Guest post by Reed Coray
The following example illustrates the issues I have with reasoning often used to argue that increasing the amount of CO2 in the Earth’s atmosphere will increase both the Earth’s surface temperature and the Earth’s atmosphere temperature. Immediately following is a direct quote from URL
http://www.school-for-champions.com/science/heat_transfer_earth.htm
“The present situation is that there has been an increase in infrared-absorbing gases in the atmosphere, such as carbon dioxide (CO2) and methane (CH4). Energy that would normally escape into space is absorbed by these molecules, thus heating the atmosphere and spreading through convection currents. The average temperature of the atmosphere has increased 0.25 °C since 1980, mainly attributed to an increase in infrared-absorbing gases in the atmosphere.”
Although the above statement makes no direct reference to Earth surface temperature, I believe it carries the implication that greenhouse gases in the Earth’s atmosphere increase the Earth’s surface temperature.
I make two comments: the first is relevant only if the above implication is valid, the second is relevant independent of the validity of the implication. First, placing matter adjacent to a warm surface such that the matter is capable of absorbing/blocking radiation to space from the warm surface can lead to a decrease in the warm surface’s temperature. Second, increasing the amount of the absorbing/blocking matter can lower the temperature of the absorbing/blocking material.
Take for example an internal combustion engine whose metal surface is exposed to a vacuum. In addition to doing useful work, the engine produces thermal energy (heat). That thermal energy will produce a rise in the temperature of the engine’s surface such that in energy-rate equilibrium the rate energy is radiated to space from the engine’s surface is equal to the rate thermal energy is generated within the engine. By attaching radiating plates to the engine’s surface, some of the energy radiated to space from the engine’s original surface will be absorbed/blocked by the plates; but because thermal energy can be transferred from the engine to the plates via both radiation and conduction, the temperature of the engine’s original surface will be lowered. This is the principle of an air-cooled engine[1]: provide a means other than radiation of transferring heat from an engine to a large surface area from which heat can be removed via a combination of conduction, convection and radiation, and the engine’s surface temperature will be lowered.
If plates at a temperature lower than the original engine surface temperature are attached to the engine, it’s true that the temperature of the plates will increase to establish energy-rate equilibrium. Once energy-rate equilibrium is established, however, increasing the plate radiating area (adding additional matter that blocks more of the energy radiated from the original engine surface) will likely lower the plate temperature.
Thus, blocking the amount of surface radiation escaping to space does not necessarily increase the surface temperature; and increasing the amount of radiation blocking material does not necessarily increase the temperature of that material. In both cases (the Earth/Earth-atmosphere and the internal combustion engine in a vacuum), the heat eventually escapes to space–otherwise the temperature of the Earth’s surface and the engine would continue to rise indefinitely. The difference isn’t that the energy doesn’t eventually escape to space (it does in both cases), the difference is in the path the energy takes to reach space. The amount of generated thermal energy in conjunction with the path the thermal energy takes to get to space determines temperatures along the path; and adding more material may increase or decrease those temperatures. To say that “Energy that would normally escape into space is absorbed by these molecules, thus heating the atmosphere…” by itself is unwarranted; because an equivalent statement for the case of adding extra plate material to the engine would be “Energy that would normally escape to space from an engine with small attached plates is absorbed by additional plate material, thus heating the plates…” For air-cooled engines, this statement is not true—otherwise the plate surface area of air-cooled engines would be as small as possible.
It’s fairly easy to visualize why (a) adding thermally radiating plates to an air-cooled engine might decrease the engine’s surface temperature, and (b) increasing the area of the radiating plates might decrease the plate temperature. It’s not so easy to visualize, and may not be true, why (a) adding greenhouse gases to the Earth’s atmosphere decreases the Earth’s surface temperature; and (b) increasing the amount of atmospheric greenhouse gases lowers the temperature of the Earth’s atmosphere. I now present one possible argument. I do not claim that the argument is valid for greenhouse gases in the Earth’s atmosphere, but I do claim that the argument might be valid, and can only be refuted by an analysis more detailed than simply claiming “Energy that would normally escape into space is absorbed by these molecules, thus heating the atmosphere.”
If we assume that (a) matter cannot leave the Earth/Earth-atmosphere system, and (b) non-greenhouse gases radiate negligible energy to space, then for a non-greenhouse gas atmosphere the only way thermal energy can leave the Earth/Earth-atmosphere system to space is via radiation from the surface of the Earth. The rate radiation leaves the surface is in part a function of both the area and temperature of the surface. For a greenhouse gas atmosphere, energy can leave the Earth/Earth-atmosphere system to space both via radiation from the Earth’s surface and radiation from greenhouse gases in the atmosphere. Suppose it is true that the density of greenhouse gases near the Earth’s surface is such that radiation emitted from low-altitude greenhouse gases does not directly escape to space, but is in part directed towards the Earth’s surface and in part absorbed by other atmospheric greenhouse gases. As the atmospheric greenhouse gas density decreases with increasing altitude, radiation emitted from high-altitude greenhouse gases can directly escape to space.
Now it’s not impossible that since (a) in addition to radiation, heat is transferred from the Earth’s surface to greenhouse gases via conduction, and (b) convection currents (i) circulate the heated greenhouse gases to higher altitudes where energy transfer to space can take place and (ii) return cooler greenhouse gases to the Earth’s surface, that the process of heat transfer away from the Earth’s surface via greenhouse gases is more efficient than simple radiation from the Earth’s surface. Many engines are cooled using this concept. Specifically, a coolant is brought into contact with a heated surface which raises the coolant’s temperature via conduction and radiation, and the coolant is moved to a location where thermal energy transfer away from the coolant to a heat sink is more efficient than direct thermal energy transfer from the heated surface to the heat sink.
One way to realize increased thermal transfer efficiency would be to use a coolant, such as greenhouse gases, that efficiently radiates energy in the IR band (i.e., radiates energy at temperatures around 500 K). Another way would be to spread the heated coolant over a large surface area. Since surface area increases with increasing altitude, thereby providing expanded “area” (in the case of a gas, expanded volume) from which radiation to space can occur, it’s not clear to me (one way or the other) that greenhouse gases won’t act as a “coolant” reducing both the temperatures of the Earth’s atmosphere and the Earth surface.
[1] It’s true that for most air-cooled engines the main transfer of heat from the engine plates is via a combination of (a) conduction of heat to the air near the plates, and (b) convection that replaces the warm air near the plates with cooler air. To aid this process, a fan is often employed, or the engine is located on a moving vehicle and the vehicle’s motion through an atmosphere provides the flow of air across the plates. Although conduction/convection may be the primary means of heat dissipation from the plates, radiative cooling also dissipates heat.
An interesting alternative take. And convection is certainly ignored in the “Standard Model”. However, I can’t see the likes of Trenberth even giving it a second glance, the science is “settled”.
Convection via thunderclouds – as Willis has noted – would fit alongside this. There is a lot of commonsense in this thinking and it deserves study. Intuitively it begins to show how part of earth’s ‘thermostat’ may function.
This ought to rattle some cages!
Getting close to a better theory.
Heat cannot be stored by these ‘so called’ GHG’s as 2nd law states that heat must be lost, an increase in entropy, but adsorbed SIR will increase the molecular kinetic energy, increasing the temperature, but this kinetic energy will be transferred to the other gasses not directly affected by the SIR. The GHG’s will radiate LIR but at a reduced energy level, the frequency change from short to long wave IR is the evidence that this happens. Also the 1st law dictates that it must. If it did not then we would be driving in cars using perpetual motion engines which violate both 2nd and 1st laws.
The reradiated heat is in fact a reduction of solar heat reaching the surface so it cannot raise the temperature more than that reached without the intervention of the GHG molecule. If the theory of reradiated heat were to be true then warm liquids placed into a vacuum flask, with its mirrored internal surfaces, would raise the liquid’s temperature by a considerable amount. We all know from experience that this does not happen but a vacuum flask only reduces heat loss slowing cooling. It insulates well but not perfectly.
We must also ask ourselves why the earth’s temperature is fairly even in that max. temperatures rarely exceed 50C nor do we get any lower than -85C. The moon, receiving the same solar insolation as earth but having zero atmosphere, achieves over 150C in sunlight and less than -150C in shadow. So much for an atmosphere increasing temperature by reradiation.
But has it really? If you look at the warming from 1900 to present, look particularly at the warming from about 1910 to about 1945. Temperatures then cooled until about 1975. Beginning in 1976, things began warming again but it took several years to warm back up to where temperatures were in the 1930’s and 1940’s. We can not really consider that period to really be overall “warming” in the context of recovery from the LIA, we can only consider that to be recovery from a spate of cooling after temperatures reached their peak in the late 30’s early 40’s. So we must wait until temperatures get back to that point before we consider that we would have any additional overall warming of the planet.
Whether or not we ever reached the levels of the 1940’s is a matter of some debate, but I would say that the period since 1980’s saw NO temperature rise attributed to CO2 and only a recovery of temperatures to NEAR what they were in the 1930’s and 1940’s. From my point of view, the second half of the 20th century actually saw no or very little “warming” at all in the context of additional recovery from the LIA. It only saw some natural variation where temperatures declined from the 1940’s through the 1970’s and then recovered.
Took a long way getting there, but I understand it in the end. Adding greenhouse gas may be like adding blades to an air-cooled engine.
If our concern is surface temperature then perhaps the “more blades” equivalent on Earth might be adding more mountains?
In a still environment you have conduction and radiation emission. The surface temperature at night will always be coolest in a vacuum won’t it? Any atmosphere, even non-greenhouse, will cause heat to linger around the surface – the buffer – which raises the average temperature at the surface. Greenhouse gases create a thicker buffer.
Just thinking of the mountain example above. Incoming radiation is constant and if we add more surface area the radiation is spread across a greater area. Each unit area absorbs less heat and hence doesn’t radiate as fast – thermodynamics says the higher the difference the faster the loss. With that in mind perhaps a flat Earth endures greater extremes than a bumpy Earth? Perhaps that applies even where more finer details are concerned e.g. trees add to the surface area.
Most heat transfer from the Earth’s surface is not directly to space, as is so often assumed in such models. You should consider clouds, which cover 70% of the Earth’s surface. Their temperature is set by the lapse rate.
I am extremely sceptical of CAGW but I have to strongly disagree with the above analysis. Adding cooling fins to a motor decreases its surface temperature because it increases the surafce area availabel to radiate that heat away, In the case of the earth the surface area is not increased. The point that energy can be lost to space from the surface at all thermal IR wavelengths and from the atmosphere but only at the GHG wavelengths is true in principle. However, because the GHG effect is so strong over the atmospheric column in effect the surface can only lose enegy at the non GHG wavelengths while the atmopshere can only lose energy to space at the GHG wavelengths. What increasing the GHG concentration does is to slightly increases the range of GHG wavelengths so the surface can lose energy over a slightly smaller range of wavelengths and the atmosphere over a slightly larger range of wavelengths. Sine the atmosphere is cooler than the surface it loses less energy than would the surface at the same wavelength. Thus the actio of the GHG increase is to slightly reduce the energy loss to space and to restore balance the temperature of the system must slightly increase.
However – what is at issue is how much. By how much does the temperature have to increase to compensate? A simple calculation shows doubling CO2 would lead to an increase of about 1C in the absence of feedbacks. That’s not serious so are the feedbacks positive or negative? This is the crux of the debate but its worth noting that every naturally stable system shows strong negative feedback and that means the rise will be less than 1C not more than it.
Where does the negative feedbakc come from here? Higher temperatures means more evaporation which must mean more rain but rain comes from low clouds so it must mean more low cloud either in density or in coverage. Both increase Earth’s albedo and reduce temperatures.
This is a very baffling post Reed, it doesn’t make sense. An air cooled engine does not cool by blocking radiation. It cools by conducting heat away from the engine into the fins and, because they provide a larger surface area, more heat is subsequently radiated away (or convected away). You have to be very careful with analogies, and this one is too contrived to be useful. The atmosphere, clearly, doesn’t work like this.
.
As an ex-motorcyclist, first hand experience leads me to agree with much of what Reed Coray writes. As a layman in the sciences I must rely on educational web sites that explain heat transfere and IR radiation and such like. I am led to believe that gases in the atmosphere can absorb OR radiate specific radiation bands dependant on the local temperature. It cannot do both at the same time. Wein’s Law will give the peak temperature at any specific IR wavelength. Using Wein’s Law to look at CO2 I find that the 2.7 micron band peaks at ~800C, the 4.3 micron band at ~400C and the 25 micron band at about -80C!! I understand only limited areas of the Earth’s surface might radiate at up to 50C so the 2.7 and 4.3 micron bands will NEVER be exited enough to absorb any energy from the surface. They might absorb a very little from the sunlight but that is working as a coolant. The so called standard surface temperature of the Earth is said to be 15C, well above the the -80C temperature level of the 15 micron band for CO2. The problem now is most of the CO2 molecules in the atmosphere will be at a temperature comensurate with the adiabatic lapse rate starting at the surface. So assuming a drop of 10C per kilometre altitude air temperature should be down to -80C at about 9.5 kilometres altitude, almost the tropopause. Only then will the CO2 molecules be cool enough to absorb radiation at 15 microns.
BUT! There is indeed nothing to stop the CO2 radiating at 15 microns and some of that radiation reaching the surface. Now another BUT! The surface, except at possibly a small area at the south pole, is well above -80C!! Any element, black body or not, does not absorb radiative energy below its peak temperature.
A CO2 molecule IS a black body with rather specific characteristics. And so is any other gas molecule in the atmosphere.
Since I am completely unable to see any ‘greenhouse’ effect in the atmosphere I need more education. Please post links that will this layman.
What about the additional LH of Vapourization drawn from the surface by both any direct additional evaporation due to CO2 GH effect but also the increased transpiration from plants as their metabolism increases due to more CO2 (double -ve feedback)? H2O vapour up into the atmoshpere causes some more GH effect , true (+ve feedback) but then recondenses as clouds ( albedo => -ve feedback) and releasing the LHV at altitude where convection/cnduction takes it upwards and into space ( -ve feedback). What is the net feedback effect? Who knows but there is no hot spot at altitude so it cannot be much.
Is Trenberth’s approval a hurdle this has to clear?
Tcha!! Educate, educate, educate!
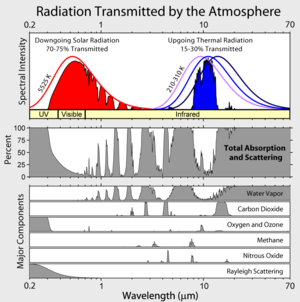
Those spectral intensity curves are wrong. The formula I = kT^4 gives the area under the curves (in W/m^2) (k = Stefan-Boltzmann constant).
Temperature Area under curve
210K 110 W/m^2
260K 260 W/m^2
310K 523 W/m^2
Solar 1367 W/m^2 at top of atmosphere.
The simple fact is that convection trumps radiation every time. Just hold you hand in front of a working ‘radiator’ and then above it. (This is why radiators are very often placed below windows, in fact).
There is no proof that I am aware of that more CO2 does not in fact cool the atmosphere by convection.
I keep looking at this: air and ground temperature recorded in the N. African desert during a solar eclipse.
http://www.shadowchaser.demon.co.uk/eclipse/2006/thermochron.gif
Could someone please show me how to use Plank’s Law of Radiation (I use Open Office) – as shown here:
http://pveducation.org/pvcdrom/properties-of-sunlight/blackbody-radiation
As I keep getting the “ultraviolet catastrophe”.
This is inline with the real null hypothesis that I posted before.
We read all over internet that the black body temperature of the Earth would have been -18C, but the actual average temperature is +15C; consequently this 33 degrees difference is supposed to be the greenhouse effect. But is this true?
Is the blackbody situation the “null hypothesis”? I don’t think so. The black body calculation assumes a sphere with a constant flux of light energy, uniformely distributed over the surface, using the Stefan Boltzman equation to derive it’s temperature like this.
But the earth is nowhere near a blackbody and if we want to really look at the null hypothesis, we would have to look at an earth without greenhouse effect, but still with an (inert) atmosphere and still rotating in 24 hrs, with seasons and all.
Now instead of using an average steady state solar radiation, we need to realize that we have the diurnal cycle with max insolation radiation at noon and no radiation incoming when the sun is below the horizon. So during daytime the earth surface warms up and much more than the according the average radiation. Equilibrium temperature at the equator in a steady state with the sun in zenith, using the full incoming 1365 w/m2 (albedo 30%) would be 360K or 87C. This follows from applying the Stephan Boltzman equation for the spot directly under the sun, instead of a uniformely distributed radiation.
So this much higher temperature of the earth surface is transmitted via conduction to the lowermost boundary layer of the atmosphere. This heated air gets is less dense, and it becomes buoyant so it rises up; Convection, the very basics of meteorology. So at daytime the atmosphere receives thermal energy of the earth. How can it lose this energy again? Remember we are in the null hypothesis, no radiation, no greenhouse effect, so the inert atmosphere cannot lose the energy by radiation.
Now, at night time the Earth does not receive radiation energy from the sun but it radiates energy out and cools quickly, obviously much more quickly in the null hypothesis even than with the greenhouse effect, which would have directed (“reflects”) some radiation back to earth. Now the cooler earth also cools the boundary layer of the atmosphere by conduction again, however there is no negative convection as the cool air gets more dense and tends to stay put; the inversion; also very basic meteorology. So despite the cooling of the earth, the missing radiation from the atmosphere prevents it from cooling at night and the next day more conducted energy is convected into the atmosphere, that stays there again.
Obviously we have an unbalance. And equilibrium can only be reached, maybe after thousands of years, when the convection at daytime has reduced so much to balance heat loss at night time via conduction back to the surface. For that the lower level atmosphere needs to be at the same temperature / density than the boundary layer would reach due to the conduction of heat from the surface.
Conclusion, in the null hypothesis, without greenhouse effect, the average temperature of the lower atmosphere would be considerably higher than the black body temperature of the surface. How much I don’t know. But the main point is that a certain portian of the temperature difference between black body and actual atmospheric temperature is not due to greenhouse effect but to the inability of the inert atmosphere to cool down by radiation.
Pretty much fits my previous contention that when molecules in the atmosphere absorb more energy then the circulation changes so as to accelerate energy to space faster.
The effect on the energy content of the system being at or near zero but the price to be paid is that circulatory change.
Then the only question is whether the circulation change from human emissions is measurable as compared to the natural changes caused by sun and oceans which gave us the MWP, LIA and current warm period.
You do realise that this quote you dug up is from an educational website aimed at schoolchildren, right? And that more detailed analyses have been being published in the scientific literature since Victorian times? Your argument is “not even wrong”. Presumably you don’t have any idea why the Moon is colder than the Earth.
If it was, then the predicted surface temperature would be about 45°C. It’s one thing to be ignorant of the science, most people are ignorant of the science. Believing in your own ignorance is another thing.
I think Reed Coray is on the ball.
The IPCC assertion that nitrogen and oxygen are not GHGs appears to conflict with the findings of Tyndall’s physical experiments (1861) which showed that N2 and O2 neither absorb nor radiate heat in the longwave infrared radiation (LIR) spectrum, even though they are transparent to incoming solar shortwave radiation. Modern spectroscopy reveals a total absence of N2 in the LIR, and barely any O2 relative to the atmospheric H2O and C2O, which dominate the infrared spectrum. Yet H2O and CO2 comprise only about 1% of the atmosphere, as against the 99% consisting of N2 and O2. Thus if the former are blanketing the earth, that is an achievement when they comprise so little of the atmosphere – most of us prefer blankets that are close to 100% wool.
Tyndall’s physical laboratory experiments found no evidence for any significant absorption of heat by nitrogen and oxygen in the longwave spectrum, and that meant for him they could not radiate heat to space. His experiments showed that air comprising only [H2O] and [CO2] both absorbed and radiated 15 times as much as air consisting only of N2 and O2:
“Air without [water vapour and CO2] produced an absorption of about 1.
Air direct from the laboratory, containing therefore its carbonic acid [CO2] and aqueous
vapour, produced an absorption [and radiation] of 15”.
(Lecture 1861:28).
Comparison of the Brazilian rainforest and the N. African Desert.
http://en.wikipedia.org/wiki/Barcelos,_Amazonas
http://www.google.co.uk/search?q=Barcelos+amazonas&num=10&hl=en&site=imghp&tbm=isch&gs_l=img.3..0l2j0i24l8.2563.8069.0.10019.10.8.0.2.2.0.532.2460.1j2j1j0j3j1.8.0…0.0.l9hxb0L6cLs&oq=Barcelos+amazonas
http://www.wunderground.com/history/station/82113/2012/5/20/MonthlyHistory.html
http://www.climate-charts.com/Locations/b/BZ82113.php
http://en.wikipedia.org/wiki/Adrar,_Algeria
http://www.google.co.uk/search?num=10&hl=en&site=imghp&tbm=isch&source=hp&q=adrar+algeria&oq=Adrar&gs_l=img.1.1.0l2j0i24l8.1151020.1152708.0.1155877.5.5.0.0.0.0.462.1453.1j0j2j0j2.5.0…0.0.PUZtKMJOlKc
http://www.wunderground.com/history/airport/DAUA/2012/5/20/MonthlyHistory.html
http://www.climate-charts.com/Locations/a/AL60620.php
For May 2012, Barcelos, Brazil (Lat: 1 South)
Temp: monthly min 20C, monthly max 33C, monthly average 26C
Average humidity 90%
For May 2012, Adrar, Algeria (Lat: 27 North)
Temp: monthly min 9C monthly max 44C, monthly average 30C
Average humidity around 0%
GHG does not block radiation, it absorbs and then re-transmits … a better term than block would be slows …
If the energy input to the Earth system (from the Sun) remained unchanged, then the fingerprint of an enhanced GHE as the culprit of tropospheric warming would be a gradual reduction in OLR at TOA. This is not what we observe. We observe the opposite. This suggests rather that the Earth system is working towards balancing an INCREASED energy input. Which is also observed. But in this case, the increased energy IN (heat gain) is clearly what causes the warming. The increased energy OUT (heat loss) is Earth’s attempt to keep pace.
Where’s the evidence of a greenhouse gas-driven warming?
Very interesting post Reed, but I have my doubts (hey, it’s what we do here).
The additional CO2 added to the atmosphere does not significantly increase its volume. A thicker atmosphere would certainly have a greater greenhouse effect (see Venus).
Keeping the volume of the atmosphere constant and increasing the surface area exposed to space (i.e. a bigger earth with thinner atmosphere) would be a more appropriate comparison to adding more metal plates to an engine.
The physical properties of the atmosphere are the sum of its constituent parts. Adding CO2 changes those physical properties. Consequently, I think a better analogy might be: adding CO2 to the atmosphere is like changing the composition of the metal (i.e. an alloy) used in the engine plates. Which is a very different situation from adding more plates.
GHG does not block radiation. They absorb and re-radiates … and they must be cooler than the hot surface to do that …
Sorry Reed, you miss the point, but you are not alone. Eli remembers eminent analytical chemists who missed the same point in print many years ago.
What happens is that effectively GHG block radiation from reaching space across most of the IR.
This includes IR emitted from the GHGs low in most of the troposphere.
Increasing concentrations of GHGs raises the altitude that GHGs can radiate to space in the blocked regions of the spectrum
Because of the lapse rate, the higher you go in the troposphere, the lower the temperature
This slows down the rate at which the Earth emits to space because it is now radiating at higher, colder altitudes
To maintain radiative balance (sun in, IR out) the entire Earth system warms until the temperature rises enough in the mid troposphere to restore the balance.
In somewhat more detail you can read about this at RR and there are links to other, more mathematical explanations there plus Science of Doom probably beat this to death somewhere
@ Richard111 says:
July 21, 2012 at 4:01 am
Try this bloke at the link Richard. Only a half dozen posts including a small pdf.
let me know what you think.
http://jinancaoblog.blogspot.com.au/2011/11/physical-analysis-shows-co2-is-coolant.html
http://www.tech-know.eu/uploads/JCao_N2O2GreenGases_Blog.pdf
Very helpful and clear. I like the focus on radiation because that is the only mechanism of interest to the warmists: they can’t account for convection and it hurts their case badly, so they ignore it. This analysis of radiation suggests that convective processes are, ultimately, like the “blades” of material on a radiator: in both cases, there is a presentation of the warmer material to as much of a cooler material as geometry or fluid transport will allow. A thunderhead is like a giant temporary radiating surface. Of course I am not giving credit to heat transport from phase changes of water in the thunderhead, but ultimately they release thermal energy through radiation to space as well. The role of water vapor in transporting heat to the upper atmosphere where it can dissipate through IR radiation is simply gigantic (see the diagram of absorption/emission spectra: CO2 and other GHGs are dwarfed by H2O).
So much of the warmists’ thinking rests on –dare I say it?– thin air.
This is a most baffling, confused post which conflates many things and adds nothing.
Andre Bijkerk says: July 21, 2012 at 4:34 am
“We read all over internet that the black body temperature of the Earth would have been -18C, but the actual average temperature is +15C; consequently this 33 degrees difference is supposed to be the greenhouse effect. But is this true?”
No, the -18C must be ground temperature while the +15C is ait temperature: comparing apples and orangutans.
“The black body calculation assumes a sphere with a constant flux of light energy, uniformely distributed over the surface, using the Stefan Boltzman equation to derive it’s temperature like this.”
And that doesn’t work because the relationship between radiation intensity and temperature is not linear. Take a blackbody “Moon” with 1400 W/m^2
on one side and 0 W/m^2 on the other side: that give two temperatures – 400K on one side and 0K on the other: average 200K. Now cast 700 w/m^2
on both sides and you get 332K on both sides: average 166K.
So the temperature of 700 + 700 W/m^2 does not equal the temperature of 1400 + 0 W/m^2 .
JeffC says: July 21, 2012 at 5:15 am
“GHG does not block radiation, it absorbs and then re-transmits … a better term than block would be slows …”
I believe JeffC to be correct. CO2 almost instantly re-radiates the outgoing IR radiation it intercepts, with around 50% of this radiated back towards the Earth’s surface. The attenuation of IR passing through CO2 does not mean the CO2 molecules have increased in temperature, but rather they have scattered the IR.
Outgoing IR radiation radiated back to the Earth’s surface could slow the radiative cooling of surface materials. However by physical experiment I have found that the effect is negligible with regard to liquid water that is free to evaporatively cool. If the grey body figure of 1.2c for radiative forcing via a doubling of atmospheric CO2 used by the IPCC is adjusted for the partial exclusion of 71% of the Earth’s surface, climate sensitivity to a doubling of CO2 would appear to be around 0.3c to 0.4c. However even the grey body figure of 1.2c should itself be in doubt given the ability of radiative gasses to radiate as IR any energy they have acquired conductively.
Arrgh! Not this again … back to basics!
@ trcurtin Isn’t the aurora borealis oxgen and nitrogen emitting in the visible spectrum? If they can emit visible light, surely they can absorb visible light.
no?
michael hammer says:
July 21, 2012 at 3:52 am
The “blocking” by GHGs would only exist as long as the stratification is not disturbed in any way. As soon as convection rears its, IMO, beautiful head, you get the wonderful heat pipe effect which transports heat from hotter to colder. Willis has in extensio presented, demonstrated via Argo temperature measurements, and discussed this.
Circulation, even when it’s horizontal, works much like convection, however we have the adiabatic lapse rate causing high pressure areas (descending air mass) with heating, and low pressure areas (rising air mass) with cooling. This might be what confuses some of the people saying we’ve got global warming. Low pressure areas are moving large quantities of heat upwards, particularly so if they’re humid air masses. On the other hand, the high pressure areas are low humidity and therefore reach higher air temperatures, but with lower heat content.
All of this regulates the heat content of the air. It is particularly noticeable here on the Gulf Coast. One is quickly disabused of the idea that air controls water temperature as soon as a south wind kicks in following a north wind. The temperatures rise quickly in the Winter, in the summer they drop and we get that wonderful “feels like” temperature measurement = hot anyway.
A couple of points.
1. Non-GHGases DO emit radiation. Both Oxygen & Nitrogen emit in the UV range, but they do lose some energy to space through radiation.
2. We’re interested in the lower atmosperic temperature (supposed to be 6 feet off the ground for weather stations) rather than earth land/water surface temperatures. I expect this makes some difference to your analysis.
A novel, “out of the box” hypothesis. Good post.
Further, data may exist to test it.
My assumption is:
For AGW models and despite atmospheric mixing, there would exist
gravitational bands of concentration for CO2. Otherwise, there is no
greenhouse. This reflecting model should show, due to reflection
a temperature at the earth side of the gravitational band a temperature
labeled x.
If this convection hypothesis is correct, the atmosphere below the gravitational
band would produce a temperature labeled y.
y would be measured to be less than x due to the convection transfer
into the gravitational band. There would be less reflected energy
available.
I may have more to say about this later, but a couple things trouble me:
Since surface area increases with increasing altitude
Area of sphere’s surface is proportional to r^2. Convection reaches up to the tropopause, for the general limit, so for an Earth diameter of 8000 miles and a tropopause of height of 10 miles, the ratio of the increase is 8010^2/8000^2, is 1.0025. What’s the average radiation, some 400 w/m^2? That means an extra watt. Worth including, but not enough.
[1] It’s true that for most air-cooled engines
This bouncing between IC engine in a vacuum and air cooled IC engine gets confusing. The plates, err fins, of an air cooled engine provide more surface area for conduction to air. Fins don’t work as radiators – you need an unobstructed view of the cold surface to work well. That why the radiators on the Space Shuttle were just flat plates on the payload doors. The air cooled engine isn’t helpful to the discussion, the radiatively cooled engine is to far outside most peoples’ experience to be a good analogy. Besides, a lot of the heat from either engine is carried away by exhaust gases. I’d be just as happy with talking about radiation from a hot plate.
Reed,
There are serious flaws in your analogy. You have your fins made from the same sort of material as the engine. Try thinking of the fins as being made of wood so you wind up with insulation rather than merely additional radiator area.
A parcel of gas at a given pressure and temperature will absorb a fraction of the power being transferred through it, essentially depending upon the ghg concentrations and not so much on its temperature. It will also radiate outward (and radiate inward) a given amount of power that depends upon its temperature and the concentrations of ghgs. If the gas is the same temperature as a surface, then the emission rate of the gas outward will be equal to the absorption rate of the original surface radiation continuum going through it. If the gas is hotter, then there will be emission lines superimposed upon the continuum energy and if the gas is cooler, there will be absorption lines.
If you add more ghgs to the parcel, there will be a stronger absorption from the continuum but there will also be a greater emission occurring for a given temperature. The gas temperature will adjust to balance the emitted and absorbed energy and that includes radiation, conduction, and convection energies. The same thing goes for adjacent or near by parcels of gas except they are emitting energy in a spectrum. Ultimately, it takes less of a T increase to radiate the same amount of power than it would if one does not take the added emissivity into account but the added absorption does require some T increase.
The top of the troposphere is the tropopause and this is where convection stops being an extremely important part of the heat transfer. Below this, convection is very significant.
The whole problem with looking just at this is one misses the bigger picture. around 62% of the Earth’s surface is covered by clouds and a goodly portion of this is on the sunlit side as a lot of the cloud cover tends to form because of sunlight and tends to dissipate at night. Clouds and particulates provide a continuum and it radiates at a continuum rate for the temperature of the material. Also, clouds and scattering and particulates provide about 80% of the Earth’s albedo which is the dominant factor in determining Earth’s temperature. It also impacts the outgoing radiation significantly, blocking surface radiation and substituting it’s own characteristic radiation temperature.
Ultimately, the ghg contributions as you are looking at and as explained and discussed everywhere is only for clear skies and when clouds are involved, it changes quite a bit.
Based on people like hansen who are at the bottom of this CAGW pyramid, one can see serious flaws and nonphysical ideas and concepts being peddled which lead to erroneous results. An example is the characteristic radiating altitude that supposedly changes with ghg concentrations which has nothing to do with reality and hence cannot provide conclusions for changes.
Reed: Eli Rabett ( http://wattsupwiththat.com/2012/07/21/some-thoughts-on-radiative-transfer-and-ghgs/#comment-1038824 ) and Arthur ( http://wattsupwiththat.com/2012/07/21/some-thoughts-on-radiative-transfer-and-ghgs/#comment-1038782 ) hit the nail on the head. The explanation that you critique is not even the best of the non-mathematical simple explanations for the greenhouse effect. Furthermore, underlying this explanation are radiative transfer calculations that show that you are wrong and the accepted explanation is correct. (The actual surface temperature of the Earth compared to the highest surface temperature it could have in the absence of an IR-absorbing atmosphere and still obey conservation of energy is also evidence that the natural greenhouse effect warms the Earth.)
And, these radiative transfer calculations are well-verified by, among other things, the modern field of remote sensing. If you want to be consistent in your skepticism, you would also have to disbelieve all of Spencer and Christy’s work reconstructing tropospheric temperatures from satellite remote sensing along with much of the other work in remote sensing (perhaps even the weather satellites).
Jerome says:
The simplest way to understand why saying the word “convection” doesn’t magically slay the greenhouse effect is to understand that convection only equalizes temperatures to a point. In particular, convection drives the lapse rate in the atmosphere down to the adiabatic lapse rate but not further. This is why convection, while reducing the natural greenhouse effect from what it would be in its absence (by close to a factor of two as I recall) , does not eliminate it. It also explains why Nikolov and Zeller in their very mistaken work ( http://wattsupwiththat.com/2011/12/29/unified-theory-of-climate/ ) were able to demonstrate that convection added to a simple model eliminated the greenhouse effect: They mistakenly added convection to the model in a way that drove the lapse rate to zero. It is easy to take the model, add convection in a more correct manner and show how it doesn’t eliminate the greenhouse effect.
Now it’s not impossible that since (a) in addition to radiation, heat is transferred from the Earth’s surface to greenhouse gases via conduction, and (b) convection currents (i) circulate the heated greenhouse gases to higher altitudes where energy transfer to space can take place and (ii) return cooler greenhouse gases to the Earth’s surface, that the process of heat transfer away from the Earth’s surface via greenhouse gases is more efficient than simple radiation from the Earth’s surface.
Not only not impossible, but true! And recognized even by the Evil Dr. Hansen in papers dating all the way back to the late 80s! It is one of the processes moderated by moisture, especially, since water vapor carries enthalpy aloft, such that the dry air adiabatic lapse rate is quite different from the moist air adiabatic lapse rate. Assuming that moist/humid air is dominant — even all the way back in Hansen’s earliest papers — leads to a much more moderate total greenhouse forcing on a doubling of CO_2.
The point is that it is really not smart to assume that climate scientists — even ones that have perhaps gotten carried away with “save the world” passion to the point where they are no longer sufficiently objective to avoid the demon of confirmation bias — are stupid, or that they don’t use the underlying physics in their models or computations. Sure, you might discover something that they’ve forgotten. And they could be wrong about what processes ARE dominant lots of ways, because nonlinear models can often “work” around multiple clusters of “reasonable” parameters describing e.g. water vapour feedback especially when we perhaps lack sufficient data to properly constrain the models. But a study of the actual physics of the greenhouse effect being used in the models will suffice to show that your idea, while correct, is hardly original, nor is it an effect being omitted altogether from the GHE-warming arguments.
Sorry,
rgb
No! Radiation absorption and re-radiation, along with convection and evapotranporation and condensation transfer the heat absorbed by the Sun to a sufficient altitude to radiate to space. If the lapse rate and albedo do not change, the only way greenhouse gases increase the temperature is by raising the average altitude of outgoing radiation to space. It increases temperature at all altitudes in the Troposphere by shifting the entire temperature profile a small amount.
“Now it’s not impossible that since (a) in addition to radiation, heat is transferred from the Earth’s surface to greenhouse gases via conduction, and (b) convection currents (i) circulate the heated greenhouse gases to higher altitudes where energy transfer to space can take place and (ii) return cooler greenhouse gases to the Earth’s surface, that the process of heat transfer away from the Earth’s surface via greenhouse gases is more efficient than simple radiation from the Earth’s surface. ”
Yes, someone finally gets it. A simple calculation shows that a parcel of air that is 1K warmer than its surroundings and rising at 1 m/sec, walking speed, carries energy aloft at a rate of 1kW/m^2. That energy eventually rises to the top of the atmosphere where it is radiated away by H2O and CO2. Increasing CO2 will increase the top of the atmoshpere radiative cooling ability proportionatly while its heating effects at the ground only increase logarithmicly with concentration.
This is a good argument but is limited by thinking of the atmosphere as one entity. A discussion about heat transport in the atmosphere very much needs to include the very different troposphere and stratosphere.
In the troposphere heat is mostly conducted by convection and the associated water evaporation and condensation. Temperature drops with altitude so warm air will rise, carrying massive amounts of heat as long as the adiabatic cooling does not reduce the temperature below that of the surrounding air. Condensing water vapor releases heat to keep a rising bubble of air warmer than otherwise and we get thunderstorms and other weather. At the tropopause, adiabatic cooling has reduced the temperature to about -55°C. Above the tropopause is the stratosphere.
The defining characteristic of the stratosphere is that temperature rises with altitude. Vertical convection effectively ceases. This is where heat transport by radiation finally becomes dominant. The dew point is very low throughout the stratosphere as it is controlled by the dew point at the tropopause.
A discussion on the effect of IR absorbing gases such as water vapor and CO2 needs to be broken down into two parts. What is the effect on convection in the troposphere? What is the effect on radiation in the stratosphere?
At a high enough altitude (40-50 km), CO2 is effectively cooling as it can emit radiation directly into deep space. Water vapor concentration is less than CO2 when the dew point reaches -55°C. http://www.dew-point.com/equivalents.html
The effect on adding CO2 in the troposphere is going to be determined by how it interacts with water vapor and changes the convection. The shear complexity of this suggests CO2 will have no effect as the atmosphere will adapt to keep entropy generation at a maximum. More paths for energy transport will reduce the effect of a constraints on a given path so complexity ensures maximum entropy generation. The CAGW believers tell us that CO2 is a constraint on radiation transport of heat but this totally ignores the dominant convection and the likely hood that CO2 will simultaneously increase convection for no net effect.
Convective heat transfer is enhanced by combined radiant + convective transfer.
For Convection, heat transfer from a surface to the air above it, the rate of heat transfer is dependent on the velocity of the air. For Natural Convection (no wind) the transfer rate is lower than for Forced Convection (windy) conditions, all other things being equal. There is a ‘boundary condition’ just above the surface where the air is barely moving, even on a windy day, and that ‘boundary’ limits the rate of convective heat transfer from the surface.
Introduce GHGs to the air above. Energy is also radiated from the surface, some photons are captured by the GHGs, and we are told, they are almost immediately ‘thermalized’, which is to say, the radiant energy is converted to thermal energy in the air. The warmer air directly above the surface now rises, increasing the Natural convection. The overall energy transfer, convection + radiant, is enhanced by the addition of GHGs. Its like the GHGs help the heat ‘jump’ the boundary layer at the surface. Rather than blocking the heat transfer, the GHGs actually would appear to speed up the rate of transfer, helping to move energy more quickly to the upper atmosphere, where it radiates out to space.
Reed, you’re post is very close to what I’ve been saying for years – but keep getting told I’m an idiot for saying it. CO2 both absorbs and radiates IR. I may be wrong, but I think it goes like this: if an unexcited molecule of CO2 is hit by a photon of the correct frequency, it will absorb the energy and move into an excited state. It will then re-release that photon in some random direction when moving back to an unexcited state.
Now, my problem is this: most of the atmosphere cannot absorb radiation from longwave IR. So as far as I can tell, it’s impossible for the re-radiated IR to heat the atmosphere directly. It can bounce around from CO2 molecule to CO2 molecule for 10 years and never “heat” the nitrogen and oxygen that makes up 98% of the air.
The question I have is this: is there another way to excite the CO2 molecule into radiating? Will conduction heat the CO2 molecule enough to cause it to radiate? If so, then would not the warm Earth impart heat to nitrogen and oxygen by conduction, which then can transfer that heat to CO2 via conduction, which will cause the CO2 molecule to fire off a photon of longwave IR on it’s own, without having absorbed one from the surface of the Earth?
Since more than 90% of the atmosphere is non-radiative in longwave infrared and does not absorb energy via radiation, most of the heat in the atmosphere is transfered there via conduction. It seems to me that a warm atmosphere will cause CO2 and H20 molecules to radiate away the atmospheric heat to space at increasingly efficient rates. The warmer the air gets, the faster those two gases radiate, regardless of what the surface of the Earth is doing, right?
Reed Coray
Re: “Energy that would normally escape into space is absorbed by these molecules, thus heating the atmosphere.”
That popular description would best be improved by noting that:
“Energy that would normally be radiated directly into space is BOTH absorbed AND then RERADIATED by greenhouse gas molecules IN ALL DIRECTIONS. This changes the atmospheric “lapse rate”.”
For a quantitative thermodynamically sound model of the atmospheric lapse rate see:
Robert E. Essenhigh (2006) Prediction of the Standard Atmosphere Profiles of Temperature, Pressure, and Density with Height for the Lower Atmosphere by Solution of the (S−S) Integral Equations of Transfer and Evaluation of the Potential for Profile Perturbation by Combustion Emissions.
