Guest essay by Ed Hoskins
Using data published by the IPCC on the diminishing effect of increasing CO2 concentrations and the latest proportional information on global Man-made CO2 emissions, these notes examine the potential for further warming by CO2 emissions up to 1000ppmv and the probable consequences of decarbonisation policies being pursued by Western governments.
The temperature increasing capacity of atmospheric CO2 is real enough, but its influence is known and widely accepted to diminish as its concentration increases. It has a logarithmic in its relationship to concentration. Global Warming advocates and Climate Change sceptics both agree on this.
IPCC Published reports, (TAR3), acknowledge that the effective temperature increase caused by growing concentrations of CO2 in the atmosphere radically diminishes with increasing concentrations. This information has been presented in the IPCC reports. It is well disguised for any lay reader, (Chapter 6. Radiative Forcing of Climate Change: section 6.3.4 Total Well-Mixed Greenhouse Gas Forcing Estimate) [1]. It is a crucial fact, but not acknowledged in the IPCC summary for Policy Makers[2].
 |
The rapid logarithmic diminution effect is an inconvenient fact for Global Warming advocates and alarmists, nonetheless it is well understood within the climate science community. It is certainly not much discussed. This diminution effect is probably the reason there was no runaway greenhouse warming caused by CO2 in earlier eons when CO2 levels were known to be at levels of several thousands ppmv. The following simplifying diagram shows the logarithmic diminution effect using tranches of 100ppmv up to 1000ppmv and the significance of differing CO2 concentrations on the biosphere:
§ Up to ~200 ppmv, the equivalent to about ~77% of the temperature increasing effectiveness of CO2. This is essential to sustain photosynthesis in plants and thus the viability of all life on earth.
§ A further ~100 ppmv was the level prior to any industrialisation, this atmospheric CO2 made the survival of the biosphere possible, giving a further 5.9% of the CO2 Greenhouse effect.
§ Following that a further 100ppmv, (certainly man-made in part), adding ~4.1% of the CO2 effectiveness brings the current level ~400 ppmv.
§ CO2 at 400pmmv is already committed and immutable. So CO2 has already reached about ~87+% of its potential warming effect in the atmosphere.
Both sceptics and the IPCC publish alternate views of the reducing effect on temperature of the importance of CO2 concentration. These alternates are equivalent proportionally but vary in the degree of warming attributable to CO2.
The IPCC have published views of the total effect of CO2 as a greenhouse gas up to ~1200ppmv, they range in temperature from +6.3°C to +14.5°C, shown below:
There are other views presented both by sceptical scientists and CDIAC, the Carbon Dioxide Information and Analysis Centre. What these different analysis show the is the amount of future warming that might be attributed to additional atmospheric CO2 in excess of the current level of ~400ppmv. Looking to the future in excess of 400ppmv, wide variation exists between the different warming estimates up to 1000ppmv, see below.

A comparison between these estimates are set out below in the context of the ~33°C total Greenhouse Effect.
This graphic shows in orange the remaining temperature effect of CO2 up to 1000ppmv that could be affected by worldwide global decarbonisation policies according to each of these alternative analyses.
Some of the IPCC data sets shows very large proportions of the temperature effect attributable solely to extra CO2. The concomitant effect of those higher levels of warming from atmospheric CO2 is that the proportion of the total ~33°C then attributable the water vapour and clouds in the atmosphere is displaced so as to be unrealistically low at 72% or 54%.
It has to be questioned whether it is plausible that CO2, a minor trace gas in the atmosphere, currently at the level of ~400ppmv, 0.04% up to 0.10% achieves such radical control of Global temperature, when compared to the substantial and powerful Greenhouse Effect of water vapour and clouds in the atmosphere?
There are the clearly divergent views of the amount of warming that can result from additional CO2 in future, but even in a worst case scenario whatever change that may happen can only ever have a marginal future effect on global temperature.
Whatever political efforts are made to de-carbonize economies or to reduce man-made CO2 emissions, (and to be effective at temperature control those efforts would have to be universal and worldwide), those efforts can only now affect at most ~13% of the future warming potential of CO2 up to the currently unthinkably high level of 1000ppmv.
So increasing CO2 in the atmosphere can not now inevitably lead directly to much more warming and certainly not to a catastrophic and dangerous temperature increase.
Importantly as the future temperature effect of increasing CO2 emissions can only be so minor, there is no possibility of ever attaining the much vaunted political target of less than +2.0°C by the control of CO2 emissions[3].
Global Warming advocates always assert that all increases in the concentration of CO2 are solely man-made. This is not necessarily so, as the biosphere and slightly warming oceans will also outgas CO2. In any event at ~3% of the total[4] Man-made CO2 at its maximum is only a minor part of the CO2 transport within the atmosphere. The recent IPCC report now admits that currently increasing CO2 levels are probably only ~50% man-made.
On the other hand it is likely that any current global warming, if continuing and increased CO2 is:
§ largely a natural process
§ within normal limits
§ probably beneficial up to about a further 2.0°C+ [5].
It could be not be influenced by any remedial decarbonisation action, however drastic, taken by a minority of nations.
In a rational, non-political world, that prospect should be greeted with unmitigated joy.
If it is so:
· concern over CO2 as a man-made pollutant can be mostly discounted.
· it is not essential to disrupt the economy of the Western world to no purpose.
· the cost to the European economy alone is considered to be ~ £165 billion per annum till the end of the century, not including the diversion of employment and industries to elsewhere: this is deliberate economic self-harm that can be avoided: these vast resources could be spent for much more worthwhile endeavours.
· were warming happening, unless excessive, it provides a more benign climate for the biosphere and mankind.
· any extra CO2 has already increased the fertility of all plant life on the planet.
· if warming is occurring at all, a warmer climate within natural variation would provide a future of greater opportunity and prosperity for human development, especially so for the third world.
De-carbonisation outcomes
To quantify what might be achieved by any political action for de-carbonization by Western economies, the comparative table below shows the remaining effectiveness of each 100ppmv tranche up to 1000ppmv, with the total global warming in each of the five diminution assessments.
The table below shows the likely range of warming arising from these divergent (sceptical and IPCC) views, (without feedbacks, which are questionably either negative or positive: but probably not massively positive as assumed by CAGW alarmists), that would be averted with an increase of CO2 for the full increase from 400 ppmv to 1000 ppmv.
The results above for countries and country groups show a range for whichever scenario of only a matter of a few thousandths to a few hundredths of a degree Centigrade.
However it is extremely unlikely that the developing world is going to succumb to non-development of their economies on the grounds of reducing CO2 emissions. So it is very likely that the developing world’s CO2 emissions are going to escalate whatever is done by developed nations.
These figures show that whatever the developed world does in terms of decreasing CO2 emissions the outcome is likely to be either immaterial or more likely even beneficial. The table below assumes that the amount of CO2 released by each of the world’s nations or nation is reduced universally by some 20%: this is a radical reduction level but just about conceivable.
These extreme, economically destructive and immensely costly efforts by participating western nations to reduce temperature by de-carbonization should be seen in context:
§ the changing global temperature patterns, the current standstill and likely impending cooling.
§ the rapidly growing CO2 emissions from the bulk of the world’s most populous nations as they continue their development.
§ the diminishing impact of any extra CO2 emissions on any temperature increase.
§ normal daily temperature variations at any a single location range from 10°C to 20°C.
§ normal annual variations value can be as much as 40°C to 50°C.
§ that participating Europe as a whole only accounts for ~11% of world CO2 emissions.
§ that the UK itself is now only about ~1.5% of world CO2 emissions.
As the margin of error for temperature measurements is about 1.0°C, the miniscule temperature effects shown above arise from the extreme economic efforts of those participating nations attempting to control their CO2 emissions. Thus the outcomes in terms of controlling temperature can only ever be marginal, immeasurable and thus irrelevant.
The committed Nations by their actions alone, whatever the costs they incurred to themselves, might only ever effect virtually undetectable reductions of World temperature. So it is clear that all the minor but extremely expensive attempts by the few convinced Western nations at the limitation of their own CO2 emissions will be inconsequential and futile[6].
Professor Judith Curry’s Congressional testimony 14/1/2014[7]:
“Motivated by the precautionary principle to avoid dangerous anthropogenic climate change, attempts to modify the climate through reducing CO2 emissions may turn out to be futile. The stagnation in greenhouse warming observed over the past 15+ years demonstrates that CO2 is not a control knob on climate variability on decadal time scales.”
Professor Richard Lindzen UK parliament committee testimony 28/1/2014 on IPCC AR5[8]:
“Whatever the UK decides to do will have no impact on your climate, but will have a profound impact on your economy. (You are) Trying to solve a problem that may not be a problem by taking actions that you know will hurt your economy.”
and paraphrased “doing nothing for fifty years is a much better option than any active political measures to control climate.”
As global temperatures have already been showing stagnation or cooling[9] over the last seventeen years or more, the world should fear the real and detrimental effects of global cooling[10] rather than being hysterical about limited, beneficial or now non-existent warming[11].
References:
[1] http://www.grida.no/publications/other/ipcc%5Ftar/?src=/climate/ipcc_tar/wg1/222.htm
[2] http://www.powerlineblog.com/archives/2014/05/why-global-warming-alarmism-isnt-science-2.php
[3] http://www.copenhagenconsensus.com/sites/default/files/ccctolpaper.pdf
[4] http://www.geocraft.com/WVFossils/greenhouse_data.html
[5] http://www.spectator.co.uk/features/9057151/carry-on-warming/
[6] http://hockeyschtick.blogspot.fr/2013/11/lomborg-spain-wastes-hundreds-of.html
[7] http://www.epw.senate.gov/public/index.cfm?FuseAction=Files.View&FileStore_id=07472bb4-3eeb-42da-a49d-964165860275
[8] http://judithcurry.com/2014/01/28/uk-parliamentary-hearing-on-the-ipcc/
[9] http://www.spectator.co.uk/melaniephillips/3436241/the-inescapable-apocalypse-has-been-seriously-underestimated.thtml
[10] http://www.iceagenow.com/Triple_Crown_of_global_cooling.htm
[11] http://notrickszone.com/2010/12/28/global-cooling-consensus-is-heating-up-cooling-over-the-next-1-to-3-decades/





Nice presentation Ed, thank you.
CO2 alone has never been the claimed problem – it was always supposed to be the knock-on affects of CO2 such as increased water vapor and and wretched Republican lifestyles that amplify the feeble CO2 effect.
notice how the author makes his case from WITHIN the accepted science.
Notice how effective the case is when you start INSIDE the accepted science..
notice that he doesnt have to resort to saying wacky stuff about the sun.
notice how he doesnt have to engage in numerology about the planets
he takes the science as given ( much like Nic Lewis does) and works from the inside
Is this anything more than a “face saving” exit plan for all the CAGW evangelists?
“Yo wagons ho!, thar be the real … (insert what you will here).
considering we really do not know what historical CO2 levels were…
…and we really have no clue what CO2 levels do to temps
and CO2 reconstructions are about as fudged as it gets
…and trying to compare any of that to fudged temp reconstructions ( is that tree right side up?)
That’s a pretty good explanation of the science if it is that way……..but then, we don’t know
Facts, we don’t need no stickin facts. We have FEAR to sell.
Rational response to CO2 is not part of the Obama administration. CO2 is a newly found political tool used by Obama’s EPA to apply control.
I’ve got to keep saying this until somebody listens. Co2 is innocent. If you fire heated gas at the surface of water the water will not accept the heat indeed even the surface itself is not affected by the heat, so the story that heat can be stored on this planet or that evaporation can be increased by the heat leaving the atmosphere doesn’t stand up to testing. Heat will not pass through the surface of water by means of convection because it is blocked by surface tension.
Surface tension is not a powerful force but is enough to get the job done so you cannot put additional heat into the ocean and the good news is you can’t boil the ocean away. AGW is utter rubbish.
I have found that your average AGWer will not admit to the Pause for the last 17 years. Nor will they accept the concept that CO2 follows temperature shown in the historical record via the ice core data. Therefore, your average AGWer will never, ever accept the fact of CO2 having an algorithmic impact on global temperatures. Any information coming from a “denier” website is instantly dismissed.
There is no enhanced GHE; that hypothesis relies on juvenile physics; any professional scientist or engineer sees almost immediately that it is a Perpetual Motion Machine of the 2nd Kind.
The way the scam works is to assume mean atmospheric emittance measured at the Earth’s surface, 333 W/m^2, is a real energy flux [2009 data]. This is not true; it is the potential energy flux the atmosphere would emit to a sink at absolute zero. Net IR flux from surface to atmosphere is [surface emittance – atmospheric emittance]; 396 – 333 = 63 W/m^2, about 1/6th of a black body.
If at constant surface temperature ‘back radiation’ increases, this net surface IR flux decreases. To give constant sum of convective, evaporative and radiative energy, the surface temperature rises. That temperature rise is 1.2 K/doubled [CO2].
The reason why the net IR is 1/6th of a black body is because the opposing emittances interact as vectors. For equal temperatures, there is zero net IR in all main GHG bands (self-absorbed). The entire IPCC logarithmic argument presented above is irrelevant.
Sorry folks; this is radiative physics 101; atmospheric sciences teach it wrongly and have done so since Carl Sagan made his science booboos in 1965. To complete that History, his assumption that the surface of Venus emitted IR as a black body was because he messed up the cloud physics and assumed about 7 times as much SW energy entering the lower atmosphere as reality.
I’m just biding my time for the first enterprising lawyer to advertise “Have you been MISS SOLD CO2” call 0800- …… so I can claim back all the bullshit £715 Vehicle CO2 emission taxes I am getting fined each year.
All these so called intelligent people running around like chimpanzees for a non problem
CO2 is so last http://www.dailymail.co.uk/sciencetech/article-2719727/Were-f-ked-Climate-change-catastrophe-mankind-study-shows-methane-leaking-Arctic-Ocean-scientist-warns.html
In the mean time the Gravy Train rolls on
http://www.dailymail.co.uk/news/article-2720903/Prescott-flies-40-000-miles-s-nearly-twice-world-five-months-lecture-climate-change.html
But in fact the so called “climate sensitivity” is
a theoretical construct relying on the tenuous assumption that lab data can be applied to the atmosphere with valid results. This assumption is looking ever more dubious. I do not think that the present flat temp trend will end before the whole of AGW theory is discredited in the eyes of all, excepting a few diehards.
Excellent essay. I really think this information needs to be repeated over and over. The facts (and IPCC’s blatant attempt to bury them) are very revealing.
If CO2 was ever a problem, it is now at worst a logarithmically decreasing problem.
Excellent description of the Law of Diminishing Marginal Returns as it applies to CO2 affecting GAST (Global Average Surface Temperature).
This sentence, however, strikes me as needing to be reworded:
”Importantly as the future temperature effect of increasing CO2 emissions can only be so minor, there is no possibility of ever attaining the much vaunted political target of less than +2.0°C by the control of CO2 emissions”
It gives the impression that it is predetermined that the arbitrarily chosen limit of +2.0°C will be crossed.
Perhaps something along the lines of:
Importantly, as the future temperature effect of increasing CO2 emissions can only be so minor; the control of CO2 emissions has no possibility of ever determining whether attainment of the much vaunted political target of less than +2.0°C is achieved.
This is, of course, ignoring that the CO2 in the tropical upper troposphere is at -17 deg C and the surface at 15 deg C, in which case, nothing radiated downward by this CO2 can warm anything. Simple thermodynamics. Any IR radiation absorbed by CO2 in the lower atmosphere is a wash in sunlight as it will be saturated and absorbing and emitting constantly. The computer models do not do night-time, at which time, CO2 and water vapor in the lower atmosphere will indeed be emitting and not absorbing, radiating IR out to space. This is why it cools down so quickly after sunset with a clear sky.
Why do the ratios for any temperature rise from Lindzen, Kondrajew & Moskalento, and Charnock & Sine always come up around 1.42 for Kondrajew & Moskalento to Lindzem, and close to 2.33 for Charnock & Shine to Lindzen? For both the figures including feedback, and the figures for excluding feedback? It’s as if all three of these agree on the magnitude of the feedback, but disagree on the magnitude of the direct effect of CO2.
Meanwhile, I have noticed that the direct effect of CO2 is widely mentioned as considered by both advocates of existence of CAGW and by skeptics as 1.1 degrees C per factor-of-2 change of CO2 (which is indeed logarythmic). This would mean before feedbacks, increase from 400 to 800 PPMV would increase global temperature by 1.1 degrees C. It’s the feedbacks where all the debate is.
In fact, according to the way I see the first 2 charts as presented, it looks as if the 2 skeptic sets of figures and the 3 IPCC sets of figures agree that the feedback is positive, and by a factor of around 4.8, causing the total effect of CO2 change to be multiplied by around 5.8. I thought skeptics considered the feedbacks to be either negative or probably negative.
Thanks, Ed Hoskins. Your article brings forth, once again, the inconvenient truth of CO2 increase having a diminishing return.
higley7 says:
“…….. The computer models do not do night-time, …….”
This statement can not be true, if it is could someone explain why these models have any validity at all?
One of my first “I smell a rat” moments in this debate was noticing that the IPCC insisted on referencing CO2’s effects based on doubling “from pre-industrial levels”. If we were currentlat at pre-industrial levels, it would make sense to quantify the effects of any given additional amount of CO2 from that perspective.
But we’re not at pre-industrial levels. Pre-industrial levels were 280 ppm. Current levels are close to 400 ppm. So in discussing the the impact of current CO2 emissions, they should be calculated against current levels of CO2.
If we accept for the moment that the direct effects of CO2 (excluding feedbacks) are indeed 1 doubling = 3.7 w/m2 = 1 degree, we can do some simple math, but instead of comparing to some distant point in time that no longer matters, lets compare to current conditions with is the only thing that matters.
At a current concentration of 400 ppm, and current CO2 increases of about 2 ppm per year…..it will take 200 years to achieve a single doubling resulting in one degree.
The second part of the IPCC shell game however is that they neglect to mention was what temperature they calculated the 1 degree of warming. Since temperature doesn’t vary linearly with w/m2, adding in 3.7 w/m2 has different effects at different temperature. We can calculate exactly what effect at what temperature using Stefan-Boltzmann Law:
P(w/m2) = 5.67 * 10^-8 * T^4 with T in degrees K.
Run the numbers and you’ll discover that 3.7 w/m2 causes a 1 degree temperature change at about -18C, which is point in the atmosphere at (IIRC) about 14,000 feet. If it is the surface temperature we are interested in, they should have done the calculation at average surface temperatures which would yield a change in temperature for CO2 doubling of about 0.68 degrees.
So, put in proper context, a doubling of CO2 from where we are now will take 200 years and will raise surface temps by only 0.68 degrees.
Even with MONSTER positive feedbacks from water vapour, that value is of little or no concern. We can be quite certain that monster positive feedbacks do not exist because if they did, the rise in temp from pre-industrial to now would be massive, and it isn’t.
The entire debate should have ended with “CO2 is logarithmic”. It has remained alive by an elaborate shell game by the IPCC. They present facts which are utterly true, and completely irrelevant. When we apply THEIR math and THEIR sensitivity and THEIR calculations to the here and NOW, their argument goes “poof” and disappears in a puff of logic.
Never before have so many been duped by such simple trickery by so few.
I misread the first of 2 non-line-graph charts, mentioning 5 different scientists/groups, by looking at it too quickly. In the first chart, the figures in the orange bands are the amount of temperature rise by increasing CO2 from 400 to 1000 PPMV, rounded to 2 decimal places. In the second chart, the bottom figures are the same, except carried out to 3 decimal places.
Meanwhile, the 5 different scientists/groups are shown as predicting effects of CO2 change ranging from .124 to 1.45 degree C per factor-of-2 change of CO2. If there is substantial evidence that this figure is much less than 1.1 degree C per 2xCO2, then I would expect Dr. Roy Spencer (drroyspencer.com) to say so, and I never seen him say so. I have yet to see him mention a figure calculated by using the MODTRAN model, which seems to me as about .8 degree C per 2xCO2. Meanwhile, MODTRAN is known to not have full resolution of the highly detailed CO2 absorption spectrum in the wavelength ranges where CO2 is partially transparent.
“The temperature increasing capacity of atmospheric CO2 is real enough,…..”
What happened during the last 17 years ?
I am actually a skeptical denier and a denying skeptic. Until somebody does dome experiments to show how the back radiation horse shit mechanism actually works I say it does not work. I have looked at CO2 lasing and CO2 lasers. That actually works. But 4 CO2 molecules out of 10,000 air molecules buzzing around at considerable speed dont impress me much. So lets have some climate warmist do the experiments.
Raymond;
This statement can not be true
>>>>>>>>>>>>>>>
And it isn’t.
davidmhoffer says in part, August 10, 2014 at 9:31 am:
“P(w/m2) = 5.67 * 10^-8 * T^4 with T in degrees K.
Run the numbers and you’ll discover that 3.7 w/m2 causes a 1 degree temperature change at about -18C, which is point in the atmosphere at (IIRC) about 14,000 feet. If it is the surface temperature we are interested in, they should have done the calculation at average surface temperatures which would yield a change in temperature for CO2 doubling of about 0.68 degrees.”
Since some of the increase of surface radiation is absorbed by greenhouse gases in the lower troposphere and reradiated back towards the surface, increasing the surface temperature increases its radiation by more than 3.7 W/cm^2 in response to a forcing of 3.7W/cm^2.
There is NO evidence that increasing CO2 in the atmosphere causes the temperature to rise. NONE. NADA. ZILCH.
That is the bottom line.
Donald L. Klipstein
Since some of the increase of surface radiation is absorbed by greenhouse gases in the lower troposphere and reradiated back towards the surface, increasing the surface temperature increases its radiation by more than 3.7 W/cm^2 in response to a forcing of 3.7W/cm^2.
>>>>>>>>>>>>>>>>>>>>>
The IPCC documentation assumes that this effect is subsumed into the 3.7 w/m2 in the first place. If you can point me to where they say otherwise, I’ll read it. AR4 on the other hand states specifically that radiative forcing cannot be directly equated with surface forcing, and then becomes rather vague as to what value surface forcing should be (but obviously less).
davidmhoffer says:
August 10, 2014 at 9:31 am
====
thanks
Nice presentation. Should be submitted to the EPA as evidence against the upcoming Power plant tailoring rules.
Yes this is an excellent piece of work.
However, I can’t help but feel frustrated by the inadequacy of this flow.
“As the margin of error for temperature measurements is about 1.0°C
….at ~3% of the total[4] Man-made CO2 at its maximum is only a minor part of the CO2 transport within the atmosphere.
concern over CO2 as a man-made pollutant can be mostly discounted.”
All things considered (which underscores the impossibility that the relative infinitesimal fossil fuel CO2 emissions have impacted our climate)
the use of “mostly discounted” just fails miserably. IMO
Up against the deliberate, calculating and institutionalized campaign of misinformation “mostly discounted” is like referring to Ted Bundy as not such a swell blind date. Or something like that?
“Mostly” invites the alarmists to embellish out whatever ginned up remaining legitimacy they need to stay the course.
Interesting to compare that with this paper from Schneider during his Global cooling period.
Schneider S. & Rasool S., “Atmospheric Carbon Dioxide and Aerosols – Effects of Large Increases on Global Climate”, Science, vol.173, 9 July 1971, p.138-141
We report here on the first results of a calculation in which separate estimates were made of the effects on global temperature of large increases in the amount of CO2 and dust in the atmosphere. It is found that even an increase by a factor of 8 in the amount of CO2, which is highly unlikely in the next several thousand years, will produce an increase in the surface temperature of less than 2 deg. K.
The EPA should be pointing this out to Obama and admitting that it needs to be downsized.
However, that is in a rational world, here in the real world where the ecoloons rule – at least they do in most of the western world. Elsewhere no one gives a rat’s unless they can see some way of using green crap to sucker some extra dough out of smug, but goofy, western countries.
http://www.telegraph.co.uk/news/politics/10949976/Smart-metersto-be-put-in-every-British-home-despite-fears-they-may-not-work.html
RMB you are largely correct (co2 doesn’t warm the ocean), but your explanation is crap.
Infrared (atmospheric radiation) is absorbed by the top few microns of the surface, which does in fact heat the ocean.
But, and this is an extremely huge BUT, the ocean primarily cools through evaporation not radiation. All atmospheric radiation does is decrease the net amount of radiation loss from the ocean surface. When that happens, evaporation simply picks up the pace a tiny little bit, and the ocean surface temperature stays exactly the same.
I have measured this non effect hundreds of times.
Here’s what I know. If I fire my heat gun at uncovered water in a bucket the water does not accept the heat. If I float something like a metal baking dish on the surface and apply the heat to the floating object the water readily accepts the heat. Try doing it for yourself and if you get the same result tell me its not surface tension. The climate guys have never tested their own hypothesis
An excellent and convincing essay. As an editor by profession, I have frequently had to restrain myself at this site on a certain point. But now I find I can’t contain myself any longer. Sorry. There are two u’s in “minuscule”.
But CO2 is very last season!
http://www.dailymail.co.uk/sciencetech/article-2719727/Were-f-ked-Climate-change-catastrophe-mankind-study-shows-methane-leaking-Arctic-Ocean-scientist-warns.html
The Earth climate system appears to be a closed loop system and the influence of carbon dioxide is limited to the extent that it changes the control input.
I have often wondered what percentage of the public who accept the AGW theory because Scientists said it is true, are aware that CO2 is a necessity.
I’m sure you’ve heard about the the petitions people like Penn and Teller passed around to get signatures to ban water, using the scientific name for it, and listing all the harm it causes as the reason the ban was needed.
I wonder what percentage of people would answer Zero in a poll asking what percentage of CO2 is acceptable in the air our children breath?
Kelvin Vaughan says:
August 10, 2014 at 10:04 am
http://www.telegraph.co.uk/news/politics/10949976/Smart-metersto-be-put-in-every-British-home-despite-fears-they-may-not-work.html
===
Crap. Last time I looked the UK national debt was about 180 bn GBP , more than half of which was due to the cost of bailing out the banks.
And they want to spend 11bn they don’t have on this kind of stupidity which will have NO possible effect on climate, even if you are dumb enought to believe the IPCC.
The second graph in this article shows it is not the logarithm that matters but the arbitrary multiplier that IPCC modellers apply to the actual calculated effect of CO2.
The so-called positive feedbacks that reduce the known and over-riding negative feedbacks that have kept climate relatively stable for billions of years, through thick and thin.
The +ve feedbacks are guesswork. Guesses that they got wrong as can be seen by their total failure to predict the post 2000 plateau in global temps.
Peter: “I wonder what percentage of people would answer Zero in a poll asking what percentage of CO2 is acceptable in the air our children breath? ”
Out or in ? I’d like to see the poll results.
Kind of highlights the fundamental dishonesty of Government funded Climatology.
Anthony, can we loose the “like” buttons , or do I have to add WUWT to my spam filters.
Steven Mosher says:
August 10, 2014 at 8:08 am
Your Team works outside science, with totally bogus, GIGO models designed to show what their programmers want shown, ie they commit the logical fallacy of begging the question. Skeptics from the outset have objected to the unwarranted, indeed shown false, assumptions of modelers about feedback effects and clouds. This post is within that tradition. Without positive water vapor feedbacks, there can be no catastrophic man-made global warming. Since there is no evidence of such feedbacks, indeed quite the opposite in a homeostatic world, your whole scam unravels like a cheap suit.
If you think the sun or modulations of its activity doesn’t influence climate, please back up this assertion using the scientific method.
Raymond says:
August 10, 2014 at 9:29 am
////////////////////
I do not know whether his comment is correct, but if it is correct, may be it is because the K&T energy budget cartoon does not do night. See for example: http://wattsupwiththat.files.wordpress.com/2009/11/trenberth-color-best.jpg
If that was the budget for planet Earth, there would be little in the way of weather since weather is generated by the fact that everything is not some hommogenous average, but rather because there are differences in energy/heat flux/pressure etc.both vertically and horizontally.
Re: “The rapid logarithmic diminution effect…”
The logarithmic response means that we need to add twice as much CO2 again to create the same amount of warming we created with the initial increase. The diminution effect may not be so rapid as the author suggests, depending on climate sensitivity and on whether intergenerational time-scales are considered.
If CO2 climate sensitivity is as low as 1.5 deg C, then increasing CO2 from pre-industrial (280ppm) to present (~397ppm), should result in an equilibrium change of 0.75 deg C. In that case, to stay under the generally accepted danger threshold of 2.0 deg C above pre-industrial temperatures, CO2 concentrations would need to peak below 700ppm. At current rates of increase, this would occur around the middle of the next century, in about 140 or so years. If we call a social generation 30 years, then this is just over 4 generations away (probably more than that before the equilibrium temperature is reached).
However, if climate sensitivity is 3.0 deg C, then the equilibrium change caused by 397ppm would be about 1.5 deg C (which we’d currently be in transit towards). In order to stay below the 2.0 deg C threshold, concentrations would need to stay below about 440ppm; a level we’d reach within the next 20 years at current rates.
Remember, these calculations take the logarithmic diminution of CO2 concentrations versus warming effect fully into account.
The absorption spectrum for CO2 makes this clear. As temperatures move up into the “atmospheric window” increased absortion of radiation decreases dramatically.
Meh. DP’s right. The argument isn’t over IR physics, it’s over feedback.
Genghis says:
August 10, 2014 at 10:11 am
RMB you are largely correct (co2 doesn’t warm the ocean), but your explanation is crap.
Infrared (atmospheric radiation) is absorbed by the top few microns of the surface, which does in fact heat the ocean.
///////////////////////
Because of the omnidirectional basis of DWLWIR, about 80% of all DWLWIR is absorbed within just 3 microns!
Does the energy absorbed in those 3 microns heat the ocean? For it to do so, it needs to be dissipated (and hence diluted) to depth at a rate faster than the energy absorbed in the top 3 microns would power/drive evaporation from the surface layer of the ocean.
The question then is how is the enormous amount of energy that is absorbed within the top 3 microns disipated to depth at a fast enough rate. Ocean overtunring is a slow mechanical process, and is largely dirurnal. So that does not look promising.
It cannot be by conduction since the energy flux is upwards (not downwards); at the very top of the ocean. the top millimetre is cooler than the ocean layers below. So unless we are mistaken as to energy fluxes and the ability of energy to ‘swim’ against the direction of flux, it cannot be by conduction.
There may be some mixing by wind and swell. Swell is a slow mechanical process, and so too is the wind when blowing at say BF3 or less. And if it is very windy (say BF8 and above), the top of the ocean becomes a divorced layer, and is not in contact with the bulk ocean below and any energy absorbed in the top 3 microns would most probably just be swept upwards into the atmosphere thereby help powering the storm raging above.
There are fundamental problems as to how DWLWIR heats the oceans given the absorption characteristics of LWIR in water, and the fact that the ocean is free to evaporate (unlike say rocks etc.).
I have never seen a convincing explanation detailing how DWLWIR heats the oceans. One needs to see an energy budget for the top 3 microns, the top 5, 10, 50, 100 microns perhaps going down to the first few metres and an explanation as to what processes are said to be going on in each of these bands, and the rate of energy transfer.
Solar does not present the same problems. According to K&T, solar is approximately 1/2 the power of DWLWIR, but wheras 80% of DWLWIR is absorbed within just 3 microns, fortunately for us, only about 1 % of solar is so absorbed.
Solar is for the main part absorbed within 1 metre (some solar finds its way past 10 metres), and this means that the energy from solar is disipated and diluted over a very substantial volume, about a million times larger than for DWLWIR. This means that solar warms the oceans without boiling it off, from the top down. But DWLWIR provides a major problem if it is truly sensible energy capable of performing sensible work since there is so much energy being fully absorbed within just 3 microns of ocean depth.
If my explanation is crap as you put it I need an explanation as to why I cannot get heat into uncovered water but if I float a metal object on the surface killing the surface tension underneath and apply the heat source to the floatin object the water heats as one would expect, explain that without surface tension. This reply is for Richard Verney.
Donald L. Klipstein says:
August 10, 2014 at 9:42 am
>>>>>>>>>>>>>>>>
Here is the money slide from AR4, figure 2.23
https://www.ipcc.ch/publications_and_data/ar4/wg1/en/ch2s2-9-5.html
Note that the bottom half of the slide shows RF (radiative forcing) from LLGHG’s at just over 2 w/m2, but that the same model when run to show SF (surface forcing) in the upper panel generates just over 0.2 w/m2. A tacit admission by the IPCC that their calculations are for the upper troposphere, and what happens on the surface (where we live) is not just a smaller number, but a number so small as to be unremarkable.
If you have 3 gases within a system 2 absorping IR at the same energy and 1 gas, inert, makes up 94.06%, a second gas 5% and the third 0.04% what will be the effect of changing the relative volumes say from 5% & 0.04% to 4% & 1.04%. Remember both absorp IR at the same energy and the IR is finite.
An excellent perspective. The entire problem is scientists who are convinced they can represent the atmosphere with mathematical equations, then project it with accuracy out 100 years.
Even if you know all the physical laws and can dial in the known numbers the solutions will only confirm your theory.
The blind spot of global climate models is clouds. Increasing low clouds and decreasing high clouds in real world observations are evidence of a negative feedback.
Increasing low level moisture is greatly underestimated by models from evapotranspiration and trillions of gallons pulled out of aquifers for irrigation and other uses. One might think that since H2O is a greenhouse gas, this would increase warming.
Observations of the US Cornbelt over the last 30 years indicate the complete opposite……….a cooling effect. During the growing season, corn plant populations have doubled during that period. This has created a micro climate, adding tremendous low level moisture from evapotranspiration as well as increasing the vegetative density of the surface.
Higher dew points and a lower lifting condensation level has increased low clouds that form earlier in the day. Less SW radiation means less daytime heating. In addition, there is a positive feedback at work. The resulting increase in rainfall goes back into the soils and are taken in by the plants which continue to contribute to the increase in evapotranspiration.
Meteorologists forecasting for this area of world are very familiar with this significant effect during the growing season.
With CO2 boosting vegetative health across the globe, this same effect is happening on a huge scale but of course with less magnitude than what we see in the Cornbelt.
The magnitude/powerful effect seen here, makes this a massive real world/outdoor laboratory that shows an underestimated negative feedback to temperatures that is occurring on a global scale.
I am often loathe to wholly discount the opinion of experts but evidently CO2 is not able in itself to be the main driver of temperature increase in the future.
I can only assume that feedback loops (eg: water vapour) included in climate models anticipating larger increases somehow multiply the impact of increased CO2. As it is evident the scientific community have only a partial understanding of how these feedbacks work then I can only conclude that there should be limited reliance placed on their projections.
Only when previous changes in climate (recent and historical) have been adequately explained and models adjusted accordingly should we give them greater credence. Even then there is a justifiable debate to be had as to whether it would be worth the economic cost to reduce, or simply adapt.
The measurement uncertainty surrounding the global average radiative forcing, the energy that heats &/or cools the earth, is +/- 5 W/sq m. That’s a total uncertainty bandwidth of 10 W and that’s about +/- 1.5% of a total 340 W/sq m.
Hold that thought.
The radiative forcing attributed to mankind’s CO2 output is 1.6 W/sq m, less than 20% of the uncertainty band. And actually less than that and diminishing.
How can anybody model or predict future warming and climate change based on a number that is completely lost in a data Oort cloud of noise, that is trivial in the normal ebb and flow of the global radiative forcing?
It’s pretty obvious by now – they can’t.
Richard Verney,
Yes all the IR is absorbed in the top three microns of the oceans surface. and the radiation difference between clear sky and a completely overcast sky is ~ 130 watts. Almost as much as the 165 watts of SW radiation claimed by NASA and Trenberth from the sun that passes through the surface.
3 microns over a square meter equals 3 cubic centimeters being heated by 130 watts. A watt is a joule per second and the specific heat is around 4 joules/cubic centimeter so the surface of the ocean should warm at a rate of .7 degrees per second or 42 degrees a minute. Just about like turning on a microwave oven.
That much heat should surely measurably warm the surface when clouds pass overhead shouldn’t it?
Well it doesn’t, I have measured it hundreds of times and there is zero difference in the surface temperature between clear and overcast skies.
The only explanation that I can think of is that evaporation increases in direct response to the increased radiation.
RMB says:
August 10, 2014 at 8:23 am
————————————
It is true that DWLWIR does not slow the cooling rate of the oceans, but your “surface tension” explanation is incorrect. LWIR is absorbed by water, but any heating of the first few microns is offset by evaporation as Gengis and Richard Verney point out.
If your explanation was valid my kitchen should be full of steam evaporating. Its not.
@ Terry says:August 10, 2014 at 11:33 am
Because the margin of error for temperature measurements is about 1.0°C
and Man-made CO2 is only 3% of the total
and because water vapor has not cooperated with the concept of fossil fuel CO2 emissions=AGW there should be zero acceptance of any climate model projections which have ultimately failed to project global temperature trend.
davidmhoffer says, August 10, 2014 at 9:51 am:
“The IPCC documentation assumes that this effect is subsumed into the 3.7 w/m2 in the first place. If you can point me to where they say otherwise, I’ll read it. AR4 on the other hand states specifically that radiative forcing cannot be directly equated with surface forcing, and then becomes rather vague as to what value surface forcing should be (but obviously less).”
The ‘lifting the effective emission height (EEH)’ version of the AGW hypothesis doesn’t seem to be dependent on the original increased forcing itself being ‘teleported’ to the surface. It is the temperature rise that’s being linearly extrapolated (along an unchanged lapse rate) to the surface from the level where the increased forcing originated.
So, very conceptually, if we move up about 17000 ft, somewhat less than halfway up the troposphere, we will find the air layer emitting Earth’s atmospheric radiation flux freely to space (it couldn’t be of course, this would have to be at/above the tropopause, but that’s different story), at a mean steady-state temperature of 255K. If we suddenly double the atmospheric CO2 content, we would increase the atmospheric optical depth for outgoing IR and hence lift this ‘effective emission height’ about 500 ft higher. To a layer at 254K, that is, one degree cooler than the ‘old’ EEH.
It is at this point that the 3.7 W/m^2 increase in forcing appears. Stated another way, this 254K layer of air presumably absorbs as much IR coming up from the layers below as before, but emits upwards 3.7 W/m^2 less than before. Somehow because of its lower temperature. Meaning, this layer will have to warm about 1 degree to restore the balance. And it does so from the imbalance itself (more in than out). Once the layer at 17500 ft has warmed by its one degree, this warming can simply be drawn down to the surface via the lapse rate.
Ta-da!
I hate to tell you guys but increasing carbon dioxide in the atmosphere has no effect whatsoever on global temperature. You are simply ignoring the real world when you go through contortions to find out what that logarithmic increase of yours might be. Fact is, there is no warming now and there has been none for 17 years while carbon dioxide steadily increased. This is an experimental observation in conflict with any and all greenhouse theories, linear or logarithmic, that predict warming. Arrhenius greenhouse theory, for example, has been predicting warming for all these years and getting nothing. If you are a scientist and your theory predicts warming but for 17 years you get nothing you are justified in tossing that theory into the waste basket of history. There is a spot reserved for it right next to phlogiston, another failed theory. This may look like not leaving us any greenhouse theory to guide us but that is not true. There is the Miskolczi greenhouse theory (MGT) that IPCC has forbidden anyone to refer to ever since it came out in 2007. Its prediction is exactly what we have now: addition of carbon dioxide to the atmosphere does not warm it. It follows that any warming observed in global temperature records is natural and not greenhouse warming. MGT differs from Arrhenius in that it is able to handle several GHGs simultaneously absorbing in the IR when Arrhenius can handle only CO2 and is incomplete. Carbon dioxide and water vapor are the most importand GHGs in the earth atmosphere. According to MGT they establish a joint optimal absorption window in the IR that they control. The optical thickness of this absorption window in the IR is 1.87, determined by Miskolczi from first principles. If you now add carbon dioxide to the atmosphere, it will start to absorb just like Arrhenius says. But this will increase the optical thickness. As soon as it starts, however, water vapor will begin to diminish, rain out, and the original optical thickness is restored. The newly added carbon dioxide will of course keep absorbing but thanks to this simultaneous reduction of water vapor it cannot cause any greenhouse warming that is imputed to it by Hansen and company. This fact should be verified by independent observations and Miskolczi did that in 2011. Using NOAA database of radiosonde observations that goes back to 1948 he studied absorption of IR by the atmosphere over time and found that absorption had been constant for 61 years. Carbon dioxide in the atmosphere simultaneously increased by 21.6 percent. Constant absorption means no warming. Hence, these data constitute an exact parallel to the warming pause/hiatus we are experiencing now. This fact has wide-ranging consequences. First, it makes a runaway greenhouse warming quite impossible. This is why the very high carbon dioxide amount in geologic history has been unable to cause any runaway warming. Hansen has been warning us that if we do not give up burning fossil fuels a runaway greenhouse effect like that on Venus will destroy us. Unfortunately he is ignorant of Venusian geology too despite having worked as an astronomer on the Pioneer Venus project. Venus has no plate tectonics. Excess radioactive heat on earth is constantly vented by plate boundary volcanism. On Venus it just accumulates beneath the crust and so weakens it that it break apart into giant slabs. These sink into the interior and an entirely new crust is formed every 300 to 600 million years. If Venus is the same age as earth there may have been as many as ten such moltings in its past. Its atmosphere is entirely a product of these giant eructations and has nothing to do with boiling oceans of Hansen’s. Secondly, the Miskolczi effect makes the enhanced greenhouse effect also impossible. This enhanced greenhouse effect is said to be the cause of anthropogenic global warming or AGW by IPCC, Hansen, and other experts. Since it is ruled out by MGT it follows that it is nothing but a pseudo-scientific fantasy, conceived by over-eager climate “scientists”to prove that the greenhouse effect is real.
Arno Arrak says:
August 10, 2014 at 12:15 pm
http://www.google.com/url?sa=t&rct=j&q=&esrc=s&source=web&cd=2&ved=0CCkQFjAB&url=http%3A%2F%2Fnige.files.wordpress.com%2F2011%2F02%2Fthe-saturated-greenhouse-effect-theory-of-ferenc-miskolczi.pdf&ei=bsXnU470BsnXiwKy6IG4Dw&usg=AFQjCNHGM-LHU1GLNqCdX3gd6mZ1w9adew&sig2=03MycldsSWC7Sh3sxecWow&bvm=bv.72676100,d.cGE
@Steven Mosher at 8.08pm. This argument is uncomfortably similar to you painting all models with in the same palette. Known knowns within physics pure does not transfer to newer forms of sub-physics, ie. climate physics.
@ Ed Hoskins
“The temperature increasing capacity of atmospheric CO2 is real enough”
—————————————-
CO2 can absorb energy from LWIR as shown by Tyndall in 1859
CO2 can also emit LWIR if conductively heated as shown by Tyndall in 1860
In our atmosphere radiative gases are emitting to space more than DOUBLE the net flux of radiative energy being absorbed at lower altitude.
The question is not the ability of radiative gases to absorb and thermalise energy, but rather their net effect in our moving atmosphere. Which is cooling at all concentrations above 0.0ppm.
A very informative piece. Equally enjoyable were all the thoughtful comments.
Of course the sun has an effect.
Excellent article. However, one point was missing:
On time scales from decades to hundreds of thousands of years, all available evidence shows that changes in CO2 are caused by changes in temperature. There is no evidence showing that ∆T is caused by ∆CO2.
The alarmist premise is that a rise in CO2 will cause global warming. But where is the empirical evidence to support that belief? So far, no such evidence has been found, despite more than 30 years of searching.
Ed Hoskins & commentators: Thank you for such a lucid explanation of the role of C02 in the AGW debate.
The oceans control Earth’s atmospheric temperature.
The amount of energy that the oceans can retain at a given level of insolation is determined by the weight of the mass of the atmosphere bearing down on the ocean surface because that is what determines the energy ‘cost’ of the evaporative change of phase from water to vapour.
At very low pressure there is little difference between the energy required to initiate evaporation and the amount of energy required for the phase change but the higher the surface pressure becomes the more energy is required by the phase change from lquid to vapour.
See here:
http://www.newclimatemodel.com/the-setting-and-maintaining-of-earths-equilibrium-temperature/
Steven Mosher,
“notice how the author makes his case from WITHIN the accepted science.
Notice how effective the case is when you start INSIDE the accepted science..
notice that he doesnt have to resort to saying wacky stuff about the sun.
notice how he doesnt have to engage in numerology about the planets
he takes the science as given ( much like Nic Lewis does) and works from the inside. ”
And although he has made his case from within the (suspect) “accepted science”, he shows that “whatever the developed world does in terms of decreasing CO2 emissions the outcome is likely to be either immaterial or more likely even beneficial”. In other words Mr. Mosher the panic that the IPCC and its adherents attempt to generate is baseless even using their own dubious assumptions. Do you agree with his conclusions and if not, why not?
davidmhoffer says:
August 10, 2014 at 9:31 am
“The entire debate should have ended with “CO2 is logarithmic””.
————————-
I agree, the entire debate should have ended 30 years ago …. but not just because of the claim that “CO2 is logarithmic”.
Now unless my logical reasoning abilities have gone completely FUBAR …. then the claim that “CO2 is logarithmic” makes no logical sense to me if it is based in/on this explanation/description of said, to wit:
—————–
Decades ago it was determined that CO2 ‘s ability to trap heat rising from Earth’s surface declines logarithmically or very rapidly (see first figure below). This means that early on, at low concentrations, CO2 does exert a significant warming of the lower atmosphere. But as the absorption bands in which CO2 captures this rising heat begin to get saturated, CO2 can capture less and less heat with each additional unit of CO2”.
Source ref: http://plantsneedco2.org/default.aspx?act=documentdetails.aspx&documentid=365
==============
Now given the above, it is therefore my learned opinion that the claim that “CO2 is logarithmic” is little more than “junk science” and anyone that believes otherwise is living proof of this quoted statement, to wit:
—————–
davidmhoffer said:
“Never before have so many been duped by such simple trickery by so few”.
===============
First of all, concerning the above explanation/description, ….. CO2 has no ability to “trap” heat or …. to “trap” thermal “heat” energy. It has the ability to absorb either “conducted” or IR “radiated” heat energy …. and/or …. the ability to emit either “conducted” or IR “radiated” heat energy.
Secondly, just what the hell does the CO2 absorption bands for absorbing IR energy have to do with the total amount of IR radiation from the earth’s surface?
Thirdly, is not the “saturation of the absorption bands” in which CO2 absorbs IR energy a direct result of the surface temperature which the surface is the source of said IR radiation?
HA, me thinks the absorption bands in which CO2 absorbs IR energy are probably pretty much saturated for 1 or 2 hours in the locales of clear skies relative to the Sun’s zenith position of “High Noon” .
And fourthly, given the fact that the CO2 is constantly emitting its absorbed IR energy …. how is it possible for it to “absorb less and less heat energy ….. just because it is “emitting more and more heat energy”?
My question is, iffen one fills and caps a 10 gallon glass container with 20% CO2 … and then directly point a 200 watt IR light source at it, from 12 inches distance, that is emitting in the “CO2 absorption bands”, …… just how “HOT” will that CO2 get ….. or will it begin to get colder after it got hotter?
davidmhoffer says in part, August 10, 2014 at 11:12 am:
“Here is the money slide from AR4, figure 2.23
https://www.ipcc.ch/publications_and_data/ar4/wg1/en/ch2s2-9-5.html”
Radiative forcing is change in net radiation flow, assuming surface and troposphere temperature do not change in reponse to the forcing. Surface forcing is the change in net radiation flow, after the surface and troposphere temperatures have responded.
Radiative and surface forcings are equal when the forcing is not affected by surface and tropospheric temperature response, such as a change in solar output.
Since an increase of surface temperature in response to an increase of GHGs causes an increase of outgoing surface radiation, it is expectable that the surface forcing from an increase of GHGs is less than the radiative forcing from GHGs.
