in areas that have high urban populations suggesting acid rain or other causes might be affecting the tree growth of more recent years. Look at the graphs included there to see the effect plotted against satellite temp records.
(c) Mann removing this bad data makes sense and is usually considered good science. He seems to have reasons to believe older data might be accurate. In any case, it doesn’t suggest dishonesty as implied by many. [see next few points]
(d) The trick is likely as harmless as using known current temp data to either replace or compensate for the bad tree growth data that deviates more and more from the last few decades of temp readings. Additionally, the trick might include plotting as the main line (possibly showing error bars) a 50 year average with 25 of those years going into the future. This explains the reason the last 25 years prior to the publishing of the paper are not included — they couldn’t be by definition.
(e) The word “trick” has many innocent meanings and is used frequently as slang in technical fields by those who practice them. Surely, many students have referred to using the “math trick” or something related in their years of study to refer to various interesting results/processes/manipulation needed to solve math problems accurately. Surely, these math tricks don’t imply there is deception going on or that the math is anything but 100% sound.
(f) A formal review was done and found Mann clear on all the serious charges.
As I said before, I have reasons to believe (and will continue to do so for now) that most of this climate science is a sane attempt if not perfect. I am willing to hear arguments against it. Even the arguments just presented against Mann, the scientist, who does not represent climate science as a whole regardless, appear to come up far short of the apparent intentions by those leveraging the accusations. Those two links were opinion papers, I think with one expressing a belief data was removed. There was nothing but circumstantial evidence suggested (anchored in part by negative interpretations of the few words in perhaps a single email). In response, I provided a link to an alternative interpretation that actually draws upon a wider context and data set rather than merely guessing bad behavior. The alternative view draws a very different picture (a positive one) and not simply a slightly less negative one.
I asked you for help in identifying links because I cannot tell what you think is a good argument. I don’t think those two links were. If you have more, you can provide them, and I will try and read them (or at least skim). [I came across one link, for example, saying that random time series gave the same “hockey stick” effect. I would have to look closer at that. Do you want to make that your next link?]
RW @ December 1, at 6:27 pm
I don’t know if I can explain it better than I did in the article, but perhaps the following comment from above may help:
http://wattsupwiththat.com/2011/10/26/does-the-trenberth-et-al-%e2%80%9cearth%e2%80%99s-energy-budget-diagram%e2%80%9d-contain-a-paradox/#comment-814677
Jose says: “Note, I did not clarify that by “massless” I meant zero rest mass only. E=mc^2 implies all energized particles have mass.”
Tim says: “Actually, you were closer to correct originally. Photons have no mass! I challenge anyone to find any authoritative reference that says photons have mass!
The full equation is not E = mc^2, but rather E^2 = (m^2 c^4) + (p^2 c^2). For particles with mass at rest, it becomes E = mc^2. For particles with no mass moving at the speed of light, it becomes E = pc. ”
Ok, if as you claim ρ²c² is massless (the kinetic term) then the rho (momentum) is zero and therefore energy is always zero. Is this really what you are saying Tim?
I think Jose_X was close here but should have used E=ρ²c² in his statement. If photons are not at rest and massless they have momentum therefore they have mass.
Oh Tim, you wanted a reference of a book… try “Gravity” (Misner,Thorne,Wheeler) p.580 on “Photon Reinterpretation of Geometric Optics” stating “Photons are particles of zero rest mass …” conversely implying “Photons are particles of non-zero mass when not at rest” or better non-zero momentum (four-momentum) demands non-zero mass.
Tim Folkerts @ December 1, at 6:47 pm
Sorry Tim but you must have been a bit hasty or perhaps over-imbibed with some good vino; let me guess; Cabernet-Merlot?
Why? Does it ever happen over the ground? (OR at sea?)
• No, according to the great prophet Trenberth, the surface (land and sea) is a black body. (emissivity =1). However, the atmosphere is not even a body, let alone a black body. The back-radiation is not a full Plancky, but follows the emission lines/bands of the emissive gases. (probably within the embrace of a partly definitive blackbody Plancky). The energy output is also reduced.
• Also, according to Trenberth, the surface emission is 396, whereas the backradiation is 333. According to you, previously, the 333 is from the total atmosphere above, whereas you now seem to be claiming that 396 is backradiated to the surface from a lesser source only 10m high. If that were the case, there would be no net heat transfer upwards.
• You have also cited MODTRAN as being definitive. Check-out my table 2 under Fig. 5, and perhaps elaborate more if you do not see a trend that is inconvenient to your claims therein
Tim, let me correct a typo before you surely catch it, that should have read E²=ρ²c², not E=ρ²c², but that’s par for me.☺
RW, further my recent post to you above, please also see the last two paragraphs in:
http://wattsupwiththat.com/2011/10/26/does-the-trenberth-et-al-%e2%80%9cearth%e2%80%99s-energy-budget-diagram%e2%80%9d-contain-a-paradox/#comment-813371
Wayne, I don’t have a copy of that book handy. Is it actually printed in the book that “Photons are particles of non-zero mass when not at rest”?
I would be surprised to see such an actual statement.
While the term “relativistic mass” has been used for “mass when not at rest”, the more typical current interpretation in special relativity follows more on the lines of wikipedia: “The term mass in special relativity usually refers to the rest mass of the object, which is the Newtonian mass as measured by an observer moving along with the object. The invariant mass is another name for the rest mass”
I was a little surprised to learn that in general relativity, the term “mass” cannot always be uniquely defined!
TIM>>For the sake of argument, let’s also assume the atmosphere in those
TIM>>first 10 m is the same temperature as the ground.
BOB>Why? Does it ever happen over the ground? (OR at sea?)
Clearly the air is sometimes warmer than the ground, and sometimes cooler. It is pretty clear that sometimes it must be the same temperature. My assumption was made to simplify the discussion so there was one less variable floating around.
TIM>> The spectrum up thru the top will be indistinguishable from the
TIM>> spectrum coming into the bottom of the column.
BOB>No … The energy output is also reduced.
Sorry, but I have to object to your objection. First, note that I am talking about the energy UP out of the top of the 10 m column (analogous to the 396 W/m^2), not DOWN into the column (analogous to the 333 W/m^2).
You seem to be suggesting that because the ground is a BB (and hence a good radiator) and the air column is not a black body (and hence a poor radiator), that the ground will send more energy into the air column than the air column sends into the ground — even when they are the same temperature (call it T(1)).
But then then there must be some temperature T(2) > T(1) that I could warm the air to such that there was no net energy flow.
But then there must be some temperature T(3) between T(1) and T(2) where there is still a net flow from the ground to the air.
Alas, we have now violated the 2nd law of thermodynamics –> there is a net flow of heat from the cooler ground (T(1)), to the warmer air (T(3))
Therefore at T(1), there must be identical flows off energy (ie identical absorption of IR photons) from the air to the ground as from the ground to the air independent of their emissivities.
And since the IR radiation from the air is isotropic, the photons the 10m tall air column emits upward must exactly match the photons it emits downward, which we just established exactly match the photons absorbed from below.
~~~~~~~~~~~~~~~~~~~~~~~~
That said, there is indeed some drop in temperature as the altitude increases. In 10 m = 0.01 km, that would be about 0.06 K cooler at the top of the column (at a typical lapse rate of 6 K/km). This will lead to a slight drop in IR radiation from the top, but a quick estimate suggest that the intensity at the top of the 10 m tall column will still be at least 99.9% as strong as at the ground in the absorption bands (and 100% in the “windows”). Of course, as you keep going up, the air keeps getting cooler and the amount out thru the top will get a bigger and bigger “bite” taken out of it.
@Jose_X…
A good book to start on is Quantum Thermodynamics, by Jochen Gemmer and M. Michel, 2004.
@Tim Folkers…
The equation C = Cp (δ) (r) is to calculate the capacity of energy storage (C) of any thermodynamic system. Cp is heat capacity, (δ is density of the system, and r is radius of the system or length of a parcel to be analyzed. It is a very simple formula that has been systematically ignored.
The results of multiplying J/kg K by kg/m^3 by m gives those square meters you are asking for.
The following formula is to calculate the time a molecule of a given substance takes to have all its excited electrons in ground state:
t =√(ΔE*(1/((m^2 kg) )) ) = √(1.345 x 10^(-20) (kg m^2/s^2) * (1/((m^2 kg) )) ) = 1.16 x 10^(-10) s
The term 1/(m^2 kg) is a correction factor derived from moment of inertia. The original formula would be:
t =√(ΔE /(m^2 kg))
In this case moment of inertia is taken as the unit 1 because inertial tensors are symmetrical.
Regarding the impossibility for a molecule of a given species to absorb the energy emitted by molecules of its own species resides in the fact of specificity of absorption spectral bands. The molecule of carbon dioxide emits quanta/waves with wavelengths pertaining to radio, such quanta/waves are not thermal radiation; consequently, they cannot be transferred as heat.
The unusable potential energy transformed in a molecule of carbon dioxide or any other molecules corresponds to sinks from where it cannot be taken to do work.
Something worth to be mentioned here is that the absorptivity of carbon dioxide at its stronger band of absorption (14 μm) is only 0.002, which is also its emissivity potential.
Jose_X, would you say that the wikipedia chart applies to quite or very moist air and that my 70% to 90% pertains too dry air?
‘Looking at this rough picture at the top section http://en.wikipedia.org/wiki/File:Atmospheric_Transmission.png, it looks like the area that is blue is much closer to 40% than to 70-90% range’.
Jose_X, my calculations came up with the following:
Total emissive power of CO2 bands = 79.8W/m^2
Emissive power entire spectrum @ 15 deg = 392W/m^2
>> A good book to start on is Quantum Thermodynamics,
Thanks for the book reference Nasif, but I am interested in your argument here and I am very unlikely to buy that book now, especially if I can get the information from elsewhere or derive it with some help. I have loads of physics books I have hardly looked at from many years back. Where the physics may have changed (I doubt it), I have google and can read up on select papers or discussions that I might find online.
>> The equation C = Cp (δ) (r) is to calculate the capacity of energy storage
OK.
It’s not clear to me what 609.7 J/m^2 K represents or how you got it.
(a) Together with the 120 picoseconds remark (which I’ll currently assume is a reasonable approximation to the length of time CO2 holds an excited state — I’m sure this value can be looked up) and
(b) noting that the units are 1/m^2,
are you saying that you figured out how much each square meter column of atmosphere on earth has of CO2 and multiplied this by how much each such CO2 can store in extra heat (as Joules) for each Kelvin degree in temperature.. and you got that 609 value?
Am I close?
If I am close, then how did you leverage this information to deduce the effect a doubling of CO2 would have on surface temperatures?
I don’t see any reference to the nonlinear time-dependent partial differential equations that must be solved on our complex earth system in order to try to try to deduce future temperature values. [This is the sort of calculation I think is done by climate models using supercomputers if necessary to perform many iterations using boundary conditions of current and past temp and other recorded values.]
>> The following formula is to calculate the time a molecule of a given substance takes to have all its excited electrons in ground state:
OK, I have a few more questions on this.
I was hoping you’d say which equations/operators lead to that very simple expression. Generally, I’d think that computing the decay times for arbitrary molecules is not something easy to do.
Also, what does delta E represent if you have multiple electrons in various excited states?
And does this equation consider LTE effects among other gas molecules? We don’t just have to deal with what CO2 can hold. CO2 will interact with other gas molecules. I heard (as mentioned already) that LTE time constants are much shorter than decay time constants so the CO2 serves as a conduit for imparting kinetic energy onto N2 and other gases.
And as mentioned above with the differential equations remark, it’s not just how much energy CO2 can hold or share with other gas molecules because excited CO2 and other ghg molecules do decay, with some of the energy going back down in the direction of the earth+oceans (which have a lot more mass). If possible, we should try to leverage as much of this information as possible to compute accurate future values.
[I think anyone wanting to check up on the predictions of these models should be taking a serious look at open source computer models, eg, GISS’ model E. We could use every extra eyeball there to make sure the models aren’t accidentally using sophisticated curve fitting that actually produces a result consistent with the past but also with pre-desired future value ranges. I think peer review probably has this covered, but a skeptical individual should really consider this path.]
Regardless of this equation, t=sqrt(delta E), or any other, I think these values have been measured and/or derived by many people already.
So my main question is still, how are you using this result to prove your claims? ..especially if it appears you are not using (generally, time-dependent) PDEs. The earth is a very complex system. We need heavy-duty calculations to get answers.
>> The molecule of carbon dioxide emits quanta/waves with wavelengths pertaining to radio,
Can you offer calculations to support this or experiments? I think much of the physics scientific community has concluded this is not an accurate statement.
>> Something worth to be mentioned here is that the absorptivity of carbon dioxide at its stronger band of absorption (14 μm) is only 0.002, which is also its emissivity potential.
I think we covered this above and Tim Folkerts presented an old paper by Hottel showing calculated tables dependent on distance-atmospheres. If we wanted to take that path to calculating some values for CO2, we need to know how much CO2 we are talking about. A huge tank of CO2 absorbs more than a little glass. The whole atmosphere absorbs much more still.
OK, I look forward to corrections and clarifications by anyone (Nasif or anyone else). This is a learning opportunity for me.
And no, I haven’t looked at the details of the climate computer models. Again, I think anyone seriously wanting to challenge details of current accepted theories needs to look at those carefully to make sure the wider community is not overlooking things and perhaps even doing elaborate curve fitting.
Robert S, again, it’s not a big deal here IMO since we aren’t doing much with the value anyway, but I was replying to what you said specifically:
[Robert Stevenson]>> This range is called ‘open radiation window’, because it is here that the least amount of absorption by water vapour and CO2 takes place. This window allows 70 to 90% of the radiation from Earth to escape into space. It includes the peak emission.
Maybe you got confused, but your calculation of CO2, which very well may be very accurate, is not the calculation we need to address the question of radiation leaving into space unimpeded. We would need to calculate similar figures for water vapor (which you even mentioned by name) and other ghgs.
The wikipedia link I think was over the total absorbed and passed through. I didn’t do any calculations, but presumably others have (or have good measurements). I think that picture addressed your question to cover “all” ghgs. That was what you seemed to be “asking” literally, even if perhaps half of your mind was thinking only of CO2 coverage (as CO2 is the main discussion gas).
This article is related to a link offered recently. It’s a fresh guest write-up by B Bickmore.
http://bycommonconsent.com/2011/12/02/global-warming-and-the-mobs
Nasif,
I am still confused about some things you say, and I disagree with others.
>absorptivity of carbon dioxide at its stronger band of absorption (14 μm) is only 0.002
Of course, absorption depends on the amount of CO2 involved. I would believe 0.2% gets absorbed in a few cm or so of CO2 in the air. Bob suggests that close to 100% is absorbed in the absorption bands within 10 m. I suggested a number more like 100 m earlier in the thread. But it is meaningless to give a number for absorption without a reference to distance & concentration.
>The molecule of carbon dioxide emits quanta/waves with wavelengths pertaining to radio …
CO2 emits strongly at ~15 um, as well as ~4 um and 2.7 um. These are definitely IR photons, not radio photons. The emission of thermal radio waves by CO2 would be insignificantly small.
>… such quanta/waves are not thermal radiation
Whether or not it is “thermal radiation” depends on the source, not the wavelength. The cosmic microwave background is thermal radiation. Microwave ovens are not thermal radiation. Visible light from an incandescent bulb is thermal; visible light from a fluorescent bulb in not thermal.
>C = Cp (δ) (r)
>Cp is heat capacity, δ is density of the system, and r is radius of the system
>For atmospheric carbon dioxide in current Earth’s atmosphere, the capacity
>of carbon dioxide to store energy during 120 picoseconds
>(1.2 × 10 ^ (-10) seconds) is 609.7 J/m^2 K.
This still greatly confuses me. I *think* you were using this equation to find the “capacity to store energy” for CO2. What values of Cp, δ, and r did you use for CO2 to calculate 609.7 J/m^2 K?
>The original formula would be: t =√(ΔE /(m^2 kg))
That doesn’t even have proper units, so it cannot possibly be correct!
Energy is in [J] = [kg*m^2/s^2]. If we divide by [kg*m^2] we get s^(-2). After taking the square root, this might be an equation for 1/t, but it is definitely not an equation for t.
Tim Folkerts,
Whether or not it is “thermal radiation” depends on the source, not the wavelength.
I think Nasif Nahle was referring to the absorption spectrum for CO2 and how that was not radio waves (which allegedly would be emitted by other CO2).
I think this is just another case of using words that may mean different things to different people at different times but generally conveys the right idea. I’d guess “thermal” meaning radiation overlapping significantly in the “short” IR range such as were CO2 absorbs.
>> After taking the square root, this might be an equation for 1/t, but it is definitely not an equation for t.
I didn’t look carefully, but yes it does appear that eqn is 1/s. Maybe wordpress blogging messed up his submission. Maybe the “1” in the numerator (of the correction factor that has moment of inertia term in the denominator) comes with units that were left out for one reason or another.
I forgot to quote above.
It’s:
****
>> Whether or not it is “thermal radiation” depends on the source, not the wavelength.
I think Nasif Nahle was referring to the absorption spectrum …
****
Also, I haven’t checked any calculations. I presume they can be done in time or looked up as necessary (especially since I don’t think the values were used).
Ah, I forgot to say one more thing [Nasif]
Looking around a bit with google, I get the impression that perhaps decay rates for excited molecules tend to be exponential, so I think that unlike the doubt expressed earlier, a simple calculation (whatever be the formula) may be reasonable.
@Tim Folkers…
“But it is meaningless to give a number for absorption without a reference to distance & concentration.”
Atmospheric carbon dioxide current partial pressure of 0.00038 atm and a column of length 7000 m.
“CO2 emits strongly at ~15 um, as well as ~4 um and 2.7 um. These are definitely IR photons, not radio photons. The emission of thermal radio waves by CO2 would be insignificantly small.”
The carbon dioxide molecule emits quantum/waves with energy E = 5.4 x 10^(-26) J, which corresponds to wavelength (λ) = 3.75 m. those are radio waves, not thermal radiation. An excited molecule of carbon dioxide has not available microstates, so it cannot absorb more energy; consequently, it only is able to emit quanta/waves with longer wavelength and lower frequency:
Serway, Raymond A., Moses, Clement J., Moyer, Curt A. Modern Physics-3rd Edition. Brooks Cole. 2005.