As a “preprint” see his similar Paper No.03F-44: Western States Section Combustion Institute Meeting: Fall (October) 2003
trccurtin
Re: “Tyndall’s physical laboratory experiments found no evidence for any significant absorption of heat by nitrogen and oxygen in the longwave spectrum.”
While small, the greenhouse contributions of O2 and 2 are NOT negligible. The greenhouse effects of O2 and are quantified by Hopfner et al.(2012) The natural greenhouse effect of atmospheric oxygen (O2) and nitrogen (N2) Geophysical Research Letters in press 2012GL051409
Willie Soon discussed this fact that O2 and N2 not negligible for greenhouse gas emissions. ICCC 7, 2012 ~ 11:30 Tuesday 22 May 2012. See also other Heartland ICCC 7 presentations
This post is utter nonsense. Why use the analogy of an air cooled engine when an air cooled radiator works by conduction/convection and the ‘whole earth’ temperature is close to a solid sphere in a vacuum problem.
Anyone who thinks they have understood this post should realise that deductive reasoning in this style can tie you up in an ugly mess. Garbage in, garbage out.
It may well be that the classical greenhouse gas model is mostly wrong – this post is completely orthogonal to that question.
Corrections. Essenhigh’s 2003 paper is at http://altmine.mie.uc.edu/nuclear/htmfile/atmcombXC.pdf
ICCC presentations at http://climateconferences.heartland.org/
Not buying it.
Adding a second blanket at night increases thermal mass and thus makes me cooler?
David Chamness
Note that “most of the atmosphere CAN absorb radiation from longwave IR” . . “the re-radiated IR CAN heat the atmosphere directly”, resulting in the average atmosphere being in effective thermal equilibrium at a given elevation. (a href=http://altmine.mie.uc.edu/nuclear/htmfile/atmcombXC.pdf>Essenhigh (2003, 2006) shows the fourth power of the reduced temperature is proportional to the reduced pressure.
Logically correct for internal combustion engines. Incorrect for Earth. To a very good first approximation, heat energy arises from incoming solar radiation (absorbed shortwave radiation). It escapes as outbound infrared (outbound longwave radiation). Again to a very good first order approximation, there is no convection or conduction of heat into a vacuum (space). There is only radiation (which is how vacuum thermos flasks work). Again to a very good first order approximation, the radiative ‘surface’ of the atmosphere, it’s TOA, does not change with temperature. Therefore it is the dynamics of ASR and OLR within the atmosphere that govern thermal change. AGW occurs (the degree, not the physics, is the question of interest) because as your chart from Wikipedia shows, GHG are less ‘transparent’ to OLR than ASR. A rise in GHG creates a temporary situation where ASR > OLR until temperature rise induces more OLR to restore equilibrium. What is interesting and provably wrong about the IPCC consensus is that they have gotten both the primary indirect feedback, rising water vapor (the most potent GHG), and the secondary indirect feedback, clouds, wrong. They provably have done so through classic selection bias in the meta-analysis that is IPCC AR4. And meta-analysis selection bias is by definiton deliberate. It therefore is tangible proof of agendas. Not for Mann and ‘hide the decline’. For the entire IPCC. I devote an 80 page chapter of a forthcoming book to irrefutable documenting this.
I think you make a good point. The distinction between fact and conjecture is sometimes blurred when the conjecture seems obvious. A case in point relayed to me by an Electrical Engineer once many years ago (yes, complete hearsay and relying on my memory from late 80’s to early 90’s). This Engineer worked for a large electronics manufacturing company in the capacitor division (60’s & 70’s). One day they (design / R&D team) were told that a customer wanted a particular capacitor model jacketed (in addition to the casing). The team of Engineers looked at each other all knowing this would increase the insulation value and potentially make the capacitor operate outside of design specifications and fail. But a customer is a customer, so they went to the lab and tried various jacket materials. Sure enough the jacketed capacitors ran hot and failed, EXCEPT ONE . They ran the experiment again and again, same results. So, they had happened upon a jacket material that wouldn’t increase the operating temperature of the capacitor. The EE told me he always wanted to go back and investigate why, but in industry answering such academic questions isn’t always (mostly not) the priority. He suspected the material increased the radiative heat loss more than it reduced the convective heat loss, but has trouble believing it considering how tiny the radiative heat loss from a capacitor is compared to the convective heat loss. The point being they all (experts in their field) thought it was a FACT that adding another layer would increase the temperature when actually it was a CONJECTURE and one that wasn’t always true.
Fact: CO2 is a GHG.
Conjecture: Adding CO2 to the atmosphere will increase the temperature.
It seems pretty obvious, but is it true? Is it always true?
The problem I have with the “standard” explanation is the equation presented for increased down welling radiation from increased CO2 concentration [F=5.35Ln(CO2f/CO2i)] has no temperature variable and yet the outgoing radiation from CO2 to space is supposedly reduced due to being “colder” due to altitude. Is temperature a variable or isn’t it? Are GHG’s more like fluorescent bulbs (non-Stefan-Boltzmann applicable) or incandescent bulbs (Stefan-Boltzmann applicable)?
According to ModTran:
280 ppm CO2 20km upward: Iout, W / m2 = 289.351
380 ppm CO2 20km upward: Iout, W / m2 = 287.53
So, a 100 ppm increase in CO2 results in a 1.821 W/m2 decrease in outgoing radiation from TOA.
280 ppm CO2 0km downward: Iout, W / m2 = 347.598
380 ppm CO2 0km downward: Iout, W / m2 = 348.226
So, a 100 ppm increase in CO2 results in a 0.628 W/m2 increase in radiation to surface.
[Leaving all other variables at default values.]
Indeed, who can forget the satellite measureing increases in outgoing IR vs. the model outputs:
http://wattsupwiththat.files.wordpress.com/2012/02/image26.png
Perhaps this is the flaw in the slaw of post normal climate science. An obvious conjecture became a fact in their minds and perhaps it is true, perhaps just not always.
100 years ago just about every scientist would have agreed with the song lyrics: Time keeps on slipping into the future; but now most would say: well, not always.
David Chamness
PS The molecule’s motion transfers heat by conduction so radiation, convection (and conduction) provide heat transfer.
Reed Coray
PS for the impact of gravity versus convection within a room see:
Lucy Skywalker: Graeffs experiments and the second law of thermodynamics
Graeff (2011) demonstrated a negative temperature gradient after stopping convection an adiabatic gas by a fine glass powder that allowed gas diffusion. The consequence is “a negative gradient of T(Gr) = – 0.07 K/ m,” i.e., gas is hotter at the bottom and cooler at the top, NOT at constant temperature throughout the adiabatic chamber – even though the environment has a positive temperature gradient (hot air rises). (This appears to be the tradeoff between gravitational potential energy and kinetic energy (ie temperature) with conservation of energy.
Eli Rabett says:
July 21, 2012 at 5:39 am
“To maintain radiative balance (sun in, IR out) the entire Earth system warms until the temperature rises enough in the mid troposphere to restore the balance.”
Eli, always nice to have someone show up to remind us of IPCC dogma. Here’s a question for you. My house is insulated with an R value of 10. But I am a rugged individualist so I have no connection to electricity or source of fuel. I don’t even occupy the house. I Iive in a tent in the back yard. But I measure and record the temperature inside the housd every hour. At the end of the year I average all the readings. But an environmentalist has convinced me that if I remove all of the insulation the average temperature inside will go down, so I do. What do you think will happen to the average temperature?
Would a black body radiate into air that is the same temperature as it is?
Count me in with Jeremy.
When I first got interested in “global warming”, the first question that came to mind was how much energy at the frequencies that CO2 absorbs was left to absorb, ie; how much was escaping to space. When the answer came back as , not that much I realized someone was trying to con me.
michael hammer said
“However, because the GHG effect is so strong over the atmospheric column in effect the surface can only lose enegy at the non GHG wavelengths while the atmopshere can only lose energy to space at the GHG wavelengths.”
This statement is false. The earth does lose energy at GHG wavelengths, it is only the transit time from surface to space which changes, not the net energy flow. GHGs absorb LIR but radiate half toward space and have back to the surface. All LIR enerrgy from the surface eventually gets to space. If your statement were true, the atmosphere would continously warm during the day as it would be trapping LIR while the sun shines. We know that the atmosphere continues to warm for only about 2 hours after peak heating from the sun and then begins to cool. Therefore, the atmosphere is radiating the energy previously received and the time lag is only due to the inherent transit time of an atmosphere.
Only a step change in GHG’s will have a transient affect on the atmospheric temperature. Eventurally, the energy transit time will be re-established and the deltaT will dissapate at a log or hyperbolic rate. GHG’s do NOT absorb LIR, they delay its transit to space. The Earth’s surface atmospheric temperature can be completely determined by the Ideal Gas Law: PV=nrT. Venus is not warmer because of more CO2 in its atmosphere, it is warmer because it is 1/3rd closer to the SUN (2.25 more watts/m2) and its atmoshpere is significantly denser than Earth’s.
GHG’s only slow the transit of enery from the surface to space, they do not prevent that transit. Half of the LIR GHGs absorb are re-radiated toward space and half back to the surface. A step change in GHG’s temporarily change the transit rate until a new equilibrium is established. Steady state GHG concentrations have no net impact on the equilibrium.
Reed, not sure how this impacts your analysis, but we do not measure the temperature of the surface of the Earth, we measure the atmospheric temperature at some nnominal distance abouve the surface. Also, for the vast majority of the energy coming from the Earth, it is just the re-emmission of the energy received from the Sun. Your analogy of an engine, where the heat is generated internally, is a bit of a stretch.
We all know that the Earth would begin to cool with 8 minutes if the Sun suddely stopped shining (forget Novas for a moment). Our atmosphere would stop us feeling it immediately, but only for a short time as all the thermal energy in the atmosphere would quickly dissipate into space. The atmoshpere does have mass so it cann store thermal energy, briefly. We would become a cold, lifeless orb in minutes, GHGs or no.
Bill
JeffC says:
July 21, 2012 at 5:15 am
GHG does not block radiation, it absorbs and then re-transmits … a better term than block would be slows …
Other papers I have read use the term ‘scatter’ which is more correct.
The only way these molecules can heat the atmosphere is if they collide with a non-radiating molecule in the very short time between being hit by the IR photon and the retransmission of that photon’s energy.
David Chamness – ” So as far as I can tell, it’s impossible for the re-radiated IR to heat the atmosphere directly. It can bounce around from CO2 molecule to CO2 molecule for 10 years and never “heat” the nitrogen and oxygen that makes up 98% of the air.”
That doesn’t hold up, David.
I went and looked at the numbers for this some time back. An excited CO2 molecule takes on average 10^-6 seconds (1 millisecond) to emit an IR photon. However, at sea level each molecule collides with other gas molecules (including O2 and N2, I’ll point out) one billion (10^9) times per second. Gas molecules are very friendly that way 🙂
Therefore at sea level pressure an excited CO2 molecule has ~1000 collisions before it can emit IR. What this means is that CO2 will share its energy with collisions, transferring rotational, vibrational, and translation energy with the air around it, and that the air will be at the same temperature as the CO2 mixed with it.
—
Unfortunately, this entire thread fails to hold up. As Eli Rabett pointed out earlier, increased CO2 raises the altitude of emission to space for GHG frequencies in the atmosphere, and the lapse rate means that the emission is from lower temperature gas – less energy leaving. And hence the entire atmosphere warms until the amount of energy leaving the atmosphere to space can balance out what comes in. I would suggest reading the following:
http://scienceofdoom.com/roadmap/atmospheric-radiation-and-the-greenhouse-effect/
For example (to give an actual reference, so you can see that all of this was used in the very earliest papers by Hansen and other climate scientists as they embarked on what became a crusade — Hansen is actually not unreasonable in this early paper) — you can probably find: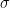 . In fact, he probably underestimates
. In fact, he probably underestimates 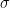 by a factor of two, at least, just from eyeballing the UAH series. This is consistent with what others (notably Koutsoyiannis) have determined analyzing the data — climate scientists for some reason consistently underestimate the variability natural or otherwise of the climate by at least a factor of two. The other is that if one concedes the starting point (which John Nielsen-Gammon pointed out to me is somewhat “cherry picked” not by intent on my part but because the paper in 1981 was predicting the behavior of the climate from 1980 on so that’s what I used) we are fairly clearly resolved at Hansen’s two sigma level for a feedback consistent with his lowest back-of-the-envelope climate sensitivity, the “no feedback” CO_2 only result.
by a factor of two, at least, just from eyeballing the UAH series. This is consistent with what others (notably Koutsoyiannis) have determined analyzing the data — climate scientists for some reason consistently underestimate the variability natural or otherwise of the climate by at least a factor of two. The other is that if one concedes the starting point (which John Nielsen-Gammon pointed out to me is somewhat “cherry picked” not by intent on my part but because the paper in 1981 was predicting the behavior of the climate from 1980 on so that’s what I used) we are fairly clearly resolved at Hansen’s two sigma level for a feedback consistent with his lowest back-of-the-envelope climate sensitivity, the “no feedback” CO_2 only result.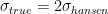 — there won’t even be a significant difference in
— there won’t even be a significant difference in 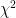 .
.
Science 213, #4511, p 957, 1981 “Climate Impact of Increasing Carbon Dioxide” by Hansen et. al.
on the internet (I did — a scanned version). Note well that he considers a variety of models from “straight CO_2, no feedback” which leads to 1.2 C increase in temperature upon a doubling of CO_2, through models that make the warming much worse when water vapor is included in certain ways (higher clouds, for example) and when he assumes all sorts of complicated macroscopic scale albedo feedbacks, e.g. melting ice caps and glaciers or dying off vegetation. Interestingly, in this early paper, his worst-case scenario warming was only 3.5 C. It’s also interesting to compare the “predictions” of his figure 7 — starting at any reasonable point in the late 70s through the early 80s — to the actual record. I actually did this, using the UAH lower troposphere data for the record in question, as I am deeply skeptical of the GISS or Hadley surface reconstructions — see http://www.phy.duke.edu/~rgb/uah-and-hansen81.jpg — and two things immediately pop out.
One is that Hansen horrendously, egregiously, underestimated
Of course that isn’t true if one shifts his curve down to look only at its slope, or equivalently starts it at different points. The trend in the curve could fit any of his proposed feedbacks if you start it or shift it or just compare the slopes, especially if one admits that
Once again, this is strong evidence that we are all, on both sides of this issues, looking for sheep in the clouds. One can look at this curve and “discover” whatever one wishes to discover. IMO the “best fit” with the different forcings Hansen examines, with complete freedom to shift the two curves vertically (but without doing the actual work as I lack his data and would have to construct some sort of numerical fit to get the curves themselves to compare) is almost certainly the 1.4C curve, but the three curves are narrowly resolved all the way out to 2010 with only 0.2C difference between the 5.8C and the 1.4C curve there! Compare this to a $\sigma_{real} \approx 0.2$ C! It is not possible to resolve this problem with 30 years of reliable data, I’m sorry.
Which is why I think that the only sensible thing to do is wait until it is possible to take any truly expensive measures to combat CO_2. There are reasons quite outside of this curve to think that the climate forcing is not Hansen’s extreme 5.6 C. Even the IPCC seems prepared to back off to 2.8 C (the middle curve) although again, resolving 2.8 from 1.4 from 1.2 from 1.0 is all but impossible on a 30 year (or even somewhat longer) base and allowing for the very real possibility that some unknown fraction of the warming and feedback comes from other causes than those considered in the models.
It might take fifty or sixty years of observations to resolve this issue, where we are only halfway there at best. It might take another ten, or twenty. It might take a full century of observations with modern instrumentation of the Sun and Earth to allow us to build a truly reliable model of the Earth and its climate, where by reliable I mean a model with predictive skill one whole decade in advance.
In the meantime, I personally do think that it is quite reasonable to take moderate public measures to minimize the production of CO_2. In particular, investing money to bring alternative energy technologies to maturity. This is not so much because I think that we are at horrible risk of Hansen’s 5+C catastrophe — I don’t. But even a 2+ C rise could have negative consequences that outweigh the benefits and besides, we need to try to establish a civilization that will last not just the next century but the next 10,000, or 100,000 years. A steady-state global civilization requires energy resources that don’t have to be dug, or pumped, out of the ground. The 21st century is clearly the century where we need to be working this out and transitioning entirely independent of the CO_2 issue! Fossil fuels of all sorts — including uranium and thorium — are good for at most 10ky (and arguably a lot less given exponentially increasing cost of recovery). How are we going to build a steady state civilization on that?
If we could only turn the public debate away from alarmism and panic (and the associated political grabs for money and power) to something like a genuine vision of a future global civilization, we might find that the entire “warmist” versus “denier” debate has been a smoke screen for the picking of our pockets and a diversion away from anything like a sober consideration of investments likely to have a good ROI over the next century and beyond. Some things are unavoidable — we are almost certainly going to reach 500-600 ppm CO_2 before it comes down — if the CO_2 cycle itself is being correctly modeled or described, which is open to debate, I agree, but either way the trend is boringly monotonous and upward at the moment so the default assumption is that this will continue until proven otherwise. IMO pure economics (plus advances in technology) will be the fundamental factor that eventually clips the rise. Depending on how a lot of unknown stuff works out, there will fairly likely be a warming that goes along with this. I doubt that it will be as large as the 2.8 IPCC AR5 estimate, and since AR-X estimates are on a decreasing trend, it seems likely that AR6 will more likely agree with me than with AR5.
Will a temperature increase of 1 C have no negative consequences at all? That’s sort of the boundary, isn’t it. A degree over a century is well within the Earth’s natural variability anyway. Even 2 C is within the range apparent in the proxy records, but that rapid a warming might have negative sequellae. It is the risk of greater warming that does, indeed, motivate at least cautious investments to ameliorate. Even if you think it is 99 to 1 against, the expectation value of the 1% risk is not zero, and deserves a nonzero investment to hedge the bets, especially when that investment is likely to have positive ROI anyway, to be a good idea quite aside from CO_2.
If there is one thing that has been coming out recently, it is the fact that most climate scientists or earth scientists are not extremists or unreasonable or stupid or venal. A lot of them are just as “skeptical” of catastrophic warming as you or I might be on this blog, and the most honest of them admit fully that we cannot be certain even within a full degree C what the temperature is likely to be in the year 2100 assuming a full doubling of CO_2 to 600 ppm and beyond. Many of them would even agree that the warmer estimates are rather UNlikely, but not vanishingly so.
One very interesting question — perhaps even worth asking on this very blog with its many skeptics — is: What do you think the likely warming due to a doubling of CO_2 (from the current, say, 400 ppm to a presumed 600 ppm that is the “doubling” most people refer to compared to a fairly arbitrary 300 ppm base)? Express this as a probability distribution of possible answers, not just the mean answer — the tails are important! What do you think the real risks (expectation value of the costs) of this much warming will be, especially for outcomes in the high end tail (say, only 5% or 10% likely)? What do you think are reasonable investments — things that are likely to have a positive ROI in any event, for example — that could positively impact the projected costs should we end up in this tail region?
The need to answer questions like this in terms of a probability distribution is evident if you play poker or backgammon and have learned to evaluate expectation value. Backgammon is a perfect example. In the game of backgammon (played for money, of course) one can at any point double the stake on one’s opponent. When should you accept such a doubling of the bet?
Curiously, it isn’t when you think you have an even or better chance of winning. It is when you have to 3 to 1 or less chance of losing. If you play four games and lose 3 (doubled) and win 1 (doubled) your expected loss is 4. That is exactly equal to your certain loss turning down all four doubles. If you your chance of losing is less than 3 to 1 — say, only 2 to 1, you will lose 2 out of every three games played identically from this point on — you should accept the double as you will only lose 2 stakes rather than the 3 you would lose if you always turned down doubles at this risk.
This is the sort of risk analysis that one has to mentally perform when looking at “climate futures”. What kind of certain loss now is justified in terms of lowered long term expectation value of cost? The answer cannot be “zero”, not in any sort of sane analysis of the problem. Nor is it 30 trillion dollars.
Sobriety and objectivity are key to making the best decision here. Let go of your passions, your anger, your belief that the world is being manipulated towards the latter investment (even if true). Sure, religious people will always frame religious propositions in terms of Pascal’s Wager, and this only works if Hell is Hell, not if Hell is Heck, or just damn hot, sometimes, with no real damage done. But what is a reasonable assessment of the probable risks? Given a 10% risk of even moderately serious negative consequences, what really is a reasonable strategy of investment in the present, while we wait for sufficient data to improve our estimates?
I’m feeling kinda warmist today, just to balance out my more skeptical days. I think our knowledge is strongly insufficient to resolve the probable temperature question within a whole degree, but I do think that 2+C is not rejectable on the basis of the data so far, and that this much warming over a century and a half is at the outside edge of what naturally has occurred in the climate record. It wouldn’t be surprising if it had negative consequences, possibly balanced to some extent by positive ones, but probably not perfectly balanced. What is it reasonable to assume are the negative and positive consequences of 1, or 2, or even 3 C warming? What are the relative probabilities of each?
rgb
No! Radiation absorption and re-radiation, along with convection and evapotranporation and condensation transfer the heat absorbed by the Sun to a sufficient altitude to radiate to space. If the lapse rate and albedo do not change, the only way greenhouse gases increase the temperature is by raising the average altitude of outgoing radiation to space. It increases temperature at all altitudes in the Troposphere by shifting the entire temperature profile a small amount.
Yeah, like this. Although the lapse rate and albedo might well change along with the water content of the atmosphere. That’s what makes the problem complex instead of just “1.2 C on a doubling of CO_2 from 300 to 600 ppm”, which is interestingly the roughly 0.1C/decade we’ve observed over the last 30 plus years, except that it should be slower than linear because it is logarithmic and hence should slow down (as David Hoffer points out) from 400 ppm to 500 ppm compared to what was observed from 300 ppm to 400 ppm.
It’s those pesky feedbacks that are the problem. Water in the atmosphere changes lapse rates and albedo both. But how? And then there are (possible) longer term feedbacks — changes in ocean temperature, icepack melting, and so on. A complex problem…
rgb
michael hammer says:
July 21, 2012 at 3:52 am
I am extremely sceptical of CAGW but I have to strongly disagree with the above analysis. Adding cooling fins to a motor decreases its surface temperature because it increases the surafce area availabel to radiate that heat away, In the case of the earth the surface area is not increased
======
MikeB says:
July 21, 2012 at 3:52 am
This is a very baffling post Reed, it doesn’t make sense. An air cooled engine does not cool by blocking radiation. It cools by conducting heat away from the engine into the fins and, because they provide a larger surface area, more heat is subsequently radiated away (or convected away).
You both appear to miss the point being made. The radiating surface area has been increased by the addition of radiating CO2 molecules that can be heated by sensible heat (conduction) both from the surface and also from N2 and O2 molecules that cannot radiate the sensible heat they have received.
This would be an extremely simple undergraduate experiment. Set up a chamber of IR transparent material with a heated base and with say 10 liters of a mixture of N2 80% and O2 20%. When the gases have been allowed to stabilize say at 80C by heating from the base release CO2 into the mixture to become 350ppm and see if there is an increase in IR radiation from the gas mixture.
An increase in radiation from the gas mixture would show that the GHG ‘increase the radiative surface’ of the Earth.
Not good to see such kind of postings on WUWT. The guy simply does not understand how the green house effect works.
May be, WUWT should put a scientific explanation of physics behind the climate. “skeptical science” does have a list of “transparent” explanations for the basic physic – but biased. I suggest, WUWT maintains a FAQ about GHG and how greenhouse works – truly scientific, showing what is basic and where are the problems.
Concerning this particular publication, the green house effect in its basic form is trivial. The average temperature at the Earth surface is defined not so much by radiation balance, but by the adiabate: the adiabate holds through the troposphere from the surface up to the tropopause. The adiabate defines the temperature lapse about 1 Grad per 100 meters here.
The visible sun radiation heats the Earth surface. The surface radiates in IR. Most of this IR radiation cannot leave directly to the space because of the GHGs. However, at some particular hight, the atmosphere becomes transparent to the IR radiation. Balancing the incoming sun radiation and the GHG radiation at this particular hight, we can find the atmosphere temperature at that hight. Then we start the adiabate from that height and calculate the temperature at the Earth suface.
Because the atmosphere density is decaying exponentially with the height, the “radiation height” depends logarithmically on the GHG concentration and so is the Earth surface temperature a logarithmic function on the CO2 concentration – we speak about temperature increase per doubling of CO2.
The above is only valid when the “radiative height” is below the tropopause. In the tropopause – there is no temperature lapse. Thus, if the CO2 concentration is so high that the radiative height is withing the tropopause (and for some bands it is already there) – we have the “saturation effect”. A further increase of CO2 levels does not lead to temperature increase at the surface.
Reed…invalid and incorrect on multiple levels. It would be a waste of time to refute all the errors in a comment section, but some info for objective readers to consider. The same “Radiation Transfered by Atmosphere” graph is on page 326 of Slaying the Sky Dragon, in the chapter by Dr Charles Anderson. One should note the top graph has no scale with 5525 K solar insolation and 210-310 K OLR presented side-by-side, implying equality. Therefore the absorbed incoming IR is 20 times the available absorbed outgoing. The absorption/emission cycle is billionth of a second. As the spectral lines indicate, water vapor and CO2 share the most active ~15 micron band and even in the driest locations, H2O molecules are 400x each CO2 molecule. Correct atmospheric physics theory and experimental proofs are posted at Principia-Scientific.org and it would be in the interest of complete scientific analysis for these ‘alternate’ views to be posted. Can there be a Slayers post at WUWT in the future ?
This is a tortured analysis. The analogy between the thought experiment and the earth breaks down because the only source of energy in the engine is internal whilst the primary source of energy for the earth is external.
Reed admits that increased greenhouse gas will absorb additional rising radiation and then emit it in a random direction. A portion of the increased rising radiation that was absorbed and emitted is now heading down and can be considered an addition to the earth’s energy budget.
Going back to the engine – consider it to be like adding an afterburner.
Lots of fun for an old volkswagen guy. It does ignore the lapse rate effect of radiating at higher altitude and therefore at lower temperature, but who knows how this balances against adding all that surface area?
Tim Curtin said:
“Tyndall’s physical laboratory experiments found no evidence for any significant absorption of heat by nitrogen and oxygen in the longwave spectrum, and that meant for him they could not radiate heat to space.”
http://wattsupwiththat.com/2012/07/21/some-thoughts-on-radiative-transfer-and-ghgs/#comment-1038788
This meant they cannot *stop* LW radiation from escaping to space, anymore than a vacuum could. This has all been explained to you at Deltoid, including quotes from Tyndall himself showing you are completely misrepresenting his work. Now you pretend you don’t know Tyndall said the following:
“No doubt, therefore, can exist of the extraordinary opacity of this substance to the rays of obscure heat: and particularly such rays as are emitted by the earth after it has been warmed by the sun. It is perfectly certain that more than 10 percent of the terrestrial radiation from the soil of England is stopped within 10 feet of the surface of the soil. This one fact is sufficient to show the immense influence which this newly-discovered property of aqueous vapour must exert on the phenomena of meteorology.
This aqueous vapour is a blanket more necessary to the vegetable life of England than clothing is to man. Remove for a single summer-night the aqueous vapour from the air which overspreads this country, and you would assuredly destroy every plant capable of being destroyed by a freezing temperature. The warmth of our fields and gardens would pour itself unrequited into space, and the sun would rise upon an island held fast in the iron grip of frost. The aqueous vapour constitutes a local dam, by which the temperature at the planet’s surface is deepened: the dam, however, finally overthrown, and we give to space all that we receive from the sun.
… Its presence would check the earth’s loss; its absence, without sensibly altering the transparency of the air, would open wide a door for the escape of the earth’s heat into infinitude.”
http://books.google.com/books?id=nO8OAAAAYAAJ&pg=PA421&source=gbs_toc_r&cad=4#v=onepage&q&f=false
You insist that Tyndall showed N2 and O2 to be the “real GHG’s”, when he explicitly said otherwise. You also claimed on that thread that A) photons are mostly fictitious B) vacuums don’t exist c) there is no vacuum between the Earth and the Sun. Have you no shame?
alex – “The above is only valid when the “radiative height” is below the tropopause. In the tropopause – there is no temperature lapse. Thus, if the CO2 concentration is so high that the radiative height is withing the tropopause (and for some bands it is already there) – we have the “saturation effect”.”
See Santer et al 2003 (http://www.sciencemag.org/content/301/5632/479.short) among others:
These so called “Thought-experiments” are steadily getting worse, –
“Take for example an internal combustion engine whose metal surface is exposed to a vacuum.”
I can only hope (I never suppose) you are referring to the said engine’s exterior surface.
If you are, then learn this: “All internal combustion engines are ultimately air cooled. Even a “water-cooled, say auto/car engine” gets its cooling water chilled in the radiator, or heat-exchanger, usually situated at the front end of the vehicle. Cooling by radiation even in a vacuum is impossible”
A thermos-flask cannot work if heat could radiate through a vacuum.
Why not do a proper experiment instead of one that only exists in your mind? In other words find a way of lighting an incandescent light bulb in a vacuum and then watch what happens to it.
I used a large glass container – one like the ones they use in kitchens all over the world. The jar had an opening large enough for an incandescent light bulb to be passed through (100 Watt is best – and brings a quick result) and a lid which was screwed down onto the top with a threaded metal ring. If the lid is made of glass (it usually is as mine was) then it is best to substitute that for a metal
one (plywood may be ok but I have never used wood before) because you need to drill two holes through the lid into each of which an engineering nipple (The type of nipple that has nuts and olives and is used by plumbers and on occasions by electricians) are to be fitted. Through one nipple an electrical lead is to be passed and a lamp holder can then be connected. The other nipple is to hold the pipe work necessary for a vacuum pump and ideally a vacuum gauge to be fitted. – Ok, this explanation is too short and maybe not easy to understand, but if you do thik up your own way of dangling a light-bulb in a vacuum then do so, in any case:
Put it all together and after adjusting the light bulb so that it is hanging free (not touching the sides or bottom of the jar, pump out the air so that the gauge, if fitted, shows a “slight vacuum”. Then light the bulb and observe. – If the bulb behaves normally then it is obvious that heat radiates away, if the bulb melts, then —
@ Baa Humbug says:
July 21, 2012 at 5:57 am
Looks like the tech-know.eu site no longer exists. Is there and alternate
link for that pdf please?
Faux Science Slayer says:
July 21, 2012 at 8:40 am
Reed…invalid and incorrect on multiple levels. It would be a waste of time to refute all the errors in a comment section, but some info for objective readers to consider. The same “Radiation Transfered by Atmosphere” graph is on page 326 of Slaying the Sky Dragon
>>>>>>>>>>>>>>>
It would be a waste of time to refute this source and principia-scientific is as sketchy a source of information as the worst warmist sites. Just because it espouses a point of view that resonates with skeptics doesn’t mean it is credible.
The notion that GHG’s serve to cool the earth is absurd. The earth is far warmer than the moon, which gets the exact same amount of insolation, but has no atmosphere. Average temperatures on Venus, which has an atmosphere, are higher than even the peak temperatures on Mercury which gets much higher insolation than Venus, but has no atmosphere.
Re: Convection vs radiance
One happens at the speed of wind. The other happens at the speed of light. Yes major amounts of energy are moved around the system by convection, but the ONLY way that energy ENTERS the system is via radiance and the ONLY way that energy LEAVES the system is by radiance.
(the purists will jump up and down and shout about tidal friction and decay of radiative materials and such and while technicaly accurate, the amounts are insignificant in comparison to insolation)
Ian W;
This would be an extremely simple undergraduate experiment. Set up a chamber of IR transparent material with a heated base and with say 10 liters of a mixture of N2 80% and O2 20%. >>>>
I would refer you to a similar actual experiment by Heinz Hug:
http://www.john-daly.com/artifact.htm
Note the link to the zip file with criticisms of the experiment which is well worth reading and shows that while the experiment is of value, it doesn’t allow us to draw firm conclusions about order of magnitude effects in the atmosphere.
Eli Rabett says: July 21, 2012 at 5:39 am
You wrote:
“Increasing concentrations of GHGs raises the altitude that GHGs can radiate to space in the blocked regions of the spectrum
Because of the lapse rate, the higher you go in the troposphere, the lower the temperature
This slows down the rate at which the Earth emits to space because it is now radiating at higher, colder altitudes”
Eli, I believe you are wrong.
First, any “slow down” in the rate the Earth emits energy to space must be transient–i.e., it can’t last forever. Otherwise, assuming an unchanged input rate of energy, a “slowed-down” energy rate implies an accumulation of thermal energy with time. At some point the outgoing rate must equal the incoming rate or all hell breaks loose. Thus, at some point the “higher/colder atmosphere” must radiate energy at the same rate as the “lower/warmer atmosphere.”
Second, If you surround an active sphere (i.e., a sphere that internally generates thermal energy at a constant rate) with a co-centered, non-touching, spherical annulus where a vacuum exists everywhere else, (a) the temperature of the active sphere will rise above what its temperature would be in isolation, (b) the altitude at which heat radiates away from the sphere/annulus system will be increased and will increase as the outer radius of the spherical annulus increases, (c) the temperature through the spherical annulus will decrease with distance from the common center (i.e., the lapse rate through the spherical annulus will have the same sign as the Earth’s atmospheric lapse rate), and (d) the increased altitude will result in a lower heat-producing-sphere temperature. As with the Earth/Earth atmosphere, Thus, it’s true that heat radiated to space from the sphere/annulus system is radiated from a higher and colder surface, but it’s also true that the surface is also larger. Depending on the thermal conduction properties of the annulus, it can be shown that if the outer radius of the annulus is larger than a threshold radius, increasing the radius of the outer annulus results in a decreasing active sphere surface temperature. The surface temperature of the active sphere won’t drop below the active sphere’s surface temperature in isolation, but as the outer radius of the spherical annulus approaches infinity, the temperature of the active sphere’s surface in the sphere/annulus system will approach the active sphere’s surface temperature in isolation.
If for the above system, you connect the active sphere and the spherical annulus with highly thermally conduction rods, not only will all of the above be true, but depending on the thermal conduction properties of the rods (and the spherical annulus), the temperature of the active sphere’s surface can be made to be lower than the active sphere’s surface temperature in isolation.
I have written a paper that I believe proves the above assertions. That paper is, however, too long (approximately 22 pages) and too mathematical for posting as a guest post. However, I’d be happy to send that paper in PDF form to Anthony (or anyone else) capable of putting the paper on the net and provide a “link” to the paper.
O H Dahlsveen;
A thermos-flask cannot work if heat could radiate through a vacuum.
>>>>>>>>>>>>>
Good to know. That being the case, the earth ought to reach absolute zero in short order as there is no way for the sun to heat the earth due to all that vacuum between the two.
@ Baa Humbug says:
July 21, 2012 at 5:57 am
Have found this page which may be the precursor to the missing pdf.
http://jinancaoblog.blogspot.com.au/2012/01/blog-post.html
Why the scientific basis of greenhouse gas warming is incorrect
“First, placing matter adjacent to a warm surface such that the matter is capable of absorbing/blocking radiation to space from the warm surface can lead to a decrease in the warm surface’s temperature.” It will always lead to an INCREASE in the surface’s temperatue..
In the case of a car radiator, and a Dimetrodon’s sail, one is in effect increasing the radiating surface area- leading to an increase in the rate of cooling. Adding greenhouse gases does NOT increase the radiating surface area of the atmosphere. The atmosphere won’t work like a radiator
gymnosperm:
That is actually not hard to estimate. The idea is to compare the fractional decrease in W/m^2 of the emission as one goes up in the atmosphere (due to the lower temperature) to the fractional increase in surface area due to the larger radius. In mathematical terms, one should compare (1/P)*(dP/dh) to (1/A)(dA/dh) where P is the intensity of emission in W/m^2, A is the surface area of a sphere, and h is the height of the emitting “surface”.
Working through the math [and using the Stefan-Boltzmann Law], one gets that (1/P)*(dP/dh) = (dT/dh)*4/T where T is the absolute temperature. (1/A)(dA/dh) is approximately 2/R_earth where R_earth is the radius of the Earth. A typical lapse rate in the atmosphere is dT/dh = -6.5 K per km and T = 255 K at the emitting level. Putting in numbers, I get that that
(1/A)(dA/dh) is about 0.00031 per km and (1/P)*(dP/dh) is about -0.10 per km, i.e., the emission per unit area decreases about 10% if the emitting level increases by 1 km while the area goes up by 0.03%.
In other words, the effect of lower temperature is about 300X as important as the effect of increasing surface area.
Bill Yarber says:
Time delays are not the correct way to think about situations that involve the continuous emission or absorption of energy at some rate. If someone turned the sun on for a few seconds and then turned it off again, you would be correct that the effect of adding GHGs would be to delay the cooling down but not the final temperature in the absence of a sun.
However, what is relevant to the case of a sun that emits energy to the Earth at a certain rate is that the Earth then has to emit energy back into space at that same rate. For a given surface temperature, GHGs reduce the rate at which energy is emitted to space (because the emission that successfully escapes to space comes from higher levels of the atmosphere where, because of the lapse rate, the temperature is colder). Thus, if you increase the levels of GHGs, the Earth will now be emitting energy at a slower rate than it is absorbing energy. This causes the Earth to warm…In fact, it warms until the radiative balance between emission and absorption is re-established.
If the theory of reradiated heat were to be true then warm liquids placed into a vacuum flask, with its mirrored internal surfaces, would raise the liquid’s temperature by a considerable amount.
This is so, so, irrelevant. The Earth isn’t a vat of passive warm liquids. It is a vat of liquids being actively heated. I know that you know this because I have told you personally over and over.
Now take your flask. Fill it with a liquid and add a heater that turns on for 12 hours a day. Consider it with and without the mirrored surface. Which one on avergage is warmer? Uh-huh. I thought so.
The point of the two figures showing insolation peaked around the visible part of the spectrum, where the atmosphere is nearly transparent, and radiative loss down in the IR is that the Earth is precisely a vacuum flask receiving additional heat every day. Surrounding it with a mirrored “surface” in the form of GHGs that don’t block the incoming heat but do block a fraction of the outgoing het absolutely causes differential warming just exactly the same way that your vacuum flask example would without any question whatsoever be warmer with the mirrored internal surface if there is heat production inside.
rgb
I don’t like analogies since they are hardly ever close enough to reality. They may tell you where to look but they don’t prove anything.
In this case the physics is very clear.
The earth ( by which I mean the earth and atmosphere combined) can only lose heat by radiation.
For the earth’s temperature to remain constant the energy from the sun absorbed by the earth must equal the energy radiated to space.
The energy radiated to space will depend on the temperature of the radiating element and it’s emissivity.
The energy radiated to space is spread across a range of wavelengths from about 3 micron to 70 microns.
If there were no atmosphere all wavelengths would be radiated from the earths surface.
Because of greenhouse gases, energy at wavelengths other than those within the atmospheric window (around 10 microns) are radiated from various levels in the atmosphere.
Wavelengths characteristic of water are radiated from all levels with an average temperature of around 250K whilst wavelengths between 14 and 18 micron (where CO2 absorbs) are radiated from the tropopause at a temperature of about 200K
The energy from the sun is in the UV/optical and very short IR range (less than 3 micron) so the presence of greenhouse gases does not significantly affect the energy absorbed so they do not alter the TOTAL amount of energy radiated either. The energy still has to balance.
Since an earth without greenhouse gases would radiate everything from the surface, the surface temperature would need to be such that it radiated the same as in the current scenario where the various wavelengths are emitted from molecules at temperatures ranging from about 288K (surface) to 200K (tropopause).
An estimate around 260K seems very plausible.
The global warming argument is that if you increase the level of CO2 further the altitude at which radiation in the 14 – 18 micron range takes place increases due to the reduced mean free path of the photon. Higher means colder so the radiation is less. Therefore the temperature at the surface has to increase to compensate.
However at the tropopause the temperature does not increase with altitude so this simple concept does not work. For AGW to be proven they will need to show a movement of the tropopause to greater altitude to allow for the temperature of the tropopause to reduce. I am not aware of any measurement demonstrating this.
So please stop trying to argue against the simple physics of greenhouse gases which is very sound and instead focus on the real issue of what the very very complex system of gases which makes up our atmosphere actually does in practice.
We need less discussion of simplistic analogies and more measurements.
And more Willis’s to do the analysis for us!
KR says:
July 21, 2012 at 9:29 am
See Santer et al 2003 (http://www.sciencemag.org/content/301/5632/479.short) among others:
Observations indicate that the height of the tropopause—the boundary between the stratosphere and troposphere—has increased by several hundred meters since 1979… This positive detection result allows us to attribute overall tropopause height changes to a combination of anthropogenic and natural external forcings, with the anthropogenic component predominating.
—————
Sorry, not very plausible. One has to monitor tropopause globally.
“Observations indicate…”
Tropopause fluctuates strongly and is at different height at different places. This paper is too weak.
Thus, if you increase the levels of GHGs, the Earth will now be emitting energy at a slower rate than it is absorbing energy. This causes the Earth to warm…In fact, it warms until the radiative balance between emission and absorption is re-established.