A couple comments on presentation:
The figure I think can be improved by rescaling the left bar so that the blue line is flat at 400 ppm (or even 350). Therefore each additional 100 ppm increase in CO2 is connected downward to the middle bar for a miniscule change in forcing and steady state temperature change.
RE: The fourth chart. Purely for psychological, social, and historical reasons, I would reverse the x axis and put IPCC on the left with rebuttals following to the right.
But my biggest problem with the fourth chart, and maybe spill over into the other charts, is this business of ~33°C total Greenhouse Effect.
I have and continue to believe that the ~33°C figure is really about 3 times too big and comes from a mathematical model blunder by treating a ~30% albedo as a one-way mirror, reflecting incoming solar energy away, but playing no part in trapping earth radiated energy. Yes, there are spectral conversion concerns, but that ~33°C figure comes from what I think is sloppy accounting of what is and is not in a GHG energy budget.
richard verney says, August 10, 2014 at 11:09 am:
(Largely, that downwelling longwave radiation hitting water is absorbed within the top 3 microns, and an increase of such radiation hitting water is heavily disiipated by an increase of evaporative cooling)
If the top 3 microns warms up without the 10 micron level warming up, then heat conduction through the extremely thin temperature gradient between 3 and 10 microns down will cool the top 3 microns faster than increased evaporative cooling. Evaporative cooling won’t outpace heat conduction until the temperature gradient expands over a much greater range of depth, at which point mixing becomes significant at transferring heat to deeper levels.
Genghis, you say with respect to the DWLWIR hitting the ocean:
“The only explanation that I can think of is that evaporation increases in direct response to the increased radiation.”
So you agree that the overall earth/ocean/atmosphere energy level is higher with this radiation than without it?
“The temperature increasing capacity of atmospheric CO2 is real enough”
Um, Please don’t speak for me. The “skeptics believe” is not true for all of us.
Below the tropopause, CO2 does exactly nothing to temperature. That is why we have a troposphere and tropopause. In that regime (where we live) water completely dominates and CO2 doesn’t get a chance to play in the radiation game. It’s an opaque atmosphere in the CO2 range.
Above the tropopause, CO2 is a net radiator to space and cools.
In no case does CO2 cause net warming.
http://chiefio.wordpress.com/2012/12/12/tropopause-rules/
Image of CO2 effect with altitude:
http://chiefio.wordpress.com/2012/12/12/tropopause-rules/stratosphere-radiation-by-species-1460/
And yes, as Mosier says, it IS effective to work inside the other guy’s paradigm, but do remember that the paradigm is wrong…
And do note that lunar tidal effects are not ‘numerology’ and account for as much cold / warm layer mixing in the oceans as the wind. That planetary orbits correlate with solar cycle changes and with lunar orbits may just be incidental. Or maybe not. Dismissing it out of hand without evidence is not a path to further understanding. While it is my opinion that the lunar tidal effect is the operative mechanism in that correlation, it is by no means proven that some solar effect is not operative as well (such as UV depth of energy deposition heating / not heating the ocean and stratosphere).
http://chiefio.wordpress.com/2013/01/24/why-weather-has-a-60-year-lunar-beat/
http://www.pnas.org/content/97/8/3814.full
So yes, as a ploy, it is useful to argue from the other side’s premisses. But just be careful you do not embrace them as true, nor imply they are accepted truth.
This s the latest in a series of very good essays on co2 by Ed.
Most of us believe in radiative physics and Also that there is some point at which adding more co2 has little additional effect on temperatures.
I wrote this piece last year in which I examined the extended Central Engand Temperature to 1538 and plotted co2 levels against it.
http://wattsupwiththat.com/2013/05/08/the-curious-case-of-rising-co2-and-falling-temperatures/
Since then I have been researching the CET record back a further 500 years. There is little doubt that there are periods in that extended record at least as warm as today and that is without entering the core years of the MWP, which may well be notably warmer than today. Not having done the detailed research on that yet I remain open to whatever the research may show.
The point is that we have considerable ups and downs over that extended period to temperatures as warm as today and much colder than today at co2 levels from 280ppm pre industrial to around 400 ppm today.
It is impossible to draw any interim conclusion at present other than superficially it appears- subject to much more research – that co2 concentrations appear to lose their ability to cause substantial warming at around the 280 ppm level
Natural variability appears to be the main driving force. Phil jones admitted a few years ago that natural variability was far greater than he had hitherto believed
Tonyb
Concerning the absorption of IR by the surface of water (top2-3 microns), there is another way to examine the matter: by rate of evaporation. A typical rate in hot climates is one cm per day; that works out to about 8 microns per minute. This figure is averaged over 24 hrs and actual daytime evaporation rates would be higher because of the much greater amount of heat involved. The point is this approach allows a calculation of the residence time of the heat in the upper two or three microns and behold, the microns are gone in a few seconds. No time for conduction to lower levels, period. A simple experiment will show that is impossible to heat water by IR.
Raymond says: August 10, 2014 at 9:29 am
higley7 says:
“…….. The computer models do not do night-time, …….”
This statement can not be true, if it is could someone explain why these models have any validity at all?
Raymond higley7’s post comes in the category of “not even wrong”(sorry I don’t know who coined the expression), Posts such as his allow warmists to categorise us realists as idiots. I’m opposed to censorship but in some things it is best e.g. the owner of this site properly forbids discussion of “*hem*rails” for obvious reasons.
Could be that I’ m wrong, but I simply cannot take decarbonization as a serious notion. For me it is not in the realm of possibilities, not even in this loony world of enviro-wackos.
mpainter says:
August 10, 2014 at 1:23 pm
“A simple experiment will show that is impossible to heat water by IR. ”
http://www.tellusa.net/index.php/tellusa/article/view/15675
Curt says:
August 10, 2014 at 12:54 pm
Genghis, you say with respect to the DWLWIR hitting the ocean:
“The only explanation that I can think of is that evaporation increases in direct response to the increased radiation.”
So you agree that the overall earth/ocean/atmosphere energy level is higher with this radiation than without it?
****************
No, the energy in the Ocean is not increased at all, because the DWLWIR never gets past the first couple of microns, before it gets evaporated away just like boiling water doesn’t get any hotter no matter how much extra heat gets applied to it. Also the ocean surface temperature acts as an upper limit on the atmospheric temperature.
Donald L. Klipstein says:
If the top 3 microns warms up without the 10 micron level warming up, then heat conduction through the extremely thin temperature gradient between 3 and 10 microns down will cool the top 3 microns faster than increased evaporative cooling. Evaporative cooling won’t outpace heat conduction until the temperature gradient expands over a much greater range of depth, at which point mixing becomes significant at transferring heat to deeper levels.
************
You have it wrong. The top couple of microns don’t heat up. There is no temperature gradient change.
Again the best analogy is measuring the temperature of boiling water while increasing the amount of heat being applied, the liquid water will stay at exactly the same temperature, increased evaporation instantly compensates for the extra heat.
TonyB: Your CET work is interesting, however: “Please note that the graphing package somewhat inflates the warmth in the decade around 1540”
Don’t blame the “graphing package” you are not centering then running average ( which is a crap filter to start with.). The reason 1540’s looks too high is because it’s too late ! It is obvious to the natked eye. Shift the “smoothed” version back 5y and both the 1540 and y2k (in fact the whole graph) will line up a lot better.
Unusually, there don’t seem to be the typical peak inversions that RM often creates, just luck of the draw in the periodiciteis present in the data.
One thing that does look wrong in the cira 2000 peak if far too pointy in realation to the unfiltered data.
Try triple running mean of half the length and it will be just as “smooth” and probably follow better.
https://climategrog.wordpress.com/wp-admin/post.php?post=989&action=edit
Mosher says
Why are you so obsessed with arguing with warmest using their play book? They are a bunch of liers trying to control you and me. When you argue inside the constraints they set they can simply say you accept their argument but misunderstand its complexities and misinterpret their results.
You let them be the authority and they can dismiss you as a cub who has strayed from the fold.
One should argue what they believe and can back up, not what is accepted by the established.
They have an agenda and could careless where the truth lies.
Genghis says:
August 10, 2014 at 1:54 pm
Donald L. Klipstein says:
If the top 3 microns warms up without the 10 micron level warming up, then heat conduction through the extremely thin temperature gradient between 3 and 10 microns down will cool the top 3 microns faster than increased evaporative cooling. Evaporative cooling won’t outpace heat conduction until the temperature gradient expands over a much greater range of depth, at which point mixing becomes significant at transferring heat to deeper levels.
************
You have it wrong. The top couple of microns don’t heat up. There is no temperature gradient change.
Again the best analogy is measuring the temperature of boiling water while increasing the amount of heat being applied, the liquid water will stay at exactly the same temperature, increased evaporation instantly compensates for the extra heat.
======
Everyone seems to love making assertions and contra assertions about this subject. Anyone got anything more concreate that back of envolop arguments and assertion?
Greg
Thanks for your comments. I always read your material.
There was a sharp peak in Cet around 2000 as can be seen in the met office Hadley CET 1772 figures.
I met up with David Parker at the Met office last year, who created the record. They haveRecently changed the stations being used as they felt they were running too warm. My estimate would be that the upwards incline to 2000 was too sharp and the decline since too exaggerated. The temperatures have started rising again but are still nowhere near their peak.
Incidentally, whilst it was an artefact of the graphing package to some extent! further research has shown that the pre 1540 period showed a sharp temperature increase. There was a sharp decline from around 1450 to 1500
Tonyb
Genghis:
I asked the question:
“So you agree that the overall earth/ocean/atmosphere energy level is higher with this radiation than without it?”
and you responded:
“No, the energy in the Ocean is not increased at all, because the DWLWIR never gets past the first couple of microns, before it gets evaporated away…”
which is not an answer to my question at all. I carefully asked about “the overall earth/ocean/atmosphere energy level”.Let’s grant, at least for the sake of argument, the the DWLWIR does not affect the temperature, and therefore the energy level, of the ocean itself. But by evaporating water from the surface, it does add the latent heat of evaporation to the overall system.
The reason I make a point of this is that there are many (and I don’t necessarily include you in this) who believe that because the liquid water body temperature does not apparently increase in the presence of increased DWLWIR, that the energy in this radiation is somehow lost. And that would be a blatant 1st Law violation.
There is substantial research showing that significantly increased or decreased temperatures reduces crop yields. That same research showed that increasing atmospheric CO2 could more than off-set the yield reductions resulting from those temperature changes.
Unless it can be shown 1) that temperatures have risen at least in part due to Man’s CO2 contributions; 2) that that rise in temperature produced an unacceptable change in climate; and 3) reducing CO2 absolutely will reduce temperatures, then the precautionary principle dictates that we must NOT reduce atmospheric CO2.
In other words: if any temperature change up or down is caused primarily by natural variation, then we will only be reducing our global food supplies by reducing CO2.
AGAIN with this absurd idea that DWLWIR can do thermodynamic work on the surface?! It’s not a heat flux, folks! If it warms the top 3 microns of the surface skin, it means it’s heat. If it provokes more evaporation, it means it’s heat. Because only ‘heat’ (and ‘work’) are real, thermodynamically working flows of energy. Actual, detectable transfers of energy. DWLWIR is not heat! Energy is transferred radiatively from the warm surface to the cool atmosphere only. As radiative heat. Period. Stop pretending DWLWIR is a separately working flux of energy, operating as if it were heat, distinct from the UWLWIR within the same, integrated radiation field. It’s not.
richard verney says, August 10, 2014 at 11:09 am:
“Genghis says:
August 10, 2014 at 10:11 am
Infrared (atmospheric radiation) is absorbed by the top few microns of the surface, which does in fact heat the ocean.
///////////////////////
Does the energy absorbed in those 3 microns heat the ocean? For it to do so, it needs to be dissipated (and hence diluted) to depth at a rate faster than the energy absorbed in the top 3 microns would power/drive evaporation from the surface layer of the ocean.”
No, Richard. It needs to be HEAT. And it’s not heat. DWLWIR is not heat and hence it cannot heat the top 3 microns of the skin layer and it cannot provoke more evaporation.
Climatology is the art of looking for trouble, finding it everywhere, diagnosing it incorrectly and applying the wrong remedies. (apologies to Groucho Marx)
Curts says
If DWLWIR is not being absorb by the oceans (which it is not) for it to raise the overall earth/ocean/atmospher energy level you would have to see a rise is atmospheric temperatures. Which is not happening. This can only mean there is an increase of energy transfer in to space.
There is no effect model of how AGW can transfer heat to the oceans and not have a corisponding rise in atmospheric temperatures at the same time.
cnxtim says:
Is this anything more than a “face saving” exit plan for all the CAGW evangelists?
“Yo wagons ho!, thar be the real … (insert what you will here).
I think the word you want may be ‘elephant’.
http://www.wordorigins.org/index.php/site/comments/elephant_to_see_the/
: >)
Curt,
The atmosphere resting on top of the solar-heated surface does of course make less energy go out from the surface per unit of time at equal temperature than if there were no atmosphere, only the vacuum of space. This is because the atmosphere has a mass. It thus has a ‘heat capacity’. It is able to warm. Space isn’t. It also, for the same reason, weighs down on the surface (space doesn’t), setting a limit to buoyant acceleration and evaporation rates at a certain temperature. This is what forces Earth’s surface to be much warmer than the Moon’s. It’s got nothing to do (it couldn’t have) with energy INPUT to the surface from a cooler place. It has to do with a smaller energy OUTPUT from the surface to this same cooler place. Because this cooler place is still warmer than space. The atmosphere still gains energy from the surface, by the simple fact of being the cooler of the two systems. It’s all a matter of how much it gains per unit of time. And this is set by the temp gradient and the weight of the atmosphere on the surface.
Come back when you can show us how the presence of radiatively active gases in our atmosphere – specifically through their radiative properties – actually reduce (or work towards reducing) the tropospheric temperature profile. If they don’t, then they don’t contribute to the warming of the surface. Simple as that.
“The rapid logarithmic diminution effect is an inconvenient fact for Global Warming advocates and alarmists, nonetheless it is well understood within the climate science community. It is certainly not much discussed.”
It is much discussed. Whenever people refer to sensitivity as 2&dseg;C/doubling, or whatever, they are invoking the logarithmic behaviour.
“CO2 at 400pmmv is already committed and immutable. So CO2 has already reached about ~87+% of its potential warming effect in the atmosphere”
The basis for this percent arithmetic (here and earlier) is not stated, and it makes no sense. A logarithmic curve has diminishing slope, but no maximum or minimum. If sensitiivity is 2K/doubling, and if 400 ppmv corresponds to equilibrium 288 K, then 400 * 2^-144 ppmv corresponds to 0K. Or 400 * 2^144 ppmv to 576 K. Of course, what that shows is simply that logarithmic behaviour is just an approximation that works in a limited range.
“The recent IPCC report now admits that currently increasing CO2 levels are probably only ~50% man-made.”
Where?
We are now at 50% of the logarithmic calculated theoritical doubling of GHGs.
50% of doubling —> +0.6C temperature increase (once you factor in the Mosher-sanctioned fake temperature adjustments).
Half-way, +0.6C.
Half-way and plants are growing much better as in yields are way-up, even your grass is growing faster.
Now we can also say the warming in the pipeline held by the oceans is only 0.2 W/m2/year after the newest results or nothing to worry about.
Full-way to doubling –> +1.2C? plus a little more once the oceans catch up that little bit and plants are more productive.
The numbers make increased CO2 a positive development for the planet. Simple as that.
Greg Goodman says:
August 10, 2014 at 2:09 pm
Everyone seems to love making assertions and contra assertions about this subject. Anyone got anything more concreate that back of envolop arguments and assertion?
+++++++++++
I am sitting in Manjack, in the Abacos this very moment with an IR gun reading the surface temperature, it is 31.4˚ C. with a clear sky, almost dead calm conditions. If conditions stay the same, when I take a reading sometime tonight, when I check the anchor, the temperature will be the same whether it is a clear sky or cloudy. In the morning it will most likely be cloudy and the same temperature.
If the wind picks up a little the surface temperature will go down. If the wind picks up a lot the temperature will stabilize at a new point, probably warmer.
I have been doing this for a couple of years now in various anchorages and I have seen the surface temp stay the same for days and even the occasional week or two. When I say the same temp I mean within a few tenths.
As a farm boy from the high deserts in Idaho, I have to admit that I was blown away by the temperature stability of the ocean surface.
So Nick Stokes has gotten permission from Hansen to reappear?
H. Grouse, John Carter and John Finn will be around any minute now.
I discussed this issue of how much infrared energy is being absorbed by CO2 with Hu McCulloch a couple months ago. He had an interesting insight. Whatever function we use to compute the radiative forcing for CO2 has got to be a bounded function. Once all the infrared in some frequency band is absorbed, adding more CO2 to the atmosphere will not result in any additional radiative forcing. Therefore, instead of ln(X), the formula for radiative forcing should something more like 1 – e^(-x).
Nick Stokes says:
August 10, 2014 at 3:06 pm
The basis for this percent arithmetic (here and earlier) is not stated, and it makes no sense. A logarithmic curve has diminishing slope, but no maximum or minimum.
Help me out with that, Nick.
Wouldn’t the minimum be either at 0% atmospheric CO2 or, at least, 1 part CO2 to the entire atmosphere; while the maximum would be at either 100% CO2 or, at most, a CO2 atmosphere with only 1 part non-CO2?
At 50% CO2 there is only one doubling left. Wouldn’t the maximum then be reached?
The bigger question would be: at what point do further doublings add only a barely measureable amount of warming?
If all of this isn’t in the “settled science” realm after all the discussions over the years, will it ever be?
Just wondering.
This is all well and good as a purely radiative argument. However, the derivation of the thermal gradient within a gravitationally bound atmosphere pays no heed to inter atmospheric radiative exchange. The tropospheric lapse rate is dT/dh=-g/Cp gives a monumentally solid depiction of the bulk if the lower atmosphere up to the point where direct atmospheric heating (solar) drives the system out of the reversible adiabatic profile. This can be derived through equating kinetic and potential energy to a dQ=0 (adiabatic) condition or thermodynamically from the gas laws.
So, theoretically, back radiation ‘heats’ the lower atmosphere with ‘special molecules’ whilst these same molecules reduce the intensity of solar energy reaching the surface and increase the upper atmospheric emissivity. Even though we haven’t used these to calculate the thermal relationship between the upper and lower troposphere, well, no further than how by mass density the molecules affect Cp. Increasing upper atmospheric emissivity cools the upper, but the upper and lower are tied by the mechanical lapse which for a given heat capacity ‘fixes’ the gradient set by gravitational containment for long term stability.
Radiative exchange never produces a thermal gradient. The exchange, reduces the thermal gradient. Gravity is responsible for the enhancement of the surface temperature and the mass aloft, as all interacting matter radiates, accounts for the necessary decrease in surface emissivity. The transmission of the atmosphere to short wave and long wave radiation is not that dissimilar, the Sun producing most of its radiative emissions in the infra red (51% IR, 37% visible) which then covers ever spectral line of every GHG. More GHG’s, less heat reaching the surface, more upper atmospheric cooling, some ‘calculated back radiation’. Net effect, zero measurable.
sturgishooper says: miskolczi.pdf
http://www.google.com/url?sa=t&rct=j&q=&esrc=s&source=web&cd=2&ved=0CCkQFjAB&url=http%3A%2F%2Fnige.files.wordpress.com%2F2011%2F02%2Fthe-saturated-greenhouse-effect-theory-of-ferenc-miskolczi.pdf&ei=bsXnU470BsnXiwKy6IG4Dw&usg=AFQjCNHGM-LHU1GLNqCdX3gd6mZ1w9adew&sig2=03MycldsSWC7Sh3sxecWow&bvm=bv.72676100,d.cGE
That looks pretty good on a first reading.
Why in years on this site have I never seen that mentioned before ?
Thanks.
Kristian says:
. Because only ‘heat’ (and ‘work’) are real, thermodynamically working flows of energy.
>>>>>>>>>>>>>>>>>>>
I hook a battery up to an electric heater. The heater gets hot. Check the wires, no temp change. Check the battery, no temp change. But the heater gets hot Apparently there IS a working flow of energy that DOES do work.
Now that you’ve made a complete fool of yourself (yet again) and your pathetic grasp of the physics is on full display, please STFU and go away.
*Standard Request* While I appreciate that people sometimes like my comments, please do not hit the like button as it fills my inbox with spam from WordPress.
..can we hit Kristian’s like button?
E.M.Smith says:
August 10, 2014 at 1:03 pm
So yes, as a ploy, it is useful to argue from the other side’s premisses. But just be careful you do not embrace them as true, nor imply they are accepted truth.
=====
…..+100
Greg
Myself and no doubt many others have referenced this work before. According to the warmists there is a glaring mathematical mistake which renders it invalid. If Nick stokes turns up he can no doubt clarify what it is. Failing that I am sure our friends at Real climate or Skeptical science have written about it.
Tonyb
Peter Miller says:
August 10, 2014 at 10:04 am
The EPA should be pointing this out to Obama and admitting that it needs to be downsized.
Given the blatant porkies he’s been uttering and amplifying, “pointing out” is taking a butter knife to a gun fight. And the EPA is itself on a power binge; why would it disarm itself?
Curt says:
August 10, 2014 at 2:19 pm
Genghis:
I asked the question:
“So you agree that the overall earth/ocean/atmosphere energy level is higher with this radiation than without it?”
and you responded:
“No, the energy in the Ocean is not increased at all, because the DWLWIR never gets past the first couple of microns, before it gets evaporated away…”
which is not an answer to my question at all. I carefully asked about “the overall earth/ocean/atmosphere energy level”.Let’s grant, at least for the sake of argument, the the DWLWIR does not affect the temperature, and therefore the energy level, of the ocean itself. But by evaporating water from the surface, it does add the latent heat of evaporation to the overall system.
==================
Yes the latent heat is increased in the atmosphere.
==================
The reason I make a point of this is that there are many (and I don’t necessarily include you in this) who believe that because the liquid water body temperature does not apparently increase in the presence of increased DWLWIR, that the energy in this radiation is somehow lost. And that would be a blatant 1st Law violation.
==================
The total energy flux in the system is exactly the same. What changes is the direction and rate.
==================
Kristian;
No, Richard. It needs to be HEAT. And it’s not heat. DWLWIR is not heat and hence it cannot heat the top 3 microns of the skin layer and it cannot provoke more evaporation.
>>>>>>>>>>>>>>>
No, it is an energy flux and just like the energy flux from a battery in my previous comment, it can and does do work. Energy can take many forms and both electric current and radiated energy are examples of an energy flux that can create heat when absorbed by a matter. In the same manner, a moving object stores energy as kinetic energy which it can turn into heat through friction. There are a plethora of examples all around you falsifying your ignorance, you only need think about it for a moment to see that you are dead wrong.
*Standard Request* While I appreciate that people sometimes like my comments, please do not hit the like button as it fills my inbox with spam from WordPress.
imS says:
August 10, 2014 at 8:28 am
I have found that your average AGWer will not admit to the Pause for the last 17 years. Nor will they accept the concept that CO2 follows temperature shown in the historical record via the ice core data. Therefore, your average AGWer will never, ever accept the fact of CO2 having an algorithmic impact on global temperatures. Any information coming from a “denier” website is instantly dismissed.
>>>>>>>>>>>>>>>>>>>>>>>>>>>>>>>>>>>>>>>>>>>>>>>>>>>>>>>>>>>>>
The default position of all “leftists” is that humans are evil, that “the selfish gene” is not a necessary requirement for life, and that we must be controlled to “sacrifice for your fellow man”. This Altruistic moral code is at the root of all hatred of industrialization and Capitalism (the restriction on government and freedom of the individual). (not to be confused with the Crony Socialism of Big Business using government to steal from the rest of us)
Allow individuals to lead productive, happy, selfish lives? NO WAY! Since you are so selfish, you must be forced into the sacrificial furnaces at the point of a gun!
Accordingly, under their moral code, any means to achieve the dreary life of sacrifice of all to all is moral. They can lie, cheat, steal and kill to achieve this goal. AND THEY DO. They can invent fictional stories about global threats, while ignoring the real threats. They have indoctrinated our children, propagandized everything from Hollywood to the media and have now gotten control of the levers of power through lies, voter fraud and more. Hopefully, the American people are waking up to the lies and machinations, of which AGW is but one facet. It seems they are.
Greg
Here is the Real Climate riposte to the 2007 and 2010 paper
http://www.realclimate.org/docs/Rebuttal_Miskolczi_20100927.pdf
Tonyb
Latitude says:
August 10, 2014 at 3:41 pm
..can we hit Kristian’s like button?
>>>>>>>>>>>>>>>>>
If your goal is to encourage science so bad that it isn’t even wrong, by all means 😉
JohnWho says: August 10, 2014 at 3:34 pm
“Help me out with that, Nick.
Wouldn’t the minimum be either at 0% atmospheric CO2 or, at least, 1 part CO2”
Zeno could have worked that out. If you cool 2°C per halving, how many times do you have to halve to get to 0%?
And what is 1 part CO2? What is special about it?
And you could say that 100% CO2 is an effective max. At 2°C/doubling, that’s more than 30°C rise.
TonyB:” My estimate would be that the upwards incline to 2000 was too sharp and the decline since too exaggerated. The temperatures have started rising again but are still nowhere near their peak.”
The almost linear ramp either side of Y2K is a result of the filter. It cam be seen by eye that the annual data are not that pointed.
Try what I suggested or post a link to exactly what data you are using there and I’ll plot it up.
Donald L Klipstein;
Since an increase of surface temperature in response to an increase of GHGs causes an increase of outgoing surface radiation, it is expectable that the surface forcing from an increase of GHGs is less than the radiative forcing from GHGs.
>>>>>>>>>>>>>>>>>
And hence, the temperature change at surface must be less than would be calculated by strictly using SB Law and RF versus effective BB temp of earth. Which was my original point.
If your goal is to encourage science so bad that it isn’t even wrong, by all means 😉
===
you know me better than that….. 😉
Hmm, and here I was, thinking that David M. Hoffer had sworn to himself and to the world to ignore me in the future. So much for that, it seems …
And he still doesn’t see that everything he says only displays his utter ignorance on thermodynamic concepts and principles. He simply doesn’t know what heat is. To him there is no difference between opposing radiant emittances and radiative heat inside a thermal exchange. They both heat in his world. We’re talking about radiative thermal exchanges, David. Where one body heats another one. Not batteries. You simply don’t get it.
You should’ve taken the hint when even Nick Stokes – not exactly a skeptic to AGW – on the Bombshell thread pointed out to you ‘the impossibility of getting work from DWLWIR’.
But no, you’re simply incapable of admitting you’re wrong, aren’t you David?
Bob Boder says: August 10, 2014 at 3:29 pm
“So Nick Stokes has gotten permission from Hansen to reappear?
H. Grouse, John Carter and John Finn will be around any minute now.”
It’s true that it’s taken a while for real scepticism to be expressed.
Care to explain the arithmetic behind
“CO2 at 400pmmv is already committed and immutable. So CO2 has already reached about ~87+% of its potential warming effect in the atmosphere.”
Or any of the % statements?
Anyone?
Kristian says:
August 10, 2014 at 2:38 pm
No, Richard. It needs to be HEAT. And it’s not heat. DWLWIR is not heat and hence it cannot heat the top 3 microns of the skin layer and it cannot provoke more evaporation.
==========
Kristian, you are correct IR radiation is not “heat’ but it can and does warm objects. It also cools objects when it is radiated and everything above 0K radiates.
When I point my IR gun at something it reads the IR radiation and converts that to an approximate temperature. It doesn’t really matter what the object is, clouds, mufflers, clear sky, coolant hoses, a fire, water, you get the idea. It isn’t always accurate but it tries : )
Nich: “And what is 1 part CO2? What is special about it?
And you could say that 100% CO2 is an effective max. At 2°C/doubling, that’s more than 30°C rise. ”
This whole argument is pointless. As you commented, the current log relationship does not apply indefinitely ( in either direction ).
The basic CO2 band is already saturated. The log relationship comes from spectral broadening. At very much lower concentrations the molecules do not mask each other ( too few ) and relationship is linear and so does not go down to negative infintiy.
The log relationship is a reasonable approximation to the regime we are in. It is not intended or expected to be pushed to mathematical extremes and still apply. But I think you realise that already.
Ok so Mosher and Stokes are back.
I’ll plead with them once again to humor this simple man.
Can one of you please give me a current articulation of how the scientific concept of
human CO2 emissions=AGW is supported by any scientific measurement or observation?
It’s been troubling to listen to the alarmists’ fallacy that nature cannot explain the supposed unusual global warming during the last 70 or so years.
That alone, on it’s face, is offensively asinine.
Forget that.
Just tell me how the human role in the greenhouse effect has been measured and determined by science.
Because the human relative contribution is infinitesimal the effect cannot be measured.
Perhaps you can dispute that the “human greenhouse gas contributions add up to about 0.28% of the greenhouse effect” ?
http://www.geocraft.com/WVFossils/greenhouse_data.html
You’re obviously a couple of believers. So why not share what it is you believe in?
You appear to be deliberately avoiding the central issue while clinging to the weeds of the matter.
Only ~3.75% of atmospheric CO2 is man-made from burning of fossil fuels
http://hockeyschtick.blogspot.com/2014/07/new-paper-finds-only-375-of-atmospheric.html
Water Vapor is not cooperating and the margin of error to global temperature is 1 degree C.
There is no Fossil Fuel CO2 warming and never was.
Kristian;
We’re talking about radiative thermal exchanges, David. Where one body heats another one. Not batteries. You simply don’t get it.
>>>>>>>>>>>>>>>>>
The heat you feel from the Sun is actually identical in all respects save frequency and wavelength from DWLWIR. It is an energy flux which is converted to heat when absorbed by matter, If your brain dead version of physics is correct then the Sun cannot heat the earth. But it does.
I used to believe that CO2 causes a limited warming too, but I am not so sure now, Ithink Venus shows that there is no effect whether 0.004% or 95% http://theendofthemystery.blogspot.com/2010/11/venus-no-greenhouse-effect.html
Genghis:
==================
The total energy flux in the system is exactly the same. What changes is the direction and rate.
==================
I don’t know if that what you meant to say but it is contradictory. If the magnitude and direction of something changes, it is not “exactly the same”, it is totally different.
Greg Goodman says: August 10, 2014 at 4:06 pm
“The log relationship is a reasonable approximation to the regime we are in. It is not intended or expected to be pushed to mathematical extremes and still apply. But I think you realise that already.”
Indeed, and I said so. But I don’t think the author of this post does.
Tonyb says:
Here is the Real Climate riposte to the 2007 and 2010 paper
http://www.realclimate.org/docs/Rebuttal_Miskolczi_20100927.pdf
Thanks, I’m wary of claims that climate is totally self regulating because it’s not:
http://climategrog.wordpress.com/?attachment_id=902
I think we should start referring to Kristian as “Murphy”, because if there is a way misunderstand a subject, he will find it and embrace it.
He manages to get stuck in the older 18th/19th century caloric-theory concept of heat as a physical entity rather than understanding heat transfer as a process resulting from various underlying physical processes.
In the case of radiative exhange, he makes a couple of critical mistakes. First, he does not realize that the radiative heat transfer between two objects is simply the result of the difference between two opposing radiative “flows” sharing a common geometry between them. He prefers the analytic abstraction of first computing a difference in radiative “potentials” computed from absolute temperatures and emissivities, then computing the net heat transfer using the geometric “view factor” between the two objects. But he mistakes this convenient abstraction for the underlying physical reality.
The second mistake he makes is thinking that his preferred method of analysis leads to a different result in computing the heat transfer. In essence, he likes to compute the heat transfer as k*(T1^4-T2^4), with k encompassing emissivities, geometry, and the SB constant. Others like to compute the transfer as (k*T1^4) – (k*T2^4). Those of us who actually remember the distributive property we learned when we were 8 years old realize that the two forms are equivalent.
davidmhoffer says: August 10, 2014 at 4:09 pm
“If your brain dead version of physics is correct then the Sun cannot heat the earth. But it does.”
No. You’re not distinguishing between heating and doing work.
The Earth’s surface radiates an amount that is roughly the sum of solar and DWLWIR, and so can stay in balance at a much warmer temperature than without DWLWIR. So to that point they are alike.
But solar can do work and DWLWIR can’t. To make heat do work, you have to collect heat at one temperature and have it pass to a cooler sink. With solar you can do this because
1. solar is time varying. At the max, you can find cooler sinks to pass the heat to. But more importantly
2. solar comes from a small fraction of the sky, and can be focussed. A collector for DWLWIR has to be exposed to the whole sky (or if not, will collect proportionately less). Since it is warmer than the sky, it must lose more than it gains. By focussing, you can collect almost all the solar, and emit very little in return.
Greg
I like the theory, but as far as I am aware miskolezi has never satisfactorily rebutted the claimed faults in his paper. it would be interesting to hear your take on it in due course
tonyb
I have been playing around in a thought experiment related to the measurement of CO2 and the data’s unique regular pumping-like behavior. Nature is a rather noisy place with ups and downs. When it turns regular, as in our slowly increasing CO2, I ponder the cause. What on Earth could be pumping like that? Surely not the vagaries of human endeavor, here today, gone tomorrow. Something bigger. The ever slightly increasing, and seasonal CO2 data set appears to me that it is reflecting CO2 being pumped into the atmosphere from a reservoir, but from where? Again, I turned away from the vagaries of human production because we have such a noisy data set globally when it comes to human endeavors because the slightly increasing pumping nature of the CO2 set makes no sense when considering our noisy lives. I am thinking bigger, and cyclical, with a good supply of CO2. I am thinking the oceans are seasonally AND at multi-century time scales outgassing it, which explains the regular pumping like nature of the measurement trend you see in both the yearly sensors and the ice age ice core measurements (there is an up and down nature to the data which is then averaged to show the ice ages).
So my thoughts have turned to the overturning global circulation. It takes approximately 600 years for that cycle to complete itself. Could it be that CO2 laden material rode the slide down to the ocean bottom, to be brought back up again and belched out? In other words, are we looking at too fine a CO2 scale when we look at Mauna Loa data, and too large a scale when we look at ice age scale ice core CO2 data? Could there be a CO2 pumping up and pumping down that coincides with the quasi-periodic overturning circulation which in term coincides with warming episodes that drive greening and then cooling episodes that kill it off, sending it to the ocean bottom?
Just my meandering thoughts on CO2 and its very cyclic seasonal nature and very regular slight increase each year. We may have errored on both sides of zooming in and out. We look too closely, and too far away.
Nick Stokes;
But solar can do work and DWLWIR can’t.
>>>>>>>>>>>>>>>
Congratulations Nick, you’ve disproved Stefan-Boltzmann Law and Planck with a single sentence. You’ve proven that microwave ovens don’t work and neither do nuclear reactors. You’ve falsified the GHE which, based on your past performance on this blog, you’ve always insisted exists. The Nobel committee will no doubt be calling you shortly.
Genghis says, August 10, 2014 at 4:06 pm:
“Kristian, you are correct IR radiation is not “heat’ but it can and does warm objects. It also cools objects when it is radiated and everything above 0K radiates.
When I point my IR gun at something it reads the IR radiation and converts that to an approximate temperature. It doesn’t really matter what the object is, clouds, mufflers, clear sky, coolant hoses, a fire, water, you get the idea. It isn’t always accurate but it tries : )”
Yes, this is the very misconception that made the AGW hype possible to begin with and why we are still discussing its merits, and why we will never be able to fully discredit it, even after it’s shown to be wrong. People simply can’t tell radiation from heat in a thermal exchange. This blind spot is more ingrained and more prevalent than I could’ve ever imagined.
Frankly I’m appalled.
Genghis. If IR radiation is not “heat”, then it cannot warm objects. Get it? Heat heats. That’s why we call it heat. Get it? Radiation (radiant emittance) in an by itself doesn’t heat. Radiation in the form of radiative heat does. DWLWIR is emittance, not heat, and therefore it cannot heat (or ‘warm’) anything. It cannot provoke evaporation either, because that would be thermodynamic work.
People, you need to learn about the laws of thermodynamics. About heat and work and internal energy. If everyone only understood those simple and fundamental concepts, we wouldn’t be having this discussion at all.
Kristian;
Genghis. If IR radiation is not “heat”, then it cannot warm objects. Get it? Heat heats. That’s why we call it heat. Get it?
>>>>>>>>>>>>>>>>>>
Electricity heats, friction heats, neither of them are heat. You’re an idiot. Get it?
davidmhoffer says, August 10, 2014 at 4:59 pm:
“Nick Stokes;
But solar can do work and DWLWIR can’t.
>>>>>>>>>>>>>>>
Congratulations Nick, you’ve disproved Stefan-Boltzmann Law and Planck with a single sentence. You’ve proven that microwave ovens don’t work and neither do nuclear reactors. You’ve falsified the GHE which, based on your past performance on this blog, you’ve always insisted exists. The Nobel committee will no doubt be calling you shortly.”
No, he hasn’t, David. Because he understands thermodynamics. Unlike you. He understands that it’s only the NET energy that matters. The heat. The heat is the only actual transfer of energy in a thermal exchange capable of doing thermodynamic work. What the presence of the atmosphere does is make the heat out from the surface smaller compared to a non-atmo situation. This is not hard, David.
TonyB: “I met up with David Parker at the Met office last year, who created the record. They haveRecently changed the stations being used as they felt they were running too warm.”
Wow, that’s unprecedented ! An adjustment that does not boost AGW.
However, the mindset is not reassuring, whichever way they tweek the data. Up a bit, down a bit until they “feel” it’s about right. Then they still call it data and not an expression of their preconceptions.
This is the same problem as the SST adjustments. They deliberately ignore the written metadata for a large number of SST logs inverting ERI to buckets and vice versa, in order to get what they consider the correct statistical mix of readings for each month in each gridbox.
ie their _speculative_ estimations of how and when change overs happened, based largely on guesswork and anecdotal evidence from the admiralty, are allowed to trump the written record.
Sadly I have little faith in anything they do any more.
Kristian;
This is not hard, David.
>>>>>>>>>>>>>>>>>
Well apparently it is because you are having a devil of a time understanding it.
Disqus puts a “settings” link at the top of its e-mails. When clicked, that ought to take you to a site where you can turn off the e-mails.
Kristian;
Do you believe that Stefan-Boltzmann Law of physics is correct?
Disqus puts a “settings” link at the top of its e-mails. When clicked, that ought to take you to a site where you can turn off the e-mails.
>>>>>>>>>>>>>>>
Thanks Roger. But I’m not logged in with Disqus, I’m logged in with WordPress. When I log out of WordPress, the site won’t let me comment unless I log back in again. Then when I do, it ignorantly tells me that “you’ve already said that”.
Given that the emission of CO2 is not as ‘effective’ in accumulating as simply emitting it, because at least 1/2 is absorbed quickly by various natural processes (half of man’s emissions) and more later. Before being able to support any of the claims for taking it to, for example, 1000 ppm, some evidence should be given that there is enough accessible ‘fossil fuels’ on the planet to create that much CO2, at a sufficient rate to overcome the absorption. This is no trifling matter.
The work above is valuable but the argument takes place within a context that, as far as I can see, assumes there is an infinite supply of fossil fuels, something fundamentally opposed, conceptually, by the very ‘environmental’ groups opposing its combustion. So after peak oil, peak gas, peak coal, peak peat, peak biomass, all of which may occur this century, where is the rest going to come from?
Tonyb says:August 10, 2014 at 3:50 pm
“Here is the Real Climate riposte “
RC also posted my earlier response
here
So the article claims that warming will be
“probably beneficial up to about a further 2.0°C+ [5].”
and that
“In a rational, non-political world, that prospect should be greeted with unmitigated joy.”
Leaving aside the politics, in what way is it rational to “greet with unmitigated joy” the sea level rise that would result from a further 2C temperature rise ?
What would the economic cost be of trying to defend major coastal cities from such a sea level rise, or the costs of failing to do so ?
At current sea level serious flooding has occurred in recent years due to storm surges in New Orleans and New York. Massive damage was caused in Japan when its coastal defences were over-topped by a tsunami. Add in higher sea level and all these risks get worse.
climatereason says:
August 10, 2014 at 4:55 pm
He hasn’t addressed it that I have seen, although he was interviewed in 2012. However, recent findings on atmospheric water vapor appear to bear out his hypothesis, despite his own couched language:
http://hockeyschtick.blogspot.com/2012/02/new-paper-supports-miskolczis-theory-of.html
http://hockeyschtick.blogspot.com/2014/02/observations-confirm-miskolczi-theory.html
The same study was featured in blog posts here.
Hi David,
Re The logarithmic response means that we need to add twice as much CO2 again to create the same amount of warming we created with the initial increase…..
I think you mean 10 times the amount based on a Log base 10 effect to get twice the temp.
Curt says, August 10, 2014 at 4:52 pm:
I think we should start referring to Kristian as “Murphy”, because if there is a way misunderstand a subject, he will find it and embrace it.
He manages to get stuck in the older 18th/19th century caloric-theory concept of heat as a physical entity rather than understanding heat transfer as a process resulting from various underlying physical processes.”
Hehe, you not getting what I’m saying, Curt, or perhaps rather willfully trying to misrepresent me, doesn’t make me wrong. I am not stuck in the 18th/19th century caloric theory. The caloric theory looked at heat an an entity residing inside an object and which could be transferred to reside in another object. This is not the ‘heat’ I’m talking about. Heat is not contained inside a body. Heat is simply the spontaneous transfer of energy from hot to cold as a result of the temperature difference. Heat is one of the fundamental concepts of thermodynamics. It is still very much in use today, extremely useful. All modern textbooks on thermodynamics uses the heat concept that I’m trying (with little success, it seems) to explain and describe to the people here. The concept of heat, the effect of a heat transfer, just like the laws of thermodynamics, don’t change even if we find out more about the microscopic processes behind it, Curt. The principle of heat applies just as much to a radiative exchange as to a conductive or convective transfer.
Nick Stokes;
But solar can do work and DWLWIR can’t. To make heat do work, you have to collect heat at one temperature and have it pass to a cooler sink.
>>>>>>>>>>>>>>>>>>
Give me a parabolic dish large enough that reflects in the IR spectrum, and I can boil a pot of water in seconds with it at night with DWLWIR. I don’t have to pass anything to a cooler sink because the surface below the parabolic reflector would cool commensurate with the reduced DWLWIR that would otherwise have been absorbed, and would be cooler than the surrounding area as a consequence. Would this be a practical way to gather energy and put it to use? Not a chance. There are far easier ways to boil a pot of water.
”
Like
Nick Stokes says:
August 10, 2014 at 4:52 pm
davidmhoffer says: August 10, 2014 at 4:09 pm
“If your brain dead version of physics is correct then the Sun cannot heat the earth. But it does.”
No. You’re not distinguishing between heating and doing work.
The Earth’s surface radiates an amount that is roughly the sum of solar and DWLWIR, and so can stay in balance at a much warmer temperature than without DWLWIR. So to that point they are alike.
But solar can do work and DWLWIR can’t. To make heat do work, you have to collect heat at one temperature and have it pass to a cooler sink. With solar you can do this because
1. solar is time varying. At the max, you can find cooler sinks to pass the heat to. But more importantly
2. solar comes from a small fraction of the sky, and can be focussed. A collector for DWLWIR has to be exposed to the whole sky (or if not, will collect proportionately less). Since it is warmer than the sky, it must lose more than it gains. By focussing, you can collect almost all the solar, and emit very little in return.
”
To do work , one uses a heat engine with a hot reservoir and a cold reservoir. Earth radiates to the universe at 2.7k. That means essentially any Earth temperature can be use in a heat engine.
DWLWIR is going into an atmospheric heat engine, increasing the water cycle. That results in more daytime low level clouds that block SW and reduce surface heating capable of penetrating ocean surfaces. Water vapor rises carrying heat upwards to the point where clouds form and radiates power outward, as cloud continuum, dymers, and individual molecules high above most of the pressure broadening and ghgs.
davidmhoffer says: August 10, 2014 at 5:25 pm
“Give me a parabolic dish large enough that reflects in the IR spectrum, and I can boil a pot of water in seconds with it at night with DWLWIR.”
No, you can’t focus DWLWIR at all. That’s just geometry.
”
davidmhoffer says:
August 10, 2014 at 5:25 pm
”
I want to see someone boil a pot of water by heating from the top.
Nick Stokes;
No, you can’t focus DWLWIR at all. That’s just geometry.
>>>>>>>>>>>>>>>>>
For gawd’s sake Nick, I can go to any decent hardware store and buy a propane heater with a parabolic reflector on it for the express purpose of focusing IR. The damn things work as anyone who has ever used one can tell you. Your detachment from the real world is alarming.
Greg Goodman says:
August 10, 2014 at 4:15 pm
Genghis:
==================
The total energy flux in the system is exactly the same. What changes is the direction and rate.
==================
I don’t know if that what you meant to say but it is contradictory. If the magnitude and direction of something changes, it is not “exactly the same”, it is totally different.
******************
You are right that didn’t come out well at all. Let me try again.
The decrease in net radiation between the clouds and the ocean surface is exactly matched by the increase in latent heat of evaporation.
I went outside and measured the ocean surface again, it is now dark at 8:40 pm, continued calm, clouds are now forming and the surface is still 31.4˚ C, exactly the same as it was a few hours ago when the sun was shining and not a cloud in the sky.
The temperature on my inlet gauge, a meter down, has declined since the sun has set as it usually does.
@ TonyB , re http://wattsupwiththat.com/2013/05/08/the-curious-case-of-rising-co2-and-falling-temperatures/
Look at the M.O. page, their graph doesn’t ramp straight up and down around y2k.
http://hadobs.metoffice.com/hadcet/
“The graph above shows annual anomalies relative to the 1961-1990 average. The red line is a 21-point binomial filter, which is roughly equivalent to a 10-year running mean.”
Ignore the up tick at the end, they are up to their old tricks of padding the data with the last value again. They usually decide this is bad practice when it goes the other way !
Through the early 2000s they were doing this on SST. When recent temps were lower than the peak and the padded filter was showing a downturn, they discovered that this was misleading. Now it’s going up for CET, it looks to be fine again. You’ll note they don’t even us a different line for padded sections.
Donald L. Klipstein says:
August 10, 2014 at 12:53 pm
/////////////////////////
It is not easy to see how the 4 to 10 micron layer is heated by conduction from the ‘heat’ absorbed in the first 3 microns of the ocean. See:http://disc.sci.gsfc.nasa.gov/oceans/additional/science-focus/modis/MODIS_and_AIRS_SST_comp_fig2.i.jpg
This is just a temperature profile, but you can see how the temperature at 10µm is warmer than say at 8µm which in turn is warmer than say at 5µm.
The 3 micron layer is cooler (presumably because of latent heat of evaporation) so how does the energy absorbed in that 3 micron layer find its way to 4 microns, then to 5 microns, then to 6 microns since to do so, if it does so by conduction, as you suggest, would require heat to be conducted against the direction of energy flux?
Unless the ‘energy’ from DWLWIR absorbed within the first 3 microns can be disipated to depth at a speed quicker than that energy powers/drives evaporation, it would appear that DWLWIR predominantly powers evaporation, and does not heat the ocean.
The manner in which DWLWIR acts over the land and the ocean may be different since the ocean is free to evaporate.
Kristian5:22pm:
1). “Heat is not contained inside a body.”
2). “the effect of a heat transfer”
If 1). is true (it is) then 2). is nonsense (it is). If heat isn’t contained inside a body (true as heat doesn’t exist), it is nonsense to write heat can transfer from that body.
Energy is contained inside a body so energy can transfer from the body. DWLWIR (aka terrestrial radiation) is contained inside the atm. so surface incident DWLWIR can be absorbed, reflected, or transmitted at the surface. If the incident object is small enough, DWLWIR can also be diffracted which is negligible on the earth and is not negligible in the moon’s powder.
Some text books do use the term “heat transfer” synonymous with “energy transfer” – you are free to chuckle at their nonsense as I do.