“Whether or not it is “thermal radiation” depends on the source, not the wavelength. The cosmic microwave background is thermal radiation. Microwave ovens are not thermal radiation. Visible light from an incandescent bulb is thermal; visible light from a fluorescent bulb in not thermal.
Thermal Radiation is the fraction of the electromagnetic spectrum between wavelengths 0.1 μm and 100 μm.
Thermal Radiation includes visible spectrum, almost the whole ultraviolet spectrum (Vacuum UV, Far UV, C-UV, Middle UV, B-UV, Near UV, and A-UV. Low UV is excluded), and almost the whole infrared (IR) spectrum, except the portion of the spectrum corresponding to wavelengths of Far-IR, from 101 μm to 1000 μm:
Pitts, Donald and Sissom, Leighton. Heat Transfer. 1998. McGraw-Hill. Pp. 289-311.
“This still greatly confuses me. I *think* you were using this equation to find the “capacity to store energy” for CO2. What values of Cp, δ, and r did you use for CO2 to calculate 609.7 J/m^2 K?”
The values found at 300 K, p of 1 atm, 1000 m and density 0.00069 kg/m^3.
It is a very known and simple formula applied in heat transfer science, everytime and everywhere; however, it has been strangely dismissed by most authors. Liquid water is very efficient on storing energy. Let’s take 10 m depth of clean water:
C = 4181.3 J / kg K * 998.2 kg m ^3 * 10 m =
41,737,736.6 J/m^2 K
And C of water vapor at 373.15 K, in a proportion of 3%, and a length of 10 m is 917.7 J/m^2 K
At height of 1000 m, for comparison with atmospheric carbon dioxide, the capacityof water vapor to store energy is 91,774.5 J/m^2 K.
Those small simple things forgotten by biased science… 😉
“>The original formula would be: t =√(ΔE /(m^2 kg))
That doesn’t even have proper units, so it cannot possibly be correct!”
Units of moment of inertia are m^2 kg.
Nasif,
>absorptivity of carbon dioxide at its stronger band of absorption (14 μm) is only 0.002 ..
> (at partial pressure of 0.00038 atm and a column of length 7000 m)
Check this reference page 144: http://dspace.mit.edu/handle/1721.1/42950
For 0.00038 atm and a column of length 7000 m = 700,000 cm, ie 266 atm*cm, we are off the top of the chart and the emissivity is above 0.2, so 20% of ALL wavelengths are absorbed. Very close to 100% is absorbed in the CO2 absorption bands. The chart might be off slightly, but your number (0.002) is simply WAY off from any possibly correct value (~1).
>Thermal Radiation is the fraction of the electromagnetic spectrum
>between wavelengths 0.1 μm and 100 μm.
I have seen “thermal IR” defined as ~ 3-15 um. But I have never seen “0.1 – 100 um” called “thermal radiation”. A more typical definition is
“Thermal radiation is electromagnetic radiation generated by the thermal motion of charged particles in matter. All matter with a temperature greater than absolute zero emits thermal radiation.” wikipedia
That is one source — Google gives many similar definitions. Thermal radiation is usually considered any radiation due to thermal energy on the early Google hits..
>C = Cp (δ) (r)
Thanks for explaining. The equation makes sense. Mostly. With somewhat odd notation.
Let Cp = specific heat capacity (at constant pressure) = (heat capacity / mass). Density is (δ) (although I usually see density as “ρ” and specific heat capacity with a lower case “c”)
.
The heat capacity, which I will call C* is then C* = Cp * m. But m = δ * V = δ * (l*w*h) = δ * (A * h) , so C* = Cp * δ * (A * h), or finally
C = (C* / A) = Cp * δ * (h)
>it has been strangely dismissed by most authors
I would instead say that most authors choose to write the equation in the more transparent form
(heat capacity) = (specific heat capacity) * (mass)
C = c * m
rather than your version which is
(heat capacity per unit area) = (specific heat) * (density) * (height)
(C/A) = c * ρ * h
>Those small simple things forgotten by biased science… 😉
I really don’t think science of any stripe has forgotten the meaning or importance of “heat capacity”. I think science was just confused by your notation.
>Units of moment of inertia are m^2 kg.
You missed the point. The left side of your equation has units of seconds. The right side is 1/seconds. There is no way such an equation can be correct.
Bob,
“Clearly, the 396, by definition, comes from the surface, but does not make-it to space. (or even to the high clouds as shown in the cartoon). The 396 and its progeny of new-generation emissions contain horizontal vector components which cannot be seen in the normal view. Thus, the vertical components must be less than the total S-B emission.”
I agree the full 396 W/m^2 does not make it to the high clouds, and in this sense Trenberth’s depiction is not accurate. But all of the 396 W/m^2 of S-B emission in the LW IR has an upward component to its direction, some of which is more horizontally or diagonally upward but it’s upward toward and in the direction of space none the less.
This is really not the biggest ‘problem’ with Trenberth’s diagram. Most importantly his depiction does not show gross net energy flux in any discernable way, and he incorrectly mixes non-radiative and radiative energy fluxes in a way that doesn’t account for the non-radiative energy returned to the surface as the temperature component of precipitation, wind, weather, etc.
RW @ December 2, at 9:29 pm
Thanks for your continued rational interest RW, and concerning your comments on part of what I’ve said.
So why does the 396 not make it up there?
Let’s approach this from a different direction, whilst simplistically ignoring the interacting thermals and evapotranspiration and stuff:
If the atmosphere were transparent, we would NOT be holding this discussion. In that imaginary situation, all the isotropic components of surface radiation would make it to space, and thus with a wide angle sensor in space, the 396 would be seen as if it were a vertical total. However, because we have an absorbent atmosphere, the critical wavelength photons from the surface are annihilated in quite short distances. When they are annihilated, they can no longer be observed.
See particularly my item 4 including Fig 3 in the article. Do you disagree that most radiation is sideways? Can you not see that the critical wavelengths are annihilated before escape to space is possible? Then please note that the horizontal vector components are persistently there as part of the isotropic emissions. (They do not go away). Are you hypothesising that when those spawning photons from the surface are absorbed that somehow their progeny after random collisional thermalization with N2 and O2 and whatnot are rotated through 90 degrees to the vertical?
@Tim Folkerts…
“For 0.00038 atm and a column of length 7000 m = 700,000 cm, ie 266 atm*cm we are off the top of the chart and the emissivity is above 0.2, so 20% of ALL wavelengths are absorbed. Very close to 100% is absorbed in the CO2 absorption bands. The chart might be off slightly, but your number (0.002) is simply WAY off from any possibly correct value (~1).”
Calculate it by yourself. The formula is as follows:
Ɛcd = [1 – (((a-1 * 1 –PE)/(a + b – (1 + PE)) * e^(-c (Log10 ((paL)m / paL)^2))] * (Ɛcd)0
A very important procedure concerns to the term e^(-c (Log10 ((paL)m / paL)^2))] and the term (Ɛcd)0.
I have the articles by Hottel, Sarofim, Leckner and Lapp and used them as references.
We cannot simply multiply partial pressure by length because it gives erroneous results, like those 266 atm*cm that you obtained as the partial pressure of the gas which is 266 times the total atmospheric pressure at sea level.
The correct procedure is to obtain the pressure that carbon dioxide would exert if it was alone occupying the whole volume of the Earth’s atmosphere; once you have obtained the pressure of the gas in each meter, you must convert atm-m to atm-cm. It is unphysical that the carbon dioxide could exhibits a pressure of 266 atm in each one of the centimeters of a column of air.
http://en.wikipedia.org/wiki/Partial_pressure
“That is one source — Google gives many similar definitions. Thermal radiation is usually considered any radiation due to thermal energy on the early Google hits..”
From Wikipedia:
“Thermal radiation is electromagnetic radiation generated by the thermal motion of charged particles in matter. All matter with a temperature greater than absolute zero emits thermal radiation.
Examples of thermal radiation include visible light emitted by an incandescent light bulb, infrared radiation emitted by animals and detectable with an infrared camera, and the cosmic microwave background radiation. Thermal radiation is different from thermal convection and thermal conduction–a person near a raging bonfire feels radiant heating from the fire, even if the surrounding air is very cold.
Source:
http://en.wikipedia.org/wiki/Thermal_radiation
Googled:
http://casswww.ucsd.edu/archive/public/tutorial/Planck.html
http://www.ptbmagazine.com/features/2009/feat1_0909.html (please, notice the big arrow which says “Thermal Radiation”. It is similar to graphs you can find in books of heat transfer.
https://docs.google.com/viewer?a=v&q=cache:J4yUwp8bBH8J:www.handbook.ifrf.net/handbook/dl.html/index.pdf?id%3D67%26type%3Dpdf+&hl=en&pid=bl&srcid=ADGEEShC7qK-yhnK8gb-xxiChSPGtN9ZnlypaeSCnvOkSX_U2Z8MgTLtLPq86vbtR-Raj3PLFAPqBnNKnoe22xOCJHZD6bTpgbO2Ck5RqUDTj2YpKSDLOOk5qeA0AmXduf5Z0LjtxUDg&sig=AHIEtbRWjWyjBUPMDrvCSNVwgOFDkMl-wA&pli=1
Etcetera, etcetera…
“rather than your version which is (heat capacity per unit area) = (specific heat) * (density) * (height) (C/A) = c * ρ * h”
Not my version… You can find it almost in all books of heat transfer.
“I think science was just confused by your notation.
I will dismiss this argument. It’s off topic and unproductive regarding science.
“You missed the point. The left side of your equation has units of seconds. The right side is 1/seconds. There is no way such an equation can be correct.”
I thought the same when I read the formula from two books of Modern Physics, but the formula was written exactly as I wrote it. Perhaps a (s^4) was dropped by the authors.
Nasif Nahle says:
December 2, 2011 at 8:29 am
The molecule of carbon dioxide emits quanta/waves with wavelengths pertaining to radio, such quanta/waves are not thermal radiation; consequently, they cannot be transferred as heat.
Nasif Nahle says:
December 2, 2011 at 3:27 pm
@Tim Folkers…
Tim: “Whether or not it is “thermal radiation” depends on the source, not the wavelength. The cosmic microwave background is thermal radiation. Microwave ovens are not thermal radiation. Visible light from an incandescent bulb is thermal; visible light from a fluorescent bulb in not thermal.”
Thermal Radiation is the fraction of the electromagnetic spectrum between wavelengths 0.1 μm and 100 μm.
Thermal Radiation includes visible spectrum, almost the whole ultraviolet spectrum (Vacuum UV, Far UV, C-UV, Middle UV, B-UV, Near UV, and A-UV. Low UV is excluded), and almost the whole infrared (IR) spectrum, except the portion of the spectrum corresponding to wavelengths of Far-IR, from 101 μm to 1000 μm:
Pitts, Donald and Sissom, Leighton. Heat Transfer. 1998. McGraw-Hill. Pp. 289-311.
Please, can we sort this out once and for all?
Visible light is not thermal, it is Light not Heat.
What applies to: “wavelengths pertaining to radio, such quanta/waves are not thermal radiation; consequently, they cannot be transferred as heat”, applies to visible light. Visible light is not thermal, it cannot be transferred as heat.
Near infrared is not thermal, it is not hot, it does not transfer as heat. It cannot heat things up any more than visible light can heat things up. BECAUSE they are not thermal radiation.
>… such quanta/waves are not thermal radiation
Tim: “Whether or not it is “thermal radiation” depends on the source, not the wavelength. The cosmic microwave background is thermal radiation. Microwave ovens are not thermal radiation. Visible light from an incandescent bulb is thermal; visible light from a fluorescent bulb in not thermal.”
Thermal depends on the the wavelength, not the source. HEAT, is thermal energy, Heat in transfer from the Sun is thermal energy in transfer by radiation, thermal energy in transfer by radiation is thermal infrared, therefore, thermal infrared is heat. Visible light is not heat. It is not thermal energy in transfer from the Sun nor from an incandescent bulb. It cannot heat stuff up.
It cannot heat stuff up because it cannot heat stuff up. It takes energy capable of moving atoms and molecules into vibrational states to heat stuff up, because that is what heat is. That is what thermal energy of the Sun is, and thermal energy from an incandescent light bulb. 95% of the energy radiated out from an incandescent light bulb is thermal infrared, heat, only 5% is light. Thermal energy, thermal infrared, heat, is what you can still feel from the light bulb after you have switched it off.
It appears to me, that you have all become horribly, and nonsensically, confused by such concepts as ‘background radiation is thermal’ and ‘everything above absolute zero emits thermal radiation’. You really need to get your concepts sorted. You’re all talking absolute gobbledegook.
Visible light as claimed in the AGWSF (AGWScienceFiction) energy budget which y’all think is logical, is ABSOLUTE bloody nonsense. Visible light cannot heat land and oceans. It cannot heat land and oceans any more than radiowaves can heat land and oceans. It does not suddenly become ‘thermal’ because it comes from the Sun, it is not thermal from the Sun any more than it is thermal from a fluorescent bulb. Fluorescence is light, not heat. It takes heat to transfer as heat.
Do try and get your heads around the difference between Light and Heat, as delineated in traditional physics. Light, such as visible and uv, do not move molecules and atoms into vibrational states, they work on the electronic transition levels, not atom/molecule vibrational levels.
For example, visible light which is not heat from a fluorescent bulb and which therefore is not heat from the Sun, is scattered by the electrons of the molecules of nitrogen and oxygen in the atmosphere. Reflected/scattered is one of effects because of how visible and uv interact with matter, because one of the four things these wavelengths have as an effect. When an electron absorbs visible light and briefly energises the electron it then comes back to norm state sending the light back out back out the way it came, this is why we have a blue sky. It’s like a pinball machine, visible light hits the electrons and gets scattered. The energy is being utilised in this, not in heating the molecules of oxygen and nitrogen. Electronic transitions do not heat matter, their energies are used in non-thermal encounters, for example, chemical energy of photosynthesis, in creating sugars, this is not creating heat it is creating sugar. Water is transparent to visible light, this means that visible light waves do not even have electronic transitions with water molecules, but are transmitted through without being absorbed. The AGWSF energy budget which you all have bought into as if real physics and not the science fiction world it is, says that visible light heats the oceans. Prove it.
You don’t even know how to prove it, or understand what I’m asking, because you either think thermal means from which source or you can’t understand that visible is not heat any more than radiowaves are heat.
Until y’all can prove that the Visible light in the KT97 and ilk claim, which is now ubiquitous in education from this junk science fiction of AGW, can actually heat land and oceans as claimed you will not understand just how ridiculous your claims and your arguments with each other are, even more garbled nonsense because you don’t understand the difference between heat and light in first principles.
Nasif Nahle:
Hi, I asked you a number of questions in http://wattsupwiththat.com/2011/10/26/does-the-trenberth-et-al-%e2%80%9cearth%e2%80%99s-energy-budget-diagram%e2%80%9d-contain-a-paradox/#comment-816466 because I find your comment incomplete and the conclusions to be against what I think is accepted science based on experiments, analysis, etc, over the years by many folks (and with proven results). If your conclusions do contradict what is widely believed and you challenge the accepted views, your conclusions have to follow from your “proof”. That “proof” is rather incomplete.
Why do I mention this? Because you continue to make those unorthodox statements apparently without support. For example:
>> The carbon dioxide molecule emits quantum/waves with energy E = 5.4 x 10^(-26) J, which corresponds to wavelength (λ) = 3.75 m. those are radio waves, not thermal radiation. An excited molecule of carbon dioxide has not available microstates, so it cannot absorb more energy; consequently, it only is able to emit quanta/waves with longer wavelength and lower frequency: Serway, Raymond A., Moses, Clement J., Moyer, Curt A. Modern Physics-3rd Edition. Brooks Cole. 2005.
Also, for the benefit of the many of us who don’t have access to that text, can you quote the part of that “Modern Physics” text you believe supports your view that CO2 is only able to emit in the radio range or that the region where CO2 absorbs does not overlap the region where CO2 emits?
>> The values found at 300 K, p of 1 atm, 1000 m and density 0.00069 kg/m^3.
OK.
I think this is what I assumed you were doing. Not having the density handy, I could not verify numbers, so I assumed you were looking at a column of atmosphere many kilometers up. So let me ask this.
Why would you only look at 1000m?
Also, as I noted in my other reply, can you please fill in the steps that take us from this observation/calculation into your conclusion that CO2 cannot lead to any greenhouse effect, in particular (to pick an example), that doubling CO2 concentrations could not possibly result in any earth surface warming (global average)?
Bob,
“So why does the 396 not make it up there?
…..because we have an absorbent atmosphere, the critical wavelength photons from the surface are annihilated in quite short distances. When they are annihilated, they can no longer be observed.”
Yes, I agree. The ‘annihilated’ or absorbed radiative power from the surface is transferred to the atmosphere via collisions, which heat the gases of the atmosphere (mostly N2 and O2). The heated atmosphere then acts as grey body, isotropically radiating in the LW IR according to its temperature like any other heated object.
“See particularly my item 4 including Fig 3 in the article. Do you disagree that most radiation is sideways? Can you not see that the critical wavelengths are annihilated before escape to space is possible? Then please note that the horizontal vector components are persistently there as part of the isotropic emissions. (They do not go away).”
Yes, I agree that most of the emission has a sideways component to its direction and is not directly perpedicular to the surface. I also agree that all the radiative emission in the atmosphere is isotropic (equal in all directions).
“Are you hypothesising that when those spawning photons from the surface are absorbed that somehow their progeny after random collisional thermalization with N2 and O2 and whatnot are rotated through 90 degrees to the vertical?”
No, not at all.
Myrrh, I think you are using your body’s biological characteristics to intuitively yet wrongly claim that visible light in not “thermal” in the sense that it can’t impart kinetic energy onto matter.
Can you find a single text book or experiment that would support your claim?
Let me ask you something. How do you think your eyes can sense visible radiation?