The problem is that it doesn’t “cause the Earth to warm”. The sun causes the Earth to warm, almost exclusively. The GHE causes the Earth to lose heat from the Sun more slowly and hence be warmer than it would have been without it.
Sorry to be picky, but half of the debate is people who do not understand that what you mean — and what a world of climate scientists mean — is not the literal meaning of the words you say. GHGs do not warm anything. They slow the cooling of something being actively warmed elsewhere, by other means, and just like the insulation in your walls makes you house warmer given a furnace inside than it otherwise would be, the Earth end up being warmer with them than it would be without them.
Clearly there are people that are confused by precisely this point. John Marshall, for example, with his “flask of warm” (but not actively warmed) liquid. Insulation doesn’t keep a house with no furnace warmer than the outside — at most it keeps at a uniform equilibrium temperature with the outside.
Yes, it gets very tedious explaining to them over and over again how the GHE works. But it isn’t made easier by claiming that it “warms” anything. It — as you do say — simply elevates the mean temperature until equilibrium is re-established between an internal source of heat delivered directly to the Earth’s surface by unblocked, non-reflected sunlight and heat loss via radiation out of an imaginary surface that contains the Earth and its atmosphere.
Space blankets “warm” humans exactly the same way. They don’t warm anything at all — no blanket does. But they do reflect back a fraction of the heat being radiated away from the human body. Since the human body continuously produces heat that has to be lost, this raises its equilibrium temperature. Insulation in the attic “warms” your house this way. It doesn’t actually warm anything — its a piece of inert spun fiberglass wool with a radiative shield — but it does slow the transmission of heat from the inside of your house to the outside, so that the inside is warmer when your furnace or other heat sources inside are turned on. It can be reduced all the way down to the heat equation itself where the mechanism of heat transmission is left completely ambiguous. If you actively heat a rod at one end, and hold the other end in a “bath” at a constant cooler temperature, then raising the thermal resistance of the pathway(s) in between by any means whatsoever — preventing any fraction of conduction, convection or radiation — will raise the temperature of the end being warmed relative to what it would have been with a better conductive pathway.
That, after all, is why we tend to cook in metal pans instead of asbestos ones. It is why a hundred watt light bulb works fine to heat an EZ-bake oven. It is why one is hotter lying around in the sun than one is in the shade, all things being equal. It isn’t rocket science, and it is utterly silly to claim that it doesn’t take place, that greenhouse gases overhead are incapable of raising the surface temperature relative to what they would have been without them, given active heating of the ground surface.
rgb
….has increased 0.25 °C since 1980, mainly attributed to an increase in infrared-absorbing gases in the atmosphere.”
Henry says
according to my sample it was +0.4 degrees C since 1980, and it was all due to natural causes….
http://www.letterdash.com/henryp/global-cooling-is-here
hint: it has to do with sun -UV-O2-O3 cycle
we still have about 33 years of cooling left….
“The average temperature of the atmosphere has increased 0.25 °C since 1980, mainly attributed to an increase in infrared-absorbing gases in the atmosphere.”
The average temperature between 1910 and 1945 increased by 0.7°C.
http://www.woodfortrees.org/plot/hadcrut3vgl/from:1910/to:1945
Until this, probably natural and twice as large increase has been reasonably explained by the models or orthodox climate scientists, please stop attributing the recent 0.3 °C increase to “greenhouse gases”.
Second, the average temperature decreased by 0.15°C since 2001
http://www.woodfortrees.org/plot/hadcrut3vgl/from:2001/to:2012
I ask where are the “greenhouse gases”?
davidmhoffer says on July 21, 2012 at 10:13 am:
“O H Dahlsveen;
A thermos-flask cannot work if heat could radiate through a vacuum.
>>>>>>>>>>>>>
Good to know. That being the case, the earth ought to reach absolute zero in short order as there is
no way for the sun to heat the earth due to all that vacuum between the two.”
=============
The Sun is radiating a form of energy that has the ability to penetrate the earth’s atmosphere. Upon hitting the top of the surface this energy interacts with the surface atom clusters or molecules. The electrons in the said atoms increase their speed resulting in increased molecular friction. A product of friction is always what we call “Heat”.
Therefore heat is a product of energy-use, it can no more be emitted as radiation than speed can.
Remember radiation cannot be seen by the human eye. Nor can it be “seen” by any modern derivative of the “Thermopile”. All that can be “seen” is the source of radiation. The heat-source can be seen as light. Radiation, at certain wave-lengths, from the Sun only turns into “Light” upon interaction with the atmosphere. – Once again read Tyndall and Fourier.
By the way, sarcasm becomes no-one.
Convection does not occur without radiation or conduction first but it is mostly conduction. Air in direct contact with a hot surface is warmed by conduction. Once it is warm, it begins to convect which pulls cool unheated air in to replace it, which is itself warmed by conduction, convects away, pulls in more unheated air, etc. Any heat loss by radiation by the surface is tiny compared to the heat lost to conduction. Radiation does not become the primary heat loss mechanism until one gets to the top of the troposphere and the air can no longer convect upwards (because the air above is warmer). BUT, what this does is in effect increase the surface area from which radiation is occurring. Consider the surface area of a sphere the size of the surface of the Earth. Now consider the surface area of a sphere the size of the tropopause. That heat is spread over much more area from which to radiate.
Henry@rgb
we had this same argument before
show me, EXACTLY, how much cooling and how much warming each GHG causes?
http://www.letterdash.com/HenryP/the-greenhouse-effect-and-the-principle-of-re-radiation-11-Aug-2011
including the amount of cooling CO2 causes by taking part in photo synthesis
which I guess has been increasing explosively since the last estimate was taken in 1974
Anyone who has done any work with flight qualified electronics, for example, knows that the rule of thumb is that you lose about 10% efficiency for every thousand feet of altitude for a heatsink. Over 10,000 feet, convection cooling is just about useless and the dominant heat loss becomes radiative. Air cooled engines don’t work well at altitude because the air becomes thin. But at the surface, heat lost due to radiation is much less than the heat loss due to conduction to the air and the air then transporting the heat away by convecting.
“Eli Rabett says:
July 21, 2012 at 5:39 am
Sorry Reed, you miss the point, but you are not alone. Eli remembers eminent analytical chemists who missed the same point in print many years ago.
What happens is that effectively GHG block radiation from reaching space across most of the IR.
This includes IR emitted from the GHGs low in most of the troposphere.
Increasing concentrations of GHGs raises the altitude that GHGs can radiate to space in the blocked regions of the spectrum
Because of the lapse rate, the higher you go in the troposphere, the lower the temperature
”
You’ve evidently spent too much time as a character in children’s stories.
first off, ghg blocking is not most of the IR or most of the energy and that’s water vapor. Increasing concentrations of ghgs increase the absorption but also increase the emission (to a slightly lesser extent). As one goes up in the atmosphere, the pressure, and hence the pressure broadening, decreases along with temperature. That affects the bandwidth of emission/absorption line so higher up there is always more absorption very close to the line center but further down there is a significant smearing of the energy over a broader bandwidth which is not going to be affected at the higher altitudes. The lapse rate is nothing more than conservation of energy so it will adapt based upon the power flow in and out of any altitude. That power flow is the total in and out for radiation, conduction, and convection.
What is absorbed in the clear sky is only a fraction of the surface emission and since line width (and peak intensity) depends upon pressure, there are contributions from all heights involved in an emission/absorption line. The net result for a 288.2k surface is about 279 W/m^2 of power leaving the Earth through clear skies (including emissions from the atmosphere). A doubling of co2 would reduce that by about 3.7W/m^2 down to 275 W/m^2. To refresh your memory, the average incoming solar power is 239 W/m^2 after accounting for about 0.3 average albedo which leaves a 40 W / m^2 deficit that must be dealt with by clouds, aerosols, and atmospheric scattering. Otherwise, our average T would have to be reduced to around 280 K for energy balance, reducing the average T to around 45 deg F from around 56 deg F.
As for some some sort of characteristic radiating altitude, it doesn’t exist. Most comes from the surface and, where present, the cloud tops. The lines include components throughout the atmosphere, including some from the stratosphere and beyond. What is actually emitted at some sort of ‘characteristic’ radiating alitude is actually almost nothing. First off, no component from the continuum away from the ghg spectral lines exists for clear skies. Next, the atmosphere above some parcel of air at this ‘characteristic’ altitude will be almost the same pressure and will absorb virtually all of the emissions and will reradiate almost the same amount and so forth as the pressure broadening and temperatures decrease with altitude.
When one looks at the system more as a whole, things get far worse for CAGW. What becomes apparent is that there is a strong net negative feedback at work which precludes the possibility of a positive net feedback.
If one tries to push watervapor as a strong feedback, they come up very short. If one claims that some slightly increased temperature will reduce the cloud cover and hence cause an albedo related positive feedback, one is faced with the severe problem of claiming we are at a maxima where decreased T results in less clouds and increased T results in less clouds and so could never have more than 62% cloud cover, despite examples like Venus which provides an example of 100% cloud cover – even though it’s not h2o vapor clouds. It also means that increased energy going into a more active water cycle results in fewer clouds despite the fact that there is no change in physics to reduce the clouds. This comes from an earlier hansen & lacis paper which ASSUMES the effect rather than the more commonly accepted situation of constant relative humidity as T rises and that has been magically transformed into the gospel.
rgbatduke says:
I said, “This causes the Earth to warm” where the antecedent of “This” that I was referring to was the fact that “the Earth will now be emitting energy at a slower rate than it is absorbing energy”. Clearly, if the Sun isn’t there, the Earth will not be emitting energy at a slower rate than it is absorbing energy.
So, I don’t see anything wrong with my statement. Nonetheless, I do agree with you on the larger point of the sort of things that do seem to trip people like John Marshall up and thus I appreciate your re-emphasis of the fact that it is necessary for there to be input from an external (or internal) energy source like the sun in order for the addition of GHGs to lead to a situation where the Earth is warmer than before.
Richard111 says:
July 21, 2012 at 4:01 am
“As an ex-motorcyclist, first hand experience leads me to agree with much of what Reed Coray writes. As a layman in the sciences I must rely on educational web sites that explain heat transfere and IR radiation and such like. I am led to believe that gases in the atmosphere can absorb OR radiate specific radiation bands dependant on the local temperature. It cannot do both at the same time. Wein’s Law will give the peak temperature at any specific IR wavelength. Using Wein’s Law to look at CO2 I find that the 2.7 micron band peaks at ~800C, the 4.3 micron band at ~400C and the 25 micron band at about -80C!! I understand only limited areas of the Earth’s surface might radiate at up to 50C so the 2.7 and 4.3 micron bands will NEVER be exited enough to absorb any energy from the surface. They might absorb a very little from the sunlight but that is working as a coolant. The so called standard surface temperature of the Earth is said to be 15C, well above the the -80C temperature level of the 15 micron band for CO2. The problem now is most of the CO2 molecules in the atmosphere will be at a temperature comensurate with the adiabatic lapse rate starting at the surface. So assuming a drop of 10C per kilometre altitude air temperature should be down to -80C at about 9.5 kilometres altitude, almost the tropopause. Only then will the CO2 molecules be cool enough to absorb radiation at 15 microns.
BUT! There is indeed nothing to stop the CO2 radiating at 15 microns and some of that radiation reaching the surface. Now another BUT! The surface, except at possibly a small area at the south pole, is well above -80C!! Any element, black body or not, does not absorb radiative energy below its peak temperature.
A CO2 molecule IS a black body with rather specific characteristics. And so is any other gas molecule in the atmosphere.
Since I am completely unable to see any ‘greenhouse’ effect in the atmosphere I need more education. Please post links that will this layman”.
Well done Richard111. As one layman to another let me say that you have a better understanding of the basic physics involved, than most climatologists. The reason why you are unable to see any “greenhouse” effect in the atmosphere is that there simply is none. The “greenhouse” effect is junk science. There can be no such effect. A zillion words have been prattled about CO2 that is based on the unquestioning acceptance of a very simple but erroneous theory. The top physicists scorn the theory – the conscientious layman has to work his way through from ground zero doing all of the tedious maths along the way.
I can’t read all the comments to see if these points were already made:
1) The conc of H20 and CO2 vary in the atmosphere; they are not homogeneously distributed.
2) The charts showing absorption clearly indicate the vast majority of the absorption is due to water vapor and not CO2.
3) In the peak of IR emission from the Earth’s surface, Water already absorbs about 2/3 of the energy where CO2 absorbes. The main CO2 band overlaps the shoulder of the main water band.
Bottom line: CO2 hardly matters. What does matter is water. And as we know, water isn’t just vapor, it’s also condensed droplets that reflect incoming radiation.
And of course, water vapor cools the surface as it evaporates. Then it rises to much higher altitude where it cools and condenses, carrying and releasing heat.
The analysis tends to be static, assumes homogeneity, is clearly incomplete, and has exceedingly poor predicitve value. Let’s just allow the Earth to do what it’s done for a few billion years without our ‘help’. You are welcome to go to the very warm place, Greenies.
If you take a planet without an atmosphere and add one to it, it will impede the outflow of radiation. The surface will necessarily heat until the point at which the incoming and outgoing energy fluxes reestablish equilibrium. That much is simple.
Now, what happens with the GHG effect as temperature increases? The radiation from the surface is sure to have the form of a blackbody radiation curve. As the peak of the curve reaches the region in which the gases absorb and re-radiate, the impedance maxes out. As the temperature moves higher, the GHG effect decreases. We reach the point of maximum warming potential for that particular gas, and the temperature stabilizes there.
A good analogy is floodgates in a dam. You place a dam across a river, and the water rises until it reaches the floodgates, water starts spilling through, and the rise is checked.
Where it gets interesting is, what happens if we add gases with lower warming potential? This is akin to opening up floodgates lower down in the dam. The water recedes. It may continue to flow out of the upper floodgates, but at a reduced rate. The more we open up the lower floodgates, the more the level of the water behind the dam recedes.
In an atmosphere with two major GHG emitters, the warming potential of the shorter wavelength emitter sets the level of the upper “floodgates”, and the warming potential of the longer wavelength emitter sets the level of the lower. If we are at a point where significant “water” (i.e., energy in the analogy) is spilling through the upper floodgates, then adding more lower floodgates will reduce the level of the water behind the dam (i.e., the surface temperature in the analogy).
In the Earth’s atmosphere, we have such a situation. The upper floodgates are set by the warming potential of CH4. Significant energy is spilling out of them. What, therefore, naturally happens when we add lower warming potential CO2 which, in effect, opens up floodgates lower in the “dam”? The retained energy, and necessarily the surface temperature, goes down.
O H Dahlsveen;
By the way, sarcasm becomes no-one
>>>>>>>>>>>>>>>>.
If Einstein was around to respond to your last diatribe he most likely would have used that classic quip “that’s not right, that’s not even wrong”. Your first statement was blatantly incorrect and got the treatment it deserved. Your subsequent response is so far divorced from reality that it hardly deserves even that.
Ken Harvey says:
I think you would be hard-pressed to find physicists who don’t accept the theory. Even among climate change “skeptics” who can be classified as physicists, like Roger Brown (commenting here as rgbatduke), Fred Singer, Will Happer, Freeman Dyson, etc., you don’t find any who reject the basic greenhouse effect theory. And, amongst the larger physics community, there is not only acceptance of the greenhouse effect but generally acceptance of the danger of AGW, as evidence by such things as the American Physical Society statement on climate change, the fact that the two textbooks that we use at RIT to teach introductory physics both discuss AGW, etc., etc.
As a physicist to a layman, let me say that both of you have very little understanding of the basic physics involved and that most climatologists, many of whom were trained in physics or closely allied fields, do.
Juray V says
Second, the average temperature decreased by 0.15°C since 2001
Henry@Juray
True. According to my sample it was about 0.2 since 2000, globally. Not a lot: I think most thermometers used at homes will not have picked up on it, as indeed most people did not. But some places are cooling down faster, like Anchorage. I have two weather stations there that are reporting cooling of about 1.5K (=1.5 degrees C) since 2000. Unfortunately, the worst is still to come.
I suppose if you live in Alaska the writing is on the wall. I would pack my bags….We still have 33 years of cooling lying ahead of us….
http://www.letterdash.com/henryp/global-cooling-is-here
joeldshore;
And, amongst the larger physics community, there is not only acceptance of the greenhouse effect but generally acceptance of the danger of AGW, as evidence by such things as the American Physical Society statement on climate change>>>>
1. Argument from authority
2. The statement by the APS is largely political in nature.
Proposing an alternative analogy.
Water makes a good analogy for all sorts of things, for example electricity. Voltage is analogous to water pressure, amperage to flow, resistance to resistance.
Imagine a lake as Earth’s energy:
Water In to the lake = Energy In to the Earth;
Water Out of the lake= Energy Out from the Earth;
Lake Level = Amount of Energy in the System which correlates to temperature of the system.
The lake’s dam is the atmosphere; it has a hole in it to allow water out analogous to IR.
The question is does adding CO2 to the atmosphere increase the energy out = increase the hole’s size thereby reducing lake level (temperature) or does it decrease the energy out = decrease the hole’s size thereby increasing lake level (temperature).
Honestly, I can see it both ways; but I look at the outgoing radiation profile with its dips that correspond to GHG frequencies and can’t help but conclude the increase in GHG decreases the outgoing radiation (makes the hole in the dam smaller). I could be wrong; the outgoing radiation may be being distributed from GHG frequencies to other frequencies in such a way as to make the others and the total higher than they would be in the absence of GHG’s.
From the beating a dead horse department: The satellite measuring increases in outgoing IR vs. the model outputs:
http://wattsupwiththat.files.wordpress.com/2012/02/image26.png
That shows at least when temperature rises, the energy out increases, which is analogous to the lake level rising increasing the pressure at the hole in the dam thereby increasing flow out which makes sense given what we know about physics (somewhat confirming the analogy); whereas the models predict analogously that as the lake level rises the water flow out decreases, obviously wrong for the analogy and I suspect for outgoing radiation as well.
However, it is radiating at a lower temperature, is it not?
Of couse, I guess there is also the work done raising that air to the tropopause. I wonder how much extra energy all this churn gets rid of.
Bart says
The retained energy, and necessarily the surface temperature, goes down.
Henry says
also wrong: it is such a pity that climate scientists have decided to ‘log” average temps. and not maxima;
there is so much to learn from maxima,
as it tells us about the energy input that we get from the sun
It follows on a natural curve – on an (apparent) 50 year warming followed by a 50 year cooling.
I strongly suspect it has to do with the sun-UV-O2-O3 cycle.
Does 7 x 7 + 1 ring a bell somewhere?
http://www.letterdash.com/henryp/global-cooling-is-here
“the heat eventually escapes to space–otherwise the temperature of the Earth’s surface and the engine would continue to rise indefinitely.”
TY, this is a point i have made in laymans terms for a long time…..IF co2 or anything else was trapping heat, meaning NOT allowing the heat to escape into space then each day the earth would be hotter than the day before and we would have been a cinder long ago…..clearly and obviously the heat energy input from the sun 24/7 does escape back into space and the greenhouse effect simply SLOWS the movement but in no way stops it or reverses it(reversal is REQUIRED for a greenhouse gas to warm the surface it MUST reverse the natural flow of the radiation from the earth towards space).
rgbatduke says on July 21, 2012 at 11:31 am:
“Yes, it gets very tedious explaining to them over and over again how the GHE works. But it isn’t made easier by claiming that it “warms” anything. It — as you do say — simply elevates the mean temperature until equilibrium is re-established between an internal source of heat delivered directly to the Earth’s surface by unblocked, non-reflected sunlight and heat loss via radiation out of an imaginary surface that contains the Earth and its atmosphere.”
=================
Have you ever considered the possibility that the idea that LWIR radiation imprisoned in GHGs will work like a blanket, may very well be totally wrong?
Yes there is a “large raft of scientist” out there who know ever so much about radiation, but can you get one of them to explain to me, and other “disbelievers”, how it is that radio-waves can penetrate a brick wall while IR and other “light-radiation” stays on the outside? – I don’t think so. – Consensus, consensus, con——.
I firmly believe that one unit of Energy Radiation (ER) from the Sun warms the surface, once and once only. The Surface’s Solar Energy Absorption Rate (SEAR) is highly variable depending on many things, color and texture being just two of them. – It is however the SEAR that gives rise to the many variations of weather and/or climate.
It only matter to perpetual motion engines, which can keep on using the same energy over and over again, how long radiation stays in the system. – Once the fuel-tank is empty, the engine stops.
The speed of convection from the surface to the top of the Troposphere is what matters to the Greenhouse Effect.
On the “Dark side of the Earth” convection only happens at and around the Urban Heat Islands (UHIs). In other words it is, in nature,non existent. On an overcast day or on the Sunny side of the Earth, convection is reduced in close relation to the amount of cloud-cover.
Regardless of the composition of its atmosphere, any arbitrary planet in equilibrium will emit the same amount of energy that it absorbs. If the portions of a planet’s atmosphere responsible for radiating IR out into space are at the same temperature as the planet, then the atmosphere will have no net effect on the quantity of IR radiated out into space, and the planet will be the same temperature as it would have been with no atmosphere whatsoever (or if it had an atmosphere with no IR-active gases). If these same radiating slices of the atmosphere are warmer than the surface, the planet will end up radiating out more IR than it otherwise would have, and in order for conservation of energy to be followed the surface of the planet will be colder than it would otherwise have been. And, finally, if the radiating segments of the atmosphere are colder than the surface, the planet will radiate less IR out to space than would otherwise have been the case, and the planet’s surface will be warmer than it would otherwise have been, allowing it to radiate out more energy to make up the deficit caused by the colder atmosphere.
The radiating portions of Earth’s atmosphere are colder than the surface.
Ergo, Earth’s surface is warmer than it would be without greenhouse gases.
…Mr. Watts, a question; do you look over these guest posts before throwing them up here? Because, honestly, it does not do much to improve your credibility when you gladly host things like this, which betray a massive lack of comprehension of very, very basic physics. It’s your site, of course, and yours to run as you see fit, but…If I were you, I might be a bit more picky about things like this post. Quite frankly, this is embarrassing.
I want to thank everyone who commented, both favorably and unfavorably, to this guest post.
To Dr. Brown (July 21, 2012 at 6:41 am). If in anyway I implied that my thoughts were original, I apologize. I’m pretty sure intelligent and knowledgeable people have looked at the issues expressed in my paper and have concluded that greenhouse gases cause global warming. At a quantitative level, the details of their reasoning are likely beyond my ability to either confirm or dispute. It would surprise me, however, to learn of the existence of an analysis that includes all non-negligible thermal transfer phenomena (conduction, radiation, convection, evaporation, etc.) over a rotating oblate spheroid with an uneven surface where the surface and atmospheric gases are heated unevenly (i.e., different rates of heat absorption as a function of location on the surface). In fact, don’t GCM models attempt to do the analysis, and aren’t they continually be adjusted to agree with new temperature measurements as they become available?
To Mike McMilllan (July 21, 2012 at 7:11 am). If the blanket you add contains tubes through which water is pumped to a radiator in the room, yes I do think the blanket adds thermal mass and will make you cooler.
To MikeB. (July 21, 2012 at 3:52 am). I don’t believe I said an air-cooled engine cools by blocking radiation. On the contrary, an air cooled engine cools despite the fact that the cooling plates absorb energy radiated from the engine’s original surface and re-radiate some of that energy back to the engine’s original surface. To me, this behavior is comparable to the claim that greenhouse gases in the Earth’s atmosphere absorb some of the surface outgoing radiation and re-radiate some of the absorbed energy back to the Earth’s surface. If the latter always produces an increase in the Earth’s surface temperature, why doesn’t the former always produce an increase in the temperature of the engine’s original surface temperature? The other issue is what happens to the temperature of the radiating plates, which corresponds to what happens to the temperature of the atmosphere. I believe that under some conditions, adding additional material to the plates will reduce the plate temperature. Thus, it doesn’t seem unreasonable to think that increasing the amount of greenhouse gas might reduce the atmosphere temperature.
To Arthur (July 21, 2012 at 4:37 am). I’ll stipulate that the “the quote I dug up is from an educational website aimed at schoolchildren.” And that “more detailed analyses have been [being] published in the scientific literature since Victorian times?” My questions to you are: Who exactly are the AGW alarmists trying to convince when they say greenhouse gases heat the Earth’s surface and that heating will lead to impending doom, scientists or the general public? And, if you’re trying to convince scientists in the field, why make simple arguments at all? I believe the primary goal of the AGW alarmists is to convince government officials (i.e., people in power) that the world is coming to an end and we’d better get on the ball. From what I’ve seen of politicians (e.g., Senator Barbara Boxer), I’ll take schoolchildren over politicians any day. Simple arguments, which I believe are at best incomplete, are used to convince the general public, who in turn it is hoped will then put pressure on the powers-that-be to implement the AGW alarmist solutions. Fair enough. By the same token, I get to present simple scenarios that seem to contradict the simple arguments. See, I do believe in my own ignorance. I also believe in the ignorance of the general public; and will continue to point out the potential flaws and/or contradictions of “simple arguments” used to sway the general public.
To JeffC (July 21, 2012 at 5:15 am). Under what conditions does the presence of greenhouse gases in the Earth’s atmosphere quit “slowing-down outgoing radiation?” I ask this because if the outgoing radiation is “slowed down” for all time, then doesn’t thermal energy accumulate within the Earth/Earth-atmosphere system for all time? Wait you say, at some point the temperatures of the Earth/Earth-atmosphere system will rise to levels where the rate energy leaves the system is the same as the rate energy enters the system. Fine, at that point “no slow-down in the rate radiation leaves the Earth” exists. But didn’t your statement imply greenhouse gases slow down radiation? The greenhouse gases haven’t gone away; and as long as they’re present, don’t they slow-down outgoing radiation. So back to my question to you, Under what conditions does the presence of greenhouse gases in the Earth’s atmosphere quit “slowing-down outgoing radiation?”
To Jeremy (July 21, 2012 at 6:09 am) who wrote: “This is a most baffling, confused post which conflates many things and adds nothing.” Ah the human condition. Some things baffle me, some things baffle you. Maybe what I wrote is baffling; but if so, I’m not sure I’d take the word of the bafflee.
Eli Rabett says:
July 21, 2012 at 5:39 am
What happens is that effectively GHG block radiation from reaching space across most of the IR.
This includes IR emitted from the GHGs low in most of the troposphere.
Increasing concentrations of GHGs raises the altitude that GHGs can radiate to space in the blocked regions of the spectrum
Because of the lapse rate, the higher you go in the troposphere, the lower the temperature
This slows down the rate at which the Earth emits to space because it is now radiating at higher, colder altitudes
To maintain radiative balance (sun in, IR out) the entire Earth system warms until the temperature rises enough in the mid troposphere to restore the balance.
=======================================================
What an absurd explanation.
The modern warmism scare is not about “the entire Earth system”, it is about the air temperatures near the surface (2m). Your “increasing concentrations of GHGs raises the altitude that GHGs can radiate to space” gives no additional energy to the surface hence the air near the surface can not get additionally warmer. This is that easy. I hope you know that that the surface warms the air by conduction and convection, so no additional warming of the surface – no additional warming of the air close to the surface. Or reduced cooling, whatever, the point is clear.
I find it hard to believe that increasing CO2 will significantly increase the ‘effective’ altitude at which energy is lost to space.
CO2 is not a major component of the atmosphere, the higher the ‘altitude’ the greater the amount of CO2 needed to increase the altitude, the ‘altitude’ at which energy is lost to space would have depth and that depth would be affected by concentration gradients. (There would be reduced density at increased altitude and a subsequent increase in the effective depth at which energy is lost – i.e. energy would still be lost from lower and warmer altitudes).
Konrad says:
July 21, 2012 at 6:11 am
Outgoing IR radiation radiated back to the Earth’s surface could slow the radiative cooling of surface materials.
====================================================
Yes, this is a notion the warmists like very much, but the problem is they can not prove it. I very patently asked them to present a link to a real scientific experiment proving that notion, but they failed (http://wattsupwiththat.com/2012/06/22/a-response-to-dr-paul-bains-use-of-denier-in-scientific-literature/). Does not look well for the concept.
Reed Coray, I have some sympathy for you trying to come up with novel ways of looking at this subject but I still agree with what Eli Rabbet, Joel Shore and some of the other posters have said about the GHG mechanism. On the other hand, most discussions on the subject seem to ignore the elephant in the room, so to speak. That elephant being the major portion of our atmosphere being the non GHG’s, nitrogen and oxygen.
The major constituents of our atmosphere are non-GHG gases with trace GHG’s being the secret sauce added to the bulk of non-GHG’s that enables the lapse rate structure of our atmosphere. GHG’s in the lower troposphere absorb LWR and due to the atmospheric density (mean time between molecular collisions being much less than the time a GHG gas molecule remains in an excited state), the absorbed energy is thermalized. So the bulk non GHG’s which do not radiate appreciably at normal temperatures are the medium of storage and transport of the heat energy apprehended by the GHG’s, The bulk non-GHG’s are the working fluid in the great thermal engine. The thermalized energy is transported to the upper troposphere by convection, the lapse rate structure being a manifestation of convection.
In the upper troposphere, GHG’s perform a predominantly emissive function. The transition from the absorptive to emissive function occurs when the density of the bulk atmosphere (not the GHG density) is such that the GHG absorption re-emission time is less than the mean time between molecular collisions. In other words, where the GHG’s are able to freely radiate to space the energy carried by the non-GHG’s.
I bring this up to emphasize that our earth’s surface temperature is a function of the earth’s atmospheric mass with GHG’s a necessary trace constituent. So when the concentration of the trace gas CO2 increases, the increase has only a miniscule effect on the height of the tropopause since it is predominantly the bulk atmospheric density that determines at what altitude GHG’s are able to radiate to space. If CO2 is the “control knob” for temperature as certain scientists have averred, then that control knob is logarithmic and there is little temperature to be gained in cranking it up.
Note also that one observes similar lapse rate structures in the Jovian and Venusian atmospheres at altitudes where atmospheric pressures are are in the range of earth’s tropospheric pressures. Yet the Jovian and Venusian atmospheres have very different atmospheric makeup both in greenhouse and non-greenhouse gases than earth’s atmosphere. I think this is a major clue that atmospheric bulk density is the major factor in earth’s climate and temperature regime with non-condensing GHG’s playing a necessary but not controlling role.
This is why I can give some credence of up to about 1C warming for a doubling of CO2 but consider the concept of amplification by water vapour or other positive feedbacks to be bogus. CO2 is a spent forcing, there is almost no warming to be eked out no matter how much coal or oil we burn. Man has little hope of averting the next ice age, either.
Thanks Anthony, a provocative post and an interesting discussion.
Arrhenius estimated that a halving of CO2 would decrease temperatures by 4-5°C and a doubling of CO2 would cause a temperature rise of 5-6°C. In his 1906 publication, Arrhenius adjusted the value downwards to 1.6°C (including water vapor feedback: 2.1°C). Recent estimates from IPCC (2007) say this value (the Climate Sensitivity) is likely to be between 2 and 4.5°C. But Sherwood Idso in 1998 calculated the Climate Sensitivity to be 0.4°C, and more recently Richard Lindzen at 0.5°C. Roy Spencer calculated 1.3°C in 2011.
I will try to make a model for the mind for those steadily repeating about thermos flasks and interpretations of laws of thermodynamics, bearing in mind rgb’s: “I may be wrong”. Imagine two similar and parallel metal planes 1 and 2. Number 1 is connected to an energy source and is radiating energy, let’s say 1000 watts, but in very short pulses each second i.e., 1000 Joule per second and only in one direction, against 2 which receives all the energy. The distance between the planes is half a light second. (Just to make it simplistic clear.) Plane1 emits a pulse against 2 which immediately absorbs the energy and reradiate (or repulsing) in both directions, one straight back to 1 and one out on the other side. This means that 500 Joule goes in each direction. Plane 1 receives, absorbs and reemits this energy in the same moment as it emits its second pulse of 1000 watts, which means it literally emits 1500 watts. This is once again absorbed and emitted in both directions from plane 2, 750 watts in both directions, which leads to a third radiation of 1750 watts from plane 1. Will it add up to infinity? No. It is an infinite geometrical progression: 1 + ½ + ¼ + 1/8… ((1/2)^n) and so on, but it has 2 as a limit. So, Plane 1 will end up emitting 2000 watts because of a feedback process between 1 and 2. Number 2 will also radiate the same but halved for both sides, so 1000 watts will go out of the system. Energy in = energy out. Have energy been created? No, the redirecting process of getting the energy out of the system has just delayed the transport so to say and made plane 1 warmer.
What if we add a third plane? If energy in = energy out, the third plane also has to emit 1000 watts out of the system from its outer side. But that means 1000 watts in both directions, in (back to 2) and out. This means that it actually emits 2000 watts. And to do so it has to receive 2000. That means that plane 2, which also radiates in both directions all together must radiate 4000 watts, which it must receive from plane 1 and 3 in this feedback process. And 1 will end up radiating 4000 watts. Another geometrical progression, but now as 1 + 2 + 4 + 8 +.. (2^n) where 1 is the outer plane and, let’s say 8, is “the ground”. Not so easy to grasp. It seems to be a bit like the hen and the egg, but this actually has a locigal solution
And this last example is what happens between different layers in the atmosphere as far as I have understood. The difference is of course that just a small fraction of the outgoing longwave radiation is absorbed by the adjacent layer. But still this process is surprisingly effective, at least in theory. The US Weather Service has a presentation of an earth radiation budget where 46% of the incoming radiation is absorbed by the surface. In the transformation 7% is sensible heat transfer, 24% is latent heat transfer, and 15 % is long wave radiation. Out of the last 9% is radiated directly to space, and only 6 % is absorbed by the atmosphere. Out of let’s say 240 w/m^2 this is about 14 watts. In a feedback process this would be enough to make the famously 390 watts that creates the 15C surface temperature through 15-20 layers of atmosphere with a reasonable amount of absorption from the GHG. But if this (which it of course isn’t, there is a convection) process alone should be responsible for the temperature upwards it would drop very quickly. The radiative laps rate is very high in the start and is far from linear. But there has to be some sort of additional radiation starting from higher altitude as well because of convection and directly absorption by the atmosphere of incoming energy. But does it happen this way?
This is a layman attempt of understanding, and instead of reading the same misinterpretations over and over again I should really like to read a post here on WUWT where these problems were addressed as comprehensive as possible, based upon an atmospheric model, and not some kind of more or less good parallel. SoD has a lot convincing about radiation, but more that it exists, and less about what I have mentioned here.
This doesn’t mean that Reed Corays post here is not interesting. I think I have read somewhere that on Neptun, the GHGs have a cooling effect, but I should have liked to have a good “traditional reference” before the alternatives are launched.
joeldshore: ” And, amongst the larger physics community, there is not only acceptance of the greenhouse effect but generally acceptance of the danger of AGW, as evidence by such things as the American Physical Society statement on climate change, the fact that the two textbooks that we use at RIT to teach introductory physics both discuss AGW, etc., etc.”
Well sure. If the earth is burnt to a crisp, then it would dangerous. Who wouldn’t agree with that? If the Rapture is tomorrow, then it would be dangerous not to be baptized today.
Now what’s your opinion?
Reed Coray says:
There is something important that you are missing here: The simple arguments that are presented to the public may be vast simplifications but they have underlying them much more complicated and detailed calculations that back up the basic conclusions. Your arguments on the other hand have nothing to back them up.
You seem to somehow be trying to say that because the scientists don’t present the public with thousands of lines of radiation code to explain the greenhouse effect but instead simple explanations, the simple explanations that you come up with that lead to very different conclusions are just as valid scientifically. I hope you can see the obvious flaw of such a notion.
Sleepalot says:
July 21, 2012 at 4:27 am
Show us what equations you are using. Or refer to my page for some ideas.
http://mc-computing.com/Science_Facts/Blackbody/Blackbody_Equations.html
Typically, most people who have problems don’t realize that the equation must be integrated!
Greg House says:
As Eli explained, the increasing greenhouse concentrations create a radiative imbalance that gives additional energy to the entire system. How that energy then gets distributed within the system is a function of the various processes that move energy around, with convection playing a dominant role in the troposphere as you have noted. In particular, since to a first approximation the average lapse rate in the atmosphere is expected to remain roughly constant (at some average between the dry and moist adiabatic lapse rates), if the temperature at a certain altitude rises by 1 C then the temperature at the surface will also rise by 1 C.
To a better approximation, there is expected to be a slight decrease in the lapse rate with increasing temperature because the lapse rate in the tropics tends to closely follow the moist adiabatic lapse rate which decreases [in magnitude] with temperature…iIe., the tropics are expected to warm more at altitude than at the surface. This is reflected in all of the climate models as the lapse rate feedback, a negative feedback. You seem to be proposing that things will warm at altitude without the surface temperature increasing at all, i.e., that the lapse rate will dramatically decrease in magnitude with temperature. However, there is no reason to expect this and no evidence that the warming is occurring in this way; if anything, there has been a discrepancy with the data showing LESS, not more warming, at altitude relative to the surface than is expected (the so-called missing “hot spot”), something that AGW skeptics have made much of (and with some serious misstatements of both the definitiveness of the data and the meaning of the discrepancy). So, it is interesting to now see skeptics such as yourself now apparently claiming that the data is so far wrong that in actual fact things deviate from the models strongly in the OTHER direction than the current discrepancy.
Furthermore, since much of the same physics controls the lapse rate feedback and part of the water vapor feedback in the climate models, models that have a larger (in magnitude) negative feedback from the lapse rate also tend to have a larger (in magnitude) positive feedback from water vapor, so that the uncertainty in the sum of these two feedbacks tends to be considerably smaller than the uncertainty in either individually.
So, to make a long story short, yes, it is to be expected that the surface will warm as the troposphere at higher altitudes warms.
Sam Yates says:
July 21, 2012 at 1:42 pm
Quoted from the end of your comment:
” … Quite frankly, this is embarrassing.”
===================
The discussions on this thread have been excellent IMO. It is likely very embarrassing for any of the believers in the CO2 arguments produced by the team. I’m not sure if your embarrassment was that or of your ending comment itself.
Maus says:
That is not what I was saying. What I was saying was that the statements by the APS and the textbooks represent the general conclusion of the physics community that the science behind the notion that temperatures will rise as a result of the anthropogenic enhancement of the greenhouse effect is solid, a conclusion that I agree with.
I used to wonder how people could wind up believing in witchcraft, blood letting, ghosts, goblins, and other manner of superstitious nonsense that could be so easily debunked by the slightest investigation of the facts combined with a bit of logic. Reading this thread I realise people still cling to superstitious nonsense in the face of facts and logic, all that has changed is the nature of the superstitions.
Thanks to rgb and joeldshore and even Eli (despite his pompous reference to himself in the 3rd person) and others for injecting some sanity into the discussion. Hopefully some of the readership will be prompted to pick up actual text books, learn to understand the calculus and the laws of physics to the point that they can apply the formulas for themselves instead of quoting drivel from web sites that throw terms around and explain away issues that for anyone who has bothered to understand the formulas and how to apply them is nothing but superstitious nonense.
Robert Austin says:
(1) I don’t think it is predominantly the bulk atmospheric density that determines this. Pressure broadening is important but the optical path length is also determined by the concentration of the greenhouse gases themselves. The effect of an increase in CO2 basically is what it is…Are you disputing the radiative calculations that have been done on this?
(2) A logarithmic dependence does not really mean that “there is little temperature to be gained in cranking it up”. It does mean that the temperature with concentration increases more slowly than linearly but it does so in a simple way already accounted for by climate scientists, e.g., it means that if the concentration has to double from 280 ppm to 560 ppm to produce a 3 C increase then it would have to double again to 1120 ppm to produce the next 3 C increase. It is why scientists talk of the sensitivity for doubling CO2 rather than the sensitivity for increasing it by, say, a constant increment of 100 ppm.
Frankly, Robert, I find your post here rather puzzling. You spend several paragraphs making all these statements casting doubt on the radiative effects of CO2. Then, in the end, you seem to admit that you agree with the consensus calculation of what the radiative effect of CO2 alone would do (warm things about 1 C if CO2 concentration doubles) but say that you don’t believe in the feedbacks magnifying that. Then you finish up with a final sentence that seems to go back to casting doubt about the conventional view of the forcing due to CO2.
I can’t figure out what sort of coherent argument you are even trying to make. Why don’t you try to very specifically explain to us what part of the conventional science you agree is correct and which part you don’t?
davidmhoffer says on July 21, 2012 at 12:56 pm:
“O H Dahlsveen;
By the way, sarcasm becomes no-one
>>>>>>>>>>>>>>>>.
If Einstein was around to respond to your last diatribe he most likely would have used that classic quip “that’s not right, that’s not even wrong”.”
===========
You’re guessing again davidmhoffer.
But since you are mentioning Einstein, think of this; “a man is initially moving along steadily at 4 miles per hour (4 mph). He then begins to increase his speed – by the time he reaches 16 mph he has quadrupled hi speed. You may say his speed is now; 4*4 or 4² mph.
However, our Albert has given us an energy equation i.e. E=mc², where E is (=) energy, m = mass and c is supposed to represent the speed of light.
So what is Einstein who believed, and went on to prove, that nothing can travel faster than light, trying to tell us?