******
Ghengis 5:49pm: You are not picking up differing IR thermometer readings with different DWLWIR amounts because in situ ocean measurements with more precise equipment (M-AERI google term) have shown about +.004K slowing in skin water cooling for each +2 W/m^2 DWLWIR increment. As you indicate, your equipment is not that sensitive in K.
davidmhoffer says: August 10, 2014 at 5:45 pm
“For gawd’s sake Nick, I can go to any decent hardware store and buy a propane heater with a parabolic reflector on it for the express purpose of focusing IR.”
A parabolic reflector focusses a parallel beam.
The feedbacks are likely to be negative. No one has even given thought to this. If I recall during the ice ages Co2 was 1000’s ppm?
Hadn’t been to the Met Office site for a while but I’ve just noticed that they no longer show the global SST time series plots. Now they prefer to show a map with lots of red bits.
http://hadobs.metoffice.com/hadsst3/
Don’t want anyone to see that SST has been flat for the last 10 or 15 years now do we?
davidmhoffer says:
August 10, 2014 at 5:45 pm
////////////////////////
Why not do an experiment?.
Get your parabolic heater and use the reflector to reflect DWLWIR from the night sky to heat say 10 ccs of water. You do not have to boil the water, just increase its temperature..
Nick Stokes;
A parabolic reflector focusses a parallel beam.
>>>>>>>>>>>>>>>>>>>>
For the purposes of this discussion, at the scale we are talking about, DWLWIR is a parallel beam. A reflector just 50 meters in radius, would not be impacted by the curvature of the earth (above it or below it) to enough extent to amount to anything more than a rounding error. Even if all it reflected was 1 w/m2 and spilled the rest because it wasn’t exactly downward and parallel, that would still be nearly 8,000 watts, plenty to boil a pot of water.
You argued that DWLWIR could not do work because of the lack of a sink source. When I pointed out that you don’t need a sink, you switched gears and tried to argue that you couldn’t focus it. Pick which ever wrong argument you wish to stick to. Or are you going to come up with yet another way to be wrong?
davidmhoffer says: August 10, 2014 at 6:27 pm
“You argued that DWLWIR could not do work because of the lack of a sink source. When I pointed out that you don’t need a sink, you switched gears and tried to argue that you couldn’t focus it.”
The issues are closely related. Here’s a proof that you can’t focus.
A parabolic reflector for sunlight can be emulated with a whole lot of plane mirror pieces, each aligned to provide a mirror image of the sun, and occluding an equivalent amount of sky (or land). It gets very hot because you see lots of suns, each sun replacing a cool; patch of sky.
What if you do that with DWLWIR. Think of a single mirror. It reflects a patch of sky, and you get the warmth from that. But you occlude a similar patch of sky, or of ground, which is warmer. You aren’t making progress. And you won’t improve with lots of mirrors.
Nick Stokes;
What if you do that with DWLWIR. Think of a single mirror. It reflects a patch of sky, and you get the warmth from that. But you occlude a similar patch of sky, or of ground, which is warmer. You aren’t making progress. And you won’t improve with lots of mirrors.
>>>>>>>>>>>>>>.
But you are. If the pot is say 30 cm in diamter, the mount of upward LW from the ground that you are occluding is a circle of earth 30 cm in diameter. But you are pointing all the DWLWIR from a 50 meter radius and focusing it on a single point.
Three times wrong Nick. Care to try for four?
davidmhoffer says:
August 10, 2014 at 6:27 pm
“For the purposes of this discussion, at the scale we are talking about, DWLWIR is a parallel beam.”
That is the problem with the discussion. It isn’t. For any point on the surface of the earth, the DWLWIR is not coming from a point source, it is coming from the entire sky.
Edward Richardson;
It isn’t. For any point on the surface of the earth, the DWLWIR is not coming from a point source, it is coming from the entire sky.
>>>>>>>>>>>>>>
Sure. But the bulk of it will be coming straight down from the perspective of a 50 meter dish.
In any event, the argument that DWLWIR cannot do work flies in the face of known physics. Arguing the minutia of how to use it to do work is just silly. Radiated energy does work, else the earth would be close to absolute zero by now. To have Nick Stokes, an advocate of the GHE, argue that it cannot do work is just jaw dropping. He wants to argue that the GHE is real, but that DWLWIR cannot do work? Talk about trying to both suck and blow….
Question: Why does CH4 track CO2 (and T) in the ice cores? –AGF
Steven Mosher says:
August 10, 2014 at 8:08 am
Steven, I don’t give special value to so called accepted science unless it is valid science. Much of the so called accepted science used by those crying wolf on global warming are using what they call consensus views to replace valid science. It is not science! Skeptics were repeatedly told that if they disagreed with what the hypothesis of AGW or CAGW claimed, they had to come up with possible alternate explanation for the heating trend of the last 150 or so years. Several did, including solar influences (which has not been falsified), long period ocean currents synchronization (PDO and AMDO), and even large long period natural chaotic variations. They did not mainly disagree with CO2 potential for warming, or even human increases, but did disagree with magnitudes, sensitivity, and feedback issues.
davidmhoffer says:
August 10, 2014 at 5:45 pm
I have kept out of this discussion so far due to the large number of not very accurate statements made by numerous comments, and responding to many would take too much effort. However, your statement clearly shows your lack of understanding on optics and radiation. A concentrated area optical source at any wavelength (visible solar or the heat from a torch) can be focused with a suitable curved geometry reflector (parabolic for distant sources, but elliptical for short object to focus distances). However, an extended source such as back-thermal-radiation (or even scattered sunlight from overcast) cannot be focused. This is a simple geometric optics issue.
Trick says:
August 10, 2014 at 6:04 pm
Ghengis 5:49pm: You are not picking up differing IR thermometer readings with different DWLWIR amounts because in situ ocean measurements with more precise equipment (M-AERI google term) have shown about +.004K slowing in skin water cooling for each +2 W/m^2 DWLWIR increment. As you indicate, your equipment is not that sensitive in K.
*****************
If I have 130 watts of additional DWLWIR (measured) then that would translate to 65 x .004K = .26K well within my .1 K equipments sensitivity. Remember that is per second of warming.
Just for you I stepped up on deck and measured the surface temp with my IR gun. It is a little after 10 pm, dead calm conditions and the sky is mixed clouds and clear, there are some big heavy clouds forming down around Marsh Harbour and the almost full moon is obscured behind some haze. The ocean surface temperature reading is still 31.4˚C. The clear sky reading is around 2˚C, and the dark clouds are close to 30˚ C and there are a lot of in-between readings.
Like I said earlier I expect it to go to full overcast by morning but who knows. At this point though my best guess is that the atmospheric radiation has increased by almost 80 watts since sunset which translates to .16K according to your reference. Obviously your reference is wrong.
Leonard Weinstein;
However, an extended source such as back-thermal-radiation (or even scattered sunlight from overcast) cannot be focused. This is a simple geometric optics issue.
>>>>>>>>>>>>>>>>>
If that is your opinion Leonard, then I accept it.
But DWLWIR still does work.
If IR can’t do work, then how am I able to get a temperature reading from my IR gun? I am not saying it does much work but it does enough to dislodge a couple of electrons from what ever chip my IR gun is using.
At room T my T gun can measure and compare fridge and freezer T. How can it do that if radiation can’t be transmitted from a cold to a hot place? –AGF
agfosterjr says: August 10, 2014 at 7:38 pm
“At room T my T gun can measure and compare fridge and freezer T. How can it do that if radiation can’t be transmitted from a cold to a hot place? –AGF”
It measures the energy transmitted from your gun to the freezer.
Nick, I measure energy from my guns by the amount of powder I put in the reloads. LOL!!!
Steven Mosher: notice how the author makes his case from WITHIN the accepted science.
Notice how effective the case is when you start INSIDE the accepted science..
I have sometimes wondered what you have meant by “science”.
davidmhoffer: At a current concentration of 400 ppm, and current CO2 increases of about 2 ppm per year…..it will take 200 years to achieve a single doubling resulting in one degree.
The second part of the IPCC shell game however is that they neglect to mention was what temperature they calculated the 1 degree of warming. Since temperature doesn’t vary linearly with w/m2, adding in 3.7 w/m2 has different effects at different temperature. We can calculate exactly what effect at what temperature using Stefan-Boltzmann Law:
P(w/m2) = 5.67 * 10^-8 * T^4 with T in degrees K.
Run the numbers and you’ll discover that 3.7 w/m2 causes a 1 degree temperature change at about -18C, which is point in the atmosphere at (IIRC) about 14,000 feet. If it is the surface temperature we are interested in, they should have done the calculation at average surface temperatures which would yield a change in temperature for CO2 doubling of about 0.68 degrees.
So, put in proper context, a doubling of CO2 from where we are now will take 200 years and will raise surface temps by only 0.68 degrees.
I think you are in the right “ballpark” as they say of coarse estimates. (1) starting now, (2) in 100-200 years, (3) cumulative effects of CO2 will be slight and (4) slightly beneficial.
Thanks to Ed Hoskins for a good essay.
Kristian, you say: “The concept of heat, the effect of a heat transfer, just like the laws of thermodynamics, don’t change even if we find out more about the microscopic processes behind it.”
You are still missing the point. You insist that people who use the underlying physical mechanisms are wrong, when they end up with the same result that you get using an analytic abstraction. This tells me that you are missing important conceptual principles.
Every thermodynamics and heat transfer textbook I’ve seen has described the process of radiative exchange (and the word “exchange” should be a clue) as two counter flows of energy with the resultant heat transfer as merely being the difference in the two flows. And yet you describe this viewpoint as completely erroneous.
@curt: Prévost’s Theory of Exchanges has misled 1000s of scientists for 228 years (from memory!). It misled me too until I went back to Planck and worked out the missing concepts.
It’s all very easy once you realise instrumental analysis does not measure what is claimed by the Climate Alchemists. Thus a radiometer, of which a pyrgeometer is a poor version, is a metal box with a hole to allow the sensor to equilibrate radiatively with the emitter(s) in the Feld of View.
Few understand the metal box is the most important part, not the sensor. It blocks the sensor equilibrating over 360 degrees so it equilibrates with the emittance from at most 180 degrees, not an energy flux but a potential energy flux. Real net flux is obtained by pointing the radiometer one way then the other and calculating the difference of the two emittances.
Hence a warm body does not emit radiant energy at the Stefan-Boltzmann rate unless it is in radiative equilibrium with the zero point energy of empty space. That is never achievable. The Earth is in radiative equilibrium with the cosmic microwave background, about 2.7 deg. K; the ‘forcing’ argument works for OLR because the opposing radiation field is so low it is negligible. The same is true for the SW energy thermalised at the earth’s surface; the Earth emits very weakly in the SW.
However, ‘back radiation’ is not real; only [surface emittance – ‘back radiation’] = (mean) 63 W/m^2 is real. The rationale is that for each wavelength, the energy flux is set by a travelling wave of the difference of the warm and cooler amplitudes. This is superimposed on a standing wave of twice the cooler body amplitude. It is all expressed by the Law of Conservation of Energy:
(monochromatic heating rate of matter/unit volume) = – ∇.(monochromatic radiative flux density)
From this you derive all the above. Climate Alchemy must change its textbooks ASAP otherwise it is condemned to remain Alchemy, not Science.
Genghis says:
August 10, 2014 at 7:33 pm
//////////////////////////////////
Your gun needs a power source.
The gun is not actually receiving energy, if it was, it would be possible to design and manufacture a gun that did not require a power source (ie., one that does not need a battery).
“””””…..The entire debate should have ended with “CO2 is logarithmic”. It has remained alive by an elaborate shell game by the IPCC. They present facts which are utterly true, and completely irrelevant. When we apply THEIR math and THEIR sensitivity and THEIR calculations to the here and NOW, their argument goes “poof” and disappears in a puff of logic……”””””
If the CO2 effect (on surface / lower tropo temperature) is logarithmic, the going from 280 ppm to 560 ppm should give the same temperature rise, as going from 1 ppm CO2 to 2 ppm; or for that matter, from one molecule of CO2 per cubic meter, to 2 molecules of CO2 per cubic meter.
CO2 temperature response IS NOT logarithmic.
Maybe it id “non-linear” ; but it ain’t logarithmic.
The logarithm function is very specific.
There isn’t any CO2 / Temperature data, that differs in any statistical sense, from linear. They often even go in the opposite direction. What kind of logarithmic function is that.
Any data there is can just as well be fitted to the function: y = exp(-1/x^2). And moreover you can use either y or x for the temperature; or for the CO2 if you like.
That function goes from 0,0 to 1,1/e, and all points beyond (both ends) but at x = y = 0, dy/dx =0
And so does all the higher derivatives. So how the hell, does it ever get to 1/e if it can never get started ??
So don’t hang you hat on any supposed logarithmic dependency on CO2; there isn’t any.
[+emphasis]
Curt, to no avail in this and other WUWT posts, others (and I) have tried to show the emphasized concept to Kristian.
I do wish you all the best in trying to get him to understand.
🙂
Matthew R Marler says: August 10, 2014 at 9:30 pm
“Thanks to Ed Hoskins for a good essay.”
I asked above if anyone could explain the arithmetic expressed at many points in % – eg
“CO2 at 400pmmv is already committed and immutable. So CO2 has already reached about ~87+% of its potential warming effect in the atmosphere.”
That seems to me to be central to the essay, and also nonsense. Can you help?
richard verney says:
August 10, 2014 at 10:05 pm
My eyeball needs a power source too, but that doesn’t mean a photon doesn’t do work on the retina. It just has to be darker than the source.
“””””…..agfosterjr says:
August 10, 2014 at 7:38 pm
At room T my T gun can measure and compare fridge and freezer T. How can it do that if radiation can’t be transmitted from a cold to a hot place? –AGF …..”””””
Radiation can go anywhere.it darn well pleases; because radiation is NOT “heat”.
So splain me how the 2.7k BB microwave background radiation, can even be detected on earth if it can’t come here, because we are much hotter that 2.7 K.
Heat is (chaotic) mechanical kinetic energy. It IS NOT electro-magnetic radiation, which is a propagating field .
george e. smith says: August 10, 2014 at 10:08 pm
“CO2 temperature response IS NOT logarithmic.”
This goes back at least to Arrhenius. Or as he puts it
“Thus if the quantity of carbonic acid increases in geometric progression, the augmentation of the temperature will increase nearly in arithmetic progression. “
@F. Ross: you have missed the key issue which is that heat transfer is not the difference in the ‘two flows’. There is only one ‘flow’ set by the integral over all wavelengths of Poynting Vectors of waves whose amplitude is the difference of the warmer and cooler amplitudes.
In simple terms, it takes two to tango. I’m glad that there is a critical mass of professionals writing on this thread who insist on this crucial bit of thinking. Climate Alchemists fixated on ‘back radiation’ have nowhere to hide because the Climate Models are classic groupthink fail.
In simple terms the heat generation rate in the atmosphere is 238.5 SW thermalisation + 333 ‘back radiation’ – 238.5 supposedly from applying Kirchhoff’s Law to the -18 deg C emitter at ToA.
The sum is 333 W/m^2, 140% of the real heating rate. That 40% is imaginary. The reason is that there is no single -18 deg C emitter. -18 deg C is the flux weighted mean emission temperature of IR sources at altitudes ranging from 0 to ~20 km. It is a virtual emitter with no physical existence.
It’s time we shut down departments which teach the false ‘back radiation’ concept, our era’s equivalent of ‘Phlogiston Theory’.
Excellent example! Wish I’d thought of it.
I didn’t see any mention of the “optical depth” of the atmosphere in the blog or comments. One can’t understand why increasing CO2 will increase warming without considering optical depth.
You still get an increase in greenhouse warming even if the atmosphere were saturated, because it’s the absorption in the thin upper atmosphere (which is unsaturated) that counts. It’s not even true that the atmosphere is actually saturated with respect to absorption by CO2.
Water vapor doesn’t overwhelm the effects of CO2 because there’s little water vapor in the high, cold regions from which infrared escapes, and at the low pressures there water vapor absorption is like a leaky sieve, which would let a lot more radiation through were it not for CO2.
@F. Ross: you detect the cosmic microwave background with a radio telescope. 2.72548 deg. K is equivalent to a peak 160.2 GHz radio wave.
davidmhoffer says:
August 10, 2014 at 7:20 pm
////////////////////
The oceans have thermal inertia, the atmosphere has thermal inertia, that is why the night time temps do not drop to say -155degC as seen on the moon (even the moon with its lengthy nights, its surface does not get down to 3K).
The difference between day and night temps, is essentially governed by the thermal inertia of the atmosphere, and where I am, even though it was clear skies tonight (thus completely open to the 3K of space) the night temp did not drop below about 24degC. If it had been a cloudy night, it would not have been any warmer (significantly warmer).
GHGs may alter the thermal inertia of the atmosphere. GHGs such as CO2 may delay surface radiated LWIR from going directly to space, bouncing it around a bit, or even a lot, thereby delaying the cooling process, but I am sceptical that they add anything of substance to the night time low.
On the other hand, when it is very humid and the atmosphere itself is carrying a lot more energy, night time temps can be kept up. In these conditions there is both more thermal lag and more energy in the atmosphere such that night time temps are kept up. In those conditions, where I am, it is possible for the nightime temps to drop not below about 28 or even possible about 30degC.
PS. I am only about 1000 metres from the sea, and the difference between high humidity and low humidity is stark. .
PPS. If the pause continues for an extended time (and even some warmists are suggesting that there may be no resumption of warming before 2030), I expect to see, not simply a re-assessment of the positive/negative feeback forcings that may amplify or attentuate the ghe of CO2, but also a re-evaluation of the basic physics underpinning the AGW theory, and one area that will get attention is the proper assessment of the GHE and wheher it truly is about 33degC, or whther it is less than that>
DWLWIR varies but is something in the order of 340 W/m-2, roughly. This value occurs at night as well. So if I walk out of my house at night into this downwelling infrared, it would seem like I should be able to feel it on my skin, since it is about the same intensity as regular sunlight at about 9 to 10 in the morning. Hmmm. I don’t feel a thing.
Paul 767:
At August 10, 2014 at 3:49 pm you write
Oh! So, Irenaeus,the 2nd century by Bishop of Lyon, was a “leftist”. And so was St. Augustine. And so was …
And you go on from that false premise about “leftists” to ascribe to them attributes which are not general to people of the left and are not unique to people of the left.
In addition to being way off-topic, your troll comments are plain daft, but they are typical of ultra-right untruths as adopted, perfected and used by propagandists for the German ultra- right in the 1930s.
Richard
Nick Stokes: I asked above if anyone could explain the arithmetic expressed at many points in % – eg
“CO2 at 400pmmv is already committed and immutable. So CO2 has already reached about ~87+% of its potential warming effect in the atmosphere.”
That seems to me to be central to the essay, and also nonsense. Can you help?
That is an interesting question and I am glad that you have come back to it. I think that it is sloppy writing, like referring to the “equilibrium” temperature of a high dimensional dissipative system that never has an equilibrium. I think there is an implicit upper bound of about 3,200 ppm (never stated, hence implicit), and the claim is that we are about 87% of the way to the effect of that implicit likely upper bound on CO2. Starting at 400ppm (an idea I work with frequently), the next doubling is to 800 (adding 1 C, give or take); the next doubling is to 1600 (adding another 1 C, give or take), and the next doubling is to the extremely high and unreachable 3200 (adding another 1C, or whatever you prefer.) Thus, 3200, or whatever, is not an asymptotic value but a reasonably realistic upper bound, probably not a least upper bound. Whatever the unit increase per doubling is, we are about 90% as far as we are going to get if the atmospheric CO2 continues to double for the next 450 years at its present rate, since only 3C (or 3 times your favorite value) will be added in 450 years.
Analogously, if I say that the scientific publications and in-house techreports are “uncountable”, I do not mean the mathematical uncountable, only that there is not likely to be sufficient manpower invested in counting them ever to come up with more than upper and lower bounds.
The asserted doubling of CO2 concentration is hypothesized to result in an increase of 3.8W/m^2 downwelling radiation, and the hypothesized increase in temperature comes from considering only the equilibrium radiative effects on a planet with a uniform surface and uniform temperature. If you consider the effects of that increased radiative energy impinging on the non-dry regions of the Earth surface, and that it takes about 660 more energy to vaporize a kilogram of water than to raise its temperature 1C, then I think it becomes clear that ignoring the evaporation effects in the non-equilibrium system leads to a serious overestimation of how much warming can be produced by a doubling of CO2. The cloud responses, not agreed upon in the peer-reviewed literature, make the idea of a constant sensitivity to doubling of CO2 a dubious concept anyway.
@richard verney: 33 K GHE is bad physics from 1981_Hansen_etal.pdf. That paper, which should never have passed peer review, claimed with no evidence that OLR comes from a single 5 to 6 km upper atmosphere IR emitter at -18 deg C in radiative equilibrium with Space.
-18 deg. C is the flux-weighted virtual temperature of a virtual emitter. Real GHE is obtained by calculating no cloud or ice albedo, 341 W/m^2, mean surface temperature and subtracting that 4 to 5 deg C from present ~15 deg C, ~ 11 K. Lindzen has come up with about 16 K GHE.
Hansen et al claimed GHE = lapse rate warming from 4 or 5 km to the surface. However, this temperature change arises purely from gravity. The 40% extra energy which comes from assuming atmospheric emittance is a real energy flux is supposedly used to increase latent and sensible heat as surface temperature rises, thereby moving upwards the -18 deg C virtual emitter. However, this assumes there are no atmospheric mechanisms bypassing the ‘CO2 – bite’ in OLR.
The real climate system keeps SW thermalised = OLR whilst minimising diurnal variation of surface temperature. The atmospheric processes mean near zero surface temperature change from well-mixed GHGs concentration change. GHE variation is solely from cloud and ice albedo change.
Scott says:
(August 10, 2014 at 5:20 pm)
“”Re The logarithmic response means that we need to add twice as much CO2 again to create the same amount of warming we created with the initial increase…..”
I think you mean 10 times the amount based on a Log base 10 effect to get twice the temp.”
_____________________
Hi Scott.
I mean in terms of doubling concentrations of CO2. If CO2 doubles from 280 to 560ppm and this causes, say, 2 deg C warming, then in order to get a further 2 deg C warming you’d need to double from 560 to 1120ppm.
The point is that just because the impact on temperature of increasing CO2 concentrations is logarithmic, this doesn’t mean that further increases in CO2 won’t cause further. They will.
James Abbott:
At August 10, 2014 at 5:19 pm you ask
Nobody suggested that the sea level rise should be greeted with anything; you introduced that red-herring.
And the economic cost of defending “major coastal cities from such a sea level rise” would be zero because any needed alterations to sea defences would be obtained as part of the normal maintenance of sea defences.
Richard
Nick Stokes: A parabolic reflector focusses a parallel beam.
Yes, and if you put an IR source at the focal point the mirror will create a parallel beam of IR.
george e. smith: Heat is (chaotic) mechanical kinetic energy. It IS NOT electro-magnetic radiation, which is a propagating field .
The concept here is that the energy in the electro-magnetic radiation can be converted to kinetic energy (and vice versa) through interaction. Two examples: radiation from the sun is absorbed by the electrons in orbits around the nuclei of diverse atoms, raising the energy levels of the orbits of the electrons, and through inter-atomic collisions the energy is transferred into the kinetic energy of the surrounding atoms; electrons in energetic “orbits” can decay into lower energy orbits, and thereby give off energy in the form of electromagnetic radiation.
Attempts by people to deny these basic concepts are astonishing. Kristian has argued that when the electrons transition from higher energy level orbits to lower energy level orbits and generate IR that way, the energy of the electrons disappears (or at least goes somewhere other than the radiation that he has not told us about.) Other people have argued that the radiant energy from CO2 molecules in the lower troposphere can’t go toward the surface because the CO2 molecules somehow know that the surface has a higher mean kinetic energy than the surrounding air; without explaining to us how the radiant energy emerging from the CO2 molecules knows that.
IF CO2 concentration is a cause of temperature, not an effect. There is nothing to support this.
“The recent IPCC report now admits that currently increasing CO2 levels are probably only ~50% man-made.”
Link with direct quotation (or chapter and page from AR5 WG1) would be very much appreciated if someone could provide this. Thanks.
F. Ross says:
August 10, 2014 at 10:27 pm
///////////////////////
I have never seen anyone argue that DWLWIR does not exist at all, ie., it is not even a signal capable of measurement. I do not consider that an issue raised by sceptics, but rather, one or more of the following:
The issue is what does DWLWIR actually do? Does the 2.7K DWLWIR from space help maintain the surface of planet Earth at its about 288K temperature? Does the 255K DWLWIR from about 17,000ft help maintain the surface of planet Earth at 288K? Etc.
Does increasing the amount of CO2 in the atmosphere actually increase DWLWIR? See: http://wattsupwiththat.com/2014/08/05/bombshell-study-shows-greenhouse-gas-induced-warming-dropped-for-the-past-14-years/ That was just one study, from which it would be wrong to seek to extrapolate a global wide effect, but the study does raise an interesting point upon which further research would be useful.
Is the radiative effect of CO2, at around today’s level of circa 280 to 400ppm, completely dwarfed by the water cycle? See the comment of E.M.Smith at August 10, 2014 at 1:03 pm.
What is the correct assessment of the GHE on planet Earth? See: AlecM at August 10, 2014 at 8:36 am. What is the evidence of a GHE on any planet/body in the Solar System?
What are the feedbacks, their correct assessment and are these positive or negative?
The fact that it appears that CO2 lags temperature change, on every time scale, and the 18 year pause seem to suggest that CO2, at a level above about 200ppm, may do little.
Whilst I understand that most people have a desire to argue from within the ‘consensus’ science, no doubt because they consider that they will be taken more seriously, or at any rate, not simply ridiculed, but it is worth rembering that history shows that consensus science is usually wrong.
Any objective observer would recognise that the AGW hypothesis has many problems with it. To what extent these are fundamental is yet to be revealed, but it may well be the case that in the next 20 or so years, scientists will have a very different take on it going beyond the climate sensitivity issue.
Cost to Europe 165bn a year for rest of this century. Wouldn’t be surprised, but where does this figure come from?
Matthew R Marler says: August 11, 2014 at 12:06 am
“I think there is an implicit upper bound of about 3,200 ppm (never stated, hence implicit), and the claim is that we are about 87% of the way to the effect of that implicit likely upper bound on CO2.”
Well, thanks, that sounds dodgy, but still doesn’t elucidate the arithmetic. What is the numerator, and what the denominator, in the 87%?
Richard
I made the observation way above that I thought 280ppm was the probable limit for an effect
http://wattsupwiththat.com/2014/08/10/the-diminishing-influence-of-increasing-carbon-dioxide-on-temperature/#comment-1706343
Can I get you to raise your estimate to that from 200ppm then we will have a 100% consensus on those that expressed an opinion? We might get it tweeted by Obama….
tonyb
Hilary
In the Stern report it is estimated there is an estimated cost of 30£ billion a year for the UK alone so that 165billion figure seems reasonable (as an estimate obviously, not as a sensible amount to spend on a non problem)
tonyb
Matthew R Marler says, August 11, 2014 at 12:30 am:
“Attempts by people to deny these basic concepts are astonishing. Kristian has argued that when the electrons transition from higher energy level orbits to lower energy level orbits and generate IR that way, the energy of the electrons disappears (or at least goes somewhere other than the radiation that he has not told us about.) Other people have argued that the radiant energy from CO2 molecules in the lower troposphere can’t go toward the surface because the CO2 molecules somehow know that the surface has a higher mean kinetic energy than the surrounding air; without explaining to us how the radiant energy emerging from the CO2 molecules knows that.”
What is astonishing is rather that people still don’t get the exceedingly simple and basic point I’m trying to make.
You are only misrepresenting what I’m saying here, Matthew. Building a straw man. No wonder people are convinced ‘I’m wrong and ignorant’.
Let me try and explain one more time.
I agree with Richard Verney. It is not at all about DWLWIR or not DWLWIR. It is about what DWLWIR is capable of doing. What is DWLWIR? And what isn’t it? Well, we all agree by now (?) that it’s definitely not heat, that is, it is not a net transfer of radiative energy to the warmer surface from the cooler atmosphere. That would be absurd.
This is a good starting point.
I will ask once more what I asked on the Bombshell thread:
“For a photovoltaic cell at ambient temperature, how do you suggest we harvest the DWLWIR energy from the atmospere above when there is always more UWLWIR moving out? How do you suggest the DWLWIR in this way will manage to increase the internal energy (U) of the photovoltaic cell? If we don’t first (artificially) cool the cell to become colder than the air above?”
Consider this. The surface (or the photovoltaic cell) is warmer than the atmosphere above. There is one radiation field between them, meaning there is one continuous thermal radiative energy exchange going on between them through this radiation field, because of their temperature difference: Q or P/A = es*(Tsfc^4 – Tatm^4). P/A (Q) is the radiative heat, the net energy, moving from the warmer surface to the cooler atmosphere. The two terms on the right-hand side are the thermal radiant emittances of the two systems involved in the exchange, the UWLWIR and the DWLWIR. Their sum is the P/A, the radiative heat.
Let’s do this as straightforward as possible. Let’s say the surface does in fact at any one time absorb the DWLWIR as a flux of radiative energy. We want to see if we can detect an actual effect from this absorption. Does the internal energy of the surface increase? Does the temperature rise as a result? It would. If it weren’t for one thing. The DWLWIR is not alone in the exchange. The surface is warmer than the atmosphere. So concurrent with the absorption of the energy within the DWLWIR, it releases more energy the opposite way, up to the atmosphere, as UWLWIR. There is no lag here. It all happens continuously, simultaneously and instantaneously.
Do we ever see the DWLWIR in fact increase the internal energy of the surface in this situation? Do we ever see it raise its temperature?
Do we ever see DWLWIR ‘heat’ the surface? Do we ever see the DWLWIR do ‘work’ on the surface?
No. Because the larger UWLWIR flux is always there countering it in real time (as it comes in).
This is why the only actual transfer of energy within a thermal exchange (a heat transfer), conductive or radiative, that we could ever detect and ever see the effect from is the HEAT, the net energy, in a radiative exchange the vector sum of the UWLWIR and DWLWIR.
I don’t understand why people get so angry when this very basic fact is pointed out to them. I’m not talking about you, Matthew. I’m talking about people like David M. Hoffer who seems to have nothing but infuriated ad homs and regurgitated talking point statements to offer, no real arguments. It’s like waving the proverbial red flag. He says I’m wrong and ignorant, but can’t and won’t point out exactly what it is I’m saying that’s so wrong, so ignorant. I’m only presenting thermodynamic concepts and principles the way they are explained and described in modern textbooks within the field. There is nothing mysterious or novel in what I’m saying.
DWLWIR has no singular effect on the surface. That is the simple truth. Yes, if the atmosphere gets warmer and the surface doesn’t, then the temperature difference between the two is reduced and the P/A (equation above) – the heat – gets smaller. That is the ONLY effect the DWLWIR term could ever have. But that is not ‘heating’ or ‘doing work on’ the surface, folks.
Can we please move on from there?
Ironically, the likely gas phase stability of carbonic acid appears to have completely slipped under the radar of climate modelling.
Arrhenius’ slip [it seems reasonable to assume he actually meant CO2, not carbonic acid] has been propagated by those who think it is too unstable to consider in the atmosphere, despite being continuously formed and decomposed during the interactions of carbon dioxide with clouds.
I once calculated the amount of CO2 dissolved in the water droplets in a typical cloud, and it’s quite small, about 7%. This neglects extra surface solubility, so it could be greater.
Nick Stokes says: August 10, 2014 at 3:06 pm
…. Whenever people refer to sensitivity as 2&dseg;C/doubling, or whatever, they are invoking the logarithmic behaviour.
Hold the ALT key down
Type 0176 on the num keys
Release the ALT key
ALT 248 works, too.
davidmhoffer says:
August 10, 2014 at 5:25 pm
Give me a parabolic dish large enough that reflects in the IR spectrum, and I can boil a pot of water in seconds with it at night with DWLWIR.
Your ignorance is astounding, if you tried your experiment, like hundreds before you, you will find that an object that is the focus of the parabolic dish COOLS.
Just Google night fridges, to save you time see
http://solarcooking.org/radiant-fridge.htm
http://littleshop.physics.colostate.edu/tenthings/SpaceFridge.pdf
Even Roy Spencer tried it with the same result, but of course being a “believer” he rationalised that without all that Radiant energy the objects in the cool box would have been as cold as space itself.
http://www.drroyspencer.com/2010/07/first-results-from-the-box-investigating-the-effects-of-infrared-sky-radiation-on-air-temperature/
Donald L. Klipstein says:
August 10, 2014 at 12:41 pm
“Since an increase of surface temperature in response to an increase of GHGs causes an increase of outgoing surface radiation,”
————–
That sounds like “perpetual energy creation”, …….best you patent it, … quickly.
Genghis says:
August 10, 2014 at 10:11 am
“Infrared (atmospheric radiation) is absorbed by the top few microns of the surface, which does in fact heat the ocean.
When that happens, evaporation simply picks up the pace a tiny little bit, and the ocean surface temperature stays exactly the same”.
([August 10, at 11:45 am] – 3 microns over a square meter equals 3 cubic centimeters being heated by 130 watts)
———————-
Genghis, concerning your above, I have a question which I am seriously interested in knowing the answer to, …. thus I would be quite pleased if you would be so kind to offer your knowledge and/or opinion to aid me in my quest.
My question is: When the above stated (H2O) evaporation occurs, is there any CO2 outgassed as a direct result of said evaporation?
Ghengis 7:24pm: “…my best guess is that the atmospheric radiation has increased by almost 80 watts since sunset which translates to .16K.”
A quick check of the noaa esrl surfrad data near New Orleans shows more like a measured drop of 415 to 380 W/m^2 DWLWIR yesterday all night. For .07K slowing of cooling of the skin T all night by DWLWIR with about 10 W/m^2 or .02K difference when cloud cover drifted by for about an hour.
In any event, your IR thermometer is picking up photons emitted from multiple bulk water depths for which bulk mass won’t change fast enough in T where the M-AERI measured skin T by interferometer vs. thermometer set a few cm.s depth. If precise measuring was as easy as your quick test, the researchers would not have gone to all the trouble for building the instrument & a month long ocean cruise taking data day and night.
george e. smith says:
August 10, 2014 at 10:08 pm
“””””…..The entire debate should have ended with “CO2 is logarithmic”. It has remained alive by an elaborate shell game by the IPCC. They present facts which are utterly true, and completely irrelevant. When we apply THEIR math and THEIR sensitivity and THEIR calculations to the here and NOW, their argument goes “poof” and disappears in a puff of logic……”””””
If the CO2 effect (on surface / lower tropo temperature) is logarithmic, the going from 280 ppm to 560 ppm should give the same temperature rise, as going from 1 ppm CO2 to 2 ppm; or for that matter, from one molecule of CO2 per cubic meter, to 2 molecules of CO2 per cubic meter.
CO2 temperature response IS NOT logarithmic.
Just got back to this thread.
@george e smith – Essenitially that is what I was suggesting when I responded way upstream to
Nick Stokes says:
August 10, 2014 at 3:06 pm
The basis for this percent arithmetic (here and earlier) is not stated, and it makes no sense. A logarithmic curve has diminishing slope, but no maximum or minimum.
Since we are talking about the amount of CO2 in the atmosphere, there must be both a minimum (0%) and a maximum (100%) atmospheric CO2 levels. The supposed logarithmic curve would not go infinitely in either direction since it would stop at both ends.
Further, there is the question of at what level of CO2 in the atmosphere do we get an effective “saturation point” where adding additional CO2 doesn’t make a difference?
The “1 degree temp increase per doubling of atmospheric CO2” just doesn’t meet the “sniff test”. As you, and I, point out, 1 part CO2/total (1 molecule) in the atmosphere wouldn’t change the temp, except, perhaps, theoretically. Doubling to 2 molecules (2 parts CO2/total) wouldn’t add 1 degree of temp. There must be a minimum amount of CO2 in the atmosphere that actually changes the atmospheric temperature by this 1 degree mark that seems important. Further, just as less than this minimum CO2 that effects a 1 degree change doesn’t meet the “1 degree for each doubling” test, wouldn’t there also be an amount at the other end of the scale where a doubling also no longer meets the “1 degree” difference? Especially since there is only a finite amount of heat to be absorbed?
Again, just wondering.
Kristian says:
DWLWIR has no singular effect on the surface. That is the simple truth. Yes, if the atmosphere gets warmer and the surface doesn’t, then the temperature difference between the two is reduced and the P/A (equation above) – the heat – gets smaller. That is the ONLY effect the DWLWIR term could ever have. But that is not ‘heating’ or ‘doing work on’ the surface, folks.
=============
Kristian, take a deep breath and relax, we are on the same side. Your functional view of DWLWIR is correct almost all of the time. The biggest part of the problem is that the warmers framed the debate the way they wanted to to amplify the affects of radiation and instead of focusing on net radiation they chose to separate it to make it appear larger and more important than it is. They love saying that the DWLWIR is 450 watts while the up welling radiation from the surface is 458 watts. 450 watts is probably more than your microwave puts out. The implication is that we are all going to roast, like a pig on a spit.
The truth though is that there is a measly net of 8 watts difference between the two objects, hardly even measurable, and certainly not something anyone could do any work with. But the 8 watts is real, can do work and can warm objects at least theoretically and there is the rub. The warmistas are only claiming a net of .6 watts. We can’t even measure the difference when there is 130 watts of real atmospheric radiation and we are supposed to run screaming to the hills because theoretically there might be .6 watts of warming?
SkepticGoneWild says:
August 10, 2014 at 11:41 pm
DWLWIR varies but is something in the order of 340 W/m-2, roughly. This value occurs at night as well. So if I walk out of my house at night into this downwelling infrared, it would seem like I should be able to feel it on my skin, since it is about the same intensity as regular sunlight at about 9 to 10 in the morning. Hmmm. I don’t feel a thing.
Obviously DWLWIR Watts are not the same as any other kind of Watts, try standing in front of a 340W electric fire, I am sure you would feel it.
Not only that but according to the Climate Balance diagram there are only about 161 Watts of sunshine at the surface, so you should feel twice as hot.
Yeah right.
Genghis says, August 11, 2014 at 6:30 am:
I appreciate it, but I think the right address for this comment of yours is David M. Hoffer and his kind who all go bananas as soon as someone as much as hints at the possibility that DWLWIR from the cooler atmosphere cannot (by the laws of thermodynamics) ‘heat’ or ‘do work on’ the warmer surface. How dare we!? As Hoffer stated: We can both measure the heat it creates and the work it does. Er. No. Only if the atmosphere is warmer than the surface (or the sensor).
Trick says:
August 11, 2014 at 6:11 am
Ghengis 7:24pm: “…my best guess is that the atmospheric radiation has increased by almost 80 watts since sunset which translates to .16K.”
A quick check of the noaa esrl surfrad data near New Orleans shows more like a measured drop of 415 to 380 W/m^2 DWLWIR yesterday all night. For .07K slowing of cooling of the skin T all night by DWLWIR with about 10 W/m^2 or .02K difference when cloud cover drifted by for about an hour.
In any event, your IR thermometer is picking up photons emitted from multiple bulk water depths for which bulk mass won’t change fast enough in T where the M-AERI measured skin T by interferometer vs. thermometer set a few cm.s depth. If precise measuring was as easy as your quick test, the researchers would not have gone to all the trouble for building the instrument & a month long ocean cruise taking data day and night.
==============
If the the top few microns of the surface is capable of absorbing 90% of the atmospheric IR then the top few microns of the surface is capable of absorbing 90% of the IR from the water below. My IR gun is picking up the same radiation as their interferometer.
They are trying to measure the gradient of the water just below the surface, which I agree is very difficult. The problem that I have with their method is that they are claiming that gradient below the surface is slowing the flux without the surface warming first.
What is wrong with my calculation regarding the joules to the surface from increased cloud radiation? My calcs are straight forward.
A C Osborn says, August 11, 2014 at 6:35 am:
“DWLWIR varies but is something in the order of 340 W/m-2, roughly. This value occurs at night as well. So if I walk out of my house at night into this downwelling infrared, it would seem like I should be able to feel it on my skin, since it is about the same intensity as regular sunlight at about 9 to 10 in the morning. Hmmm. I don’t feel a thing.
Obviously DWLWIR Watts are not the same as any other kind of Watts, try standing in front of a 340W electric fire, I am sure you would feel it.
Not only that but according to the Climate Balance diagram there are only about 161 Watts of sunshine at the surface, so you should feel twice as hot.
Yeah right.”
DWLWIR could be 1,000,000 W/m^2 and if you emitted 1,000,050 W/m^2 worth of UWLWIR, you wouldn’t feel it any more than if the DWLWIR were a mere 300 W/m^2 and your UWLWIR 350 W/m^2. Because the radiative HEAT in both situations would be 50 W/m^2 moving from you to the air. You would cool to the air in both scenarios.
Only the net energy matters in a heat transfer.
george e. smith says:
August 10, 2014 at 10:16 pm
“Heat is (chaotic) mechanical kinetic energy. It IS NOT electro-magnetic radiation, which is a propagating field .”
This is argument by definition. The word ‘heat’ as noun and verb and the Roman equivalents ‘caloricum’ and ‘calentare’ were around long before physicists defined “electromagnetic energy” and “specific heat.” To insist that the sun does not radiate ‘heat’ is a tyrannical abrogation of lexical authority, like claiming a dromedary is not a true camel. Since molecules and electrons never really touch you have in essence defined the verbal use of ‘heat’ out of existence.
How about it: does the sun ‘warm’ a camel’s back? Does it heat it? Let’s not make up our own words and definitions. –AGF
AlecM 12:11am: “Real GHE is obtained by calculating no cloud or ice albedo, 341 W/m^2, mean surface temperature and subtracting that 4 to 5 deg C from present ~15 deg C, ~ 11 K”
That 11K is just using your different definition of GHE. Satellites measure Tmean = 255K in thin atm. at their orbit and surface thermometers in Earth thick surface atm. measure Tmean = 288K. This is not disputed unless you can find a basic physics error in one of them. Which would make the evening news.
10:26pm: “…the heat generation rate in the atmosphere is 238.5 SW..”
Heat doesn’t exist in nature. There is no energy generated in the atm. either as the atm. uses up no fuel. This comes from your confusion over applying the generalized 1st law in non-atm. text books from your 10:02pm:
“(monochromatic heating rate of matter/unit volume) = – ∇.(monochromatic radiative flux density)”
Your 1st term for the atm. is = 0 as no energy is generated within the atm. and the term on the other side for the atm. is (net energy in from sun – net energy out from surface) for the volume of interest. For the surface volume balance, the mass of the atm. radiates a real flux incident on the surface all the time at all frequencies at all of its temperatures and has to be included as in nature.
And again, can anyone tell me why CO2 tracks methane in the ice cores? –AGF
@agfosterjr: the warming of the camel’s back from solar SW energy is by the physical process called ‘thermalisation’. This is the absorption of SW energy quanta by raising electron orbitals to higher energy states followed by emission of lower energy quanta, a process called fluorescence.
The lower energy quanta cause the energy to be transferred to greater molecular motion; heat.
CO2 lags warming, Stupid! Not the other way round. Why is this shite even here?
A C Osborn says: Not only that but according to the Climate Balance diagram there are only about 161 Watts of sunshine at the surface, so you should feel twice as hot.
Yeah right.
Please tell me you are not serious. The “Climate Balance” diagram displays 24 hour averages. Per Wikipedia, “The Sun’s rays are attenuated as they pass through the atmosphere, thus reducing the irradiance at the Earth’s surface to approximately 1000 W /m2 for a surface perpendicular to the Sun’s rays at sea level on a clear day”
DWLWIR is electromagnetic radiation just as the sun’s radiative spectrum is electromagnetic radiation. The frequencies are just different. Watts are watts in the electromagnetic spectrum. 430 watts of DWLWIR at the earth’s surface should produce something my skin would feel. But I walk out into the night and feel no warmth on my skin. What’s up with that?.
Kristian: Well, we all agree by now (?) that it’s definitely not heat, that is, it is not a net transfer of radiative energy to the warmer surface from the cooler atmosphere. That would be absurd.
The radiant energy of radiation is transferred (or converted) to kinetic energy through interactions.
In a parcel of air, the temperature is proportional to the mean kinetic energy of the molecules: some have way above average energy, some have way below average kinetic energy; in a parcel of the surface, the temp is proportional to the mean kinetic energy of the molecules, and some are above average, some below. When the temp of the air increases, the fraction above a certain kinetic energy level increases. Net heat flow is always from warmer to cooler parcels, but the highest energy molecules in the air radiate in all directions, including downward, and when the temp of the air increases, the fraction of the molecules radiating downward (as well as other directions) increases, slowing the net transfer of energy from the warmer surface to the cooler air. That isn’t illogical or contrary to known physical laws; you just have to remember that not every molecule capable of emitting or absorbing radiant energy is at the mean kinetic energy of its environment. Warming of the surface is caused by the radiant energy of the sun; the net warming effect of CO2 is caused by the reduction in the surface cooling rate.
Nick Stokes: Well, thanks, that sounds dodgy, but still doesn’t elucidate the arithmetic. What is the numerator, and what the denominator, in the 87%?
the dodginess results from the lack of explicitness in specifying the range of the CO2 concentrations over which the logarythmic relations holds. 87% is a little more than 5/6, representing a little over 5 doublings out of 6 in the range. Like the hypothetical equilibrium climate sensitivity (and whatever it represents in the real climate), it can’t be estimated to 2 significant figures. 12ppm to 800 ppm is 6 doublings, of which 5 have occurred already. We can’t really tell what the correct upper and lower bounds are for this estimation.
David G says:
August 11, 2014 at 8:20 am
CO2 lags warming, Stupid! Not the other way round. Why is this shite even here?
==================================================================
On the chance that David G was responding to my question, I would refer him and any others to this graph: http://en.wikipedia.org/wiki/Milankovitch_cycles#mediaviewer/File:Vostok_420ky_4curves_insolation.jpg
which is a colorized version of figure 3 here: http://geoweb.princeton.edu/people/bender/lab/downloads/Petit_et_al_1999_copy.pdf
And while David G is not likely to grasp the problems entailed by Vostok’s close tracking of CO2 and CH4, others may be. If so I’d like to hear from them. –AGF
Kristian,
“DWLWIR could be 1,000,000 W/m^2 and if you emitted 1,000,050 W/m^2 worth of UWLWIR, you wouldn’t feel it any more than if the DWLWIR were a mere 300 W/m^2 and your UWLWIR 350 W/m^2. Only the net energy matters in a heat transfer.”
One little experiment you can try is to place your hand near a source of radiant heat such as a grill (or broiler). Choose a distance where the initial feeling is hot but not uncomfortable. Each second that passes your hand feels hotter and hotter, then burning hot.
What is happening? Why doesn’t your skin register the pain in the first tenth of a second, the time for the nerve impulse to reach the brain?
In the first second more energy is being received by your skin than is radiating outwards – equilibrium has not been reached – and at this outgoing radiation flux, your skin has warmed to a comfortable temperature. After another second, in order to reach equilibrium, more energy is being radiated back from your skin and this can only happen at a higher temperature. The process continues until the outgoing radiation from your skin equals the incoming radiation – or the temperature becomes unbearable and you remove your hand.
If you replace your hand with a slice of bread you can relax and watch it toast. It does so because when the bread radiates at the same flux density as the incoming radiation from the grill, the temperature to make this happen is very high – which is why you couldn’t leave your hand there.
Now, what about a situation where you was radiating and receiving 1,000,050 W/m^2. The net energy transfer is zero but I wouldn’t like it, would you? The temperature would be hot enough to chargrill you.
Matthew R Marler says, August 11, 2014 at 8:39 am:
“Net heat flow is always from warmer to cooler parcels (…)”
There is no such thing as ‘net heat’ in a thermal exchange between two objects at different temperatures. There is ‘net energy’ and this net energy is defined as the HEAT. This might seem like pure semantics, but it’s not. It is a very important distinction.