Something in the visible light has to communicate with your eyes. There is an energy transfer. Your eyes are tuned in (via evolutionary changes) to accept visible light photons while rejecting most other types of photons.
Myrrh, sorry. I didn’t finish reading all of your comment.
So you think visible light photons can cause electron transitions in molecules but don’t impart any kinetic energy? What makes the visible light photons different than other types of photons?
CO2 has limited ability to accept photons, but a body full of molecules interacting with each other can exhibit a very different and wide range of abilities to absorb photons.
So, specifically, why do you think the earth and oceans (and most solids, especially in large amounts) cannot absorb visible light? Where do you think is the limit of what the earth can absorb.. and why do you think that?
Myrhh, http://en.wikipedia.org/wiki/Attenuation explains that light (eg, visible light) can lose its intensity as it passes through some material. See also http://en.wikipedia.org/wiki/Beer-Lambert_law
So if you have less light intensity at the end, what happened to that energy that was lost?
If that energy excited electrons, then do those electrons stay excited forever? If not, what happens to this energy?
Also, what do you think of this: http://en.wikipedia.org/wiki/Black_body
When you say people here are talking “gobbledegook”, do you realize that physicists have been talking this same gobbledegook for many years?
@Jose_X…
I am sorry, José, but I will not respond questions of this tone:
“Why do I mention this? Because you continue to make those unorthodox statements apparently without support. For example:
“text you believe supports your view that CO2 is only able to emit in the radio range or that the region where CO2 absorbs does not overlap the region where CO2 emits?”
It is not my fault that you have not enough knowledge on quantum thermodynamics as to understand that those are not my assertions or beliefs, but well demonstrated science.
I will only answer the following question:
can you please fill in the steps that take us from this observation/calculation into your conclusion that CO2 cannot lead to any greenhouse effect, in particular (to pick an example), that doubling CO2 concentrations could not possibly result in any earth surface warming (global average)?
And here my answer:
ΔT = (5.35 W/m^2 (LN (([CO_2]_f * 2) / [CO_2]_∞)) / (4 * σ * (T_st)^3) = 0.65 K.
That would be the change of temperature of only the fraction of carbon dioxide that absorbed that load of thermal radiation (5.35 W/m^2).
It is evident that 5.35 W/m^2 is not constant because it depends on the intensity of thermal radiation emitted by the source; additionally, the absorptivity of the absorbent system, carbon dioxide in this case, depends on its temperature and the change of temperature that such system exhibits before and after further absorptions. For example, carbon dioxide absorptivity collapses at partial pressures below 0.1 atm m at temperatures below 500 R and above 1500 R.
Myrhh, where do you draw the boundary between electromagnetic radiation that can impart kinetic energy and that which cannot? Why do you draw the boundary there?
And do you think it is impossible that a molecule excited/energized by radiation (photons of some wavelength) can then pass on some of that energy to a different molecule?
If you do think an energized molecule can impart some of that energy to another molecule, might that not apply to any molecule excited? and wouldn’t this mean that a visible light photon exciting some material can then later have that energy turn into kinetic energy gains on nearby molecules? So then wouldn’t this just be “thermal energy” being imparted thanks to the visible light?
[Bob Fernley-Jones]>> Are you hypothesising that when those spawning photons from the surface are absorbed that somehow their progeny after random collisional thermalization with N2 and O2 and whatnot are rotated through 90 degrees to the vertical?
Why do you think that the Trenberth diagram shows 396 at 90 degrees vertical rather than 396 above the horizontal plane (ie, into the upper hemi-space)? If you wanted to suggest “upper hemi-space” why would you absolutely not use an arrow pointing up? In other words, why does arrow pointing up have to mean 90 degrees up only [or some other reduced range around 90 degrees]?
Nasif Nahle:
>> ΔT = … = 0.65 K.
>> That would be the change of temperature of only the fraction of carbon dioxide that absorbed that load of thermal radiation (5.35 W/m^2).
So you are saying that a part of the atmosphere is hotter than everything else and remains so constantly?
Nasif Nahle:
[Jose_X]>> [why do you believe that] CO2 is only able to emit in the radio range or that the region where CO2 absorbs does not overlap the region where CO2 emits
[Nasif Nahle]>> It is not my fault that you have not enough knowledge on quantum thermodynamics as to understand that those are not my assertions or beliefs, but well demonstrated science.
Nasif Nahle, so you are saying that CO2 absorbs within one range of photon wavelengths and emits within another?
Considering the structure of molecules, in simple terms a reader of this blog might be likely to understand, would you do us a favor and communicate what electron and nucleus behavior is going on to justify that absorption and emission happening at different frequencies?
Now, I did some googling, and I came upon a website apparently maintained by Harvard University (USA). Look at what it says in part:
https://www.cfa.harvard.edu/~jbattat/a35/cont_abs_em.html
“So a cloud of cool gas that absorbs certain colors of light will also emit those same colors. If we look at the cool gas without the blackbody in the line of sight, we will see an emission line spectrum, and the colors of the lines we see are exactly the same colors that were missing from the absorption line spectrum.”
It seems to me they are roughly saying that absorption and emission happens in the same range of frequencies for a given gas.
From University of California: Riverside, http://physics.ucr.edu/~wudka/Physics7/Notes_www/node107.html
“When heated every element gives off light. When this light is decomposed using a prism it is found to be made up of a series of “lines”, that is, the output from the prism is not a smooth spectrum of colors, but only a few of them show up. This set of colors is unique to each element and provides a unique fingerprint: if you know the color lines which make up a beam of light (and you find this out using a prism), you can determine which elements were heated up in order to produce this light.
“Similarly, when you shine white light through a cold gas of a given element, the gas blocks some colors; when the “filtered” light is decomposed using a prism the spectrum is not full but shows a series of black lines (corresponding to the colors blocked by the gas); see Fig. 8.3…”
Then it clarifies:
“…For a given element the colors blocked when cold are exactly the same as the ones emitted when hot.”
I am sure I can find many more universities to make such statements.
Nasif says: “It is unphysical that the carbon dioxide could exhibits a pressure of 266 atm in each one of the centimeters of a column of air.”
You are mis-interpreting the units. “266 Atm-cm” means “the equivalent amount of gas that would fill a container 1 cm thick to a pressure of 266 Amt”.
OR a container 266 cm thick to a pressure of 1 atm.
OR a container 266 cm/ 0.00038 thick to a pressure of 0.00038 atm.
So a container with a CO2 partial pressure of 0.00038 atm would have to be 7000 m tall to absorb this well. (Actually, it would have to be a bit taller, since the pressure will drop considerably as you get above sea level. Of you could set the column horizontally to avoid this problem.)
There is nothing unphysical about 380 ppm CO2 spread thru a column 7000+ m tall in the atmosphere — in fact, it sounds very much like the true physical conditions of the atmosphere. Other than a few details like pressure broadening of the absorption/emission lines, all three columns of air have the same amount of CO2 and would absorb IR the same amount.
Aggh, Jose_X, I lost my post to you in trying to get a link to a previous post..
Jose_X says:
December 3, 2011 at 7:28 am
Myrrh, I think you are using your body’s biological characteristics to intuitively yet wrongly claim that visible light in not “thermal” in the sense that it can’t impart kinetic energy onto matter.
Can you find a single text book or experiment that would support your claim?
Let me ask you something. How do you think your eyes can sense visible radiation?
Something in the visible light has to communicate with your eyes. There is an energy transfer. Your eyes are tuned in (via evolutionary changes) to accept visible light photons while rejecting most other types of photons.
Jose_X says:
December 3, 2011 at 7:44 am
Myrrh, sorry. I didn’t finish reading all of your comment.
So you think visible light photons can cause electron transitions in molecules but don’t impart any kinetic energy? What makes the visible light photons different than other types of photons?
CO2 has limited ability to accept photons, but a body full of molecules interacting with each other can exhibit a very different and wide range of abilities to absorb photons.
So, specifically, why do you think the earth and oceans (and most solids, especially in large amounts) cannot absorb visible light? Where do you think is the limit of what the earth can absorb.. and why do you think that?
Jose_X says:
December 3, 2011 at 8:12 am
Myrhh, http://en.wikipedia.org/wiki/Attenuation explains that light (eg, visible light) can lose its intensity as it passes through some material. See also http://en.wikipedia.org/wiki/Beer-Lambert_law
So if you have less light intensity at the end, what happened to that energy that was lost?
If that energy excited electrons, then do those electrons stay excited forever? If not, what happens to this energy?
Also, what do you think of this: http://en.wikipedia.org/wiki/Black_body
When you say people here are talking “gobbledegook”, do you realize that physicists have been talking this same gobbledegook for many years?
==========================================
That’s the problem. That’s why you have GIGO. AGWSF has changed the basic properties and given to visible, Light, what is the true characteristic of thermal infrared, Heat.
I have already gone through this in above posts, please read them first and if you still have any of these questions at the end of that, post again.
What I am giving you is traditional science:
Real traditional physics as still taught. All the heat we feel from the Sun is thermal Infrared.All that thermal infrared is what is heating the Earth’s lands and oceans and atmosphere.
Thermal infrared direct from the Sun is powerful, without the Water Cycle the Earth would be 67°C, but water is the great absorber of thermal infrared and takes this heat away from the Earth through evaporation, water vapour rises with it even faster, it’s lighter than air anyway, and in the colder regions it gives up its heat and condenses into rain. (And, by the way, taking carbon dioxide out with it, all pure rain is carbonic acid because carbon dioxide spontaneously combines with water).
And, we feel the heat because it warms us up inside. That’s how thermal infrared saunas work. When we get too heated up from the thermal infrared from the Sun our bodies work to get rid of some of it, we sweat. Water is a great absorber of Heat, thermal infrared radiation, and its high heat capacity means that it holds on to the heat longer – our bodies are around 20% carbon and the rest mainly water, we are great absorbers of thermal infrared.
Follow the link in my posts above to the wiki page on http://en.wikipedia.org/wiki/Transparency_and_translucency
Note the differences between electronic transmissions which is how Light energies act, visible and uv, and thermal infrared heat energy – it takes movement of the whole molecule into vibrational states to get kinetic energy, visible light isn’t capable of this, it works on electronic transmission levels. You’ll need to get some perspective here, the scale of these energies, visible is tiny and highly strung, it gets knocked about the sky by the electrons of the molecules of oxygen and nitrogen, it doesn’t heat them, it doesn’t even get to the electrons of water molecules, water is a transparent medium and visible is passed through, transmitted.
@Jose_X
Read from the sources I provided and make calculations. You cannot rely exclusively on Internet information.
The transfer of energy from a quantum/wave to a particle of matter is an irreversible process:
http://www.brighthub.com/engineering/mechanical/articles/4616.aspx
And laws of classical thermodynamics apply also in quantum thermodynamics:
http://en.wikipedia.org/wiki/Second_law_of_thermodynamics
Many things you cannot explain and test through classical thermodynamics is well explained and can be tested through quantum thermodynamics.
What could you do with the following formula? Just a question, not pretentious:
λ = (1.986 x (0^(-25) J.m) / E
Notice the term E, which implies the energy emitted by a particle of matter is not the same as the absorbed energy by such particle.
And the following formula lets you know how much of the energy absorbed is emitted by the absorbent system:
E = (hc)/λ
ΔE = E_f – E_i
If ΔE is negative the shift would be towards blue; if ΔE is positive, the shift would be towards red. The effect is measurable and it has been measured.
Black bodies do not exist in the known universe:
http://en.wikipedia.org/wiki/Black_body
http://www.pnas.org/content/106/15/6044.full
http://www.iki.rssi.ru/asp/pub_sha1/Sharch06.pdf
And what you are describing as a molecule that absorbs the whole energy transported by a quantum/wave and emits the same amount of absorbed energy is a black body, i.e. something hypothesized (an entity) that does not exist in nature.
@Tim Folkerts…
“You are mis-interpreting the units. “266 Atm-cm” means “the equivalent amount of gas that would fill a container 1 cm thick to a pressure of 266 Amt”. OR a container 266 cm thick to a pressure of 1 atm. OR a container 266 cm/ 0.00038 thick to a pressure of 0.00038 atm.”
No, you are misinterpreting what partial pressure of a gas is. Again:
You are saying that carbon dioxide, if it was alone in the atmosphere, would exert a pressure 266 times higher than the whole column of air on the surface. Consequently, it is unphysical.
Anyway, I did such calculations taking into account the numbers you give and the results are the same, i.e. total emissivity of carbon dioxide is 0.002 at its current proportion in the atmosphere.
Please, do the calculations by yourself so speculations stop here. The formula is as follows… go on:
Ɛcd = [1 – (((a-1 * 1 –PE)/(a + b – (1 + PE)) * e^(-c (Log10 ((paL)m / paL)^2))] * (Ɛcd)0
I don’t know if I am coming across as impolite. I don’t know what to do.
Scientists supposedly publish things as a team. You have various work together to recheck the path they are taking because individuals have many lapses and weak spots. They take a bunch of time. After a paper appears done, it goes by editors and others. In the end, the idea is that if a paper passes, it is a team effort. A whole team was fooled if there are problems. Effort was made to avoid errors, but if the error fooled many, then it was not an easy error to avoid, generally.
I have thought about not commenting, but I feel like (a) there is a learning opportunity (for me and/or for others), and (b) I think I see attacks on a whole industry of people and livelihoods and using unsound arguments (obviously if I thought the argument was ok I would have little to complain about).
I would like to be part of a team here, but this seems like a very adversarial environment. I’m not sure what to do.
As to the use of the word “thermal radiation”, this is mostly matter of semantics.
I have no problem saying that “most thermal radiation” or “typical thermal radiation” is between 0.1 – 100 um, but I would not use this as a definition. I would use a more physical definition and say any photons emitted to to the temperature of an object make up the “thermal radiation”
* Lasers operate in the range 0.1 um – 100 um, but are not thermal radiation.
* Hot stars (40,000 K) emit about 1/2 of their “thermal radiation” below 0.1 um.
* the microwave background is thermal radiation that is mostly above 1000 um.
(If Myrrh wants to give a different definition of “thermal radiation” he is welcome to do that too. )
RECAP:
* Not all radiation between 0.1-100 um is thermal radiation.
* Not all thermal radiation is between 0.1-100 um.
@Jose_X…
I hope this message to you does not bother the administrators of this blog.
As you are questioning scientific knowledge, I think you are already part of WUWT team.
The only part I don’t go with concerns to personal references which cast doubts into the integrity of professionals posting here.
For example, if one made a mistake on writting a statement, references to his mental capabilities or knowledge are plainly unpleasant.
@Administrators… I sincerely apologize if I got out of bounds on this answer.
>You are saying that carbon dioxide, if it was alone in the atmosphere,
>would exert a pressure 266 times higher than the whole column of air
>on the surface. Consequently, it is unphysical.
NO! I am not saying that at all! Read what I am saying.
I am saying that (pressure)*(length) determines the absorption by gases. The same amount of gas will fill a column 1 cm deep @ 266 atm or fill a column 266 cm deep @ 1 atm or fill a column 7000 m deep @ 0.00038 atm. (assuming of course, constant temperature and the same area for all the columns).
All of these have the same value of P*L = 266 atm*cm.
All of these have the same amount of gas in them.
All of these have the same absorption of a beam of IR light by that gas.
@Tim Folkerts…
It is not semantics, but delimitation.
Going simpler:
Thermal radiation is thermal energy in transit due to a spatial difference of internal thermal energy density. It can be transferred as heat or work.
I think that Myrrh refers to the capability of visible light to cause changes of temperature on thermodynamic systems.
I have to leave this dialogue for awhile because I am a bit busy on an experiment. Please, understand my position.
Jose_X says – there isn’t really a team here, but individuals sharing their points of view. There is a general background that AGW is junk science, because we’ve had just so much evidence on so many different fronts it’s an unavoidable conclusion, but within that – I’m arguing here against both pro and anti AGW, because I’m saying both have got their basic physics wrong in the energy budget. I don’t have a team, there may well be many who agree with me.., I don’t know. It’s a great place for learning, just don’t expect ‘consensus’ opinion, just work out what you think and who you agree with and don’t agree with and why and and argue the points and you’ll do fine.
@Tim Folkerts…
This will be my last message on this issue.
I recommend you to make the calculations using your numbers and find that the result I obtained is correct.
A couple years ago I made the calculations in several ways and found the same results. There are other formulas to obtain the total emissivity of atmospheric gases, although a bit more complicated; for example, the following formula which takes into account the time a photon takes on colliding with molecules of carbon dioxide and mean free path length of quantum/waves in an atmosphere composed exclusively by carbon dioxide at its current concentration:
ε = (1-e^(t * (-1/s)) / √π
The results are the same, i.e. total emissivity of carbon dioxide is 0.002.
Nasif Nahle, I haven’t read the details of your last couple of comments on this issue, but of CO2 emissivity, but without a reference to the model used to derive these formulas, you and anyone else can make a mistake and others would not be able to correct them. Can you quote a paper or something for these equations. Surely you don’t believe you are flawless in understanding the details of these formulas or that whoever came up with it is also flawless.
I also want to note that Tim was using a table from a published paper that specifically included those values he is quoting which are much closer to 0.2 for very large sections of the atmosphere. I think he provided a link (he did earlier) and the table is somewhere between page 10 and 30 I think. If you are interested in looking at it, I’ll look up the specifics again.
Myrhh:
>> Aggh, Jose_X, I lost my post to you in trying to get a link to a previous post..
That is a horrible feeling. I wish this blog page could be refreshed without trashing what one was writing. I try to use a text editor all the time, but sometimes I forget and then have an accident. Aggh is right.
>> NASA: Far infrared waves are thermal. In other words, we experience this type of infrared radiation every day in the form of heat! The heat that we feel from sunlight, a fire, a radiator or a warm sidewalk is infrared.
This page is designed for young students. You should not expect them to use the same meaning of “heat” and “thermal” as you might use in a more advanced physics course. These words are probably used in their more casual meanings than their more technical counterparts in thermodynamics.