As far as I am concerned c² = the speed of light squared is not only not possible, just like your EM radiation idea, but it is also likewise quite useless.
– Unless, of course, Einstein was trying to tell us that we will never work out what E for energy really is. – And that is what I think he did.
Think for yourself davidmhoffer, do some experiments and tell us about your own conclusions. Some parrots can utter human words, but do they therefore think like humans?
Do the simplest experiment experiment possible davidmhoffer. Do the experiment indoors where the air is still and ambient temperature is more uniform. – Take a piece of, say plywood, paint it matt black and lay it on the floor – you now have the very best absorber of IR radiation laying down there. Invert and suspend a hotplate, one that gives off IR and not red radiation, approximately one meter(1m) above the “ideal absorber of IR” and line it up precisely with the piece of black plywood. Measure the temperature (T) of the matt black absorber. Turn the hot-plate on and watch T increase dramatically.
If matt black plywood T does not rise, then I- if I were you – would reject the back radiation from GHGs six miles up theory all together.
joeldshore says:
July 21, 2012 at 3:14 pm
As Eli explained, … … So, to make a long story short, yes, it is to be expected that the surface will warm as the troposphere at higher altitudes warms.
====================================================
He did not say that, so your long story misses the point and obfuscates the matter.
That narrative of his I referred to in my previous comment is only designed to avoid the embarrassment of the main “back radiation” concept of warmists. Like I said, no additional warming of the surface – no additional warming of the air close to the surface. This is a high school level stuff.
joeldshore says:
July 21, 2012 at 3:17 pm
==================
If a conclusion is wrong but published in a book, then does that make it right? I guess that the use of that as a textbook was done by consensus. Specifically, by whom and when?
There is a lot more focus now on the so-called ‘science’ consensus of the past. The future’s greatest skeptics will arise from learning that the textbooks were wrong. Actually today’s greatest skeptics arose from learning that the consensus was wrong. Whether in the form of a textbook or a press release, saying it, writing it, or by consensus doesn’t make it so.
TA: “And 1 will end up radiating 4000 watts. Another geometrical progression, but now as 1 + 2 + 4 + 8 +.. (2^n) where 1 is the outer plane and, let’s say 8, is “the ground”.”
Sorta. Consider a radiating plate, as you had it, and two receiving plates with no distance between them. Assume some fraction of the radiation passes the prior plate but is absorbed by the second plate. What radiation is re-emitted from the latter plate in the sandwich is largely meaningless. When a photon is dumped it will either travel generally toward or away from the prior plate in the sandwich. And if it is toward the prior plate, reaches the prior plate, and is absorbed by the prior plate, then the process repeats. Just as you have it generally.
But place a gap, in a vacuum for convenience, between the plates of the sandwich. Such that a photon generally exiting one bulk and headed toward the other bulk cannot be absorbed by anything. Then the only thing that can or does change is the lateral displacement of a photon leaving one bulk to the other. The entire system is unchanged otherwise. However it’s meaningless so long as we’re positing infinite plates. Lateral displacement is of no interest as there is no worth worrying about where it gets off to as the plates are infinite, parallel, and uniformly irradiated.
Now make it concentric spheres radiated from a external point source. Now, given the geometry, the the lateral displacement matters greatly. For if the enclosing sphere dump a photon across the gap to the enclosed sphere then it is going to get itself off to a point on the enclosed sphere that receives less radiation; by a maniacal average. Nothing fancy about it at all.
Now add a third sphere, between the previous two, and with a gap on either side of it. Any back-radiation from the uppermost sphere will laterally transfer to a point on the sphere underneath it. And should any back-radiation be emitted from that, it will laterally transfer again. Again nothing fancy. But given the geometry, gaps, width and material of the plates, and a given temperature profile for each of then across their surface and depth: Then what are the odds that a given statistical photon, of a chosen wavelength, will exit the system the t time?
It’s really two questions: What are the odds that a given statistical photon will exit its bulk towards an enclosed sphere in t time? And what are the odds that it will exist its bulk towards an enclosing sphere and/or the universe in t time?
This all matters only for reducing your ‘2 in the limit’ value. Which is interesting and perhaps important to model. But it is a purely academic exercise, a ‘toy model’, in that there are numerous heterogenous layers, that are each hetergenous but considered to be homgenously mixed. They are gaseous, have convective currents, and their are no vacuum gaps. All except the lower most sphere, or course. The absorpta spectra are not continuous and is a non-linear relationship with temperature when measured as such. And the whole thing rotates with respect to the point source.
And clouds, of course.
You’re on the right track. But even to get the toy-model off the ground we have to acknowledge that while black-bodies are useful, we do not have one. That geometry matters a great deal. And that energy exchange models via statistical photons are not synonymous with ‘temperature’ as we measure it on airport tarmacs.
joeldshore: “What I was saying was that the statements by the APS and the textbooks represent …”
Apologies. So would your authority agree with the conditional: If the Rapture is tomorrow, then it would be dangerous not to be baptized today?
Reed Coray,
Thanks for a very interesting article!
Robert Austin says regarding CO2:
“…that control knob is logarithmic and there is little temperature to be gained in cranking it up…. This is why I can give some credence of up to about 1C warming for a doubling of CO2 but consider the concept of amplification by water vapour or other positive feedbacks to be bogus.”
Exactly. The planet is just not acting as if CO2 is controlling anything. It may cause a small amount of [entirely beneficial] warming. But with every passing day it becomes more obvious that the CO2=CAGW conjecture is nonsense. Scientific skeptics understand this, but the alarmist contingent is still stuck on stupid; CO2 is not a problem, it is a benefit. More is better.
I am surprised at the number of those posting comments that don’t seem to understand some of the basic physics behind the greenhouse effect. There is little argument among skeptics and alarmists who do understand the physics, concerning whether the greenhouse effect is real or not. The only big arguments I see are over the effect of doubling CO2 and how much of the recent CO2 increase is anthropogenic.
I think it would be of benefit to everyone if someone good at explaining physics to students were to submit an explanation, that Anthony can use as “The Greenhouse Effect” reference page here on “Watts Up With That”. This could be a starting point, that could be commented on and continually improved by the feedback provided by everyone else. The combined expertise of the physicists that post here, together with the questions and comments from those having trouble understanding the greenhouse effect, form a team that could do wonders toward developing a better text on the subject.
The whole idea of GHG “absorbing heat” is erroneous. CO2 absorbs a photon, goes into the “bending” mode of molecular vibration and almost immediately radiates the photon which it absorbed. It cannot give up any fraction of this energy; there is no state between this 667 wavenumber excited state and its vibrational ground state. It cannot “warm the air” The effect of this re-radiation of 15 micron IR is to take upward directed radiation from the surface of the earth and aim one half of it back to the earth;s surface. Same for any GHG. The downward re-radiated IR then becomes part of the surface radiation budget. At the “top” of the atmosphere, all of this must equal the solar radiation absorbed by the surface (average=~238W/sq. meter). Night sky radiance shows about 20% of IR that would otherwise go directly into space is backscattered back to the surface. Let f be the fraction of surface IR absorbed by the atmosphere (~65%) and bs = the fraction backscatterd. Then radiative eq. implies
238=I(earth)*f/2+(1-bs)(1-f)*I(earth) => I(earth)=393w/sq.meter~ 15.5C
I(earth)*f.2=>temp. ay tropopause=128W/sq. meter=>-55.3C
(1962 Standard Atmosphere values for surface and tropopause are obtained by f=.645 and bs=.195)
This gives correct values! Only derivation that does that I have seen. (Yes , it is mine)
Considering the proper physical mechanism gives right answer
(Tropopause height, wet and dry adiabatic lapse rate can be similarly calculated using conservation of energy)
@ Richard111 says:
July 21, 2012 at 9:33 am
I have a copy on file. Send an email to supportATjoannenovaDOTcomDOTau and ask them to forward it on to me. I’ll send you the pdf
Alan D McIntire says: July 21, 2012 at 10:37 am
Alan wrote (bold text below):
“First, placing matter adjacent to a warm surface such that the matter is capable of absorbing/blocking radiation to space from the warm surface can lead to a decrease in the warm surface’s temperature.” It will always lead to an INCREASE in the surface’s temperatue..
(Emphasis mine)
It will? Take a sphere of radius 1 meter, place the sphere in cold space, put a constant thermal energy source symmetrically just below the sphere’s surface, wait for thermal energy rate equilibrium, and measure the surface temperature. Now surround the sphere with material identical to the material of the sphere. Wait for energy-rate equilibrium, and measure (a) the surface temperature of the expanded sphere and (b) the temperature of the sphere at its original radius. The surface temperature of the expanded sphere will be lower than the surface temperature of the original sphere because the area of radiation has been increased. With the same rate of input thermal energy, the increased area implies a reduced surface temperature at energy rate equilibrium. Now, if the material making up the original sphere (and the added material) is highly thermally conducting, the temperature at the radius of the original sphere will be governed primarily by the thermal conduction properties of the materal and can be made to be approximately the same as the temperature of the expanded sphere’s surface. Thus, it seems to me we can placing matter capable of absorbing/blocking radiation to space adjacent to a warm surface and the temperature of the “surface” at the original radius will be lowered relative to its original temperature in isolation.
And to the objection that with the expanded material the original surface doesn’t exist, I respond by saying don’t cover the entire surface of the original sphere. Leave a small area uncovered for a small height–i.e., leave a small cavity in the added material. Put a gas in the cavity. Now (a) we have a portion of the original surface, and (b) because the gas is material that is placed next to the original surface, I believe your claim implies that the area of the original surface exposed to the gas will be warmer than the original surface in isolation. I don’t believe it. Because the sphere is highly thermally conducting, I believe the exposed surface area will be much closer to the temperature of the sphere at nearby points, which can easily be made to be lower than the original sphere’s temperature in isolation.
Can GHGs in the atmosphere receive conducted energy from non GHGs, (I think this is how they form a LTE,local thermal equilibrium) and then radiate that energy away?
It the answer to this question is yes, then are not those CO2 molecules (the ones which receive conducted energy from non GHGs) accelerating the loss of energy to space, which, in the absence of GHGs, would not be able to leave the atmosphere?
John West says:
July 21, 2012 at 7:26 am
Fact: CO2 is a GHG.
Conjecture: Adding CO2 to the atmosphere will increase the temperature.
It seems pretty obvious, but is it true? Is it always true?
========================================================
John, it is much worse than that.
That “fact” is not a fact, it is a conjecture. And they change their narratives if necessary to obfuscate that. All what they have in the hand is the Tindall’s experiment, the rest is conjectures. They do not have any (not fake) experiment proving CO2 ability to warm the surface.
Pochas asks Eli
My house is insulated with an R value of 10. But I am a rugged individualist so I have no connection to electricity or source of fuel. I don’t even occupy the house. I Iive in a tent in the back yard. But I measure and record the temperature inside the housd every hour. At the end of the year I average all the readings. But an environmentalist has convinced me that if I remove all of the insulation the average temperature inside will go down, so I do. What do you think will happen to the average temperature?
Eli responds: since this is a normal house, sunlight will enter through the windows. The insulation will slow the flow of the energy deposited by the sunlight out of the house, thus the interior will heat, as in a car. If the insulation is removed (or the house is leaky), the temperature on the inside will be lower without insulation, but still higher than the outside average. Both convection and radiation work on temperature difference.
Dave (and others) talk about re-radiation by green house gases.
Eli responds: Thee is a relatively rapid transfer of vibrational to kinetic energy in ghg molecules that absorb photons so essentially all greenhouse gas molecules that absorb photons do not re-radiate the energy (the radiation rate is five to six orders of magnitude slower, so only one in a million will reradiate promptly. OTOH there will be some ghg molecules that are excited by other collisions, the proportion being controlled by the local temperature. Some more details
Reed writes
“First, any “slow down” in the rate the Earth emits energy to space must be transient–i.e., it can’t last forever.”
Damn right, and the way the Earth re-establishes equilibrium is by heating up, so that the emission from the top of the atmosphere matches the incoming.
cba accuses Eli of being a character in a childrens novel and throws much detail against the wall which really does not shift the argument much. cb, if you want detail go read the science of doom articles on the greenhouse effect that KR provided. The mechanism remains what the Bunny pointed to.
http://scienceofdoom.com/roadmap/atmospheric-radiation-and-the-greenhouse-effect/
joeldshore says: (July 21, 2012 at 2:56 pm)
Reed Coray says:
There is something important that you are missing here: The simple arguments that are presented to the public may be vast simplifications but they have underlying them much more complicated and detailed calculations that back up the basic conclusions. Your arguments on the other hand have nothing to back them up.
You seem to somehow be trying to say that because the scientists don’t present the public with thousands of lines of radiation code to explain the greenhouse effect but instead simple explanations, the simple explanations that you come up with that lead to very different conclusions are just as valid scientifically. I hope you can see the obvious flaw of such a notion.
No, I’m not saying and didn’t say any such thing. Nor did I “seem to somehow be trying to say…”. I mentioned at least twice that greenhouse gases might warm the Earth’s atmosphere. I just don’t know. I never said that the argument that greenhouse gases cool the atmosphere was scientifically valid. What I said was, the arguments presented in the referenced article (and many other places–see Eli Rabett’s comments on this post) imply conclusions for which counter examples can be constructed. Such arguments are at best incomplete; and if used in an attempt to convince the general public that societal changes having major impacts to mankind must be immediately implemented, then I say shame on the people making those arguments.
Here is a fairly accurate simulator you can run yourself the shows that if anything, without GHG the atmosphere would be warmer at altitude than at the surface.
ferdberple says:
July 22, 2012 at 12:28 am
New jar with updated source. Still seeing negative gradient. Which is interesting. One possible explantin is that GHG actually cools the atmosphere, not warms it. Which explains the hot thermosphere where GHG is rare.
Added time correction at boundaries and increased molecules to 2000. Made the speed and gravity controls exponential to increase their range. Decreased the low range and increased the high. Changed the grid which appears to smooth the motion.
http://www.filedropper.com/gas20120721
Also fixed a few bugs. On occasion the code was throwing NAN’s where there were lots of collisions
rgbatduke says:
July 21, 2012 at 8:08 am
I personally do think that it is quite reasonable to take moderate public measures to minimize the production of CO_2. … we need to try to establish a civilization that will last not just the next century but the next 10,000, or 100,000 years. …If we could only turn the public debate away from alarmism and panic (and the associated political grabs for money and power) to something like a…
========================================================
“We need to try to establish a civilization that will last not just the next century” ??? (shock) This is the most extreme example of alarmism I have ever seen.
And then later in the same comment you said “if we could only turn the public debate away from alarmism and panic“… Your “end of civilization” IS alarmism and panic.
TA. says (July 21, 2012 at 2:37 pm): [snip]
TA, your setup resembles Willis Eschenbach’s “Steel Greenhouse”, still one of my favorite articles on WUWT. If you haven’t already, check it out and see if it answers your questions:
http://wattsupwiththat.com/2009/11/17/the-steel-greenhouse/
I want to thank Anthony for allowing publication of this article. Until now, I assumed that all but a very small minority of WUWT visitors accepted the physics of the basic “greenhouse effect”. The comment thread suggests the number of “doubters” is higher than I thought. 🙁
Robert Austin says:
So the bulk non GHG’s which do not radiate appreciably at normal temperatures are the medium of storage and transport of the heat energy apprehended by the GHG’s,
About six percent of all CO2 at STP is vibrationally excited by the local thermodynamic equilibrium (2 fold degenerate, ~700 cm-1 vibrational energy, and 300K is ~200 cm-1), so essentially 6% is always ready to radiate, of course which molecules are excited is a constantly changing dance, but the amount of emission measured at various altitudes is in accord with this BOE,
I’m not sure you’re right about this.
A larger sphere has more surface area than a smaller sphere.
An earth without any GHGs represents a warm sphere the size of the earth which is radiating IR into space.
Adding GHG’s to the earth creates a larger warm sphere (the earth plus its atmosphere) radiating IR into space. Hence, “the surface area available to radiate the heat away” is increased.
The Sun is radiating a form of energy that has the ability to penetrate the earth’s atmosphere. Upon hitting the top of the surface this energy interacts with the surface atom clusters or molecules. The electrons in the said atoms increase their speed resulting in increased molecular friction. A product of friction is always what we call “Heat”.
Therefore heat is a product of energy-use, it can no more be emitted as radiation than speed can.
Remember radiation cannot be seen by the human eye. Nor can it be “seen” by any modern derivative of the “Thermopile”. All that can be “seen” is the source of radiation. The heat-source can be seen as light. Radiation, at certain wave-lengths, from the Sun only turns into “Light” upon interaction with the atmosphere. – Once again read Tyndall and Fourier.
By the way, sarcasm becomes no-one.
Perhaps not, but Adam Sandler fans will recognize the following:
Mr. Madison, what you’ve just said … is one of the most insanely idiotic things I have ever heard. At no point in your rambling, incoherent response were you even close to anything that could be considered a rational thought. Everyone in this room is now dumber for having listened to it. I award you no points, and may God have mercy on your soul.
Radiation cannot be seen by the human eye? Sheesh. Radiation from the sun only turns into light in the atmosphere? For the love of God, man, go steal a physics book and read it — or visit the ones I’ve put on the web for free — before ever opening your mouth in a public forum discussing it again.
rgb
davidmhoffer says on July 21, 2012 at 3:33 pm:
“I used to wonder how people could wind up believing in witchcraft, blood letting, ghosts, goblins, and other manner of superstitious nonsense that could be so easily debunked by the slightest investigation of the facts combined with a bit of logic. Reading this thread I realise people still cling to superstitious nonsense in the face of facts and logic, all that has changed is the nature of the superstitions.”
============
Dear davidmhoffer.
Please tell me why does CO2 absorb LWIR radiation from the ground but not from other GHGs? – You see if your nice and neat theory is correct there can be no end to warming by GHGs never mind how few they are.
I know it is fashionable to adjust the GHG theory as you go along, i.e. it’s not a greenhouse – it’s a blanket. – GHGs don’t heat the surface it just retains the heat that was here yesterday. – Or, the best and most convincing one of them all: I’m, or we are, not doubting that CO2 has a positive radiative heating effect in our atmosphere, due to LWIR re-radiation, that is well established by science.”
Well established by science? – Who’s science? – a science that turns a blind eye to “conduction” rejects convection and still clings to the theory that beauty is a thing that can be radiated across the room”
Keep your witchcraft, blood letting, ghosts and goblins in your own poxy cupboard oh righteous one.
davidmhoffer says:
July 21, 2012 at 9:47 am
The notion that GHG’s serve to cool the earth is absurd. The earth is far warmer than the moon, which gets the exact same amount of insolation, but has no atmosphere.
==========================================================
Unfortunately you have committed a logical fallacy. Even if you had made a correct comparison “with atmosphere – without atmosphere” (the other conditions being equal), you could have only conclude something on atmosphere, no more than that. You comparison however does not prove anything about any specific part of the atmosphere.
rgbatduke says on July 21, 2012 at 4:55 pm:
“Perhaps not, but Adam Sandler fans will recognize the following:
Mr. Madison, what you’ve just said … is one of the most insanely idiotic things I have ever heard. “
============
So, you call on Adam Sandler fans to recognize something somebody else said.
When are you warmists and Co2 fans going to say things like: “I know, because I have done the necessary experiments?” – And when are you going to stop hiding behind the “somebody else said” quotations?
You AGW lot have got no personal experience of science, no personal opinions and no personalities. STOP QUOTING OTHER PEOPLE, LET US HEAR WHAT YOU HAVE GOT TO CONTRIBUTE!
I may admit I may not always be right but sure as hell you lot do not even know the people you are quoting.
rgbatduke says:
July 21, 2012 at 11:31 am
GHGs do not warm anything. They slow the cooling of something being actively warmed elsewhere, by other means, and just like the insulation in your walls makes you house warmer given a furnace inside than it otherwise would be, the Earth end up being warmer with them than it would be without them.
=======================================================
I am very surprised, I thought the “insulation” argument was dead, but no…
Apparently your GHGs do not work like a house nor like a greenhouse. Even Wikipedia has abandoned this narrative recently.
An enclosed space stays warmer only when being heated and second because the warmer air can not escape and be replaced by colder air from the outside. If you mean it is because of “back radiation”, then you need to prove it, and you know very well that neither you nor other warmists have ever presented a real experimental proof. All what we have seen are either fakes or unrelated stuff or “thought experiments”.
You might get a better example than your engine block for heat transfer problems from this document. It’s a very interesting read.
http://www.projectrho.com/public_html/rocket/supplement/Presby_Engineer_Degree_Thesis.pdf
“We need to try to establish a civilization that will last not just the next century” ??? (shock) This is the most extreme example of alarmism I have ever seen.
And then later in the same comment you said “if we could only turn the public debate away from alarmism and panic“… Your “end of civilization” IS alarmism and panic.
Piffle. Civilization requires energy. Lots of it. In fact, a direct measure of the growth of civilization — perhaps the direct measure of the growth of civilization — is the per-capita availability of energy, its cost per capita. If energy is abundant enough, we can desalinate the oceans and make the deserts bloom. If energy is abundant enough, the standard of living of every person in the world can be increased to that of today’s wealthiest individuals and beyond, just as today’s lower-middle-class first world citizens are wealthier in all the ways that matter than the kings of the world a mere century or two ago. Energy poverty is the worst poverty of all, a kind of poverty from which there is no escape.
I’m not talking about things in my own lifetime — I’ll be dead long before any of these things matter to me or to my own children or possibly even my grandchildren (who are just now starting to be born). But fossil fuels were and are never going to be more than a stopgap, a stepping stone, a boosting point that we can use to uplift human civilization to a state where energy scarcity isn’t the fundamental scarcity, the one that dominates and limits all other scarcities. You can mine coal and oil and natural gas all you want and — assuming that you are correct and CO_2 truly isn’t any sort of risk whatsoever — you will still run into issues of scarcity and political and economic control that make them undesirable fuels to use in the very long run.
We once heated our homes with wood. Wood seemed inexhaustible — until we cut down all the trees from from measurable fractions of the surface area of the world to burn for fuel and were still hungry for more. Coal then seemed inexhaustible — and to some extent still does — if one neglects the risk and hidden costs of mining it. Oil was plentiful, but oil fields proved finite and ever more expensive to find and exploit, and our thirst for it is inexhaustible because everybody wants to drive cars — not just in the US, everybody in the world, all 7 billion of them (including the children too young to drive). Everybody wants their own car, and fuel for their car so cheap that they can go anywhere they like. There are no limits on our desire for cheap energy and our ability to put that energy to work to make our lives better.
I (as a physicist) can see precisely two energy resources capable of sustaining the human race ‘indefinitely’ — long enough that we will have recognizably evolved long before we run out. One is solar energy. The other is thermonuclear fusion. So both are thermonuclear fusion. Geothermal and hydroelectric are distant runners up — inexhaustible in reasonable time frames, but also scarce and far from ubiquitous. Indeed, solar isn’t truly ubiquitous, although we may be able to solve transport problems — to give us total control, we have to master fusion.
I’m not saying these things to institute a panic. How could they? I’m talking about things that will be necessary over centuries, that I think we should very deliberately start working on now. I might say “I think we should work on colonizing the planets” and it is hard to see how that would be causing a panic either. Or that I think it would be truly nifty if we work on computers that interface directly with the human brain and can understand speech and thought. Does that cause you to panic and run screaming that yes we should, or no we shouldn’t? No, it opens up a dialogue where people can contribute and agree or disagree.
So let me state again, in terms that are clear enough not to be misunderstood. I think — that is to say in my opinion — a worthy goal for the human race in the 21st century, that is to say, now, is to pursue two goals before all others. One is freedom from religion. The other is complete energy independence, the establishment of a steady state civilization that does not rely on a fundamentally scarce resource that more or less guarantees a kind of creeping erosion of wealth as it is slowly exhausted. I’ll go one step further and note that the only possible reasonable basis for currency that is somehow more than empty promises and an act of faith is to have a currency that is backed by energy, as the fundamental scarcity. Only such a currency is proof against inflation.
We can debate the need to be free from religion and all of its mythologies and absurdities another time, but I think that it is difficult to argue that your life doesn’t beat time to the tune of energy prices in countless ways, and price is always at some point an expression of scarcity and demand.
rgb
An enclosed space stays warmer only when being heated and second because the warmer air can not escape and be replaced by colder air from the outside. If you mean it is because of “back radiation”, then you need to prove it, and you know very well that neither you nor other warmists have ever presented a real experimental proof. All what we have seen are either fakes or unrelated stuff or “thought experiments”.
Ah, Greg. How exactly can one provide proof to someone who is so ignorant that the actually state that an enclosed space only stays warmer when being heated and because warmed air cannot escape? A real experimental proof is that the Earth — from which warmed air cannot escape and which is constantly being heated — doesn’t become infinitely hot. Now stop being silly.
Also stop being silly about “real experimental proofs”. Open up any physics book and you can read about conduction, convection and radiation as being ways that warmed objects cool. There are mountains of literature on radiative cooling, most of it completely disconnected from climate science. Why do you think physicists silver the inside of Dewar flasks (from the time of Dewar on)?
I mean seriously. It’s just embarrassing. Why not try to learn something before making silly — and false — statements?
Personally, I can “experimentally” observe back radiation as you like to call it just sitting out on a shaded porch next to a sunny piece of pavement, or getting into a heated car. Not that there is any difference between back radiation and the other kind, since there is no other kind, radiation is radiation.
rgb
rgb
wobble says:
You missed this post http://wattsupwiththat.com/2012/07/21/some-thoughts-on-radiative-transfer-and-ghgs/#comment-1039067 where I showed that a reasonable estimate of the two effects (radiation decreases with height of emission layer because it gets colder and radiation increases with height of emission layer because surface area increases) shows that the first effect is about 300 times greater than the second.
Getting very confused here …
rgbatduke: “GHGs do not warm anything. They slow the cooling of something being actively warmed elsewhere”
Ok.
Camping in the “Outback”. Nights get cold much more rapidly under clear skies than cloud cover, even allowing for “bioclimatic comfort zone”, eg evaporative cooling less, RH higher.
(Not hard to check these days – $50+ temp/RH data logger?)
O H Dahlsveen: “On the “Dark side of the Earth” convection only happens at and around the Urban Heat Islands (UHIs). ”
Don’t think so. My observation (of tropical UHI) is that convection continues to occur after dark.
With TUHI the problem is that absorbance (by concrete in particular) throughout the day can take longer to re-radiate than it takes for the sun to return. Re-radiation is of course inwards as well as outwards. 200mm exposed concrete walls at 19°S can take 7 months to cool down.
Bitumen gets much hotter but cools down faster. Cattle camp on unfenced bitumen roads early, but move off well before dawn (watch your speed approaching the brow of a hill).
rgbatduke says:
July 21, 2012 at 5:41 pm
someone who is so ignorant…stop being silly…stop being silly…embarrassing… silly…
====================================================
What a high level, I am impressed.
Reed Coray says:
If you find the arguments incomplete, then the best solution is to search out more detailed arguments or to look at the online radiation codes or what have you. If someone in chemistry tells me of a fundamental accepted concept in chemistry and gives me simple arguments that I don’t find entirely convincing, I would not go off and write blog posts saying that I think it is wrong and presenting lots of counterarguments and examples. First, I would endeavor to understand in more detail the evidence and calculations and theoretical understanding that has gone into coming up with that conclusion. And, I would not say that the person making the argument should be ashamed of himself for not inundating me with lots of chicken-scratch chemical formulas up on the blackboard.
Besides which, in my experience dealing with those who deny the reality of the greenhouse effect, I have found that the issue is not with the quality of the arguments that are presented to them. The issue is that they have a mental block and are unable to process scientific notions that conflict so strongly with their worldview.
My very simple question for Joeldshore and Eli Rabett, can non condensing radiative gasses such as CO2 radiate as IR energy they have acquired conductively? All that is required is a simple yes or no answer. The name “Pierrehumbert” need not be invoked. Just yes or no.
Bucky Cochrane says:
July 21, 2012 at 4:12 pm
The whole idea of GHG “absorbing heat” is erroneous. CO2 absorbs a photon, goes into the “bending” mode of molecular vibration and almost immediately radiates the photon which it absorbed. It cannot give up any fraction of this energy; there is no state between this 667 wavenumber excited state and its vibrational ground state. It cannot “warm the air”
Sorry Bucky but it does warm the air. The CO2 molecule is always vibrating from collisions with other air molecules. Due to these collisions, translational motion and vibratory motion are freely and very quickly exchanged at the gas pressures and temperatures typical of the lower atmosphere. This is precisely why gamma, the ratio of specific heats of gases comprised of triatomic molecules differ from diatomic molecules which differ yet from single atoms. The difference is due to the additional degrees of freedom to vibrate that effectively hide some heat energy from contributing to pressure and temperature. Rapidly cool the gas and, as long as there are collisions, the vibrational energy is released along with the reduction in translational energy. This process is very quick and fully reversible. There is no difference between vibration due to a collision and that due to absorbing a photon.
joelshore says:
“…in my experience dealing with those who deny the reality of the greenhouse effect, I have found that the issue is not with the quality of the arguments that are presented to them. The issue is that they have a mental block and are unable to process scientific notions that conflict so strongly with their worldview.”
If it were not for psychological projection, joel shore wouldn’t have much to say.
A ‘worldview’ is not the same as real world evidence. Empirical [real world] evidence shows clearly that GHG’s have no measurable effect.
eyesonu says:
I think that how the RIT Physics Department chose those particular textbooks is largely irrelevant since they are not obscure textbooks but among the top few textbooks, used by hundreds if not thousands of universities throughout the U.S. and the world. Besides which, those aren’t the only textbooks that talk about it; in fact, I know for a fact that others do. I was just using it as an example of how ubiquitous and accepted the concept of the greenhouse effect and of anthropogenic global warming is in the physics community.
First of all, my original response was directed at someone who claimed that “top physicists scorn the theory” [of the greenhouse effect]. You may not like argument from authority (although authorities in science are usually authorities for a reason); however, it is even worse when the person using such an argument is making false statements about what the authorities say. So, I was correcting that.
However, your views also incorporate a few fallacies. The first is that because the most famous people in science went against a consensus, that means that most of the attacks on consensus are correct and we can thus safely ignore the consensus. The actual fact is that for every Galileo or Einstein, or whoever AGW skeptics imagine themselves to be, there are probably a thousand people of various levels or seriousness or crackpot-ness that challenge the scientific consensus and are wrong.
A second point is that even when a consensus changes, it seldom sweeps away all of the past views. For example, we still teach Newton’s Laws to our students even though Einstein (and whoever you want to credit for the creation of quantum mechanics) showed that there are regimes in which these laws are no longer an accurate approximation for the behavior of objects.
A third point (closely related to the 1st and 2nd) is that although the scientific consensus might at any time be incorrect, it still represents the best way that we know of to represent the current understanding of the science that we have. If we decide that public policy should ignore science that we don’t like (because, say, it conflicts with our ideological or religious beliefs) then this is a recipe basically to no longer have science help inform public policy, a recipe that most of us scientists (and presumably many non-scientists) find quite repugnant. If you think the current scientific consensus is wrong, it is your job to change the consensus by convincing the scientists of the correctness of your views. If you instead choose instead to influence policy by making either ignorant or deceptive arguments to the public, then scientists will generally conclude that you are trying not to change the consensus but basically just to subvert the role of science in our society when the current scientific consensus disagrees with what you want it to say.
rgbatduke says:
July 21, 2012 at 5:41 pm
I can “experimentally” observe back radiation as you like to call it just sitting out on a shaded porch next to a sunny piece of pavement, or getting into a heated car. Not that there is any difference between back radiation and the other kind, since there is no other kind, radiation is radiation.
=========================================================
What you are trying to make people think you “observe” and what it really is are two different things. And if you claim they are the same you need to prove it. I know, these unproven claims can already be found in some recent “textbooks”, this is very sad.
Your notion about warming back radiation is a misunderstanding from the 19th century and it was easily debunked experimentally by professor Wood in 1909. Look, some warmists have already switched to “radiating from a higher altitude to the space”, they even do not mention back radiation. Some however still do. You guys have a really wide range of conflicting narratives.
So, radiation from a colder body directed to the warmer body is radiation, but no warmist has been able to present a real falsifiable scientific experiment proving that this sort of radiation can warm the warmer body (or slow down it’s cooling, whatever).
Now, your main narrative is that a -18C cold surface radiates IR and some portion of it gets returned by the “greenhouse gases” and warms the surface by 33C. IR cameras have no difficulties however to see through the “greenhouse gases”, hence the most IR escape the “trap”. Given that the small part produces so much warming, just turn off your freezer, open it and enjoy the heat. Be careful, if your hypothesis is correct, you can easily get burned by the freezer’s heat.
This is how ridiculous your warmist theory is. I also hope a notion of reductio ad absurdum is familiar to you.
LET US HEAR WHAT YOU HAVE GOT TO CONTRIBUTE!
I may admit I may not always be right but sure as hell you lot do not even know the people you are quoting.
You not only aren’t “always right” in regard to radiation theory, you are so infinitely wrong that you are, quite seriously, almost stunning in any conversation. Worse, you haven’t a clue that you are clueless, and make your vastly incorrect statements to correct somebody that actually has a clue.
Here’s what I have to contribute. Light is electromagnetic radiation. Go on, look it up. The entire electromagnetic spectrum is light. Radio waves are light. Microwaves are light. Infrared radiation is light. Visible light is a narrow band of light. Ultraviolet radiation is light. X-rays are light. Finally, gamma rays are light. The only thing that differentiates a gamma ray from a radio wave is its frequency and wavelength, and those aren’t even invariant properties — one can in principle doppler shift a radio wave into an x-ray by moving through it fast enough.
Second, the only thing the human eye can see is light. I mean good God, man, why do you think they call it turning on the lights when you enter a dark room?
Third, radiation from the sun does not, for the most part “turn into light” only when it reaches our atmosphere. Again, this is so wrong it is difficult even know how to begin. Children understand this better than that. Sunlight is emitted as light by our very hot sun. It travels as light — both visible and invisible, an entire spectrum of light — through the near-vacuum in between the Sun and the Earth. When it reaches the Earth, in very crude terms some of it is reflected at some point or another by the atmosphere without losing (much) energy, some of it is transmitted, and some of it is absorbed. How much of each depends on a host of things — clouds reflect more energy back to space than clear dry air, but clouds and water vapor also absorb more on the way to the ground than clear dry air. Of the radiation that reaches the ground, some is reflected and again passes more or less completely out of the atmosphere without significant loss, and the rest is absorbed. Of the radiation that reaches the ocean, some is reflected at or near the upper surface, and virtually all the rest is absorbed.
Fourth, if you want to understand the way electromagnetic radiation is created, transmitted, absorbed, scattered, you have to begin by learning Maxwell’s Equations. Maxwell’s equations are the classical partial differential equations that describe the electromagnetic field. They aren’t complete — they are classical and atoms and molecules are really quantum mechanical — but to even think of understanding quantum electrodynamics it helps to start with classical electrodynamics. To understand classical electrodynamics, it would really help you to take a class in introductory physics one day, assuming that your calculus background is up to the task. Even in a first year intro physics course in E&M, like the one I am teaching right now, you would learn all of the things I listed above and more besides — I generally try to teach my students that transmitted electromagnetic power is the flux of the Poynting vector through the specified surface, for example, which is entirely apropos of the current conversation.
If you cannot afford a physics textbook, feel free to use the ones I’ve written — they are available for free online here:
http://www.phy.duke.edu/~rgb/Class/intro_physics_1.php
http://www.phy.duke.edu/~rgb/Class/intro_physics_2.php
and if you want to try to tackle real graduate level electrodynamics, you can try:
http://www.phy.duke.edu/~rgb/Class/Electrodynamics.php
but be warned, it isn’t for the faint of heart and you’ll need a reasonable proficiency with partial differential equations and non-Abelian algebras and Lie groups to get through the book. A knowledge of tensors would also be very useful, but sadly few students (even physics graduate students) have much of one so the book tries to be self-contained in this regard. It is also intended to be the second semester of a two semester series, so it presumes you’ve already mastered the Poisson equation and spherical decompositions and magnetostatics and are ready to get on with Maxwell’s equations and true Electrodynamics.
Now “we lot” — by which I assume you means “warmists” used as a pejorative term — sometimes do know very, very well precisely of what we speak. I, for example, do. And I’m not a “warmist”, for that matter. That smacks of religion, and I can and do justify my opinions about almost anything all the way down to the microscopic level — or admit ignorance.
So it is from a state of very much non-ignorance that I repeat — your previous statement, criticizing the entirely correct statement of Mr. Hoffer who is also no warmist, merely a rational skeptic who doubts the alleged magnitude or importance of the GHE, not its very existence — was something that left anyone who read it very slightly dumber. I could feel my own brain cells reeling in shock from it. Radiation turning into light only when it hits the atmosphere? Eyes unable to see light? It made me feel that my entire professional career, spent teaching people far better than that, has been wasted. How is it even possible for a high school education to turn you out into the world that ignorant? I knew better in grade school.
So your statement was not only not a rebuttal of David Hoffer — it was an open insult to the entire US educational system. It was unamerican! Do you want the entire world to laugh at us?
Hence my unaccustomed vigor in striking down your contribution, which, you will note, I am continuing. I’m quite serious. You owe it to yourself, you owe it to simple honesty to crack a physics book and at least try to understand what electromagnetic radiation is before again entering a public debate on the subject and attempting to correct people that have actually studied it, or teach it.
But of course you won’t, will you? Neither will Greg House, or any of the others that make absurd statements about radiation being unable to be reflected back to a warm surface and thereby slow its cooling. It’s so startlingly ignorant a statement that it makes one want to simply throw one’s hands up in despair. Not even my suggestion to go buy a space blanket and wrap yourself in it to gain firsthand experience of “warming” by trapping your own body’s radiation — an “experiment” you can actually perform at home — will actually get you to do it. Or taking an ordinary light bulb and placing it in front of a sheet of plastic wrap, then in front of a sheet of aluminum foil, to see which one reflects more heat (and note well — reflects heat from something much cooler than the light bulb filament). I could probably think up a half dozen other table top experiments to demonstrate radiative heating and cooling — they are elementary school science fair stuff — but of course to you they can’t exist because you know radiation only turns into light when air molecules experience friction or some other long line of complete, utter, absurdities.
I do declare, with people like you “helping” the skeptical “cause”, it doesn’t need to be opposed — the real warmists of the world can just point at you and wait for people to stop laughing themselves to death. Which is a logical fallacy, of course — you can disbelieve in CAGW because a pink unicorn came to you in a dream and told you to and still be right, just as they can be supported by not entirely implausible arguments and still be wrong, and wise people look at the arguments themselves and not individuals — but it does make it all to easy for sensible skeptical arguments to be dismissed when there exist “skeptics” whose arguments are only a hair better than pink unicorns.
rgb
Michael Hammer,
“Adding cooling fins to a motor decreases its surface temperature because it increases the surafce area availabel to radiate that heat away,…”
You seem to be forgetting that parallel finning at a right angle to the surface being cooled irradiates themselves mostly!!! Without air flow and conduction/convection finning has limited cooling ability. Motorcycles without circulation fans are notoriously finicky about being stopped and idling in hot weather!!
By the time the world runs into scarcity issues the world may no longer be interested in coal, oil, and natural gas.
Then why did you include it in your list of energy sources which will “run into issues of scarcity” that would make it an “undesirable fuels to use”?
Oh, so the problem isn’t a scarcity issue. Your claiming that the problem is with the risk and hidden cost to mine it. Well, first, what’s the risk of mine coal. What risk are you talking about, and isn’t this risk already priced into coal? And what energy source will be risk free? Second, what’s the “hidden” cost of mining coal? Is there a cost of mining coal that we don’t know about because it’s been hidden from us?
And do the “risk” or “hidden” cost have anything to do with CO2? If so, then you’re using circular logic.
What?? When did this happen, and why didn’t anyone tell me that all the trees have been cut down. Wait…I just saw trees today…actually I saw lots of trees…what are you talking about??
Peak oil is so last decade, Dude. Get up to speed. And if oil is so expensive, then it will price itself right out of the energy market, right?
As a physicist you should know that far too little solar energy/area hits the surface of the earth for it to be broadly useful – even if conversion was 100% efficient.
Feel free to explain why fission doesn’t make your list – even your runner up list. Cookie cutter plants that are inexpensive to regulate and operate.
(Obviously, you mean independence from fossil fuels not domestic independence.)
Fine, so initiate the project, but utilize efficient project management principles. Don’t irrationally attempt to accelerate lab experiments into infrastructure builds that we all know will be inefficient and deliver an negative EROEI. That’s pure stupidity.
If fact, we shouldn’t over expend our precious resources on developing energy technologies faster than what’s efficient. Don’t enlist the services of 9 women in your attempt to produce a baby in 1 month. It’s expensive and won’t work anyway. It will still take 9 months.
You even said, that we have centuries to solve this problem. It doesn’t make sense to pretend that we only have 20 years – unless you’re worried about a CO2 tipping point – which after everything you’ve written – seems to be your obvious concern.