“Warming of the surface is caused by the radiant energy of the sun; the net warming effect of CO2 is caused by the reduction in the surface cooling rate.”
There exists no such net warming effect from CO2, Matthew. Only on a theoretical level and in closed glass boxes in lab experiments. Not in the real dynamic surface/atmosphere system. For the simple reason that more CO2 in the atmosphere can never reduce the atmospheric temperature gradient up from the solar-heated surface and hence reduce the rate of energy loss from it.
Kristian, maybe you can address my question no one responded to. What about CO2 and “optical depth”. That is that as CO2 density increases, it is delivered higher into the atmosphere. Higher up is colder with less or no water vapor to slow outward IR. The newly added CO2 now does that slowing. How do you understand this?
Vince Causey says, August 11, 2014 at 9:08 am:
OK, I see that you don’t understand at all what I’m saying.
AlecM 12:11am: “Real GHE is obtained by calculating no cloud or ice albedo, 341 W/m^2, mean surface temperature and subtracting that 4 to 5 deg C from present ~15 deg C, ~ 11 K”
That 11K is just using your different definition of GHE. Satellites measure Tmean = 255K in thin atm. at their orbit and surface thermometers in Earth thick surface atm. measure Tmean = 288K. This is not disputed unless you can find a basic physics error in one of them. Which would make the evening news.
10:26pm: “…the heat generation rate in the atmosphere is 238.5 SW..”
Heat doesn’t exist in nature. There is no energy generated in the atm. either as the atm. uses up no fuel. This comes from your confusion over applying the generalized 1st law in non-atm. text books from your 10:02pm:
“(monochromatic heating rate of matter/unit volume) = – ∇.(monochromatic radiative flux density)”
Your 1st term for the atm. is = 0 as no energy is generated within the atm. and the term on the other side for the atm. is (net energy in from sun – net energy out from surface) for the volume of interest. For the surface volume balance, the mass of the atm. radiates a real flux incident on the surface all the time at all frequencies at all of its temperatures and has to be included as in nature.
******
Ghengis 6:53am: There is a difference; not my research interest to figure out how exactly.
Nick Stokes says:
August 10, 2014 at 4:05 pm
“Care to explain the arithmetic behind
[quoting] “CO2 at 400pmmv is already committed and immutable. So CO2 has already reached about ~87+% of its potential warming effect in the atmosphere.”
Anyone?”
—————-
Explaining the arithmetic …. is easy.
Trying to explain one’s belief that CO2 ‘s ability to “trap” heat declines logarithmically …. is not easy.
Especially given the FACT that there is no known entity in the universe that is capable of “trapping” thermal (heat) energy other than the proverbial Black Holes that exist at the center of galaxies.
Thermal (heat) energy can be converted to mass [e=mc2], but it can’t be trapped. (Unless one invents a container whose inside surface is a 100% perfect reflector of IR/thermal (heat) energy)
And without said “trapping” ability being possible, ……. then said “logarithmically decline” is an impossibility.
If one uses an instrument to release a “2 second burst of IR from the surface” …. it does not simply disappear after traveling 2 or 3 feet through the air as a result of being “trapped” by the CO2 molecules therein.
A “point” measurement of the IR intensity decreases the farther it travel from its source, but that is due to its “scattering” …. and not because it is being “trapped”.
What’s good for the “IR” goose …. is good for the “IR” gander, …. regardless of whether it is a H2O vapor “goose” …. or a CO2 “gander.”
Now iffen you are talking “buckets under a leaking roof”, then “yes”, the ability of the buckets to “trap” rainwater decreases logarithmically as the buckets fill up.
Samuel C Cogar, I haven’t heard anyone say, or mean to say, CO2 “traps” heat. My layman understanding is that upward IR heat is like ball in a pinball machine. CO2 is the bumpers.
climatereason says:
August 11, 2014 at 3:26 am
///////////////
Tony
When I typed my comment, I initially had 280ppm, but before sending it, I reduced the figure, perhaps i should have reduced to about 255/260ppm rather than 200ppm. That said, I would not forcefully argue against using 280ppm as the base figure.
RMB says:
August 11, 2014 at 8:52 am
If my explanation is crap as you put it I need an explanation as to why I cannot get heat into uncovered water but if I float a metal object on the surface killing the surface tension underneath and apply the heat source to the floatin object the water heats as one would expect, explain that without surface tension. This reply is for Richard Verney.
+++++++++++
Well I am not Richard, but I am the one that said your explanation was crap : )
Surface tension allows evaporation which cools the surface. Increased airflow increases evaporation. Evaporation always takes energy from the surface of the water and transfers it to the air.
IR is only capable of penetrating a few microns into the water which increases the rate of evaporation. Are you starting to get the picture here? Surface tension isn’t really a factor in this.
Surface tension does inhibit convection and diffusion through the water though which makes it harder to heat the water under the surface.
Basically the only way the oceans are warmed is by SW radiation from the sun which penetrates many meters down into the ocean.
Genghis, aren’t you leaving out the sloshing, tradewind furrowing, la nina piling, and ocean conveyer effect for mixing warm water down and forcing cold water up from the thermocline? You put cold water in a tub, then warm water on top of it, then put a kid in, the warm and the cold will get mixed.
AlecM says:
August 11, 2014 at 8:10 am
@agfosterjr: the warming of the camel’s back from solar SW energy is by the physical process called ‘thermalisation’. This is the absorption of SW energy quanta by raising electron orbitals to higher energy states followed by emission of lower energy quanta, a process called fluorescence.
The lower energy quanta cause the energy to be transferred to greater molecular motion; heat.
=================================================================
Again, the word ‘heat’ was around before ‘molecules’ were identified. Your neologism ‘thermalization’ is the same as ‘heating,’ and I don’t think we need it (your new word). Why do you feel the need to eliminate the gerund–and verb? –AGF
Vince Causey says:
August 11, 2014 at 9:08 am
/////////////////////
Now apply that to the real world.
You can cook your steak say 10 inches above a BBQ, but you cannot cook it 10 inches from the side of the BBQ, still less 10 inches below the BBQ. But if you measure the IR budget, it is the same below the BBQ as above it.
In the real world other energy exchanges dominate other the radiative budget, and that is why it is likely that the radative energy budget is unlikely to explain how the climate on planet Earth works.
Indeed, if the energy budget was as K&T depict, ie., some homogenous whole, there would be little in the way of weather. It is only because the system is not dynamically balanced and it is not some homogenous whole, that we get weather patterns as energy is distributed around the oceans and the atmosphere (bother laterally and vertically).
Richard Verney, My IR gun uses batteries because the display and chip in it require more energy than is produced by the IR source.
It so happens that my primary use of the IR gun is to monitor the various engine temperatures. One of the amazing things is how well all the radiant IR sources manage to heat up my engine room and make hot water for me.
If you really want a demonstration of IR’s effectiveness at heating objects I would invite you to spend an afternoon working on my engine with me 🙂
Kristian,
“OK, I see that you don’t understand at all what I’m saying.”
Believe it or not, I have read all your posts twice and I agree with everything you have said. If my response sounds pedantic it is because it is the only thing I could argue with. 🙂
A C Osborne;
Your ignorance is astounding, if you tried your experiment
>>>>>>>>>>>>>>>
I already admitted my mistake upthread when Leanard Weinstein pointed it out to me. Whipping a dead horse?
As for the rest of it, nothing much changes. Kristian has claimed that only heat can make something else hotter. I gave him two examples that a ten year old can understand that falsify that claim. When I did so, he started complaining he was talking about heat transfer. That’s precisely the problem. We’re talking about radiated energy which is not heat. It only turns into heat when it is absorbed by something.
The amount of energy any given body radiates is governed by Stefan-Boltzmann Law:
P=5.67*10^-8*t^4
Notice that this formula has no term in it for the temperature of the ambient surroundings. A body at 15 C in an atmosphere at -40 C radiates 390.1 w/m2. A body at 15 C in an atmosphere at +40 C radiates 390.1 w/m2. The difference is that one will, over time, be cooling off and the other will be, over time, warming up. But at that point in time, the body is radiating 390.1 w/m2.
The important take away is that what the body is radiating is not heat. It is energy carried by photons (which have no mass and hence cannot carry “heat”). But when the photons are absorbed by matter, the energy is then converted to heat. Since two bodies radiating toward each other cannot change the amount of energy they radiate to be anything other than in accordance with SB Law, that energy has to go some where and turn into heat when absorbed.
So put a cold object next to a warm object, and the cold object will warm and the warm object will cool based on the net transfer of energy between them. But make no mistake about it, there is energy going both ways, with the net satisfying the requirements of both 2nd Law of Thermo and Stefan-Boltzmann Law.
RMB says:
August 11, 2014 at 8:52 am
/////////////////////////
First of all, I should just point out that I am not the one who is calling your explanation crap. That is not my style of writing. I was simply quoting and expanding upon a comment made by another commentator, which other commentator although largely agreeing with you, called your expllanation crap.
Second, surface tension may play a role, but how effective a role is moot. The ocean is rarely as calm as a mill pond, and wind will tend to rip apart the very top of the ocean and in the process this may weaken the effect of surface tension. Surface tension may inhibit convection, but probably has little effect on conducton, but I have not seen experiments that truly deal with that issue.
Third, we know that LWIR pentrates water. We know from this that the surface tension of water does not cause an impenetrable barrier to LWIR. However, the optical absorption of LWIR in water is such that it becomes fully absorbed within a very short distance. See http://scienceofdoom.files.wordpress.com/2010/10/dlr-absorption-ocean-matlab.png from which you will note that 60% of LWIR is fully absorbed in just 4 microns. This is of course a vertical distance, and because of the omnidirectional nature of DWLWIR such that about 10% of it intersects with the ocean at a grazing angle of 10deg or less, 20% at a grazing angle of 20deg or less, 30% of it at a grazing angle of 30deg or less, etc I suggested that about 80% of all DWLWIR is full absorbed within 3microns of vertical depth (that figure may be may be a little too high). So theoretically IR is capable of heating water. But does it cause only the very top (ie., the first few microns) to evaporate or can it in some way heat the bulk of the ocean below? My post was about heating the bulk, and I was suggesting that it cannot be by conduction since the energy flux is upwards (the very top of the ocean is cooler than the 10 micron layer which is cooler than the 15 micron layer, ie., the temperature profile of the ocean is warmer at shallow depth ( see: http://disc.sci.gsfc.nasa.gov/oceans/additional/science-focus/modis/MODIS_and_AIRS_SST_comp_fig2.i.jpg ). I suggested that unless the energy absorbed in the first few microns can be dissipated and diluted to depth at a speed greater than the speed at which DWLWIR absorbed in the first few microns would power/drive evaporation, then it would be difficult for it to heat the oceans; it would predominatly drive evaporation rather than raise water temperature.. I suggested that ocean overturning was a slow mechanical process and therefore that does not look a promising mechanism.
Fourth, I suspect that what you see with your IR heat gun is that it cannot heat the bulk liquid since it simply boils off the top because nearly all the energy is absorbed within just a few microns, causing rapid evaporation from the very top down. To the extent that it warms the bulk this is probably because the container is being warmed, and the warmed container warms the water by conduction. I agree that water that is free to evaporate is very difficult to heat by LWIR coming from above.
Fifth, your example of floating a steel plate is an example of heating by conduction. That is something rather different. Floating a steel plate on top of water and heating the steel plate from above involves much the same process as heating water in a metal sauspan placed on a cooker element and heating the base of the sauspan from below, only that it is less efficient since convestion wasista in carrying heat upwards.,
The upshot is that Ghengis and myself are to some extent with you in that we both see difficulties in DWLWIR effectively heating the ocean. We both consider that it is likely that to the extent that it is doing anything, it is predominantly driving evaporation.
We both agree that it is solar that heats the ocean; fortunately the optical absorption of solar is very different such that solar energy is diluted and dissipated over a volume about a million times larger than that at which DWLWIR is absorbed, with the effect that solar does not burn off the ocean from the top down, but rather it gently warms it at depth.
Richard Verney, I’m trying to understand these concepts as a layman. You said” ocean overturning was a slow mechanical process and therefore that does not look a promising mechanism” for warming the ocean. This makes me think of a description of la nina I saw awhile back. It said that trade winds blow toward the western Pacific pushing warm surface water along with it. The warm water “piles up” in the western Pacific and weighs and pushes warm water down on the thermocline. With the thermocline pushed down in the western Pacific, it rises in the eastern Pacific delivering cold water to the surface. This has been used as an hypothesis for the “pause”. Seems altimetry supports this idea as the sea level is shown to be higher in the western Pacific with a falling incline to the east.
@richard verney: for atmospheric temperature <= sea surface temperature, net LWIR direction is surface to atmosphere; it cannot heat the sea surface.
@davidmhoffer: A body creates a propagating electromagnetic field. When it interacts with an opposing field, net EM energy transfers from the hotter to the cooler body at the rate:
Net EM Flux = σ(εhot.Thot^4 – εcold.Tcold^4) (parallel plates). The heat transfer rate to the hotter body is the negative of the EM flux, so it cools. At the cooler body, the absorbed net EM energy thermalises to kinetic (heat) energy.
A single S-B equation implies that Tcold = 0 deg K, so it is a potential, not a real energy flux.
This is standard radiative physics. On average there is zero EM flux from cooler to hotter. You have to consider thermal incoherence, but it averages to zero.
@agfosterjr: thermalisation is a specific physics term which is used when energy quanta are converted to molecular motion. It is the process of producing heat, not heat itself.
@Trick: satellites measure mean 255 deg K OLR emission temperature but that is the flux-weighted mean of the partial spectral temperatures.
There is no single -18 deg C/255 deg K at 5-6 km as claimed by Climate Alchemy; it is a virtual construct with no physical existence.
Therefore 33 K GHE does not exist as a physical entity. The real GHE is set by albedo change but is superimposed on cyclical temperature changes from solar induced ocean heating and cooling. There is near zero warming from well-mixed GHEs, as is being proved experimentally.
Hence the change of GHE from 2 K at the last glacial maximum to the present 11 K is from biofeedback reducing albedo.
“DWLWIR could be 1,000,000 W/m^2 and if you emitted 1,000,050 W/m^2 worth of UWLWIR, you wouldn’t feel it any more than if the DWLWIR were a mere 300 W/m^2 and your UWLWIR 350 W/m^2. Only the net energy matters in a heat transfer.”
Not exactly. If DWLWIR transfer efficiency is 20% and UWLWIR transfer efficiency is even just 20.5%, you’d absolutely notice the difference. Spectral analyses matter!!
Kristian says:
August 11, 2014 at 9:12 am
I can’t make a lick of sense out of that. Do you claim precedent for these defintions (if so please cite authorities) or do you just make them up as you go along? –AGF
Just noticed another howler: ‘So put a cold object next to a warm object, and the cold object will warm and the warm object will cool based on the net transfer of energy between them. But make no mistake about it, there is energy going both ways, with the net satisfying the requirements of both 2nd Law of Thermo and Stefan-Boltzmann Law.’
WRONG: for purely radiative heat transfer between the two bodies, the heat energy lost by the hotter body equals the heat energy gained by the cooler body; the EM energy transfer is one way only on average (thermal incoherence superimposes an oscillation about zero mean)
Since nobody seems to agree on what heat is might I suggest we distinguish between radiating transfer of energy (over long distances) and molecular transfer of energy (over short distances)? Call them T1 and T2 just for the purposes of this thread (they’re ultimately the same thing). –AGF
Matthew R Marler says: August 11, 2014 at 8:48 am
“12ppm to 800 ppm is 6 doublings, of which 5 have occurred already.”
So a lower bound of 12ppm has been plucked out of the air as well? I actually think his denominator involves the 33°C. But that’s the effect of all GHG, not just CO2.
But the thing is, no-one knows what the arithmetic of % that is basic to the essay actually means. Yet you thought it was a good essay?
Samuel C Cogar says: August 11, 2014 at 9:36 am
“Explaining the arithmetic …. is easy.”
But no-one can do it?
StuL says:
August 10, 2014 at 4:14 pm
I used to believe that CO2 causes a limited warming too, but I am not so sure now, Ithink Venus shows that there is no effect whether 0.004% or 95% http://theendofthemystery.blogspot.com/2010/11/venus-no-greenhouse-effect.html
i want to this site and it is very interesting. I don’t have the time now but if correct this should hold true for every planet with an atmosphere in the solar system if so it is definitive and irrefutable.
Maybe someone that has a little more time can take a look at this.
Nick Stokes says
I am not a big fan of Nicks nor do I typically agree with him but he is correct the basic % statement made makes no sense.
Nick Stokes: Yet you thought it was a good essay?
Sure. Good books have been written that contain detailed elaborations of the equilibrium model of the Earth, even though Earth doesn’t have an equilibrium climate. All the laws are approximations, and little filling in helps to clarify the author’s meaning in this case. It would be helpful if he would make the case more explicitly, but it isn’t a terrible flaw.
What did you think about my propositions that the next 3 doublings, at current rates, would take about 450 years and produce around 3C of warming? If that happens, only 6/9th or about 67% of the warming achievable through the next 450 years (starting long ago) will have occurred already. Some people have written that another doubling after 800ppm can not occur from fossil fuel burning, in which case more than 80% of the achievable heating has already occurred.
The author is a little loose, but the idea is hardly mysterious.
A point I’ve been trying to make, with little success, by describing the atmospheric resistance to radiation as a set of resistors in series rather than a single resistor. The marginal change to the resistance near the Earth’s surface, where the resistance is the largest is much smaller than the marginal change at height, where the resistance is the smallest. Because there are processes that “short circuit” the radiative loss channel — notably convection and convection carrying latent heat — even this distributed, nonlinear shift in total resistance in the radiative channels is diminished in its impact by the shunt resistance. GCMs appear to be largely incorrect in their coarse-grained treatment of this — the vertical slab size is too large to see the one, and far, far too broad to see the others, so both are replaced with ad hoc linearized approximations for the entire cell(s).
Not to mention the fact that this all still assumes that one can break the net heat transport down in terms of linearized “forcings” at all in an open nonlinear chaotic system.
In the end, it all comes down to the GCMs. Either this problem is solvable with our currently accessible computational resources, or else it is not. If it is, all well and good but sooner or later in this case the models will need to come way down off of their literally too high horses and start corresponding to the actual observed climate history as it evolves into the future. If not, it is sadly rather probable that it will never be solvable, because of the many, many orders of magnitude more compute power that should be necessary to solve the problem correctly, according to what we already know about problems of this type.
We cannot predict the weather more than a few weeks into the future, and fail to predict even very simple, large scale numbers like “number of hurricanes expected this year” or “whether or not El Nino will form” a mere six months in advance with any significant accuracy, and what accuracy we do have comes as much from human art as it does from any specific computational model.
What is truly amazing is that the modellers have managed to sell the world the idea that even though the models do not generate predictions (if they did they would be instantly falsified) and even though their projections are falsified anyway, even though we know that the weather models that are the direct antecedents of the climate models are not integrable over years such that they get anything but noise almost fully decorrelated from the actual weather, we should still take them seriously even as the world’s actual climate does something completely unpredicted by the models. As we might have predicted! It would have been amazing if the climate models had worked, but it is completely unsurprising that they are not working.
Beyond that, the computing “forcings” and “feedbacks” and so on is all model dependent, and we know — or at this point, should know — that the models we have are rather unreliable and hence so are all assertions of forcings, feedbacks, and other attempts to linearize the problem into politically usable terms!
Humans just aren’t going to respond well to the fact that hey, it could actually cool some over the next five years — or not — storms could get worse — or better — we could have great droughts — or normal rainfall — or massive floods — or some mix of all three — but what the heck, we cannot predict any of what will happen with any reliability, so give me five percent of your annual income every year anyway to help solve the problem that won’t be solved by any measures we take to solve it using that money, according to those models, even if the models we have that aren’t working turn out to work after all.
In fact, they might just say: Keep your damn hands off of my wallet unless and until you have at least one model that manifestly works, not just over its reference period (training set) but works to predict — not project — the actual future in such a way that the actual future doesn’t immediately falsify the robust prediction.
And then, of course, we could start worrying about thirty years, or fifty years. Or not.
rgb
Oops: 3200 is the 8th doubling starting at 12.5, so if 8 doublings is the maximum achievable, 6/8 = 75% of total accumulation has already occurred if 3200 is the maximum physically achievable CO2 concentration.
matayaya: Samuel C Cogar, I haven’t heard anyone say, or mean to say, CO2 “traps” heat. My layman understanding is that upward IR heat is like ball in a pinball machine. CO2 is the bumpers.
CO2 “traps” heat in its orbital electrons: physically, the orbital sizes are such that the specific wavelengths of LWIR cause the electromagnetic oscillations to interact with the electrons and transfer energy from radiant to orbital energy. The reverse process occurs when electrons “decay” from higher energy orbits to lower energy orbits and release the energy as electromagnetic oscillations.
Me: I went into a store to buy an item. I gave the cashier $20 for the item, and he gave me $15 in change.
Kristian/AlecM: No, no, no! You’ve got it all wrong! You PAID $5 for the item! You don’t understand how to do accounting!
Standard science: The earth’s surface averages about 396 W/m2 upward radiative power flux density in the longwave infrared. The earth’s atmosphere averages about 333 W/m2 downward radiative power flux density.
Kristian/AlecM: No, no, no! You’ve got it all wrong! The heat transfer from the earth to the atmosphere at 63 W/m2! You don’t understand how to do physics!
Matthew R Marler:
At August 11, 2014 at 1:24 pm you say
No, that is an atomic effect which is trivial in the atmosphere.
CO2 is a greenhouse gas (GHG) because a CO2 molecule can absorb a photon of appropriate wavelength, and the absorbed energy of the photon becomes vibrational, rotational or stretching energy of the molecule.
A CO2 molecule (O-C-O) can bend so vibrate.
An O2 molecule (O-O) has no angle to bend so cannot vibrate.
CO2 is a GHG but O2 is not.
Richard
Kristian: There is no such thing as ‘net heat’ in a thermal exchange between two objects at different temperatures. There is ‘net energy’ and this net energy is defined as the HEAT. This might seem like pure semantics, but it’s not. It is a very important distinction.
Yes on the net “energy” transfer instead of net “heat” transfer. I get sloppy sometimes and use “heat” for “energy”. That aside, what else that I wrote do you dispute?
1. That the molecules in a region have a distribution of energies, instead of all being at the mean energy?
2. That some of the molecules in that region can absorb radiation concurrently with other molecules in that region emitting them?
3. That the change in electron orbital energies transfers energy to the electromagnetic waves that are emitted?
4. That that radiant energy is conserved, and transferred to kinetic energy and orbital energy when it is absorbed?
5. That a region of molecules emitting radiation radiates in all directions, including downward toward a surface of potentially higher temperature?
6. That CO2 molecules absorb and emit radiation in the LWIR portion of the spectrum?
If you put these all together, then an increase in atmospheric CO2 concentration will result in an increase in downwelling LWIR at the surface, thus reducing the cooling rate of the surface; and some concomitant increase in the vaporization rate of surface water.
richardscourtney: CO2 is a greenhouse gas (GHG) because a CO2 molecule can absorb a photon of appropriate wavelength, and the absorbed energy of the photon becomes vibrational, rotational or stretching energy of the molecule.
And a CO2 molecule “can absorb a photon of appropriate wavelength” why? Because the wavelengths are tuned to the electron orbits? Because the wavelengths are tuned to the C-O bond lengths? I think you are correct that it is the second. Thank you for your reminder.
The bonds are electron orbitals, but the distinction you made is still important, and I wrote the matter incorrectly.
Matthew R Marler says: August 11, 2014 at 1:16 pm
“Good books have been written that contain detailed elaborations of the equilibrium model of the Earth, even though Earth doesn’t have an equilibrium climate.”
But they at least explain their numbers and approximations. People know what they mean. And the numbers aren’t just made up.
“What did you think about my propositions that the next 3 doublings, at current rates, would take about 450 years and produce around 3C of warming?”
There is currently about 800 Gtons C in the air and we’re emitting nearly 10 Gt/year, rising rapidly. So the first doubling will be well short of 80 years. As to 3°C, that’s coming back to CS. I think you’re saying no feedbacks; I think water vapor at least is certainly a positive, at least 2x.
“””””…..agfosterjr says:
August 11, 2014 at 7:15 am
george e. smith says:
August 10, 2014 at 10:16 pm
“Heat is (chaotic) mechanical kinetic energy. It IS NOT electro-magnetic radiation, which is a propagating field .”
This is argument by definition. …..”””””
Suit yourself agfosterjr, I gave no formal “definition”. I simply said what “heat” (noun if you must) is, and what it is not. I don’t see why we grant the Romans the right to tell us now what “heat” or “heating” is; they had not the foggiest idea what it is. And you shouldn’t be guilty of “argument by definition.”
I’ve actually calculated how much heat you could conduct from the sun to the earth, using a (93 million miles long) rod made of type II-a diamond. And even if you waited a year, it would not warm a thimble full of water, up to where you could measure the Temperature rise. And in any case, the diamond close to the sun, would evaporate, and break the connection; not to mention that type II-a diamond, only has its super high thermal conductivity, at 100 K temperatures.
Dunno, what “electro-magnetic energy” and “specific heat” definitions have to do with anything.
But I make it a point to never stand between someone, and a cliff they are determined to jump off.
So I’ll hold your coat; but keep on going.
g
george e. smith says:
August 11, 2014 at 1:45 pm
You most certainly did give a formal definition: radiant energy is not heat. This is tantamount to saying the sun is not hot. And you remain oblivious to the violence you do to the English language. Heat, to heat, heating, warm, to warm, warmth, hot, calor, caliente, etc. These words were all around before the atom and thermodynamics, and we all knew what they meant until a bunch of bossy pedants came along and told us we didn’t. If you want a word to mean something for a specific time and purpose, go ahead and provide a provisional definition. But don’t go burning dictionaries and telling us we were wrong all along. –AGF
Nick Stokes: People know what they mean.
I don’t think many people know what the “equilibrium” means in a system like the Earth that has no equilibrium. The positive water vapor feedback follows from the equilibrium assumption because it assumes that the extra 3.8W/m^2 increase persists long enough for an equilibrium to occur in the water column. If instead you look at the non-equilibrium case of day/night temperature differences and seasonal differences, and the hydrological cycle (with clouds) the case for a positive water vapor feedback disappears, because the 3.8W/m^2 does provide enough energy both to vaporize the water and raise surface temperature.
The equilibrium model is tractable, but its relationship to reality is unclear.
But I did ask for your opinion, and I thank you for it..
Matthew R Marler, “equilibrium” to me can refers to the top-of-atmosphere measurement of energy coming in and energy going out. Right now there is more energy coming in than going out; i.e., disequilibrium. That energy churns around in countless ways once it is inside the system, but it is the top-of-atmosphere measurement that is our primary equilibrium concern.
I’m actually encouraged that many of the posters here DO understand the difference between EM radiation and “heat” (noun). Both are forms of “energy”, so is electricity. So is mass (per Einstein).
And the can be interconverted; but here’s the rub.
“Heat” has a special handicap as a repository of MECHANICAL ENERGY.
When you whack your thumb with a hammer; every single molecule in your hammer, is travelling towards your thumb, in essentially the same direction; they all operate in concert. That’s why it hurts so much.
Don’t hit your thumb with a hammer; HEAT IT instead.
In the case of “heat energy”, which I have said is mechanical energy (like your hammer), the damn molecules can’t agree among themselves, which way to go. So they don’t co-operate in squishing your thumb.
“Heat energy” is the garbage dump of mechanical energy.
The sun can heat (verb) the earth; BUT we get NO “heat” (noun) from the sun.
We make it ALL here on earth, by wasting most of the good Cadillac style (maybe Mercedes) electro-magnetic radiant energy (photons) that we DO get from the sun.
But we don’t waste ALL of it.
Much of it, is turned into grass or wood, or phytoplankton, or oil and coal,instead of heat,
Gee whizz, these days, we can even turn some of it (EMR) into electricity, by means of PV cells.
Wind is NOT as good as PV solar cells. Whyzatt ?? Well to get wind turbine energy, you first have to make “heat energy” (noun). So you put the good stuff (EMR) into the garbage dump , to let it rot and become “heat energy” (maybe phonons), and then you start from that load of crap, to make wind.
Very bad form.
Most forms of energy can be turned into waste “heat” (noun), with almost 100% efficiency; but “heat energy” can not be turned by any process, into ANY other form of energy, with 100% conversion efficiency. Entropy things, and the Carnot efficiency, set a limit to that type of conversion.
BUT “hat energy” (noun) does have a trump card (the Joker) to play.
ONLY HEAT ENERGY (NOUN) CAN CHANGE THE TEMPERATURE. Of anything.
So why don’t we let Mother Gaia take care of turning good pristine solar EMR energy into stored chemical energy, like phytoplankton, grass, wood, coal, gas and oil (unless gravity makes those last two), and then we can all enjoy those fossil and notyetfossilized chemical energy fuels, that MG provided for us, and continues to do so.
g
rgbatduke;
A point I’ve been trying to make, with little success, by describing the atmospheric resistance to radiation as a set of resistors in series rather than a single resistor. The marginal change to the resistance near the Earth’s surface, where the resistance is the largest is much smaller than the marginal change at height, where the resistance is the smallest.
>>>>>>>>>>>>>>>>>>>>>.
An interesting approach. Are you attributing the steadily increasing size of the resistors as you approach earth surface solely to the energy flux required by SB Law, or are you also allowing for increased water vapour which doesn’t know up from down and hence the larger concentrations closer to earth surface must resist the downward flux from above just as they resist the upward flux from below?
agfosterjr says, August 11, 2014 at 11:44 am:
“I can’t make a lick of sense out of that.”
Doesn’t surprise me in the least. Seeing that it looks as though there is a 97% consensus on the comment threads of this blog that “We shall not get that most basic of thermodynamic concepts that is ‘HEAT’. We shall rather do out utmost to confuse it as much as possible, so as to make AGW seem plausible.”
When Climate ‘Science’TM burst onto the stage with its Great Green Confusion Brigade and hijacked normal physics with its ‘back radiation heating’ story, epitomised by the K&T97 Earth energy budget diagram (and all its successors), the clear distinction between what heat is and isn’t, and what heat can do that things that aren’t can’t, successfully ended up completely jumbled in people’s minds.
What the ‘back radiation heating’ story and these diagrams are all about, after all, is to let people think that the ‘back radiation’ down from the cooler atmosphere to the warmer surface is actually an extra, positive energy INPUT, able to raise the internal energy of the surface and thus its temperature by 33 degrees beyond a pure solar radiative equilibrium. In other words, even though it is of course never stated in plain words, the goal is to make people think that the ‘back radiation’ in itself will be able to heat the surface, meaning, people are deliberately tricked into conflating radiation (radiant emittance), like the DWLWIR and UWLWIR, with actual radiative heat transfer, like the solar flux. All arrows in the K&T type diagrams are actual heat fluxes EXCEPT those megathick DWLWIR and UWLWIR arrows. Is this pretty important distinction ever mentioned anywhere? Nope. Of course not. That’s the trick. They don’t come out and say it, that they expect the ‘back radiation’ to result in a direct thermal effect AS IF IT WERE HEAT. All they need to do is leave the two radiation arrows there just next to all the heat arrows and let the impression, the implicit notion sink in that they all represent the same thing, energy transfers equal in properties and abilities.
And people have fallen for it hook, line and sinker. And that’s why we are where we are today.
The result? No one seems to know (or care about) what the actual physical definition of heat is anymore. Even though it is still one of the most basic and most useful concepts of thermodynamics, incorporated into its very Laws, and one of the most easily sensed and experienced physical phenomena there is.
Heat is the reason you feel warm next to a fire and cold when opening the door to your freezer.
Why do you feel warm next to a fire? Because the fire is warmer than you, therefore transferring energy to you AS HEAT. Why do you feel cold in front of the open freezer? Because you are warmer than the freezer, therefore transferring energy to it AS HEAT. Your internal energy (U) increases next to the fire, but decreases next to the freezer. Making you warmer/colder. Simply because of the different heat transfers.
Just like you cool to your freezer, the global surface of the Earth cools to the atmosphere. Because the surface is warmer than the atmosphere, it transfers HEAT to it. It doesn’t get any heat back. Seems like people are in bad need of a reeducation on this.
In a thermal exchange (a heat transfer), what isn’t heat doesn’t heat. In the radiative exchange between the surface and the atmosphere, neither the DWLWIR nor the UWLWIR are heat. Therefore, none of them will cause anything to warm (or cool). ONLY the net of the two, the net energy moving from the surface to the atmosphere, the HEAT, will cause warming/cooling. Because that is the amount of energy which is actually at any one time brought from the surface to the atmosphere. The surplus, so to say. It all happens in one continuous process, after all. The reduction in surface internal energy equals the increase in atmospheric internal energy. 398 W/m^2 UP, 345 W/^2 DOWN? Doesn’t matter. They’re not heat. They’re EM radiation. Radiant emittances. Within one integrated radiation field. [398-345=] 53 W/m^2 UP is what matters. The radiative heat.
Climate ‘Science’TM has all but managed to make us forget about this basic knowledge.
Heat is defined as the energy transferred spontaneously from hot to cold as a result of the temperature difference. When it comes to radiative heat transfer, the heat is the ‘net energy’ always moving only from the hotter to the colder object. It is a unidirectional flow of energy. Just like a waterfall, like wind or an electric current. Always from high to low potential. Spontaneously.
This definition is not controversial at all. Well, here for some reason it seems extremely controversial. I’ve been called an ignorant and an idiot for daring to discuss it and its corollary.
The ‘net heat’ term comes from the First Law. The Q term is the sum of heat IN and heat OUT of a system. But the heat OUT is not going to where the heat IN is coming from. They do not directly oppose each other. ‘Heats’ never do. It’s in the definition. They are not part of the same heat transfer, but of two different ones. The central system absorbs heat (heat IN) from its heat source (hot reservoir), a hotter place. It then also emits heat (heat OUT), but to a colder place, its heat sink (cold reservoir). If the heat IN equals the heat OUT, we can say that the ‘net heat’ is zero.
The surface of the Earth gets its heat in from the Sun, a hotter place, its heat source/hot reservoir. At the same time it sheds heat out to the atmosphere, a cooler place, its heat sink/cold reservoir. These two need to balance for the temperature to remain the same. The only thing the atmosphere could ever do in this three-body situation is limit the heat OUT from the surface, meaning the energy transferred away from it per unit of time. It can limit its cooling. It can never increase its heating, that is, warm it directly. Only the Sun warms it directly. The atmosphere suppresses its cooling rate at a certain surface temperature to let the surface balance its heat IN with its heat OUT.
How?
The atmosphere has a mass. That means it has the ability to warm. And it will and does warm. Simply from being conductively > convectively coupled with the solar-heated surface. The atmosphere is able to warm. Space isn’t. Therefore the presence of the atmosphere will reduce the heat OUT from the surface and force it to equilibrate at a higher steady-state temperature. Simple as that.
george e. smith says:
August 11, 2014 at 2:41 pm
“The sun can heat (verb) the earth; BUT we get NO “heat” (noun) from the sun.”
See, this is prescriptive lexicography. And it’s on mighty shaky ground. Try teaching that to a first grader. And I guarantee, I’ll be siding with the first grader. But I’ll give you an ‘A’ for style. –AGF
“””””…..agfosterjr says:
August 11, 2014 at 1:59 pm
george e. smith says:
August 11, 2014 at 1:45 pm
You most certainly did give a formal definition: radiant energy is not heat. This is tantamount to saying the sun is not hot. …..”””””
Well ag, sorry to tell you; NOTHING is “tantamount” to SOMETHING ELSE.
That is why we even have language. Words have meaning, and “other” words, have “other” meaning.
“””… radiant energy is not heat. This is tantamount to saying the sun is not hot….”””
What on earth does saying “” radiant energy is not heat.””, have anything to do with the sun ??
Last time I thought about it,
A DEFINITION tells you what SOMETHING IS ; it does not tell you what something is not.
We would use up all the trees in the world for paper, if we simply wrote down a complete list of things that RADIANT ENERGY IS NOT.
I would call that an “undefinition”, but then that would be “other words.”
But I’ll stand aside so you can keep going.
“””””…..agfosterjr says:
August 11, 2014 at 2:52 pm
george e. smith says:
August 11, 2014 at 2:41 pm
“The sun can heat (verb) the earth; BUT we get NO “heat” (noun) from the sun.”
See, this is prescriptive lexicography. And it’s on mighty shaky ground. Try teaching that to a first grader. And I guarantee, I’ll be siding with the first grader. But I’ll give you an ‘A’ for style. –AGF …..”””””
Shifting the discourse from Physics to “lexicography” on this site, is likely to get people to confuse you with Eli Rabett. He likes to use “other words” too.
g out
Kristian,
That same debate has been going on here for years. I don’t think it will be resolved today.
My 2¢: There is a small book, not much more than a hundred pages, by Atkins called the Four Laws. The book is cheap if bought used on Amazon; well worth the time and money. It explains thermodynamics from the Zeroth Law through the 3rd Law very well. It’s not a ponderous tome, or a textbook.
I have my own views on whether heat flows only one way, or whether it is a statistical situation where the net transfer is what matters. But I’ve learned that those who agree with me don’t need convincing, and those who think differently will not change their minds. So I’ll lurk on this thread.☺
matayaya: Matthew R Marler, “equilibrium” to me can refers to the top-of-atmosphere measurement of energy coming in and energy going out.
The calculations based on equilibrium assume that the the Earth surface has a uniform temperature, uniform illumination, and uniform texture. The TOA energy fluxes averaged over the year and each radial angle balance closely, but at any given time the energy flux into a region may exceed the energy flux out, which is how your neighborhood may warm in the day time and cool at night, and have greater mean temp in summer than in winter.
Sorry, Ed Hoskins,
You have managed to take a simple mathmatical concept, the logarithmic response, then convert it to an obfuscatory jumble of numbers.
The pictorial use of linear portions of CO2 concentration, like 400-500, 500-600 ppm etc., is particularly confusing.
Some fundamental concepts are not even addressed. For example, what are the measured CO2 concentrations at altitudes where the alleged GHG heating is supposed to do its work? Is top of atmosphere more germane than top of Mauna Loa or just above the surface of land or sea?
There is little place for non-physical % units such as ‘remaining CO2 effect on temperature’.
Finally, do try to avoid emotional words like ‘greeted with unmitigated joy.’
Kristian says: August 11, 2014 at 2:48 pm
“What the ‘back radiation heating’ story and these diagrams are all about, after all, is to let people think that the ‘back radiation’ down from the cooler atmosphere to the warmer surface is actually an extra, positive energy INPUT, able to raise the internal energy of the surface and thus its temperature by 33 degrees beyond a pure solar radiative equilibrium.”
I agree with a lot of what you’ve said about nett energy fluxes etc. But we part ways there.
If you had a conductive heat path, you can only measure nett flow, unless you get down to a really microscopic scale. And you characterize the path by its thermal conductivity.
With radiative transfer, it is possible to measure flux both ways, even though there is an obligate linkage. So, with S-B in mind, people do. And the measure that replaces conductivity is the back radiation. That is how the impeding effect of CO2 is expressed. Quantitatively, it works just fine. But you could convert it to an effective conductivity if you want.
This is just radiative physics. Climate scientists didn’t invent it.
dbstealey says, August 11, 2014 at 3:23 pm:
“Kristian,
That same debate has been going on here for years. I don’t think it will be resolved today.
(…) I’ve learned that those who agree with me don’t need convincing, and those who think differently will not change their minds.”
Yeah, strange how that works … I guess I’m a fool for being an eternal optimist.
rgbatduke says:
August 11, 2014 at 1:17 pm
—————————————-
Your comment in part –
“Because there are processes that “short circuit” the radiative loss channel — notably convection and convection carrying latent heat — even this distributed, nonlinear shift in total resistance in the radiative channels is diminished in its impact by the shunt resistance”
– seems to echo the wise words of Sir George Simpson from his 1938 response to Callendar –
“..but he would like to mention a few points which Mr. Callendar might wish to reconsider. In the first place he thought it was not sufficiently realised by non-meteorologists who came for the first time to help the Society in its study, that it was impossible to solve the problem of the temperature distribution in the atmosphere by working out the radiation. The atmosphere was not in a state of radiative equilibrium, and it also received heat by transfer from one part to another. In the second place, one had to remember that the temperature distribution in the atmosphere was determined almost entirely by the movement of the air up and down. This forced the atmosphere into a temperature distribution which was quite out of balance with the radiation. One could not, therefore, calculate the effect of changing any one factor in the atmosphere..”
You further state –
“In the end, it all comes down to the GCMs. Either this problem is solvable with our currently accessible computational resources, or else it is not.”
To which I would respond with a qualified “yes”, if the problem is stated as “will adding radiative gases to our atmosphere cause measurable warming or cooling?” Currently GCMs focus their CFD capability on the horizontal and parametrise too much in the vertical (computational resources are too limited for high resolution in both for long runs). If this ratio were reversed with the horizontal parametrised and CFD dedicated to the vertical, better results could be achieved.
As to whether these results would be considered “better” by those currently relying on GCMs to push their case is another question entirely 😉
george e. smith says:
August 11, 2014 at 2:54 pm
“A DEFINITION tells you what SOMETHING IS ; it does not tell you what something is not.”
==================================================================
Well let’s look at the word “define.” ‘fin’ means ‘end’ or ‘limit’ so that the word means to delimit or draw boundaries, that is indeed, to determine what it is not, or how far it goes, much like determining a flight envelope. So yes, a large part of defining a word is in telling what it does not mean, as you have done above. When asserting that the sun does not heat anything you are in fact defining the word heat as something that has nothing to do with the sun. In so doing you divorce the verb ‘heat’ from the adjective ‘hot,’ and this is something that a first grader, or 12th grade debate student or freshman logic student might have a hard time swallowing.
It is of course nonsense, reflecting uncharacteristically on your usually insightful observations. Sorry. –AGF
dbstealey: Thanks for recommending the Atkins book, “Four Laws That Drive the Universe” (Oxford University Press, 2007). You beat me to it. It is very good for a variety of levels of technical background.
There is a quote in it on the definition of heat that impressed me enough that I typed it out a while ago, so I will show it here:
“In everyday language, heat is both a noun and a verb. Heat flows; we heat. In thermodynamics heat is not an entity or even a form of energy: heat is a mode of transfer of energy. It is not a form of energy, or a fluid of some kind, or anything of any kind. Heat is the transfer of energy by virtue of a temperature difference. Heat is the name of a process, not the name of an entity.
Everyday discourse would be stultified if we were to insist on the precise use of the word heat, for it is enormously convenient to speak of heat flowing from here to there, and to speak of heating an object. The first of these everyday usages was motivated by the view that heat is an actual fluid that flows between objects at different temperatures, and this powerful imagery is embedded indelibly in our language. Indeed, there are many aspects of the migration of energy down temperature gradients that are fruitfully treated mathematically by regarding heat as the flow of a massless (“imponderable”) fluid. But that is essentially a coincidence, it is not an indicator that heat is actually a fluid any more than the spread of consumer choice in a population, which can also be treated by similar equations, is a tangible fluid.
What we should say, but it is usually too tedious actually to say it repeatedly, is that energy is transferred as heat (that is, as the result of a temperature difference). To heat, the verb, should for precision be replaced by circumlocutions such as ‘we contrive a temperature difference such that energy flows through a diathermic wall in a desired direction’. Life, though, is too short, and it is expedient, except when we want to be really precise, to adopt the casual easiness of everyday language, and we shall cross our fingers and do so, but do bear in mind how that shorthand should be interpreted.”
Kristian says
Kristian this is the point that Harry Hoffman makes in his comparison of the earths atmosphere and Venus’s atmosphere. His claim is esstially that if you compare earths atmoshepher and Venus atmosphere in areas of the same density and factor out the difference in the amount of energy received from the sun, you should get the same temperature in the atmosphere. The point being that the only factor that is relivent for temperature is the mass/density of the atmosphere and the amount of energy available coming in. The specific composition of the atmosphere in his argument is irrelevant.
I have not had time to investigate his results but they appear to be consistent with your argument and thus would be a real world example of what you are saying.
Curt 7:22pm: Thanks for that. I note the word “heat” does not appear in the top post. Heat is still in search of a definition.
The contortions of language gone thru to put corporeal (tangible) existence to nonexistant in nature “heat” has hit a new high; dictionary.com being the previous record holder in my experience. We now are told:
heat is…
1) not an entity (not n., something that has real existence)
2) not a form (not a n., configuration) of energy (yet heat is usually & mysteriously always configured with units of joules)
3) a transfer of energy (something not a form energy not an entity was over there and now the not a form of energy not an entity is over here – ghost like travel)
4) a name (I should have picked that moniker)
5) not an imponderable fluid anymore (RIP)
6) although not an entity or form of energy “heat” can be conducted anyway: ‘a temperature difference such that energy flows through a diathermic (adj., capable of conducting heat) wall in a desired direction’.
This on top of dictionary.com where heat is in thermodynamic context (thereby excludes: the Police & pistol)…
7) a state
8) a condition
9) a quality
10) a degree (n., step)
11) a sensation
12) a nonmechanical (adj., non-machine) not a form of energy but energy transfer nevertheless
13) added energy causing a temperature rise. (…except at the surface of earth per Konrad, AlecM & Kristian et. al.)
To that add the independent defn.s Kristian has posted. Now when someone writes the term “heat” which defn. do YOU pick? This is sort of like dividing by 0, once an eqn. does that, further answers can be anything a writer wants.
Folks. Listen & learn. Make progress. Stop using the term “heat” if you want to be clear; otherwise if you don’t care about being obtuse (adj., not sharp), fine – use the term “heat”. Confusion WILL always commence around the “heat” term; if misused hoffer might have some sort of conniption fit & Trick will be even more irreverant….
For those of you might think heat is really nonmechanical, put thimbleful of water in a test tube with thermometer immersed, cork it, put on insulated gloves, shake for all you are worth – until you sweat gobs, that thermometer will increase in reading.
Trick: I agree that we should banish the word “heat” from these discussions for the sake of clarity.
Kristian,
~~~~~~~
“Heat is defined as the energy transferred spontaneously from hot to cold as a result of the temperature difference. When it comes to radiative heat transfer, the heat is the ‘net energy’ always moving only from the hotter to the colder object. It is a unidirectional flow of energy. Just like a waterfall, like wind or an electric current. Always from high to low potential. Spontaneously.”
~~~~~~~
That’s essentially what my reference text says, but without your pedantic spontaneity or “net energy” disclaimer:
============
heat (q) “A means by which energy is transferred from a hot body to a colder body when the two are place in thermal contact with one another.”
– Principle of Modern Chemistry, 4th Edition, glossary
“The amount of energy transferred between two objects inititally at different temperatures is called heat or thermal energy. When a hot body is brought into contact with a colder body, the two temperatures change until they become equal. This process is sometimes described as the “flow” of heat from the hotter to the colder body. Although this picture is useful, it is somewhat misleading because it implies that heat is a substance that is contained in matter. Instead, heat (like work) is a way in which energy is exchanged between a system and its surroundings.”
– Principle of Modern Chemistry, 4th Edition, p.209
=========
Kudos for your tenacity.
When a hot body is brought into contact with a colder body
>>>>>>>>>>>>>>>
That would be conduction, not radiated energy.
“”””….agfosterjr says:
August 11, 2014 at 6:10 pm
george e. smith says:
August 11, 2014 at 2:54 pm
“A DEFINITION tells you what SOMETHING IS ; it does not tell you what something is not.”
==================================================================
Well let’s look at the word “define.” ‘fin’ means ‘end’ or ‘limit’ so that the word means to delimit or draw boundaries, that is indeed, to determine what it is not, or how far it goes, much like determining a flight envelope. So yes, a large part of defining a word is in telling what it does not mean, as you have done above. When asserting that the sun does not heat anything you are in fact defining the word heat ……”””””
Well now you done it. Nowhere in my post did I ever say the sun does not heat anything.