From http://www.thefreedictionary.com/heat , note the difference in the physics meaning and the biology meaning:
heat [hiːt]
n
1. (Physics / General Physics)
a. the energy transferred as a result of a difference in temperature
b. the random kinetic energy of the atoms, molecules, or ions in a substance or body Related adjs thermal, calorific
2. (Life Sciences & Allied Applications / Physiology) the sensation caused in the body by heat energy; warmth
Anyway, you appear to trust NASA. I found another page http://helios.gsfc.nasa.gov/qa_gp_ht.html and a different quote which I believe contradicts your view of visible light:
> Yes, it is possible (and relatively easy) to change the wavelength of electromagnetic waves. As you have pointed out, visible light from the Sun can heat matter and that matter then radiates as infrared waves, which our eyes cannot see but our bodies can sense as “heat”.
So NASA stated that visible light from the Sun can heat matter.
Lastly, if you go back to the last of the comments I made that you quoted, I provide a link to wikipedia page on attenuation. I then asked how you would explain what happened to that missing energy? If molecules get excited with visible light and presumably through that mechanism remove energy from the light shining through it, then what happens to that energy in those excited molecules in that solid? It’s not to hard to suspect that some of that energy turns into vibrational energy that heats up the matter.
Anyway, I think the NASA quote above supports the view that visible light can help heat the planet.
Nasif Nahle, I was looking at the paper by Hottel http://dspace.mit.edu/handle/1721.1/42950 (direct link: http://dspace.mit.edu/bitstream/handle/1721.1/42950/02748698.pdf?sequence=1 ) for clues on how to interpret that table.
The data table that shows approximately the 0.2 emissivity for CO2 is table 1.4.1-2 on page 29.
On page 24 it says:
“In order to check the consistency of the different independent experimenters and to check the agreement between the present computed emissivities and the experimental results it was necessary to reduce all data to a common basis of Pc = 0 and Pt = 1 atm.”
This normalization comment and tables such as table 4.2.2.5-1 on page 113 suggest that a single table (1.4.1-2) was constructed from values obtained at different atm. The table allows one to lookup path length easily.
I believe they use some theoretical procedure to make the conversion, so the experiment data is “corrupted” by this procedure, but it does suggest that what Tim Folkerts was saying is correct.
Let’s say CO2 in our atmosphere is at a partial pressure of .00038 atm. If we consider a height of say 1000000 cm (ie, 10 km) in our atmosphere, we multiply this by .00038 atm to get 380 cm*atm. We then look up 380 (cm*atm) on the table 1.4.1-2. The row with nearest PL has the entry 300 on the far left. Then on the next column over (300 Kelvin) we find .215. That value would be the emissivity for CO2 according to that table. That value represents a normalized result of numerous experiments calculating emissivity of CO2 at different temperatures, for different path lengths, at different atmospheres.
Nasif Nahle:
This paper “Effective Atmospheric Emissivity under Clear Skies” ( http://www.patarnott.com/atms411/pdf/StaleyJuricaEffectiveEmissivity.pdf ) is from March 1972 Journal of Applied Meteorology.
Fig 1 on page 352 (pg 4 of pdf) shows “effective atmosphere emissivity of CO2” at various pressures and for 3 mixing ratios. I think the mixing ratios (in g/kg) relate to fractional composition of CO2 in the atmosphere and would then appear to be in the ballpark of our current CO2 in the atmosphere (at about .04% in terms of number of molecules). All 3 curves suggest a 1013 mbar (1 atm) value of approximately between 0.17 and 0.2.
As a second example, Table 1 on pdf page 6 is for the 1013 mbar case and has CO2 at .19.
If these figures (and those from Hottel’s table 1.4.1-2) are in the ballpark (and if I am understanding properly from skimming the paper), they suggest the emissivity of CO2 in our atmosphere is on the order of tenths of 1 rather than thousandths of 1.
Jose_X @ December 1, at 9:45 pm
I was going to ignore your comments, but it keeps coming back to me that it is very sad that you are so gullible and naïve as to believe for example the source that you quote:
You say that you want to study and learn, but when you are recommended to read FREE information, ….. Oh, I’d better stop there….. but will instead quote something that Wayne commented to you:
I could show more proof that the hockey-team and others are corrupt, but it is really up to you to study and learn, and it is off-topic.
Nasif Nahle, googling around for various things, I came across an article written by you, I’m assuming.
I will link to it here since it involves exactly the calculations and results you mentioned here for CO2 emissivity. http://jennifermarohasy.com/2011/03/total-emissivity-of-the-earth-and-atmospheric-carbon-dioxide/
I’ll try and go over it carefully tomorrow if possible (and maybe even look at the reference in the comments to Schwarzchild’s equations.)
Bob Fernley-Jones, your last comment didn’t make much sense to me. Is there a “free” link you want me to read? Is there a problem you found in Barry Bickmore’s write-up? Do you have a bias against Bickmore and automatically assume his write-up is garbage?
Nasif, I’m lost with the equation:
>> Ɛcd = [1 – (((a-1 * 1 –PE)/(a + b – (1 + PE)) * e^(-c (Log10 ((paL)m / paL)^2))] * (Ɛcd)0
I don’t know the meanings of:
1. a
2. b
3. PE
4. c
5. paL
6. m
7. (paL)m
8. (Ɛcd)0
Also, is the beginning:
1. a minus (1 times 1) minus PE
or
2. (a minus 1) times (1 minus PE)
Does “((paL)m / paL)” reduce to “m” or is (pal)m a distinct variable?
@ Nasif Nahle
This is probably my last post too. You have mentioned a few calculations you have done to find emissivity.
You suggest the the emissivity of the CO2 in the atmosphere as a whole is ~ 0.002 — ie that well under 1% of the IR energy passing thru the CO in the atmosphere is absorbed
* The published reference I linked to disagrees
* The satellite data posted at the top of this page disagrees
* This simple experiment disagrees: http://www.youtube.com/watch?v=Ot5n9m4whaw
* The entire science behind global warming disagrees.
When such and array of data and theory are weighed against your calculations, I will believe these results and questions your calculations.
Nasif, I found the book Modest, Michael F. Radiative Heat Transfer-Second Edition. 2003. Elsevier Science, USA and Academic Press, UK. on googlebooks: http://books.google.com/books?id=lLT-aKLTxkQC&pg=PA826&lpg=PA826&dq=Modest,+Michael+F.+Radiative+Heat+Transfer-Second+Edition.+2003.+Elsevier+Science,+USA+and+Academic+Press,+UK.&source=bl&ots=7k4Sxqi87s&sig=DqLHm-TPB9S-a3ICO2YSyvX-Yko&hl=en&ei=nsfaTvGiFZDAgQf154GpBg&sa=X&oi=book_result&ct=result&resnum=3&ved=0CC0Q6AEwAg#v=onepage&q=paL&f=false
This is a book you referenced in the http://jennifermarohasy.com/2011/03/total-emissivity-of-the-earth-and-atmospheric-carbon-dioxide/ article for the formula you used [the one having a, b, c, PE, and some other values].
On page 342 there is a formula that looks sort of like what you provided, but the formula is set equal to a ratio and is used as an adjustment where you look up the base emissivity and then apply the formula if you are working under non 1 atm environment. The book also provides a table of the meanings of the various constants/variables (many of which look like what you provided).
The book also leverages the Hottel data (and the Leckner fixes, which are similar) and mentions that some of his extrapolated data is a bit off, but I think the deviations are on the order of some percentage points and mostly for the higher temp cases.
I applied the formula 10.145 (although I didn’t have to for 1 atm environment, as the calculations below will show) using values of .00038 bar for CO2 partial pressure and 300 Kelvin and total pressure of 1 bar. The result of the ratio was very close to 1 (just like in the book example 10.11 that uses different scenario). Because the ratio is about 1, the emissivity of CO2 would be the reference one, which basically can be estimated from Hottel’s work as given on figure 10-25 on page 342 of the google book. Those values are shown as curves and resemble the Hottel table Tim Folkerts linked to. Each curve corresponds to a row in the table. The “y” value of the curve is the emissivity and the “x” values would be the temperature. These curves in figure 10-25 seem to almost overlap the table in emissivity values.
Notes:
formula 10.145 with values already substituted:
emiss /(emiss base) = 1 + ((.000057 / 1.8001) * exp((-1.47) * (log(.6)^2))) = 1.00002945;
using t=.3, a=1.57, b=.23, c=1.47, (PaL)m / (PaL)0=.6, PE=1.0001 (see table 10.5 on page 344 of google book).
Recap: We can look up the values for CO2 emissivity for a given depth * partial pressure at some temp using the Hottel experimental results done at about 1 atm. The ratio formula (10.145) is used as a correction factor if we were calculating for a pressure other than 1 atm. [so that formula was not useful for the earth atmosphere climate case; it would be useful for lab experiments and any system under different enough pressure from 1 atm.] Conclusion, the CO2 emissivity can be taken (more or less) from the table on page 29 of the 1972 Hottel paper linked to earlier. That is what the Modest, Michael F. Radiative Heat Transfer-Second Edition appears to be doing around page 342+.
BTW, this and the last prior comment I made resourcing Modest, Michael F. Radiative Heat Transfer-Second Edition and performing a calculation (equal to 1) with a formula that looks a fair amount like the formula used by Nasif when deriving .002 emissivity of CO2, and where that book appears to reference Hottel’s and Leckner’s work, will probably be my last comment (following Tim and maybe Nasif’s lead) debating the emissivity value of CO2. The result appears to be pretty much what Tim Folkerts had concluded numerous comments earlier upon first(?) referencing Hottel’s 1972 work on CO2 and H2O emissivity. Going several kilometers up the atmosphere, the CO2 emissivity appears to be near 0.2.
Oh… Now, I see. The formula in the book is supposed to be the one Nasif stated.
To answer a prior comment…
First, the formula is wrong in that the parenthesis are off (probably due to copy/paste formatting slip-up).
As per Radiative Heat Transfer-Second Edition:
Ɛcd = [1 – [(a-1)*(1-PE)/(a + b – 1 + PE)] * e (-c (Log10 (p_CO2_L_m / p_CO2_L_0) )^2) ] * Ɛcd_0
.. and not //// XXXX Ɛcd = [1 – (((a-1 * 1 –PE)/(a + b – (1 + PE)) * e (-c (Log10 ((paL)m / paL)^2))] * (Ɛcd)0
Ɛcd = emissivity. The formula is useful mainly when the total pressure under consideration is not 1 bar (aka, 1 atm) since otherwise we likely get approximately Ɛcd = Ɛcd_0.
Ɛcd_0 = emissivity value at 1 bar. For CO2, we can look up from the Hottel table.
T = temperature in kelvin; eg, 300 K.
T_0 = 1000 K.
t = T / T_0; eg, 0.3 .
a = 1 + ((.1) / (t^1.45)) for CO2; eg, 1.57 .
b = .23 for CO2.
c = 1.47 for CO2.
p = total pressure in bar (1 bar is about 1 atm); eg, 1 bar.
p_CO2 = partial pressure of CO2 in bar; eg, .00038 bar is about partial pressure of CO2 in atmosphere.
PE = (p + .28 * p_CO2) / (1 bar) for CO2; eg, 1.0001
p_CO2_L_m / p_CO2_L_0 = 0.054 / t^2 for CO2 and t<0.7 according to the book table, but this may need adjustment using the actual definition of p_CO2_L_m and with P_CO2_L_0 = 1 bar cm.
Jose_X @ December 3, at 4:38 pm
YAWN; I REPEAT part of what I said with added bold: I could show more proof that the hockey-team and others are corrupt but it is really up to you to study and learn, and it is off-topic.
As you have repeatedly stated that you want to study and learn, and you do appear to be quite an intelligent chappie with much divergent thinking, it would be appropriate if you were to follow your declared principles, and read and digest the stuff recommended to you. (although I find it distressing that you cannot afford the valuable journalistic research of the IPCC corruptness in the downloadable Laframboise digital book at $4.99)
Oh BTW, did you ever hear about the Amman and Wahl fiasco involving the IPCC, Mann, McIntyre, journal rejections, editorial staff changes, and different versions and dates of their papers used for various naughty purposes, and substantial other whatnots. Here follows a nice lucid and pleasantly readable account, and it’s FREE:
http://bishophill.squarespace.com/blog/2008/8/11/caspar-and-the-jesus-paper.html
You won’t like it of course, because it does not fit with your dogma. Nevertheless, you might benefit from the high quality of the English prose.
If it weren’t so serious, the A & W fiasco would be funny.
Jose, BTW, please read my Andrew Montford (Bishop Hill) reference just above, carefully and with an open mind. I don’t know if language might be an issue here but I guess that your native tongue might be Spanish or Portuguese. If that is the case, I sincerely complement you in your good English, as a second tongue, but wonder if you need to be more cautious in what you say.
Perhaps draft what you want to say in a word processor, and then come back to it later, preferably the next day, and examine it for ambiguities. This was something I as an Englishman by birth commonly did in the past in industry, especially with tricky issues.
With your divergent thinking you may be able to contribute here, but please be more careful.
Jose_X says:
December 3, 2011 at 8:20 am
Myrhh, where do you draw the boundary between electromagnetic radiation that can impart kinetic energy and that which cannot? Why do you draw the boundary there?
I draw the boundary here because traditional practical real world tried and tested and well understood physics has already categorised it, it is the difference between Heat and Light, the science fields of optics and thermodynamics. Since the AGWSF manipulation of the education system this distinction has been so blurred that older papers can’t be understood properly by most now and typically we have all the above confusion as seen in these arguments, for example Tim who makes out that heat is either something esoteric that can’t be understood or it’s all a matter of semantics so use the word as you want. Tim is typical here in AGWSF influence in denying that heat is as traditional physics describes it, as he has done a few posts up, and, typical in denying that thermal infrared direct from the Sun heats the land and oceans, going with the science fiction KT97 ilk produced by AGW pushers to dumb down the population, that visible light heats land and oceans, in the real world this is impossible. Visible light is Light, not Heat. OK, let’s start with traditional understanding of Heat.
So, lots of confusion created by AGWSF to better sell its propaganda, it has reduced the physical world to a one dimensional reality, nothing in it has any properties or characteristics; not as here in the meme ‘all electromagnetic energy is the same’ and ‘all absorption of energy heats’, nor in the properties of molecules where ‘oxygen, nitrogen and carbon dioxide are ideal gases’. You really need to appreciate what I’m saying here, in traditional physics you have to put those properties, and so the differences between them, back into the picture. Molecules have weight and volume and attraction and electromagnetic waves have size and so on.
These are simple, basic differences, don’t be distracted into making it more complicated than it is, even a primary school child should be able to understand the following, and certainly by secondary school level. It is now longer easy to find traditional physics taught, you have to go into the applied science world where it you’ll find it because real physics is a description of the real world and they learn how real things work.. For advanced take dscott’s advice here: http://wattsupwiththat.com/2011/09/29/1-k-or-not-1-k-that-is-the-question-2/#comment-755585
For basics, solid understanding of basics without which you cannot build anything and understanding of which will stop you from going off into dead end muddled tangents as Tim et al do, and, which will enable you to see where Tim and ilk confuse read this:
Italics as used in the piece.
Further:
So. What we feel is heat created by the mechanical energy in rubbing our hands together, and, heat is thermal energy in transfer. There’s no confusion here. Why not? Because it’s one and the same thing.
The only confusion is in Tim et al (sorry Tim, just using you as example here because you’ve posted), confusing the situation because they don’t understand this. Because they don’t understand it they make up their own claims about what it is, and then they extrapolate from them to claim, to them it appears logical, that visible light from the Sun heats land and oceans, but get stumped when asked to prove it, because they can’t. All they can do is obfuscate more. As dscott put it so well: “What we have here is the classic false proof, where the assumption at the outset of logical thought process is incorrect. In typical fashion you scientific types run with the flawed assumptions, apply flawless logic and then end up with a false conclusion.”
So remember, it’s not semantics, it’s a knowledgeable use of the word heat by people who understand what it is and can tell by context when others understand what it is, and can see when they don’t:
“Thermal energy and heat are often confused. Rightly so because they are physically the same thing. Heat is always the thermal energy of some system. Using the word heat helps physicists to make a distinction relative to the system they are talking about.”
So, the great thermal energy of the Sun is heat in the Sun; it is heat in radiative transfer to us as it flows from the Sun in straight lines and reaches us in around eight minutes; it is this heat we feel when our bodies absorb this thermal energy transfered to us by radiation, (one of the three ways of heat transfer) as it heats the water in our bodies; the heat from the Sun in transfer by radiation is heat; this the invisible thermal infrared, which is heat, which is heat energy, which is thermal energy in transfer by radiation, which is why it is called thermal.
It is not visible light, visible light is not thermal. Near infrared is not thermal.
Recall the kid’s page from NASA – the difference in size between the not hot near infrared and thermal infrared, near infrared microscopic, thermal infrared, the invisible heat we can feel, the size of a pin head.
So back to: “where do you draw the boundary between electromagnetic radiation that can impart kinetic energy and that which cannot”
The link I gave from the wiki page on transparency and translucency has a section which gives the basic basics of the difference, where the line is drawn between thermal and non-thermal infrared is that near is not thermal, it is reflective, and from mid infrared on it is thermal, absorptive. Near classified with Light not Heat, think cameras. Just as an ordinary camera captures the light reflected from objects so does the near infrared, it is what is reflected by our bodies, not as thermal infrared cameras capture, the heat energy radiating out from our bodies. Near infrared can penetrate deeper than visible before being reflected out, visible can penetrate deeper than uv, uv doesn’t make it past the first of the three layers of the epidermis. Highly energetic = powerful? Put a shirt on to block it..
From the wiki page:
That’s the cut off point, the boundary.
Now, read on from there, UV-Vis: Electronic transitions, bearing in mind that this will also apply to near infrared which is not hot, which is reflective, think cameras, as are visible and uv (there are plants deeper in the ocean than visible red can reach which use near infrared light for photosynthesis, they’re a sort of violet colour, the colour they don’t absorb at those deeper levels reflected out as the green reflected out generally by plants).
There are four bullet points listed: When photons (individual packets of light energy) come in contact with the valence electrons of atom, one of several things can and will occur:
NB, can and will occur.