But you already admitted that coal isn’t really scarce so this ruins your conclusion. Try again.
rgb,
You have more patience than I would under the circumstances. Much appreciated.
rgbatduke says:
July 21, 2012 at 6:27 pm
Neither will Greg House, or any of the others that make absurd statements about radiation being unable to be reflected back to a warm surface and thereby slow its cooling….space blanket … trapping your own body’s radiation — an “experiment”
==================================================
Of course, it is an “experiment”, but not the experiment.
A blanket reduces or prevents convection and you feel a warmer air therefore, warmed by your own energy, this is what people knew before there was the word “physics”. Then 150 years ago the first warmists came up with the idea of “back radiation”. By the way, the father of warmism Tyndall also had an idea about “cold radiation” inducing cold. This has a potential. After the warmism is eventually dead the “climate scientists” can use it. The “physics” is very simple and obvious: just hold the hand above a frozen chicken and you will feel cold, this must be “cold radiation”! I am sorry, but this is the level the warmism functions on.
rgbatduke say:
Indeed.
Yeah…It is a logical fallacy. But, I think it also does illustrate an important point which is that no matter how good the science is on some particular matter, you will have people not believing it simply by virtue of the fact that it goes against what they want to believe. In particular, it illustrates the fallacy in the claim that the various arguments that you see here and at other websites demonstrate that the science of AGW is clearly too unsettled (to take any policy action), or, to put it another way, it demonstrates the dubiousness of claims to the effect that “If scientists could provide sufficiently strong evidence of AGW then I would be convinced. The fact that I am not convinced demonstrates that the science is not sufficiently strong.”
Faux Science Slayer says:
July 21, 2012 at 8:40 am
Therefore the absorbed incoming IR is 20 times the available absorbed outgoing.
=================================================
Could you please elaborate on the issue of incoming IR? Because warmists have made the solar IR disappear, thus avoiding the necessity to account for, let us say, “inverted CO2 effect” of CO2 letting less solar energy arrive at the surface thus contributing to cooling.
joel shore says:
“If you think the current scientific consensus is wrong, it is your job to change the consensus by convincing the scientists of the correctness of your views. If you instead choose instead to influence policy by making either ignorant or deceptive arguments to the public, then scientists will generally conclude that you are trying not to change the consensus but basically just to subvert the role of science in our society when the current scientific consensus disagrees with what you want it to say.”
Exactly. And as it happens, the scientific consensus states overwhelmingly that CO2 is harmless, and beneficial to the biosphere:
That statement was co-signed by more than 31,400 scientists, all with degrees in the hard sciences — including more than 9,000 PhD’s.
That is the true consensus regarding the effect of CO2 on the biosphere. A small minority in the alarmist crowd pretends they represent the consensus. But clearly, they do not: numerous attempts to obtain as many signatures on their alarmist counter-petitions have ended in abject failure. The total number of signatures attempting to dispute the OISM Petition is but a very small fraction of the OISM numbers. Even if the OISM co-signers were reduced by two-thirds, they would still heavily outnumber the alarmist so-called ‘consensus’. And most of the same names appear repeatedly on the various alarmist counter petitions, so their number is smaller still.
Therefore, the real consensus regarding the effects of CO2 is heavily in favor of the OISM Petition as stated above. There is no scientific evidence showing that CO2 is harmful. In fact, at both current and projected concentrations, more CO2 is better. There is no downside.
If joel shore wants to try and convince the consensus of scientists that he is right and they are all wrong, he has an uphill battle. As shore says: “If you think the current scientific consensus is wrong, it is your job to change the consensus by convincing the scientists of the correctness of your views. If you instead choose instead to influence policy by making either ignorant or deceptive arguments to the public, then scientists will generally conclude that you are trying not to change the consensus but basically just to subvert the role of science in our society when the current scientific consensus disagrees with what you want it to say.” That all applies directly to joel shore, who constantly makes deceptive statements. The onus is on him, not on the true consensus of scientific skeptics.
joel shore is trying to subvert the role of science in our society, because the current scientific consensus disagrees with what he wishes it would say. The true consensus is heavily on the side of scientific skeptics — as is the scientific evidence, which challenges the mistaken conjecture that CO2 causes any harm. It does not, as the lack of any supporting evidence shows.
I don’t know if I should laugh or cry.
Every day, hundreds of thousands, perhaps millions, of engineers all over the world use the exact principles and equations that rgb is explaining to design everything from boilers to ovens to nuclear reactors to freezers…. the list is endless. These things work because that’s how the physcis works and if it didn’t, the designs would fail. But they don’t. Yet House et all still insist that the physics is wrong. Do they suppose that hundreds of thousands of engineers world wide are secretly using completely different physics than what is in the text books? Do they think that engineering is somehow not physics?
If so much were not at stake, this would simply be amusing. But the point is that there IS much at stake, so I find it as frustrating and aggravating as rgb (though he is much more eloquent in his expression of disgust 😉 ).
The research that has been done to arrive at the equations is public. The experiments used to verify the equations is public. The results have been summarized in text books that are used by theoretical physicists and applied physicists (engineers) are public. The products that are designed and built upon these very physics are all around us, every single day, by the tens and hundreds of millions. How much more can it take?
Unfortunately, crass and willful stupidity are also public.
Here’s your answer: Less than what the world is spending now.
Try to remember that technological advances accelerate over time. If you believe that the equivalent of $30 trillion is a reasonable bet against probability weighted negative effects of warming and that such warming will start to negatively impact the world’s wealth within 30 years, then it’s irrational to start spending $1 trillion per year now.
Again, it’s cheaper to hire one woman and allow for 9 months to have a baby. Throwing excessive resources in an attempt to overly accelerate technological development is a waste of resources. Acceleration will occur naturally, and, more importantly, resources can be better focused at a time when they are more valuable (later in time) after more promising directions are identified.
Also, it’s strange that you framed the question of resource spend from a game “winning” perspective when it’s quite clearly more appropriate to use an insurance and/or risk management framework. Do you know anything about these?
joeldshore says:
July 21, 2012 at 6:20 pm
If you think the current scientific consensus is wrong, it is your job to change the consensus by convincing the scientists of the correctness of your views.
========================================================
The problem with the alleged “scientific consensus on climate change” is that it does not exist. This can be very easily derived from a well known study: http://wattsupwiththat.com/2012/04/30/consensus-argument-proves-climate-science-is-political/#comment-972119
davidmhoffer says:
July 21, 2012 at 7:25 pm
Yet House et all still insist that the physics is wrong… hundreds of thousands, perhaps millions, of engineers…The experiments used to verify the equations is public…
====================================================
Physics is just fine. Warmism is not.
“Experiments used to verify the equations is public”… What experiments? All the warmists presented was unrelated stuff like “space blanket” or fakes like the recent one from Al Gore.
Why would Al Gore resort to a fake if he could present a genuine one? The only rational answer would be: he desperately needed one but had no other choice.
Come on, do not beat around the bush with your “millions of engineers”…
Because Mr House, Mr Gore is as incompetant as you.
Physics is just fine. Warmism is not.
>>>>>>>
LOL
ROFLMAO
Greg House inquires about a comment from Faux Science Slayer
July 21, 2012 at 8:40 am
Therefore the absorbed incoming IR is 20 times the available absorbed outgoing.
=================================================
Greg, this starts with a confusion between IRs. IR from the sun (the solar spectrum) extends from ~0.8 to 2.5 microns or so. The region between 0.8 and ~2 microns is call the near IR usually written NIR. There is very little overlap with the region where the greenhouse gases absorb, 2.5 to 20 microns, often called the fingerprint region or mid-IR. Beyond 20 microns you have the far IR (FIR, who said chemists were very original), where water vapor rotational lines are strong.
This is compounded by the usual error of comparing the solar insolation at the sun with the solar insolation at the surface or at the top of the atmosphere. When you do so, the incoming solar, less outgoing reflection (albedo), matches the outgoing IR. If the incoming NIR were really 20 x the outgoing mid/far IR, then the Earth would be hotter than Venus.
Now it’s not impossible that since (a) in addition to radiation, heat is transferred from the Earth’s surface to greenhouse gases via conduction, and (b) convection currents (i) circulate the heated greenhouse gases to higher altitudes where energy transfer to space can take place and (ii) return cooler greenhouse gases to the Earth’s surface, that the process of heat transfer away from the Earth’s surface via greenhouse gases is more efficient than simple radiation from the Earth’s surface.
—————–
It is impossible, assuming I have penetrated the meaning of the obfuscating language.
The green house gases radiating heat into outer space are less efficient at radiating heat than the solid surface is. They do the best job they can but exposing the surface directly to space would be better.
It’s because the heat from the surface has to be transported aloft by convection, the gas cools in the process and so CO2 at -30C and H2O at 0C is radiating heat to space. If there were no atmospheric green house gases the surface at 20C can radiate to space and hotter means more efficient.
Try thinking about adding more matter to your engine in the form of insulation, or just restricting air flow through the engine bay, or adding more matter in the form of rust to your engine cooling system. The engine still has to get rid of the same amount of heat, but the engine core gets hotter.
The surface area argument is also not enough. The atmosphere is a very thin layer relative to the radius of the earth, so the gain in radiating area going from surface to top of atmosphere is minuscule.
For anyone still paying attention at the end of this blog…
The other day I put together a visual of 393 ppm CO2. This number reduces quite nicely to 4 parts per ten thousand. So I bought a plastic bottle with 10,000 bright yellow airsoft BB’s and removed its cardboard central piece to reveal an unobstructed view of all 10,000 BB’s to the viewing world. I then added 4 bright blue airsoft BB’s of the same size.
You can guess the result. Every now and then a single or partial blue will appear or can be identified as one shakes and turns the container. But it is an empty barren field overwhelmed by a sea of yellow. And the blue represents the concentration of a near inert gas. Are highly government grant paid “climate scientists” serious about this? What a joke!! Ask any other scientific discipline, unless their pay is now contingent on the success of the findings after these grants filter through the various “independent” institutions, what their thoughts are on this point.
Think of this as $4 vs $10,000. Or think of it in any equivalent way, the result is the same. How much influence does the expenditure of $4 have on $10,000?
CO2 is rising yes, in 1960 CO2 was somewhere around 300 ppm. I guess that means that in fifty years I can add one additional blue airsoft BB? Maybe it screams to 500 ppm in half that time. Do I add the single additional BB in 25 years? Lets see that would be near 2040, is that a cataclismic date for some? Check the jar. What do you see? Check the non visual models, what are you told.
Konrad says:
July 21, 2012 at 6:11 pm
My very simple question for Joeldshore and Eli Rabett, can non condensing radiative gasses such as CO2 radiate as IR energy they have acquired conductively?
If you mean by conduction, T-V (translational to vibrational) energy transfer, yes.
Greg House says
“Experiments used to verify the equations is public”… What experiments? All the warmists presented was unrelated stuff like “space blanket” or fakes like the recent one from Al Gore.
—————
You simply have not looked far enough. Rather than look on the Internet, which has to much crap and you will not be able to sort out what is crap or not, I suggest you go to a library.
The Al Gore experiment was rubbish. It’s more difficult to do properly than a naive view would expect. But it can be done. Why don’t you do it?
I like the idea of having 7 bottles labeled 1960, 1970, . . . , 2010, and 2020. Then, each should have the appropriate number of blue BBs. This can be used as a visual display of the increasing CO2 problem.
Can you provide a link to the type of container that you purchased?
joeldshore says:
July 21, 2012 at 6:20 pm
==============
I will give you an opportunity to carefully read and edit your comment. Seems to be somewhat of a ramble. Seems to me that you have switched roles and wrote a comment that would be more appropriately directed to the comment you wrote.
dp says:
July 21, 2012 at 5:28 pm
You might get a better example than your engine block for heat transfer problems from this document. It’s a very interesting read.
http://www.projectrho.com/public_html/rocket/supplement/Presby_Engineer_Degree_Thesis.pdf
==============
Interesting. Lotsa deep comprehension required. The heat pipe was the limit for me at one sitting. Very interesting concept there. I’ll add the rest of the paper to my vast wealth of useless knowledge later. It’s that need to know kind of thing!
BTW.
Try it yourselves. Its quite revealing and a lot of fun. Start with a jar of 10,000. (I should have taken four yellows out, but I didn’t – you can). The main point of this exercise is that you will have your atmosphere jar, tuned to CO2 influence.
It is probably pointless to ask you this, but have you never observed, on a moonlit night, the growth of a beautiful cumulus cloud over a distant mountain far away from any possible urban heat island?
I’m not certain why I am still hanging around this thread, but Policy Guy has finally posited an objection that has a certain amount of merit. If you’re prepared to be open minded Policy Guy, I will attempt to explain by extending your analogy.
Imagine for a moment that your jar is the same diameter it is now, but 50,000 feet tall. The blue bb’s are mixed in the exact same ratio as they were before. If I guess that your original jar was say 1 foot tall, that would be 200,000 blue bb’s in the jar. Still only four in ten thousand, but in this use case, scale matters.
What would the cross section be of a single bb? We know that 10,000 fill a 1 foot tall jar, so clearly the cross section of the jar would be something less than 10,000. Let’s take a wild guess and say that it takes 100 bb’s to “cover” the bottom of the jar. Clearly, in a one foot tall jar, four bb’s could don’t even come close to that. But in the 50,000 foot jar, we’ve got 200,000 blue bb’s.
Now imagine a photon going from bottom of the jar to the top in a straight line. The rule that the photon has to follow is that if it hits a yellow bb, it goes straight through. But if it hits a blue bb, it has to stop and take off again in a random direction.
Obviously, in the one foot tall jar, the chance of a photon encountering a blue bb is almost zero. But in the 50,000 foot jar, the possibility that a photon will travel in a straight line without encountering a single blue bb is about zero. The area of “coverage” of 200,000 blue bb’s exceeds the cross sectional area of the bottom of the jar by many, many, many time. By chance, perhaps they are all stacked up in a row on one side? Not likely.
So you can imagine that poor photon trying to escape. Even though Mr Photon only needs to avoid 4 in 10,000 blue bb’s, the chance that there is a straight line through 50,000 feet is pretty much nil. That photon will hit many bb’s and change direction many times before finaly escaping from the top of the jar.
Now double the number of bb’s from 4 per 10,000 to 8 per 10,000. In one foot of jar, that’s almost meaningless. In the 50,000 foot jar, the number of collisions that photon will have with blue bb’s just went way up. There will be many more collisions, and it will take longer for the photon to get out.
Police Guy says:
You might try a similar visual to show how, say, a few parts per million of plutonium in the air would certainly not cause you any harm!
Smokey says:
It is amusing that you think that consensus in science is determined by designing a Soviet-style election whereby only “YES” votes are recorded. And, furthermore, when no attempt is made to determine the qualifications of the signers….Oh wow, they have some sort of degree in “the hard sciences” (rather loosely defined)…Boy is that ever impressive! I love how you guys quibble about the details of how a rigorous poll of scientists is conducted but will then believe something like this that is about as far from scientific as you can possibly get.
That is probably because attempts to circulate such petitions have actually involved the novel idea of only including signers that are clearly qualified to have an informed opinion on the subject…and because organizations representing many, many more than 31000 scientists have made their position on the science crystal clear.
The process of using science to inform public policy actually has defined ways of doing it, of producing consensus documents, that have served our society well. The fact that you even put the OISM petition in the same category is embarrassing.
rgbatduke says:
Indeed.
Yeah…It is a logical fallacy. But, I think it also does illustrate an important point which is that no matter how good the science is on some particular matter, you will have people not believing it simply by virtue of the fact that it goes against what they want to believe. In particular, it illustrates the fallacy in the claim that the various arguments that you see here and at other websites demonstrate that the science of AGW is clearly too unsettled (to take any policy action), or, to put it another way, it demonstrates the dubiousness of claims to the effect that “If scientists could provide sufficiently strong evidence of AGW then I would be convinced. The fact that I am not convinced demonstrates that the science is not sufficiently strong.”
I’ve another simple question for Joel Shore etc : If ”non- radiative” gasses such as N2 and O2, H2 can’t radiate thermal energy in the IR that they have acquired through conduction then a jet of N2 or O2 at 15c emitted by a spacecraft in a vacuum wouldn’t show up on an IR thermal camera would it (but a jet of C02 at 15c would)? The simple experiment’s been done, hasn’t it?
Couldn’t we then use O2,N2,H2 to store vast amounts of energy so long as they were in deep space and as you say they “can’t radiate” much? Just imagine, we could heat up H2 to a million degrees and hardly no heat would radiate from it.
Smokey:
As a public service, let me give you a tip to help you evaluate the quality of your argument. Imagine the reverse situation: Let’s say that the overwhelming fraction of the peer-reviewed papers say that the human increase in CO2 levels is harmless or even beneficial. Let’s say that this opinion is also expressed in statements by the National Academy of Sciences in the U.S. and the analogous bodies in all the G8+5 nations, by the councils of the AGU, APS, AMS, etc.
However, let’s say that there are environmentalists who disagree and argue that it is just grant funding that is biasing the scientists who are then acting as gate-keepers of the journals, that the NAS and other bodies’ statements just represent a small fraction of their members, etc., etc. As evidence, they cite a petition produced by Greenpeace that bombarded the mailboxes as scientists at academic institutions (and maybe other scientific institutions) across the country (world?) with a propaganda piece arguing how the rise in CO2 is dangerous was and then asked them to sign a petition to this effect. And, let’s say 31000 of scientists did and that they supposedly all had some sort of “hard science” degree.
Can you honestly tell me that you would conclude that there is a consensus that the rise in CO2 is dangerous?
Process does matter, Smokey. It is not always about warping reality to fit your ideological worldview.
My it’s quiet isn’t it. It must be getting late.
davidmhoffer says:
July 21, 2012 at 8:52 pm
If I guess that your original jar was say 1 foot tall, that would be 200,000 blue bb’s in the jar. Still only four in ten thousand, but in this use case, scale matters… Now imagine a photon going from bottom of the jar to the top in a straight line…
=======================================================
Very nice calculation. Now imagine instead of “a photon” let’s say 999,999,999,999,999,999,999,999,999,999,999,999,999,999,999,999,999,999,999,999,999,999,999,999,999,999,999,999,999,999,999,999,999,999,999,999,999,999,999,999,999,999,999,999,999,999,999,999,999,999,999,999,999,999,999,999,999,999,999,999,999,999…… photons. A little bit closer to reality than your “a photon”, you know.
I am looking to your scientific explanation of the destiny of these poor photons.
And please think of a freezer or a frozen chicken as a heating device (see above).
Joel,
Darn it, I lost your comment but the jist was, ” you seem to be arguing that at some point the atmosphere increases in temperature.”
I would agrue that.
Carbon dioxide seems to be getting into the stratosphere. I doubt anyone knows how. Ozone holes? The tops of those thunderheads that dome in? As you undoubtedly know at the tropopause the lapse rate is inverted and temperature increases with altitude, eventually reaching levels nearly as warm as the surface.
I’m going to leave the math to you, but the trouble with, “It’s not difficult to calculate”, and, “It’s simple physics”, is that the reality seems not to be easy or simple.
One could argue for Occam if the empirical evidence for warming suggested anything nearly proportional to CO2 emissions…
David,
First, I am very open minded about your response. I’m not sure that I agree, but I am glad that you posted.
By doing so, you have opened the door for different responses from a whole host of many. I am very interested in how to best interpret my small attempt to represent current data as experimental hands on observations.
OK you want to talk about a 100,000 foot jar vs my 1 foot jar, the concentration will be the same. Why is that different from a warming viewpoint?
Again, I am trying to come up with a visual presentation of the current, past and future concentrations of atmospheric CO2. How can I make this attempt better?
Thank you
davidmhoffer says (July 21, 2012 at 8:52 pm): “I’m not certain why I am still hanging around this thread…”
For what it’s worth, those of us still reading (yes, both of us) appreciate the efforts of both you and RGB. 🙂
davidmhoffer says:
July 21, 2012 at 8:52 pm
Is there a way we could talk some more? Can this site facilitate a connection if you agree?
Policy Guy says (July 21, 2012 at 9:45 pm): “OK you want to talk about a 100,000 foot jar vs my 1 foot jar, the concentration will be the same. Why is that different from a warming viewpoint?”
davidmhoffer was illustrating that a short column of the earth’s atmosphere may transmit most of the IR radiation passing through it, but a column the height of the atmosphere may be essentially opaque. The real life situation is illlustrated in the diagram accompanying Reed’s article, which shows 100% of the earth’s outgoing thermal radiation absorbed by the atmosphere at certain wavelengths (mostly by water vapor and carbon dioxide).
Policy Guy;
OK you want to talk about a 100,000 foot jar vs my 1 foot jar, the concentration will be the same. Why is that different from a warming viewpoint?
>>>>>>>>>>>>>>
Imagine that the yellow bb’s are instead invisible. Look at the jar from the side. You’d see a spec of blue here and there, but you’d have to really look closely to find them. Now look at the jar from the top looking straight down. You would see solid blue.
By doubling the number of blue bb’s from 4 in 10k to 8 in 10k, that blue would become thicker. Photons coming up from the bottom, each carrying a tiny parcel of energy, would spend a lot more time working their way up to escape, and some of them would actually wind up going back down and hitting the bottom of the jar, raising itz temperature higher than it otherwise would have been. So, looking at the blue bb’s from the side makes them look insignificant. Looking at them distributed randomly in a vertical column 50,000 feet tall makes them suddenly look pretty thick, and doubling the number from 4 in 10k to 8 in 10k should obviously make a difference to how long it takes any given photon on average to escape, and also raises the chances that any given photon might bounce around to the point that it gets all the way back down, raising the temperature of the surface.
I think it gets tricky to use this analogy beyond that. By doubling the number of blue bb’s, the math regarding how many times any given photon does what get’s tricky. The point here is that if one starts with 4 and adds 4 to make 8, one would get an effect of X. Will adding 4 more for a total of 12 make it 2X? NO! To get 2X we’d need to add 8 for a total of 16. This is why the IPCC refers to 1 degree of warming per doubling of CO2. They explain in detail that pre industrial concentrations were 280 ppm, and so doubling that would raise the temperature one degree. What they gloss over is that it has been more than a century since we were at 280 ppm. The concentration today is close to 400 ppm. So, to get one more degree out of CO2 from where we are TODAY, we ‘d need to add another 400 ppm which at current rates will take about 2 more centuries. To get 2 degrees, we need to get to 1600 ppm which would take a rather long time.
How to represent that with blue and yellow bb’s, I’m not sure. My original point was to show that scale matters, and makes even a trace gas significant when you consider the entire path from surface to top of atmosphere. To extend the analogy further, we’d want to add red bb’s, about 400 of them per 10,000 yellows, to represent water vapour, because water vapour also absorbs (though not as well) in the same spectrum as CO2. To make matters more complicated still, we’d want to vary the concentration of red bb’s from 400 at the bottom, to a decreasing number of almost zero at the top, because as we rise in altitude, temperature declines, and the amount of water vapour that the atmosphere can hold declines with temperature.
Then, we’d want some way to add still more red bb’s in some ratio over time, because in theory, as the temperature rises, the holding capacity of atmosphere for water vapour also increases, so adding blue bb’s means a bit layer in time we’d have to add some more red ones as well.
Where things seem to fall apart for the warmist side is that the red bb’s aren’t increasing in concentration with temperature as expected. So even though warmer air CAN hold more water vapour, it doesn’t mean that it will, and the latest NASA data seems to show that it is holding LESS not more. I could introduce other aspects of the photons path to space that we could explain with the bb analogy, but as you can see it is getting pretty complicated already and we’ve only scratched the surface in regard to all the factors at play.
My contention is that the real achiles heel of the warmist meme is threefold.
1. The logarithmic nature of CO2 (1 degree of warming per doubling of CO2) means that, based on CURRENT concentrations, we’d have to burn stupid amounts of fossil fuel for centuries to get just a couple of degrees of warming.
2. The IPCC assumes feedbacks from increased water vapour and other factors that give a 3:1 boost to the effects of CO2. We’re certainly not seeing anything close to that, and in fact there is growing evidence that the feedbacks may in fact be negative.
3. All the historical and geological records point to the biosphere being most productive at temperatures warmer than we have today, and human civilization thriving in those temperatures and suffering greatly at lower ones. We can’t eat ice! I’m not afraid of a warmer earth, I embrace it. Unortunately I don’t think we’re capable warming it up enough to matter with CO2 even if we were doing it on purpose.
joeldshore says (July 21, 2012 at 9:25 pm): “However, let’s say that there are environmentalists who disagree and argue that it is just grant funding that is biasing the scientists…”
Um, Joel, there’s no “grant funding” for saying everything is fine, nothing to see, move along… Of course, that hasn’t stopped some “environmentalists” from saying there is. Anthony, how big was this month’s check from Big Coal? 🙂
Policy Guy says:
July 21, 2012 at 10:17 pm
davidmhoffer says:
July 21, 2012 at 8:52 pm
Is there a way we could talk some more? Can this site facilitate a connection if you agree?
>>>>>>>>>>>>>>>
mods, please feel free to pass my email address to Policy Guy.
davidmhoffer says:
July 21, 2012 at 7:25 pm
Every day, hundreds of thousands, perhaps millions, of engineers all over the world use the exact principles and equations that rgb is explaining to design everything from boilers to ovens to nuclear reactors to freezers…. the list is endless. These things work because that’s how the physcis works and if it didn’t, the designs would fail.
—–
Ironically this is one of the reasons why I do not believe the GHG radiative warming effect exists (or if it does exist it only very marginal). If the physics is there why hasn’t an engineer designed an engine to take advantage of the effect?
I challenge anyone – create an experiment which will create a ‘Runaway Greenhouse Effect’ using a synthetic atmosphere which will take advantage of the GHG radiative warming effect. Create any experiment which will take advantage of GHG’s to create an engine which will run on radiative energy alone.
Eli Rabbit writes “This slows down the rate at which the Earth emits to space because it is now radiating at higher, colder altitudes”
Easy to say but harder to prove. At that increased altitude it is still the CO2 molecules that radiate according to a probability they have sufficient energy to do so. If you have more of them (as is the assumption that the atmosphere is well mixed) then you have more of them radiating.
Eli writes at his blog “Decreasing temperature slows down the rate at which each molecule can emit while decreasing density means there are fewer greenhouse gas molecules available to absorb or radiate the energy.”
Fewer than down lower thats true, but still more than there were previously at that altitude. so whilst they may be radiating less often, you cant escape the fact there are more of them doing so and its not at all intuitive what the net result is.
Dear RGB, please excuse my poorly articulated (Layman terminolgy) questions in the last paragraph of this post. Please follow the few posts to understand the questions in the last post, and, if you please, provide an answer, or answers if possible.
Can GHGs in the atmosphere receive conducted energy from non GHGs, (I think this is how they form a LTE; local thermal equilibrium) and then radiate that energy away?
If the answer to this question is yes, then are not those CO2 molecules (the ones which receive conducted energy from non GHGs) accelerating the loss of energy to space, which, in the absence of GHGs, would not be able to leave the atmosphere?
====================================================
Eli responds: Thee is a relatively rapid transfer of vibrational to kinetic energy in ghg molecules that absorb photons so essentially all greenhouse gas molecules that absorb photons do not re-radiate the energy (the radiation rate is five to six orders of magnitude slower, so only one in a million will reradiate promptly. OTOH there will be some ghg molecules that are excited by other collisions, the proportion being controlled by the local temperature.
=================================
Robert Austin says:
So the bulk non GHG’s which do not radiate appreciably at normal temperatures are the medium of storage and transport of the heat energy apprehended by the GHG’s,
About six percent of all CO2 at STP is vibrationally excited by the local thermodynamic equilibrium (2 fold degenerate, ~700 cm-1 vibrational energy, and 300K is ~200 cm-1), so essentially 6% is always ready to radiate, of course which molecules are excited is a constantly changing dance, but the amount of emission measured at various altitudes is in accord with this BOE,
===============================
Eli Rabett says:
July 21, 2012 at 8:23 pm
Konrad says:
July 21, 2012 at 6:11 pm
My very simple question for Joeldshore and Eli Rabett, can non condensing radiative gasses such as CO2 radiate as IR energy they have acquired conductively?
If you mean by conduction, T-V (translational to vibrational) energy transfer, yes
======================================
So, RGB the answer appears to be yes, but poorly quantified….”About six percent of all CO2 at STP is vibrationally excited by the local thermodynamic equilibrium (2 fold degenerate, ~700 cm-1 vibrational energy, and 300K is ~200 cm-1), so essentially 6% is always ready to radiate…”, OR…”so only one in a million will reradiate promptly. OTOH there will be some ghg molecules that are excited by other collisions, the proportion being controlled by the local temperature.”
So my questions are as follows. How much of the non GHG energy is radiated to space via collision with GHG molecues? If the GHG molecues were not present, how much longer would this energy stay within the atmosphere if it could only be conducted and convected about, but not radiated to space. And, as additional GHG molecues speed the escape of conducted Non GHG energy, would not this reduced residence time of conducted non GHG energy have to be subtracted from the increased residence time of IR energy raqdiating from the surface, and backradiating from the GHG molecues? TSI incoming is a consistent flow, so the energy gained or lost by either radiating conducted non ghg energy out, or keeping surface energy within the atmosphere is porportional to the residence time of the energies affected.
Konrad writes “CO2 almost instantly re-radiates the outgoing IR radiation it intercepts, with around 50% of this radiated back towards the Earth’s surface.”
Not so. At sea level, the CO2 almost instantly gives up its absorbed energy to the rest of the atmosphere due to a collision. Collisions happen on the order of every 10^-7 seconds whereas it takes on average 10^-3 seconds to radiate. CO2 at sea level is a warming agent for the atmosphere and it occurrs right at ground level within tens of meters.
Ref : Pierrehumbert’s “Infrared radiation and planetary temperature”
http://geosci.uchicago.edu/~rtp1/papers/PhysTodayRT2011.pdf
Much further up in the atmosphere where collisions are less frequent this changes and is a fundamental property of the atmosphere that is not mentioned when it comes to the average altitude of radiation to space. It should be though.
Eli Rabett says:
July 21, 2012 at 5:39 am
“To maintain radiative balance (sun in, IR out) the entire Earth system warms until the temperature rises enough in the mid troposphere to restore the balance.”
But since Mainstream Climate Science’s predicted Tropical Tropospheric “Hot Spot” has not eventuated, then according to “the physics” of Mainstream Climate Science’s CO2 = GW hypothesis: there has been no “entire earth system” GW and in particular, no Global Mean Temperature increase.
David says
Now imagine a photon going from bottom of the jar to the top in a straight line. The rule that the photon has to follow is that if it hits a yellow bb, it goes straight through….
Henry says
Well, this where you all go wrong. Carefully look at the picture that this post starts with. Ozone re-radiates more sunlight then earthshine. Water vapor re-radiates strongly in the IR coming from the sun, as does CO2.
Greg is right. You have to make a balancesheet and show us how much warming and how much cooling is caused by each GHG.
Look at water vapor and CO2 around 2 um and see how that makes a dent in the incoming solar radiation. Notice that the ozone shields us from ca. 15-20% of all sunlight by absorbing and re-radiating in the UV region. In fact, if you really grasp what you are seeing in this graph/ representation (from a cloudless day), you would realize that without the ozone and CO2 and H2O and other GHG’s you will get a lot more radiation on your head. In fact, you would probably fry.
For comprehensive proof that CO2 is (also) cooling the atmosphere by re-radiating sunshine, see here:
http://www.iop.org/EJ/article/0004-637X/644/1/551/64090.web.pdf?request-id=76e1a830-4451-4c80-aa58-4728c1d646ec
They measured this re-radiation from CO2 as it bounced back to earth from the moon. So the direction was sun-earth (day)-moon(unlit by sun) -earth (night). Follow the green line in fig. 6, bottom. Note that it already starts at 1.2 um, then one peak at 1.4 um, then various peaks at 1.6 um and 3 big peaks at 2 um. You can see that it all comes back to us via the moon in fig. 6 top & fig. 7. Note that even methane cools the atmosphere by re-radiating in the 2.2 to 2.4 um range.
This paper here shows that there is absorption of CO2 at between 0.21 and 0.19 um (close to 202 nm):
http://www.nat.vu.nl/en/sec/atom/Publications/pdf/DUV-CO2.pdf
There are other papers that I can look for again that will show that there are also absorptions of CO2 at between 0.18 and 0.135 um and between 0.125 and 0.12 um.
We already know from the normal IR spectra that CO2 has big absorption between 4 and 5 um.
So, to sum it up, we know that CO2 has absorption in the 14-16 um range causing some warming (by re-radiating earthshine) but as shown and proved above it also has a number of absorptions in the 0-5 um range causing cooling (by re-radiating sunshine). This cooling happens at all levels where the sunshine hits on the carbon dioxide same as the earthshine. The way from the bottom to the top is the same as from top to the bottom. So, my question is: how much cooling and how much warming is caused by the CO2? How was the experiment done to determine this and where are the test results? (I am afraid that simple heat retention testing might not work here, we have to use real sunshine and real earthshine to determine the effect in W/m3 / [0.03%- 0.06%]CO2/m2/24hours).
I am doubtful of the analysis of the spectral data. I have not seen any work that convinces me. In the case of CO2, I think the actual heat caused by the sun’s IR at 4-5 could be underestimated, i.e. the radiation of the sun between 4 and 5 may be only 1% of its total energy output, but how many Watts per m2 does it cause on earth? Here in Africa you cannot stand in the sun for longer than 10 minutes, just because of the heat (infra-red) of the sun on your skin.
In all of this we are still looking at pure gases. The discussion on clouds and the deflection of incoming radiation by clouds is still a completely different subject.
CO2 also causes cooling by taking part in the life cycle. Plants and trees need warmth and CO2 to grow – which is why you don’t see trees at high latitudes and – altitudes. It appears no one has any figures on how much this cooling effect might be. There is clear evidence that there has been a big increase in greenery on earth in the past 4 decades. Therefore, there is a good chance that the total net effect of more carbon dioxide in the atmosphere could be close to zero. But unless we come up with the right test methods and measurements, we will never know for sure. For more on why it is considered highly unlikely that CO2 is a contributory cause to global warming, see here:
http://www.letterdash.com/henryp/global-cooling-is-here.
Hint: plot the development of the speed of warming (maxima).
which now stands at
+ 0.036 K per annum from 1974 (38 years)
+ 0.029 K per annum from 1980 (32 years)
+0.014 K per annum from 1990 (22 years)
-0.016 K per annum from 2000 (12 years)
Henry, your post appears correct. Any solar spectrum chart shows that about 98% of that energy lies between about 250 nm in the UV and 4.0 microns; with the remaining as 1% left over at each end. Such graphs often have superimposed on them the actual ground level (air Mass once) spectrum; that shows the amounts of that energy taken out by primarily O2, O3, and H2O, in the case of H2O which absorbs in the visible and near IR perhaps 20% of the total solar energy is capture by water VAPOR (clear sky) clouds are an additional loss over and above that. So as WV increases there is a corresponding reduction in TSI reaching the surface, and therefore a reduction in LWIR leaving the surface.
However, certainly all this is quantified in the climate models? I know that Steve McIntyre has been requesting an engineer style describtion of the GHE for some time. regarding my question here, (David says: July 21, 2012 at 11:15 pm) do you have any thoughts on how much non GHG energy is conducted to GHGs and leaves (radiates to space) the earth atmosphere system faster then it would if their was less GHG?
TimTheToolMan says: July 21, 2012 at 11:22 pm
———————————————————————
I have seen two basic theories of the greenhouse effect;
A- CO2 scatters 50% of outgoing LWIR it intercepts back to the surface slowing its rate of cooling.
B- CO2 directly heats the air molecules around it on intercepting outgoing LWIR.
I have found through empirical experiment that incident LWIR can slow the cooling rate of some materials, but not liquid water that is free to evaporatively cool. This rules out option A as a mechanism for CAGW.
You have chosen option B, stating “Not so. At sea level, the CO2 almost instantly gives up its absorbed energy to the rest of the atmosphere due to a collision. Collisions happen on the order of every 10^-7 seconds whereas it takes on average 10^-3 seconds to radiate”
If this rate of molecular collision directly equated to the speed of equalisation of energy states between molecules in air this would make air highly conductive and entirely unsuitable for use in double glazing.
Of course either option A or B as the primary CAGW mechanism matters little to the central question raised by Reed Coray’s post. No matter how CO2 is supposed to cause global warming, its ability to warm will be an inverse logarithmic function of its concentration in the atmosphere. However its ability to radiate to space energy it has acquired from conductive contact with Earth’s surface or atmosphere is a linear function of its concentration in the atmosphere.
The real problem lies within the physics themselves, it is the application of the Stefan Boltzmann planetary temperature to Earth (AKA -18C incident to 240W/m^2 insolation avged/spread globally), you’re pretending the Earth has no thermal capacity and behaves in accordance to a blackbody surface recieving 240W/m^2 insolation, or something close to the above behavior. In reality you have a ROTATING SPHERE, recieving 480W/m^2 insolation over 1/2 the spherical area when applying Holder’s inequality…the energy recieved via the Sun is mostly retained at ‘night’ by the Ocean/Atmosphere system..the oceans feature NO Diurnal Cycle and are for all intents and purposes a greenhouse fluid retaining heat with an enormous capacity.. and conduct heavily to an atmosphere composed of 99.8% non-emitting Oxygen/Nitrogen molecules (Though O2 absorbs heavily in the UV Spectrum).
Most of the 33C warming over the planetary Stefan Boltzmann temperature is via retention..in fact it is H2O-laden convection (AKA cloud albedo)that reduces the retention-driven planetary warming..it should be clear that retention is the issue as the high-temp of the “day” never occurs at noon rather you’ll see it occur around 3PM…same goes for the seasons at the poles, only the lag is 8 weeks.
The concept of equilibrium between the surface and atmosphere above the surface is also nonsense. GHGes which compose an avg of 0.2% of the atmosphere have no discernable impact on temperature..if anything the effect is negative given the enhancing of the water cycle/general convection.
Third and most important..if the Greybody temperature is 255K (and this mythical emission height exists at 14kft), then in that case the stratopause should not avg -55C or the conservation of energy is violated..this is, however, for another time.
Konrad writes “If this rate of molecular collision directly equated to the speed of equalisation of energy states between molecules in air this would make air highly conductive and entirely unsuitable for use in double glazing. ”
I’m not quite sure what you’re getting at there. The energy states of molecules in the atmosphere varies as per the Maxwell-Boltzmann distribution. There will always be some molecules at the energy state that CO2 needs to radiate but they wont all be CO2 molecules(!) and individually they wont stay there for long either.
That doesn’t make air a good conductor…
David says
However, certainly all this is quantified in the climate models?
Henry says
You are joking?
The so-called climate experts haven’t even figured out yet that there is a natural 50 year warming cycle followed by a 50 year cooling period –
Israel apparently knew about it (7×7 + 1 jubilee year) and I suspect Moses picked it up from the Egyptians, the pyramid builders, who were experts on everything that happened on the sun.
Did you do the plot and did you find the roots of the binominal (parabolic ) plot?
I am reasonably convinced that this cycle is caused by the sun-UV-O2-O3 cycle. The scare about the ozone falling was the greatest in the nineties when ozone was at its lowest, and it picked up since 1995, as can be expected by my theory….
Lester Via says:
July 21, 2012 at 6:13 pm
Bucky Cochrane says:
July 21, 2012 at 4:12 pm
The whole idea of GHG “absorbing heat” is erroneous. CO2 absorbs a photon, goes into the “bending” mode of molecular vibration and almost immediately radiates the photon which it absorbed. It cannot give up any fraction of this energy; there is no state between this 667 wavenumber excited state and its vibrational ground state. It cannot “warm the air”
Sorry Bucky but it does warm the air. The CO2 molecule is always vibrating from collisions with other air molecules. Due to these collisions, translational motion and vibratory motion are freely and very quickly exchanged at the gas pressures and temperatures typical of the lower atmosphere
Sorry, Lester, it does not warm the air. Explain yourself specifically without quoting specific heat ratios. etc. and vigorously waving your hands. The 010 state is a radiative decay state; look at CO2 laser diagrams. It would give over 1000C kinetic energy to a molecule if the 010 state were entirely converted to translational KE. How can all of the vibrational energy of CO2 molecules with atoms vibrating in opposite directions be converted to unidirectional KE? How is momentum conserved in such an interaction? PLUS, my model yields correct calculation of earth surface temperature. Remember, this ~.08 ev must be released in ONE interaction; there are no intermediate states. (Rotational states are in the micro wave region and will warm the gas, but we are not talking about microwave radiation) I am interested in your explanation if it is not a bunch of PY 101 platitudes.
JPeden says:
Your statement is very confused. Even if we assume you are correct about the data not showing the “hot spot”, that does not mean that things have not warmed. It just means that the places at altitude in the tropics that were expected to warm more rapidly than the surface have not warmed more rapidly than the surface. The most direct consequence of such a fact would be that the lapse rate feedback, a negative feedback in the climate models, shouldn’t be there and thus that the models may be UNDERESTIMATING the climate sensitivity a little bit.