I said we get no heat from the sun; we make it all here on earth; largely from solar radiant energy.
So I’m going to start a very long analysis of the OED; unexpurgated version. And I will keep count of what fraction of all the words in the OED, actually tell what that word does not mean. Then you can go argue with them.
Squirm all you like; you are toast. Virtually all other energies, than heat energy, can eventually be turned 100 % into HEAT.
HEAT on the other hand, can NOT ever be turned 100% into any other form of energy. That is the distinction. And as I also said, HEAT is the only form of energy that can change a temperature; either up or down. And that is inherent in the thermodynamic temperature scale.
Electricity does not have a temperature; gravity does not have a temperature, the potential energy of humpty dumpty sitting on a wall, is unrelated to temperature.
Only heat, has any relationship with temperature.
And stop putting words in my mouth; I specifically said the sun might heat (verb) the earth, but it does so without sending us any heat.
An asteroid crashing into earth does not bring us any heat. certainly it brings kinetic energy, but that isn’t heat, because all of the molecules of the asteroid, are acting in concert; all moving in the same direction like the hammer that mashed your thumb.
But when it gets here, the KE of the asteroid will be turned into heat, and other things; but that all happens right here.
And in the field of science, many words, that have every day common language meanings, may also have specific scientific meanings, and we should always use the correct words, in science, if we expect to be understood.
I suppose you also teach your first grade kids, that Ohm’s Law says E = RI. It does not, in case you were wondering; it says R = constant.
There is a very simple way to prove radiative energy transport from surface to equal temperature (T) atmosphere is one way, at a rate equal to the difference between the two opposing emittances at the plane. Assume emissivities are εs and εa.
Scenario 1 (the IPCC story): atmosphere emittance to the surface 333 W/m^2 = σ.εa.T^4 AND this is the radiative energy transfer rate; surface emittance to the atmosphere 396 W/m^2 = σ.εs.T^4. AND this is the radiative energy transfer rate. Standard physics** shows radiation entropy production rate = 4u/rT where u is the radiation flux. Therefore the total radiation production rate for this scenario s1 = 4(σ.εa.T^4 + σ.εs.T^4)/3T. Simplifying; s1= 4.σ.T^3(εa + εs)/3.
Scenario 2 (standard physics): surface emits to the atmosphere at a rate = Δ[emittance at the plane] = (σ.εs.T^4 – σ.εa.T^4) = σ.T^4(εs – εa). Radiation production rate s2 = 4 σ.T^3(εs – εa)/3.
Thermodynamics predicts that at equilibrium, a thermodynamic system always acts to minimise rate of production of entropy. s2 is much less than s1; Scenario 2 must be correct. QED
**see Eq. 6 in: http://www.bnl.gov/envsci/pubs/pdf/2010/BNL-81482-2008-JA.pdf
Curt says:
August 11, 2014 at 9:48 pm:
“Trick: I agree that we should banish the word “heat” from these discussions for the sake of clarity.”
Of course you do. To perpetuate the confusion in people’s minds on this topic. So that you can continue in peace to conflate heat and radiation, duping people into thinking (without having to come out and say it) that it is in fact possible for ‘back radiation’ by itself to actually create a detectable increase in surface internal energy (meaning, raise its temperature and/or provoke evaporation), even though it’s not ‘heat’ at all (as opposed to the solar flux) but merely one part of a continuous, integrated radiative thermal exchange where the net energy spontaneously moves up and away from the surface because of the temperature difference – heat.
Knowing and utilizing the concept of heat and heat transfer brings clarity, Curt. It makes one see through the confusion engendered by the notion that energy flying around in all directions will heat everywhere it arrives, no matter what. ‘It’s energy, so it heats (or does work).’ Like Hoffer believes.
You apparently didn’t read that last paragraph you quoted. I’ll highlight it for you:
“What we should say, but it is usually too tedious actually to say it repeatedly, is that energy is transferred as heat (that is, as the result of a temperature difference). To heat, the verb, should for precision be replaced by circumlocutions such as ‘we contrive a temperature difference such that energy flows through a diathermic wall in a desired direction’. Life, though, is too short, and it is expedient, except when we want to be really precise, to adopt the casual easiness of everyday language, and we shall cross our fingers and do so, but do bear in mind how that shorthand should be interpreted.”
“… energy is transferred as heat (that is, as the result of a temperature difference).”
Exactly. So when the surface is warmer than the atmosphere above, energy is transferred as heat from the higher temperature surface to the lower temperature atmosphere. This happens spontaneously in nature. It doesn’t matter if we have radiation in both directions. The actual ‘radiative transfer of energy’ goes from the surface to the atmosphere only. And so the surface ‘heats’ the atmosphere. It cools to the atmosphere. Not the other way around.
Yes, I use the noun ‘flow’ and the verb ‘to heat’ so as not to “stultify” my language and my explanation of what this transfer of energy is and does. It doesn’t make the distinct and quite palpable physical phenomenon that is ‘heat’ any less real.
davidmhoffer says, August 11, 2014 at 10:10 pm:
“When a hot body is brought into contact with a colder body
>>>>>>>>>>>>>>>
That would be conduction, not radiated energy.”
Sigh. You don’t give up, do you?
Two bodies being in ‘thermal contact’ simply means they are able to exchange energy through the process of heat. This will also occur across a vacuum.
Heat is heat whether the energy is transferred by radiation or conduction, David.
PS What we are discussing here is whether the modern equivalent of the Phlogiston Theory, the IPCC claim that radiative emittance at a plane is a real energy flux, is true. If so, by some miracle, the Earth’s surface adds the atmosphere to surface emittance to the real net radiative flux = SW energy thermalised – (conducted + evapo=transpired heat) = 160 W/m^2 – 97 W/m^2 = 63 W/m^2.
Therefore the IPCC claims real surface to atmosphere flux = 333 W/m^2 + 63 W/m^2 = 396 W/m^2., its emttance. However, I show above this is thermodynamically precluded because radiation entropy production rate = ~(1 + .84)/(1 – 0.84) = 11.5 x higher than reality! (assumes surface emissivity is unity; 0.84 atmospheric emissivity is an experimental average.)
The only way this would be possible would be if the atmosphere does emit to the surface according to Scenario 1 above AND the surface had the magic property of absorptivity = 0, emissivity = 1, hence the imaginary atmosphere to surface radiative flux ‘bounces off’.
Such a material is the province of Science Fiction: let’s call it Saganite in honour of H G Wells’ invention in his 1901 ‘The First Men on the Moon’, of Cavorite, a substance which turns gravity flux back on itself! Sorry IPCCers, what you are attempting to purport is pure science fiction, worthy of the Greats. It has nothing to do with Real Science, net surface IR flux = 63 W/m^2 and contains no CO2 15 micron band IR or that of the main H2O bands. This is an absolute fact.
george e. smith says, August 11, 2014 at 11:22 pm:
“I said we get no heat from the sun; we make it all here on earth; largely from solar radiant energy.
(…) I specifically said the sun might heat (verb) the earth, but it does so without sending us any heat.”
Exactly the opposite. It does so precisely by ‘sending us heat’. Because the Sun is (much) hotter than the Earth, it transfers energy to us by radiation AS HEAT.
Heat is a thermodynamic ‘process function’, not a ‘state function’, meaning heat is not ‘something’ that is contained within objects (systems). It only moves between them.
http://hyperphysics.phy-astr.gsu.edu/hbase/thermo/heat.html
Trick said “There is no energy generated in the atm. either as the atm. uses up no fuel.”
Yes it does – in my _unqualified_ humble opinion, it oxidizes methane.
Methane is produced by bateria over the entire damp surface of the land, and all the pooh thereon, and exposures of coal and shale. (I don’t know if the oceans produce methane.)
I estimate (and someone more knowlegeable should check) that if every sq metre of the surface produced 0.004 moles per day (4ml or 4cc or 64mg per day) and that was oxidized, it would be equivalent to 1 W/m2.
Kristian:
I have been observing this debate with astonishment. It seems you have invented your own physics and are insisting that everybody should adopt your invention.
Energy exists in many forms. These forms include heat, EM radiation and mass.
And energy can be moved from one place to another. For example, the energy which is the mass of an apple moves towards the center of the Earth when the apple falls from its tree, and the energy which is a photon of EM radiation may move towards the center of the Earth when the photon leaves the Sun.
And energy can be transformed from one form to another. For example, mass can be transformed to heat and that is why A-bombs work. Similarly, an EM photon absorbed by the Earth is transformed to heat and that is how the Sun heats the Earth.
But mass and EM radiation are not heat. Mass, EM radiation and heat are each forms of energy.
Think of energy as being like modelling clay. A piece of modelling clay can have the form of a car, a mouse, or a tree but it is the same piece of clay whether it has the form of a mouse or a house. A unit of energy can have the form of matter, or of EM radiation, or of heat, or etc. but it is the same unit of energy.
A unit of energy can travel from Sun to Earth as EM radiation, be absorbed by the Earth’s surface and thus be transformed into heat. But it was not heat when it was EM radiation. Similarly, a unit of energy is not heat when it is mass.
However, at August 12, 2014 at 12:02 am you insist
No, Kristian, the Sun loses energy e.g. as EM radiation which may impinge on the Earth where it may be reflected (e.g. by ice or clouds) or be absorbed to become heat.
Richard
richardscourtney says, August 12, 2014 at 12:44 am:
“Kristian:
I have been observing this debate with astonishment. It seems you have invented your own physics and are insisting that everybody should adopt your invention.”
This is just getting silly. I post links, other people quote from textbooks, showing how HEAT in physics is specifically defined as “a transfer of energy by virtue of a temperature difference”. This is NOT a controversial issue in physics. Then people like you come along with your ancient caloric view of the world, where heat is something that is created and resides ONLY inside bodies. As if you hadn’t opened a book on thermodynamics in a century or two. Heat is not a ‘state function’. It’s a ‘process function’. Go read a book on the subject.
Sorry, I appreciate most of what you write on this blog, Richard. But this is just too silly.
Apologies: in the radiation entropy post above, I made a typo.
Radiation entropy production rate = 4.u/3.T where u is the radiative flux.
Kristian:
OK I tried to help but at August 12, 2014 at 1:23 am you say you refuse to listen because I am talking about an “ancient caloric view of the world”.
Please note that I was discussing photons which – by definition – are quantum events.
It seems to me that the difficulty is your assertion saying
and you think from that definition says all energy can ONLY be transferred by temperature difference.
So, in hope of progress, perhaps you would explain your view of how according to you a photon IS “heat”, and how mass IS “heat” because – as I said – each can move from one position to another but their altered position is not a transfer of heat to their new position.
If what you mean were explained then perhaps it would be possible to understand what you are trying to say because – at present – it makes no sense of any kind.
Richard
@Kristian: there is clearly a big problem in physics’ teaching if here are people out there who claim that EM energy transfer is a form of heat transfer. The difference between the two is profound.
It’s because the entropy change when the total internal energy of a body increased by dQ is dQ/T. This comes from Clausius. However, radiation has the property of exerting pressure. Hence its contribution to entropy for a given energy transfer dQ is 4dQ/3T. Therefore, you cannot consider transfer of energy by EM radiation as a form of heat exchange. Anyone who does so is teaching junk physics.
As a discussion point, the radiation entropy production rate via OLR is about twice that for the thermalisation of the same SW energy. If the radiation entropy were not a function of transfer temperature, because the same amount of energy is transferred, there would be the same entropy production rate and the heat engine that comprises the Earth’s atmosphere would be 100% thermodynamically efficient, an impossibility because of the 2nd and 3rd Laws of thermodynamics……
This is exactly what I’m talking about.
When people live by the notion that ‘heat’ is something that is created inside a body whenever it absorbs ‘energy’ of some kind, any kind, then there is no longer any reason to doubt that ‘back radiation’ (being energy in the form of … radiation) will also create ‘heat’ inside the surface system.
This fundamental misunderstanding of basic thermodynamic principles lies at the core of the whole ‘heating by back radiation’ travesty.
Kristian, just a layman trying to follow this discussion and learn, but you said one thing I think I understand enough to comment on. You said that the flaw in back radiation thinking is their saying that “back radiation’ (being energy in the form of … radiation) will also create ‘heat’ inside the surface system”. That is not how I understand AGW theory. No one is saying heat is being created inside the system but that heat is being prevented from leaving the system.
Kristian:
At August 12, 2014 at 1:54 am I asked you
And at August 12, 2014 at 2:42 am you have replied saying in full
OK. I understand your reply to say that your understanding of “heat” has nothing to do with concepts from physics but is an excuse you have constructed to deny “heating by back radiation”.
I am content to accept the understandings of physics derived from empirical observation and to ignore your political constructs.
Richard
@Kristian: ‘back radiation’ is a measure of the apparent temperature of the atmosphere. The pyrgeometer instrument internally converts the temperature measurement to the flux the atmosphere would emit to a sink at absolute zero.
Physicists call this the emittance or exitance. It is not real. Think of it as a potential energy flux adding as a vector to all the other potential energy fluxes at the point or plane where you want to establish the real energy flux.
At the Earth’s surface, net surface IR flux = [surface emittance – atmospheric emittance].
You get this from Maxwell’s Equations via Poynting Vectors. An emittance is simply the assembly over all wavelengths of the individual Poynting Vectors comprising the radiation field of the Planck dissipative oscillators that can perform the quantised exchange of heat energy to EM energy and vice versa. This is the Law of Conservation of energy: qdot = – ∇.Fv where qdot is the monochromatic rate of heat generation per unit volume of matter and Fv is the monochromatic radiation flux density.
In short ‘back radiation’ is not energy or heat flux, simply a measure of the maximum energy flux the Planck oscillators could supply to a sink at absolute zero for their temperature.
AlecM says, August 12, 2014 at 3:20 am:
“@Kristian: ‘back radiation’ is a measure of the apparent temperature of the atmosphere. The pyrgeometer instrument internally converts the temperature measurement to the flux the atmosphere would emit to a sink at absolute zero.
Physicists call this the emittance or exitance. It is not real. Think of it as a potential energy flux adding as a vector to all the other potential energy fluxes at the point or plane where you want to establish the real energy flux.
At the Earth’s surface, net surface IR flux = [surface emittance – atmospheric emittance].”
Indeed.
Compare the pure Stefan-Boltzmann equation (1) with the general radiative heat transfer equation (2):
(1) P/A = εσT4
(2) P/A = εσ(Th^4 – Tc^4)
The pure version portrays an ideal situation where the radiating object ejects its energy into a perfect (0 K) heat sink. There is a maximum/ideal/largest possible temperature difference between object and surroundings. Hence, there is only the temperature of the object radiating to consider.
The composite version (the general radiative heat transfer equation) reflects a situation where there is no longer just an empty void surrounding the radiating object*, but rather surroundings/other objects with an ability to absorb and store energy, and therefore possessing a temperature. In other words, it’s no longer enough to simply consider the temperature of the radiating object itself. One also needs to take into account the temperature of its surroundings. The temperature difference is no longer the largest possible and so the radiative heat escaping the radiating object (P/A, equal to the more familiar Q) is less than the maximum/ideal value.
*Or, more relevant on Earth, surroundings/objects much, much colder than the radiating object.
http://hyperphysics.phy-astr.gsu.edu/hbase/thermo/cootime.html
Note, in both (1) and (2) above, the left-hand side of the equation is the solution, the value we’re looking for, of the actual physical phenomenon being studied. The right-hand side merely shows us how this value is mathematically derived, based on the temperature (and emissivity/absorptivity) of the objects involved in the thermal exchange (heat transfer). The only radiation ever detected within a thermal exchange is always the ‘heat’ (P/A). It’s the only actual ‘transfer of energy’ (from hot to cold).
In (1), the single mathematical term on the right-hand side simply happens to equal the heat on the left-hand side. In (2) there are two opposing terms on the right-hand side and the heat is therefore only the net (the sum) of the two. In this case, each single term on the right-hand side only signifies a potential transfer of energy. They would only be real (detectable, that is, thermodynamically working) transfers of energy if they were facing a perfect (0 K) heat sink like in (1), that is, if they were completely thermally isolated from one another.
This is an extremely important point, because the entire ‘Climate Establishment’ base their rGHE/AGW argumentation on the idea that these two opposing terms (in (2)) in fact do represent physically real fluxes of energy, each operating separately and distinctly from the other, as if alone, inside one single, integrated radiation field.
It’s an appallingly naive, simplistic and, quite frankly, absurd view on how things work in the real world. But it has still effectively managed to infect the minds of practically every person alive today. The hypothetical construct claiming the reality of an ‘atmospheric radiative greenhouse effect’ (the rGHE) warming the surface of the Earth is simply taken at face value. It is taken for granted as ‘fact’. By all. It is never questioned in the least, there is no critical thinking whatsoever directed at its fundamental premises and tenets.
And in the end, it bases itself wholly on a profound misrepresentation of reality.
The idea is that the atmosphere needs the so-called ‘GHGs’ to radiate a (real, working) flux back down to the surface for it to become warmer. Without these radiatively active gases in the atmosphere, there would allegedly be no such flux and the surface could not become as warm as it is. It’s ridiculous. Like you say, it’s the TEMPERATURE of the atmosphere that determines the rate of energy loss from the surface, not the radiative properties of the so-called ‘GHGs’. The fact that the massive atmosphere is able to warm while the ‘non-massive’ vacuum of space isn’t, makes all the difference. You don’t need gases to absorb IR for this. You don’t need them for the atmosphere to warm All you need is a solar-heated surface and a convective/evaporative response. You do however need them for the atmosphere to cool to space.
The warming (insulating) effect of an atmosphere is very real indeed. The global surface of the Moon absorbs on average 295 W/m^2 from the Sun, nearly 80% more than the global surface of the Earth (165 W/m^2 on average). Still the latter one has a mean temp 90K higher than the former!
It simply doesn’t have anything to do with ‘trapping’ outgoing radiation. It has all to do with the mass (the ‘heat capacity’ and the weight) of the atmosphere.
Nick Stokes says:
August 11, 2014 at 12:02 pm
“Samuel C Cogar says: August 11, 2014 at 9:36 am
“Explaining the arithmetic …. is easy.”
But no-one can do it? ”
……………………………………….
“CO2 at 400pmmv is already committed and immutable. So CO2 has already reached about ~87+% of its potential warming effect in the atmosphere.”
——————
Nick, I can explain the arithmetic of ……. 2 + 3 = 5 ….. and it is easy to do.
But explaining the reasoning via which said logarithmically derived ~87+% was obtained …. is not easy to do because it is simply illogical.
Now there is a lot of mathematical calculations being touted by various posters … with the/their math being correct, ….. but many of the numerical figures being cited are simply “imaginary”. Thus, said math results of “87+%” is meaningless.
Nick, just as soon as everyone starts using their calculated percentages, associations, correlations, proxy data, etc. …. as reference information only, …. instead of factual entities, ….. then climate science will take a great leap forward.
Matthew R Marler says:
August 11, 2014 at 1:24 pm
“CO2 “traps” heat in its orbital electrons: physically, the orbital sizes are such that the specific wavelengths of LWIR cause the electromagnetic oscillations to interact with the electrons and transfer energy from radiant to orbital energy. The reverse process occurs when electrons “decay” from higher energy orbits to lower energy orbits and release the energy as electromagnetic oscillations.”
—————–
But, but, but, ….. iffen that heat is “trapped” in the orbiting electron ….. then how is it possible for the electron to “decay” to a lower energy orbit?
Curious minds would like to know.
@Kristian: the real explanation of the heating AND cooling effect of the poorly mixed GHG in the atmosphere, water vapour, has ben completely missed by Climate Alchemy.
richardscourtney says, August 12, 2014 at 2:58 am:
“OK. I understand your reply to say that your understanding of “heat” has nothing to do with concepts from physics but is an excuse you have constructed to deny “heating by back radiation”.
I am content to accept the understandings of physics derived from empirical observation and to ignore your political constructs.”
OK. Whatever you say, Richard. “Political constructs.” Good one. Yeah, that’s what I’m doing here. I’m promoting an evil ideological agenda by pushing ordinary thermodynamics on the masses.
AlecM 3:20am: “…‘back radiation’ is not energy or heat flux, simply a measure of the maximum energy….”
Huh? No. Commonly back radiation is energy radiated by the mass of the atm. with a vector direction incident on Earth surface which is then reflected, absorbed, or transmitted by the L&O surface, not “thermalised”. Now you are so confused as to have written back radiation is both “not energy” and “energy”.
“This is the Law of Conservation of energy: qdot = – (div).Fv”
In part only, you leave out many terms in the generalized law in non-atm. text books & applying the generalized law to the atm. need set qdot = 0 as no energy is generated within the atm. since the atm. uses up no fuel (i.e. “qdot is the monochromatic rate of heat generation per unit volume of matter” = 0 in 1st law applied to any atmosphere.)
AlecM 2:03am: “Therefore, you cannot consider transfer of energy by EM radiation as a form of heat exchange. Anyone who does so is teaching junk physics.”
Transfer of energy by EM radiation is energy exchange. Anyone teaching it is a form of heat exchange is teaching confusing physics as heat is nonexistant in nature therefore cannot be exchanged.
The top post is correct; there is diminishing effect on Earth surface Tmean = 288K of increasing CO2 concentrations since the upcoming surface emitted photons are in essentially the same supply due balance with sun exists and eventually fewer single CO2 can find unmarried surface born photons for nuptials.
Trick, so what if those “unmarried” CO2s migrate higher in to the atmosphere where they have space to get married?
richardscourtney 12:44am: “Energy exists in many forms. These forms include heat..”
No.
That was a concept served its purpose (like slide rules) and science has now moved on. If you encounter a paper for example on say “Ocean Heat Content” simply chuckle, replace “heat” term with energy and proceed understand the paper. There is zero “heat” in the ocean but plenty of energy.
Sleepalot 12:39am: Tries to explain atm. generates energy using an ~adiabatic process as an example.
An adiabatic process is one in which Q=0; often said (although not by me) to be a process in which “no heat is absorbed”. Consider the combustion of hydrogen mixed in oxygen in an insulated, sealed container. A chemical reaction occurs, and the temperature of the reaction products is higher than that of the initial gas mixture.
Yet this process, as close to adiabatic as the cylinder is to completely insulated by anyone’s reckoning, is often described as one in which “heat is absorbed” (from where is anyone’s guess) or “heat is generated”. To where? Confused? You have every right to be. Contradictions such as this one, which are rife in thermo. and on blogs are an impediment to understanding & correctly discussing nature.
I am not in the grip of an irrational hatred just irreverence in trying to show typical confusion sown by playing fast and loose with the term “heat”. As was Dr. C. Bohren in his 1998 text book on Atmosphere Thermodynamics.
Khwarizmi 10:04pm: Adds heat is:
14) a means
15) an amount
16) a flow
17) (implies) a substance
18) a way
Confused? You have every right to be. The immense unrelenting contortions in the struggle to put corporeal (tangible) existence to nonexistent in nature “heat” continues. Folks – stop it. Always to be precise & clear use “energy” term instead. It is always possible to do so. E=mc^2. Ocean Energy Content.
@Trick: heat does exist in nature. It is a form of energy stored in the vibrational or translational motion of atoms, ions or molecules, or due to phase change. Its useful characteristic is that it can do mechanical work.
You wrote: “No. Commonly back radiation is energy radiated by the mass of the atm. with a vector direction incident on Earth surface which is then reflected, absorbed, or transmitted by the L&O surface, not “thermalised”. Now you are so confused as to have written back radiation is both “not energy” and “energy”.
“This is the Law of Conservation of energy: qdot = – (div).Fv””
Sorry, you have got it very wrong. ‘Back radiation’ is the emittance of the atmosphere, the energy flux detected by a cooled sensor (bolometer, IR spectrometer) or the pyrgeometer which uses a kludged ambient sensor. It cannot transfer any energy to a body at the same or higher temperature.
As for the Law of Conservation of Energy applied to matter and the EM continuum, look it up in Goody and Yung, ‘Atmospheric Physics’. It’s absolutely basic in that it shows the exact equivalence of the rate at which energy is transferred to or from matter by radiation, and the rate of change of radiative energy flux in that volume.
@matayaya: at equilibrium, no thermalised SW energy is stored in the atmosphere; it all goes out as OLR. There is energy stored as a transient in the seas as OHC and ice as latent heat. The idea that GHGs trap heat is ludicrous because virtually zero net IR is emitted by the surface in the main GHG bands, and what is emitted in the non self-absorbed H2O bands goes mostly to Space because the absorptivity and emissivity are low in those bands.
AlecM, I think it is well understood that CO2 does not “absorb” IR. In my layman understanding, it’s more like CO2s are the bumpers in a pin ball machine, IR being the ball. It’s hard to ignore the Vostok ice core showing a half million years of temperature and CO2 running in relative tandem. It’s a stretch to say it is coincidence.
“It’s hard to ignore the Vostok ice core showing a half million years of temperature and CO2 running in relative tandem. It’s a stretch to say it is coincidence.”
Higher temperatures bring more CO2, no coincidence… you would expect CO2 levels to have a clear relationship with temperature. It’s a stretch to say the CO2 is causing the temperature changes, IMHO.
Kristian;
When people live by the notion that ‘heat’ is something that is created inside a body whenever it absorbs ‘energy’ of some kind, any kind, then there is no longer any reason to doubt that ‘back radiation’ (being energy in the form of … radiation) will also create ‘heat’ inside the surface system.
>>>>>>>>>>>>>>>>>>.
You’ve been given several examples of exactly that. Electric current creates temperature rise (heat in your terminology) inside a resistor. So, with a single example, I have shown you that
people who live by the notion that ‘heat’ is something that is created inside a body whenever it absorbs ‘energy’ of some kind, any kind, then there is no longer any reason to doubt that ‘back radiation’ (being energy in the form of … radiation) will also create ‘heat’ inside the surface system are perfectly correct to do so .
Several other examples have been given upthread, all of which also falsify your assertion. You are contradicted by facts that a 10 year old can understand. Your argument rests on nothing more than your ability to define your own terminology and your own physics that exist only in your pretend world. Simple observation falsifies you. But you won’t quit because you know that you can fool some of the people some of the time, and so you continue throwing around physics that is 90% correct and 10% completely made up. To what end, I do not know.
@davidmhoffer: to use your analogy with a resistor, ‘back radiation’ is a measure of potential, equivalent to the potential at one end of the resistor. It does not generate electric current. To do that you need a potential difference.
In the case of the Earth’s surface, the resistance is the process by which its heat is converted to electromagnetic energy. The rate of conversion of heat to EM energy is set by the difference of opposing radiative emittances (potential difference) at the surface plane.
Just remember this: ‘back radiation’ is like measuring a voltage. By itself it can do no thermodynamic work.
Matayaya says
The energy is already in the system the amount of energy leaving the system is the same how does having it bounce around in the system make any difference? The energy contained in the system is dependent on the mass of the system and the input of energy to the system.
Bob Boder,
Not sure I follow that. You say “The energy is already in the system the amount of energy leaving the system is the same.” Your phrasing. I thought the premise of more energy coming in than going out was not in dispute.
Ah, so much to reply to, so little time. Let’s start with:
The wavelengths in question are many orders of magnitude larger than the physical dimension of the molecule. The molecules can absorb photons of the appropriate wavelength because the electron clouds have quantum levels which, when mixed, have an electrical dipole (or sometimes other multipole) moment. The dipole in question has a “natural” (quantum) frequency associated with the energy difference between the two levels, such that . If the molecule (or atom) is driven at resonance by a light wave at this frequency, it causes transitions from the lower level to the upper one and vice versa. If there are many molecules in a small volume (less than a wavelength) and the light is sufficiently intense, the response to this driving light is likely to be semi-classical — it will simply oscillate into and out of the excited state at resonance, occasionally fluorescing via spontaneous emission to scatter some of the light sideways. If the molecules are widely separated and/or the light intensity is low, the interaction will involve single (hence distinguishable) photons — the molecules will simply absorb a single photon and remain in the excited state until it either collides with something, absorbs an additional photons to get kicked into a third level, or spontaneously emits the radiation to fall back into the ground state.
. If the molecule (or atom) is driven at resonance by a light wave at this frequency, it causes transitions from the lower level to the upper one and vice versa. If there are many molecules in a small volume (less than a wavelength) and the light is sufficiently intense, the response to this driving light is likely to be semi-classical — it will simply oscillate into and out of the excited state at resonance, occasionally fluorescing via spontaneous emission to scatter some of the light sideways. If the molecules are widely separated and/or the light intensity is low, the interaction will involve single (hence distinguishable) photons — the molecules will simply absorb a single photon and remain in the excited state until it either collides with something, absorbs an additional photons to get kicked into a third level, or spontaneously emits the radiation to fall back into the ground state.
In the specific case of CO_2, the spacing between the molecules is large enough and the field weak enough that the photon picture is more appropriate. The resonant photons absorbed carry both energy and momentum, so when the molecule catches the photon it recoils in the direction the photon was travelling, with some of the energy of the photon transformed into kinetic energy. The lifetime of the excited state(s) are usually much longer than the mean time between collisions of the molecule and the surrounding gas (O_2 and N_2 molecules). These collisions irreversibly transfer enough of the kinetic energy of recoil to randomize the quantum state of the CO_2 molecule two ways — by kicking it into a superposition of many states (and losing still more of the original photon energy) and in the process by “broadening” the quantum transitions involved between the participating states (very crude description, phase interruption leading to a spread Fourier transform). These collisions, which occur even when the molecule is in the ground state, make it somewhat easier to accomplish the original resonant capture by allowing the molecule to have a decent probability of absorbing photons in a band of nearby energy/wavelengths, not a single infinitely sharp wavelength. The spontaneous emission process does the same thing, giving each of the lines a “natural width” that can be broadened further by interactions and collisions. Finally, the molecules are all moving relative to the frame of reference where the photons were emitted, and this introduces a small random doppler shift of the photons relative to the molecule, further broadening the lines relative to the light.
Spontaneous emission and collisional/pressure broadening are usually referred to as “homogeneous broadening” and this is the dominant process at higher pressures near the ground. High up in the atmosphere, collisions become comparatively rare and doppler “inhomogeneous” broadening can be dominant. Even this creates a problem for model builders, because the broadening of the molecular lines causes nearby lines to overlap, creating bands of frequencies wherein absorption is very likely within some distance/time known (on an exponential average basis I won’t explain) as the “optical depth” of the gas. The gas is effectively opaque over a few optical depths — nearly 100% of any emissions in the CO_2 absorption bands will be absorbed as the intensity of the light is exponentially attenuated, transferring its energy to the surrounding gas (warming the O_2 and N_2, which otherwise would be transparent to this energy and hence unwarmed by it). The CO_2 molecules themselves remain in quasi (or rather, dynamical and local) thermal equilibrium with the surrounding (open system) gas, and radiate energy away via thermal radiation — but not a pure blackbody spectrum, rather one convoluted with the strongly coupled bands.
At ground level, the air remains “close” to ground temperatures both because of conduction/convection and radiation all three. It radiates energy back towards the ground in the GHG-linked LWIR bands at a “blackbody” temperature characteristic of the ground. You can take a look at figures from Petty reproduced here:
http://wattsupwiththat.com/2011/03/10/visualizing-the-greenhouse-effect-emission-spectra/
to get a feel for the spectrographs at different locations and conditions. Note that on the ground looking up (e.g. figure 8.1 comparing Nauru and Barrow) the CO_2 band and Ozone band are particularly clearly displayed, and the contrast between the two figures illustrates nicely the role that water vapor (a factor at Nauru, not so much at Barrow) plays. The measured back radiation spectra has resonant peaks/bands at the characteristic temperature of the ground, plus a much smaller general background at the temperature of the height (in the case of water vapor at Nauru) where the radiation is emitted. The radiation observed at the top of the atmosphere looking down has a complementary structure. There, radiation in the absorptive bands is observed at the temperature of the top of the troposphere where the atmosphere finally becomes transparent to in-band LWIR, where elsewhere the radiation is “direct” from the ground at ground temperatures.
These figures are as direct evidence as one could possibly ask for of the atmospheric radiative effect, badly misnamed the greenhouse effect. They clearly show (what one can verify at will with simple handheld IR detectors) that there is a substantial radiative energy flux, even at night, downward from the LWIR GHG-coupled bands, and during the day this flux is added to direct solar flux in the visible. Whether one wishes to count this energy as “heat”, or “radiative energy” or anything else one might want to call it, it is part of the energy budget of the dry-land surface and has to be accounted for in the energy flow equations that ultimately determine the dynamic equilibrium temperature of the surface and surface layer of the atmosphere. But ultimately it is the lapse rate as well as this process at all heights from the ground to where the thinning atmosphere becomes transparent in the bands that determines the effective reduction in outgoing flux in the resonant bands.
The lapse rate itself is a complicated thing established by a mix of vertical “slow” convection in both directions and radiative transfer. Because the Earth is unevenly heated and cooled, the atmosphere is highly unstable, nearly always moving from one place to another and one height to another. In the rare cases where it becomes stagnant, or when a warm front overlays cooler air underneath, an inversion can occur where the lapse rate is partially negative over the troposphere. Finally, wet air and dry air behave very differently and establish different lapse rates, and wet air can transfer far more energy via direct transport of latent heat. Convection, especially when laden with moisture, represent an additional “channel” for heat delivered to the surface to leave the surface (cooling it) and be carried aloft, where it can more effectively and rapidly be radiated a way. For all practical purposes, though, only incoming solar radiation warms the Earth (with tiny contributions from other sources of free energy) and only outgoing thermal radiation cools it.
Note well that nobody should care in the slightest what one calls this process, but there is absolutely overwhelming evidence that the process exists almost exactly as I’ve described it above, and because we can see how the process varies with the concentration of at least water vapor (as a GHG) at various heights, humidities, and from surfaces at different temperatures, we can affirm that the atmospheric radiative effect results in a surface that is warmer than it would be, all things being equal, if the atmosphere were composed only of N_2 and O_2 with no H_2O or CO_2 or CH_4 or O_3 or… Furthermore, there is really little doubt that the linearized response in temperature to increased concentrations of these gases in the atmosphere is at least initially positive — more water vapor in a water vapor free atmosphere is going to (comparatively, remember) warm the ground, more CO_2 in a CO_2 free atmosphere is going to warm the ground.
It is less clear what will happen when the gases are saturated to where the atmosphere is optically thick in their absorption bands, in part because some of the bands overlap — note well the overlap between CO_2 and H_2O at Nauru or the TOA looking down tropical Pacific — and in part because nonlinear phenomena and latent heat modify this process substantially by directly modifying the albedo at different heights looking down. That is, in addition to water vapor carrying heat up through much of the troposphere, once there it tends to condense into clouds that have a very high albedo. Albedo reduces ground insolation before absorption and any need to participate in the greenhouse cycle, and does so with an extremely negative derivative, with a much stronger cooling effect than the combined greenhouse effect during the day and as a potential active and passive transport “short circuit” in the greenhouse process at night.
Once again, the atmosphere proves to be highly unstable over oceans, where a truly complex process of absorption, re-radiation, evaporation, and condensation all occur, and all occur nonlinearly and differently as things like surface wind speed, time of day, overhead humidity, overhead cloud cover, height of overhead cloud cover, temperature profile of the atmosphere, temperature profile of the ocean all vary, with clouds forming and disappearing, spreading and raining out, with water vapor losing the latent heat it carries at much warmer temperatures than the “bare” DALR plus GHE computation would have it. Here it is by no means clear that the partial derivative of average temperature in response to changes in saturated CO_2 is strongly positive, or even positive at all. Overall, we know that the atmosphere has powerful negative feedbacks because it does not exhibit runaway warming from water vapor — to put it bluntly, the slope of the partial derivative of the greenhouse effect from water vapor alone manifestly has a zero point, preventing exponential or sub-exponential growth of temperatures. If it were not so, given 70% of the Earth’s surface made up of oceans, we would be Venus.
That, in turn, raises the interesting question concerning the second order terms that are generally neglected in linearized descriptions of the GHE. Changing CO_2 from (say) 300 ppm to 400 ppm in an otherwise perfectly dry atmosphere might well produce some warming. And yes, warming given an ocean might well be expected to produce an increase in humidity. But these assertions are orthogonal, or inconsistent. If you already have humidity that is in balance, such that increases in humidity cause net cooling (necessary to prevent runaway warming, as the ocean is always happy to make things more humid as it warms) and add CO_2 to the atmosphere, it could well be much like adding a strong acid to a buffer (metaphorically speaking, of course) — instead of dramatically decreasing the pH, it finds it cheaper to rearrange the much less expensive equilibrium of the buffer. To re-express this in context, one does get some warming from the CO_2, but only a fraction of what you would expect because the system finds it nonlinearly simpler to shift the H_2O equilibrium, which is already at the zero point and becomes negative with more H_2O to cancel most of the warming until a new mutual equilibrium is established.
This sort of mechanism has been suggested in the literature (Milosovic?) and is actually rather plausible, made likely to be the dominant response to more CO_2 by the simple observation of pre-existing water-vapor equilibrium, which by definition means that the partial derivative of global temperature with respect to water vapor variations must be (on average) zero, and further more must change sign as water vapor varies around the zero to push it back towards equilibrium and not away! However…
This is still only a tiny part of the story. We haven’t discussed the dual role of aerosols — to directly reflect incoming energy and to nucleate clouds, further and nonlinearly increasing the albedo and indeed enabling nonlinear feedback conditions to cause net cloud coverage growth (monkeying with the aforementioned water vapor equilibrium and keeping it from ever being simple), soot and particulates, lateral transport of heat, the effect of the Earth’s tilt, the fact that the tilted Earth is in an eccentric orbit that causes TOA insolation itself to vary by over 90 W/m^2 from perigee to apogee, in a way that is projected onto the slowly precessing axial tilt so that different continents and oceans receive maximum insolation at different times of the year, nonlinearly affecting net heat uptake on a grand scale because of the different mean albedo and albedo feedbacks at the different latitudes and seasons. Oh, and the sun itself varies by a possible significant hair over the solar cycle, which is then superimposed onto this and the possibly coupled decadal oscillations in general patterns of atmospheric circulation, which are (again necessarily) net warming and net cooling changes in order for the Earth to remain in approximate dynamic equilibrium…
Which it doesn’t! And that’s the damnedest thing, isn’t it. In even the very limited high frequency thermometric record, the Earth’s temperature and other “climate” measures vary substantially on a decadal or longer scale, with numerous visible global temperature shifts on the order of 0.2 to 0.5 C over times as little as 10 to 20 years, both with and (key!) without the help of rapidly increasing CO_2. Furthermore, the temperature has proven to be quite capable of not rising along with CO_2 on similar timescales, again suggesting that the global climate system has substantial multivariate buffering capacity that in all probability substantially reduces the expected CO_2-only Beer-Lambert warming. But no simple CO_2-is-the-only-knob system can explain the geological climate record — indeed, models that attempt to do so majorly fail to describe only the Holocene, let alone the Wisconsin, Younger Dryas, or general Pleistocene. The models of CMIP5 fail to describe HADCRUT4 outside of the reference interval singly or collectively over only the last 150 years, as illustrated by figure 9.8a of AR5.
And honestly, that’s no surprise. Nick has stated that he is “pretty sure” that the feedback from water vapor is positive and will roughly double CO_2 only warming. I’m at least equally certain that it is negative. I think my argument is better than his, because he has to explain how CO_2-driven warming will increase the water vapor warming by increasing the water vapor concentration while increases in water vapor at the current dynamical equilibrium temperature decrease the temperature — on average — or else the climate would have a runaway warming catastrophe from positive feedback in the water vapor greenhouse channel. His argument presumes a knowledge and ability to compute all of the nonlinear phenomena associated with the water cycle in the climate in models that are too coarse grained to even represent much of that dynamics. Indeed, it is generally acknowledged in many published papers that we don’t really know how to compute or account for the water cycle — what is used in the models is basically a coarse grained approximate guess, a guess that then has to be empirically balanced against other gain and loss terms as a guess in the reference period and that might not work outside of it.
My argument does not. It relies only on the observation that the climate empirically is locally stable against water vapor fluctuations because if it weren’t we’d be Venus — a positive fluctuation would grow instead of shrink if the average feedback past the set point were not negative, including all of the computable and non-computable effects of everything else.
rgb
Samuel C. Cogar: But, but, but, ….. iffen that heat is “trapped” in the orbiting electron ….. then how is it possible for the electron to “decay” to a lower energy orbit?
Good question. First, note that Richard S. Courtney corrected my misrepresentation: the absorbed electromagnetic radiation is converted to (rotational and vibrational) kinetic energy. The word “trapped” need not mean “permanently trapped”: when I trap squirrels, for example, a few are able to escape from the traps. As far as I am aware, how the energy in the molecule is released to EM radiation is not known; the energy may be transferred to other molecules via collision (especially at lower altitude/ higher pressure where the density is higher and collisions happen faster than the orbital decay; the rate of decay (half-life, as with radioactive decay) of each species at each energy level can be measured; but the exact mechanistic description has a hole in it (at least as far as my reading has taken me.)
So perhaps you could say “trapped and released” as in wildlife studies. All the macro world analogies fail at some point when describing the molecular level processes.
Kristian:
At August 12, 2014 at 6:59 am you deny my reply to you at August 12, 2014 at 2:58 am that said
Your denial of that says in total
Unfortunately your denial omits to mention any inaccuracy and/or error in what I wrote. Perhaps you can address this omission?
Richard
PS I have just seen the post by rgbatduke at August 12, 2014 at 9:21 am and I commend that you read it and learn from it.
@rgb: I have explained above why there is near zero net IR energy emission from the Earth’s surface to the atmosphere in all self-absorbed GHG bands. Because of this, your detailed exposition of the GHE falls at the first hurdle.
How do I know? I am one of the relatively few who has measured coupled convective and radiative loss of heat from solids, specifically sheets of metal in process plants. This is standard process engineering with data from the best part of a century in engineering handbooks. You need to get to ~100 deg C before the radiative flux exceeds natural convection for 0.9 emissivity.
To claim that the Earth’s surface emits net IR energy to the atmosphere at the black body rate creates ~40% more heating than reality in the models. It would be much higher were it not for the ludicrous claim that OLR comes from a single -18 deg C emission zone at 5 to 6 km. I have discussed with modellers their claim that you get the same flux as OLR but negative in the two-stream approximation. Plain stupid: the sum is 238.5 + 333 – 238.5 = 333. Yup, you’re left with the imaginary ‘back radiation’ flux as the heating!
Climate Alchemy, because Carl Sagan made two crucial mistakes, has blundered into a scientific rabbit hole of immense depth with the daft idea that radiative emittance is a real energy flux when it is a potential flux to a sink at absolute zero.
Near zero heating of the atmosphere by surface IR means no ‘back radiation’, no ‘positive feedback’. The World is starting to cool. The Great CAGW scare is on its last legs. The practitioners are dashing for cover so they won’t be the ones to be blamed for this catastrophic failure of science.
rgb at duke: The wavelengths in question are many orders of magnitude larger than the physical dimension of the molecule. The molecules can absorb photons of the appropriate wavelength because the electron clouds have quantum levels which, when mixed, have an electrical dipole (or sometimes other multipole) moment. The dipole in question has a “natural” (quantum) frequency associated with the energy difference between the two levels, such that \Delta E = \hbar \omega. If the molecule (or atom) is driven at resonance by a light wave at this frequency, it causes transitions from the lower level to the upper one and vice versa.
How does this affect the basic narration/causal analysis? It is still true that the CO2 molecules are “tuned” (so to speak) to the specific narrow band of IR wavelenghts/frequencies, right? And that the energy of the EM radiation is absorbed by the CO2 molecules, right? And that the the increased energy in the molecules can be transferred to other molecules via collisions, right? And that the energy may instead be radiated by the molecule later, right? And that from a volume containing CO2 molecules, the radiation will be omnidirectional, right? And that there are lacunae in the description/explanation of how the absorption and emission occur, such as exactly when you need to recall the wave nature of light, and when you have to recall the particle nature of light, right?
Recall that some commentators have asserted that the radiation can not be converted to the kinetic energy of molecules, it’s a “signal” not an energy transfer. There is no reason to take that assertion seriously, right?
I am not disputing your description; I think it is more detailed than what I wrote in response to the correction from Richard S Courtney.
I think you say so with this: The gas is effectively opaque over a few optical depths — nearly 100% of any emissions in the CO_2 absorption bands will be absorbed as the intensity of the light is exponentially attenuated, transferring its energy to the surrounding gas (warming the O_2 and N_2, which otherwise would be transparent to this energy and hence unwarmed by it). The CO_2 molecules themselves remain in quasi (or rather, dynamical and local) thermal equilibrium with the surrounding (open system) gas, and radiate energy away via thermal radiation — but not a pure blackbody spectrum, rather one convoluted with the strongly coupled bands.
but I think that you mean “convolved with” for “convoluted with”.
the whole post is worthwhile, I think. consider this: And honestly, that’s no surprise. Nick has stated that he is “pretty sure” that the feedback from water vapor is positive and will roughly double CO_2 only warming. I’m at least equally certain that it is negative. I think my argument is better than his, because he has to explain how CO_2-driven warming will increase the water vapor warming by increasing the water vapor concentration while increases in water vapor at the current dynamical equilibrium temperature decrease the temperature — on average — or else the climate would have a runaway warming catastrophe from positive feedback in the water vapor greenhouse channel.
In the dynamic system operating in real time, that is with daily warming and cooling, the warming happens too fast for water vapor equilibrium, which produces clouds and rainfall; then the temp falls below the hypothetical equilibrium temperature because some energy was expended in vaporizing the water instead of warming the surface. The equilibrium model is tractable but inaccurate; the dynamical model is intractable, but a semi-quantitative analysis suggests that the equilibriium model overestimates the surface warming because it underestimates the non-radiative transfer of the latent heat from the surface to the upper troposphere.
Does it seem from our writings that you and I are in disagreement? I don’t think so.
AlecM 9:02am: ”You have got it very wrong. Back radiation’ is the emittance of the atmosphere…”
Concur. The top post is still correct. Your atm. emittance (the birth of a photon) when incident on the L&O surface is either absorbed (death of photon), reflected, or transmitted (both are life of photons) by the terrestrial surface. No photon is “thermalised”.
“…energy flux detected by a cooled sensor…”
Agreed. Various sensors can detect the amount of commonly termed back radiation from atm. photonic energy absorbed, reflected and transmitted. Cooling a pyrgeometer will increase its sensitivity at a great expense for greater accuracy. The accuracy expense which is generally unneeded by NOAA ESRL surfrad. experiments for atm. physics study is avoided. If want to go to great detail for an application, yes, make the expenditure, get a cooled pyrgeometer as onboard say Kepler for space IR studies.
Goody & Yung is a great ref. been there read or skimmed it, the authors show how the optical depth of the atm. can modulate surface Tmean with back radiation energy in formal math. Shows the 1st law simplified formula for surface balance: net solar energy into minus net terrestrial energy out of surface volume = 0 = qdot for approx. long term balance. Goody & Yung show solar energy into surface volume is net of albedo & outgoing terrestrial radiative energy is net of back radiation terrestrial energy.
Here is a non-atm. on line ref. for generalized radiative transfer that defines the general 1st law eqn. 10.70, the simplified 1st law as you post at 3:20am eqn. 10.75 and defines your qdot as “the heat (sic, meaning energy, chuckle) generated in the medium” p. 297 which is = 0 for an atmosphere.
http://books.google.de/books?id=J2KZq0e4lCIC&pg=PA298#v=onepage&q&f=false
So I have got it right at least so far as the text goes. If you can show precisely where the text has “got it very wrong” please advise further.
The top post is still correct.
“Trick: heat does exist in nature. It is a form of energy stored in the vibrational or translational motion of atoms, ions or molecules,”
That form of energy would be kinetic energy not heat.
“…or due to phase change…”
That would be latent enthalpy.