The second is how the difference between reflective and absorptive comes about in the general difference between Light and Heat, light being reflective, heat being absorptive. Here light is absorptive in a light context, not as a comparison in heat and light differences which is reflective visible, absorptive on atomic/molecular level thermal.
Remember, visible light is even smaller than near infrared, light works on an electronic transition level, and in reflection/scattering it hits the electron and its energy briefly absorbed and is bounced back out as bullet 2 explains. This is what is happening in the atmosphere, the blue sky we have is because blue light is even more energetic than red and so is more easily bounced around by the electrons of the molecules of oxygen and nitrogen, think pin ball machine.
The atmosphere, therefore, is wrongly claimed by AGWSF to be transparent to visible light, as in the ‘greenhouse’ descriptions, but it isn’t, because it is absorbed on an electron level. Bear this in mind, because the next point is a description of a real transparent medium to visible light.
The third bullet point describes this: An electron cannot absorb the energy of the photon and the photon continues on its path. This results in transmission
Water is a real transparent medium to visible light, it is not absorbed by the electrons but transmitted through.
AGWScience Fiction claims that visible light heats water. How? It takes moving the whole atom and molecule into vibration to heat matter which is not in the level of capability of visible light, which are in Electronic Transitions not Vibrational (kinetic) modes of operation.
So be aware, AGWSF misuses the word ‘absorption’ as it does the word ‘heat’; that oceans are described as ‘absorbing different colours to different depths’ does not mean visible light is heating the water, it can’t, it can’t even get to the electrons of water as it does in the atmosphere because it is transmitted through transparent mediums. Again, don’t get yourself distracted into tangents that try to obscure this basic difference in well known tried and tested real world physics fact..
If you can get these simple basics clear, then you’ll be able to spot more easily where AGWSF takes liberties with real physics elsewhere; by taking laws out of context, by giving the property of one thing to another, as here giving the properties of thermal infrared to visible light, by stipping all properties to hide the differences and so the actual effects, as in ‘carbon dioxide well mixed in the atmosphere and able to accumulate’ because ‘the atmosphere is empty space with all the molecules zipping around at tremendous speeds’ and not the real world fluid gaseous heavy volume pressing down on us a ton/sq ft, where we have sound.., and so on.
I am convinced the confusing manipulations of terms is deliberate, because the more I explored this the more I saw it takes someone with a real grounding of physics to be able to create the confusion by these fiction sound bites, and produce the so called ‘proofs’ which on examination turn out to be more sleight of hand, as in the ‘heating experiments’.
Remember the real boundary in these arguments:
As dscott put it so well: “What we have here is the classic false proof, where the assumption at the outset of logical thought process is incorrect. In typical fashion you scientific types run with the flawed assumptions, apply flawless logic and then end up with a false conclusion.”
p.s. sorry, for clarity:
“The link I gave from the wiki page on transparency and translucency has a section which gives the basic basics of the difference, where the line is drawn”
From “where the line is drawn” is separate point to the info on the link, best put in brackets.
Jose_X @ December 3, at 8:28 am
Well for a start the maths would be a bit tricky. How do you subtract a downward facing hemisphere from an upward facing hemisphere? OK, Trenberth does it as 396 – 333 = 63. Is this net heat transfer of 63 a hemisphere, full sphere, a weird shape, or vertical? Oh, and the horizontal stuff does not have a relative sign BTW.
Another bit of fun for you is that surface radiation can only be hemispherical, whereas the 333 comes from the atmosphere which gives spherical radiation. Thus there is another 333 “upwards”. So, that means that there is a total “upwards” of 396 + 333…….
Bob Fernley-Jones >> you want to study and learn
Yes, but I have not the time nor desire to follow and absorb every link someone posts. I have to be judicious in how I spend my time. As is, I have a lot more things I would like to be doing unrelated directly to posting comments related to climate science.
You provided a link loaded with links. I read over that page to get an idea, but I am not going to follow all the links of every link someone gives. I am not going to read hundreds of comments on an article. If you have done the reading yourself, you should be able to point out the more important points and lay out your argument in a bit more detail. [Obviously, if I were really into this or saw things from your perspective more, I would not need much prodding at all.]
>> although I find it distressing that you cannot afford the valuable journalistic research of the IPCC corruptness in the downloadable Laframboise digital book at $4.99
I can’t control your level of distress, duh. I was honest about some of my views towards paying for literature because why kid you? There are many great pieces of fiction and non-fiction, and, in another lifetime, I might be able to read them. I don’t make a lot of money and have IMO much more important things — free reading — I still have not found time to read. I haven’t even read the free IPCC WG 1 report (only bits and pieces) or anything but a super-duper, teeny-weeny, tiny fraction of formal papers on this subject [and don’t intend to read except as necessary). I am certainly not caught up on the physics (which I do find much more interesting than office politics or other people’s dirty laundry). I am not passionate about bringing down the IPCC.
To repeat, I am no one’s keeper. These are not my heroes. If any specific individual is proven a fraud, that will lead to a bit of re-evaluation, but it should not be that much. Even if they were honest, the science should speak for itself (though obviously there is much you or I can’t read, understand, or confirm). I don’t work in this industry and am not affected very much at all no matter which way the coin falls. I am affected as any other human might be if the news is bad. I certainly don’t think humans are a negligible force on this planet. I do care about the integrity of science generally. This is one reason why I both want to help improve it if I can and avoid having it be tarnished unnecessarily. Science works by bringing in something that works better.
OK, I will post this comment and then go and read the “Caspar and the Jesus paper”.
[FWIW, I catch some language mistakes after I post. Yes, I review what I write but maybe not as much as I should. And my understanding of (English) grammar only goes so far. The biggest reason my skills fall far short of those of Shakespeare has little to do with English not being my native language. You become skillful in areas where you spend time. I put in enough time so that I can be understood generally and at times even entertain a bit, but I don’t go too far beyond that. This is not a literature forum. A great poem or story is not as important here IMO as a fair dissection of some science related point. Although it might be true that my expectations are misplaced.]
Bob Fernley-Jones, don’t interpret what I just wrote to mean that I think I am always understood, but in the end what someone might understand someone else won’t. There is no science. I think I have shown I am eager to understand and be understood, even if it takes numerous comment exchanges.
Bob Fernley-Jones, ..and I do care about abuse and exploitation, but an unjustified attack on the science is what would really be an abuse and exploitation. I want to make sure we get the science right.
Jose,
Don’t get me wrong, I wish my Italian and nearly forgotten Spanish was half as good as your English.
BTW, congratulations for posting comment #600
Bob Fernley-Jones, that was a neat article. I did not read the whole thing. What I got out of it during this run was that some scientists very possibly overplayed the value of their paper and they probably had some help from people high up in facilitating a path for that paper to make cut offs that should then not have been met. Both of these things (to the extent true) obviously have some repercussions, but in the big picture it “probably” isn’t much of a blow at all to the science (although if it is, then so be it) nor to most people. We mostly all gain from more accurate science.
I liked the statistics related coverage because I want to learn more about some of those basics covered in the article (so I will go back to read with more care and do some cross reading).
I like the fact that a paper that perhaps likely should be put in its place will be so put in time if not immediately and that predictions will probably be adjusted to reflect what we currently know and based on our statistics/probabilities. But in the end little may change if the statistics of that one experiment were a little short of meeting some cut-off. [Maybe my statistics ignorance is showing here.] It appears this is a case where the potential costs to individual careers and/or pride is much higher than to the science. I am not a fan of punishing individuals (you can invoke here the “cast the first stone” and “take the plank out of your eye first” mentality), but I do care about restricting the damage individuals can cause.
Sorry, if there were more serious issues I missed. I did read fast in order to get a taste of what was there. Have thoughts you want to share on this preliminary “summary” I just wrote?
Bob Fernley-Jones, I have not given much of an opinion on the 333; however, a simple diagram can convey useful information while still having many limitations. There is no need to think that a given diagram must present material in a way where certain complex or precise calculations must be achievable.
And it can even have “insider” meaning to the extent it might specify the value of mathematical variables of some model. The values would be useful to someone who knows the details of such a model. [I am not a climatologist and don’t “get” this inside angle that may exist.]
I do like the theme of this last reply of yours. I may think a bit about the math of hemi-planes and may also think more about that 333. Actually, I am still trying to understand atmosphere physics, so the 333 will have to wait. The 396 doesn’t seem so problematic to me at this point in my understanding.
>> Don’t get me wrong, I wish my Italian and nearly forgotten Spanish was half as good as your English.
Don’t be too impressed. I essentially grew up in the US… um, please don’t take that as an attack on the US educational system. 😀
>> BTW, congratulations for posting comment #600
It’s hard to miss key numbers like that when I post 80 comments in a row!
OK, not quite 80.
Speaking of cherry-picking, did I post #500 and #400?? How about #444 or #555????
Bob Fernley-Jones, reading over http://wattsupwiththat.com/2011/10/26/does-the-trenberth-et-al-%e2%80%9cearth%e2%80%99s-energy-budget-diagram%e2%80%9d-contain-a-paradox/#comment-818852 , I could find problems with how I conveyed my thoughts.
For one, the tone might be taken to be aggressive even though I was trying to be more “factual” than complaining in tone (maybe I was a little defensive but mostly just trying to clarify) and was not angry at all.
>> and have IMO much more important things — free reading — I still have not found time to read.
More accurately:
and have IMO much more important things I still have not found time to read (or do), including a great lot of reading which happens to be free.
Finding and fixing the other problems with that comment (if any) will be left as an exercise for the reader.
[ .. have I hit #666 yet?]
Tim Folkerts @ December 2, at 6:14 am
Tim, sorry for the delay.
One of the fundamental oversights in your various hypotheses’ which I’m not going to grind through, is that if we accept the numbers of the great prophet of cartoons, in fig 1, then there are four basic heat transfer happenings from the surface. They are, in his terminology and W/m^2;
Thermals = 17; Evapotranspiration = 80; Surface radiation absorbed by atmosphere = 23; & surface radiation direct to space = 40. (Total = 160)
Thus the portion of thermal activity that you were discussing was only ~14 % of the total. However, you cannot sensibly ignore the other processes which happen interactively.
>> and was not angry at all
Well, a little bit of frustration that goes along with writing almost any “defensive” comment was surely there and an inkling of anger might necessarily follow frustration.
But I can’t remember.
How’s that laptop holding up, Bob?
..Am I at 888 yet?
[OK, sorry, I had trouble resisting this single followup comment. Sorry.]
Bob, I definitely agree that “However, you cannot sensibly ignore the other processes which happen interactively.”
I am not ignoring them per se. I do, however, think that they will be relatively small in most cases. Or more specifically, I think that there will be very little CHANGE in distances on the order of 10 m or 100 m.
Of course, we will not resolve the magnitudes of these numbers just discussing them here. But in any case, this is a very different idea than “most of the IR is sideways rather than upward”. I think interplay among the different sorts of energy transport could be interesting and possibly fruitful to better understand energy flows. I don’t feel the same about your horizontal/vertical arguments.
But apparently we will also not resolve that in this setting either. So it is time to sign off from this part of the discussion, too, and get back to other things.
Myrhh:
I will use “>” to mark off quotes your used from another source and “>>” for what you say.
> The atoms and molecules in the metal of the burner are moving very rapidly because the electrical energy from the wall outlet has increased the thermal energy in the burner.
If you agree with that, why do you object to this:
The atoms and molecules on the earth are moving very rapidly partly because the electromagnetic energy from visible light and from other photons from the sun have increased the thermal energy on the earth.
Why can electricity warm the conductor but visible light (or another form of radiation) can’t warm its own “conductors”? In each case you have a source of energy that gets used up to some degree (battery or sun) as energy flows from source to sink through a medium.
What evidence can you offer that only radiation of a certain wavelength can warm things, in general?
Also, what happens to the energy of the visible light hitting the earth’s surface.. say black surfaces? What about photons above visible?
> When you put your hand over a hot stove you can feel the heat.
It is not saying that only if you can feel it, is it heat. It simply says that if we feel something, there is heat.
> Thermal Energy: A specialized term that refers to the part of the internal energy of a system which is the total present kinetic energy resulting from the random movements of atoms and molecules.
Why do you assume that visible light cannot impart energy to molecules?
Myrrh>> the science fields of optics and thermodynamics.
These are just two approaches optimized to solve a different set of problems.
What does that thermo theory say happens when light strikes a surface? If it doesn’t cover light very well, then the theory is incomplete.
So we have theories that say a lot about “heat” but little about “light”.
We have theories on light which say a lot about light but perhaps don’t say much about “heat”.
And then there are theories that say a fair amount about both “light” and “heat”.
Tim is basing his discussion on some of these last group of theories. Those theories say things the others do not. They fill in blanks left by the other theories.
So, yes, light does more than just “reflect”, “refract”, etc. Yes, “heat” can turn into “light” and vice-versa. There are experiments to support these broader theories. Perhaps we should be talking more about these experiments.
Let me ask this again:
> Lastly, if you go back to the last of the comments I made that you quoted, I provide a link to wikipedia page on attenuation. I then asked how you would explain what happened to that missing energy [from sunlight that is attenuated as it passes through shades]? If molecules get excited with visible light and presumably through that mechanism remove energy from the light shining through it, then what happens to that energy in those excited molecules in that solid?…
What does thermo say about what happens to that energy that excited the molecules as that sunlight was attenuated?
… What do you think about NASA saying that light can turn into heat?
>> don’t be distracted into making it more complicated than it is, even a primary school child should be able to understand the following
Does a child understand the general theory of relativity? Some theories, in order to do a better job explaining phenomenon that simpler theories fail to answer, are more complex.
>> the heat from the Sun in transfer by radiation is heat; this the invisible thermal infrared, which is heat, which is heat energy, which is thermal energy in transfer by radiation, which is why it is called thermal.
Why do you want to exclude visible light and other frequencies from what is capable of transferring heat?
Can you find a single source that says that if we can’t feel something as heat with your hand, then it is not heating us and imparting us with thermal energy, that is, increasing the kinetic energy of our molecules?
Our bodies have different types of sensors, but that doesn’t mean visible light can’t be absorbed and heat things up just because we can’t feel it.
Should I ask again, where does the energy from visible light go when that light passes through sunglasses and comes out with less intensity?
Also, keep in mind that visible light is a very narrow range of frequencies. There is a lot more overall infrared energy than pure visible energy.
Of course, if our biological “heat” sensors aren’t tuned to react with our brain in a coordinated way when pure visible light hits them (let’s assume), then we won’t feel the heat imparted to us.
>> near infrared microscopic, thermal infrared, the invisible heat we can feel, the size of a pin head
Yes, the wavelengths are different, but the question I have is why do you say that visible light cannot be absorbed and heat things, generally?
Why do you appear to say that if we can’t feel it with our skin then it isn’t heat?
What happens when visible light hits a black substance? Where does the light go? Where does the energy go?
If electrons are excited, what happens when they lose their excitation state? Where does the energy go?
Myrhh, this is not a climate science page:
http://hyperphysics.phy-astr.gsu.edu/hbase/mod3.html
>> The different parts of the electromagnetic spectrum have very different effects upon interaction with matter. Starting with low frequency radio waves, the human body is quite transparent. (You can listen to your portable radio inside your home since the waves pass freely through the walls of your house and even through the person beside you!) As you move upward through microwaves and infrared to visible light, you absorb more and more strongly. In the lower ultraviolet range, all the uv from the sun is absorbed in a thin outer layer of your skin. As you move further up into the x-ray region of the spectrum, you become transparent again, because most of the mechanisms for absorption are gone. You then absorb only a small fraction of the radiation, but that absorption involves the more violent ionization events. Each portion of the electromagnetic spectrum has quantum energies appropriate for the excitation of certain types of physical processes. The energy levels for all physical processes at the atomic and molecular levels are quantized, and if there are no available quantized energy levels with spacings which match the quantum energy of the incident radiation, then the material will be transparent to that radiation, and it will pass through.
(cont)
> Visible Light Interactions
> The primary mechanism for the absorption of visible light photons is the elevation of electrons to higher energy levels. There are many available states, so visible light is absorbed strongly. With a strong light source, red light can be transmitted through the hand or a fold of skin, showing that the red end of the spectrum is not absorbed as strongly as the violet end.
> While exposure to visible light causes heating, it does not cause ionization with its risks. You may be heated by the sun through a car windshield, but you will not be sunburned – that is an effect of the higher frequency uv part of sunlight which is blocked by the glass of the windshield.
Did you note that: “visible light causes heating”
This is not “climate science”.
Do you remember what NASA said? Visible light can turn into heat.
Myhrr:
>> So, lots of confusion created by AGWSF to better sell its propaganda, it has reduced the physical world to a one dimensional reality
The following doesn’t sound 1 dimensional to me:
http://www.oar.noaa.gov/climate/t_modeling.html
> How do we model climate?
> Climate models are systems of differential equations derived from the basic laws of physics, fluid motion, and chemistry formulated to be solved on supercomputers. For the solution the planet is covered by a 3-dimensional grid to which the basic equations are applied and evaluated. At each grid point, e.g. for the atmosphere, the motion of the air (winds), heat transfer (thermodynamics), radiation (solar and terrestrial), moisture content (relative humidity) and surface hydrology (precipitation, evaporation, snow melt and runoff) are calculated as well as the interactions of these processes among neighboring points. The computations are stepped forward in time from seasons to centuries depending on the study.
Jose_X @ December 4, at 6:18 pm
Actually, my laptop is becoming painfully slow, in this very long thread, and to make matters worse I’m heading to the mountains for a couple of days, where I may get a poor radio signal or maybe even none at all.
I’m rather surprised that the comment count as given at the head of comments has reached just over 600, you hitting #600, although it has wandered a bit off-topic. Why 888, or did you mean 666?
I hope you can find time to carefully read the Bishop Hill article in full, because there are many important revelations in there. It may be a while before I can respond to your other issues, but I hope to get back to you soon. Meanwhile, I should mention something to Myrrh that he may have overlooked in his long-standing crusade to radically change quantum theory and stuff.
Myrrh,
I see you are still crusading that EMR in the visible, near IR, and UV is not thermal radiation. I take it that you still do not accept that sea water absorbs sunlight mostly by around 100m depth, and that you did not conduct an experiment with IR blocking glass. Etc, etc and etc.