However, the reality of the situation is that the data for the multidecadal trends in the tropics is not really good enough to conclude definitively whether the “hot spot” is missing or not. It is also noteworthy that the expected amplification of temperature variations with altitude does occur for temperature fluctuations over monthly to yearly time scales, severely constraining any explanations of how the models could be wrong in the basic prediction.
gymnosperm says (in reference to my comment here http://wattsupwiththat.com/2012/07/21/some-thoughts-on-radiative-transfer-and-ghgs/#comment-1039067 ):
Yes, CO2 gets into the stratosphere; I don’t think there is any argument about that. However, the mean emitting level of the Earth, i.e., the level at which most of the radiation emitted can successfully escape to space is still well within the troposphere. So, the vast majority of the emission to space occurs from the troposphere. Hence, one is justified in assuming the lapse rate that I assumed. Any corrections to that by doing the calculation more rigorously might change things a little bit…but even if my original calculation was off by a factor of 3 (which seems unlikely), we are still talking about the cooling with the decreasing emitting layer temperature effect being 100 times as important as the increasing emitting layer area effect.
”
Eli Rabett says:
July 21, 2012 at 4:35 pm
cba accuses Eli of being a character in a childrens novel and throws much detail against the wall which really does not shift the argument much. cb, if you want detail go read the science of doom articles on the greenhouse effect that KR provided. The mechanism remains what the Bunny pointed to.
http://scienceofdoom.com/roadmap/atmospheric-radiation-and-the-greenhouse-effect/
”
*************************
Perhaps you should try harder to make sense of it. The argument doesn’t have to shift much at all. A change of only from 3-5 deg C warming per co2 doubling down to around 1 deg C rise per co2 doubling changes things from CAGW to AGW or CGW to GW.
I didn’t have time to go completely over your reference – whose political affiliations are prominantly contained in the name of the website. I did notice that all the graphs were done using /cm instead of wavelength which tends to be more like speaking in ebonics rather than in english – quite understandable to a small fraction of the populace but almost meaningless to the majority. Since the graph shapes up differently with power per unit /cm versus per unit wavelength, it does help the graph look scarier and allows for much smaller bandwidths to be displayed, thus making the apparent effect of ghgs look more significant. I also didn’t see any mention of A. Eddington in the presentation of the radiative xfer approximations – but then maybe I was just scanning over it too rapidly.
As RGB has pointed out, CAGW extremists have been known to actually use legitimate physics in preparation of their hysterical claims. Of course it doesn’t take a long time to find that some, like hansen, sometimes quickly depart from that and invent new things like ‘characteristic’ radiating altitude – where in reality nothing of any significance is actually radiating into space from that altitude because its either radiating through there from the ground at wavelengths unaffected by any ghg molecules or its being absorbed by higher layers except for the slight decrease in line width due to a reduction in pressure – which also increases the peak’s ability to absorb/emit.
Radiative transfer can get a rather good estimate of how much power is absorbed or transferred through clear skies – but not through clouds. It cannot tell you the sensitivity of Earth’s temperature to a change in the amount of power transferred / absorbed. The vast majority of measurements intended to determine this have ignored albedo variation which can be several times that of the change in co2 power absorption. Simple averages, mostly of real numbers can give you a real average sensitivity and that is a far cry from the usual estimates, being less than than straight stefan’s law estimates which means there’s net negative feedback present. It also shows just how far off you people are when it comes to the dellusions of massive amounts of positive feedback that have some sort of tiny relative stability. Also, your big h2o vapor feedback bugaboo is simply BS. Even a 5 deg C shift in T for surface and atmospheric column at constant RH would net you scarcely 30% and h2o is every bit a log function just like co2 and is roughly linear over almost a dozen halvings just like co2. Hint, even a 30% increase in absolute humidity for h2o provides less additional power absorption than a co2 doubling at the tropopause.
Thanks to all that have seriously participated in the discussions on this thread. I has been very interesting. There is certainly no consensus here. To some it may appear that no progress has been made. To me it appears that a lot has been made as ideas are debated, yet far from any settled consensus. This has been real peer review.
Some have argued for a specific point (possibly correctly in that right) against another supporting the same point but with the inclusion of a related concept which led to arguments of minute details where they were basically in agreement. Seemed a little chaotic at times. But the entire discussion of climate is actually a chaotic discussion of a complex and chaotic system. There are just too many variables effecting every little piece of the many interrelated variables involved to ever come close to any settled conclusions with regards to the overall concept of understanding climate. Simply put, chaos can’t be fully explained. It is just that, chaos. But it is a real intellectual challenge to try to learn as much about the chaos as possible. That’s why most of us are here. Then there is always the token trolls and those with an agenda.
Again, thanks to all.
Joel says
your statement is very confused
Henry says
it is you who is confused
a) you have no proof that the net effect of more CO2 is warming rather than cooling, as I requested you to give to me in previous postings
b) you have no tests or measurements that you have collected yourself, so you rely on others
c) JPeden asked about the temperature drop that we note since the beginning of the new milennium, to which you obviously have no reasonable explanation – seeing that CO2 is still rising.
You are most certainly not the (truthful) prophet Joel from the bible to whom you were named after.
http://www.letterdash.com/HenryP/more-carbon-dioxide-is-ok-ok
”
joeldshore says:
July 22, 2012 at 6:43 am
gymnosperm says (in reference to my comment here http://wattsupwiththat.com/2012/07/21/some-thoughts-on-radiative-transfer-and-ghgs/#comment-1039067 ):
Carbon dioxide seems to be getting into the stratosphere. I doubt anyone knows how. Ozone holes? The tops of those thunderheads that dome in? As you undoubtedly know at the tropopause the lapse rate is inverted and temperature increases with altitude, eventually reaching levels nearly as warm as the surface.
I’m going to leave the math to you, but the trouble with, “It’s not difficult to calculate”, and, “It’s simple physics”, is that the reality seems not to be easy or simple.
Yes, CO2 gets into the stratosphere; I don’t think there is any argument about that. However, the mean emitting level of the Earth, i.e., the level at which most of the radiation emitted can successfully escape to space is still well within the troposphere. So, the vast majority of the emission to space occurs from the troposphere. Hence, one is justified in assuming the lapse rate that I assumed. Any corrections to that by doing the calculation more rigorously might change things a little bit…but even if my original calculation was off by a factor of 3 (which seems unlikely), we are still talking about the cooling with the decreasing emitting layer temperature effect being 100 times as important as the increasing emitting layer area effect.
”
*********************************
It’s nonphysical. There is no level where most of the radiation emitted can successfully escape. 70% of the radiation from the surface under clear skies makes it through the atmosphere, some of it after being absorbed and reradiated numerous times. No layer absorbs or emits significant amounts of power. You are dealing with hansen’s dellusions based on the amount of power radiated from a blackbody or greybody at a given temperature and that doesn’t exist above the surface under clear sky conditions.
The actual result is that your emitting altitude factor is no more important than your area increase effect. One is very small but meaningful, the other is meaningless because it doesn’t exist. Each layer has contributions to emission and absorption but it takes a 1 dimensional model to determine it as it is a function of pressure as well as temperature that affects the line shape.
****
Eli Rabett says:
July 21, 2012 at 5:39 am
Increasing concentrations of GHGs raises the altitude that GHGs can radiate to space in the blocked regions of the spectrum
Because of the lapse rate, the higher you go in the troposphere, the lower the temperature
This slows down the rate at which the Earth emits to space because it is now radiating at higher, colder altitudes.
****
Why does the altitude that radiation escapes get higher? I don’t see it. As GHGs increase in concentration, their absorption/emission-bands widen, and so more of the earth’s total IR emission comes from the tropopause relative to warmer, lower altitudes. So the surface IR “window” (that gets past GHGs) must increase its emission (surface gets warmer) to get back to ~equilibrium. Fine. But I still don’t see why the emission height must change. At the tropopause the lapse-rate has gone to zero due to increasing absorption of UV by ozone. Raising the emission height there won’t have any effect from altitude change. If the emission height got even higher, it would get into warmer, stratospheric air, which would be an anti-greenhouse effect.
joeldshore says:
July 22, 2012 at 6:30 am
==========================
Curious post. Joel Shore appears to be saying that the missing hot spot is either “A” an indication that the lack of overall atmospheric heat is an indication that it is : worse the we think”, or “B” the measurements are wrong. When this is your only two possible answers I think the potential for confirmation bias is greatly enhanced.
Joel, perhaps you could put the models numbers on these questions…
Any solar spectrum chart shows that about 98% of that energy lies between about 250 nm in the UV and 4.0 microns; with the remaining as 1% left over at each end. Such graphs often have superimposed on them the actual ground level (air Mass once) spectrum; that shows the amounts of that energy taken out by primarily O2, O3, and H2O, in the case of H2O which absorbs in the visible and near IR perhaps 20% of the total solar energy is capture by water VAPOR (clear sky) clouds are an additional loss over and above that. So as WV increases there is a corresponding reduction in TSI reaching the surface, and therefore a reduction in LWIR leaving the surface.
However, certainly all this is quantified in the climate models? I know that Steve McIntyre has been requesting an engineer style describtion of the GHE for some time. Regarding my questionS here, (David says: July 21, 2012 at 11:15 pm) do you have any thoughts on how much non GHG energy is conducted to GHGs and leaves (radiates to space) the earth atmosphere system faster then it would if their was less GHG?
This is complicated. Tim the tool man states …At sea level, the CO2 almost instantly gives up its absorbed energy to the rest of the atmosphere due to a collision. Collisions happen on the order of every 10^-7 seconds whereas it takes on average 10^-3 seconds to radiate. CO2 at sea level is a warming agent for the atmosphere and it occurrs right at ground level within tens of meters. so several question come to mind. How much of the energy in CO2 which it is “giving up” via conduction to non GHGs, came from conducting cooling, non GHG molecues? In which case it is neither warming or cooling, just acting as if it was another non GHG molecue. However, it does still radiate at times? How often? If it radiates towards space is it not potentially radiating conducted “non GHG energy” away from the planet faster then that energy would leave if it only encountered non radiating non GHGs.? How much more energy is moved via convection if the intial affect is primarily warming? How much energy is absorbed via an acceleration of the hydrological cycle? How do these ratios change at diaparate elevations, as the higher up in the atmosphere one goes, the more time is required between conduction via collisions, and a higher percentage of energy is radiated?, Numbers numbers numbers.?????
David says:
July 22, 2012 at 7:55 am
==========
😉
This is really dopey:
“So, radiation from a colder body directed to the warmer body is radiation, but no warmist has been able to present a real falsifiable scientific experiment proving that this sort of radiation can warm the warmer body (or slow down it’s cooling, whatever).”
Does this clown think that the warmer body is transparent to the radiation from the colder body? Does it pass through without any effect? Or it detours around the warmer body? Bounces off?
Suspend a heating element in a vacuum chamber and heat it to a constant temperature of 500 F. Suspend another element near the first and heat it to 300 F. Work on that.
Henry@David
Just to clarify: I am watching with some amusement a lot of scholar discussions on the green house effect as I realised again that the people that I encounter on most scientific blogs don’t understand the chemistry principle of absorption and subsequent re-radiation. In fact very few people do understand it because if they did they would have raised the alarm bells ringing long time ago. But they all got stuck at Tyndall and Svante Arrhenius. …
They know that CO2 (carbon dioxide) “absorbs” in the 14-16 um region. Most people think that what it means is that the molecules absorbs photons here which then subsequently get transferred as heat to neighbouring molecules. Then it absorbs again, and so on, and so on…and all the absorbed light is continuously transferred to heat…
Although this may happen up to a certain saturation point as soon as the light or radiation hits on the gas, that is in fact not what is causing the heat entrapment.
I happen to be familiar with spectrophotometry. You have to understand what actually happens when we put a beam of light of certain wavelength on a sample of liquid or gas.
We have various spectrophotometers that can measure the various ranges of UV-visible -IR etc. Usually you have the option to vary the wavelength of the beam of light, either manually or automatically.
If the gas or liquid is completely transparent, we will measure 100% of the light that we put through the sample coming through on the other side. If there is “absorption” of light at that specific wavelength that we put through the sample, we only measure a certain % on the other side. The term “extinction” was originally used but later “absorption” was used to describe this phenomenon, meaning the light that we put on was somehow “absorbed”. I think this was a rather unfortunate description as it has caused a lot of confusion since. Many people think that what it means is that the light of that wavelength is continually “absorbed” by the molecules in the sample and converted to heat. If that were true, you would not be able to stop the meter at a certain wavelength without over-heating the sample, and eventually it should explode, if the sample is contained in a sealed container. Of the many measurements that I performed, this has never ever happened. Note that in the case of CO2, when measuring concentrations, we leave the wavelength always at 4.26 um. Because the “absorption” is so strong here, we can use it to compare and evaluate concentrations of CO2.
The best way to experience re-radiation for yourself is to stand in a dark forest just before dawn on a cloudless night. Humidity must be high. Note that water vapour also absorbs in the visible region of the spectrum. So as the first light of sun hits on the water vapour you can see the light coming from every direction. Left, right, bottom up, top down. You can see this for yourself until of course the sun’s light becomes too bright in the darkness for you to observe the re-radiated light from the water vapour. This is also the reason why you will quickly grab for your sun glasses when humidity is high, because even with the sun shining for you from your back and driving in your car, you can feel on your eyes that the light from the sun is re-radiated by the water vapor in the atmosphere.
A third way to experience how re-radiation works is to measure the humidity in the air and the temperature on a certain exposed plate, again on a cloudless day, at a certain time of day for a certain amount of time. Note that as the humidity goes up, and all else is being kept equal, the temperature effected by the sun on the plate is lower. This is because, like carbon dioxide, water vapour has absorption in the infra red part of the spectrum.
We can conclude from all these experiments that what actually happens is this:
in the wavelength areas where absorption takes place, the molecule starts acting like a little spherical mirror, the strength of which depends on the amount of absorption taking place inside the molecule. We may assume that at least 50% of a certain amount of radiation is sent back in a radius of 180 degrees in the direction where it came from. (However, because the molecule is very small and therefore might behave more or less like a sphere, it could be up to ca. 62,5% ). This re-radiation in the sun’s spectrum and in the earth’s spectrum is the cooling effect, or warming effect, respectively, of a gas that is hit by radiation. An effect that is very similar to this, is also observed when car lights are put on bright in humid, moist and misty conditions: your light is returned to you!!
Unfortunately, in their time, Tyndall and Arrhenius could not see the whole picture of the spectrum of a gas which is why they got stuck on seeing only the warming properties of a gas (i.e. the closed box experiments).
If people would understand this principle, they would not singularly identify green house gases (GHG’s) by pointing at the areas in the 5-20 um region (where earth emits pre-dominantly) but they would also look in the area 0-5 um (where the sun emits pre-dominantly) for possible cooling effects. If you really want to understand what happens in the atmosphere, this rough graph / representation (on a cloudless day) is very important:
http://albums.24.com/DisplayImage.aspx?id=cb274da9-f8a1-44cf-bb0e-4ae906f3fd9d&t=o
….now carry on at to my previous post to davidmhoffer……sorry about the confusionswith the two Davids…
Joel,
What of density? If your construct of “mean radiating altitude” has any physical basis it would be the density of radiating molecules, yet ultimately more radiating molecules means more photons escaping to space.
Joel Shore:”Your statement is very confused. Even if we assume you are correct about the data not showing the “hot spot”, that does not mean that things have not warmed. It just means that the places at altitude in the tropics that were expected to warm more rapidly than the surface have not warmed more rapidly than the surface. The most direct consequence of such a fact would be that the lapse rate feedback, a negative feedback in the climate models, shouldn’t be there and thus that the models may be UNDERESTIMATING the climate sensitivity a little bit.
However, the reality of the situation is that the data for the multidecadal trends in the tropics is not really good enough to conclude definitively whether the “hot spot” is missing or not. It is also noteworthy that the expected amplification of temperature variations with altitude does occur for temperature fluctuations over monthly to yearly time scales, severely constraining any explanations of how the models could be wrong in the basic prediction.”
At the risk of pulling this thread off-topic, I have heard you make this statement before and I can’t see how it could be true. You seem to be arguing that the only way that the hotspot to be missing is for there to be no more condensation of WV on average than when the air was drier. It seems to me much more likely that the models are wrong about how efficiently those levels of the atmosphere radiate heat to space(for whatever reasons). If those levels are much more efficient in this regard than the models assume, then it is possible for the hotspot to be missing and the lapse rate feedback to be present. Further, since it seems to be highly unlikely that there is no lapse rate feedback, I would say that this second alternative is much more likely to be taking place (assuming there is no hotspot).
Cheers, 🙂
****
Sam Yates says:
July 21, 2012 at 1:42 pm
…Mr. Watts, a question; do you look over these guest posts before throwing them up here? Because, honestly, it does not do much to improve your credibility when you gladly host things like this, which betray a massive lack of comprehension of very, very basic physics. It’s your site, of course, and yours to run as you see fit, but…If I were you, I might be a bit more picky about things like this post. Quite frankly, this is embarrassing.
****
So far, I’ve increased my understanding from some of the replies in this thread (however incrementally). So it certainly isn’t “embarrassing” to me in any way, and I’d wager most others, too. Jeesh….
OK, to answer some of my own questions, I looked at an atmospheric rad-spectrum, and the large CO2 “chunk” out of it centered at 667 cm-1 is at around -50C (~-60F). The altitude where the majority of the CO2 GHE is therefore where the atmos temp is -50C. Looking at various atmos temp profiles, -50C is close to, but not quite at the tropopause. OK, if the radiation height of the CO2 increases, there’s still alittle room for it to get colder, but not much. And the zero lapse-rate height would be a “limit” to how much it could rise to increase the insulation effect by that means.
But my question remains: Why would the altitude increase?
Beng says: “So far, I’ve increased my understanding from some of the replies in this thread (however incrementally). So it certainly isn’t “embarrassing” to me in any way, and I’d wager most others, too. Jeesh….”
But why should you have to wade thru a poor top post and hundreds of poor (and/or off-topic) replies in order to find the few replies that actually produced “incremental” increases in understanding. A well-written, on-topic top post would have provided more learning for more people with less effort and less possibility for continued misunderstanding of basic physics.
It’s cool that so many people are so passionate about science, but it is disappointing that so many of them are rather clueless (yet still feel they are expert enough to teach others). I’m sure a few people learn well by being told lots of wrong things and a few right things, but not most people.
beng – “…my question remains: Why would the [tropopause] altitude increase?”
Because the height of the troposphere, the altitude of the tropopause, is set by the top layer of convection. Maximum tropopause altitude (west equatorial Pacific for example, ~17.5km) is where there are high surface temps and lots of convection, low tropopause where there is less convection (Antarctica for example, ~8km, where tropospheric mixing is mostly due to frontal system uplift).
And as the atmosphere heats up, there’s more convection, and the tropopause gains in altitude. There’s no hard “limit” to tropospheric altitude.
beng;
But my question remains: Why would the altitude increase?
>>>>>>>>>>>>>
part of the reason is simply the math regarding the % chance that any given photon radiated in an upward direction will escape directly to space. The denser the CO2 the higher up that photon has to have even a chance of seeing a free path to space.
the other part of the reason has to do with water vapour which has absorption bands overlapping co2 (see chart in article above). At sea level in the tropics, water vapour is at 40,000 ppm, and completely overwhelms the effects of CO2. Going from 400 ppm to 800 ppm at sea level is as a consequence nearly meaningless. But at higher altitudes, colder temperatures reduce the amount of water vapour to very low amounts, so suddenly CO2 effects are much more pronounced compared to what would have happened otherwise.
Michael Tremblay;
Create any experiment which will take advantage of GHG’s to create an engine which will run on radiative energy alone.
>>>>>>>>>>>>
Given the number of times in this thread alone that it has been specified that the physics being explained is reliant upon an external energy source, one can only wonder if people like you even bother to read the explanations.
Shawnhet says:
Are you saying that they are somehow radiating more heat than one would expect given their temperature? Furthermore, the thermal structure of the tropical atmosphere is dominated by convection. The radiative transfer is already such as to maintain a larger lapse rate than exists but convection occurs and reduces the lapse rate approximately to the moist adiabatic lapse rate.
Sure, if the laws of radiative physics break down and they somehow emit more than allowed for by the laws of radiative physics, I suppose that is possible but seems rather unlikely (and calling it the “lapse rate feedback” would be a misnomer…probably want to call it the “magical emission feedback”). However, if we assume, as per the laws of radiative physics, that there is the expected dependence of emission on temperature then if you don’t have higher temperature at altitude then you won’t have the necessary emission to have the lapse rate feedback. [What the lapse rate feedback say, essentially, for those who don’t know is that if the atmosphere at altitude warms faster than at the surface, the surface doesn’t have to warm up as much in response to a radiative imbalance (due to, say, increasing greenhouse gases) as would otherwise be the case in order for the emission to increase enough to re-establish radiative balance.
cba says:
You are just picking nits. We know that the concept of a single emitting layer is an approximation. But doing the calculation more precisely is not going to change the factor of 300 down to anything remotely close to 1.
gymnosperm says:
No…That is not how it works with radiative transfer in gases. It is complicated but a simplified way to think about it is to imagine that you have a solid surface at some altitude that emits according to the Planck function. In the real system, density will have effects in terms of the exact distribution of emission with altitude but you are not going to magically get more emission than you would by imagining a spherical surface of emissivity 1.
eyesonu:
And, here we have the requisite comment expressing the logical fallacy that because one can find people willing to argue anything on the web, that means the science isn’t settled. In fact, as I noted, what this thread really demonstrates (at least to those who actually know the greenhouse effect is real) is how there are some people who will refuse to accept the scientific evidence no matter how strong when it leads to conclusions that conflict with what they want to believe for other reasons, a fact we already really knew from the evolution – creation debates.
It is a cautionary tale from which much can be learned.
joeldshore says:
July 22, 2012 at 6:30 am
JPeden says:
But since Mainstream Climate Science’s predicted Tropical Tropospheric “Hot Spot” has not eventuated, then according to “the physics” of Mainstream Climate Science’s CO2 = GW hypothesis: there has been no “entire earth system” GW and in particular, no Global Mean Temperature increase.
Joel replies:
“Your statement is very confused. Even if we assume you are correct about the data not showing the “hot spot”, that does not mean that things have not warmed.”
Exactly: according to “Mainstream Climate Science’s” own predictions, the subsequent empirical data effectively reduces to absurdity “the physics” contained within the GCMs – involving the specific hypothesis that [increasing] CO2 = GW, and likewise predicting the empirical existence of a tropical tropospheric “hot spot”.
To begin with, Mainstream Climate Science’s own GCM “physics” did not manage to produce a distinct measurable empirical effect as advertised from increasing CO2 concentrations, while GMT did in fact increase over the period of ~1975-1998.
Likewise, there has been no empirical effect produced or detected from “the physics” of CO2 = GW during the subsequent ~15 yr., as manifestd by the failure of GMT to increase over this period as per “the physics” of continued increases in CO2 concentrations, a failure to warm which was specifically predicted by GCMs to not occur under the CO2 = GW hypothesis – unless of course CO2 concentrations did not increase.
“It just means that the places at altitude in the tropics that were expected to warm more rapidly than the surface have not warmed more rapidly than the surface.”
Yes and finally, the empirical data shows that the CO2 = GW hypothesis failed according to its own [lapse rate + water vapor abetted] predictions from its own “physics” – according to which there also shouldn’t have even been any GW from any cause, because the now claimed to be ‘nonspecific’ sign of any GW “hot spot” simply did not occur as predicted by the mainstream GCM “physics”!
Bottom line in these two cases, Joel: Mainstream Climate Science “physics” hasn’t been able to provide or detect any empirical warming from “the physics” of its CO2 = GW hypothesis.
Henry at Tim
I did not encounter too many experts here, but I am glad you are here! Perhaps you can help me. In the graphic that this post starts off with, we see the spectrum of CO2, but obviously the presentation of the graph looks as if the CO2 causes almost all the absorption at 14-16 in the atmosphere. In reality this cannot be true. The concentration of water vapor alone is ca. 15x greater than CO2, so at the very most, the CO2 cuts off only a little corner of that area of radiation from earth not covered by the absorption by water vapor.. Apart from that, if you look very closely, you can see that there is also absorption of oxygen and ozone in the 14-16. Now I have been posting that it is the variation in ozone that seems to be the cause of global warming and its increase is now the cause of global cooling. The observed global warming and subsequent global cooling all follows on nice parabolic curves, as proven from my results, as reported in my earlier posts here. The question I have is this: is the (weak) absorption by oxygen/ozone at 14-16 caused by the ozone or by the oxygen?
Konrad says:
July 21, 2012 at 6:11 am
JeffC says: July 21, 2012 at 5:15 am
“GHG does not block radiation, it absorbs and then re-transmits … a better term than block would be slows …”
I believe JeffC to be correct. CO2 almost instantly re-radiates the outgoing IR radiation it intercepts, with around 50% of this radiated back towards the Earth’s surface….
_______________________________
That is one of the fallacies. 50% is not radiated back towards earth. Think in 3 dimensions not in 2 dimensions because the CO2 molecule and the earth are three dimensional. Also energy can be transferred via collision to other molecules so the energy headed in the direction of the earth is less than 50%.
The second point brought up by Sleepalot @ July 21, 2012 at 4:21 am, is the day/night cycle of off-again/on-again solar insolation and the rapid response of the air and land temperatures to the “switching -off” of the sun’s energy. Sleepalot’s link
Sleepalot @ July 21, 2012 at 4:53 am then points out the actual effects of the GHG water vapor on the temperature by comparing high vs low humidity.
Take a good hard look at those two pieces of real world data and ask yourself what it is telling you.
#1. The solar eclipse data tells you the earth & air temperature response (in low humidity) to a change in solar energy is FAST!
#2 The effect of the addition of water vapor (~ 4%) is not to raise the temperature but to even the temperature out. The monthly high is 10C lower and the monthly low is ~ 10C higher when the GHG H2O is added to the atmosphere in this example. The average temperature is about 4C lower in Brazil despite the fact that Algeria is further north above the tropic of Cancer. Some of the difference is from the effect of clouds/albedo but the dramatic effect on the temperature extremes is also from the humidity.
I took a rough look at the data from Brazil. Twelve days were sunny. I had to toss the data for two days because it was bogus. The average humidity was 80% for those ten days. The high was 32 with a range of 1.7C and the low was 22.7C with a range of 2.8C. Given the small range in values over the month the data is probably a pretty good estimate for the effects of humidity only. You still get the day-night variation of ~ 10C with a high humidity vs a day-night variation of 35C without and the average temp is STILL going to be lower when the humidity is high.
This data would indicate GHGs have two effects. One is to even out the temperature and the second is to act as a “coolant” at least if the GHG is H2O.
The latent heat of evaporation could be why the average is 4C lower when in Brazil vs Algeria. As one of the commenters here at WUWT mentioned using temperature without humidity to estimate the global heat content is bad physics.
davidmhoffer, I think this jar would absolutely show the silliness of the CO2 concern. Even 8 blue BBs in a jar of 9,592 yellow BBs will look incredibly sparse in comparison to the 400 red BBs.
Now, create four jars:
Jar #1: 4 blue BBs + (400 + σ) red BBs
Jar #2: 8 blue BBs + (400 – σ) red BBs
Explain that Jar #1 is displaying base case CO2 with slightly higher than average water vapor.
Explain that Jar #2 is displaying the supposed catastrophic case for CO2 with slightly lower than average water vapor.
Now, ask the observer if it makes sense that CO2 could have a measurable effect (even 1°C) on sustained global temperatures given the fact that it’s quite obvious that any conceivable amount of blue BBs is a rounding error compared to the red BBs.
Ask if it makes any sense to conclude that earth’s climate system could be that sensitive to the blue BBs despite the fact that the system demonstrates relative stability during periods that the red BBs constantly fluctuate on an enormous scale (in comparison).
But an environmentalist has convinced me that if I remove all of the insulation the average temperature inside will go down, so I do. What do you think will happen to the average temperature?
Do you have big, transparent, south-facing windows with a thin IR-blocking film in them so that sunlight streams in every day, heating the floors? Because if you do, I suspect the environmentalist might be right.
The Earth’s surface and atmosphere aren’t heated from the top down (outside in) — they are heated from the inside out because a significant fraction of sunlight directly heats the surface after passing through the atmosphere without being either absorbed or reflected. Which makes most of the metaphors that have been proposed in this thread completely wrong.
Treat sunlight delivered to the surface correctly as a heat source heating an object inside a vacuum flask and cooling only by radiation, and it becomes quite clear that absorbing from a high temperature black body (the Sun) in the visible band, but radiating from a much cooler black body (the Earth) in the IR band, creates a situation where adding insulation in the form of atmospheric CO_2 that blocks direct radiation from the surface in a chunk of the IR spectrum can only raise the temperature of the surface to compensate until equilibrium is maintained, energy in equals energy out.
The Earth is not a closed system. It receives a huge amount of energy in the form of sunlight every day. It loses all of this energy, every day! At least on average. If you reduce the rate of loss in one channel without altering the input, you MUST increase the rate of loss in all other channels until the two balance. The only thing that can increase the rate of radiative loss in the unblocked channels is higher temperature, since the outgoing radiation is at least mostly thermal in the first place.
rgb
wobble
you totaly and completely missed the point of the explanation
Here’s your answer: Less than what the world is spending now.
Try to remember that technological advances accelerate over time. If you believe that the equivalent of $30 trillion is a reasonable bet against probability weighted negative effects of warming and that such warming will start to negatively impact the world’s wealth within 30 years, then it’s irrational to start spending $1 trillion per year now.
And with this, I agree! In fact, I make the same argument! That was easy, right?
My usual argument is that the best thing to do about CAGW even if it might be right is almost nothing! Now, at any rate. Continue to invest heavily in research that can bring alternative energy resources to maturity, because this sort of research will almost certainly have a positive ROI regardless of CAGW, and it a trivial fraction of your trillion a year. Invest modest amounts in pilot projects that help us work out the scaling and true costs of these technologies as they go along. Is a solar updraft tower a viable, competitive, feasible energy producer? It will be difficult to say without building not just one, but several of them, using what we learn from one to build the next one. Is a solar updraft tower built at the top of a mountainside collector a viable competitive energy producer? Maybe, but first one has to build a few regular towers without the mountainside to learn the engineering to find out.
These sorts of things cost at most a few billion a year, and many of them have trillion dollar payoffs if they hit. What I don’t like — and at least some climate scientists who believe in AGW and at least some negative sequellae aren’t thrilled with either — is carbon trading, large scale subsidies of immature technologies that don’t have a clear positive ROI, and so on.
Personally, I think that Solar PV technology is going to be a no-brainer within roughly 8 to 10 years, with or without further government funded research, faster with than without. I do argue with those that for some reason seem to oppose it (I can’t imagine why). Solar is damn close to break even as far as ROI is concerned even without a subsidy, and a factor of 2 improvement in cost per watt will put it solidly into the black for a large fraction of the United States without a subsidy. Or a breakthrough in energy storage would do it. Or both would do it fast, with a lot of money on the line. Even if I’m wrong what’s the cost? A teensy fraction of the money pissed away in Iraq, money that could have funded a HUGE amount of solar energy (even at a subsidized loss) for a far greater ROI. Solar should be implemented when implementing it is profitable; I just think it will be profitable soon.
If it is, then who cares about CAGW? Long before carbon trading has any useful effect (probably infinitely long given a latter date of “never”) the problem will be resolving itself simply because it is cheaper that way and makes people more money, or the evidence for CAGW will be unmistakable and we’ll be taking more directed (and more justified) action.
rgb
Try this: in a jar the height of the atmosphere put clear BBs and blue BBs. 8 blue BBs for every 10,000 clear. Look at the jar from the top. Kinda blue, ain’t it? Do you suppose there is any way a photon originating at the earth’s surface could reach space without colliding with a blue BB, or two?
The top physicists scorn the theory – the conscientious layman has to work his way through from ground zero doing all of the tedious maths along the way.
They do? Damn, why didn’t I know that. Who are these “top physicists” that scorn the reality of the greenhouse effect?
Not that there probably aren’t one or two — finding a dozen physicists that agree on anything complex can be challenging. But if you showed any of them the TOA spectrographs they’d change their minds. I mean, physicists aren’t usually stupid.
rgb
That’s an inaccurate assumption. I didn’t miss anything. I understood your point about a long jar being opaque looking from the top down, and I certainly didn’t say that you were wrong. I merely stated that your jar would absolutely show the silliness of the CO2 concern.
wobble;
I merely stated that your jar would absolutely show the silliness of the CO2 concern.
>>>>>>>
In that case, you totaly and completely missed the point.
Please Bucky Cochrane, QuantumPhysicistPhil, RGB, say more. This seems to play into the misinterpretation of Tyndall’s experiment that began this entire mess of misinterpretation. If any one will read just page 13 and 14, just two pages, and get a vivid mental image of Tyndall’s experiment you will see that he merely proved that carbon dioxide and other ‘greenhouse’ gases absorb and emit LW radiation. That is a fact I think anyone versed in physics agrees.
http://books.google.com/books?id=RT9-bP9p9zQC&pg=PA13&dq=Tyndall
But look deeper, if Tyndall would have used a rock salt 3 foot by 3 inch tube instead of the tube of IR absorbing glass, that same experiment would have reported entirely different results, the gas in the tube would have cooled, not warmed. Then only a portion of the LW from the source copper plate would have been reaching the far thermo-piles on either side of the far copper plate.
Now I admit since the atmosphere is more or less homogenous horizontally, but radiation is line-specific unlike the glass that absorbs then re-emits in a gray body type spectrum redistributing that energy spherically in any direction so the factor of conversion to thermal would be greatly different in the two cases.
See anything there?
Don Monfort says:
July 22, 2012 at 8:49 am
Does this clown think that the warmer body is transparent to the radiation from the colder body? Does it pass through without any effect? Or it detours around the warmer body? Bounces off?
Suspend a heating element in a vacuum chamber and heat it to a constant temperature of 500 F. Suspend another element near the first and heat it to 300 F. Work on that.
=========================================================
Yeah, I know this tale: http://www.drroyspencer.com/2010/07/yes-virginia-cooler-objects-can-make-warmer-objects-even-warmer-still/ .
Inexperienced readers might get the impression that it is real, but it is not. It is just a so called “thought experiment” with no reference to a real genuine falsifiable scientific experiment. Which leads to the conclusion that that notion has never been proven. It was just an “explanation” of a fiction.
As for “Work on that”, it looks rather typical for warmists: when asked for experimental proofs of their claims, they either say it is not necessary because it is so in theory or suggest the opponents do the experiment. Or they give “explanations” of what they have yet to prove. Unbelievable.
So, radiation from a colder body directed to the warmer body is radiation, but no warmist has been able to present a real falsifiable scientific experiment proving that this sort of radiation can warm the warmer body (or slow down it’s cooling, whatever).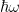 in the energy of each and every photon, and those photons are indeed absorbed by pretty much anything capable of absorbing photons at those frequencies, even the cold liquid helium, by scattering light back onto an object, adds energy to it compared to the energy that would be there if there were no cold mirror, no cold helium.
in the energy of each and every photon, and those photons are indeed absorbed by pretty much anything capable of absorbing photons at those frequencies, even the cold liquid helium, by scattering light back onto an object, adds energy to it compared to the energy that would be there if there were no cold mirror, no cold helium.
Since I’m not a “warmist”, technically this is true. However, I’ve suggested two or three real falsifiable scientific experiments that you can do in the privacy of your own home that show that reflected radiation can lead to a higher equilibrium temperature of a body being warmed and in dynamic equilibrium with radiative heat loss.
But you don’t want to actually perform such an experiment, not even if it is simple enough for a high school student to perform.
Here’s an even simpler experiment. Visit this website:
http://en.wikipedia.org/wiki/File:2_Helium.png
The picture, you will note, is alleged to be a small container of liquid helium, at a presumed temperature of perhaps 4K. It is, you will note, really really cold.
Yet we can see it! Oh, my, gosh! Photons of visible light are somehow being scattered by this helium and making it to our eye, where they — gasp — warm the eye relative to the temperature it would have if the helium were replaced by nothing. In fact, we can see a fire and feel its heat even if the light we see, spectrally emitted from a hot system is reflected from a very cold mirror indeed. Since the light we see reflected in a cold mirror or scattered from cold liquid helium carries
Greg, sooner or later you’re going to have to realize that you can actually do experiments that falsify your bizarre belief that cold things can’t affect the rate that things cool (and hence, affect their equilibrium temperature when they are being warmed). Personally, I don’t understand how you could really end up thinking that. Spend a single windless night in a desert, sleeping under the stars, then come to North Carolina and spend a windless night in my back yard at the ocean, sleeping under the same stars with a relative humidity of maybe 60% or 80%. Look at the temperature differential almost anywhere at night with and without overhead clouds. I learned about the greenhouse effect in boy scouts at the age of 12, back in the 60s when there wasn’t the slightest bit of concern about global warming and if anything, there was talk of a return of glaciation. It was expressed as a simple rule — nights with cloud cover are almost always warmer (on the ground) than clear, dry nights.
In both cases the air overhead is cooler than the ground, Greg. The clouds are cooler. The air is cooler. You have to go up to the thermosphere before you start to find air that is warmer, where the “warmer” air is so thin that nobody cares. Yet we have a readily observable, systematic, reproducible temperature difference on the ground, one that transcends wind, conduction, convection, evaporation — all things being measurably equal on the ground, a cloudy night will be warmer than a cloudness night. I don’t believe you could have lived long enough to learn to write without observing this yourself countless times.
This one systematically falsifiable observation is all it takes to refute you: So, radiation from a colder body directed to the warmer body is radiation, but no warmist has been able to present a real falsifiable scientific experiment proving that this sort of radiation can warm the warmer body (or slow down it’s cooling, whatever).
Except that colder clouds overhead seem to warm the ground or slow down its cooling or whatever. They also backscatter even visible light. Mere humidity — it doesn’t even require actual clouds — warms the ground or slows its cooling or whatever. Water is a powerful greenhouse gas — much more important than CO_2 at least once the CO_2 warming (which isn’t very great) is saturated.
It is an undeniable fact that radiation trapped, reflected, or scattered by a colder body carries energy. It is a fact that energy, steadily being delivered to an object, will cause it to be warmer than it would without the delivery of energy, all things being equal. There is nothing in the second law of thermodynamics applied to open, non-equilibrium systems that prevents heat from flowing from a warm object to a cold one more slowly if the cold object warms up (but remains colder than the warm object). Indeed, even straight up conduction will proceed more slowly and the warm object will be warmer at all times starting from identical initial conditions even without an active heat source there.
I fail to see your argument. What you are asserting is falsified by simple, everyday experience and common sense. Of course a colder body can affect the cooling rate of a warmer body, a statement that is logically identical to the things that you claim no real experiment can show. They show little else!
How they affect it and by how much they affect it requires analysis of the physics involved. But you can’t even start to participate in the grown-up arguments until you get over your childish assertion that the heat equation itself is somehow wrong, that modulating the temperature of a cold reservoir in contact with a warm reservoir doesn’t affect the rate that the warm reservoir cools.
Once you accept the fact that a cold gas can most certainly affect the rate of radiative cooling of a warmer surface the same as any other physically coupled thermal reservoir, we can move on. And that will happen when, in 2050? When hell freezes over? When you are fiddling with your car battery and it delivers a powerful shock across your frontal lobes that shocks you momentarily into sanity a la Stanley Stupid? When you find yourself stranded in your car on a -20F night and your space blanket — in spite of being a thin sheet of aluminized mylar more or less in equilibrium with the -20 F outside air, in spite of the fact that the car is closed and there is basically no difference in convective cooling with or without the blanket — saves your life by reflecting your own body heat back at your body, thereby slowing your rate of energy loss?
Who knows?
So Dr, Spencer is a warmist too. You are thick, Greg. Get yourself two radiant heaters from Costco. Turn one on high and the other on low. Stand between them. They both warm you. Right Greg? Remove yourself from between the heaters. Now they are warming each other. Oh, but that can’t be. That would dispel your willful ignorance. Thick types like you are the best friends the warmists have.
rgbatduke says:
July 22, 2012 at 4:22 pm
Greg, sooner or later you’re going to have to realize that you can actually do experiments that falsify your bizarre belief that cold things can’t affect the rate that things cool …
=======================================================
Yeah, first of all you omitted the word “warmer”: “warmer things”. Of course, it is just a coincidence that you have distorted the central point you apparently can not prove.
As for my alleged belief, I am completely open to any conclusion. All you need is to prove your point. Until now you have failed to provide any link to a real genuine falsifiable scientific experiment. Maybe you can not for whatever reason, but let us hope (other) warmists will come to help. Or you can keep trying to sell your narratives as science, no problem, people are not stupid and will understand what is going on and what “climate science” is based on. Please, do not go away, we need you.
michael hammer says:
July 21, 2012 at 3:52 am
The 8th comment in the thread. Should have stopped reading, after that one.
rgbatduke says:
July 22, 2012 at 4:22 pm
I’ve suggested two or three real falsifiable scientific experiments that you can do …But you don’t want to actually perform such an experiment,
=======================================================
This is very funny, because I just wrote in my previous comment: “it looks rather typical for warmists: when asked for experimental proofs of their claims, they either say it is not necessary because it is so in theory or suggest the opponents do the experiment. Or they give “explanations” of what they have yet to prove. Unbelievable.”
Than you for the confirmation.