@ rgbatduke says:
August 12, 2014 at 9:21 am
“Ah, so much to reply to, so little time. ..”
Whoa – most excellent post.
Took me some time to go through it, and I’ll admit there is a lot I don’t understand, but overall from what I did understand, I feel good knowing that while there may be a lot we don’t know regarding “climate science”, what we do know in the “Physics” world often is either overlooked or deliberately ignored when applied to the Earth’s climate.
Probably overlooked by some skeptics and probably deliberately ignored by Alarmist/Warmists, IMHO.
Your post is worth a “cut & paste” for reference.
Thank you, Dr. Brown.
Samual C Cogar: But explaining the reasoning via which said logarithmically derived ~87+% was obtained …. is not easy to do because it is simply illogical.
Now there is a lot of mathematical calculations being touted by various posters … with the/their math being correct, ….. but many of the numerical figures being cited are simply “imaginary”. Thus, said math results of “87+%” is meaningless.
The author repeats the standard that CO2 has to date raised the mean temperature of the Earth (or the “equilibrium temperature”) by 33 C. The next 3 doublings may raise the temperature of the Earth by 4.5 C, (insert your favorite here), to 37.5 C. If that is the maximum achievable temp raise (because, perhaps, of the finite supply of fossil fuel), then 33/37.5 = 88% of the possible temp increase has already occurred.
This is no mystery, but the author ought to clarify exactly how the obtained the 87% figure.
Matthew R Marler says:
August 12, 2014 at 10:36 am
The author repeats the standard that CO2 has to date raised the mean temperature of the Earth (or the “equilibrium temperature”) by 33 C.
Wait:
Isn’t the first degree of temp from atmospheric CO2 at 20ppm?
If so, 0-20 = 1 degree
Doubling:
20-40 = 2 degrees
40-80 = 3 degrees
80 – 160 = 4 degrees
160 – 320 = 5 degrees
320 – 640 (where we are currently at about 400ppm) would only be 6 degrees.
Maybe that 33 C is inclusive of all the Green House gasses?
Kristian: The atmosphere has a mass. That means it has the ability to warm. And it will and does warm. Simply from being conductively > convectively coupled with the solar-heated surface. The atmosphere is able to warm. Space isn’t. Therefore the presence of the atmosphere will reduce the heat OUT from the surface and force it to equilibrate at a higher steady-state temperature. Simple as that.
You have come around to the standard equilibrium argument. Does the surface temperature equilibrate? Has it ever equilibrated? At the warmer equilibrium temperature, does the rate of water transfer (with its latent heat) from the surface to the upper troposphere increase, with the subsequent increase in rainfall? Or does the water vapor eqiilibrate (approximately as the Clausius-Clapayron relationship, perhaps?) without any increase in the rate of the hydrologic cycle? Does the water vapor change amplify or damp the temperature increase caused by the increased CO2?
@Trick: heat and enthalpy are different. The former is molecular vibration and translation. The latter, a thermodynamic potential, includes heat, latent heat energy and PV for the system.
Latent heat is the energy released or absorbed by a substance when it undergoes a phase transition at constant temperature.
AlecM;
AlecM says:
August 12, 2014 at 9:18 am
@davidmhoffer: to use your analogy with a resistor
>>>>>>>>>>>>>>>>
It wasn’t an analogy. It was an example of something that Kristian claims doesn’t exist.
@ Matthew R Marler: the water cycle substantially reduces diurnal and annual surface temperature variation. it also keeps warming from well mixed GHGs near zero.
There are hundreds of thousands; maybe even millions of Engineers on this planet; no they are not scientists, but they deal with real down to earth heating (verb) and cooling(verb) all the time.
If you put together your own PC computer; maybe with Intel inside (or AMD), one of the first things, you will have to do, is to slap some thermally conductive goo onto your CPU package, and then attach the multifinned “heatsink” (noun), to transport thermal energy (chaotic molecular kinetic energy) that is generated from electric currents flowing in dynamic resistances, in the CPU chip. That multifinned sink, will also have a fan to blow air across those fins, to remove the air that is heated (verb) by the fins. That thermal energy, may be conveyed to those fins by conduction through a copper slab, or an aluminium one (cheap), or it could be carried by convection, in the transport of a fluid through some tubes. That fluid, might also be a phase change medium, that has a high latent heat (noun) of freezing / melting, or even a vapor, having a low boiling point temperature, and high phase change latent heat (noun).
One thing that ALL engineers, specially electronics ones, know, is that these heat (noun) transports are very slow processes. “Heat” (noun) , simply oozes from one place to another.
By the time your shower water gets to a comfortable temperature, you already finished your shower.
There’s a very good reason, that heat (noun) travels slowly; the molecules can’t make up their mind, which direction to move in, so they pass the baton back and forth in every direction, in a chaotic process, that actually makes a circular Italian firing squad, look like a well organized operation. The KE that molecules exchange between colliding pairs; presumably elastic collisions, simply does not propagate in any organized fashion or direction.
It is this problem, that prevents heat (noun) from being 100% converted to mechanical work.
As the sorcerer’s apprentice furiously carries water buckets trying to empty the tank; the broom bric a brac, simply dumps more water from the well, negating the efforts of the apprentice.
And some posters here, would have you believe, that the sun is sending you heat (noun) in such a disorganized fashion.
At least the members of the Italian firing squad, are at least trying to all kill the same target, and not each other. The “customer” will no doubt be dead quickly; almost at the speed of light, one might say.
If you buy into this; EM radiation is heat, stupidity, don’t bother applying for a job as a heating (verb) / cooling (verb) engineer. I certainly wouldn’t hire you, as a Butler, to run a bath for me.
Alec M: @ Matthew R Marler: the water cycle substantially reduces diurnal and annual surface temperature variation. it also keeps warming from well mixed GHGs near zero.
That does not take us very far. What is “near” 0: a 4K rise above a base mean temp of 288K? I am not saying you are wrong, but I think that here we step into the realm of what I call “intractable”.
And as of yet, I have not encountered a single word (so far), in the Oxford English Dictionary, (unabridged), that is defined by stating what that word does not mean.
Maybe I’ll try Webster’s next.
After reading RGB I almost decided to retire, but with GES’ last I’ll have to put another dig in. I guess the sun is not capable of “heat transfer” even through conventional defined means.
To recap, Nick Stokes says the IR gun “measures the energy transmitted from your gun to the freezer.” G E Smith counters: “Radiation can go anywhere.it darn well pleases; because radiation is NOT ‘heat’.” This anti-definition of heat seems to be motivated by his interpretation of the second law: heat does not flow from cold to hot places (energy can). Smith goes as far as to claim that although the sun can heat the earth, no heat is transferred. (In fact “heat transfer” as typically defined includes radiation [over macro-distances], conduction [intermolecular radiation], and convection.)
We have three possibilities:
1) Energy is in fact transmitted from the freezer to the gun (Smith).
2) Energy is transmitted from the gun to the freezer (Stokes).
3) Energy is transmitted in both directions, with a net transfer toward the freezer (everybody?), the only question being what is the gun measuring exactly.
While the word ‘heat’ seems to be confusing everyone (especially Kristian), I think Smith is right, whether or not we call radiation heat: it can go anywhere it darn well pleases. Energy/heat/whatever can radiate from a cold to a hot place; it just radiates more from a hot to a cold place.
And of course Singer is justified when he deplores the atrocious physics of those skeptics who argue that CO2 cannot in principle warm the earth. –AGF
PS, feel free to weigh in, RGB et al.
@agfosterjr: “And of course Singer is justified when he deplores the atrocious physics of those skeptics who argue that CO2 cannot in principle warm the earth”
If there were no negative feedback process, the 3 W/m^2 reduction of OLR for doubled [CO2] would, by increasing ‘forcing’, reduce net surface IR by the same power. To maintain constant convection, evapo-transpiration and IR, the surface would rise in temperature by 1.2 K.
However, strong negative feedback by the water cycle must reduce this temperature rise to zero, on average. Any competent engineer sees how the control system works. Only the atmospheric scientists and poor physicists who believe in photons shooting out at the S-B rate would persist i the dumb IPCC argument….:o)
Kristian:
You are still missing the larger point. Whether you regard radiative heat transfer as resulting from the differential of two opposing radiative power fluxes, or as a single unidirectional thermal flux, you end up with the same result in energy balance calculations. It simply does not matter.
To put numbers on it (the precise values are not important), whether you regard the radiative transfer between the earth’s surface and atmosphere as the difference between 396 W/m2 radiative power flux upward from the surface and 333 W/m2 radiative power flux down from the atmosphere, or simply as a 63 W/m2 upward thermal flux, your energy balance works out the same.
If the atmosphere were completely transparent, you would get the same result whether you considered it 396 up and ~0 down radiative power fluxes or just a 396 up thermal flux. In either case, there would be a resulting heat transfer flux of 396 W/m2 upward, which is far more than the earth/atmosphere system receives from the sun, so present temperature levels could not be maintained.
This has been pointed out to you many times, and yet you continue to hijack thread after thread with what is (at best) semantic nitpicking.
@curt: you have got the wrong end of the stick.
Only the 63 W/m^2 is real. However, the real scam is to assume a single -18 deg C OLR emitter at 5-6 km. This does not exist and the assumption (Kirchhoff’s Law) that there is a DOWN negative flux in the two stream approximation increases energy input by 40% over reality.
There’s another sam in hind-casting. The enhanced GHE does not exist. Negative feedback by the water cycle reduces CO2-AGW to zero.
Matthew R Marler says: August 12, 2014 at 10:36 am
“This is no mystery, but the author ought to clarify exactly how the obtained the 87% figure.”
It’s not just the 87%. The first table has several %s. In the text we have 77%, 5.9%, 4.1%. Below the green block figure, there is a whole table of them. The blue column table has a % in each column. But no-one knows what they mean.
The mystery is that anyone takes the essay seriously.
AlecM 10:54am: “Trick: heat and enthalpy are different.”
Concur. And the top post is correct. To understand this difference in science, please go back to the combustion experiment I posted 8:30am: “Consider the combustion of hydrogen mixed in oxygen in an insulated, sealed container. A chemical reaction occurs, and the temperature of the reaction products is higher than that of the initial gas mixture.”
Consider two ways of describing that ~adiabatic combustion of hydrogen and oxygen: 1) the temperature and enthalpy is higher following combustion; 2) heat is generated.
Notice the difference between these two descriptions. The first is a concrete statement about a measurement that can be made directly with a thermometer. The second is abstract, invoking a paranormal hypothetical quantity that doesn’t exist in nature, forcing a struggle to be defined in at least 17 ways in this thread alone that can at best be inferred indirectly only from temperature measurements. In science, description 1) is best.
Saying that a temperature increase is a consequence of metaphysical “heat generation” is word jazz (prevalent on blogs), not a physical explanation. From 1LOT the total energy of my container is constant, and hence rearrangements of molecules in combustion result in a decrease of internal PE (remember this is energy associated with separations between atoms), which must be compensated for by an increase of internal KE, manifested macroscopically by a temperature increase. There is also a p*V increase so total enthalpy is affected and, really, enthalpy is the conserved quantity. There is no “heat” term in the equation for enthalpy.
”…heat is…molecular vibration and translation”
Heat from AlecM is now: this definition # 18), the struggle continues & grows. As I wrote above to you 10:24am, NO, “That form of energy would be kinetic energy not heat.” Get a quorum of molecules together and a thermometer will measure their temperature from “..molecular vibration and translation”. Temperature is not heat.
It is very easy to deal with the defn. of heat: Heat does not exist in nature. Curt is correct banish what does not exist from concrete science discussion.
*****
matayaya 12:13pm: Yes as the thick dance of couples (of all atm. species) at the surface goes ever higher, the couples & singles thin out and the newborn & older single photons have easier time to escape the dance unmarried.
@matayaya: what you must realise is that because CO2 and H2O atmospheric IR emission switches off surface IR emission in the same bands, there is zero surface IR energy to have to pass the slalom.
The real GHE would be from the OLR effect, but it is exactly offset by atmospheric processes involving the water cycle.
Once you see how this works, you are really attracted to the ideas of intelligent design!
AlecM 1:12pm: ”To maintain constant convection, evapo-transpiration and IR, the surface would rise in temperature by 1.2 K.”
No. Convection is not conserved. Neither is paranormal, hypothetical, non-existent heat. Total system energy (enthalpy) is conserved by 1LOT.
”Only the atmospheric scientists and poor physicists who believe in photons shooting out at the S-B rate..”
And Max Planck. The folks you mention apply Planck distribution successfully as he wrote specifically it is applicable to a surface with an atm. above. The atm. scientists and physicists only get into trouble with his nature when they consider radiating objects not having positive radii and diameters on the order of the wavelength of interest. As he wrote in his original paper, diffraction is ruled out & is negligible for earth system, not the moon system.
Trick: “Notice the difference between these two descriptions. The first is a concrete statement about a measurement that can be made directly with a thermometer. The second is abstract, invoking a paranormal hypothetical quantity that doesn’t exist in nature, forcing a struggle to be defined in at least 17 ways in this thread alone that can at best be inferred indirectly only from temperature measurements. In science, description 1) is best.”
Although I agree with you about temperature’s being “a measurement that can be made directly with a thermometer,” there is a subtle difference among temperature definitions that DeWitt Payne and Paul Birch seized upon a couple of years ago to derail my attempt to set Dr. Brown straight, as you and I have tried to a couple of times now, about what I’ve come to think of as the Brown-Eschenbach Law of Conservation of Lapse Rate (the “B-E Law”).
I am particularly mindful of that at the moment because, frustrated at the failure by scientists among this site’s readers to point out how bizarre the B-E Law is, I submitted a proposed post laying the statistical mechanics out myself a few weeks ago. Unfortunately, Mr. Watts spiked the piece, dismissing Velasco et al. as “junk.”
@Trick: heat is kinetic energy. Learn it.
The average translational energy of a freely moving particle at temperature T is 3kBT/2 where kB is the Boltzmann constant.
@Trick: I meant constant sum of heat loss from the surface by convection., evapo-transpiration and radiation. This is conservation of energy.
[I thought I was pedantic!]
Curt says: August 12, 2014 at 1:34 pm
“You are still missing the larger point. Whether you regard radiative heat transfer as resulting from the differential of two opposing radiative power fluxes, or as a single unidirectional thermal flux, you end up with the same result in energy balance calculations. It simply does not matter.”
I agree. I’ve supported Kristian’s insistence that the flows cannot be separated, so that the back flow can’t act as a power source. They have to be coupled for a proper entropy accounting. But as a practical matter computing separate fluxes has been done since Boltzmann, and works perfectly well for figuring heat transfer. It certainly isn’t peculiar to climate science.
When Kristian wants to define flux from h as
(2) P/A = εσ(Th^4 – Tc^4)
the immediate query is, what is c? eg for the atmosphere. And how do you define Tc? It just isn’t helpful.
AlecM 1:56pm: “@curt: you have got the wrong end of the stick. Only the 63 W/m^2 is real.”
The top post title is ok. For what you write to be natural, the atm. would not be radiating at all (zero.zero). However all matter at all temperatures radiates at all frequencies at all times. Just look at the Planck distribution to confirm this is true; the Planck distribution formula is never zero.zero at any temperature or any frequency interval for gas, liquid, solid, plasma.
“…a single -18 deg C OLR emitter at 5-6 km. This does not exist…”
This is not in dispute; the atm. radiates at all its temperatures at all heights; that a certain atm. height above ground happens to be -18C is perfectly natural. It is referred to in texts as effective not actual emission height.
“Negative feedback by the water cycle reduces CO2-AGW to zero.”
Prove it. You will make the evening news. This is the actual dispute.
******
Joe Born 2:23pm: As a vet of that discussion, Velasco ~1996 (one molecule) has been superseded by Verkley 2004 (total column of molecules) then Akmaev ~2006 (precision added to Verkley) papers which is the last I looked into that discussion, not sure if more work has been done. Try working with those updated papers for WUWT – google will work for you.
I can’t thank you enough for this……
“I think my argument is better than his, because he has to explain how CO_2-driven warming will increase the water vapor warming by increasing the water vapor concentration while increases in water vapor at the current dynamical equilibrium temperature decrease the temperature — on average — or else the climate would have a runaway warming catastrophe from positive feedback in the water vapor greenhouse channel. His argument presumes a knowledge and ability to compute all of the nonlinear phenomena associated with the water cycle in the climate in models that are too coarse grained to even represent much of that dynamics. Indeed, it is generally acknowledged in many published papers that we don’t really know how to compute or account for the water cycle — what is used in the models is basically a coarse grained approximate guess, a guess that then has to be empirically balanced against other gain and loss terms as a guess in the reference period and that might not work outside of it.
My argument does not. It relies only on the observation that the climate empirically is locally stable against water vapor fluctuations because if it weren’t we’d be Venus — a positive fluctuation would grow instead of shrink if the average feedback past the set point were not negative, including all of the computable and non-computable effects of everything else.
rgb “
Trick: “Velasco ~1996 (one molecule) has been superseded by Verkley 2004 (total column of molecules)”
Velasco et al. argues from statistical-mechanics first principles, and I’ve gone through the math. Verkley does not. I’ve seen nothing in the math (except one harmless typo in an integration limit in its predecessor paper) that gives me any reason to drop it in favor of a thermodynamics treatment. My experience is that thermodynamics makes smart people say dumb things.
Incidentally, Velasco et al. applies to any number of particles, so your reference to “one molecule” is unclear.
AlecM 2:25pm: “@Trick: heat is kinetic energy. Learn it.“
None of this subtracts from the top post. NO, heat is not kinetic energy measured by temperature which is not heat. KE exists in nature thru conserved 1LOT enthalpy, heat does not. If heat were KE, there would be plenty of heat in the oceans but on inspection find there is zero heat in the oceans & plenty of (KE + PE + p*V) = conserved enthalpy.
Rub your hands together vigorously. Feel the temperature increase. The mechanism by which the temperature of your hands increased is beyond our understanding based on the kinds of macroscopic measurements that are possible. Simply using word jazz and calling this mechanism generation of heat adds nothing to our understanding.
In fact, that subtracts from our understanding by deluding us into thinking that we have explained what we observed whereas all we have done is invoke a nonexistent paranormal nonentity “heat”. At the macroscopic level, all we can say is that one hand exerts a force on the other through a distance, resulting in working, which raises the internal energy of both of them, and this is manifested macroscopically by a temperature increase.
I surmise a retort saying generation of heat is just a short way of summarizing this process. An equally short scientific way is to say that temperature increases.
For a critical discussion of the confusing and contradictory uses of the word “heat”, see Zemansky 1970: The Physics Teacher, Vol. 8 pp. 295-300. If you take the time to read the seminal papers by our illustrious predecessors like Max Planck, J.C. Maxwell, you will be moved by the clarity and individuality of their writing, both of which seem to be banished from blog comments at times.
******
Joe 4:46pm – My point is discussing a more recent paper might get you in the door at WUWT to open that Pandora’s box again.
I won’t go further except to quote this from Velasco 1996: “The following microcanonical single-particle distributions for an f-dimensional ideal gas in a gravitational field have been derived…” m is the mass of one particle and then the paper discusses adding N particles to infinity using mostly word jazz. Verkley uses eqn.s and Akmaev completely eliminated the word jazz.
rgbatduke says:
August 12, 2014 at 9:21 am
Thanks for another very informative post.
Trick says:
August 12, 2014 at 5:21 pm
Are you saying there is not such thing as heat? I suggest you define it first, though you might find it difficult to define the nonexistent. And this:
“The mechanism by which the temperature of your hands increased is beyond our understanding based on the kinds of macroscopic measurements that are possible.”
What rubbish. Benjamin Thompson abandoned Caloric Theory on account of his observation of cannon boring friction–and he measured the “heat” produced. Where do you come up with all this crap? –AGF
Trick: “using mostly word jazz.”
Not quite sure what that means. I went by the equations, not the verbal characterizations.
Mathematically, Velasco et al. (in the Roman et al. paper they reference) compute as a function of a first particle’s altitude and momentum the size of the phase space permitted to the subsystem consisting of all the other particles. That gives the relative density of the probability that the first particle will have that momentum at that altitude. Since there is no reason to suppose that the probability-density function thus determined for one one particle is any different from any of the others, that function gives the molecular-population density as a function of altitude and momentum and thus the mean kinetic energy as a function of altitude.
No need to keep track of those treacherous tacit assumptions that plague thermodynamic treatments, which is what Verkley and Akmaev are. So I find Velasco et al. the most persuasive.
looncraz says:
August 12, 2014 at 7:12 pm
“It’s hard to ignore the Vostok ice core showing a half million years of temperature and CO2 running in relative tandem. It’s a stretch to say it is coincidence.”
====================================================
Where did that come from (date/time–Yahoo destroyed by Firefox search tool)?
You’re right of course–that’s why I ask: why do CO2 and CH4 track in the cores? I see no possibility other than that they are forced by a common agent: temperature, or rather, albedo/ice sheet extension. –AGF
Nick Stokes: I’ve supported Kristian’s insistence that the flows cannot be separated, so that the back flow can’t act as a power source. They have to be coupled for a proper entropy accounting. But as a practical matter computing separate fluxes has been done since Boltzmann, and works perfectly well for figuring heat transfer. It certainly isn’t peculiar to climate science.
I like that.
agfosterjr 6:45pm: “Are you saying there is not such thing as heat?”
Yes. Unequivocally. Very irreverently. And also I note the diminishing influence of increasing carbon dioxide on surface mean temperature; CO2 increasing has NO effect on heat as heat does not exist in nature. When you read someone writing “heat” just substitute “energy” to see if they are making sense.
“What rubbish”
True, the Count dissed heat existed as caloric also & measured the “mean temperature of the water” from the kinetic energy of the molecules on his 4 mercurial Fahrenheit thermometers; Ben did NOT measure heat. There is no such thing as heat. Very thankfully this was not important as The Count still was able to give us the drip coffee pot. His “cold rays” have been discontinued in science also along with heat.
To make sure no stray radiation came in, he closed the shutters (admits opening them only for a moment! to read the thermometers) & eliminated “currents of air”.
http://rstl.royalsocietypublishing.org/content/94/77.full.pdf+html
Interesting story though about the cannon boring. Clifford Truesdell tells us from where The Count’s interest stems. The Austrians boring cannons (in vats of water) for the French to use on the Germans created quite a public stir when it was first noticed that water could be boiled without fire during the turning process. The public came from far around to watch the marvelous vats boiling of water with no fire for energy input. Meant no more gathering wood for fire!!
It may have been the Count spoiled things by irreverently pointing to the horses out back turning the lathe. No free energy; the crowd disbursed back to gathering wood and posting blog comments.
Trick says:
August 12, 2014 at 7:34 pm
But how can you say heat does not exist if you don’t know what it is? Tell us what it is (or is not, if G Smith will forgive me), so that we can decide for ourselves. Something like ‘heat’ –a four letter word without meaning which people used to believe in before the earth froze. If there is not such thing as heat then there must be no such thing as warmth or warming either, so that you can disprove global warming simply by proving there is no such thing as heat. This too should make the evening news. –AGF
Go for it AG; I’m way too long in the tooth to worry about a little cow poop being slung around in my presence. But please try to use MY words, when you quote me.
Remember; other words, have other meaning.
Two notable groups of parasites, are famous for trying to put “other words” into people’s mouths.
NorthEastWestSouth “reporters” are the commons types, and lawyers are the royalty of the species.
But count me in your camp AG, as believing that “heat” (noun) really does exist; but damn little of it between here and the sun.
Now you can’t even count on solar charged particles or meteoric dust to bring us any heat; remember, for a charged particle to get here from the sun, it has to follow a rather restrictive path to get here. But “heat” will waffle around so much, we’ll all be dead, before it finds its way to the earth.
But the big bang radiation, still makes it here; thumbing its nose at the second law scofflaws; it must get lonely on the trip.
I do like your polarizing sun glasses by the way !
I’ve been had. I’m gonna hit the sack.
“””””…..“The mechanism by which the temperature of your hands increased is beyond our understanding based on the kinds of macroscopic measurements that are possible.”
What rubbish. Benjamin Thompson abandoned Caloric Theory on account of his observation of cannon boring friction–and he measured the “heat” produced. Where do you come up with all this crap? –AGF …..”””””
Sic him AG; personally my hands contain a whole lot of water; there’s not much to me except a lot of water, and a few spoons of cheap chemicals. That’s why some people say I’m all wet.
Well that water does like to warm me, when I’m out in the sun. Don’t get much caloric from basking in the CO2, sans the sun though. Is that bad grammar, lexicologically speaking, of course ?
AGF 8:03pm. “But how can you say heat does not exist if you don’t know what it is? Tell us what it is (or is not, if G Smith will forgive me), so that we can decide for ourselves.”
The best clear definition for heat is still: Heat does not exist. Why waste time and effort defining something that does not exist?
If two macro bodies are placed in thermal contact, one body with a higher temperature than the other at the interface, the temperature of the hotter body always decreases whereas that of the colder body always increases.
When you abandon all attempts to identify heat as an entity, you can think more clearly & eliminate much befuddlement about thermodynamic problems. “Heat” term is so much misused – and completely unnecessary term.
Others try to entice us into thinking heat exists by increasingly fantastic measures to try & make noncorporeal heat tangible, a summary of the above if you haven’t been up on the counting:
Curt posted Atkins says heat is…
1) not an entity (not n., something that has real existence)
2) not a form (not a n., configuration) of energy (yet heat is usually & mysteriously always configured with units of joules)
3) a transfer of energy (something not a form energy not an entity was over there and now the not a form of energy not an entity is over here – ghost like travel)
4) a name (I should have picked that moniker)
5) not an imponderable fluid anymore (RIP)
6) although not an entity or form of energy “heat” can be conducted anyway: ‘a temperature difference such that energy flows through a diathermic (adj., capable of conducting heat) wall in a desired direction’.
These using dictionary.com where heat is in thermodynamic context (thereby excludes: the Police & a pistol)…
7) a state
8) a condition
9) a quality
10) a degree (n., step)
11) a sensation
12) a nonmechanical (adj., non-machine) not a form of energy but energy transfer nevertheless
13) added energy causing a temperature rise. (…except at the surface of earth per Konrad, AlecM & Kristian et. al.)
Khwarizmi 10:04pm: Adds heat is:
14) a means
15) an amount
16) a flow
17) (implies) a substance
18) a way
AlecM writes heat is kinetic energy as measured by thermometers. But thermometers are well known not to measure heat.
I mean to increase clarity, decrease befuddlement: Heat does not exist.
Try it, you’ll like it.
@Trick: stop this silly posturing.
Thermometers measure temperature. The average translational kinetic energy of a freely moving particle in a system with temperature T is 3.kB.T/2, where kB is the Boltzmann constant. The Specific Heat Capacity (J/Mol) of an ideal monatomic gas at temperature T is 3.R/2 where R is the Ideal Gas Constant.
As temperature rises, the heat in an assembly of such molecules increases. This heat increase due to increased molecular motion causes pressure to rise at constant volume. This does work = ΔP.V, units Force x Distance = work.
Heat is work. Work is heat. Read this Wikipedia article about the mechanical equivalent of heat.
Curt says, August 12, 2014 at 1:34 pm:
“Whether you regard radiative heat transfer as resulting from the differential of two opposing radiative power fluxes, or as a single unidirectional thermal flux, you end up with the same result in energy balance calculations. It simply does not matter.
(…)
To put numbers on it (the precise values are not important), whether you regard the radiative transfer between the earth’s surface and atmosphere as the difference between 396 W/m2 radiative power flux upward from the surface and 333 W/m2 radiative power flux down from the atmosphere, or simply as a 63 W/m2 upward thermal flux, your energy balance works out the same.
If the atmosphere were completely transparent, you would get the same result whether you considered it 396 up and ~0 down radiative power fluxes or just a 396 up thermal flux. In either case, there would be a resulting heat transfer flux of 396 W/m2 upward, which is far more than the earth/atmosphere system receives from the sun, so present temperature levels could not be maintained.”
I’m amazed you don’t see it yourself, Curt. That – from what you propose here – the two approaches specifically do NOT give the same result. This is EXACTLY the confusion you promote! This is exacly where it leads to. The idea that the purely S-B calculated 396 W/m^2 UWLWIR is in fact a real flux of energy that the surface sends out, only to be countered by an equally real DWLWIR flux of energy coming down (derived only from subtracting the actually detected ‘radiative heat’ from the S-B calculated UWLWIR). And that this downward flux is ONLY there because there are radiatively active gases in the atmosphere. Without them, no DWLWIR and the UWLWIR would be alone inside the radiation field and thus cool the surface way too much.
This is such an absurd, upside-down approach to a real-world problem that I can’t but laugh! But this is where ‘Climate ScienceTM’ and its great green confusion brigade has taken us all.
The mean upward transfer of energy by radiation from the global surface would be 63 W/m^2 in both cases, Curt. And only that. As long as the average incoming from the Sun is still 161 W/m^2 and the mean conductive/evaporative energy loss rate is 97 (98) W/m^2, the surface can’t put out more energy than that by radiation. Otherwise you would have a situation where the atmosphere radiated back down a flux to the surface directly ‘heating’ it, more INPUT (derived from the original output), increasing its internal energy, making it warmer in absolute terms, forcing its OUTPUT to increase also.
There’s an atmosphere on top of the surface, Curt. Not a vacuum. This is not a purely radiative situation.
What happens in the real world is rather, as the atmosphere starts warming (and it would warm with or without the absorption of IR, Curt), less energy is going OUT, the OUTPUT from the surface is reduced, the INPUT isn’t increased. That would be energy transferred as HEAT from cool to warm.
Let’s look at it in the simplest possible way:
# First the Sun warms the surface – 200 W/m^2 of mean radiative INPUT.
# Then the surface emits that same energy to the atmosphere (its heat sink/cold reservoir) by virtue of its attained temperature, warming the atmosphere – 200 W/m^2 of mean radiative OUTPUT.
# The atmosphere then, after absorption, takes half of this same energy and emits it BACK DOWN to where it came from, the surface, its hot reservoir/heat source, making this energy warm the (still warmer) surface a second time, to an even higher temperature than during the first round – 100 W/m^2 of mean radiative INPUT.
In effect, the surface heats itself. This is like a heat engine discarding its residual heat – after doing work – to its cold reservoir, but having half of it come back in the process .. to heat it some more or to make it do more work. Directly reducing the entropy.
It’s completely mental. Totally warped. This cannot and does not happen. Nature can’t work like that. Our Universe would lose all its natural order if energy could just fly around and arbitrarily heat (make stuff warmer) in all directions, even from cold to hot.
Look, the effect is real. It violates no thermodynamic laws. Your description of the process, your explanation of how the effect comes to be, however, clearly does.
In your scenario, the solar input does NOT increase. And the initial surface output does NOT decrease. There is ONLY the increased input from the cold reservoir, the atmosphere, adding directly to the internal energy of the surface (its hot reservoir), making it warm in absolute terms, forcing its output to become larger, not smaller, in the process! The other two ‘fluxes’ don’t help. The extra heating of the surface arises solely from the extra (recycled) atmospheric input.
This is where you’ve taken physics. You say:
“To put numbers on it (the precise values are not important), whether you regard the radiative transfer between the earth’s surface and atmosphere as the difference between 396 W/m2 radiative power flux upward from the surface and 333 W/m2 radiative power flux down from the atmosphere, or simply as a 63 W/m2 upward thermal flux, your energy balance works out the same.”
Yup. Indeed. So why not just show the 63 W/m^2 upward flux!? The actual radiative transfer of energy detected between the surface and the atmosphere. If it’s all the same? Why do people make such a point out of putting up two fat potential transfer (radiant emittance) arrows between surface and atmosphere, with the one much bigger than the solar flux, pointing directly down to the surface, just next to a bunch of actual transfer (heat) arrows? If the end result will be the 63 W/m^2 upward transfer anyway?
If it weren’t specifically to visually confuse people into thinking that the fat ‘back radiation’ (DWLWIR) arrow – not a result of the atmospheric temperature apparently, but only of the atmospheric presence of your so-called ‘GHGs’ – works exactly like the neighboring and much slimmer solar flux arrow on the surface? Heating it.
I realise that I’ve got a pretty hard row to hoe on this one, just to disentangle your mess. I also realise that what I write here will arouse a cognitively dissonant response of fear and loathing in most people. So this is as far as I’ll go …
The cooler atmosphere warms the warmer surface by itself being warm, reducing the surface energy OUTPUT per unit of time (its cooling rate) as a consequence, Curt, not by increasing its energy INPUT (its heating).
http://okulaer.wordpress.com/2014/08/11/why-atmospheric-radiative-gh-warming-is-a-chimaera/
(Feel free to read, although I’m sure you won’t.)
Sorry, Specific Heat Capacity units are J/Mol.K
Kristian,
When you calculate your flux from the surface to the atmosphere:
(2) P/A = εσ(Th^4 – Tc^4)
what is Tc? How is it measured?
Trick:
You say you reject the concept of “heat”.
I am wondering if your difficulty in understanding why that rejection is wrong results from one of the misunderstandings you assert at August 12, 2014 at 8:41 pm, You write:
Absolutely not!
The temperature of the hotter body may reduce as it loses heat but the temperature of the colder body may not increase in temperature because the addition of that heat to the colder body may alter its state and not its temperature. One such example of a colder body would be a mixture of ice and water where the change of state is melting of ice with resulting alteration to the proportions of ice and water.
Richard
Kristian:
I note that you are still posting to this thread but have overlooked the request for clarification in my post addressed to you at August 12, 2014 at 10:13 am. This link jumps to my post that stated my understanding of your reply to me and provided this request:
Thanking you in anticipation of your correcting the omission
Richard
richardscourtney: “The temperature of the hotter body may reduce as it loses heat but the temperature of the colder body may not increase in temperature because the addition of that heat to the colder body may alter its state and not its temperature.”
This is beyond my pay grade, but I’m told that stars and black holes have negative heat capacities: as they gain energy they lose temperature. I can’t explain it, but that’s what the smart guys tell me.
richardscourtney 1:30am: “…mixture of ice and water…”
Absolutely so! In your example the well stirred bodies are at the same temperature which does not change while the ice is melting.
You are misled thinking the melting ice is being heated, hence we are supposed to believe the state of motion changes. But the temperature does not change, and hence we are supposed to believe its state of motion does NOT change.
Confused? You should be. Heat does not exist; reduce your befuddlement eliminating that nonexistent heat concept from your vocabulary, dictionary.com notwithstanding, rise above it. Science was right to remove heat from corporeal existence.
Kristian 1:05am: “There’s an atmosphere on top of the surface, Curt. Not a vacuum.”
According to Max Planck right in his original paper, vacuum or atm. over surface makes no difference to his distribution per his extensive testing.
@Trick: as i wrote above. heat can do work and work can be converted to heat. Its unit, the Joule, is approximately the amount of energy to raise the temperature of 1g of water in an open container by 0.24 K.
The mistake you are making is to confuse heat with energy which can exist in many other forms than kinetic motion.
For a closed system, one version of the first law of thermodynamics states that the change in internal energy ΔU of the system is equal to the amount of heat Q supplied to the system minus the amount of work W done by system on its surroundings.
Trick:
At August 12, 2014 at 8:41 pm, you wrote:
Note that you wrote “the temperature of the hotter body ALWAYS decreases whereas that of the colder body ALWAYS increases”.
Your assertions are plain wrong! They are untrue. They are false!
So, at August 13, 2014 at 1:30 am I wrote refuting your assertion saying
Have you thanked me for pointing out your error? No, you replied saying this
Absolutely so! In your example the well stirred bodies are at the same temperature which does not change while the ice is melting.
You are misled thinking the melting ice is being heated, hence we are supposed to believe the state of motion changes. But the temperature does not change, and hence we are supposed to believe its state of motion does NOT change.
NO! YOU claimed “the temperature of the hotter body always decreases whereas that of the colder body always increases”. I denied that nonsense.
Clearly, you are an idiot who does not understand what he writes and claims others have made his errors.
Richard
Trick:
The formatting went wrong at the end of my post August 13, 2014 at 5:15 am.
The corrected ending is as follows:
==================
{snip}
Have you thanked me for pointing out your error? No, you replied saying thisbold
NO! YOU claimed “the temperature of the hotter body always decreases whereas that of the colder body always increases”. I denied that nonsense.
Clearly, you are an idiot who does not understand what he writes and claims others have made his errors.
Richard
richardscourtney says: August 13, 2014 at 1:30 am
“One such example of a colder body would be a mixture of ice and water where the change of state is melting of ice with resulting alteration to the proportions of ice and water.”
I do not endorse Trick’s athermality. But this example doesn’t work. The only way ice can be melted is if heat is supplied to replace latent heat. The only way that heat can be supplied is by conduction or equivalent transfer along a temperature gradient. So the temperature does have to rise while the ice is melting. You can’t have melting in a body uniformly at 0°C. Heat has to be transferred.
Nick and richard – The well mixed ice and water are at the same temperature until all the lh in ice is used up and the ice is water.
Trick says: August 13, 2014 at 7:20 am
“Nick and richard – The well mixed ice and water are at the same temperature until all the lh in ice is used up and the ice is water.”
No. Melting ice consumes h**t. If isothermal, h@@t cannot move.
@ Trick August 13, 2014 at 7:20 am
Really?
What if the overall ambient temp is 0° C?
If the water and ice are pure, would the entire mixture stabilize as water that is almost ice or ice that is almost water?
Either way, both the ice and water will ultimately stabilize to 0° C.
It would seem that if that is precisely the freezing point, then all will become ice, but if it is just slightly below the freezing point then wouldn’t all become liquid?
Just trying to think logically.
Nick Stokes:
At August 13, 2014 at 1:30 am I wrote
At August 13, 2014 at 7:06 am you say of that
Say what?!
There is a temperature gradient from the hotter to the colder body (that is what hotter and colder mean). And there is a transfer of heat down that gradient (that is why the ice melts).
But there is no overall temperature rise of the cooler body BECAUSE – as you say – “The only way ice can be melted is if heat is supplied to replace latent heat”.
The hotter body loses heat and the colder body gains heat but – as I said – there is no rise in temperature except very locally and that very local heating induces convective mixing of the water.
Richard
RMB:
No, it is not surface tension so you have been told yet again and, therefore, you have no need to again repeat your assertion.
Richard
I’m not pushing a theory. I find that when I try to heat water through the surface the water completely rejects the heat. If I float a metal pan on the surface and apply the heat to the floating object the water accepts the heat as one would expect. Uncovered water will not accept heat covered water will. Try it for yourself. The floating object kills the surface tension.
AlecM says:
August 12, 2014 at 9:02 am
“Sorry, you have got it very wrong. ‘Back radiation’ is the emittance of the atmosphere, the energy flux detected by a cooled sensor (bolometer, IR spectrometer) or the pyrgeometer which uses a kludged ambient sensor. It cannot transfer any energy to a body at the same or higher temperature.”
——————–
HA, iffen there is “Back radiation” then there has gotta be “Front radiation” and “Sideways radiation” ……. so why is no one ‘accounting’ for them in their calculations?
And me was thinking that energy transfer between 2 different entities was irrespective of the temperature of either one. Thus, if one entity is absorbing more energy than it is emitting its temperature will increase. If is emitting more energy than it is absorbing its temperature will decrease
Matthew R Marler says:
August 12, 2014 at 9:57 am
“The word “trapped” need not mean “permanently trapped”: when I trap squirrels, for example, a few are able to escape from the traps.”
————–
That’s probably true for bleeding-heart liberals that use cages and box-traps, but when I trap squirrels I use a steel-trap and no of them get away.
So, from now on, only use the word “trap” when you are trying to catch squirrels, or mice, etc.
When talking climate science, words such as …. trap, greenhouse effect, greenhouse gases, forcing, backfeeding, feedbacks, sensitivity, consensus of opinions, etc., only serve to “brain-wash” and/or confuse the ell out of the science uneducated and/or miseducated populace and thus give credence to the “junk science” rhetoric being propagated by the proponents of CAGW.
===============
Matthew R Marler says:
August 12, 2014 at 10:36 am
“The author repeats the standard that CO2 has to date raised the mean temperature of the Earth (or the “equilibrium temperature”) by 33 C.
This is no mystery, but the author ought to clarify exactly how the obtained the 87% figure.”
—————
“DUH”, the author CAN NOT clarify exactly how the (he/she) obtained the 87% figure ….. simply because he/she can not provide any actual evidence or proof that the “to date” increase in atmospheric CO2 has caused one iota of measurable increase in near surface temperatures.
matayaya says:
August 12, 2014 at 12:48 pm
“AlecM, I think it is well understood that CO2 does not “absorb” IR. In my layman understanding, it’s more like CO2s are the bumpers in a pin ball machine, IR being the ball.”
——————
Well fer sure, it is not well understood by you, …. cause youse got it wrong.
It is not like the CO2s are baseball player’s bats (bumpers) that reflect the baseball (IR) away when contact is made.
In actuality, the CO2s are like the baseball player’s gloves that temporarily ABSORB the baseball (IR) ….. which the baseball player then flings (emits) back into the “game”.
Nick 7:32am: “No. Melting ice consumes h**t. If isothermal, h@@t cannot move.”
To understand why the top post is correct, this discussion helps set a solid & natural science foundation in place for CO2 surface temperature physics discussion without need for heat to exist.
Added defn.s by Nick. Heat is:
20) consumed
21) moving
Nick adds to the contortions to get heat to have corporeal existence. Heat doesn’t exist in nature, therefore heat cannot be consumed or be moving. You are being deluded b/c of your dependence on LH and SH being explanations when they do not exist in nature b/c heat does not exist. They are both logically enthalpies in nature. Other authors do a better job writing it out, see their work in detail, more than here. Nick & yes, JohnWho, thinking logically with natural terms will increase understanding of top post.
What the ice block has in nature is a set amount of conserved enthalpy = PE+KE+p*V. Draw a control volume around the ice. Account for enthalpy being conserved.
Temperature in the mixture at interfaces remains constant as melting occurs, by high school science level test. PE reduces as the macro ice molecules reduce in volume from solid to liquid state, this means KE+p*V terms have to increase by 1LOT in the control volume to keep same amount of enthalpy conserved. Think thru the melting process with naturally existing & explainable terms. And seek out, find other authors detail work.
I know the PE + p*V term is important b/c it leads to ice expanding in the reverse freezing process – the harsh winter froze & cracked open my expensive cast iron well pump. This was logically due to the PE + p*V terms not KE enthalpy term and certainly not due to nonexistent LH or SH.
………………………………………………. On abolishing (unspecific) ‘heat’
1) What about traditional equivalents in other languages–what does the rest of the world care what we do? English has not exactly replaced Latin.
2) What about “heat transfer,” “heat flow,” and “specific heat”?
3) What about ‘calories’ –“warmicules”?
4) The distinction between science and general experience is artificial. Any vocabulary which attempts to make such a distinction will also be artificial.
5) The phlogiston and caloricum were well defined before they were abandoned. To abandon an undefined concept hardly constitutes progress. A large number of meaningless 4-letter words–26^4 in languages using the Roman script–could be outlawed and we would be no better off than before.
5) To deny any traditional meaning of the word ‘heat’ is absurd. Whether noun, transitive or intransitive verb, adjective (‘hot’), babes and beasts understand the fundamental concept. Hot, warm, luke warm, cool, cold, freezing–these words constituted T measurement before F and C existed.
6) The problems seem to arrive when people try to tell us what heat is not–not what it is. A dromedary is a camel. Because you can’t count humps should we abolish the word? Heat may not be phlogiston or caloricum; there may be no such thing as either, but heat will endure for at least another 15 billion years, no matter what we call it.
To the stake with Trick…and a few others. –AGF
@Samuel C Cogar: there is an appalling lack of knowledge about radiative physics.
All bodies have a radiation field, detected by having a metal box with a hole in the front. Inside the box is a sensor, usually cooled. Because the metal box doesn’t have a hole in the back, the sensor detects the apparent temperature of the emitter in its field of view. Use this in the Stefan-Boltzmann equation and you get the emittance. Divided this by the black body emittance to get the emissivity
The emittance, expressed in W/m^2 is not the ElectroMagnetic energy emitted in the absence of the detector; It is the EM energy it emits to the cooled sensor where it is converted to heat. Because the sensor is cooled, it does not emit much EM energy to the emitter in the view angle; what you estimate is the potential energy flux from the emitter to a sink near absolute zero.
Turn the sensor around by 180 degrees and you get the emittance of the emitter that was to the rear of the original measurement. Subtract this signal from the first and you get the net EM energy transferred from the first emitter to the second.
If you do this at any angle in the atmosphere with no temperature gradient, the results will always be the same; up-down; sideways. For the case of the atmosphere near the Earth’s surface, the signal is between two equal temperature emitters but they have different emissivities. This is because some weakly absorbing H2O band IR and atmospheric window IR goes to Space unimpeded by the atmosphere..
Net mean IR flux from the Earth’s surface to the atmosphere is 63 W/m^2, about 1/6th of a black body. None of the atmospheric emittance, 333 W/m^2, carries energy to the surface; it simply offsets same band surface emission; no net surface IR in ‘self-absorbed’ IR bands.
I hope this explains the real physics; the ‘back radiation’ bouncing backwards is puerile nonsense paid for by Obama and his predecessors to pretend CO2.is dangerous; it ain’t because the atmosphere makes CO2-AGW exactly zero on average, the same for any well-mixed GHG.
AGF 10:09am –
1) Even in other languages heat cannot be made to exist; replaced with enthalpy which is widely used in science of all languages.
2) See above, if heat is not anywhere it cannot transfer, cannot flow, specific heat is really specific enthalpy in science.
3) Calories are a unit not heat.
4) any general experience encounters heat, it really encountered enthalpy.
5) Phlogiston and caloricum were not well defined; they would still exist if so. Contortions of science were made also to keep them around longer than necessary.
5 again) to define something that doesn’t exist is fantasy, which has a role other than science.
6) Heat ceased to exist in nature last millennium, heat didn’t even make it to this one & well defined enthalpy will still be around in the next one.
To the stake with heat. Leave it buried in the past science where it served out its purpose; no need for reincarnation, leave it lie. RIP.
In English ‘heat’ is an abstract conceptual term.
It refers to the transfer of energy which can raise temperatures or enact a phase change. It refers to the energy being transferred with the aforementioned properties, no matter in which state it resides.
Friction generates heat. The sun generates heat. It need not be important that the heat comes in various forms of energy until the time when it is the focus of the discussion.
The following phrases are equally correct, one is just more pedantic than the other:
Heat from the sun travels through the atmosphere, warming it, and hits the surface, which holds some of the heat until nightfall. At night this heat is slowly released, with some bouncing off the atmosphere and hitting the surface again, keeping temperatures warm until the sun comes back up again.
—
Solar radiation, in various wavelengths, interacts with atmosphere where Raleigh Scattering occurs to differing degrees based on atmospheric composition, pressure, temperature, albedo, mass, electron orbital spin moment, and various spectral qualities. A portion of this solar radiation manages to reach the surface where it will either by absorbed (and then randomly remitted) or reflected by the surface at varying depths and angles in accordance to the albedo and spectral qualities of the surface and incoming solar radiation. The solar radiation will impart molecular and atomic excitement where absorbed which can be macro-measured in terms of T, otherwise known as temperature. The solar radiation interacting with the surface shall now have two terms: “reflected radiation” – for the solar radiation which was reflected by the surface, and “surface radiation” – for solar energy which interacted with the surface and has been emitted towards the atmosphere OR towards other surfaces.
During the time of greatest insolation, the surface will typically convert a significant portion of incoming solar radiation into molecular and atomic momentum, excitement, and/or motion. The portion not held in said manner will become surface radiation of a longer, lower energy, wavelength when emitted from the surface – also exhibiting the primary characteristics of Raleigh Scattering. During times of lower insolation this process will provide energy which continues to excite the lower atmosphere, keeping its macro-measured excitement property (temperature) from declining as rapidly as it would without a surface present. Likewise, the atmosphere will scatter, via Raleigh Scattering, some of this surface radiation back to the surface, where the energy is, potentially, once again in a position to re-excite the surface. This process serves to prevent both the surface and the lower atmosphere from moving to a lower average excitement level (temperature).