Here anew for your consideration is a graph of the spectrum of sunlight, which follows quite closely a Planck curve as if the sun were a black body:
http://en.wikipedia.org/wiki/File:Solar_Spectrum.png
Notice that the Y axis has the same parameters for ALL wavelengths. Notice also that according to this graph, the far IR comprises a rather small proportion of the depicted energy output from from the the sun.
Are you able to explain why you think that this Planck curve is wrong, and at what wavelength is the boundary between thermal EMR, and some other undescribed form of EMR in the same units, that you allege is not thermal?
Tom Folkerts, does that Utube experiment infer that the entire infra red spectrum is absorbed by CO2? This cannot be true, particularly of the 5 to 12 micron waveband.
* This simple experiment disagrees: http://www.youtube.com/watch?v=Ot5n9m4whaw
Robert
The video doesn’t say what wavelengths of IR the camera picks up. Presumably it is tuned to pick up only (or at least primarily) in a band that is absorbed by CO2.
Tom Folkerts says – ‘ Presumably it is tuned to pick up only (or at least primarily) in a band that is absorbed by CO2.’
In other words it is ‘cherry-picked’ for the AGW cause; it is far more potent than the hockey stick!
Robert
One man’s “cherry-pick” is another man’s “highlight”. Since I see no intent to deceive, I have no problem with them showing that CO2 does indeed block some of the IR, which is exactly what is claimed by scientists when explaining the “greenhouse effect”. I really don’t expect a 2 minute video clip for the general public to show any greater detail than this.
The “hockey stick”, however, does bother me a bit. It appears that they selected data not based only on how good the data was, but also partly on how well it supported their hypothesis. That is poor practice in anyone’s book (other than a defense attorney’s).
Tom Folkerts says – “One man’s “cherry-pick” is another man’s “highlight”. Since I see no intent to deceive, I have no problem with them showing that CO2 does indeed block some of the IR, which is exactly what is claimed by scientists when explaining the “greenhouse effect”.
A greenhouse blocks all of the IR which is not the same thing. That camera in the video clip should be tuned to the wavelengths that CO2 does not block to give a balanced view. I learnt this when I was first studying physics at 14. So I could never accept the “greenhouse effect” as presented by the warmists. Most folks watching that clip believe that all IR is absorbed by CO2 .
Robert Stevenson,
That youtube experiment was not “natural” unfortunately. It was created for demo purposes of CO2 absorbing potential. The CCD used to view the image was not at a realistic frequency and computer enhancement was rather tuned to that narrow range. It may have been partly to oversell global warming, maybe, I don’t know. It may have been partly as many science demos are for young students, to attract them to science. However, the title is rather straightforward and shows that effect. One can assume it is not a regular camera.
I had come across a page on the web that specified those details, but I can no longer find that same discussion.
Robert.. an update. The page doesn’t specifically mention computer manipulation (maybe I just assumed they used computers to do digital filtering), but you can read that they use filters and focus on a tight range in order to show the effect. http://www.creative-science.org.uk/hollywood15.html .
Jose_X, – So reading between the lines you seem to agree with me on this point then Jose. Incidentally are you going for the world blogging record; can’t be far looking at the above posts.
Jose_X, says:
Robert Stevenson,
That youtube experiment was not “natural” unfortunately.
Viewing the clip for a second time I must say it is a charlatan’s trick to inflict that experiment on schoolboys. They’ll all go home and ‘lecture’ their parents about the evils of carbon dioxide and catastrophic, hyperfeedback, ice-cap melting, man-made, global warming; becoming distorted scientific thinkers at an early age. Unless they see through it of course.
Robert >> So reading between the lines you seem to agree with me
Yes, I think it overplays the effect. Doing so is an significant issue depending on context. Backers will say that it is better to be safe than sorry, that it’s a partially inaccurate presentation of an accurate effect intended to warn of a believed important global issue. The planet is not candle hot, surely, but a few degrees of warming can be very serious (the latter is currently accepted science regardless of what non-experts think).
I don’t like seeing inaccuracies. You can for a very long time have misconception about physical effects because of being mislead by an experiment.
I think the key is to explain how the experiment was done without overselling it (ie, this is a show of x effect, period). This would include toning down the dialog in a couple of places.
I’m not appalled by it, but, especially in the current environment, there might be many who would be angered.
BTW, my objection is at the very beginning and later on where it is suggested that the actual atmosphere blocks a great deal of radiation that perhaps it does not. However, I agree with Tim, that you can only get so precise in such a short demo. The page I linked to can serve as the needed disclosure.
So the CO2 absorbed in the 4 micro range not the 15 micro range. That is roughly 750 K. Bad experiment.
mkelly, I think you are generalizing too much based on peak wavelength notions. The radiation at 300K is very broad. What was chosen could have been at the other end of the spectrum in a different part of CO2 absorption band (implying something much colder than average daily temperatures). The chosen focal point was done based on available technology. It needed to be sharp so that the effect would stand out. In many ways, the actual narrow slice is arbitrary, and the experiment wouldn’t be too much better or worse if chosen elsewhere.
Jose_X, I went to the link you provided. The experiment does not show that Co2 absorbs in the 15 micro range to show the effects of AGW . It is an exaggerated experiment, so bad.
Mkelly & Jose
This wonderful “experiment” reminds me of OVE HOEGH-GULDBERG from University Queensland, an extreme activist prophesying the death of the Great Barrier Reef. He appeared on a Richard Attenborough BBC Movie where he demonstrated that by blowing air from his lungs through a tube into a container of seawater, the alkalinity dropped slightly. THERE! Death by acidification! What a fruitcake! I wouldn’t mind his job though!
Then in that recent Al Gorathon, Anthony Watts showed that in the “experiment” heating a jar with CO2 in it, that it was done with naughty video techniques
The Utube experiment is typical of the global warming bias on BBC TV, a public broadcaster funded by a licence fee payable (£145 pa) by every household in the UK. The BBC is a politically driven organisation with a strong left wing bias; for instance a play commissioned by the BBC entitled ‘The Falklands Play’ was denied a prime time slot because it was allegedly deemed too sypathetic to the former prime minister Mrs Thatcher.
The experiment was akin to the apalling film made by Al Gore; I watched that film on TV and was amazed by just how blatantly flawed it was, not one bit of it rang true.
etc….
Robert, Jose & mkelly
What annoys me about these crap experiments is that some people are impressed by them, and it is intended to scare the pants off them, what with gross exaggeration, and even irrelevance.
Furthermore, the silly thing is that sceptics mostly agree that there is global warming, (well, up to about a decade ago), but that there is no empirical evidence that CO2 is a significant factor in it, or that it is unprecedented. Many influential people still believe or claim that the hockey-stick proves that there was no MWP, despite overwhelming evidence to the contrary, and proof that the scientists involved were & are, very naughty indeed.
Coming back to that Youtube experiment, it was only 1 minute long so I watched it. One thing that puzzled me was that the candle, with a colour temperature of over 1,000 C, emits a lot of visible light, yet the CO2 was demonstrated to extinguish that light. Doesn’t the greenhouse theory depend on CO2 not absorbing visible light?
Tim Folkerts,
I think you made some assumptions about the Youtube experiment you cited.
I’ve just watched that video again, and ’tis strange but the infrared camera monitors the visible light from the candle fine, until the CO2 is pumped in!
Bob Fernley-Jones says:
‘Coming back to that Youtube experiment, it was only 1 minute long so I watched it. One thing that puzzled me was that the candle, with a colour temperature of over 1,000 C, emits a lot of visible light, yet the CO2 was demonstrated to extinguish that light. Doesn’t the greenhouse theory depend on CO2 not absorbing visible light?’
Bob, you are quite correct a greenhouse lets in most of the sun’s spectrum but blocks all of the LWIR. The atmosphere of course doesn’t do that, it blocks only in certain wavelengths, CO2 for instance doesn’t absorb in the 5 to 12 micron band, and it certainly doesn’t absorb in the visible range 0.38 to 0.76 microns. In fact standing outside of a greenhouse you would still see the candle flame but not get any heat from it. Well spotted its more of a charlatan trick than I thought!
People, maybe I am misunderstanding the last few comments above, but did you read the link I provided to the page that explains details about that experiment? The only thing happening is that a camera initially shows the image it picks up in the 4 micro-m range. When you add the CO2, most of that reception disappears. There is no visible light issue anywhere. The experiment demonstrates the effect of CO2 absorbing in that 4 micro range.
The problem with the video (as clarified below) is arguably suggests that CO2 traps a very wide range of radiation (when it does not). This flaw is made explicit when the host broadly states at the end that the candle “warmth” was trapped rather than state that the experiment specifically only covered a very narrow slice of the full spectrum and that the atmosphere covers much more but still much less than the full spectrum of the candle or of any body.
This is the rough transcript:
“I can show you how carbon dioxide affects us(?) climate using this heat sensitive of(?) infrared camera which is pouring away here, a candle, this glass tube which is hooked up to this (otherlouse?) canister of carbon dioxide gas. Now, if I light the candle you’ll see that on the monitor the camera picks up the flame perfectly. Look at that — the hot spots of glowing white. Now watch what happens when I turn on the carbon dioxide. Just keep your eye on the flame. The gas is invisible so you don’t see it filling the tube, but, as it comes in, you should see the candle itself disappear. There it goes. Look at that! What’s happening is that the carbon dioxide in the tube is effectively trapping the heat. The candle’s warmth no longer reaches the camera. ..instead it is absorbed by the carbon dioxide inside the tube.”
The first sentence says the experiment shows us how carbon dioxide affects the climate. This is not true 100%. That is, the experiment does not replicate the climate in every way and to proper degrees; however, it does show that CO2 can absorb heat, and current accepted science (by almost all experts in the field) states that this absorption has an effect in warming the climate.
That sentence also specifically states we are talking about an infrared camera.
The next sentence is basically correct although we can argue that no camera “picks up” anything “perfectly”.
“The hot spots of glowing white” is a descriptive phrase perhaps accurately describing the white on the monitor which refers to hotter parts of the flame.
“The gas is invisible” is essentially correct (to the naked human eye).
“You should see the candle itself disappear” likely refers to the image of the candle being shown to the audience, and it does largely disappear presumably as the CO2 fills the tube.
“What’s happening is that the carbon dioxide in the tube is effectively trapping the heat” and “instead it is absorbed by the carbon dioxide inside the tube”: “Effectively trapping the heat” sounds reasonably accurate to me, as the amount of radiation being picked up by the camera has apparently gone way down.
“The candle’s warmth no longer reaches the camera.” This is only true when referring to the 4 micro-m section. That statement and the introduction appear to refer to a much broader range of infrared being in play. That part is clearly misleading.
Yes, as you guys have said, there is the additional point that the particular band chosen to show off the effect is not the relevant one for the greenhouse effect.
However, the effect is already hacked to a narrow band, and picking one slice over another is not a major issue (at this point). It may call into question if in fact absorption works the same when you change bands, but science agrees, I think, that the essential physics doesn’t change when you change frequencies. I don’t know why the right peek wasn’t chosen. I assume the equipment was chosen to keep costs down since I think this is expected to be a demo for teachers to use in their science classes.
Bob & Robert,
You both seem to be mistaken about the camera being used. What difference does it make that the candle emits visible light in addition to IR??? The hot flame will emit both visible light and IR from roughly the same hot areas, so it would be about the same size & shape whether viewed with IR or visible light.
The camera is clearly an IR camera, and in fact the link Jose gave earlier discussed that the camera was indeed tuned to ~ 4 um IR so that the CO2 effect would be strongly noticeable. The fact that the candle also creates light of 0.5 um or 10 um photons is immaterial to this experiment. I consider constructing an experiment to show a specific feature of nature while eliminating extraneous details to be a standard, time-honored approach in science. Robert apparently thinks only charlatans would do such a thing. My only objection to the video is that, to be a little more accurate, a few times they should have said “some”. (eg “some of the IR is absorbed by the CO2”) to be a little more accurate. OTOH, I don’t expect a 2 minute TV demo to provide all the details.
If Robert wants to be that picky, then I could get picky about what he said.
>A greenhouse blocks all of the IR
No. Only some. Both CO2 and glass block SOME IR. Both Robert & the video gloss over this; Robert is just more emphatic about being inaccurate.
>a greenhouse lets in most of the sun’s spectrum but blocks all of the LWIR
Much better. NIR & SWIR get thru very well. MWIR gets thru somewhat. LWIR (8-15 um according to standard definitions) is almost entirely blocked by glass as claimed.
>you would still see the candle flame but not get any heat from it
Myrrh’s objections not withstanding, any wavelength of light transfers energy and hence “heat”. So the visible light would give you “heat”. The NIR & SWIR & MWIR would also certainly give you “heat” through the glass and could be picked up by cameras sensitive to these wavelengths.
~~~~~~~~~~~~~~~
These details can be important if you are trying to do calculations, but for a general discussion it is good enough to know that both glass and CO2 block some IR while letting thru essentially all visible light, and that this fact helps explain the observed warming. (PS Of course, in a real greenhouse, the blocking of air flow is typically more important than the blocking of IR)
Well it looked a bit fishy to me. My wife looked it and said obviously the CO2 had extinguished the flame.
correction…… looked at it…..
Re: Candle flame.
I agree with Robert; Well it looked a bit fishy to me.
The image of the flame monitored by the infrared camera looked rather similar to what I’ve just checked indoors in both bright and dim daylight with my own candle and a long matchstick. I always thought that infrared photography gave different colours and contrasts to what can be seen in natural light. (As is employed in many galleries of such art photography).
Bob says: “I always thought that infrared photography gave different colours and contrasts to what can be seen in natural light. (As is employed in many galleries of such art photography).”
There are two very different sorts of IR imaging. The “traditional” version used “near IR” just beyond red (~ 0.7 – 1 um). This sort of IR is typically reflected IR from the sun that passes thru most glass easily (and is sometimes referred to as “reflected IR”). So a standard camera with film sensitive to near IR can pick it up, creating an image that looks sort of like black and white, but with odd contrasts. Furthermore, many digital cameras can pick this up (the CCD is sensitive to wavelengths beyond 0.7 um) so they can “see” remote controls that operate in this range.
The the other sort of imaging uses longer wavelengths of “thermal IR” that are produced by the heat of objects. This requires a more specialized camera (like the one in the video) that does not respond to visible light.
Google images of “infrared images” and you will get both types of images and you can generally tell the two apart quite easily.
Jose,
OK, this has apparently become an open thread after over 600 comments. (phew!)
You seem to have declared that exaggeration in AGW stuff is OK, providing that it is taken in context for appropriate observers, whoever they may be. However, I feel that when such crap is aimed at gullible people, especially including the young, that it is inexcusable.
AGW exaggeration often involves the deliberate deletion or omission of “inconvenient data”, such as in “hide the dendro-decline”, (= the divergence problem), but let me give another example:
The young glaciologist; Jason Box, was a co-author for IPCC AR4 WG1 Chapter 4.6.2.2.1 concerning the popular alleged disastrous melting of the Greenland icesheet:
http://www.ipcc.ch/publications_and_data/ar4/wg1/en/ch4s4-6-2-2.html
Yet, his earlier joint authorship of a paper suggested that Greenland air temperatures around 1930 – 1940, were on average higher, especially in summer, versus at the time of AR4. (IPCC 2007 report). I think he attributed the ~60-year cycle of the PDO as a significant correlation. However such inconvenient data had NO MENTION in that chapter.
OK, maybe rather than following many other IPCC contributing authors, perhaps he did not want to spruke his own paper. However, there were some later papers that were more definitive and confirmative, that could well have been employed into the report had the IPCC wanted them. (That is going by their acceptance of other post-cut-off, post-final-draft inclusions, when it suited the IPCC ideology and their UN charter to confirm CAGW)
Plugging 1000 C into my Planck/Wien’s Law spreadsheet I do indeed observe that the bulk of the emitted IR is in the 2.3 to 3.0 micron and 4.0 to 4.8 micron ranges with wery little in 15 micron range as Jose pointed out vis a vis the U tube experiment. The graphical printout confirms this and gives the peak intensity at 2.3 microns.
So tuning your IR camera to these wavelegths would give you the disappearing conjuring trick candle flame. However integrating the areas under Planck’s curve gives a me a relatively small percentage of the total emissive power that is absorbed by CO2.
I would re-do the experiment to reflect this fact. Clearly this would make people more sceptical
concerning the catastrophic etc as I have found when I explain this simple fact in discussions.
Jose_X says:
December 4, 2011 at 9:04 pm
What evidence can you offer that only radiation of a certain wavelength can warm things, in general?
Jose, I’m sorry, I don’t have the time to got through this further with you as you’re still getting distracted from the basics – I’ve given you the mechanism to your question above, the difference between electronic transitions and atomic/molecular vibrational:
I can see that you have a great facility for thinking about all this, from the range and volume of your questions, but until you have a thorough grasp of this basic difference you will continue to fall into the traps of terms distorting meaning, for example, “absorbed” means different things according to context. Visible light’s energy is not absorbed by the molecules of water, water is a tranparent medium for visible light, bullet point 3 on the list on that page, so that “absorbed” in ‘visible light is absorbed by the ocean because it disappears at different levels’ can’t be referring to the same meaning of “absorbed” in the difference between absorption and transmission of visible light in the fluid gaseous atmosphere above us, where it is briefly absorbed by the electrons of the molecules of oxygen and nitrogen before being reflected/scattered out, bullet point 2, and not being absorbed on electronic transition level by the water molecules, because it is transmitted through without being absorbed, bullet point 3. Neither of these are using the energy to heat up the molecules, which takes greater energy than they possess, to move whole atoms and molecules into vibration, which is heat, thermal energy.
Now, which bullet point on that list describes what happens to visible light which is neither reflected nor transmitted on encountering matter but its energy absorbed as chemical energy in photosynthesis? This too does not create heat, it creates sugars.
“Absorbed” does not automatically mean that energy creates heat.