So far, I’ve increased my understanding from some of the replies in this thread (however incrementally). So it certainly isn’t “embarrassing” to me in any way, and I’d wager most others, too. Jeesh….
I’ll also add that Anthony is a professional weather scientist in his own right, and author of a book (which I proudly own) that will teach you to predict the weather, within reason, from a knowledge (that he provides) of atmospheric dynamics and physics. Very cool.
Without wishing to speak for him, I believe that he has indicated on countless threads that neither he nor most of the professional scientists who participate in these discussions “deny” that there is a greenhouse effect, that there has been global warming on average (not monotonic) since roughly 1850, or that human emissions of CO_2 may not have contributed some fraction of that warming. Very few scientists (climate or otherwise) or weather professionals would argue with any of this because there is direct evidence that he can read just as well as you or I for the greenhouse effect being “real”, direct thermometric evidence for the warming, and a very plausible argument that humans have contributed some of the increase in atmospheric CO_2 observed over time (although just how much is still the subject of some discussion, since very different — but all physically motivated — models are capable of explaining the increase with very different human inputs) and that this increase can plausibly be responsible for some fraction of the observed warming. I certainly don’t.
On the other hand, that does not automatically mean that I agree that most of the warming from any multidecadal interval in the record is due to CO_2 (and there has only been roughly 1.5 C of warming total across all 150 years with an increase in CO_2 concentration of around 1 decibel). If 100% of that warming were due to the CO_2, it would suggest that we could get a maximum of 3 more degrees C from the next two decibels (to a doubling). If 1 C were due to the CO_2 it would suggest 2 more degrees. If (most reasonably) 0.4-0.6 C were due to CO_2 it would suggest at most around 1 more degree. I think a lot of skeptics would agree that we could observe around 1 C more of total CO_2 induced warming by the time we reach 600 ppm, which IMO is likely to be at or very close to peak CO_2 before it starts to go down because people simply aren’t burning fossil fuels much any more not because of impending catastrophe but because they are too expensive and dangerous to mine and use and suffer from strictly increasing scarcity and risk of recovery (making them fairly predictably more expensive).
David Hoffer has an even smaller estimate, but not even the majority of climate scientists are calling for even 3 C any more (let alone the more egregious of the high-side estimates of Hansen’s papers past). AR5 will pick 2.8C as the median rise predicted by their collective GCMs IIRC, but there are plenty of climate scientists now that think it will be less, and the general trend is very much to lower the prediction. Who knows, skeptics and mainstream climate scientists might even converge in a year or five on 1.5 C or the like — non-catastrophic.
But Anthony does not censor even bad science out of the blog, and only rarely makes direct comments about bad science that shows up. In that I think he is wise. For one thing, to do so he’d have to make personal judgements about what is and isn’t bad science, and it is better to permit some really, really bad science in than to reject some good science by accident or ignorance. The debates usually reveal what is clearly bad, what is clearly good, and what might go either way, what isn’t really known or is up for discussion.
It is also, as you note, very educational even for non-participants in these discussions to hear the issues hammered out. You too have to form your own judgments as to what is and what isn’t a valid argument, who is and who isn’t well enough educated in the basic science involved to be at least approximately trustworthy, but you are better off doing so without Anthony’s peremptory intervention. Two of the other climate blogs that I can think of offhand engage in direct censorship and intervention, and both of them are seriously the worse for it.
rgb
Don Monfort says:
July 22, 2012 at 4:46 pm
So Dr, Spencer is a warmist too.
===========================================
This is a purely technical term. Technically it looks like he is, yes, but certainly not a radical one. By the way, being a warmist does not automatically mean being wrong. If they scientifically prove what they claim I will certainly become a warmist, too.
I would appreciate it to no end if Greg House would post links to experiments that prove:
1. The earth is round.
2. That the earth orbits the sun.
TIA
Don Monfort says:
July 22, 2012 at 4:46 pm
Get yourself two radiant heaters from Costco. Turn one on high and the other on low. Stand between them. They both warm you. Right Greg? Remove yourself from between the heaters. Now they are warming each other.
========================================================
Can you prove that “now they are warming each other”? Proving it is necessary, just rephrasing the same claim alone does not constitute a scientific proof.
No problem if you can not, until now no warmist has been able to prove it, so you are not alone. The more important aspect is that they can not prove their key assertion. Looks bad for warmism. Scientifically bad, I mean, the propaganda machine is still working.
rgbatduke says:
July 22, 2012 at 4:57 pm
…there is a greenhouse effect, that there has been global warming on average (not monotonic) since roughly 1850, or that human emissions of CO_2 may not have contributed some fraction of that warming. Very few scientists (climate or otherwise) or weather professionals would argue with any of this…
==================================================
This is so not true. Even a well known study on “consensus” in fact proves the opposite: http://wattsupwiththat.com/2012/04/30/consensus-argument-proves-climate-science-is-political/#comment-972119 .
The concept of equilibrium between the surface and atmosphere above the surface is also nonsense. GHGes which compose an avg of 0.2% of the atmosphere have no discernable impact on temperature..if anything the effect is negative given the enhancing of the water cycle/general convection.
Well, er, or one could look at the top of atmosphere spectrographs. I would think a quantum physicist could read them, Phil. I’d think they could do a mental integral of the flux of the Poynting vector implicit in the data. I think they’d conclude that GHGs have a discernable impact on temperature. They damn sure have a discernible impact, at a concentration of 0.03% for CO_2 alone, on the TOA power spectrum, one that fits damn near perfectly (as real data goes) with blackbody curves. It’s also wise to look at simultaneous spectrographs taken from TOA looking down and bottom of atmosphere looking up. That way you can see where a measurable fraction of the missing energy goes — right back down to the surface.
With that said, I agree that one need to account for rotation, the heat capacity (especially of the 70% of the Earth that is ocean) and a lot of other things, but bear in mind that none of these things mean that CO_2 isn’t an important factor, especially the initial concentration that is not yet saturated. Right now CO_2 is saturated (the atmosphere is optically thick) so that the effect of varying the concentration of CO_2 is rather open to debate and yes, it may be that the full nonlinear coupling between CO_2 derived variations of temperature and water derived variations of temperature may turn out to cancel as easily as add, but that kind of question is what science is for. Evidence and argument first, conclusion later. It’s better that way.
Also, I wouldn’t assume that all of the GCMs ignore things like rotation, heat capacity, and so on. Rather the contrary. I don’t actually think that any of them are deliberately leaving physics out, even though they may be getting physics wrong (or being mistaken when they neglect something they think is negligible).
I just think it is a hard problem. I also think that we are decades short of having enough reliable data pulled from modern instrumentation that is even approximately global in its coverage. Anthony has discovered rather huge problems with straight up thermometric sampling, even with modern digital electronically recording thermometers. And this is, or should be, our most reliable data out of the entire 150+ year thermometric record. Which speaks sadly for the certainty or reliability of any gross conclusion drawn from the data.
UAH lower troposphere I trust, at least to be what it is, and fairly consistent. I’d even allow that it can be extended a decade or so with soundings. Surface and oceanic temperatures — the latter especially — are dubious even in the present, and doubly so in the past, although the ocean we may finally be sampling reliably if not sufficiently densely. Who knows, by 2030 or 2050, we might understand some things!
rgb
Is that like a “last and final” boarding call at the airport? Redundant much?
Sorry, this thread needed a detour.
You have more patience than I would under the circumstances. Much appreciated.
Thirty years of teaching physics from intro through graduate level stuff, twenty five of that spent as an active theorist and computational physicist. And I have kids. Yes, I do get tired of trying to get Greg House to look at TOA/BOA spectrographs and try to predict them without using the physics of the GHE, and I very quickly got tired of SkyDragon’s inability to look at the pictures at the very top of this very article and note that the Sun emits the vast bulk of the energy the Earth absorbs in or near the visible part of the spectrum, at the very least at wavelengths clearly resolvable from those the Earth emits as it cools, but SkyDragon clearly does not understand (cannot derive) the blackbody radiation curves in the first place any more than Greg can. I can. I have — all physics students have to, usually while they are undergrad majors, as it is where quantum theory was really discovered. SkyDragon thinks that “heat radiation” is all in the IR part of the spectrum. So do a number of other posters. Apparently they never actually look up at the Sun, or look at an incandescent light bulb, or look at hot glowing coals, or…
I have no idea what Greg thinks about anything, since his entire argument seems to be “you can’t make me accept (some unstated proposition that varies from time to time) as proven. Prove the GHE with falsifiable data, he says. Look at the TOA/BOA spectra, I say — falsifiable data galore. No, he says, prove it. Prove that cold CO_2 can warm the ground. I proceed to offer proof after proof, including proofs he can prove at home. No, he wants a double blind, placebo controlled experiment that the actual atmosphere is warming the ground — back to the TOA/BOA spectra. But that’s not a proof, he wants… well, it’s not entirely clear what he wants, and since he has no hypothesis of his own to offer, falsifiable or not, the conversation gets rather circular. At that point I usually give up, but damn, on the next thread I try again. Some people never learn.
I’m talking about me, of course. I should learn not to get drawn in to hopeless cases.
The smart thing to do would be to pretend that he doesn’t exist at all. After all, maybe he doesn’t? Could he provide me with falsifiable experimental evidence of his own existence? That would be funny. He could send me a photograph, and I could say “but this isn’t really evidence of your existence, any more than TOA spectrographs are evidence of the GHE’s existence”. He could try to point to places where he’s had some effect — such as annoying me by pretending that my replies to his requests haven’t occurred, or aren’t “really” evidence — and say that if I really doubt it I could come visit him. I could then say aHA, so you can’t actually provide evidence (that doesn’t involve me doing something like looking). Then he could try again with a photograph, or birth certificate, and we could have a jolly old time.
rgb
As a physicist you should know that far too little solar energy/area hits the surface of the earth for it to be broadly useful – even if conversion was 100% efficient.
Are you shittin’ me? As Samuel Johnson once said:
http://en.wikipedia.org/wiki/File:Solar_land_area.png
“Thus I refute you.”
The problem isn’t that there is far too little solar energy — hundreds of watts per square meter is plenty. The problems are (in order) cost per watt for the cells themselves, storage, and transportation. Of these cost per watt of the cells is paramount — if it is less than $1/watt and one can dump surplus energy back into the grid, the grid effectively stores and transports it, at least locally, and it becomes break even to win a bit to cover your very own house with a solar collector, at least in the US Southwest and much of the South. Other parts of the world it is even simpler as it is even sunnier — Australia, North Africa, Mexico, India, Arabia, all have plenty of sunlight, lots of silicon, and vast empty/desert spaces that nobody would miss if they were covered with solar cells.
But it is pretty simple to do the math. At $1.75/watt, $1750 buys a kilowatt. A kilowatt hour costs between $0.06 and $0.15 in different parts of the US. A rooftop collector can produce roughly 7 or 8 kWh a day — call it $1’s worth of electricity in high rent states, maybe $0.50 in the cheapest states. It takes 3 to 6 years to recover your investment — or would, except that you also have to buy an inverter for several thousand dollars and at least some buffering battery capacity even if you will automagically sell your daytime surplus back into the grid to help subsidize your nighttime consumption. Last time I looked it cost a bit over $11000 to buy 5 kW of rooftop capacity, installed — call it $2.25/watt. I live in a cheap-electricity state, which kicks the number of years to positive ROI on an amortized investment up to maybe 13 to 15, just barely not worth it on a twenty year basis, as $11,000 invested conventionally is probably worth about as much.
Drop the cost of cells alone to $1/watt, and the cost installed drops to $1.50/watt, including labor and inverter and battery. The $11000 drops to maybe $8000 for 5 kW, and I recover my investment even in NC in less than a decade. I then proceed to double it over the next decade. Assuming a 20 year lifetime for the cells and inverter — probably underestimates for both — it is at least as good an investment as anything I could do with my money that is relatively low risk. Even allowing something for maintenance and/or replacing the battery, the gamble of probably getting 30 years, over 20 of them actually making me money every year makes it a good bet, every bit as good as my replacement of my last heater/AC with a high efficiency unit that has halved my energy bills (but will take at least a decade or more to pay for its additional cost nevertheless).
Normal humans can do this math. It does not depend on government subsidy, although with the subsidy I could probably win a bit already even at current cell costs.
Corporate conversion is already at $1/watt per at commercially scaled prices. Power companies have a slightly different cost profile — they have to pay for land and labor where my rooftop is “free” to me — but they also realize a number of economies of scale, and in expensive-power states solar is already an attractive investment with or without subsidy. In all states roughly south of the Mason-Dixon line, dropping the consumer cost to $1/watt and the scaled cost to power companies to $0.50 to $0.75/watt would almost certainly suffice to start a major round of investment in solar conversion both on private rooftops and in corporate farms, not because it is good for the environment, but because it is a good investment. Other economies of scale would have some impact as well, as would other technological advances. Batteries, for example. Cheap enough battery capacity to allow solar to actually replace, rather than augment, conventional power would have a profound effect on the profitability.
As for fission, I just love fission, given sufficient care to avoid its known problems. The problem is that there isn’t enough Uranium/Thorium to last 10,000 years, although Uranium eked out by solar probably could easily last this long or even another 10,000 years. Definitely not a million years, or 50,000 years, though. The Earth is just too damn old, losing Uranium all the time. Still, I totally agree — if the powers that be, including the environmentalists, truly are worried about CO_2 and don’t want to destroy civilization (which is what pulling the plug on fossil fuels would do) in order to “save” it, they could put their money where their mouth is and provide every possible incentive to build nukes, ideally Thorium nukes where are much more bomb-proliferation-proof than Uranium nukes and (in at least some of the designs I’ve seen) nearly meltdown-proof as well.
Still, in the long run — and as noted I mean the truly long run, the steady state human civilization long run — the only fossil fuel that can stay the course is Deuterium. Deuterium is practically inexhaustible. Helium 3 would also be groovy but there isn’t enough of it; tritium too short-lived and dangerous. Without fusion, in 50,000 years the human race will be down to solar, wind, hydroelectric, geothermal — all of those currently despised technologies (although why they attract so much rancor is beyond me — burning stuff for energy is an expensive, dirty, pain in the ass even if CO_2 is so wholesome that we should all be concentrating it and sniffing it as a vitamin — speaking of which I like to whiff my own vitamins of this sort using a decoction of naturally fermented barley, water, and hops, pressurized by yeast in the bottle, and I do indeed have a bottle of very cold homebrew in my fridge that is calling to me, so I’ll cut this short).
This probably won’t be civilization ending, but it will make living in the northern temperate zone difficult, especially given that we’ll almost certainly be in an major glaciation period by then. After all, the Ordovician-Silurian ice age happened in spite of CO_2 at 4000 ppm, ten times what it is today, in the middle of its glaciation (it started when CO_2 was more like 7000 ppm) and nobody has convinced me that the same thing that started that ice age isn’t responsible for the current one, and that it has nothing whatsoever to do with CO_2. Indeed it might be nice to save some of the coal and oil for this era — but we won’t do that. Or rather, we probably will, because burning stuff for energy is an expensive, dirty pain in the ass, whether it is firewood in your fireplace or charcoal in your grill or gasoline in your gas tank or — ok, I’ll make an exception for methane through propane.
rgb
David asks
So my questions are as follows. How much of the non GHG energy is radiated to space via collision with GHG molecues?
Essentially all of the IR radiated to space from the atmosphere is a result of collisional excitation of vibrational modes.
If the GHG molecues were not present, how much longer would this energy stay within the atmosphere if it could only be conducted and convected about, but not radiated to space.
The atmosphere would be much colder because the surface would be colder (somewhat technical argument related to the derivation of the adiabatic lapse rate starting with an average surface temperature). If you allow no ghgs, there would be no water vapor or clouds.
You can get an idea from this figure. Everything below the 320 K line is a result of ghgs re-radiating after collisions.
You raise the interesting question of what the energy of such an atmosphere would be. The temperature at each level would be given by the dry adiabatic lapse rate, the energy per unit volume given by CvT. where Cv is the effective specific heat.
<i.And, as additional GHG molecues speed the escape of conducted Non GHG energy, would not this reduced residence time of conducted non GHG energy have to be subtracted from the increased residence time of IR energy raqdiating from the surface, and backradiating from the GHG molecues? TSI incoming is a consistent flow, so the energy gained or lost by either radiating conducted non ghg energy out, or keeping surface energy within the atmosphere is porportional to the residence time of the energies affected.
This is somewhat backwards. GHGs (and increases in same) SLOW the escape of IR energy from the Earth surface and atmosphere together. Without GHGs the escape of IR energy from the surface is much faster, and if you look at energy balance, the speed up exactly matches the deficit from wiping out the ghgs.
Greg House says:
July 22, 2012 at 5:14 pm
Don Monfort says:
July 22, 2012 at 4:46 pm
Get yourself two radiant heaters from Costco. Turn one on high and the other on low. Stand between them. They both warm you. Right Greg? Remove yourself from between the heaters. Now they are warming each other.
========================================================
Can you prove that “now they are warming each other”?
Yes, measure the temperature of each when a) they are directly facing each other and b) when they are facing away from each other. Next.
Don Monfort says: referring to Greg
July 22, 2012 at 8:49 am
Does this clown think that the warmer body is transparent to the radiation from the colder body? Does it pass through without any effect? Or it detours around the warmer body? Bounces off?
Yes, Greg House is a believer in intelligent photon theory
Police Guy says:
The other day I put together a visual of 393 ppm CO2. This number reduces quite nicely to 4 parts per ten thousand. So I bought a plastic bottle with 10,000 bright yellow airsoft BB’s and removed its cardboard central piece to reveal an unobstructed view of all 10,000 BB’s to the viewing world. I then added 4 bright blue airsoft BB’s of the same size.
Add a drop of ink to a liter of water. Better, add 0.4 gm of a strong dye to a liter.
The bunny says: “Greg House is a believer in intelligent photon theory.”
And bun-bun is a believer in intelligent molecule theory: that sneaky CO2 is making the Arctic melt, and leaving the Antarctic alone.
Yes there is a “large raft of scientist” out there who know ever so much about radiation, but can you get one of them to explain to me, and other “disbelievers”, how it is that radio-waves can penetrate a brick wall while IR and other “light-radiation” stays on the outside? – I don’t think so. – Consensus, consensus, con——.
Any physicist — no, any physics major, by their third year, can easily explain this. You too could understand it if you tried, assuming you are moderately competent in calculus through partial differential equations and complex variables and are willing to take a year or two to learn it.
So, are you willing to try?
I’ll even take the time, on list, to reduce the explanation as much as I can to simple things that you can understand without necessarily using calculus, but it is really not that difficult. Some materials are transparent, some are opaque, because of their quantum molecular structure, in some frequencies but not others. Good conductors e.g. metals are usually not only opaque, but typically strongly exclude electromagnetic fields and hence reflect incoming E&M radiation — but still not necessarily in all frequencies. Insulators are much more variable. Brick, microscopically, is a good insulator and hence has a large skin depth for low frequency radiation, but the small sand particles are made of molecules that can absorb and reradiate optical frequencies quite well. Glass, on the other hand, often has a large skin depth in optical frequencies and only slowly attenuates visible radiation. Water is an amazing substance in and of itself — transparent almost precisely in the visible band, but rather opaque all around it, and with a transparency and reflectivity that varies with its state and structure. Sea water is moderately transparent to visible light, but as a good conductor blocks/reflects many frequencies but, curiously enough, not very long wavelength radio waves.
But the main things you’ll need to learn to get a first order understanding of this are skin depth (explained/derived in my online book on Electrodynamics) as a generic response of charged matter to incident radiation, subject to dispersion, and quantum mechanics, which explains the details of the dispersion and predicts the actual interaction between electromagnetic radiation and atoms or molecules of various kinds.
If, on the other hand, you aren’t really interested in trying and are just trying to assert that physicists have no idea why brick walls let radio waves through but not visible light or IR, then as previously noted, you need to stop wasting everybody’s time. The only person’s time you should be spending is your own, studying. You remember studying? It involved getting out textbooks, finding mentors, taking classes, and working your ass off, the same as everyone else who ever learned the material. No shortcuts, no you’re not so intelligent that you can do it without hard work by means of your own sheer brilliance and intellect, and yes every single step along the way is individually confirmable in laboratory experiments.
You stand on the backs of giants, sir, whether or not you wish to acknowledge them, and you couldn’t even type out your responses on this blog on your computer and have them be transmitted over an electronic network and be transformed in due time into light and dark patches on your screen if physicists and engineers could not only answer your questions, but answer them in detail. The evidence is literally at your fingertips as you read this. Do you really want to pretend that this isn’t the case, that we really don’t know how to make electrons sit up and dance?
rgb
rgb
Beng says:
Why does the altitude that radiation escapes get higher?
Because absorption is proportional to the number density of the absorbing molecules. When the number density is high enough, essentially direct radiation to space is blocked, the emission from the ghg molecule lower down gets absorbed higher up and does not escape to space.
I very patently asked them to present a link to a real scientific experiment proving that notion, but they failed (http://wattsupwiththat.com/2012/06/22/a-response-to-dr-paul-bains-use-of-denier-in-scientific-literature/). Does not look well for the concept.
And I provided him with a link to a real scientific experiment that produced real spectrographs that proved that notion. His response then, as now, is to pretend that this never happened, or that it doesn’t prove the notion, it isn’t clear which. But don’t take my word for it — follow the links and compare TOA and BOA spectrographs taken at the same location and time, and observe the complimentary greenhouse hole at the top and backscattered radiation in the exact same greenhouse bands at the bottom. If direct measurements of the radiation isn’t evidence, then — to Mr. House — nothing will be, as he isn’t really interested in the evidence, he’s interested in repeating a claim over and over again in spite of the evidence.
rgb
paulinuk says:
July 21, 2012 at 9:01 pm
I’ve another simple question for Joel Shore etc : If ”non- radiative” gasses such as N2 and O2, H2 can’t radiate thermal energy in the IR that they have acquired through conduction then a jet of N2 or O2 at 15c emitted by a spacecraft in a vacuum wouldn’t show up on an IR thermal camera would it (but a jet of C02 at 15c would)? The simple experiment’s been done, hasn’t it?
Couldn’t we then use O2,N2,H2 to store vast amounts of energy so long as they were in deep space and as you say they “can’t radiate” much? Just imagine, we could heat up H2 to a million degrees and hardly no heat would radiate from it.
Clever enough :), but the sun is a plasma, not a gas and in any case you would need some gravity to hold the damn thing together.
Again, I thank everyone for responding to this guest post.
Those who claim this post is embarrassing may be right. But if so, it’s primarily an embarrassment to me and maybe secondarily to Anthony. I can’t speak for Anthony, but I can stand whatever embarrassment you ascribe to this post.
I infer from some of the comments on this thread that some people believe (a) that greenhouse gases have a negligible effect on the rate the Earth/Earth-atmosphere absorbs solar energy, and (b)atmospheric greenhouse gases “slow down” outgoing radiation, and it is this “slow down” that warms both the Earth’s surface and atmosphere. In an earlier comment (July 21, 2012 at 5:15 am) I asked the question “Under what conditions does the presence of greenhouse gases in the Earth’s atmosphere quit slowing-down outgoing radiation?” As far as I can tell I never got an answer to that question. So to those who believe/claim (a) and (b) above, I repeat the question. If the answer is: “As long as there are greenhouse gases in the atmosphere, they never stop ‘slowing down’ outgoing radiation”, then I’d appreciate an answer to the obvious follow-up question: “If (a) the presence of greenhouse gases in the Earth’s atmosphere doesn’t change the rate radiation (energy) is absorbed by the Earth/Earth-atmosphere system, (b) in the absence of greenhouse gases the energy-rate equilibrium temperature is T, and (c) greenhouse gases always ‘slow down’ outgoing radiation which causes the temperature to rise, then why doesn’t the temperature increase without bound (or at least until the greenhouse gases go away or the temperature changes so that the temperature is high enough that radiation from the Earth/Earth-atmosphere is negligible in the greenhouse gas absorption bands)?”
Eli Rabett says:
July 22, 2012 at 7:05 pm
Can you prove that “now they are warming each other”?
Yes, …
===================================================
Yes you can! Yes you can! Yes, this is hilarious…
“Can you prove it?” in that context means “Prove it!”, you know, it was not a real question, the question form is rhetorical. And “Prove it!” does not imply your telling me to prove it. You guys should prove your claims, not we.
Now I am looking forward to a link to the scientific falsifiable experiment proving that. What, you have nothing? I thought so.
davidmhoffer says:
July 22, 2012 at 11:44 am
Michael Tremblay;
Create any experiment which will take advantage of GHG’s to create an engine which will run on radiative energy alone.
>>>>>>>>>>>>
Given the number of times in this thread alone that it has been specified that the physics being explained is reliant upon an external energy source, one can only wonder if people like you even bother to read the explanation.
>>>>>>>>>>>>>>
That comment was totally uncalled for.
I have read most of the explanations and I know that they are talking about an external energy source.
You seem to hold a misconception that all engines are run by an internal energy source. This is simply not true – I have sitting on my desk a small working model of a Stirling Heat Engine. It is run by using an external energy source.
My suggestion was that someone could get out of their scientific ivory tower working with theoretical physics and apply them to a practical purpose by making a physical model to quantify the effect.
Add a drop of ink to a liter of water. Better, add 0.4 gm of a strong dye to a liter.
Damn you sir! I wish I’d thought of that. But David’s original answer wasn’t bad either. And food coloring is even readily available!
I wish I could find good IR photographs in the CO_2 band. They should illustrate image attenuation quite dramatically. Do you know of any? I’d think they might convince — no, wait, who am I fooling.
I’m tired, though, and have just written another 10k words or so. Time to drink heavily (that one whole beer I mentioned above, my contribution to the GHE for the day) and either work on a real book or go to bed. Or play poker with my sons. I suppose I could play poker.
rgb
rgbatduke says:
July 22, 2012 at 7:22 pm If direct measurements of the radiation isn’t evidence,
=====================================================
Of course it is not. You need to prove you key assertion that radiation from a colder body can warm a warmerbody. Radiation is very nice, but you guys apparently can not present any link to a real scientific experiment proving this alleged warming effect, not just radiation.
I hope now after I told you that like for the 999th time (just my feeling) you will understand the point.
No, I didn’t miss anything, but it’s becoming obvious that you missed mine.
So, you want to invest resources now in order to solve energy problems beyond 10,000 years.
This isn’t rational.
HenryP writes “The question I have is this: is the (weak) absorption by oxygen/ozone at 14-16 caused by the ozone or by the oxygen?”
I’m hardly an expert! But my interpretation of the “Oxygen and Ozone” components in the diagram would be that the Oxygen is primarily an absorber in the UV range where it disassociates the O2 which reforms to become O3 in some cases and combined they capture even more UV.
And the O3 would be the absorber in the IR range because O2 (and N2) are negligible absorbers in those ranges themselves. So the short answer which should be taken with a grain of salt is O3.
Michael Tremblay;
You seem to hold a misconception that all engines are run by an internal energy source.
???????????
No, I said that the physics in question was reliant upon an external energy source.
Michael Tremblay;
My suggestion was that someone could get out of their scientific ivory tower working with theoretical physics and apply them to a practical purpose by making a physical model to quantify the effect.
>>>>>>>>>>>>>>
The various examples you provided in your original comment are impossibilities that prove nothing at all in regard to what IS possible in regard to the radiative physics being explained. If you want a physicist to descend from the ivory tower and provide practical examples of physical models, then I would direct you to the multiple examples of same provided by rgbatduke upthread.
Eli writes “When the number density is high enough, essentially direct radiation to space is blocked, the emission from the ghg molecule lower down gets absorbed higher up and does not escape to space.”
I dont think so Eli. Real density matters too not just the proportion of GHGs in that volume except at saturation perhaps.
rgbatduke;
David Hoffer has an even smaller estimate
>>>>>>>>>>>>>
Well for clarity, I just note that the IPCC calculates CO2 doubling = 3.7 w/m2 = +1 degree. But they also represent the average temp of earth surface as being 15C. Since it would take 5.5 w/m2 to raise temp from 15C by one degree, these numbers seem to contradict one another. 3.7 w/m2 would raise the temp of -20C by one degree.
Upon investigation, it turns out that the calculation is done not against earth surface temp, but against “effective black body” temp of earth, which as it turns out, is about -20C.
Regardless of how one extrapolates the 3.7 w/m2 modeled at TOA to earth surface, one simply cannot come up with a temp change of 1 degree at surface. It would be more like 0.7 degrees, and my assumption (though I have no way of knowing this) would be that even if feedbacks are positive, they would scale in the same manner at surface.
So… my point is that IPCC numbers, when put into their proper context, do not support +1 degree at earth surface, only +0.7 for doubling of CO2. By extension, feedbacks that would result in 3 degrees at effective black body temp of earth would be more like 2.1 degrees at surface.
Given that the IPCC AR5 is supposedly going to scale back their median estimate even further, to 2.6 including feebacks, my assumption being that this number also is calculated at effective blackbody temps, translates into only 1.8 degrees at surface.
I find that the IPCC documents are consistant in terms of finding the worst possible context to present the numbers, and are extremely vague in that regard to mask the spin they are putting on the issues, and this is a fine example of same. We live on earth surface and that is the temperature we care most about, so those are the number which in my opinion the official literature should be discussing.
davidmhoffer says:
July 22, 2012 at 8:23 pm
The various examples you provided in your original comment are impossibilities that prove nothing at all in regard to what IS possible in regard to the radiative physics being explained. If you want a physicist to descend from the ivory tower and provide practical examples of physical models, then I would direct you to the multiple examples of same provided by rgbatduke upthread.
>>>>>>
That is better than your previous remark. My first remark was an attempt to describe why I have serious doubts about the description of the radiative physics – that being, that if it is as extreme as some people think it is, some engineer would have exploited it to make an engine out of it. My remark was off topic but it did not call for the remark you made.
I might suggest that, if you don’t agree with someone else’s comments, that you either not respond, or respond in a manner which is more constructive rather than insulting and attempting to belittle them. You have to remember that most of the people that follow WUWT are not experts in the various scientific disciplines which are required to understand climatology, and are seeking answers.
I have been following rgbatduke’s threads and find them instructing and helpful. He also exhibits a large amount of patience.
Reed Coray says:
July 22, 2012 at 7:45 pm
Embarassing? Whoever is embarassed by this thread needs to be embarassed more, IMO. While Joel may feel we are sheepherders, he is actually learning too. While Anthony may be embarassed by the passion, what a relief from the political drivel.
rgbatduke,
It might help explaining Greg House that the IR from the colder body is not actually warming the warmer body directly, but rather indirectly by reducing its rate of heat loss. Thereby no thermodynamic law is violated and we can all move on …
Joel Shore:”Are you saying that they are somehow radiating more heat than one would expect given their temperature? Furthermore, the thermal structure of the tropical atmosphere is dominated by convection. The radiative transfer is already such as to maintain a larger lapse rate than exists but convection occurs and reduces the lapse rate approximately to the moist adiabatic lapse rate.”
No, my personal position is that the condensation in atmosphere that causes the lapse rate feedback is also accompanied by *another* negative feedback namely increased cloudiness. Thus, my relationship is increased water vapor leads to lapse rate feedback and (negative) cloud feedback that in turn cause the (near) absence of the tropical hotspot. You, OTOH, argue that if the hotspot is indetectable then the *only* possible interpretation is that there is no lapse rate feedback. I doubt that this is the only interpretation one can draw from the absence of the hotspot but it is possible I am mistaken.
Cheers, 🙂
There have been many interesting comments here, but not a lot of hard facts that support the AGW hypothesis and its proponents like Eli and Joel. Here are a few that do not.
Although AR4 WG1 at FAQ 1.1 (i.e. Trenberth) states that the reason the earth’s surface is as warm as around 14oC “is the presence of greenhouse gases [GHGs], which act as a partial [sic] blanket [sic] for the longwave radiation coming from the surface [OLR]. This blanketing is known as the natural greenhouse effect. The most important greenhouse gases are water vapour and carbon dioxide. The two most abundant constituents of the atmosphere – nitrogen and oxygen – have no such effect…Human activities intensify the blanketing effect through the release of greenhouse gases”, mainly by the combustion of hydrocarbon fuels like coal, gas and oil. That implies there should be an observable reduction in OLR in line with the rising atmospheric concentration of CO2. But the NOAA NCEP reanalysis dataset shows the following UPWARD trend in OLR between 1948 and 2011:
y=0.1369x+226.88
R² = 0.8019
Source: NCEP Reanalysis Produced at NOAA/ESRL PSD at http://www.esrl.noaa.gov/psd/data/timeseries/ Date submitted: 7/16/2012 at 01:53
Similarly, satellite data in 1970 and 2003 showing temperatures (in oK) by altitude over the Tropical Pacific reveal no observable increase at all, in fact the curves and their trends are virtually identical:
1970:y=-6.2945x+300.62
R² = 0.7518
2003:y=-6.296x+300.72
R² = 0.7538
Then there are the trends for the level of water vapor (per cm.) by altitude, which according to AR4 and all should have increased in line with AGW but actually declined between 1970 and 2003, confirming that there was no warming over the tropical Pacific:
1970:
y = -1519.5x + 18498
R² = 0.6781
2003:
y = -1431x + 17219
R² = 0.6162
See the Appendix Tables A1 and A5 in Griggs & Harries (JoC 2007), Comparison of Spectrally Resolved Outgoing Longwave Radiation over the Tropical Pacific between 1970 and 2003 Using IRIS, IMG, and AIRS.
These results appear to confirm the nul that CO2 plays NO role either in reducing OLR or in raising temperatures and water vapor at any altitude (from 1000 hPa to 4.606).
Reed Coray asks
“Under what conditions does the presence of greenhouse gases in the Earth’s atmosphere quit slowing-down outgoing radiation?”
Henry@ Reed
First of all they must get the definition of a GHG right. The definition should not be that the substance is a GHG if it has absorption in earthshine wavelengths. The definition must be that if the net effect of more of it in the atmosphere is one of warming, we can call it a GHG. Like in the case of ozone, it has absorption at 9-10 (see your graph that you start of with) and that indeed does leave a dent in earth’s outgoing radiation. So it is classified a GHG. But ozone also deflects a lot of sunlight 0-0.5 um, which is radiation of high energy. I maintain that an increase in ozone (as indeed we are currently observing) will lead to a net cooling effect. My results show earth has started getting less energy exactly since ozone started moving up. So really, they should classify ozone an IHG. (Ice house gas). In fact, oxygen would probably then also be an IHG.
In the case of CO2, its radiative cooling effect and its cooling effect by taking part in photosynthesis cannot be ignored. A paper in in 1974 estimated that about 0.2 -0.3 % of incoming sunlight is consumed by vegetation. With everybody wanting more crops and more trees I am willing to bet that it has gone up substantially since then. I would not be surpised if it is eventually found that the warming effect of CO2 is more or less cancelled out by its cooling effects.
Either way, it has been cooling since 1997 or 1998 even though CO2 has continued to rise. In fact, in Baring (Alaska) it appears to me that CO2 also stopped increasing. Which is all what we could have expected if some people like Hansen and Gore had not jumped on the AGW wagon. There is only GW (CO2 gasses out from the oceans) and GC and until 2045 we are now in GC mode.Sorry. Better get some extra cloths and if you can move to lower latitudes.
http://www.letterdash.com/HenryP/more-carbon-dioxide-is-ok-ok
@ Ken Harvey says:
July 21, 2012 at 12:35 pm
Thank you for your kind words sir. 🙂
@ Baa Humbug says:
July 21, 2012 at 4:15 pm
I have recieved the file. Many thanks for your help.
I have read it twice and have been confused by some points.
Example: at one point (in a thought experiment) he claims because CO2 can radiate it will cool down to 0K.
It seems to me that CO2 can only cool to 193K and then need help from other gases that can radiate below that temperature level.
Musn’t discuss that here though, a bit OT.
Eli Rabbet: “Clever enough :), but the sun is a plasma, not a gas and in any case you would need some gravity to hold the damn thing together”.
But the National Radio Astronomy Observatory guys say that all matter emits thermal radiation, be it a lump of stone or iron, plasma or gas because charges are moving in relation to one another. As a gas has molecules and they contain a charge( protons and electrons), then as the molecules jostle about, the charges in one molecule move in relation to other molecules. In the case of a plasma the charges are free and this emission is called “Free-free emission”. This has nothing to do with absorbtion bands of molecules. The radiation emitted is broadband and is called blackbody radiation. At 15c the infrared radiation emitted peaks at 10.1um.
The National Radio Astronomy Observatory explanation:
http://www.nrao.edu/index.php/learn/radioastronomy/radiowaves#blackbody
@ rgbatduke:
Thank you for your patience and descriptions of radiative transfer. (ps – forgive my apparent ‘misspellings’ – I use Canadian English)
A lot of my problems with the concepts of radiative transfer come from misunderstandings. For example; the entire effect is labeled the Greenhouse Effect (GHE), whereas it has very little to do with how a greenhouse works actually works; Greenhouse Gases (GHG) are defined as those gases which absorb Infrared Radiation (IR) which is only a small portion of the entire spectrum of Electromagnetic Radiation (EMR) which covers all of radiative energy. IR is directly associated with heat when it is only a form of radiative energy which is emitted by objects within the temperature range that fall within the IR spectrum. Finally, with Colour Temperatures (an aesthetic description), red is considered a warm colour while blue is considered a cold colour – in direct opposition to their respective energy levels and temperatures. I think that these basic misunderstandings are true for a lot of people, including well educated scientists.
I thank God for the so-called GHE, since without it Mankind would not exist. Rather than call it the GHE though, I would rather call it the Atmospheric Effect (AE) since I believe that the protective effect of the entire atmosphere is far more important than the effect of GHGs.
First, it is important to remember that most of the theoretical discussions I have encountered only consider that the atmosphere is mixture of gases. As you are certainly aware, this is not true. The atmosphere can more accurately be described as a mixture of gases with suspensions of liquids and solids. Most people assume that clouds are water vapour, again, this is not true. Clouds are suspensions of liquid and solid water in the atmosphere – water vapour is completely transparent, if you can see it it is either liquid or solid. Liquid water and solid water have completely different radiative absorption and emission properties, as well as reflective properties, for the entire solar spectrum when compared to water vapour. As a retired Steam Engineer, I have an appreciation for the thermodynamic properties of water which, I suspect, is far greater than most climatologists – It takes a lot of energy to convert water from one phase to another and when it reverts from gas to liquid to solid it releases a lot of energy, a property which makes it among the best for converting energy into work.
The atmosphere also has other suspended liquids and solids within it which can also have an effect on the reflective, scattering, and absorptive properties for radiative energy. Most of the descriptions of these effects are lumped together by those people creating climatic models as a variable in order to ‘accurately’ model their effects, but for the most part these variables are guesses and increase the inaccuracy of the model depending on their interpretation (sensitivity?).
I followed the link to Dr. Spencer’s site (http://www.drroyspencer.com/2010/07/yes-virginia-cooler-objects-can-make-warmer-objects-even-warmer-still/) and I understand the concept that a ‘cooler’ object can ‘make warmer objects even warm still’. I have a problem with his experiment when it relates to the Earth’s atmosphere. His ‘hot’ plate is constantly radiating at 150F because it is having energy constantly applied to it. This only occurs to the Earth during the daytime (with energy coming from the Sun), so if the experiment is accurate, the Earth’s surface temperature will increase due to the radiative effect of the atmosphere during the day or if the temperature of the atmosphere is higher than the Earth’s surface. At night the temperature of the Earth’s surface will decrease, and the atmospheric temperature will increase, until they reach thermal equilibrium. I believe that the real AE at night is that the RATE of energy transfer is reduced, not that the atmosphere is warming the surface due to back radiation.
Another problem that I have with climatologists’ interpretation of the AE is that they forget that the atmosphere also reduces the RATE at which the Sun heats the Earth. During the daytime, radiative energy is intercepted (absorbed) by the Earth’s atmosphere. Oxygen, Nitrogen, and Ozone (non-GHGs) absorb EMR at shorter wavelengths and use this energy to split the molecular bonds of O2, N2, and O3 while at the same time the energy is converted to heat when the molecular bonds reform – one of the (theoretical) reasons the Stratosphere is warmer than the upper Troposphere. If this shortwave radiation reached the Earth’s surface it would ionize molecules and atoms at the surface and be absorbed by the solid and water surfaces and converted to heat, raising the surface temperature even further. As far as I am concerned the daytime AE is far more important since if you can reduce the rate of heating it doesn’t matter if you reduce the rate of cooling. I know that this radiation is not IR, but since shortwave radiation has much more energy than IR, isn’t the absorption of high energy EMR (UV) more important than the absorption of low energy EMR (IR) in order to prevent a rise in temperatures?
Finally, I would like clarification on this point – If a CO2 molecule (or any molecule for that matter) is absorbing a photon of a specific frequency and re-emitting a molecule of the same frequency, where is the energy transfer? From my perspective, in order for there to have been an increase in temperature (heat energy) or in kinetic energy, the emitted photon has to be at a lower frequency – what is really happening when a gas molecule absorbs a photon?
Eli Rabett says:
July 22, 2012 at 6:59 pm
David asks
So my questions are as follows. How much of the non GHG energy is radiated to space via collision with GHG molecues?