—
Seriously, which is better? And, please, let’s not be pedantic about little mistakes 😉
Come off it Trick, enthalpy is not heat. It is a defined thermodynamic potential, designated by the letter “H”, that consists of the internal energy of the system (U) plus the product of pressure (p) and volume (V) of the system.
Internal energy can be explained in microscopic terms by two virtual components; the microscopic kinetic energy due to the microscopic motion of the system’s particles (translations, rotations, vibrations). This is heat. The other is the potential energy associated with the microscopic forces, including chemical bonds and static rest mass energy.
So, enthalpy is a much more complex beast; stop trying to confuse it with heat energy please, which is what does external work.
AlecM 10:54am – Good progress. Major progress actually in understanding top post.
“This is heat.”
No. What “this”is is kinetic energy measured by thermometers reading calibrated temperature not “heat”, now I’ve pointed that out 3 times; a component of nature’s enthalpy. Heat ceased to exist in nature last millennium. PE is also a component of natural enthalpy as you write can be chemical like my hydrogen and oxygen combustion example.
Enthalpy is not at all complex: PE + KE + p*V and is the actual conserved quantity in 1LOT.
Heat energy? That is like writing joules joules. Only need energy once measured in joules.
Energy can do external work using up a fuel (f*d joules); heat cannot do work though kinetic energy can. Heat has no such tidy definition, as heat doesn’t exist in nature past the last millennium.
looncraz 11:27am: “Friction generates heat. The sun generates heat.”
Not a little mistake to understand top post. Explained above by using up a fuel friction generates KE which is not “heat”. Sun generates plenty energy using up a fuel but zero “heat” which expired last century.
“Heat from the sun travels.. holds some of the heat..slowly released..”
Heat does not travel as it doesn’t exist anywhere to travel from and something that doesn’t exist “heat” can’t be held or released. Last two paragraphs – better science. I would add the surface can transmit also with the surface can either reflect or absorb. Earth surface can do all three for incident radiation and surface diffraction is negligible.
But if ‘heat’ is in any way interchangeable with ‘enthalpy,’ and we blacklist this dirty little 4-letter (Germanic) word in favor of the euphemistic (Latin) ‘enthalpy,’ we merely kick the can a few yards down the road. The euphemism will quickly gather the former connotations of the banned word and will have to be replaced with something else. ‘Scat’ by any other name would smell as bad.
And of course if enthalpy is really heat, and there is no such thing as heat, then there is no such thing as enthalpy either. –AGF
Trick:
If it pleases you to believe that heat does not exist then do. But please keep your beliefs to yourself and stop befouling WUWT threads with them. They are a distraction.
Richard
oops, that’s a Greek word for heat we be resurrecting. Who knows, maybe 2ky from now the dead word ‘heat’ will be resurrected to enhance clarity. –AGF
AGF 12:07pm: Thanks for the discussion. Energy replaces the “heat” word in this century for general reading to improve clarity & discover faults, both are joules, energy exists.
If you are interested in what is conserved to understand more detail like the melting of ice or especially in a gas (like the atm.) for understanding details of “temperature increasing capacity of atmospheric CO2” in top post, use more precise enthalpy. Energy can mean the KE, the PE or the p*V term, enthalpy covers the waterfront.
richard 12:13pm: “..keep your beliefs to yourself…”
That heat does not exist in nature is not a belief or view, it is solid, basic science discussed even since the 50’s. The top post generates a lot of dispute; that dispute will thin out when the solid, basic, tested science becomes well understood generally thru blog give & take.
You are of course welcome to post using the caloric theory “heat” and try to understand nature from that basis. Science has long since moved on, I am just pointing out the obvious, “heat” is past its expiry date on the warning label.
There’s a lot more than aerosols flying around on this thread.
This leads me to restate, one of my guiding beliefs. You MAY quote me.
” Ignorance, is NOT a disease; we are ALL born with it !
But stupidity, has to be taught, and sadly there are a great many people willing and able to teach it ! ”
It is a great disservice, to those who do not have an extensive science background, and come here to Anthony’s great learning site, (like me) to have to wade through screeds of; well crap. AGF called it that, and he’s a pedant for lexicological exactitude.
And that crap, is coming from some truly stupid people; who would teach it to others.
Now I have fessed up, to NOT being a quantum mechanic. I’m largely a classicist, due to a lack of education.
But a very famous PhD Physicist, who is an even more famous Medico; Surgeon and Anesthesiologist; who was recently taking QM classes at Stanford (he is North of 90 years old), told me to forget about QM, if classical Physics would suffice. QM “only confuses things”, he said. You only know there’s a certain probability that you are correct.
I’m not going to name him; but probably most of the people, on this planet, all saw him on their T&V in the late 1960s to early 1970s.
I’ve lost my connection with him, now, and I sure regret that lost learning asset. I also got to drink a beer, in a shady home garden, with a Physicist Canuck, who got a Physics Nobel Prize for proving that the putative guts of an atomic nucleus, actually exists; at least to the extent that we know anything really exists. Much as “heat” (noun), really does exist.
So what about the Second Law of Thermo-dynamics, that litters these threads.
I am partial to the statement of that principle, that was allegedly given (presumably in German), by the great German Physicist, Rudolph Clausius, who lived for about 2/3rds of the center portion of the 19th century.
To whit : “No cyclic machine, can have no other effect, than to transport HEAT from a source, at one Temperature, to a sink at a higher Temperature.”
Now even AGF must agree, that is one piece of lexicological legerdemain. Hopefully, the original German, is equally obtuse.
Maybe one of our German friends, can find us the German original.
So what the hey, is Clausius telling us ??
Well first notice that little word “cyclic”. Words have meaning !
The second law presumes that HEAT ENERGY can travel in BOTH DIRECTIONS, but there is a net transport, only in the hot to cold direction. That is it in a nutshell.
So now what about electromagnetic field propagation, and radiant energy. That Poynting Vector business ??
Only two of the four forces of nature, are infinite in range, and can go anywhere (and everywhere) they darn well please. Gravity, and electro-magnetism.
The other two, the weak force , and the strong force, are doomed to remain imprisoned, in the atomic nucleus. The strong force stops that gang of protons, from blowing the whole thing to smithereens.. The weak force is involved in beta decay. I’ll face the wrath of Prof .rgb, if I’m wrong about that second case of confinement.
So back to the EM force and fields, and flocks of photons.
Well Rudolph Clausius, also had something to say about that. Specifically, Clausius at a quite early historic date, derived the so-called “Optical Sine Theorem”.
The OST says that in any EM field propagation, the quantity N.H.Sin(U) is conserved (invariant).
N is the refractive index of the medium, that the field is in, H is the height of a ray or image (or object), and U is the angle that the ray makes with the propagation axis.
A rigorous proof of the conservation everywhere in the field, can be found in “Born and Wolf”.
I have not been able to confirm that Wolf was German; Max Born certainly was.
The OST for small values , so called paraxial optics, becomes n.h.u where it is commonly referred to as the “Lagrange Invariant.”
For thermodynamassistants, nhu is a description of the radiance (brightness (bad swear word)) of a source of EM radiant energy. What Black Bodies emit; that Stefan-Boltzmann business.
So what Clausius, basically said, was that ” no optical system, can form an “image”, that is brighter than the source. There I used that swear word. Use “Radiance” or “luminance”, depending on radiant versus visual units. (Light is a figment of the humane eye/brain, in response to radiant energy in the circa 400-800 nm range). Ergo, we get no light from the sun, either; it’s all in our head !
So Clausius postulated an optical system, that could create, an “image” having a higher radiance than the “.source”.
So he chose a source that is a black body radiator , that is at a Temperature Ts, and produces a Lambertian radiance of (sigma).Ts^4 / pi , W / sr / m^2.
At the image of his optical system, he now has a radiance of s.(sigma).Ts^4, where s is a factor >1.0 per Clausius asserted condition.
So we now put a second black body at the image, to capture all of the radiant energy emerging from the optical system.
At equilibrium, the receiving black body, will be at a Temperature Ti, and will be emitting at a Lambertian radiance of (sigma).Ti^4, which must at equilibrium just equal the received energy, so we must have (sigma).Ti^4 = s.(sigma).Ts^4, and since s we defined as being greater than 1.0, so we must have Ti > Ts by s^0.25
This violates the second law, so our postulate of a radiance gain, is impossible; no optical system, can make an image brighter (oops) than the source.
Now since optics (at least geometrical optics) is bidirectional, so if I switch source and image, then the Clausius forbidden optical system would make an image that is dimmer than the source.
Nah ! can’t do that either. Ergo, the Lagrange invariant, or OST, is conserved.
A corollary to that rule, there is an add on Optics theorem, which says; “No optical system, can make an image that is brighter (ouch) than that made by an “Aplanatic” optical system.
Aplanatic, is a three dollar word, that means, corrected for both “spherical aberration”, and also corrected for “coma.” Coma, makes star images look like little comet shaped ice cream cones. So you can’t tell exactly where in the image, the star actually is.
Astronomers prefer Ebola, to coma.
But back at the EMR is not heat thing; if a cooler entity is radiating towards a higher Temperature object, and that radiation is all absorbed by the presumably isolated receiving object, and eventually ripples down to the heat basement, the source will find that it is in the line of fire of the hotter object, which is radiating harder than the cooler object, and if everything persists, the hotter higher Temperature source will win.
Mickey Mouse cannot keep up with all the broomicles in trying to empty the flooded sorcerer’s Alchemy shack.
But how the heck can a rotating earth ever reach equilibrium (Temperature equality), with the sun.
That blow torch beam, that is beating down on me now, in California, will be over Mauna Loa, in a few hours, and I will get a chance to cool down, before getting enshined again tomorrow. And this damn M$ editor tried to correct my speeling, and enshrine me. Too late for that.
So radiant energy emitters and receivers, do not escape the wrath of Rudolph Clausius, if they keep on looking at each other; but they aren’t trading “heat” (noun) back and forth. They simply conform to the optical sine theorem; and can’t outshine each other.
You
Heat (as a noun) is defined as being any of the following:
1. the quality of being hot; high temperature
2. heat seen as a form of energy arising from the random motion of the molecules of bodies, which may be transferred by conduction, convection, or radiation.
3. the amount of heat that is needed to cause a specific process or is evolved in such a process.
#2 is of most interest here. Heat travels… in the form of radiation.
No no no. Go back to a thermo book and look at phase diagrams. Contemplate ice-water mixtures, which remain at the temperature of ice (when heated or cooled quasi-statically by a WARMER or COOLER sink). In fact, that’s how they originally defined the reference temperatures — water boiling at 1 atm remains at 100 C as long as there is water to boil, ice/water mixtures were used to define 0 C for the same reason.
Well, a qualification for the no — yes, the ice water has to be heated or cooled by a reservoir at a higher or lower temperature than the ice water mixture, but provided that the ice-water mixture is heated slowly enough to remain in (local) thermodynamic equilibrium, the well-mixed ice and water are at the same temperature. Heat entering the system goes preferentially into latent heat, shifting ice across the phase boundary either way.
You can remind yourself of this stuff here:
http://www.physics.rutgers.edu/~wdwu/351/Lecture15.ppt
Note well slide 5, which shows S(P). See the places where S jumps discontinuously at constant P? That’s basically heat transfer at constant temperature — the entropy change is \Delta Q_latent/(T = 273.16) — the absorption/rejection of the latent heat at constant temperature at the first order phase boundary.
rgb
In other words, you are saying that when we teach physics (or chemistry) courses on thermodynamics, we no longer teach the first law of thermodynamics:
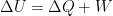
 is the heat — defined a variety of ways, but essentially as internal energy spontaneously transferred and hence no longer available to do work) because “heat” doesn’t exist?
is the heat — defined a variety of ways, but essentially as internal energy spontaneously transferred and hence no longer available to do work) because “heat” doesn’t exist?
(sign convention U is internal energy OF system, W is positive work done ON system,
All of those section discussing heat in contemporary physics textbooks are meaningless? The classical thermodynamic definition of entropy change at constant temperature (useful in the consideration of phase change/latent heat and in analyzing heat engines) as:
is meaningless?
I think you misunderstand, big time. Heat is a perfectly well understood concept, even as it is fully recognized in macroscopic thermodynamics textbooks that there is no such thing as a well-defined “amount of heat in a body”, only an amount of internal energy in that body. Since heat transfer depends on path, thermodynamics books often represent its differential with a bar through it to remind is that one cannot integrate it to find an absolute amount, even though in motion it is a perfectly reasonable idea.
I think you are referring to statistical mechanics. In stat mech, heat per se does not exist and entropy is the log of the missing information. When one does the averages, though, stat mech solutions for large systems always obey the laws of thermodynamics.
With that said (and it needed to be said) I agree that heat is often misused in the discussion of climate, but, I suspect, not in a way that causes any violations of the laws of thermodynamics as long as Slayers are not involved. I’m guilty of it myself — it is very easy to conflate heat and internal energy in casual discussion, and talk about the heat flowing into a system as if it all goes into increasing temperature (internal energy) rather than some into increasing temperature and some into doing work. Especially in macroscopic scale climate systems, where the net work over any long time average is manifestly (almost) zero.
rgb
Arrgh. Should have previewed first, sorry. Let’s try:

 is the heat flow into the systems in the statements above.
is the heat flow into the systems in the statements above.
and that
rgb
“I have not been able to confirm that Wolf was German; Max Born” Emil Wolf: Czech then Brit. My (sixth-edition) version says he was teaching at the University of Rochester. I think Born & Wolf was an English-language expansion of Born’s “Optik.”
No, you did fine. Note well that the source of light-borne energy is the sun, surface temperature 5500 K, so basically there isn’t even the faintest possibility of paradox associated with heat flow in the greenhouse effect at temperatures over an order of magnitude lower here, though.
Note also that the most common abuse of the Clausius principle is to assert that the existence of an intermediate LW (IR) absorptive layer between the Earth’s SW (Visible) sun-warmed surface and the very cold indeed 3 K of mostly empty space cannot cause the surface to warm relative to the temperature it would attain in dynamical equilibrium without it because it is colder than the surface. This is simple nonsense, mere piffle, moonshine, ignorance in motion, and violates the common principles of radiative physics and the laws of thermodynamics, which without any doubt show that the sun-warmed surface will indeed warm further if an interpolating LWIR absorber later exists between the surface and cold infinity.
So be sure you don’t encourage the growth of absurdity. A simple mirror at 3 K is perfectly capable of reflecting energy back at a heated source so that its temperature rises compared to what it would be if there were no mirror. This is the basic idea of the so-called “space blanket”, used to preserve the warmth generated by electronics in cold, cold perfectly absorptive space.
rgb
Um, you do know about phase transitions, right? The melting ice is indeed being heated, but its temperature does not change because heat is not the same thing as internal energy! In particular, you are ignoring the change in entropy. The organization of the solid is completely different from the organization of the liquid. The information required to specify the state of the solid is much less than that required to specify the state of the liquid. If you look at coexistence curves with entropy as an axis this is all perfectly clear.
Maybe you should take a thermo course at some point? I’m just sayin’…
rgb
richardscourtney says: August 13, 2014 at 7:51 am
“There is a temperature gradient from the hotter to the colder body (that is what hotter and colder mean). And there is a transfer of heat down that gradient (that is why the ice melts).”
Yes. And part of that gradient is within the cooler body. It can’t be isothermal.
In fact, phase change takes place at an interface. Heat has to be brought to that interface (or taken away). In melting, that generally requires a temperature gradient through the liquid phase. As melting proceeds, heat has to travel further, so overall the temperature rises.
“””””…..Joe Born says:
August 13, 2014 at 3:22 pm
“I have not been able to confirm that Wolf was German; Max Born” Emil Wolf: Czech then Brit. My (sixth-edition) version says he was teaching at the University of Rochester. I think Born & Wolf was an English-language expansion of Born’s “Optik.”…..”””””
Thanx Joe. I did find a reference that cited some award of a Czech medal, but nowhere did anyone say he was Czech. I did consider it a possibility.
My B&W is buried around here somewhere, and I wish I had thought of checking there. I need to disinter it anyway, to keep it closer at hand.
Your Great Uncle, sure wrote one hell of a fine textbook.
george e. smith says:
August 13, 2014 at 4:03 pm
Max Born, as in Olivia Newton-John’s granddad? So, Joe’s cousin?
“””””…..looncraz says:
August 13, 2014 at 2:39 pm
Heat (as a noun) is defined as being any of the following:
1. the quality of being hot; high temperature
2. heat seen as a form of energy arising from the random motion of the molecules of bodies, which may be transferred by conduction, convection, or radiation.
3. the amount of heat that is needed to cause a specific process or is evolved in such a process.
#2 is of most interest here. Heat travels… in the form of radiation……”””””
Well I would wager that that is not a definitive definition, from some well recognized thermo-dynamics text book. If it is, then give me the name of it, so I don’t make the huge mistake of buying it.
“””…#2 is of most interest here. Heat travels… in the form of radiation……”””””
Well of equal interest, is that heat is transferred by means of grocery shopping cart, as well as the conduction, convection and radiation that you cite. You simply fill the cart with Presto logs, or Kingsford BBQ briquettes; maybe you can even buy coal at the local hardware store or feedlot store.
Simply put a match to it to open it up and let all that heat leak out of the cart.
It is ENERGY that is conveyed by radiation. And here on earth, you can turn it into algae, or grass or trees, and even now, photo-electricity. You don’t really have to waste it by throwing it into the trashcan of heat.
“Max Born, as in Olivia Newton-John’s granddad? So, Joe’s cousin?”
All my ancestors are German, but my uncle, who is indeed a physicist, tells me there’s no known connection with Max Born.
Max Born, tends to get a bit too underrated. I think he was a giant.
But what competition. The German physicists of the late 19th, and early 2oth century; were an incredible bunch, as George Gamow, writes in his fascinating book; ” Thirty Years, that shook Physics. ”
Incidentally, the physicist / MD, I mentioned above, was once a student of George Gamow;
Thanx Joe.
Joe Born says:
August 13, 2014 at 4:32 pm
Your uncle is lucky to be blessed with such a great name for a scientist. The American Paul Ehrlich, not so much.
Kristian (August 13, 2014 at 1:05 am):
Every time I think you cannot get more confused, you manage to amaze me. You say:
“The mean upward transfer of energy by radiation from the global surface would be 63 W/m^2 in both cases [transparent and radiatively active atmospheres], Curt. And only that. As long as the average incoming from the Sun is still 161 W/m^2 and the mean conductive/evaporative energy loss rate is 97 (98) W/m^2, the surface can’t put out more energy than that by radiation.”
You are completely missing the very elementary fact that if the atmosphere cannot transfer any energy to space (which it could only do by radiation), it cannot on any ongoing basis get transferred to it energy from the earth’s surface. So to say that it would accept an average 97 W/m^2 on an ongoing basis from the surface when it radiates 0 W/m^2 to space is simply ridiculous. That you would assert something so trivially wrong indicates that you cannot perform the most simple thermodynamic analysis. You would be flailing by the second week of an introductory thermodynamics class.
If the atmosphere absorbed nothing in the visible spectrum as well as LWIR, the surface would absorb an average 240 W/m^2. Over the long term, the atmosphere would neither transfer energy to or from the earth’s surface, the only entity it could exchange energy with. The earth’s surface would need to be able to reject an average of 240 W/m^2, and the only avenue for it to do so is by radiating to space (which has an effective radiative temperature of ~0K, so it does not matter whether we look at it as uni-directional or bi-directional). So it would have to have a temperature of 255K (or less if varying) to do this.
If the atmosphere absorbed 79 W/m^2 of solar radiation (with 161 W/m^2 absorbed by the surface) but had no way of radiating LWIR to space, the earth’s surface would still need to radiate 240 W/m^2 to space, with the atmosphere transferring 79 W/m^2 to the surface (so there would be a temperature inversion in the atmosphere).
If you cannot perform these most basic energy balance calculations, you have nothing — NOTHING — to contribute to these discussions.
Nick Stokes:
Your post at August 13, 2014 at 3:54 pm selectively quotes from my post at August 13, 2014 at 7:51 am
It says
Yes, and that is why I wrote but you chose to ignore and not quote
However, that could be overcome by experimental design.
The point of my illustration was that two bodies at different temperatures which are thermally connected do not ALWAYS experience cooling of the hotter body and warming of the colder body.
An illustration needs to be appropriate for its intended recipient. The ice/water illustration is appropriate for someone with the limited knowledge displayed by Kristian. It would not have been appropriate for me to have provided an illustration such as the black hole described by Joe Born at August 13, 2014 at 2:00 am.
Your pedantic point can be refuted in that context by amending the case to being:
when the hotter body cools to 0°C when the ice/water mixture stabilises to 0°C so there is no eventual warming and – at best – the cooler body only experiences temporary warming which exists only in theory because it is too small to be measured.
Richard
I’ve always wondered if the IR content of solar radiation makes much of a contribution towards saturation of CO2 absorption bands.
Trick:
At August 13, 2014 at 12:48 pm you wrote saying to me
NO! You are wrong! And your belief is a denial of basic scientific principles.
I do not need to detail your errors because rgbatduke has already done it in a series of excellent posts to this thread. At August 13, 2014 at 3:09 pm he writes saying to you
I commend all that post to you and its addendum. This link will take you straight to it.
Richard
richardscourtney says: August 14, 2014 at 1:33 am
“Your pedantic point can be refuted in that context by amending the case to being:
when the hotter body cools to 0°C when the ice/water mixture stabilises to 0°C so there is no eventual warming and – at best – the cooler body only experiences temporary warming which exists only in theory because it is too small to be measured.”
No, it’s not a pedantic point. I have dealt a lot with heat flow over the years, and I think Trick’s original point was right. If you put two bodies of different (uniform) temperatures together, the warm one will cool and the cool will warm. One can devise ways in which the change in sensible heat could be made small, but not zero.
When you bring two bodies together, there is a temperature discontinuity. That is, a very steep gradient, and an initial very large flux. That early part has a similarity solution. Heat will rapidly pass from one body to the other.
Then a melting front progresses. You can make it complex by stirring, but it is a Stefan problem. Heat flux with a temperature gradient is an essential part.
I thought your original objection was pedantic. But my response was, it doesn’t work.
Nick Stokes:
re your post at August 14, 2014 at 2:31 am.
If it makes you happy to think you were right then do, but your point was wrong.
As I said,
I spell out one simple example.
The ice at 0°C could be a few atoms thickness coating the inside of a container at 0°C enclosing water at 0°C.
The thermal transfer would be from a small amount of warmer water in which the container is immersed. The intial transfer of heat from the container wall would be to the surface layer of ice molecules and entropy would power it not temperature difference. That initial thermal transfer would result in melting not temperature rise.
But all of this pedantic interaction is pointless. As I said to you,
So, if it makes you happy to think you were right then do.
Richard
Curt says, August 13, 2014 at 11:24 pm:
“Every time I think you cannot get more confused, you manage to amaze me.”
*Sigh* That’s because you’re incapable of grasping what I’m telling you.
I should hardly bother to write this response, but here goes.
You have a weird approach to reality. This is not a radiative problem at all, Curt. That’s what’s wrong with the energy balance that ‘Climate ScienceTM’ sets up for Earth’s surface?
It’s a convective problem.
You and the establishment pretend that this is a radiative balance problem for the surface. It’s not. A heated surface surrounded by a fluid in a gravity field can NEVER (do you hear? NEVER!) achieve a pure radiative equilibrium with its heat source. Why? Because it will always preferably lose energy by conductive>convective heat transfer to the fluid. As long as the fluid’s there and the heat is coming in to the surface from its heat source, this will be the case.
You are absolutely blind to this fact, it seems.
The pretend surface radiative equilibrium that the Great Green Climate Brigade has devised for us is nothing but a chimera.
This is how the real world works, Curt. Read it carefully:
#1) The Sun heats the surface. It transfers energy as heat to the surface by radiation. The transfer is 165 W/m^2 on average.
#2) The surface absorbs the radiative heat flux from the Sun, its internal energy increases and it warms as a result.
#3) The surface in turn rids itself of the absorbed energy and transfers it to the surface air layer above (and space). It does so by evaporation, conduction and radiation. At dynamic equilibrium (steady state), the mean energy shed equals the mean energy absorbed: 165 W/m^2. This is split like this (global average): evaporation 88 W/m^2, conduction 24 W/m^2, radiation 52-53 W/m^2 (of which 32-33 go to the atmosphere and 20 directly to space). (From Stephens et al. 2012.)
#4a) The surface energy transferred to the surface air as heat through conduction and radiation, is quickly absorbed, warms the air as a result, expanding it, making it less dense. Almost all of the (289K) surface radiation either goes directly to space via the atmospheric window or is absorbed near the surface by water vapour, a little bit also by CO2.
#4b) The surface energy transferred to the surface air as ‘latent heat’ through evaporation, makes the air lighter and buoyant by the injection of H2O molecules.
#5) 4a+b automatically and naturally initiates convective uplift. In other words, the original surface energy is brought up and away from the surface air layer, into the atmosphere at large, by the process of convection – the bulk movement of air. This is, in simple terms, how energy is transported through a fluid: Heating (energy in) at the bottom > energy moving with the fluid from bottom to top > cooling (energy out) at the top.
#6) Climbing up through the tropospheric column towards the tropopause, the surface-heated air warms the surrounding air by doing work on it (expanding into it). At the same time, the ‘latent heat of vaporisation’ held inside the WV is progressively released as it condenses upon cooling. In fact, the main tropospheric ‘warmer’ is water vapour condensing, moving upwards by way of convection:
http://i1172.photobucket.com/albums/r565/Keyell/HeatamprainJRA-25_zpsda38e24a.png
#7) Finally the surface energy is radiated to space from all levels of the troposphere, from wherever it finds a line out, mostly (naturally) from the upper levels. The main (almost exclusive) ‘cooler’? H2O in all its forms and all its glory:
http://chiefio.files.wordpress.com/2012/12/stratosphere-radiation-by-species-1460.jpg
#8) The absorptivity of the air + the natural mechanism of buoyant/convective uplift upon warming (relative to surroundings) precludes ‘back radiation’ from ever having a noticeable influence back down towards the surface. The radiation is ‘forced’ to leak upward towards space, because that’s the way the convective conveyor belt moves. This is a fine-tuned machinery designed for the Earth to be able to rid itself of as much energy as it gets in.
#9) The tropospheric temperature gradient (the environmental lapse rate) is maintained globally fluctuating around the ideal adiabatic lapse rate by the tight interaction between solar surface heating and the direct convective/evaporative response. There is nothing internal thermal radiation can do to upset this large-scale balancing act. The closest thing would be if it weren’t able to cool (radiatively) from the top.
So you see, Curt, the 52-53 W/m^2 (or your 63) is the amount of energy that goes out from the surface by radiation to begin with. No more, no less. That’s radiation’s contribution to the surface energy balance between IN (from the Sun) and OUT (to the atmosphere/space). That’s all the energy it has at its disposal. Nothing of this energy returns to the surface. It is absorbed by the surface air, brought up by convection and emitted at altitude to space. Just like the conductive heat and the latent heat. That’s the way the energy from the Sun moves through the Earth system.
If the atmosphere were transparent to IR, the 52-53 W/m^2 would simply go straight through to space in its entirety. The lapse rate wouldn’t become any steeper making the surface any colder. Conduction>convection would still bring surface energy into the troposphere, warming it. In fact, the opposite would happen. With a radiatively inactive atmosphere, the Earth system could not adequately shed its absorbed energy to space. And it would heat up. No radiatively active gases, no stability.
The radiatively active gases (the so-called ‘GHGs’) do not enable the atmosphere to warm from the surface. It is warmed through convection. They enable it to cool to space. Because this can only happen through radiation.
Bye.
Kristian:
I write to again remind you that you have not answered my question. I iterate the matter for your benefit because I am trying to help you to start to understand the scientific issues of which you display such great ignorance and misunderstanding.
I remind that I first asked you to clarify the matter in my post at August 12, 2014 at 10:13 am which is here.
It said
Richard
Kristian says, August 14, 2014 at 3:44 am:
“A heated surface surrounded by a fluid in a gravity field can NEVER (do you hear? NEVER!) achieve a pure radiative equilibrium with its heat source. Why? Because it will always preferably lose energy by conductive>convective heat transfer to the fluid. As long as the fluid’s there and the heat is coming in to the surface from its heat source, this will be the case.”
A red hot piece of metal lying somewhere in the open on Earth can of course be approximated as a ‘pure radiator’. Conductive/convective loss can be ignored. At its surface. But this is of course not what I’m talking about. Such a piece of metal is not directly coupled to the atmosphere the way the surface of the Earth is. It can never raise its temperature, establishing a steady temp gradient up through it in the process. And it’s very hot indeed. Not -18C. I assume people will appreciate the difference. But yes, to be completely precise, the “NEVER” in the previous comment does come with this caveat.
richardscourtney says, August 14, 2014 at 3:58 am:
“Kristian:
I write to again remind you that you have not answered my question. I iterate the matter for your benefit because I am trying to help you to start to unders …”
No, Richard. Sorry, but I’m not interested in having this conversation with you. You accuse me of inventing physics for some obscure ideological reason when the physics I promote is something you can find presented and explained in any textbook on thermodynamics. Just open one and you’ll see.
Your idea that ‘atmospheric back radiation’ can and does ‘heat’/do ‘work’ on the surface is your delusion. Not mine.
Kristian:
At August 14, 2014 at 4:14 am you say to me in total
Ah. I see. You know you are spouting bollocks so you are “not interested in having this conversation”.
I “accused” you of nothing. At August 12, 2014 at 2:42 am you wrote that your reason for pretending heat does not exist is
That is a purely political statement. So, YOU said you are pretending heat does not exist for reasons of pure politics that have no relation to science. I accused you of nothing but accepted your statement of your reasoning.
I said nothing about ideology: that is an other of your untrue imaginings.
The nonsense you promote is refuted by every textbook on thermodynamics. Indeed, this was explained in detail in this thread by rgbatduke in this thread at August 13, 2014 at 3:09 pm and his explanation (with addendum) can be read here.
I have no “delusion” about backradiation. I know that EM radiation can transform to heat when absorbed and radiation is emitted in all direction from within the atmosphere so about half of this emission travels down towards the Earth’s surface where it can be absorbed to become heat. That is empirical reality. Your denial of reality seems to be deliberate.
In summation, your post I am answering consists solely of a series of blatant lies attempting to justify your promoting falsehoods that you know are untrue.
Richard
I doubt if much back radiation takes place. An IR photon from the heated land that happens to be absorbed by a GHG excites the molecule producing stretching, bending and rotation. The excited molecule is likely to collide with another gas molecule before it has a chance to re-emit a photon. The collision will pass on energy as kinetic energy (speed of the molecule). If a parcel of air has its kinetic energy raised in this way it will possess a higher temperature than before. It will probably expand, doing work against adjacent parcels and also rise by convection.
@Schoedinger’s Cat:
(1) There is near zero net surface IR in the self-absorbed GHG bands, hence no GHG-absorbed IR in those bands (the 23 W/m^2 is non self-absorbed H2O bands, ~kilometres optical depth).
(2) In the Tyndall Experiment, the GHG-absorbed then gas-phase thermalised energy is balanced by equal emission from the local gas volume of the same IR energy, thermalised at the inner surface of the brass tube. If that did not happen, the absorptivity of the gas would be greater than the emissivity, not permitted by Kirchhoff’s Law of Radiation for Local Thermodynamic Equilibrium.
This science has slipped under Climate Alchemists’ Radar. There can be no ‘back radiation’, no Enhanced GHE. I shall leave the discussion as to why there is zero CO2-AGW for another time!
Kristian says, August 14, 2014 at 3:44 am:
“So you see, Curt, the 52-53 W/m^2 (or your 63) is the amount of energy that goes out from the surface by radiation to begin with. No more, no less. That’s radiation’s contribution to the surface energy balance between IN (from the Sun) and OUT (to the atmosphere/space). That’s all the energy it has at its disposal. Nothing of this energy returns to the surface. It is absorbed by the surface air, brought up by convection and emitted at altitude to space. Just like the conductive heat and the latent heat. That’s the way the energy from the Sun moves through the Earth system.”
The nonsense about the Earth’s surface having to radiate directly according to its temperature is based solely on a fundamentalist – and ultimately flawed – interpretation of the Stefan-Boltzmann equation: P/A = es*T^4. This equation specifically describes a situation where a black (gray) body is radiating into a perfect heat sink at 0 K. It is only applicable when the object radiating can be considered a ‘pure radiator’; either it faces no temperature at all or it is much, much hotter than its surroundings so that surrounding temperatures can be ignored (approximated as zero). In this case all we need to know is the temperature (and the emissivity) of the object radiating. Output (P/A) and temp is directly related.
Output and temp is NOT directly related when the object radiating is surrounded by temperatures fairly close to its own. Then the radiative heat transfer equation applies: P/A = es*(Th^4 – Tc^4). The energy (radiative heat) output from the radiating (hot) object in this case (like with Earth’s surface) is directly related rather to the temperature DIFFERENCE between it and its surroundings.
When, on top of this, energy loss mechanisms other than radiation are also available, the direct relation between EM emission & temp becomes even more diluted.
Mathematically, though, it’s still there. Hence the two opposing terms on the right-hand side of the radiative heat transfer equation. They do, however, not represent real (detectable) fluxes of energy, only potential fluxes, emittances, if the object(s) radiating did so into a perfect heat sink at 0 K, that is, thermally isolated from the other one.
The only thing we ever actually detect in a thermal exchange is always the heat (going out or coming in, depending on the temperature of the surroundings relative to you/the sensor). The actual transfer of energy between the systems. The other two terms (UWLWIR and DWLWIR) need to be calculated from this and temperature readings. In the case of Earth’s surface, we first detect the heat going out and measure the temperature of the surface (assumed to have an emissivity of 1). From this we can then first calculate the surface emittance (its potential flux to 0 K) that is the UWLWIR term and from this we can finally simply subtract the detected heat and get the atmosphere emittance (its potential flux to 0 K) that is the DWLWIR term. The mathematical ‘net’ (sum) of these two terms is then P/A, the radiative heat. Circular accounting in a way, starting and ending with the same value.
This is why, in order to get a direct reading of the atmospheric radiative spectrum from ground level, we need to turn it into a heat source to our instrument sensor, making the incoming heat flux as pure as possible. To do this, we cool our sensor to very, very low temperatures.
If we could just freely detect (isolate) cool downward atmospheric radiation regardless of the coincident/coexistent presence of warm upward surface radiation within the same radiation field, then no sensor would have to be cooled to freezing temperatures like this to find it. We have to turn it into radiative ‘heat’ and make the sensor resemble its near-perfect heat sink.
rgb 3:09pm: “…delta Q is the heat — defined a variety of ways, but essentially as internal energy spontaneously transferred…”
Then it is better to teach modern science as delta Q is the internal energy spontaneously transferred…just skip over the “heat” word as it doesn’t exist in science anymore & adds confusion in science students.
There is no need to add the extraneous word “heat” which is simply a hold over from caloric theory in the last millennium.
If you propose “heat” in joules has a separate existence in nature from energy in joules then devise a test to measure heat separately from total energy say in a glass of near boiling tap water. Absent that formality “heat” does not exist. Energy exists. We already know the water molecular kinetic energy mean can be measured by a mercury thermometer which reads out in temperature (F, C or K) not joules. It is widely generally accepted and held that temperature is not heat.
Trick Q: Which contains more “heat”: 1) a near boiling glass of 12oz. tap water or 2) a Great Lake on Jan. 1?
The correct answer is critical to prove a thorough understanding of the top post title & text.
@Trick: what is the difference of total energy of a 12 oz glass of water at 70 deg C (a) at sea level and (b) at the top of Everest?
Answers on a post card please.
Kristian 6:28am: “This (S-B) equation specifically describes a situation where a black (gray) body is radiating into a perfect heat sink at 0 K.”
No, 0K sink is not needed as none exist so this would be a disaster if really req.d. Again, you are differing from Max Planck’s own words. Find and read Max Planck’s orig. paper. Planck distribution of which the S-B is the integral over any freq. interval is exactly the same whether the object in question radiates to a vacuum or is covered by an atm. Planck says so in his own words setting the stage – based on his extensive testing.
Planck distribution & S-B depend only on frequency interval and temperature & some major constants of nature. That’s it. Those are never zero in nature. Thus all matter radiates real energy at all frequencies at all temperatures at all times.
The only assumptions in Max Planck paper is the distribution applies to bodies of macro positive radii and wavelengths much larger than the diameter of the object in question, otherwise diffraction also becomes important to understand nature’s radiation field.
You will need this Planck paper reading to understand the top post.
Kristian:
You have spent a huge amount of time countering arguments I did not make, but did not respond to the arguments I did make.
Earlier you had argued that on a planet with an atmosphere transparent to LWIR, the atmosphere would absorb a mean 79 W/m^2 from solar radiation and 63 (or whatever) W/m^2 conductive/convective power from the planetary surface. You argued that this would be a steady-state condition, but provided no means for such an atmosphere to reject any power. I asked you how this could be, and I ask this again. There is no point in going further until you provide a convincing answer.
Curt says, August 14, 2014 at 7:54 am:
“Earlier you had argued that on a planet with an atmosphere transparent to LWIR, the atmosphere would absorb a mean 79 W/m^2 from solar radiation and 63 (or whatever) W/m^2 conductive/convective power from the planetary surface. You argued that this would be a steady-state condition, but provided no means for such an atmosphere to reject any power. I asked you how this could be, and I ask this again. There is no point in going further until you provide a convincing answer.”
It seems you’re incapable of not misread (or rather, misrepresent) what I say, Curt. It appears to be one of your favoured debate tactics.
Where did I say what you claim I’ve said here? Can you please provide the correct quote plus the time and place?
In fact, I know I haven’t said it, because the 63 figure is specifically NOT from conductive/convective heat transfer, but from LWIR heat transfer. And the 79 solar figure would also not be absorbed by the atmosphere if this happened to be radiatively inactive, but rather go straight down to the surface.
Why do you feel you have to ‘misunderstand’ what I say all the time to build an argument against me, Curt? It happens ALL THE TIME. Is it because you’ll rather let me spend my time sorting your misunderstandings and straw men out rather than focus on your lack of a coherent argument?
If the atmosphere absorbs say 100 W/m^2 from conductive/convective heat transfer on a daily basis from the surface, but cannot radiate any of it away to space, how do you suggest the Earth system gets rid of it?
So, does longwave radiation “flow” both ways?
Don’t argue endless Eisteinian “thought experiments” about theorectical endless vertical tubes in a perfect gas world. Measure the longwave radiation.
Place 6 thin steel plates (A, B, C, D, E, and F), each 1 meter x 1 meter x 2 mm thick, in a vacuum chamber.
At that thickness, the temperature of the farside (side 2) will be the same as the temperature of the nearside (side 1).
Each plate is 18 mm distance from its neighbors.
At those dimensions and distances, there is almost no radiation from the enclosure walls at all into plates B, C, D, nor E. It doesn’t matter what temperature the chamber is, although a low emissive chamber wall will further reduce extraneous effects in the final plate.
In a vacuum, there will be no complicating convection nor evaporation effects; the plates do not touch each other, so there is no conduction losses. All energy transmitted from Plate A to Plate E can only come from the emitted LW radiation from the next hotter plate.
Now, heat the plate A electrically on the outside (side 1) .
The heat energy from the current will increase the temperature of Plate A, side 1 and side 2. And both side 1 and side 2 of Plate A will begin radiating the heat away from both of its surfaces via LW radiation. Conventional theory holds that side 1, Plate A, will radiate back towards the chamber wall.
But Side 2 Plate A will radiate its heat energy into the side 1 Plate B, which will begin heating up side 2, Plate B.
Plate B heats up, which radiates heat from both side 1 and side 2. Side 2 Plate B radiates towards Side 11 Plate C, which then heats up Plate C. Which radiates heat energy to Plate D, etc.
Next, measure the intermediate temperatures and the final temperatures of each plate as they approach equilibrium.
RMB says, August 14, 2014 at 8:46 am:
“I’m not pushing a theory. I find that when I try to heat water through the surface the water completely rejects the heat. If I float a metal pan on the surface and apply the heat to the floating object the water accepts the heat as one would expect. Uncovered water will not accept heat covered water will. Try it for yourself. The floating object kills the surface tension.”
No, RMB. You simply move from trying to warm a static volume of water by radiative heat transfer (highly inefficient) to trying to warm it by conductive heat transfer (rather more efficient).
If its as inefficient as I.m finding whats all the fuss about.
RMB:
You have had a response from Kristian.
A debate between the two of you should be wildly entertaining so I will only watch and enjoy.
For maximum fun you may want to expand your discussion by inviting participation of the Guy who thinks the world is expanding.
Richard
I am not putting forward a theory. What I am saying is a demonstrable fact. Now try heating water through the surface and get back to me.
AlecM 7:00am: Very good, sir. Back of Postcard delta = mgh
Samuel C. Cogar: In actuality, the CO2s are like the baseball player’s gloves that temporarily ABSORB the baseball (IR) ….. which the baseball player then flings (emits) back into the “game”.
That’s not so different from “trapping and releasing”. When I release animals back to the wild, I expect most of them get eaten by predators and few survive. I don’t see how that’s worse than feeding worms and bacteria. But you right in the unquoted recommendation to use the words “absorption” and “emission”, and only use words like “trap” and “fling” with the technical terms immediately in parentheses.
HA, iffen there is “Back radiation” then there has gotta be “Front radiation” and “Sideways radiation” ……. so why is no one ‘accounting’ for them in their calculations?
Why do you say that no one is accounting for sideways and upward radiation? All the heat flow diagrams (Trenberth and Fasullo, for example) account for upward and downward radiation; sideways radiation that is not absorbed becomes upward radiation, and is accounted that way.
AlecM 6:26am: “…the same IR energy, thermalised at the inner surface of the brass tube. If that did not happen…”
This (“thermalised”) did not happen and yet Kirchoff stuff is ok. The IR photonic energy is either absorbed, reflected or transmitted when incident on a highly polished inner surface of a lab brass tube.
Understanding this leads to improved foundation for top post.
george e. smith says:
August 13, 2014 at 1:58 pm
======
It is such a joy to read your writing. Thanks for what you do. I greatly appreciate it.
@trick: the IR absorptivity of a polished brass tube is ~1.0.
Try again.
PS Thermalisation of absorbed EM energy can only occur at heterogeneous surface. it can never take place in a gas. The reason is that the IR density of states in a gas is determined solely by its temperature; the Law of Equipartition of Energy.
Kristian:
You have gone beyond ridiculous now. Let’s recap:
In your comment on August 13, 2014 at 1:05 am, you said:
“The mean upward transfer of energy by radiation from the global surface would be 63 W/m^2 in both cases [transparent and radiatively active atmospheres], Curt. And only that. As long as the average incoming from the Sun is still 161 W/m^2 and the mean conductive/evaporative energy loss rate is 97 (98) W/m^2, the surface can’t put out more energy than that by radiation.”
In my comment on August 13, 2014 at 11:24 pm, I cited the time and date of your comment and explicitly quoted you, adding the words in the square brackets you see above so that others could get the context.
I responded with the following comment:
“You are completely missing the very elementary fact that if the atmosphere cannot transfer any energy to space (which it could only do by radiation), it cannot on any ongoing basis get transferred to it energy from the earth’s surface. So to say that it would accept an average 97 W/m^2 on an ongoing basis from the surface when it radiates 0 W/m^2 to space is simply ridiculous.”
I even gave you the benefit of the doubt by analyzing two cases for an atmosphere transparent to LWIR, one where it is not completely transparent to solar radiation (reading you literally) and one where it is (what you may have really meant):
“If the atmosphere absorbed nothing in the visible spectrum as well as LWIR, the surface would absorb an average 240 W/m^2. Over the long term, the atmosphere would neither transfer energy to or from the earth’s surface, the only entity it could exchange energy with. The earth’s surface would need to be able to reject an average of 240 W/m^2, and the only avenue for it to do so is by radiating to space (which has an effective radiative temperature of ~0K, so it does not matter whether we look at it as uni-directional or bi-directional). So it would have to have a temperature of 255K (or less if varying) to do this.”
“If the atmosphere absorbed 79 W/m^2 of solar radiation (with 161 W/m^2 absorbed by the surface) but had no way of radiating LWIR to space, the earth’s surface would still need to radiate 240 W/m^2 to space, with the atmosphere transferring 79 W^2 to the surface (so there would be a temperature inversion in the atmosphere).”
Despite my very careful practice to quote you precisely, you accuse me of not doing so.
And now you have the unmitigated gall to accuse ME of making the mistake YOU made and I called you on. You said in your comment of August 14, 2014 at 8:21 am:
“If the atmosphere absorbs say 100 W/m^2 from conductive/convective heat transfer on a daily basis from the surface, but cannot radiate any of it away to space, how do you suggest the Earth system gets rid of it?”
Re-read the top quote of your comment in this post. YOU made the claim that a transparent atmosphere could absorb a steady flux of conductive/convective heat transfer from the surface even though it would have now way of rejecting that power anywhere, not ME. I pointed out how erroneous this analysis was, and now you accuse ME of the error!
Last year at Jeff Id’s blog, I made this comment about arguing with folks like you:
” You point out an inconsistency in their arguments, and they treat it as your inconsistency.”
You’ve just provided another example of this. You have brought this down to the level of the Monty Python “argument” sketch.
“You are completely missing the very elementary fact that if the atmosphere cannot transfer any energy to space (which it could only do by radiation), it cannot on any ongoing basis get transferred to it energy from the earth’s surface.”
Are you saying that the atmosphere can’t get heat from the surface by any means other than radiation? Because that is axiomatically false. The only reason the atmosphere can only lose energy to space in the form of radiation is because it is effectively a vacuum so no meaningful convection can occur… but I’d wager that some actually does occur (along with outgassing).
Alec M 1:57pm: No. The reflectivity of a polished brass tube is near unity – most of the incident photons live on, the rest of the photons are absorbed ceasing to exist, their energy living on, none are transmitted, none are “thermalised” in the lab.
rgb 3:31pm: “Um, you (Trick) do know about phase transitions, right?”
Yes.
“The melting ice is indeed being heated…”
Um, you do know heat doesn’t exist, right? Have you missed the events of the demise of the caloric theory? Heat is deceased, it has ceased to exist in modern natural science. Though like Monty Python’s dead parrot, some portray heat as being alive to make a sale. Energy & enthalpy live on.
“its temperature does not change because heat is not the same thing as internal energy!”
Um, you do realize per what you wrote 3:09pm this really is in modern science:
…temperature does not change because internal energy is not the same thing as internal energy!
You will want to re-think & restate that after thoroughly consulting with a thermo. prof.
“In particular, you are ignoring the change in entropy.”
True, as it is well known entropy increases as ice melts; I did not ignore the change in conserved enthalpy which is the explanation needed.
“Maybe you should take a thermo course at some point?”
Done. Passed. I recommend you catch up on your study by reading Bohren 1998 Atm. Thermo. text for thermodynamic internal energy and the 1st law with regard to demise of heat & follow-up on ice/water mixtures p. 22-28, p. 221-2 for ice-water equilibrium, then apply your learning to the freezing of lakes p. 355 for the thickness of ice with time. Very interesting and informative thermo.
looncranz: You ask: “Are you saying that the atmosphere can’t get heat from the surface by any means other than radiation?”
No! Please read what I said carefully. It was said in response to Kristian’s claim upthread that with a transparent atmosphere, the surface would still have a “mean conductive/evaporative energy loss rate of 97 (98) W/m^2” to the atmosphere.
I was pointing out that if the atmosphere had no way of rejecting an equivalent power flux, it could not continue to receive this from the surface. (If it started out colder than the surface, it could do so for a while, but eventually it would become as hot as the surface and these transfers would stop.)