That however is the claim of the AGWSF energy budget, KT97 and ilk, which is how they say visible light, the shortwaves, directly convert land and ocean to heat, directly heat up land and ocean, and, they say that the Sun’s actual thermal energy doesn’t play any part in this, they say it doesn’t even reach the surface. But we know in traditional physics that the heat we feel from the Sun, its thermal energy on the move to us via radiation (contrasting with heat transfer by conduction and convection) is the invisible thermal infrared.
So, we know, in traditional physics and not the science fiction physics of AGW promotion, that visible light’s energy works on electronic transition level, this energy utilised differently depending on the physical circumstances, as plants utilising it for creation of sugars or electrons of molecules scattering and reflecting it as in the atmosphere, and we know that it takes a bigger more powerful energy to move the whole molecule into vibration which is what heat, the invisible thermal infrared wavelength which is thermal energy, does. That is how water molecules are heated, by heat, when the whole molecule absorbs the invisible thermal infrared heat energy from the Sun the water in our ocean is heated. Visible light on electronic transition level doesn’t have the power to do this, its power is so miniscule that it doesn’t even get to be absorbed by the electrons of water molecules, but is transmitted through, transmitted being the correct technical term for this process.
Now, if you’d just concentrate on this, the very basic real physical differences between visible wavelengths, and the short of the AGWSF energy budget, and thermal infrared, you’d see why I keep asking for proof that “visible and shortwave solar directly heat land and oceans” – as in the claim in the cartoon above.
Until you deal with my question, the question I’m actually asking here, you will continue to get distracted by questions that you will be able to answer yourself when you fully grasp what this difference is.
Have a go. Prove the claim in the cartoon, prove that visible light heats land and oceans.
Unless you can do so, and you are actively defending that claim so it is up to you to prove it when I’ve asked, I see your agility of mind as merely avoiding the challenge.
I have shown you from traditional physics why it can’t.
Tom Folkerts says:
I am simply saying that “evaporative cooling” is synonymous with the 80 W/m^2 of energy leaving the surface. The oceans are the ‘wet t-shirt around the earth’.
The oceans also absorb/dissolve an awful lot of CO2 far more than one would expect from Henry’s Law constant.
CO2- Water Solubility
Henry’s-law constant for CO2-water solutions is 1.42×10^3 at 20 C . At equilibrium the oceans therefore should hold only 30% of the 2,900 giga tonnes of the atmospheric CO2 if simple Henry’s-law solubilities were applied not 50 times which is the actual figure.
Myrrh, you have not shown me “from traditional physics why it can’t”. Traditional physics contradicts your viewpoint.
For starters, you keep ignoring what nasa and other respectable websites have said directly, that sunlight can be turned into heat naturally. You have not explained the “blackbody” effect as observed non-ideally in all sorts of objects and as taught by virtually any university. Here, I’ll add another quote from a harvard webpage, https://www.cfa.harvard.edu/~jbattat/a35/blackbody_color.html , “[i]f we had infrared goggles, we would be able to see objects at room temperature by their emitted light.”
You tried to use a thermo website, but it never said anything about visible light not being turned into IR or “heat”. In fact, thermodynamics supports the view that many and/or most natural processes generate heat.
From http://en.wikipedia.org/wiki/Irreversible_process
> All complex natural processes are irreversible… A certain amount of “transformation energy” will be used as the molecules of the “working body” do work on each other when they change from one state to another. ..During this transformation, there will be a certain amount of heat energy loss or dissipation due to intermolecular friction and collisions; energy that will not be recoverable if the process is reversed.
The quotes you took from various wikipedia pages also fail to claim or iirc even suggest that visible light cannot be turned into IR.
Q1 — Let me ask a question, what do you theorize happens to the vast quantity of the sunlight absorbed by objects on earth?
[This is an extension of the sunglasses example you haven’t addressed yet.]
According to the very Trasparency and Translucency wikipedia page you used for quotes in your recent comment most sunlight appears to be absorbed:
> When light strikes an object, it usually has not just a single frequency (or wavelength) but many. Objects have a tendency to selectively absorb, reflect or transmit light of certain frequencies. That is, one object might reflect green light while absorbing all other frequencies of visible light. Another object might selectively transmit blue light while absorbing all other frequencies of visible light.
Q2 — I have another minor question, were you suggesting that plants can absorb sunlight and store that energy without generating “heat”? Wouldn’t that violate the second law of thermodynamics?
From the same http://en.wikipedia.org/wiki/Irreversible_process
> Many biological processes that were once thought to be reversible have been found to actually be a pairing of two irreversible processes. Whereas a single enzyme was once believed to catalyze both the forward and reverse chemical changes, research has found that two separate enzymes of similar structure are typically needed to perform what results in a pair of thermodynamically irreversible processes.
Q3 — ..and I’ll repeat, why do you keep ignoring what most universities are teaching (eg, blackbody)?
These are all well established multi-disciplinary physics theories, not “climate science” theories.
Bob, didn’t Tim just show that you are not infinitely precise either? Has it ever been in doubt that I (as likely most people) believe that being imprecise and not knowing everything is a fact of life? I said that I think the demo presentation should be adjusted and that the disclosure page should be clearly marked (a part of doing experiments/demos in connection with science is that you reveal a fair amount of your “secrets”).
Also, it is you that is going against established science in denying man has a non-negligible effect on the environment a la “global warming”. Pointing to a few potential mistakes here or there in a ton of information doesn’t prove you right or me negligent for accepting the science. I clearly don’t accept unconditionally, but the volume of the evidence is not on your side. To take a point I conditionally accepted, that one of Mann’s paper should not have received the value that it got, that doesn’t prove anything against climate science except that a few data points and one study are under question.
It seems you are criticizing aspects of climate science. That is good and healthy.
Bob>> Chapter 4.6.2.2.1 concerning the popular alleged disastrous melting of the Greenland icesheet
The conclusion from that section on Greenland:
> Assessment of the data and techniques suggests a mass balance for the Greenland Ice Sheet ranging between growth of 25 Gt yr–1 and shrinkage of 60 Gt yr–1 for 1961 to 2003, shrinkage of 50 to 100 Gt yr–1 for 1993 to 2003 and shrinkage at even higher rates between 2003 and 2005. Lack of agreement between techniques and the small number of estimates preclude assignment of statistically rigorous error bounds. Interannual variability is very large, driven mainly by variability in summer melting, but also by sudden glacier accelerations (Rignot and Kanagaratnam, 2006). Consequently, the short time interval covered by instrumental data is of concern in separating fluctuations from trends.
Basically, it says the jury is still out but enough evidence apparently supports that the rate of ice melting in certain parts (Greenland Ice Sheet) has been growing.
>> However, there were some later papers that were more definitive and confirmative, that could well have been employed into the report had the IPCC wanted them. (That is going by their acceptance of other post-cut-off, post-final-draft inclusions, when it suited the IPCC ideology and their UN charter to confirm CAGW)
You might be right that the Greenland section may have been a section where the draw of the papers submitted and accepted on time could have affected the conclusions. [I would entertain links if you have them.] I do note that a number of quoted studies were rather recent (post 2005 range).
As concerns global warming, the main claims are about global averages. Locally, any number of atmosphere/land/water variations (plus overall global cycling and plain old statistical anomalies) might lead to some parts cooling over that time. It’s easier to get the little details wrong over 100 year “forecasts” than the main trends.
>> Greenland air temperatures around 1930 – 1940, were on average higher.. I think he attributed the ~60-year cycle of the PDO as a significant correlation. However such inconvenient data had NO MENTION in that chapter.
What do you mean? If there is correlation with a cycle, that would support the view of not over-emphasizing the air temps then. The ice melts over a prolonged period is much more significant especially given how water absorbs much more heat than air.
Of course, did you realize the title of that section?
“4.6.2.2 Measured Balance of the Ice Sheets and Ice Shelves” is not about air temperatures. It is more than reasonable to neglect discussion of air temperatures.
Myrrh, sorry I have misspelt your name (it seems some of the time I spell it myrhh).
Q: How do you justify how we use electrical energy to heat up an oven very hot? The heat comes from a glorified electrical battery.
Transducers are all around us. Energy is always changing forms. [We eat apple created via sunlight and use that converted energy to chop down the tree for extra warmth.]
If energy can so readily change forms, why is it so hard to accept that excited electron states in molecules (because of visible light and other non-IR radiation) can be turned into IR radiation?
Maybe I’ll research the quantum mechanical description of just this process — widely used physics, not merely “climate science”.
Jose_X,
Without commenting on the accuracy of Myrrh’s statements, let me just say that a few others have already tried reasoning with Myrrh in other threads, presenting many of the same sort of arguments you have given. So far, no one has been able to convince him that he has made any significant errors in logic or science.
Jose_X @ December 8, at 9:24 am
Sorry Jose, I don’t have the links, but if you look around for Box, Chylek, Polyakov, or Lesins, + Greenland, you should find something.
I take exception to this:
Here is one of Jason Box’s co-authored papers, where in the extract, they seem to think that it’s all about air temperatures being a consideration, and pointing to the big warming in 1920, which apparently Box and other IPCC authors somehow omitted:
Global Warming and the Greenland Ice Sheet by Chylek P.1; Box J.E.2; Lesins G.3 2004
http://www.ingentaconnect.com/content/klu/clim/2004/00000063/F0020001/05140445
Jose_X says:
December 8, 2011 at 9:09 am
Myrrh, you have not shown me “from traditional physics why it can’t”. Traditional physics contradicts your viewpoint.
No it doesn’t. I’ve given you traditional physics, the difference between Heat and Light.
For starters, you keep ignoring what nasa and other respectable websites have said directly, that sunlight can be turned into heat naturally.
And I have warned of the infiltration of this junk science into the education system. What NASA used to teach was that the heat we feel from the Sun was the invisible thermal infrared, now it teaches that thermal infrared doesn’t even reach the surface of the Earth.. Do you see the disjunct here? Which is why I keep trying to get you to concentrate on the very basics, the difference between Heat and Light.
You have not explained the “blackbody” effect as observed non-ideally in all sorts of objects and as taught by virtually any university. Here, I’ll add another quote from a harvard webpage, https://www.cfa.harvard.edu/~jbattat/a35/blackbody_color.html , “[i]f we had infrared goggles, we would be able to see objects at room temperature by their emitted light.”
If you keep being distracted before you have a firm grasp of the basics then you’ll continue to be confused.
You tried to use a thermo website, but it never said anything about visible light not being turned into IR or “heat”.
It didn’t have to… 🙂 I began, by giving you traditional science about Heat. Until and unless you understand that how will you understand the difference between Heat and Light?
In fact, thermodynamics supports the view that many and/or most natural processes generate heat.
From http://en.wikipedia.org/wiki/Irreversible_process
> All complex natural processes are irreversible… A certain amount of “transformation energy” will be used as the molecules of the “working body” do work on each other when they change from one state to another. ..During this transformation, there will be a certain amount of heat energy loss or dissipation due to intermolecular friction and collisions; energy that will not be recoverable if the process is reversed.
The quotes you took from various wikipedia pages also fail to claim or iirc even suggest that visible light cannot be turned into IR.
It didn’t have to say that, it was describing Light… 🙂 What I gave was the basic mechanisms of what happens to visible, shortwave, on meeting matter, electronic transitions. If you had bothered to understand what was being said about Heat and that it takes vibrational energy to move molecules to this, you would have a grasp of the difference by now between that and electronic transitions, get some scale perspective here. The difference in size between near infrared, not hot, and thermal infrared is between microscopic and pin head size, visible light is even smaller than near infrared. You’re still not concentrating.., you’re not bothering to give this any thought as in ‘getting a feel’ for it, but instead without understanding what I’ve said already you continue to confuse yourself by bringing in irrelevancies or questions that you could answer yourself if you’d bothered to spend time digesting what traditional physics says of the differences.
Q1 — Let me ask a question, what do you theorize happens to the vast quantity of the sunlight absorbed by objects on earth?
[This is an extension of the sunglasses example you haven’t addressed yet.]
According to the very Trasparency and Translucency wikipedia page you used for quotes in your recent comment most sunlight appears to be absorbed:
> When light strikes an object, it usually has not just a single frequency (or wavelength) but many. Objects have a tendency to selectively absorb, reflect or transmit light of certain frequencies. That is, one object might reflect green light while absorbing all other frequencies of visible light. Another object might selectively transmit blue light while absorbing all other frequencies of visible light.
I don’t have to theorize what happens to the vast quantity of the sunlight absorbed by objects on earth, we know what happens, light enables us to see the world via reflection, pigmentation, and is utilised for photosynthesis. Those four bullet points show what is actually happening in the processes of Light contacting matter, and note, not all absorbed as the third bullet points out, transmission means that light is not absorbed, as in light travelling through a transparent medium, water. Bullet four covers photosynthesis, some of the light is absorbed for chemical changes and some reflected back out, which takes back to bullet 2.
Q2 — I have another minor question, were you suggesting that plants can absorb sunlight and store that energy without generating “heat”? Wouldn’t that violate the second law of thermodynamics?
Plants are not storing that energy, it is being utilised as chemical energy to produce sugars, not to produce heat. The laws of thermodynamics have nothing to do with this, light isn’t hot…
Q3 — ..and I’ll repeat, why do you keep ignoring what most universities are teaching (eg, blackbody)?
These are all well established multi-disciplinary physics theories, not “climate science” theories.
Irrelevant. I’m talking about the difference between real Light and real Heat..
Bringing this into the ‘explanations’ for the AGWSF energy budget is a distraction from real physics in these arguments, by eliminating the real differences between the different wavelengths, claiming that ‘all energy is the same and all create heat’, etc. Get back to the basic differences between wavelengths, their different properties relative to each other such as size, character, etc., the different processes, such as the difference between electronic transitions on an electron scale and thermal vibration on an atomic/molecular. Then, for example, you could move on to looking at the differences between these in the ionising and non-ionising categories, such as UV has both. Sets are useful tools here, as you’ve given above, http://hyperphysics.phy-astr.gsu.edu/hbase/mod3.html, different wavelengths do different things because they are different.
The category I’m covering is the difference between Heat and Light. Because, the claim from the AGWSF junk energy budget says that visible light directly heats land and oceans, and this is physically impossible because of visible light’s properties and processes.
Now, unless you or anyone else can prove that visible light direct from the Sun can actually heat land and oceans, you shouldn’t be using that cartoon as the basis for this real world’s energy budget.
Tim Folkerts says:
December 8, 2011 at 12:48 pm
Jose_X,
Without commenting on the accuracy of Myrrh’s statements, let me just say that a few others have already tried reasoning with Myrrh in other threads, presenting many of the same sort of arguments you have given. So far, no one has been able to convince him that he has made any significant errors in logic or science.
🙂 No more argument from you then..
Jose_X – here’s a recent reply from me to one such still trying to convince me that I’ve significantly erred in logic or science:
http://wattsupwiththat.com/2011/12/02/foia-is-not-enough-why-not-legally-mandate-transparency-in-climate-research-a-modest-proposal/#comment-822762
Bob >> “Here is one of Jason Box’s co-authored papers”
Abstract > This suggests that the Greenland ice sheet and coastal regions are not following the current global warming trend.
Interesting. I wonder how knowledgeable the author was on the Greenland ice sheet back in 2004. I hear that ice melting is one of those areas climatologists apparently have been underestimating. Maybe there are more ideas and measurements to shake out in the upcoming years in this area.
This one paper’s possible surmising doesn’t suggest to me that a section of the report on ice sheets should include a discussion on surface temperatures, especially if this issue were to have been unsettled (for example, perhaps there has been genuine confusion among researchers over surface temperatures possibly not correlating well with ice sheet melt). If unsettled, they might not mention it and keep the discussion to what they are most sure about. And, of course, this author could simply have been wrong in the paper on that point.
Personally, if that view were to have been representative (air temp being linked to ice melt), I would think they would want to mention it. To judge better, we would have to look at a representative selection of relevant papers and then armchair quarterback.
I can’t see foul play or suspect biased judgement on so little info, but it’s an interesting observation nevertheless.
Myrrh,
Here’s another one for you. It was a warm day yesterday ~30C, and I popped up to the shops without footwear. Big mistake: I had to negotiate some tarmac (blacktop), and it was painfully hot, yet strangely, paler pavements in the sun were OK. Now I wonder why that should be! No need to reply this time either, I’m just having fun.
Mods – I posted a reply to Jose_X before I posted to Tim, it hasn’t appeared.
[Reply: I checked the spam folder. Sorry, it’s not there. Please re-post. ~dbs, mod.]
Jose_X @ December 8, 9:24 am
Actually, I feel that the biggest problem in so-called climate science is exaggeration by the alarmist “scientists” commonly by exclusion of “inconvenient” data, and more importantly the political roll-on from there.
The MBH 99 hockey-stick was the charging white stallion in the 3AR (2001) IPCC report with six (or seven?) prominent displays within the report and even larger wall versions behind subsequent rostra.
If I remember correctly, in the 4AR 2007 report, the IPCC wisely buried MBH 99 amongst I think it was eleven spaghetti graphs all purporting to verify MBH 99. There were a large number of “scientists” involved. This is the junk that is used to scare policymakers etc, that 20 th Century had unprecedented warming.
Incidentally, in addition to hiding the decline of tree-ring correlation with instrumental temperatures since around 1940 or 1960 there are a bunch of other naughties in all these spaghetti graphs. One of the worst is probably what caught the eye of Severinghaus, before he knew about hide the decline, that there is a strange flattening of the curves in about the last 15 or 20 years. In fact, there is another cheat here in that in a 30-year CPA smoothing as is required for time-series smoothing, the smoothed line should stop 15 years short of the end raw data for lack of 15 years of future data beyond the centre-point. No worries, these “scientists” simply add non-existent data/ fiddle, (that I could elaborate), to show what they want.
I don’t want to overload you with references, but perhaps after you have finished and digested the Casper and Jesus paper stuff, you could get some more context here:
http://wattsupwiththat.com/2011/12/05/tim-barnett-on-the-hockey-stick-statistics-were-suspectthe-rest-of-the-team-knew-of-problems-with-manns-reconstruction/
CLARIFICATION: I’m not saying above that 30-year smoothing as in the IPCC 2007 spaghetti graphs is a requirement, and in fact MBH 99 employed 40-year smoothing. The issue is that a Centre Point Average, (CPA), by definition must end at before the end of the raw data at half the span of the smoothing time-span that is arbitrarily selected.
Myrrh says:
December 8, 2011 at 5:31 pm
Mods – I posted a reply to Jose_X before I posted to Tim, it hasn’t appeared.
[Reply: I checked the spam folder. Sorry, it’s not there. Please re-post. ~dbs, mod.]
Ah, it seems to have appeared now. Thanks.
Bob Fernley-Jones says:
December 8, 2011 at 5:07 pm
Myrrh,
Here’s another one for you. It was a warm day yesterday ~30C, and I popped up to the shops without footwear. Big mistake: I had to negotiate some tarmac (blacktop), and it was painfully hot, yet strangely, paler pavements in the sun were OK. Now I wonder why that should be! No need to reply this time either, I’m just having fun.
Reminds me of the difference between sands, Cancun’s I recall was cool underfoot..
Myrrh @ December 8, 8:24 pm
I seem to remember that they had record low temperatures in the Cancun fest. Rather disappointing to all and sundry
Myrrh, this is starting to look like a real journey of discovery. Please, join me. I need your specific input in order to conquer this jungle efficiently. After the upcoming intro/rant, I get right back to asking questions I’ll need.
>> If you keep being distracted before you have a firm grasp of the basics then you’ll continue to be confused.
You aren’t making sense. You don’t offer any proof about what you claim is real science. You simply state that visible light can’t turn into “heat”. Then when I point out that the wider world out there doesn’t agree with you, you pretend they are all wrong. Yet, you get back to repeating that it is you who is teaching me real science (even though it disagrees with what most scientists apparently believe).
What proof do you have that visible light doesn’t turn into “heat”?
NASA sent people to the moon. Harvard and all the universities together have pumped out a great bunch of inventions and educated a bunch of people who have gone on to create further inventions.
What have you built up with your theories?
Your models of light and heat appear to be rather useless in comparison to those used by Caltech, Harvard, MIT, and a great many other schools and centers of research.
I am very much so allowing my self to be distracted by those who can explain things and create things. I am not (hopefully) allowing myself to be distracted by you or anyone who is pushing theories that are incomplete or just incorrect. This isn’t religion. Until you can prove your view that light cannot turn into heat or until you can create something more amazing than what these other folks are creating, I think I will continue to accept their clean models which appear rather consistent and powerful.
Don’t get too lonely, Myrrh. You can always open your eyes and join the rest of the world here when you get tired of pushing weak science that provides you with limited tools.
>> If you had bothered to understand what was being said about Heat and that it takes vibrational energy to move molecules to this, you would have a grasp of the difference by now between that and electronic transitions
You said, “it takes vibrational energy to move molecules to this”. Can you find a quote from that thermo website or from wikipedia or from anywhere that supports this statement you just made?
Here comes question 4. It’s a set of questions. If you want also answer the question I just posed in the prior paragraph.
Q4 — What do you define as “vibrational energy”, and what is the “this” molecules need to move to? And how are they “moving” there? How do you define “electronic transitions”? Do you believe in the concepts of atoms, electrons, associated potential and kinetic energies, and conservation of energy?
You can answer simply or refer to existing definitions/models/etc. Essentially, I eventually want to figure out how you track energy among different forms (eg, vibrational, electron transitions, kinetic, etc), and if you even believe that such tracking (quantifying) is useful and relevant.
>> I don’t have to theorize what happens to the vast quantity of the sunlight absorbed by objects on earth
Yes, you explained a number of things in that paragraph, but not one of them was what happens when visible light is absorbed by an opaque rock. That very wikipedia page you quoted explains that most visible light is absorbed by opaque objects. They didn’t say “reflected”, “refracted”, “scattered”, etc. They said “absorbed”. So again:
Q1 — .. what do you theorize happens to the vast quantity of the sunlight absorbed by objects on earth?
Your theory is weaker than the one used by NASA because you can’t explain this and they can.
I have a related set of questions since you brought up pigments.
Q1b — Are you saying that the light hitting a rock is absorbed by rock “pigments”? What happens to that energy of excitation in those pigments’ electrons? Surely, you don’t believe electrons get excited and keep getting more and more excited for perpetuity? They have to release that energy at some point if they aren’t accumulating an infinte amount. And have you isolated these rock pigments in order to test your theories?
Now, for a set of questions about photons and their energies (part a).
Q5a — Are you saying visible light photons don’t have energy? Are you saying they can’t provide a driving force for other parts of nature to move? Where do the chemicals in photosynthesis get all of their energy if none of it is from visible light? What prompts them to do anything with the visible light if the light is not imparting any energy? Let me offer you some tentative alternative theories: do you believe that sunlight “heat” gives plants all the extra energy they might need and not yet have (eg, provide “activation energy”) in order to carry out photosynthesis? Have you carried out an experiment to prove this hypothesis, that it’s the sun’s IR that totally enables photosynthesis action? So if IR provides all of this energy, why do you even say that visible light is necessary for photosynthesis? What is the point of visible light if it imparts no energy, if it has no capability of producing stimulus?
>> Plants are not storing that energy, it is being utilised as chemical energy to produce sugars, not to produce heat.
Out of curiousity how does the visible light turn into “chemical energy”? Do you have details on this? How do you define “chemical energy” and how is visible light turned into it? [I won’t bother to label this question.]
OK, you are on record as saying that none of the energy from visible light is stored by plants. This may or may not be true. FWIW, I don’t know if anyone knows this. However, I will interpret your comment then to mean that work is being done using some energy from visible light in order to put sugars together.
Photons and their energies (part 2):
Q5b — Do you believe that visible light carries energy? If so, does your theory quantify this value? Can you share the formula with us? For example, you might call the photon energy of visible light to be hv (Planck’s constant times frequency). Can you also give a formula for the energy of IR? Also, does your theory (a) of light and your theory (b) of heat have the concept of a “photon”? Can you give some details for each?
>> The laws of thermodynamics have nothing to do with this, light isn’t hot.
Can you quote from a website that defines “light” and “hot” and perhaps states that only “hot” things obey the laws of thermodynamics. If you can’t find a quote, this isn’t a major point anyway.
Q2b — Do you think plants (or chlorophyll/chloroplasts) are “hot”? I was talking about plants creating something and producing heat during that process. Did you really mean to say that thermodynamics doesn’t apply to plants and their constituent parts?
OK, to dig deeper into this issue (Q2), it would help me a lot to know your answers to Q5a,b.
>> Irrelevant. I’m talking about the difference between real Light and real Heat..
Blackbody, as the example I mentioned in parenthesis, is not irrelevant or otherwise why would the model exist? The model is an idealization of how many objects are believed to behave. Surely, if the model speaks of an ideal body that can absorb visible light and many other non IR and likewise emit them in certain very precise proportions as relates to temperature, then, surely, at least some real objects (if not virtually all) are believed by the proponents of that model to also absorb and emit _at_least_some_ quantities of both “light” and “heat” (ie, of photons in the visible and in the IR parts of the spectrum) if not the exact amounts dictated by Plank’s formula. In fact, these university website go right out and essentially state that they believe many real bodies approximate that photon distribution’s shape.
Q3b — So do you want to update your “irrelevant” remark and answer why (a) the blackbody model and (b) these related beliefs, of how actual objects can approximate this model, are taught in potentially all legitimate universities that teach physics?
Q3c — Can you give me the name of a single university that agrees with you, that teaches either of the views (a) that visible light cannot be converted into heat or (b) that when visible light is absorbed by a rock or by most substances that in fact those visible light photons won’t “turn into” (aka, “lead to the creation of”) any amount of heat?
Myrrh, keep in mind that I am not out for super technical meanings of words. I can work with whatever is simplest that will allow me to ask the next question. Assuming you want to join me on this journey, you probably want to keep things as simple as possible to get by. If I need more details, I’ll ask and try to explain why I want more details (or it should be clear that more details would be needed in order to resolve something). I am hoping to gain a better appreciation of nature and a better idea of what experiments and analysis have been done (or rejected).
Bob, thanks for formally making the thread open topic.
3333 comments, here we come!
And may the force be with all your laptops.
Bob, thanks for the leads to McIntyre/Mann. I’m sure it’s all over parts of the Internet, but I’m fairly new to this. I am looking through a handful of blog postings McIntyre put up in August 2008 that are related, so I may comment in time.
McIntyre sounds very credible (it seems to me his story is generally accepted and not challenged), but, if I continue to stay motivated, I will spend time learning some relevant statistics in order to judge better.
Some of his words:
From http://climateaudit.org/2008/08/13/pielke-jr-discusses-the-bishop-and-the-stick/
> I didn’t argue that it turned AGW theory upside down, but neither was it a nothing… I said very clearly that if I had been a manager or principal of the next IPCC report, I would have wanted to understand very clearly what, if anything, was wrong with it, and how we could avoid such mistakes in the future.
To the extent Mann (I’ll use past tense for simplicity) was respected, put up a wall defense, and was relied upon by others without much questioning, outsider McIntyre’s nontrivial mathematical argument questioning not just trusted data points but potentially certain underpinnings of some data analysis methods is something that you should expect has disturbed and threatened a number of people. This is normal reaction, and really didn’t start to take effect until some time after the late release of Wahl/Ammann’s famous Supplementary section in mid-late 2008. Some of the emails that touch upon this would be covering then relatively recent (and perhaps somewhat disorienting) developments for those writing the email blurbs.
With hockey stick not being so hockey stick perhaps, the urgency should die down somewhat. You already pointed out evidence this might be happening on the IPCC’s part.
A view I have is that no matter how much we might be contributing to creating a warming situation for the planet it would be somewhat (not totally) coincidence that we would reach the age of computers and satellites are around the same time we were near a peak of sorts (eg, sun/earth cycles would have to coincide). Yes, everyone wants to live at a key moment in history, but that won’t always be the case. However, if we aren’t at an absolute high in temp over the last few thousand years (something we might never know), we might still be setting up for serious problems perhaps not even decades from now. [Well, we already have a lot of pressing issues to worry about globally.] I think it is important to take this issue seriously, especially since our CO2 levels appear to be rather unnatural and we certainly appear to be at least near if not at temperature highs over last several centuries. Many people find different ways to contribute and wouldn’t necessarily be more productive if we remove some issues from our collective global problems plate. We can certainly tackle numerous important issues at one time.
Robert Stevenson @ December 8, at 2:34 am
Hi Robert, I hope you are still around here, and sorry for delayed response. I was expecting Tim Folkerts to declare his wisdom on your interesting observations, (concerning candle flame emissions), but maybe Tim is busy elsewhere?
Robert and Tim,
I’ve just done a quick sniff around on Planck-Curves WRT to the colour temperature of a candle flame, and one of the frustrations has been the blogosphere uses a variety of units in the X axis of graphs WRT chemistry, thermodynamics and whatnot other interests. Nevertheless, you may find the following site interesting if you feed-in a temperature of say 1373 K, into that Plancky calculator. (a candle typically = 1,100C),
http://lamp.tu-graz.ac.at/~hadley/ss1/emfield/blackbody.php
This following stuff is also rather interesting:
http://itg1.meteor.wisc.edu/wxwise/AckermanKnox/chap2/planck_curve.html
http://scienceofdoom.com/2010/07/25/the-sun-and-max-planck-agree-part-two/
Have fun!
Robert Stevenson >> So tuning your IR camera to these wavelegths would give you the disappearing conjuring trick candle flame. However integrating the areas under Planck’s curve gives a me a relatively small percentage of the total emissive power that is absorbed by CO2. ..I would re-do the experiment to reflect this fact. Clearly this would make people more sceptical concerning the catastrophic etc
I think this is a neat experiment but to demonstrate the idea of absorption. Neat experiments/demos are those that isolate extraneous effects in order to more clearly see a particular physical law at play.
If “global warming” is mentioned, it should be in the context that the experiment exploits a very narrow band that is not reflective of the wider atmosphere. The experiment might naturally mention global warming in passing if say the theme covers “greenhouse effect” that allow our planet to stay warmer at night. In this latter context, CO2 might be used but the entire warming effect in our atmosphere comes from various other gases as well.
The debate over how much the climate is likely to change under various scenarios and what important effects that will have is a separate and much more complex issue than the basic physical effects. You would address that issue fairly only with much more comprehensive coverage and of transparent science.
In short, I think this experiment is a neat experiment to have in a classroom setting, but I would rework the dialog a little (very few words would need adjusting). If I wanted to fall back to this video, I would make sure to qualify it (aka, provide a disclosure of the details and discuss the overall picture more accurately).
Bob – Scroll down to beaches:
http://www.cancunbeachconditions.com/
Jose_X says:
December 9, 2011 at 6:21 am
You aren’t making sense. You don’t offer any proof about what you claim is real science. You simply state that visible light can’t turn into “heat”. Then when I point out that the wider world out there doesn’t agree with you, you pretend they are all wrong. Yet, you get back to repeating that it is you who is teaching me real science (even though it disagrees with what most scientists apparently believe).
Shrug, so prove it. You’ve shown me nothing from the “wider world” which actually explains how visible light creates heat, how it heats land, how it heats water as per the claim. And that is what I am asking for. Prove that this junk science claim produced by the AGWSF department’s brainwashing section that visible heats the oceans – just that one.
What proof do you have that visible light doesn’t turn into “heat”?
NASA sent people to the moon. Harvard and all the universities together have pumped out a great bunch of inventions and educated a bunch of people who have gone on to create further inventions.
What have you built up with your theories?
LOL Now you’re just bsing. Come on, give me all the inventions and uses of visible light heating water and matter produced by Harvard and all these great universities…
Yeah, right NASA sent men to the moon – and NASA had to junk StefanBoltzmann to do it..
And now? If they’re teaching that visible light heats the oceans then there’s no scientists left there, they couldn’t find their way through a door let alone get to the moon.
Your models of light and heat appear to be rather useless in comparison to those used by Caltech, Harvard, MIT, and a great many other schools and centers of research.
Yeah, yeah.., come on show me how they teach this!! Show me how they prove it! Show me!
I am very much so allowing my self to be distracted by those who can explain things and create things. I am not (hopefully) allowing myself to be distracted by you or anyone who is pushing theories that are incomplete or just incorrect. This isn’t religion. Until you can prove your view that light cannot turn into heat or until you can create something more amazing than what these other folks are creating, I think I will continue to accept their clean models which appear rather consistent and powerful.
You’re still bsing, show me exactly how they teach this, the experiments which prove it, the classes outlining the studies, the great things they are creating in their fantasy world where visible heats oceans.. you’re the one(s) claiming this in your cartoon energy budget – I don’t have to provide any information – it is encumbent on you to prove it. Or don’t use it. And certainly don’t demand that we believe you’re right when you haven’t shown anything which actually proves visible light heats water. Water is a transparent medium for visible light
– it can’t physically do this! YOU prove it.
Don’t get too lonely, Myrrh. You can always open your eyes and join the rest of the world here when you get tired of pushing weak science that provides you with limited tools.
🙂 Not lonely with the sane. You could always try going to these great universities and bastions of scientific integrity and ask them to prove the claim of the cartoon KT energy budget, do report back. I could do with a laugh.
Here’s something else for you to think about: the AGWSF energy budget claims that the atmosphere is transparent to visible light, but, the mechanism behind reflection/scattering is electronic transitions, which means the electrons of the molecules of oxygen and nitrogen are absorbing visible light from the Sun – so the atmosphere is not transparent to visible you (generic fictionados) claim. So answer this:
How much is visible light travelling direct from the Sun to Earth heating the oxygen and nitrogen in the atmosphere as it is being absorbed by their electrons in reflection and scattering?
…..
? Look, I’m not interested anymore in finding you anything, you should be concentrating on providing me with irrefutable science to your claims. You’re defending the AGWSF KT97 and its ilk energy budget which claims visible light heats land and oceans. Prove it.
Convince me!
Actually, I’ll give you a few tidbits in no particular order which need to be considered in this.
So note well, in the real world where heat and light are understood, it is simply a physical fact that infrared, heat energy on the move, is what goes through a window heating the room inside, and golly, industry produces filters to keep heat energy out while allowing non-heat creating visible in to light up the room.
In your fanasy world where visible light heats matter, you would have to use blinds to exclude it if you wanted to keep your room cool… 🙂 And so, as now, you’d be sitting in the dark.
On the same theme:
Think about this. That cartoon energy budget you claim is science fact says that it is shortwave radiation from the Sun which directly heats land and oceans. This is a massive amount of heating to create. If that was really science fact it would be stated everywhere that visible light heats the Earth’s land and oceans – you should have no problem finding me proof. Because as you see, in real phyics, that is not one of the properties of visible light.
And on that page also: http://www.antonine-education.co.uk/physics_gcse/Unit_1/Topic_5/topic_5_what_are_the_uses_and_ha.htm – scroll down to hazards
– no hazards for visible light, no heating effect from it, no danger. Visible light is benign.
And note very well – what’s the prevention against infrared heating? Reflective surfaces. Remember that when some brainwashed by AGWSF gives you the ‘two cars’ experiment…
Just to make that really clear. Since visible light is incapable of heating the inside of any car no matter what the car’s colour, any measurements of differences between white and black cars in sunlight will be physically relating back to the energy which does heat the interior – thermal infrared. This experiment says NOTHING about visible light. This is typical AGWSF sleight of hand to confuse those ignorant of real world basic physics.
As in my posts above, it’s heat which is molecules and atoms on the move, and heat which this produces, thermal infrared.
(the vibrational v electronic transmissions from the wiki page) –
“Vibrational: Resonance in atomic/molecular vibrational modes. These transitions are typically in the infrared portion of the spectrum.”
From my post above:http://wattsupwiththat.com/2011/10/26/does-the-trenberth-et-al-%e2%80%9cearth%e2%80%99s-energy-budget-diagram%e2%80%9d-contain-a-paradox/#comment-818283
This is to aid you in answering my questions – I’m not alone..
Prove that visible light from the Sun heats the water of the oceans or have the courtesy to admit you’re talking b*ll*cks.
And that goes for all of you, all, who claim that this cartoon correctly presents real world basic physics on visible and thermal infrared.