In our atmosphere, the (poorly named) “greenhouse gases” have the ability to radiate power to space, so the atmosphere in general stays cooler than the surface and can absorb power from the earth’s surface conductively/convectively on an ongoing basis. But these same radiatively active gases keep the earth’s surface from radiating directly to space at many wavelengths.
Okay, so you are saying surface directly to space, the /other/ way of interpreting the quoted statement 😉
Very well, carry on good sir.
Curt says, August 14, 2014 at 7:43 pm:
“You have gone beyond ridiculous now.”
You always have to start out with an insult, don’t you, Curt? Just to try and establish some kind of superiority. Your game here is pretty transparent, you know that?
You have no case. You studiously avoid reading what I’m saying, the substance of my argument, rather picking small parts here and there that you feel you can argue against and stick to that, so as to divert from the main thrust of what I’m saying as much as possible.
You clearly know all the alarmist tricks.
Curt says, August 14, 2014 at 8:51 pm:
“In our atmosphere, the (poorly named) “greenhouse gases” have the ability to radiate power to space, so the atmosphere in general stays cooler than the surface and can absorb power from the earth’s surface conductively/convectively on an ongoing basis. But these same radiatively active gases keep the earth’s surface from radiating directly to space at many wavelengths.”
No, this is exactly what I’m pointing out to you that you seem completely blind to. It’s the very presence of the mass of the atmosphere that keeps the Earth’s surface from radiating its entire flux directly to space, Curt. Because it prevents the surface from ever reaching a purely radiative equilibrium with its heat source, the Sun. Because a large portion (the main portion, in fact) of the surface energy is transferred away through conduction/evaporation > convection. The surface energy is automatically brought up into the troposphere by convective processes. This is why Earth’s surface itself cannot radiate the final system flux to space.
The IR-active gases are just there, to do the job.
“It was said in response to Kristian’s claim upthread that with a transparent atmosphere, the surface would still have a “mean conductive/evaporative energy loss rate of 97 (98) W/m^2″ to the atmosphere.
I was pointing out that if the atmosphere had no way of rejecting an equivalent power flux, it could not continue to receive this from the surface. (If it started out colder than the surface, it could do so for a while, but eventually it would become as hot as the surface and these transfers would stop.)”
Yes, this is one of the great, poorly concocted fairy-tale stories of ‘Climate (Pseudo)ScienceTM’: If the atmosphere can’t cool by radiation to space, then this means in the end that the Earth system will become cold, not hot, because, because, at some point conductive>convective transfer from the surface will just stop – gone into thin air – and it will rather simply start radiating it all directly to space, cooling back to a steady-state temperature of 255K. Yippee!
This is what is beyond ridiculous, Curt! This story makes so many un-physical, unreal assumptions to get where it wants, that even a schoolchild should have no problems spotting them.
Addressing a few of these assumptions:
# How and why would the conductive/convective transfer of energy from surface to atmosphere just stop at some point? When is this point reached? And, more importantly, how do we stay there? You know that the atmopshere is free to expand, don’t you? That the air away from the solar-heated surface gets progressively thinner and therefore naturally colder the higher you go? You know also that the Sun never stops shining down on the surface? Why would the transfer stop before the surface had grown so hot and the atmosphere had grown so far as to start eroding into space?
# At the point where this peculiar non-conductive situation is seemingly reached, does the Earth system simply switch to full radiative loss from the surface? Does this situation all of a sudden turn into a pure radiative situation? Conductive/convective transfer to the fluid is forever eliminated as a mechanism for transfer of energy? How does this work? How is this maintained? The atmosphere is still there. The Sun is still shining. The surface still naturally ‘wants’ to shed energy to the atmosphere.
# So when the surface now all of a sudden is able to radiate the entire solar flux to space, does the enormous surplus of energy already in the atmosphere slowly convect/conduct back down, initially turned into a second heat source for the surface, by being warmer than the directly radiatively cooled surface, then finally reaching the same isothermal temperature of 255K? So at this point NO energy from the Sun passes from the surface at 255K to the atmosphere at 255K? As if the atmosphere weren’t there at all!
You can’t just block conductive/convective energy loss from a constantly heated surface surrounded by a fluid and expect to be rid of it, simply set it to zero and say “Now it’s gone, out of business.” and that now this situation is suddenly a purely radiative one, like a BB radiating directly into space, as if the massive atmosphere itself were gone all of a sudden, Curt.
You seriously don’t see the absurdity in this story? It’s just a story you throw out without even thinking it through. You’ve created a hyothetical bubble world, Curt, where even for the surface in the end nothing matters but radiation. Because the CO2 story NEEDS for this to be the case. It’s not. Reality doesn’t work like this. It’s all about convection. The mass of the atmosphere. You cannot ignore it. Come down from your pink little radiant cloud, down to Earth.
I explained to you how the world really works, Curt, here:
http://wattsupwiththat.com/2014/08/10/the-diminishing-influence-of-increasing-carbon-dioxide-on-temperature/#comment-1708851
I’d rather you address this. A much more interesting (and hopefully fruitful) discussion. Rather than your convenient dabbling in hypothetical scenarios that you clearly have no grasp of, but which can never be fully resolved because they can never be properly tested, so you can just go on and on with your nitpicking.
Listen. Energy is released from the solar-heated surface into the surface air layer through three different mechanisms: conduction, radiation and evaporation. But when the energy in this way is transferred to the air, convection takes over and transports it up through the tropospheric column towards the tropopause, from where it can be radiated to space. It’s as simple as that.
Curt, meet reality.
For a fluid in a gravity field and heated from below: Heating at the bottom (energy IN) > energy moving with the fluid up > cooling at the top (energy OUT).
Curt says, August 14, 2014 at 8:51 pm:
“In our atmosphere, the (poorly named) “greenhouse gases” have the ability to radiate power to space, so the atmosphere in general stays cooler than the surface and can absorb power from the earth’s surface conductively/convectively on an ongoing basis.”
You clearly don’t even understand the corollary of what you’re saying here.
Here’s the gist: Since convection is the ultimate cooling mechanism of a solar-heated surface beneath a massive atmosphere, somehow blocking for it will cause heating, not cooling. The IR active gases strengthens the convective cooling of the surface by radiating the surface energy at altitude to space. If you then take out these gases, efficient convective cooling of the (still) solar- heated surface would be impeded, and solar energy would thus automatically accumulate, making the surface warmer to reestablish (maintain) the temperature gradient. It wouldn’t respond by simply letting radiation take over. Even worse, by cooling below the original temperature (with convection working) as it did. No, the surface would naturally get hotter and hotter in order to strengthen convection to prevent the temp gradient from buckling, the atmosphere progressively expanding in the process. You see, the surface will automatically work this way, desperately trying to cool conductively/convectively to the atmosphere directly on top and will therefore accumulate the solar energy coming in until it can.
This ongoing nonsense about blocking for convection, the ultimate cooling mechanism of a heated surface surrounded by a fluid, and then get this surface to COOL (not heat) by just switching to radiation through the fluid instead, no more convection needed, is quite frankly sad to behold. It’s the perfect radiative delusion.
AlecM says:
August 13, 2014 at 10:29 am
“@Samuel C Cogar: there is an appalling lack of knowledge about radiative physics.”
—————-
I agree with your above, …… but I also think you really should have addressed the rest of your commentary to, … to wit:
matayaya says:
August 12, 2014 at 12:48 pm
Matthew R Marler says:
August 14, 2014 at 10:43 am
“That’s not so different from “trapping and releasing”. When I release animals back to the wild, I expect most of them get eaten by predators and few survive”
—————
Don’t be talking silly at me.
The Manager would throw that baseball player outta the game like “lickety split” iffen he held onto that baseball glove’s “absorbed” ball for more than a “New York instant”.
“HA”, and probably do the same iffen that baseball player emitted a “wild” throw or wildly threw it into the “wild”.
Kristian says, August 14, 2014 at 3:44 am:
“#8) The absorptivity of the air + the natural mechanism of buoyant/convective uplift upon warming (relative to surroundings) precludes ‘back radiation’ from ever having a noticeable influence back down towards the surface. The [radiative energy] is ‘forced’ to leak upward towards space, because that’s the way the convective conveyor belt moves. This is a fine-tuned machinery designed for the Earth to be able to rid itself of as much energy as it gets in.”
“#5) 4a+b automatically and naturally initiates convective uplift. In other words, the [energy originally transferred from the surface] is brought up and away from the surface air layer, into the atmosphere at large, by the process of convection – the bulk movement of air. This is, in simple terms, how energy is transported through a fluid: Heating (energy in) at the bottom > energy moving with the fluid from bottom to top > cooling (energy out) at the top.”
“[52.4 W/m^2 is] all the energy [radiative transfer from the solar-heated surface] has at its disposal. [None] of this energy returns to the surface. It is absorbed by the surface air, brought up by convection and emitted at altitude to space. Just like the conductive heat and the latent heat [from the surface]. That’s the way the energy from the Sun moves through the Earth system.”
Consider a pot full of water heated externally from the bottom and assume that the only way this body of water could ever rid itself of energy is through radiation.
How would this work?
The energy is coming IN from the external heat source at the bottom, absorbed by the lowermost layer of water in the pot, which would automatically warm, get slightly less dense and rise as a result. An upward convective current would thus naturally be established through the water column. The external energy originally brought into the volume of water from the heat source at the bottom would be – after initial absorption – transported up and away from the lowermost layer to spread through (mixed into) the volume at large, creating a temperature gradient in the process. In this way, the total volume of water would slowly be heated, not just the lowermost layer.
From wikipedia:
http://en.wikipedia.org/wiki/Natural_convection
“In natural convection, fluid surrounding a heat source receives heat, becomes less dense and rises. The surrounding, cooler fluid then moves [in] to replace it. This cooler fluid is then heated and the process continues, forming a convection current; this process transfers heat energy from the bottom of the convection cell to top. The driving force for natural convection is buoyancy, a result of differences in fluid density.”
But, you could say, there is also radiation going on all the time. All matter with a temperature radiates according to this temperature. And this radiation would be emitted in all directions – sideways, back, forth, up and down.
So the actual heated bottom of the pot would not just conduct energy as heat to the water. It would also radiate energy as heat.
Sure. The point is, the water would always absorb this radiation immediately. Liquid water is practically 100% opaque to IR (yes, it can move a bit further if the source is red hot, but certainly not much).
So what happens?
Yes, the water molecules always try to radiate (and they do it too), but as soon as the radiation is emitted, it’s reabsorbed and moved a bit higher through the parallel convective uplift going on. It can’t get anywhere by itself, either to the sides, down or up. Likewise the water molecules will try all the time to conduct energy away through collisions, to the sides, up and down. And they indeed do it. But it amounts to nothing. Conduction doesn’t manage by itself to actually move the energy anywhere, because as soon as it’s transferred from one molecule to the next, both molecules are moved higher no matter what happens, by the parallel convective uplift through the water column.
In other words, the actual movement of energy through the volume of water, away from the heating at the bottom, is done by bulk movement – convection. That’s a specialty of fluids. Microscopic radiative and conductive attempts at propagating some energy back down against this steady upward flow are futile. The natural and automatic process of convection simply sees to it that even these microscopic energy transports are always biased towards a relentless ‘leaking’ upwards, away from the source of the heating. Only the heating rate at the bottom and the cooling rate at the top ultimately matters to how fast the fluid/energy moves.
At the top of the water column, the convective current can’t move any further up. The radiation (and conduction) has simply been along for the ride up until this point. But here we’ve finally reached the ‘top of convection’. There is no more liquid water above to immediately absorb the radiation emitted by the water molecules. So the radiation being emitted UP from this level will be able to escape the volume altogether, out from the top surface. Into ‘space’.
As the water thus ‘dumps’ its energy at the top surface, it cools and sinks back down to the sides as a result, making way for new warm water coming up from below, becoming part of the already established convective cell.
This is in principle exactly how our atmosphere works also. Only the IR opacity is less complete (a fair share goes through the atmospheric window directly from the heated surface to space). AND, the atmosphere is 1) gravitationally getting less dense the higher up you go, and 2) capable of expanding upon heating, the body of water hardly at all.
I would once again encourage curious readers to peruse this article by the Chiefio – an excellent, empirically based exposition of how the atmosphere really operates, what matters and what doesn’t inside the troposphere:
http://chiefio.wordpress.com/2012/12/12/tropopause-rules/
I would also like to repeat what Sir George Simpson of the Royal Meteorological Society pointed out to Guy Callendar in 1938. It’s quite pertinent to what I’ve been saying here (and to Chiefio’s post above) about radiation vs. convection in the troposphere:
“…but he would like to mention a few points which Mr. Callendar might wish to reconsider. In the first place he thought it was not sufficiently realised by non-meteorologists who came for the first time to help the Society in its study, that it was impossible to solve the problem of the temperature distribution in the atmosphere by working out the radiation. The atmosphere was not in a state of radiative equilibrium, and it also received heat by transfer from one part to another. In the second place, one had to remember that the temperature distribution in the atmosphere was determined almost entirely by the movement of the air up and down. This forced the atmosphere into a temperature distribution which was quite out of balance with the radiation. One could not, therefore, calculate the effect of changing any one factor in the atmosphere …”
(Thanks, Konrad.)
Kristian says:
August 14, 2014 at 6:28 am
Kristian says, August 14, 2014 at 3:44 am:
“So you see, Curt, the 52-53 W/m^2 (or your 63) is the amount of energy that goes out from the surface by radiation to begin with. No more, no less. That’s radiation’s contribution to the surface energy balance between IN (from the Sun) and OUT (to the atmosphere/space). That’s all the energy it has at its disposal. Nothing of this energy returns to the surface. It is absorbed by the surface air, brought up by convection and emitted at altitude to space. Just like the conductive heat and the latent heat. That’s the way the energy from the Sun moves through the Earth system.”
The nonsense about the Earth’s surface having to radiate directly according to its temperature is based solely on a fundamentalist – and ultimately flawed – interpretation of the Stefan-Boltzmann equation: P/A = es*T^4. This equation specifically describes a situation where a black (gray) body is radiating into a perfect heat sink at 0 K. It is only applicable when the object radiating can be considered a ‘pure radiator’; either it faces no temperature at all or it is much, much hotter than its surroundings so that surrounding temperatures can be ignored (approximated as zero). In this case all we need to know is the temperature (and the emissivity) of the object radiating. Output (P/A) and temp is directly related.
Output and temp is NOT directly related when the object radiating is surrounded by temperatures fairly close to its own. Then the radiative heat transfer equation applies: P/A = es*(Th^4 – Tc^4). The energy (radiative heat) output from the radiating (hot) object in this case (like with Earth’s surface) is directly related rather to the temperature DIFFERENCE between it and its surroundings.
No the full equation is:
P/A = es*(Th^4 – To^4)+kconvection.s(Th-Tc)+kConduction.s(Th-Tc)
To and Tc aren’t the same, Tc would be the temperature in contact with the object, To could be at a distance. In the case of the Earth Th^4-To^4 is much larger than Th-Tc
A classic application of this is a thermocouple in a flame in a furnace, Tw=Th.
The thermocouple measures a lower temperature than the gases it is immersed in because of the losses, convection to the gases and conduction to the support wires both depend on the diameter of the wire and so the true temperature is approached as the wire diameter is decreased so one way to assess the error is to measure with different sized thermocouples in close proximity and extrapolating to zero diameter. That still leaves the radiative losses, these are dealt with by putting a radiation shield around the TC(a quartz tube for example), this reaches a much higher temperature than the distant wall and the error is much reduced.
In the case of the Earth there is only radiative loss so direct application is possible, the complication is that not all wavelengths are emitted from the same ‘surface’, that is the role of the GHG. The losses from the actual surface are more complicated.
“In short, they forgot to identify what actually happens before they made their ‘mental model’ and played with it.”
Curt. That’s you he’s talking about.
AlecM says:
August 14, 2014 at 1:57 pm
@trick: the IR absorptivity of a polished brass tube is ~1.0.
Try again.
PS Thermalisation of absorbed EM energy can only occur at heterogeneous surface. it can never take place in a gas. The reason is that the IR density of states in a gas is determined solely by its temperature; the Law of Equipartition of Energy.
No, for a gas molecule in equilibrium when it absorbs a photon it becomes out of equilibrium and then collisionally gives up energy to its neighbors thereby returning to equilibrium i.e. thermalizes
george e. smith says:
August 10, 2014 at 10:08 pm
If the CO2 effect (on surface / lower tropo temperature) is logarithmic, the going from 280 ppm to 560 ppm should give the same temperature rise, as going from 1 ppm CO2 to 2 ppm; or for that matter, from one molecule of CO2 per cubic meter, to 2 molecules of CO2 per cubic meter.
CO2 temperature response IS NOT logarithmic.
Maybe it id “non-linear” ; but it ain’t logarithmic.
The logarithm function is very specific.
As I’ve explained here several times before the CO2 effect is not logarithmic at all concentrations, at the range we see in the present atmosphere it is a good approximation. At low concentrations CO2 is a weak absorber an has a linear response, as the concentration increases it becomes a moderate absorber and the effect is approximately logarithmic, a further increase to a strong absorber and you have a square root effect (as in the case of methane now). That’s one reason why the graph in the original post is misleading because it shows an asymptote instead of the transition to square root (steeper) dependance.
“””””…..Phil. says:
August 15, 2014 at 11:18 am
george e. smith says:
August 10, 2014 at 10:08 pm…..”””””
I know you have explained it several (many) times Phil.
Al low ? concentrations, linear (roughly), at medium concentrations logarithmic (roughly), and at high concentrations, square root (also roughly).
So in the transition zones between low, medium, and high; just what mathematical functions does it follow (roughly) ??
And over the presumably reliable period of the Mauna Loa recorded data, the CO2 has gone up about 26-27% of one doubling, for which the logarithm function is not appreciably different from linear, and the recorded data is not capable of discerning which.
I’ll agree it is non-linear, and follows (accurately), no known elementary function.
What purpose is served by asserting ANY function, which is not followed by measured data ??
We don’t have any observed atmospheric “low” abundance data, nor do we have any observed atmospheric “high” abundance data. So what relevance are those non existent regimes ??
“””””…..August 14, 2014 at 7:55 pm
Alec M 1:57pm: No. The reflectivity of a polished brass tube is near unity – most of the incident photons live on, the rest of the photons are absorbed ceasing to exist, their energy living on, none are transmitted, none are “thermalised” in the lab……”””””
The reflectivity of a polished brass tube, is nowhere near unity. Freshly evaporated silver, with a quartz overcoat is about 98% reflectivity, for central visible wavelengths, and 2-10 micron IR.
Protected Gold is around 97% from 0.8 to 2.0 microns, and only 94% at 0.7-0.8 microns.
Protected Aluminum, is 85% for 0.4 to0.7 microns.
Copper is worse than Aluminum, and brass mimics copper (polished). Both tarnish rapidly in air.
george e. smith 12:47pm: “The reflectivity of a polished brass tube, is nowhere near unity.”
No. Various sources put emissivity of polished brass tested at 0.03. Since no photon is transmitted, the reflectivity is 0.97 which is near unity not “nowhere near unity” as you write. Reflectivity + absorptivity + transmitivity = 1.0. .97+.03+0.0=1.0
Kristian 9:04am: For some reason you leave out Callendar 1938 replying to Simpson in agreement with the top post:
“..in replying (Callendar) realized the extreme complexity of the temperature control at any particular region of the earth’s surface and also that radiative equilibrium was not actually established, but if any substance is added to the atmosphere which delays the transfer of low temperature radiation, without interfering with the arrival or distribution of the (energy) supply, some rise of temperature appears to be inevitable in those parts which are furthest from outer space.”
@george e. smith 8/15 12:26 pm
We don’t have any observed atmospheric “low” abundance data, nor do we have any observed atmospheric “high” abundance data. So what relevance are those non existent regimes ??
We have evidence of “high” abundance regimes in the geological history of the Earth. Depending on your definition of “low” we have evidence of low abundance regimes, too. Getting a handle on the uncertainties associated with different extrapolations of a variety of potential functions of CO2 absorption strength might benefit work in paleoclimate studies. It would at least be a reminder of how much we don’t know.
@ Kristian’s quoted statement: August 15, 2014 at 9:04 am
“that it was impossible to solve the problem of the temperature distribution in the atmosphere by working out the radiation”
———–
Which is pretty much exactly what I have been saying for the past 10+- years, which is, to wit:
“I do not believe it is possible for anyone to measure the heating (warming) effect of the lesser quantity of gas (CO2) in a mixture of two different gases when the quantity of the greater volume of gas (H2O vapor [humidity]) is constantly changing from hour-to-hour, day-to-day and/or month-to-month.
Especially when said greater volume of gas (H2O vapor [humidity]) has a potentially 100+ times greater “warming” potential (40,000 vrs. 400) for said mixture than does the lesser volume of said gas (CO2) in said mixture”
——————-
@ Kristian — I really don’t have a problem in reading and understanding your commentary and/or your verbiage use therein simply because I read it “in context” as to what I perceived your intent was when you penned it …… and not “in context” of what I think you should have written nor the exact verbiage that I think you should have used when writing it.
Everyone knows the meaning of the words “hot”, ”cold”, “warm” and ”heat” simply because they are four (4) of the first six (6) words that they were nurtured the meaning of before they quit wearing diapers. The other two (2) are “ma-ma” and ”da-da”. And all of those words and their meanings are still “written” in the DNA of their brain’s neurons and the person still uses them quite frequently “in context” depending on who they are conversing with.
Anyone who dislikes and/or objects to the use of the word “heat” ….. should also dislike and/or object to the use of the word “greenhouse” …… because they are “two peas in the same pod”.
To the majority of the populace the word “greenhouse” means the same thing as the word “heat”.
george e. smith says:
August 10, 2014 at 10:08 pm
If the CO2 effect (on surface / lower tropo temperature) is logarithmic, the going from 280 ppm to 560 ppm should give the same temperature rise, as going from 1 ppm CO2 to 2 ppm; or for that matter, from one molecule of CO2 per cubic meter, to 2 molecules of CO2 per cubic meter.
That is what I was pointing out to Nick Stokes way upstream in this thread.
CO2’s effect may be close to logarithmic from approx. 20 ppm to, perhaps, 2560 ppm and then begin to trail off.
This appears to be just one more item of “settled science” that is not settled.
Moreover, this CO2 effect is being described in a purely hypothetical, perfect laboratory environment. Can the effect ever be totally realized in the ever churning, swirling gaseous atmosphere while under varying levels of cloud cover and cloud reflectivity, solar fluctuations, orbital variances, cosmic radiation, etc.?
I’m not saying there isn’t some effect, but it simply will not stand still long enough for us to measure it.
@ george e. smith says: August 15, 2014 at 12:26 pm
“Al low ? concentrations, linear (roughly), at medium concentrations logarithmic (roughly), and at high concentrations, square root (also roughly).
So in the transition zones between low, medium, and high; just what mathematical functions does it follow (roughly) ??”
—————
Keep asking those “good” questions, george e., ……… cause I enjoy watching them “wiggle n’ squirm” when attempting to think up an actual, factual and/or intelligently logical “answer” ….. to justify and/or explain the mimicry of their nurtured “religious” beliefs.
Saw this only now:
AlecM says, August 12, 2014 at 9:18 am:
“@davidmhoffer: to use your analogy with a resistor, ‘back radiation’ is a measure of potential, equivalent to the potential at one end of the resistor. It does not generate electric current. To do that you need a potential difference.
In the case of the Earth’s surface, the resistance is the process by which its heat is converted to electromagnetic energy. The rate of conversion of heat to EM energy is set by the difference of opposing radiative emittances (potential difference) at the surface plane.
Just remember this: ‘back radiation’ is like measuring a voltage. By itself it can do no thermodynamic work.”
Very well put.
And Hoffer’s response?
davidmhoffer says, August 12, 2014 at 11:04 am:
“It wasn’t an analogy. It was an example of something that Kristian claims doesn’t exist.”
Where do I claim that the POTENTIAL doesn’t exist, David? That’s exactly WHAT exists. The DWLWIR flow, the DWLWIR transfer of energy is what doesn’t exist as a real entity. Because, as Alec points out, you need a potential DIFFERENCE to generate flow. Just like an electric current. Or like wind. And the flow in the case of surface/atmosphere thermal exchange will then be the HEAT – the net of the opposing potentials. But you didn’t read this last part, did you David? Too much cognitive dissonance to handle for one day, I guess …
george e. smith says:
August 15, 2014 at 12:26 pm
“””””…..Phil. says:
August 15, 2014 at 11:18 am
george e. smith says:
August 10, 2014 at 10:08 pm…..”””””
I know you have explained it several (many) times Phil.
Al low ? concentrations, linear (roughly), at medium concentrations logarithmic (roughly), and at high concentrations, square root (also roughly).
So in the transition zones between low, medium, and high; just what mathematical functions does it follow (roughly) ??
Be my guest George:
http://www.physics.sfsu.edu/~lea/courses/grad/cog.PDF
And over the presumably reliable period of the Mauna Loa recorded data, the CO2 has gone up about 26-27% of one doubling, for which the logarithm function is not appreciably different from linear, and the recorded data is not capable of discerning which.
I’ll agree it is non-linear, and follows (accurately), no known elementary function.
What purpose is served by asserting ANY function, which is not followed by measured data ??
The log function is a result of the measurement for the concentration range present in the atmosphere.
We don’t have any observed atmospheric “low” abundance data, nor do we have any observed atmospheric “high” abundance data. So what relevance are those non existent regimes ??
Our atmosphere includes the results of the linear phase as well as the log phase, graphs such as the one showed in the original post here get it wrong because they assume the logarithmic dependence for all concentrations of CO2 and are therefore wrong.
Phil. says:
August 16, 2014 at 7:06 am
george e. smith says:
August 15, 2014 at 12:26 pm
“””””…..Phil. says:
August 15, 2014 at 11:18 am
george e. smith says:
August 10, 2014 at 10:08 pm…..”””””
I know you have explained it several (many) times Phil……”””””
Thanks for the math Phil; I have tucked it away for safe keeping.
One thing immediately stands out.
The entire outcome rests on the assumption of a unidirectional one dimensional path through a uniform slab of absorber, and presumes a Beer’s law Transmission.
Well, I think originally, Beer’s law was about absorption, and not transmission, so the transmission assumption, assumes that the photons, stay absorbed.
But they don’t, in the atmosphere. The absorbed photon is later re-emitted, perhaps frequency shifted, but in the same general neighborhood, and that re-emission is isotropic, not uni-dimensional, so the absorbed photons do not stay dead.
So it is the same case as a fluorescent absorbing medium. The incident spectral component, may be attenuated, but the net energy transmission is very much greater, because the photons are re-incarnated, and in the case of the CO2 and presumably other GHGs, the resurrection spectrum, is not greatly different from the incident one.
Which is why I say, the Beer-Lambert Law is invalid, for materials that re-radiate.
In the limit, where the absorbed energy is converted to heat (I don’t understand the QM of that ), some of the energy will propagate as thermal radiation due to the Temperature rise, and of course some of that heat would conduct or diffuse in all directions as well.
So the theory is pretty. It would be nice to know of any actual experimental observation, of a transmission conforming to that math, for ANY material example; even hydrogen. I can’t even imagine how one would experimentally verify such a transmission formula, in the lab.
But I do thank you for the paper. Sometimes I wonder what those line broadening expressions actually are.
For small values of (x), ln (1+x) = x , and also (1+x)^0.5 = 1+ x/2
So neither one is appreciably different from linear, for the range of x we have observed :
400/315 =1.27 ; ln (1.27) = 0.24 1.27^0.5 = 1.127
This is at least as close to linear as we can depend on data since IGY, 1957/58.
Kristian:
If you want to understand what effect something has, you need to understand what would happen without it. If you want to be able to start to analyze complex systems, you must be able to successfully analyze simple systems. This is why it is good to think about what would happen in a world with an atmosphere completely transparent to electromagnetic radiation.
You say:
“For a fluid in a gravity field and heated from below: Heating at the bottom (energy IN) > energy moving with the fluid up > cooling at the top (energy OUT).”
But for an atmosphere without radiatively active gases, there is no energy OUT. It has no way of transferring any energy out to space. This means, by absolutely trivial logic and calculation, this means that in the steady-state condition, there can be no energy IN.
You ask:
” How and why would the conductive/convective transfer of energy from surface to atmosphere just stop at some point? When is this point reached? ”
Do you really not know the answer to this? This point is reached when the temperature of the atmosphere, at the surface at least, reaches the temperature of the surface. When they are at the same temperature, heat transfer stops. If the atmosphere started out cooler than the surface, it would warm until it got to this temperature. If the atmosphere started out warmer than the surface, it would cool until it got to this temperature.
You then ask:
“You know also that the Sun never stops shining down on the surface? Why would the transfer stop before the surface had grown so hot and the atmosphere had grown so far as to start eroding into space?”
Again, do you really not know the answer to this? It’s because the surface, unlike an atmosphere without radiatively active gases, has the ability to radiate power to space. Fundamentally, the surface under these conditions would rise to the temperature at which its output radiative power flux matches the solar input power flux.
Once again, you manage to get things completely backwards, believing that a radiatively inactive atmosphere has the ability to transfer power to space, but a radiatively active surface with close to blackbody emissivity does not!
Next you ask:
“At the point where this peculiar non-conductive situation is seemingly reached, does the Earth system simply switch to full radiative loss from the surface? Does this situation all of a sudden turn into a pure radiative situation? ”
Have you never solved any coupled system of differential equations? Let’s review what goes on for this planet with a transparent atmosphere. The radiative power loss from the surface is (approximately) proportional to difference in the 4th powers of the surface temperature and the effective temperature of deep space. The conductive/convective power loss from the surface is (approximately) proportional to the difference in (the 1st powers of) the surface temperature and the atmospheric temperature at the surface.
A transparent atmosphere’s only power transfer mechanism is the conductive/convective one with the surface. If it is cooler than the surface, it will accept power from the surface, and its rate of change of temperature is this amount of power divided by its thermal capacitance. Because the power transfer is proportional to the difference in temperature, as the atmospheric temperature approaches the surface temperature, the rate of change of temperature decreases gradually, not “all of a sudden”. (As the solution to a first-order differential equation, it decreases on a negative exponential curve.) When atmospheric temperature reaches the temperature of the surface, there is no more power transfer and no more temperature change.
The surface has both the conductive/convective transfer with the atmosphere mentioned above (but any energy gain by the atmosphere is energy loss by the surface) and the radiative power transfer with space, so it is a little more complicated. But still, computing a steady-state temperature in response to a constant solar input is not difficult.
You continue:
“How is this maintained? The atmosphere is still there. The Sun is still shining. The surface still naturally ‘wants’ to shed energy to the atmosphere.”
This state is maintained because the surface radiative losses to space match the incoming insolation and the atmospheric temperature matches the surface temperature. When this happens, the surface no longer “wants” to shed energy to the atmosphere.
Next you ask:
“So at this point NO energy from the Sun passes from the surface at 255K to the atmosphere at 255K? As if the atmosphere weren’t there at all!”
The answer to your question is simply YES — no energy passes from the surface at 255K to the atmosphere at 255K. Why would it, when there is no temperature difference? You know the equations — USE THEM! This should be an absolutely trivial point, but you get it completely wrong. And yes, in the steady state it is “as if the atmosphere weren’t there at all!”
Then you assert:
“It’s the very presence of the mass of the atmosphere that keeps the Earth’s surface from radiating its entire flux directly to space.”
Now you’re just making s** up. The mass of the earth’s atmosphere keeps radiation from leaving like a black hole does???
You say:
“Consider a pot full of water heated externally from the bottom and assume that the only way this body of water could ever rid itself of energy is through radiation.”
and go on to explain how convection cells form. And this would explain the behavior of an atmosphere that could NOT rid itself of energy just HOW????
I could go on and on and on, but there really is no point. Virtually every assertion of yours is based on a fundamentally flawed analysis. If you get the analysis of simple systems completely wrong, you cannot hope to start on the analysis of complex systems like the earth where many things are going on.
And yes, in the earth’s system, net convective/conductive power losses from the surface are highly significant. And analyzing how they might vary with changing radiative properties of the atmosphere is not at all easy or obvious, despite the claims of the alarmists.
But until you realize that these net transfers can only occur because of the radiative absorption of the atmosphere, and serve to lessen but not eliminate the temperature increase due to that radiative absorption, you cannot even get started on that analysis. And you allow the alarmists to say that skeptics don’t even understand basic physics. And that is what is so sad.
Curt says:
August 17, 2014 at 8:35 am
“If you want to understand what effect something has, you need to understand what would happen without it. ….. This is why it is good to think about what would happen in a world with an atmosphere completely transparent to electromagnetic radiation.
But for (a world with) an atmosphere without radiatively active gases, there is no energy OUT. It has no way of transferring any energy out to space. This means, by absolutely trivial logic and calculation, this means that in the steady-state condition, there can be no energy IN. ”
———————
Careful bout that now, ….. your above statement would only be true iffen the surface of said “world” was a perfect reflector of electromagnetic radiation.
But the earth’s surface is not a perfect reflector of electromagnetic radiation, but on the contrary, a pretty good absorber of said. Thus, there is energy IN and the surface temperature increases. And the surface is also a good emitter of IR, thus there is energy OUT.
Thus if the earth’s atmosphere was devoid of any radiatively active gases the aforesaid IR emissions would propagate directly into outer space. But the earth’s atmosphere will still “warm up” as a result of direct conduction of the thermal energy from the surface to the other gas molecules in the atmosphere (O2, N2). And those gas molecules in the atmosphere can “cool down” via conduction of their thermal energy back to the surface …… to be rid of via the aforesaid IR emissions. To wit:
———————-
“4. Conclusion
In summary, if there is no radiation source, CO2 approaches 0 K because of its emission;
absorption of the thermal radiation from the earth ground surface rises CO2 temperature from
-273.15°C to -78°C only. Carbon dioxide gains heat by molecular collision from nitrogen
and oxygen, and dissipate the gained heat by radiative emission. Considering gases are far
more effective than bulky objects in heat dissipation by emission, one would not surprise to
realise that it is non-radiative nitrogen and oxygen gases that award the Earth a warm liveable
near surface atmosphere”. http://www.tech-know-group.com/papers/JCao_N2O2GreenGases_Blog.pdf
Do we know why it is hot enough on the surface of Venus to melt lead?
Because of the planet’s extraordinary albedo, which makes it one of the brightest “stars” in the sky, less solar heat reaches the surface of that planet than reaches the surface of the Earth, per square foot.
The atmosphere of the planet, however, is over 90% carbon dioxide.
All this would seem to challenge that thesis that increasing concentrations of atmospheric CO2 have decreasing effects on global temperature.
“All this would seem to challenge that thesis that increasing concentrations of atmospheric CO2 have decreasing effects on global temperature.”
It would if Venus didn’t have such high atmospheric pressures. Model the earth’s atmosphere at 93 bar and see what happens 😉 oh, and add some nice 200MPH+ constant winds for some nice adiabatic forcing.
Warmists like to claim that Venus’s atmosphere was once like Earth’s but that a runaway greenhouse effect made the planet what it is today… but I know of not a single model that can account for that or that would say that Venus /should/ have had an earth-like atmosphere at any point. In science, after-all, the evidence is suppose to create the theories.
Samuel:
You misquote me by inserting the clause “a world with”, and then claim I am in error!
I said, “But for an atmosphere without radiatively active gases, there is no energy OUT.
The is no mechanism for energy OUT of the atmosphere to space. And that is correct.
Now that this thread has reached nearly 500 responses and has slowed down, I would like to ask my question again, as I’d really be interested in finding an answer.
Hoskins says this in his original essay:
“The recent IPCC report now admits that currently increasing CO2 levels are probably only ~50% man-made.”
Does anyone know where in AR5 (chapter, page) this 50/50 admission can be located?
@ Curt:
I inserted that clause in parentheses therefore I was not misquoting you. My reason for doing so was to clarify your use of the word “atmosphere” for those spectators who might not be learned in/on the subject being discussed.
To wit:
at•mos•phere – noun: atmosphere; plural noun: atmospheres
1. the envelope of gases surrounding the earth (world) or another planet.
Jesse Fell says:
August 18, 2014 at 5:34 am
“Do we know why it is hot enough on the surface of Venus to melt lead?”
—————
“Yes”, we do know.
And one (1) of the primary factors responsible for the temperature of Venus’s atmosphere ….. to add to what ….. looncraz said: August 18, 2014 at 7:49 am
is the axial rotation of the planet Venus ….. Or the length of one (1) Venus day.
It takes 243 Earth days to rotate once on its axis ….. which means that +-50% of Venus’s atmosphere is being subjected to …. 121.5 earth days of continuous intense solar radiation.
Just imagine how “HOT” it would get in New York City or Miami iffen they were subjected to 121.5 continuous 24 hour days of intense bright Sunshine (solar radiation).
Samuel, I’m not sure I find that explanation convincing, for the following reasons:
1) While one side of Venus is continuously exposed to sunlight for 243 days, the other side is in darkness. If, owing to the length of Venusian days, one side of the planet is always smoking hot, wouldn’t the other be cold — and wouldn’t there be at least some exchange of temperatures between the day and the night of Venus? At any rate, the length of the Venusian day has no effect on the total amount of heat that it receives from the sun.
2) Owing to the density of Venus’ cloud cover, the amount of solar heat reaching the surface of the planet is a small fraction of the amount of heat that reaches the surface of the Earth — even though Venus is much closer to the sun. The albedo effect of Venus’ dense cloud cover — which makes it the brightest “star” in the sky — is extraordinary — like nothing on Earth.
3) Do you really think that the tremendous concentration of CO2 in the atmosphere of Venus (98%) can be dismissed as a factor in making Venus hot enough to make iron glow dull red?
At any rate, I kind of wish I didn’t know any of this stuff about Venus. “Perelandra” will never read the same again.
@ Rick Cina
Sorry, but I can answer your question.
I have never read any of the IPCC reports …. and have no inclination of ever doing said.
@ Jesse Fell:
“Climate and weather
Winds of about 224 mph (360 kph) keep the clouds of Venus in constant motion. Though the planet spins slowly, only once every 243 Earth days, the clouds zip around the top of the planet every four days. But wind speeds drop closer to the surface, where they only move a few miles per hour.
On Earth, seasons change based on the planet’s tilt; when a hemisphere is closer to the sun, it experiences warmer regions. But on Venus, most of the sun’s heat fails to make it through the thick atmosphere. As such, the planet not only doesn’t experience significant temperature changes over the course of the year, it also keeps things constant from night to day.”
http://www.space.com/18527-venus-atmosphere.html
Jesse, …… “most of the sun’s heat” …… doesn’t mean “all of the sun’s heat”, …. and “fails to make it through the thick atmosphere” means it doesn’t make it directly to the surface of the planet.
Samuel, That’s true. Midday on Venus looks like twilight here, so at least some light is getting through the atmosphere. But I’ve read that the amount of solar heat that reaches the surface of Venus per square unit of measure is a fraction of what reaches the surface of the Earth. If that is the case, how can it be hot enough to melt lead on the surface of Venus? The only explanation that I have read about — and it’s a very plausible one — is that atmosphere of Venus, being 98% CO2, creates a terrific greenhouse effect.
“The only explanation that I have read about — and it’s a very plausible one — is that atmosphere of Venus, being 98% CO2, creates a terrific greenhouse effect.”
The same reason only a portion of the sunlight reaches the surface is the same reason the surface is so hot. The surface can’t convect or radiate its heat away. This would be true of pretty much any 93 bar atmosphere, I’d think. Imagine our atmosphere at 1300 PSI (and also more mass). Sulfuric acid clouds are effectively opaque, so UV gets in, does its thing, downshifts in frequency, and can’t back get out.
I read a detailed examination once that normalized Venus to Earth pressures and couldn’t discern if CO2 was responsible for the heat (gases act differently as different pressures and concentrations). The lack of water vapor remaining prevents the positive feedback mechanisms claimed, and Earth’s wealth of water and magnetic field would prevent that from happening here, though many try to equate the two planets for their own agendas.
Curt says, August 17, 2014 at 8:35 am:
“But for an atmosphere without radiatively active gases, there is no energy OUT. It has no way of transferring any energy out to space. This means, by absolutely trivial logic and calculation, this means that in the steady-state condition, there can be no energy IN.”
Haha, I see Curt is still on with his magic pink dragons and unicorns climate physics.
How does he expect this particular (expandable) system to ever reach a steady state before it’s blown off into space, if it is not able to get rid of the energy constantly provided to it? Heat stops flowing (steady state) between two systems when there is only the two systems involved, Curt. When there ISN’T energy constantly being added from a third system, an external heat/power source, to the one system.
Curt is practicing the classical circular AGW way of doing ‘science’ by postulating his own premise as fact in order to show himself to be correct. Yup, there will be a steady state (because I say so), and FROM THAT you can see that I’m right. Priceless!
It’s not like the Sun stops shining, Curt. It’s not like the surface will all of a sudden find itself in a stable, purely radiative (BB) situation if you simply put a stop to conduction>convection. You can’t just set conductive/convective losses to zero and be rid of them. The real world doesn’t work like a mathematical equation; just strike out conduction/convection to get where we wanna be – radiation only. If you suppress natural conductive>convective transfer of energy from a constantly heated surface surrounded by a fluid, you will get warming, not cooling. Why? Because the energy from the heat source will accumulate at that surface until such transfer is restored. Naturally. Automatically. It’s what happens in the real world, Curt.
Energy from the surface could only completely bypass the atmosphere through radiation if it were much, much hotter than the atmosphere. It could hypothetically do this because conductive/convective loss grows linearly while radiative loss grows exponentially. But such a situation could never occur. The Earth couldn’t reach a state where its surface is at say 1000K and its atmosphere at a mere 300K. Because the two are directly convectively coupled. There would naturally be a gradual temperature gradient between them. Either there wouldn’t be an atmosphere in this case or the surface would have to be much, much cooler.
The surface energy, however, could not completely bypass the atmosphere through radiation if it were cooler, at the same temperature, or just a little bit warmer than the atmosphere above … In these cases the conductive/convective mechanism would be way too significant. The first two conditions would be highly unstable and could not be maintained, the latter one is similar to the one we have on our real planet, where our surface could at most let 52-53 W/m^2 bypass the atmosphere through radiative heat loss directly to space.
Kristian says, August 20, 2014 at 6:57 am:
“The Earth couldn’t reach a state where its surface is at say 1000K and its atmosphere at a mere 300K.”
Or 255K vs. 75K for that matter.
Hehe, sorry, forget about my two last posts (not the third last!). They refer to the situation where the temperature of an object’s surroundings can be ignored in a radiative exchange.
No, a heated surface surrounded by a fluid simply needs to be very hot, period, for radiative loss to dwarf conductive/convective loss to such an extent that it can be considered a pure radiator. Compare to a bonfire or a candle flame.
Jesse Fell says:
August 20, 2014 at 1:20 pm
The surface pressure of Venus’ atmosphere is about 93 times Earth’s. Mars’ atmosphere is also mainly CO2, but only with 0.06% Earth’s mean atmospheric pressure, so it’s a lot colder there, more than just lower insolation can account for.
At the point in Venus’ atmosphere where its pressure is one bar, its temperature is about the same as Earth’s average.
sturgishooper 1:33pm: “At the point in Venus’ atmosphere where its pressure is one bar, its temperature is about the same as Earth’s average.”
As the top post indicates, there is a diminishing influence of increasing ppm CO2.
And it is well known for long time, there is a big difference in general temperature between Earth & Venus at 1bar. Check out the 1 bar T difference shown in Fig. 1 here, T profile defined in papers cited in SI S.6 as early as 1997.
http://faculty.washington.edu/dcatling/Robinson2014_0.1bar_Tropopause.pdf
IGL P=density*R*T can be used further since the local density v. height was measured by Venus satellite radio occultation experiments. If input the measured density differences at 1bar, then find the same T since ideal gas law (IGL) works close approximation at both sites as should be expected, no big surprises.
******
Jesse 1:20pm: “The only explanation that I have read about — and it’s a very plausible one — is that atmosphere of Venus, being 98% CO2, creates a terrific greenhouse effect.”
The above paper should add factors to your “only” explanation for Venus optical depth increased over that of Earth.
The paper explains that an atm. optical depth tau is increased by density, mass extinction coefficient, and mass mixing ratio of the atm. constituents, eqn. S14. Venus’ optical depth becomes so thick at surface due these factors, find Venus’ DWIR net of UWIR ~15,000 W/m^2 at surface pressure; generally Venus has no true atm. windows at IR wavelengths > 3 microns.
A greenhouse effect is taking place on Venus; the question is simply what causes it.
The article cited seems to imply that the composition of the atmosphere on Venus is relatively a matter of indifference; it is the density, mass extinction coefficient, etc. that render the atmosphere opaque to outgoing IR.
Granted, the extraordinary density and pressure of the atmosphere of Venus create a situation radically different from that on Earth. Still, can density, pressure, etc. be the only effective circumstances that bottle up the heat the reaches the surface of the planet?
In other words, other factors can be ruled in; but how is it that the properties of CO2 with respect to IR can be ruled out — especially given that the atmosphere is Venus is CO2 and very little else?
“In other words, other factors can be ruled in; but how is it that the properties of CO2 with respect to IR can be ruled out — especially given that the atmosphere is Venus is CO2 and very little else?”
They’re certainly not ruled out, but CO2 on Venus isn’t always even a gas, but a super-critical fluid exceptionally good at conducting heat with poor convective/radiative cooling from the atmosphere keeping that fluid nice and toasty no matter which side is facing the sun. Near-surface winds are very low as a result (effectively non-existent, since the surface is actually a super-critical fluid, no wind, more like a gentle current). This situation means there is very little convective cooling, and the sulfuric acid clouds mean there is very little radiative cooling.
Mars’ total warming as a result of having a nearly pure CO2 atmosphere: 5C. But that, of course, is without positive feedbacks.
@ Jesse Fell says: August 20, 2014 at 1:20 pm
“If that is the case, how can it be hot enough to melt lead on the surface of Venus?”
———————-
Jesse, the simple answer is, ….. the “mass density” of Venus’s atmosphere … and it actually has nothing to do with the type of gases in the atmosphere ….. as long as it isn’t H2O vapor.
Just kinda think of Earth’s atmosphere as being a cubic yard (meter) of fluffy feathers …. and Venus’s atmosphere of being a cubic yard (meter) of solid steel.
Or better yet, …. just read-up on ….. the effect of Urban Heat Islands, to wit:
http://www.epa.gov/heatisland/about/index.htm
==============
@ Jesse Fell says: August 21, 2014 at 1:22 am
“but how is it that the properties of CO2 with respect to IR can be ruled out — especially given that the atmosphere is Venus is CO2 and very little else?”
—————–
Now Jesse, me thinks you are assuming that all of the IR being emitted by the CO2 in Venus’s atmosphere is being DIRECTED toward outer space. Well now, … sorry bout that, …. said IR is being radiated in all directions …. plus the CO2 molecules are CONDUCTING thermal energy between each other, …. up n’ down n’ sideways throughout the entire atmosphere.
Thus, with a 98% CO2 concentration and a 93 bar atmospheric density ….. only the high altitude CO2 is radiating a portion of its absorbed IR into space.
And ps, Jesse, kinda ferget about that “greenhouse affect” because it doesn’t actually exist anywhere except within the confines of the enclosure of an actual greenhouse.
@looncraz 8/21 6:34 am
…. CO2 on Venus isn’t always even a gas, but a super-critical fluid exceptionally good at conducting heat with poor convective/radiative cooling
Thanks for that. I didn’t realize CO2 a Venus surface was in the super-critical phase.
An interesting phase diagram showing CO2 and H2O from 0K to 650 K, 1 mbar to 1 kbar
http://upload.wikimedia.org/wikipedia/commons/thumb/4/40/Comparison_carbon_dioxide_water_phase_diagrams.svg/1280px-Comparison_carbon_dioxide_water_phase_diagrams.svg.png