——————————————————————————————————————————-
Eli says…
Essentially all of the IR radiated to space from the atmosphere is a result of collisional excitation of vibrational modes.
———————————————————————————————————-
My response… First thank you for engaging my questions. But, is not some of the energy radiated to space, first recieved, not by conduction, but by radiation in the correct WL?
Question two
If the GHG molecues were not present, how much longer would this energy stay within the atmosphere if it could only be conducted and convected about, but not radiated to space?
=============================================================
The atmosphere would be much colder because the surface would be colder (somewhat technical argument related to the derivation of the adiabatic lapse rate starting with an average surface temperature). If you allow no ghgs, there would be no water vapor or clouds.
You can get an idea from this figure. Everything below the 320 K line is a result of ghgs re-radiating after collisions.
You raise the interesting question of what the energy of such an atmosphere would be. The temperature at each level would be given by the dry adiabatic lapse rate, the energy per unit volume given by CvT. where Cv is the effective specific heat.
===================================================
Thanks again Eli, but I think you did not answer my question. With an equally dense, non GHG atmosphere, would it not be more transparent to incoming, and not just outgoing TSI, and therfore would not the surface insolation be higher? Of couse the non GHG on a water planet would be impossible, becuase the increases surface insolation would clearly lead to increased W/V. but, for the sake of a thought experiment, let us assume no increased W/V., but equally dense atmosphere. Would the surface T not rise higher in relationship to increased surface insolation? Would the surface not lose energy by both conduction to non GHG molecues, as well as through radiation to space, admittedly zipping past the non GHG atmosphere to space? However, any energy which conducts (not radiates past) to a non GHG atmosphere must stay in that atmosphere longer then it would in a GHG atmosphere, as they only way such an atmosphere can lose energy is by conduction to the surface where from there it can radiate to space. So my question remains unanswered, but it appears logical to me that GHG slow the loss of raidated energy from the surface, but accelerate the loss of conducted eneregy from the surface. I was hoping to see the ATMOSPHERIC residence time of CONDUCTED energy from the surface compared to a non GHG atmosphere verses a GHG atmosphere. I inderstand that radiated energy has a shorter residence time in a GHG world, but I suspect that conducted energy has a longer residence time in a non GHG atmosphere. I would like to see this quantified.
=======================================
My next question
<i.And, as additional GHG molecues speed the escape of conducted Non GHG energy, would not this reduced residence time of conducted non GHG energy have to be subtracted from the increased residence time of IR energy raqdiating from the surface, and backradiating from the GHG molecues? TSI incoming is a consistent flow, so the energy gained or lost by either radiating conducted non ghg energy out, or keeping surface energy within the atmosphere is porportional to the residence time of the energies affected.
————————————————————
Eli answers…
This is somewhat backwards. GHGs (and increases in same) SLOW the escape of IR energy from the Earth surface and atmosphere together. Without GHGs the escape of IR energy from the surface is much faster, and if you look at energy balance, the speed up exactly matches the deficit from wiping out the ghgs.
————————————————————————————————————-
Thank you, but again I think you are not answering my question. I would like to first correct your above statement. Wherever you say "IR energy" please change that to RADIATED IR energy and we have no problem. However my question concerned the atmospheric residence time of CONDUCTED surface energy. The surface, when it is losing heat, is all the time losing heat by radiation and conduction. The atmosphere can only lose heat to space by radiation. A non GHG atmosphere must therfore have a longer residence time for conducted energy then a GHG atmosphere.
trccurtin,
You said:
“That implies there should be an observable reduction in OLR in line with the rising atmospheric concentration of CO2. But the NOAA NCEP reanalysis dataset shows the following UPWARD trend in OLR between 1948 and 2011”
Yup, both the NOAA Interpolated, ISCCP-FD and ERBE data show increasing rather than decreasing OLR at TOA during the modern warming era. If there has been no increase in the energy input (from the sun) during this period, then this is the direct opposite of what one would expect. There is no real sign at TOA that we’re strengthening the insulating power of the troposphere. It’s nice to hypothesize about what should and could occur if so and so, even with sound, coherent physics and numbers to back up your theory, but once in a while it’s a good idea to take a look out your window to verify if your isolated process has or has not a detectable NET effect on the actual, real-world system. If not, but you’re still confident that it must be real, then it means it’s not alone. There are forceful counter-effects and/or the show is actually run by different and much more potent processes, totally overwhelming it.
If there HAS been an increase in energy input (which the observations suggest), then THIS is the cause of the warming and the increased OLR at TOA simply Earth’s lagged response.
Kristian says:
July 22, 2012 at 10:22 pm
It might help explaining Greg House that the IR from the colder body is not actually warming the warmer body directly, but rather indirectly by reducing its rate of heat loss. Thereby no thermodynamic law is violated
=================================================
Yeah, I know this narrative, I formulated the problem both ways a few times.
The warmists problem is that they can not produce any real genuine falsifiable scientific experimental proof of that “reducing its rate of heat loss”. They just keep “explaining”, rephrasing and mostly suggesting I should prove their claim.
Michael Tremblay says:
July 23, 2012 at 1:04 am
I followed the link to Dr. Spencer’s site (http://www.drroyspencer.com/2010/07/yes-virginia-cooler-objects-can-make-warmer-objects-even-warmer-still/) and I understand the concept that a ‘cooler’ object can ‘make warmer objects even warm still’. I have a problem with his experiment when it relates to the Earth’s atmosphere. His ‘hot’ plate is constantly radiating at 150F because…
====================================================
No, there was no hot plate and there was no experiment. It was a fictitious experiment with fictitious plates, fictitious temperatures and fictitious conclusions. All fiction, nothing real. The problem is many readers think it was real, because otherwise it would have been outrageous to present that as science, so people subconsciously rule this option out and just BELIEVE.
Joeldshore writes “In other words, the effect of lower temperature is about 300X as important as the effect of increasing surface area.”
I think this is incorrect Joel. Surely it has nothing to do with surface area and instead has to do with CO2 concentration at that altitude. If the CO2 levels increase then, at the average radiating altitude there will be more CO2 and hence more energy is radiated.
If you say that the altitude has to increase because more CO2 above “captures” the radiation then that is a statement and not a fact. If you only use models and theory to back up that statement then you’re not making a satisfactory scientific argument.
paulinuk says: July 23, 2012 at 12:17 am
“But the National Radio Astronomy Observatory guys say that all matter emits thermal radiation …
True enough, but this is where you need deeper knowledge. They also say only a theoretical “black body” emits as well as possible; all true materials emit less well, and they emit differently at different wavelengths. It turns out (for reasons that are theoretically well understood) that CO2 (and all molecules with at least 3 atoms) will emit certainly wavelengths of IR quite well, but emit other wavelengths EXTREMELY poorly. It also turns out that symmetric diatomic molecules emit EXTREMELY poorly at ALL IR wavelengths.
Yes, if you look closely enough you can find IR from room temperature N2 and O2, but it is orders of magnitude less intense than the IR from CO2, O3, CH4, etc.
Greg House says: “so people subconsciously rule this option out and just BELIEVE.”
Yes. In the same way that I rule out the option that the next time I drop a rock it will fall UPWARD. I “just BELIEVE” it will fall downward. If I do a “fictitious experiment” and say “suppose I let go of a rock in mid air ..” we don’t need to say “Which rock? Have you tested THAT rock before to make sure it will fall? Have to dropped it lots of times to see if it falls every time?”
Science is about finding consistent rules that accurately predict what will happen, based on previous experiments. So I don’t HAVE to do every possible variation of an experiment to be quite sure of the outcome.
Thousands (millions!) of experiments have measured the nature of electromagnetic radiation.
Thousands (millions!) of experiments have measured the IR properties of gases.
Thousands (millions!) of experiments have confirmed the laws of thermodynamics.
It would be perverse to expect a rock to disobey well-established rules of physics and suddenly fall upward.
It would be perverse to expect the hot-plate, etc. to disobey well-established rules of physics and suddenly quit conducting/convecting/radiating in the expected manner.
Sure, it would be nice to have a specific experiment measuring a specific hot plate, but thousands (millions) of practical devices operate every day within the rules of physics, and engineers don’t have to wonder about every device they design, worrying that the laws of physics might not apply.
Tim the Toolman says: “If you only use models and theory to back up that statement then you’re not making a satisfactory scientific argument.”
But don’t you see, you ALSO were only using “models and theories” when you assumed that “If the CO2 levels increase then, at the average radiating altitude there will be more CO2 and hence more energy is radiated.” I think that Joel is right that the average radiating level will rise — there are lots of analogies that would lead to this conclusion (not to mention hard math and physics). Your model seems to be that “average radiating height” is a fundamental feature of the earth, independent of where the molecules emitting the IR radiation are found.
And in my book, “using theories” is a VERY scientific approach to trying to discern what will happen. (Of course, that assume you have a solid grasp on the theories so that you can reason out what they are telling you.)
****
davidmhoffer says:
July 22, 2012 at 8:41 pm
****
Funny that joelshore and the rabbit aren’t addressing your important point. Apparently they don’t want to go there….
Reed Coray says:
July 22, 2012 at 7:45 pm
I asked the question “Under what conditions does the presence of greenhouse gases in the Earth’s atmosphere quit slowing-down outgoing radiation?” As far as I can tell I never got an answer to that question. So to those who believe/claim (a) and (b) above, I repeat the question. If the answer is: “As long as there are greenhouse gases in the atmosphere, they never stop ‘slowing down’ outgoing radiation”, then I’d appreciate an answer to the obvious follow-up question: “If (a) the presence of greenhouse gases in the Earth’s atmosphere doesn’t change the rate radiation (energy) is absorbed by the Earth/Earth-atmosphere system, (b) in the absence of greenhouse gases the energy-rate equilibrium temperature is T, and (c) greenhouse gases always ‘slow down’ outgoing radiation which causes the temperature to rise, then why doesn’t the temperature increase without bound (or at least until the greenhouse gases go away or the temperature changes so that the temperature is high enough that radiation from the Earth/Earth-atmosphere is negligible in the greenhouse gas absorption bands)?”
Because the outgoing radiation increases as the fourth power of temperature whereas the effect of CO2 is logarithmic. Consequently the stationary point where incoming energy equals outgoing energy goes up but with bounds.
rgbatduke says:
July 21, 2012 at 11:31 am
Thus, if you increase the levels of GHGs, the Earth will now be emitting energy at a slower rate than it is absorbing energy. This causes the Earth to warm…In fact, it warms until the radiative balance between emission and absorption is re-established.
The problem is that it doesn’t “cause the Earth to warm”. The sun causes the Earth to warm, almost exclusively. The GHE causes the Earth to lose heat from the Sun more slowly and hence be warmer than it would have been without it.
Sorry to be picky, but half of the debate is people who do not understand that what you mean — and what a world of climate scientists mean — is not the literal meaning of the words you say. GHGs do not warm anything.
Robert I agree with most of what you say but since you are being picky I’ll reciprocate. GHGs do warm something, they warm the atmosphere.
Michael Tremblay says: July 23, 2012 at 1:04 am….
Michael, I appreciate that your background as a steam engineer gives you some great insights, but I think you underestimate climate scientists’ understanding of basic science.
“Rather than call it the GHE though, I would rather call it the Atmospheric Effect “
This is simply semantics, not science. We all need to get past the names and think about the concepts. (And I have a problem with “Atmospheric effect” since the atmosphere has MANY effects. The “Greenhouse effect” deals with the IR properties of the atmosphere. If anything, you would want to use terms like “Atmospheric IR effect” and “Atmospheric albedo effect” and “Atmospheric evapooration/condensation effect” ….
“First, it is important to remember that most of the theoretical discussions I have encountered only consider that the atmosphere is mixture of gases.”
That is because that is a simple starting point. Sort of like in most discussions people will say “the boiling point of water is 100C”. But any expert in the field knows that impurities and pressure affect boiling point; any expert will know that there are indeed suspended liquids and solids in the atmosphere.
For example, one of the standard models for radiation in the atmosphere is “MODTRAN” — “MODTRAN® is a “narrow band model” atmospheric radiative transfer code. The atmosphere is modeled as stratified (horizontally homogeneous), and its constituent profiles, both molecular and particulate, may be defined either using built-in models or by user-specified vertical profiles. The spectral range extends from the UV into the far-infrared (0 – 50,000 cm-1), providing resolution as fine as 0.2 cm-1.
“Another problem that I have with climatologists’ interpretation of the AE is that they forget that the atmosphere also reduces the RATE at which the Sun heats the Earth. ”
Again, this is not true. Even the simple “Trenberth cartoon” shows 78 W/m^2 of sunlight getting absorbed by the atmosphere before reaching the earth. And they include reflection by the atmosphere. If the simplest models include the atmosphere reducing incoming sunlight, I am sure more advanced models will too.
“If a CO2 molecule (or any molecule for that matter) is absorbing a photon of a specific frequency and re-emitting a molecule of the same frequency, where is the energy transfer?”
That is subtle, but let me try an explanation.
* A CO2 molecule can gain vibrational energy by absorbing a photon
* A CO2 molecule can gain vibrational energy by colliding with nearby molecules
* A CO2 molecule can lose vibrational energy by emitting a photon
* A CO2 molecule can lose vibrational energy by colliding with nearby molecules
It is important to note that a CO2 molecule could gain energy from a photon and lose that energy via a collision, or vice versa.
If the CO2, the nearby molecules and the source of incoming IR photons are at the same temperature, then these processes will all be in some sort of equilibrium. The CO2 molecule is as likely to gain energy from a photon as it is to lose energy from a photon. It is as likely to gain energy from a collision as it is to lose energy from a collision.
Now suppose you warm up external the source of IR photons. Now there are more incoming photons (at every specific wavelength). Now the CO2 molecule is more likely to gain vibrational energy than lose energy via IR photons. Consequently, these energetic vibrations mean the CO2 is also more likely to lose energy via collisions than to gain energy (since they have more average energy than the surrounding molecules.)
Or think of it this way. Suppose a parcel of N2 is at 250 K and the ground is at 300 K. The CO2 in that parcel is “thermally connected” to the ground via IR photons. The CO2 is also thermally connected to the N2 by collisions. The photons try to warm the CO2 to 300 K (or “try to warm the vibrational mode of CO2 to 300K” if you want to be picky); the N2 tries to cool the CO2 to 250 C. The temperature of the (vibrational modes) CO2 will be somewhere in between. Thus the “warmer” CO2 will warm the cooler N2.
I believe one of the best illustrations of the effect of carbon dioxide on the atmosphere is a graph presented in the Wikipedia article on ‘Radiative Forcing.’ This is a line by line calculation by the MODTRAN webtool hosted by the University of Chicago. It shows the calculated difference between energy escaping the troposphere when the CO2 concentration is 300 PPM, the green curve, and what it would be for 600 PPM, the blue curve. For 95 to 97 percent of the graph, the blue curve runs right over the the green curve indicating almost no difference in heat energy escaping the Earth for a full doubling of the CO2 content. The MODTRAN program was originally developed by the Air Force to calibrate their equipment. It shows the ‘raw’ effect of CO2 on thermal energy escaping the troposphere, given exactly the same atmospheric conditions and surface temperature.
Ref: http://upload.wikimedia.org/wikipedia/commons/9/9c/ModtranRadiativeForcingDoubleCO2.png
I think this plot tells it all. End of story.
Bucky Cochrane says:
July 21, 2012 at 4:12 pm
The whole idea of GHG “absorbing heat” is erroneous. CO2 absorbs a photon, goes into the “bending” mode of molecular vibration and almost immediately radiates the photon which it absorbed. It cannot give up any fraction of this energy; there is no state between this 667 wavenumber excited state and its vibrational ground state.
Absolutely wrong, there are many rotational states between the excited vibrational state and the ground state. During the lifetime of the excited state it endures on average thousands of collisions with neighboring molecules, each of these are capable of reducing the molecule’s energy by an amount equal to the energy difference between energy states. These collisions effectively ‘chip away’ the excitation energy before the molecule can radiate it away and at the same time slightly increase the energy of the collision partners. It’s also possible for the excited molecule to radiate as the result of a rotational transition, i.e. in the microwave range.
It cannot “warm the air” The effect of this re-radiation of 15 micron IR is to take upward directed radiation from the surface of the earth and aim one half of it back to the earth;s surface. Same for any GHG. The downward re-radiated IR then becomes part of the surface radiation budget.
Clearly it does, near the surface the re-radiation only occurs for a small fraction of the excited molecules, at a higher altitude the balance changes.
“You need to prove you key assertion that radiation from a colder body can warm a warmerbody. Radiation is very nice, but you guys apparently can not present any link to a real scientific experiment proving this alleged warming effect, not just radiation.”
Greg thinks that radiation is very nice. He likes radiation from a warmer object more than he likes radiation from a cooler object. He thinks that radiation from a warmer object can warm things, but radiation from a cooler object can’t. Well, maybe he ain’t quite that dumb. Maybe he thinks that radiation from a cooler object has no effect on a warmer object, because the radiation from a cooler object knows it ain’t supposed to warm a warmer object.
Radiation is radiation, Greg. There isn’t one kind that warms things and another kind that don’t. Radiation warms the things that it meets up with, period. It is called radiative heat transfer. People use this stuff to make the world go round, Greg. Try to catch up:
http://www.engineeringtoolbox.com/radiation-heat-transfer-d_431.html
You see that formula for net radiation loss rate, Greg? You better contact the engineers and inform them that they don’t have a clue.
That is all the time I have to waste on you, Greg.
For what it’s worth. The figure entitled “Radiation Transmittd by the Atmosphere” that appears at the start of this post was not not included in the material I sent to Anthony. I’m not sure why Anthony included it; but it’s his blog. As he was kind enough to post my thoughts, I make no complaint. My only intent is to clarify.
Gail Combs says:
July 22, 2012 at 12:48 pm
Konrad says:
July 21, 2012 at 6:11 am
JeffC says: July 21, 2012 at 5:15 am
“GHG does not block radiation, it absorbs and then re-transmits … a better term than block would be slows …”
I believe JeffC to be correct. CO2 almost instantly re-radiates the outgoing IR radiation it intercepts, with around 50% of this radiated back towards the Earth’s surface….
_______________________________
That is one of the fallacies. 50% is not radiated back towards earth. Think in 3 dimensions not in 2 dimensions because the CO2 molecule and the earth are three dimensional. Also energy can be transferred via collision to other molecules so the energy headed in the direction of the earth is less than 50%.
Firstly, JeffC is wrong, CO2 doesn’t almost immediately re-radiate the outgoing IR radiation, in terms of the collision frequency (~10^9/sec) it takes an age to re-radiate which means most of the CO2 molecules near the surface don’t re-radiate.
Secondly, thinking in 3-D for an atmospheric layer 10km thick around a planet with a diameter of 8000km means that ‘around 50%’ is radiated back towards earth.
Tim Folkerts says:
July 23, 2012 at 7:03 am
Yes. In the same way that I rule out the option that the next time I drop a rock it will fall UPWARD. I “just BELIEVE” it will fall downward. If I do a “fictitious experiment” and say “suppose I let go of a rock in mid air ..” we don’t need to say “Which rock? Have you tested THAT rock before to make sure it will fall? Have to dropped it lots of times to see if it falls every time?”
========================================================
I am afraid you thoroughly misunderstood my point about “ruling out the option”. I meant the option that the Spenser’s “experiment” was NOT a description of a real experiment, but was just a fictitious construction with no basis in real experimental physics. Note, he did not even refer to ANY real falsifiable scientific experiment of the kind, this is an indication that NONE ever existed.
Tim Folkerts says:
July 23, 2012 at 7:03 am
Thousands (millions!) of experiments have measured the nature of electromagnetic radiation.
Thousands (millions!) of experiments have measured the IR properties of gases.
Thousands (millions!) of experiments have confirmed the laws of thermodynamics.
…It would be perverse to expect the hot-plate, etc. to disobey well-established rules of physics and suddenly quit conducting/convecting/radiating in the expected manner.
=======================================================
Again: a colder body does radiate. But there is no law of thermodynamics nor a “well-established rule of physics” saying that a colder body can warm (or reduce cooling of) a WARMER body, in our case by means of radiation.
But it might be possible to make this discovery, why not, just do it and present the scientific falsifiable experimental proof. Spenser and others failed to do that. And please, do not beat around the bush, the point is clear.
Hoser says:
July 21, 2012 at 12:43 pm
I can’t read all the comments to see if these points were already made:
1) The conc of H20 and CO2 vary in the atmosphere; they are not homogeneously distributed.
2) The charts showing absorption clearly indicate the vast majority of the absorption is due to water vapor and not CO2.
3) In the peak of IR emission from the Earth’s surface, Water already absorbs about 2/3 of the energy where CO2 absorbes. The main CO2 band overlaps the shoulder of the main water band.
Point 1 is mostly true, much less so for CO2 than H2O.
Point 2 is not true, it’s certainly not a ‘vast’ majority.
Point 3 is false. See the following for example.
http://i302.photobucket.com/albums/nn107/Sprintstar400/Atmos.gif
Tim Folkerts says:
July 23, 2012 at 7:03 am
“Thousands (millions!) of experiments have measured the nature of electromagnetic radiation.
Thousands (millions!) of experiments have measured the IR properties of gases.
Thousands (millions!) of experiments have confirmed the laws of thermodynamics.
…It would be perverse to expect the hot-plate, etc. to disobey well-established rules of physics and suddenly quit conducting/convecting/radiating in the expected manner.”
Right, Tim. But we have a group of stubbornly ignorant clowns, who think that our understanding of radiative physics has been imposed on us by manipulative climate scientists. It is obvious that climate scientists are not that clever. They couldn’t fool the engineers. And the engineers have been using this stuff for a long time.
http://www.engineeringtoolbox.com/radiation-heat-transfer-d_431.html
Greg House says:
July 21, 2012 at 2:30 pm
Konrad says:
July 21, 2012 at 6:11 am
Outgoing IR radiation radiated back to the Earth’s surface could slow the radiative cooling of surface materials.
====================================================
Yes, this is a notion the warmists like very much, but the problem is they can not prove it. I very patently asked them to present a link to a real scientific experiment proving that notion, but they failed
Not only are there experiments but there are products based on this, I’ve given real world examples of it here before. Here’s two examples of the application that are widely used.
Radiation shields for thermocouples, particularly in flame temperature measurements. I first encountered this in 1970 in graduate-level classes, a key reference being a NACA report from the 50’s (forerunner of NASA). We not only learned about it but conducted experiments on the effect and used their heat transfer correlations in the analysis. Such devices are routinely used by engineers.
Light bulbs with an IR reflective coating which causes the filament to heat up and emit more visible.
http://hirheadlights.com/USPTO%20HIR%20bulbs.pdf
http://www.pegasuslighting.com/par38-halogen-ir-light-bulbs-48w-flood-25-degree.html
Come on you guys. Stay sober and stay skeptic. Just look at the results. In my tables.
http://www.letterdash.com/henryp/global-cooling-is-here
No need for any “deeper knowledge” and endless discussions about whether GHG’s cause warming or a delay in cooling. Simple observations at your own weather stations show:
From ca. 1945 the input of energy has been increasing. As has ozone been decreasing.
From ca. 1995 the input of energy has been decreasing. As has ozone been increasing.
There is no AGW. There never was. And the scare of ozone disappearing in the nineties due to anthropogenic activities also was never true. Everything follows on natural parabolic -like curves.
If it gets too cold, pack up, and go, live at a lower latitude. That’s life. It has been.
http://wattsupwiththat.com/2012/07/13/coldest-july-in-history-for-anchorage/#comment-1039049
Phil. says: July 23, 2012 at 7:53 am
Because the outgoing radiation increases as the fourth power of temperature whereas the effect of CO2 is logarithmic. Consequently the stationary point where incoming energy equals outgoing energy goes up but with bounds.
Phil, thank you for your response.
However, I’m not sure I follow your answer. You say the outgoing radiation increases as the fourth power of the temperature whereas the effect of CO2 is logarithmic. It’s not clear what you mean by effect. I’ll assume you mean: “Temperature is proportional to the logarithm of the amount of CO2 in the atmosphere.” If my assumption is incorrect, please let me know; and clarify your meaning. If my assumption is correct, then in the first part of your statement you relate the behavior of the outgoing radiation to the fourth power of the temperature. In the second part of your statement you relate temperature to CO2 levels. Mathematically:
Outgoing radiation = “constant one” times T^4
T = “constant two” times logarithm(CO2 levels)
Doesn’t this imply
Outgoing radiation = “constant one” times [“constant two” times logarithm(CO2 levels)]^4
which to me implies that outgoing radiation should increase (although slowly because of the logarithmic function) with increasing CO2 levels.
In any event, I believe your statement implies that at some combination of CO2 level and temperature, the outgoing radiation (rate of energy loss) equals the incoming radiation (rate of energy absorption). I agree. Since part of my question included the caveat that the rate of incoming radiation is unaffected by the CO2 level, when energy-rate neutrality is reached, CO2 doesn’t “slow down” outgoing radiation. I.e., at best the CO2 “slow down” was transient.
Tim Folkerts “Yes, if you look closely enough you can find IR from room temperature N2 and O2, but it is orders of magnitude less intense than the IR from CO2, O3, CH4, etc”.
Thanks Tim, but you havn’t given me experimental evidence to back that up.The experiment I was considering was impracticable but this is more manageable: create a vacuum in a metal chamber cooled down to near absolute zero (like deep space); introduce a jet of diatomic N2 at 15c into the chamber and measure the watts of IR coming off the the jet . Do the same for triatomic Co2. You say CO2emission of IR is orders of magnitude higher, could be, I’m not sure about that.
Greg says: “But there is no law of thermodynamics nor a “well-established rule of physics” saying that a colder body can warm (or reduce cooling of) a WARMER body, in our case by means of radiation. ”
Ah … “proof by bold assertion”. But I can just as well assert that there IS such a rule. And since the three PhD physicists that I know who have been participating in this discussion all think the cold object CAN slow the cooling of a warmer body (and have given varying degrees of explanation), I go with the bold assertions of the PhDs.
The “rule” is actually quite simple. The warm object emits radiation as a function of its own temperature (independent of anything else going on around it: P_out = (epsilon)(sigma)(Area)(T^4) ). As you admit, the cold object does radiate at least a bit. And at least some of that radiant energy gets absorbed by the other (warmer) object: Call it P_in_from_cold. In the absence of the cold object, the warm object would be receiving from that direction a smaller amount of energy: call it P_In_from_3K_Space.
The net change with the cold object nearby is
[P_in_from_cold – P_out ] (a negative number = cooling]
The net change without the cold object nearby is
[P_in_from_space – P_out ] (a bigger negative number = morecooling]
The cool object keeps the warm object from cooling as rapidly as it would without the cool object nearby. (or with a heater like the sun present, the equilibrium temperature is higher with the cooler object nearby than it would be with the even colder outer space nearby.)
~~~~~~~~~~~~~~~~~~~~~~~~~~~~~~~~~~~~~~~~~~~~~~~~~~~
Maybe that is the best way to think of it. The earth is being warmed by the sun. It could be cooled by either
* surrounding it by ~250 K atmosphere
* surrounding it by 3 K outer space.
Anything warmer than 3 K (the background radiation of space) will keep the earth warmer than it would be with that 3 K background instead. (Like your skin staying warmer when surrounded by 20 C air rather than -20 C air).
Phil. says:
July 23, 2012 at 9:21 am
Not only are there experiments…
==============================================
Yeah, just present a link to a real genuine falsifiable scientific one. It is not enough to say “they are there”.
Phil. says:
July 23, 2012 at 9:21 am
…but there are products based on this, …Light bulbs with an IR reflective coating which causes the filament to heat up and emit more visible.
http://hirheadlights.com/USPTO%20HIR%20bulbs.pdf
http://www.pegasuslighting.com/par38-halogen-ir-light-bulbs-48w-flood-25-degree.html
=================================================
Look, just multiplying the claim is no proof of the claim being correct.
The manufacturer CLAIMS his bulb is more efficient, the same goes for the patent. Now, WHERE IS THE SCIENTIFIC PROOF FOR THAT? Nowhere, apparently.
We are talking about science here, not about marketing.
Tim>> you can find IR from room temperature N2 and O2, but it is orders
Tim>> of magnitude less intense than the IR from CO2, O3, CH4, etc”.
Paul> Thanks Tim, but you havn’t given me experimental evidence to back that up.
Sorry — I considered this “common knowledge” for people interested in IR. I didn’t feel i needed to give evidence.
The simplest evidence is the image at the very top of this post, showing absorption of various wavelengths by various gases. They didn’t forget about N2 — it simply is not important at visible or IR wavelengths.
You can also study the quantum mechanics of molecular energy levels. They are quite good at predicting the spectra of molecules. And the theory says molecules that can bend (3+ atoms) or that have a dipole moment (eg HF) will have strong IR lines. Molecules with no bending and no dipole moment (eg N2) will have no IR lines. (In reality there can be weak induced dipole moments in N2, so tit can have weak IR lines).
House, it’s time to put it to rest.
It appears obvious to me that you may be a troll trying disrupt this thread.
Greg House has still not responded to my challenge to produce links to verifiable experiments proving that the earth is round and the earth circles the sun.
Tim Folkerts says:
July 23, 2012 at 9:57 am
Greg says: “But there is no law of thermodynamics nor a “well-established rule of physics” saying that a colder body can warm (or reduce cooling of) a WARMER body, in our case by means of radiation. ”
Ah … “proof by bold assertion”. But I can just as well assert that there IS such a rule. And since the three PhD physicists that I know who have been participating in this discussion all think the cold object CAN slow the cooling of a warmer body (and have given varying degrees of explanation), I go with the bold assertions of the PhDs.
==================================================
I do not need to prove anything. You guys failed to present an experimental proof for your assertion. Even on the theoretical level you are just generally talking about lows and rules without proving that they support your assertion.
RE: Greg House:(July 23, 2012 at 8:45 am)
Again: a colder body does radiate.
That is not quite true . . . The colder body just does not radiate as much. The standard IPCC thermal budget diagram for the Earth shows 390 W/m² leaving the surface with 324 W/m² returning from the cooler atmosphere above so that the surface sees a net loss of 66 W/m², by radiation to the upper atmosphere. The atmosphere is actually radiating 265 W/m² to outer space. The extra 200 W/m² or so is partially delivered from the surface by convection and transpiration and, based on this diagram, about 67 W/m² is from direct solar heating.
Of course, the carbon dioxide band is dead, but again, that is like a one foot diameter tree in the middle of a ten-foot wide stream. Water vapor appears to be a leaky greenhouse gas, probably due to condensing accelerated convective activity.
REF: http://www.windows2universe.org/earth/Atmosphere/images/earth_rad_budget_kiehl_trenberth_1997_big.gif
Also, a colder body can reflect heat from a warmer body without being warmed by the reflected radiation.
Tim Folkerts says:
July 23, 2012 at 9:57 am
the cold object does radiate at least a bit. And at least some of that radiant energy gets absorbed by the other (warmer) object: Call it P_in_from_cold. …The cool object keeps the warm object from cooling as rapidly as it would without the cool object nearby…
=======================================================
You do understand, that a repetition or rephrasing of the same unproven claim is not a scientific proof that this claim is scientifically correct, don’t you?
OK. So O2 and N2 do not absorb IR energy- they are considered transparent to visible and IR in “conventional” NASA-GISS-CAGW theory. And, apparently, in the calculations used in descriptions of their CAGW models.
A question not addressed yet.
Assume 1370 watts are present at the top of atmosphere at the equator.
Approximately 360 watts are absorbed by the atmosphere before reaching the ground. What absorbed that 360 watts that are NOT transmitted, and where does that energy “go” if O2 and N2 do not radiate or absorb IR energy?
Greg House;
You do understand, that a repetition or rephrasing of the same unproven claim is not a scientific proof that this claim is scientifically correct, don’t you?
>>>>>>>>>>>>>>>
Since your entire diatribe in this and other threads consists of responding to all explanations to you with “that’s not proof, I asked for proof” your statement is rather amusing. And you still haven’t responded to my challenge to produce verfiable proof of the same sort you have asked for that the earth is round and circles the sun. If you can’t, I presume you to be of the opinion that the earth is flat and that the sun circles the earth.
I have, by the way, posted multiple times, a link to an experiment that shows exactly what you are asking for. You don’t comment on it because you don’t have the basic understanding of physics required to even begin discussing if it proves my point or not. In this case the experiment I linked to is in fact valid. But your understanding of physics is so primitive that you don’t actually know, so you don’t comment. I could have chosen an experiment that was totaly bogus in this regard, and you would have ketp your yap shut on that one because you wouldn’t understand it either, and so you’d simply ignore it just like you do the dozens of actual proofs that you’ve been presented with. Your objections are rooted in blinding ignorance which you display a remarkable determination to maintain.
Set/GregHouse=Ignore
Spector says:
July 23, 2012 at 10:28 am
The colder body just does not radiate as much. The standard IPCC thermal budget diagram
=================================================
There is no need to care about the “IPCC thermal budget diagram” because the warmism is stuck with its key assertion (colder body reduces cooling of warmer body by radiation) apparently not being proven experimentally.
It is just a narrative, rephrasing, repeating and so on.
RE; RACookPE1978:(July 23, 2012 at 10:35 am)
“Assume 1370 watts are present at the top of atmosphere at the equator.”
I believe that is the value at noon. Most of these calculations of this type are averaged over a full daily cycle. When albedo is also taken into account, (depending on what assumptions are made) I think the result can be close to 293 W/m² for the tropics.
Spector says:
That is not quite true . . . The colder body just does not radiate as much.
>>>>>>>>>>
I think this is the fundamental point that confuses a lot of people. In the absence of the colder body, what is there? People confine their thinking to the exchange of energy between the warm surface and the cold surface. We need to take one additional step and ask a simple question. If the cold surface was not there, what would be?
Answer: NOTHING!
Question: What is the temperature of “nothing”?
Answer: 2.7 degrees K, roughly -270 degrees C.
So, if we have a warm object at say 100 C exposed directly to space, it cools rather rapidly. If we insert a “cold” object at say -20 C in between the warm object and space, the warm object will obviously cool more slowly. The cooler object is only cooler relative to the warm object. But compared to NOT being there, the cool object is blazing hot. The cool object at -20 C is still 250 degrees C warmer than “nothing”.
Spector says:
July 23, 2012 at 10:58 am
RE; RACookPE1978:(July 23, 2012 at 10:35 am)
“Assume 1370 watts are present at the top of atmosphere at the equator.”
I believe that is the value at noon. Most of these calculations of this type are averaged over a full daily cycle. When albedo is also taken into account, (depending on what assumptions are made) I think the result can be close to 293 W/m² for the tropics.
>>>>>>>>>>>>>>>
No, it is the value presented by the sun to the earth if earth was a flat plane directly facing the sun. Adjust for albedo, subract 30%. Adjust for curvature of earth and rotation of earth divide by four. That gives you a rough average which is useless except for the most basic of discussions. I suggest
http://eos.atmos.washington.edu/erbe/
For actual data on relative energy flux at different latitudes going both up and down. Note that the result in the tropics is WELL above 293 w per m2.
RACookPE1978 Asks:
“What absorbed that 360 watts that are NOT transmitted, and where does that energy “go” if O2 and N2 do not radiate or absorb IR energy?
Look at the figure at the very top of this post: “Radiation Transmitted by the Atmosphere”.
* Ozone absorbs most of the UV.
* gaseous water absorbs a bunch of solar IR
* Rayleigh scattering sends some of it back to space
* Its not on the diagram, but aerosols absorb some, too.
All together, that would add up to about 360 W/m^2. Most of it would heat the atmosphere directly; some is lost back to space
<davidmhoffer says:
July 23, 2012 at 11:03 am
So, if we have a warm object at say 100 C exposed directly to space, it cools rather rapidly. If we insert a “cold” object at say -20 C in between the warm object and space, the warm object will obviously cool more slowly. The cooler object is only cooler relative to the warm object. But compared to NOT being there, the cool object is blazing hot. The cool object at -20 C is still 250 degrees C warmer than “nothing”.
davidmhoffer, I know you’re frustrated with the amount of time you’re having to spend on this issue, but I think it’s time well spent.
You’re getting better and better at explaining your point and probably getting more converts than you think.
Greg House – “The warmists problem is that they can not produce any real genuine falsifiable scientific experimental proof of that “reducing its rate of heat loss”. They just keep “explaining”, rephrasing and mostly suggesting I should prove their claim.”
Greg, I would suggest you look at the spectra linked/shown at the top of the page (http://upload.wikimedia.org/wikipedia/commons/thumb/7/7c/Atmospheric_Transmission.png/300px-Atmospheric_Transmission.png). The smooth curve on the right represents the spectra of a ‘blackbody’, the most efficient thermal radiator possible. The jagged blue spectra shows the actual emissions from our atmosphere to space, shaped by the various GHG’s in our atmosphere.
That emission spectra is lower than the blackbody spectra, meaning that the Earth must be at a higher temperature to radiate the same energy (the area under the spectral curve). As observed from space, the IR emissivity of the Earth is about 0.612, 61% as efficient as a blackbody. Feed that into the Stephan-Boltzmann equation (one of the best established relationships in physics):
Power = emissivity * SB Constant (5.670 373(21)×10^−8 W·m^−2·K^−4) * T^4
240 = 0.612 * SBc * T^4
T = 288.37 K = 15.2 C
And the shape of that spectra changes as we add more GHG’s, in a fashion clearly measurable from space, (Harries et al 2001, https://workspace.imperial.ac.uk/physics/Public/spat/John/Increase%20in%20greenhouse%20forcing%20inferred%20from%20the%20outgoing%20longwave%20radiation%20spectra%20of%20the%20Earth%20in%201970%20and%201997.pdf). We’ve observed the atmosphere emit IR less and less efficiently.
That’s the greenhouse effect in the simplest terms – increasing GHG’s decrease the amount of energy radiated at any particular temperature, creating an imbalance between incoming/outgoing energy, and hence the world warms in compensation – until that imbalance is cancelled out.
—
You have repeatedly demanded that folks show you the experiments to prove their case. They have. Thermal radiation, cooler objects (yet warmer than the background) adding to the temperature of warmer objects, and the 33 C higher temperature of the Earth than would be seen without GHG’s – those have all been proven. And the various experiments you have been pointed to (which all have been done in high schools across the world) are simply opportunities for you to observe the same thing yourself. Your repeated demands are, therefore, rather disingenuous.
Greg House:”Again: a colder body does radiate. But there is no law of thermodynamics nor a “well-established rule of physics” saying that a colder body can warm (or reduce cooling of) a WARMER body, in our case by means of radiation.”
Ok, so let’s see if we can unpack this a bit. If a colder body does radiate, and we can hopefully agree that radiation *can* warm(or reduce the cooling of) something what is it about the *radiation* that the colder body emits that stops it from doing so. Are you arguing that there are two types of radiation: radiation from cooler bodies and radiation from warmer ones or what?
It seems to me that one of the obvious consequences of your line of thought is that a warm object in a vacuum would cool *less* slowly than an object in the atmosphere. The warm object will radiate at the same rate in the atmosphere as in a vacuum but in an atmosphere it can also cool due to convection and conduction. OTOH, if we allow that cool objects can slow the cooling of warming ones, we can easily see how objects in an atmosphere will stay warmer than the same object in a vacuum, even though an object in a vacuum cannot cool through conduction and convection(and one in the atmosphere can).
Cheers, 🙂
yes davidmhoffer:
“So, if we have a warm object at say 100 C exposed directly to space, it cools rather rapidly. If we insert a “cold” object at say -20 C in between the warm object and space, the warm object will obviously cool more slowly. The cooler object is only cooler relative to the warm object. But compared to NOT being there, the cool object is blazing hot. The cool object at -20 C is still 250 degrees C warmer than “nothing”.”
The problem that the intransigent dopes have is that they have heard that heat always travels from a warm object to a cooler object. Actually, there is an interchange of heat/radiation. The colder object receives more radiation from the warmer object than it gives. Net effect for the colder object is that it gets warmed up. The warmer object cools less rapidly, because it is receiving some radiation from the colder object. At lest that is the crazy idea that physicists and engineers dreamed up, long ago.
davidmhoffer says:
beng says:
Fine…I will address it. It is based on a simple misconception: The ~1 degC temp change at the surface is obtained by taking the ~1 degC temperature change at the TOA and extrapolating down to the surface under the assumption that the lapse rate remains constant as the atmosphere warms.
Is that assumption a good one?
(1) It is not perfect, but it is not too bad. In fact, the lapse rate is expected to decrease a little bit with increasing temperature because the moist adiabatic lapse rate decreases with increasing temperature.
(2) It doesn’t matter.
Now, (2) might seem a bit strange of a claim, but here is the reason: The models all correct for the fact that the lapse rate decreases with temperature by including a feedback called the “lapse rate feedback”. It is a negative feedback that accounts for this.
So, what David Hoffer is arguing about comes down to just a matter of definition: The climate scientists talk about the zeroth-order temperature change being what you get if you assume a constant lapse rate and then to the extent that the lapse rate is not constant, it gets corrected, lowering the temperature change at the surface. By contrast, David Hoffer wants to take this correction and put it into the zeroth-order temperature change that gets talked about.
Either way you do it, you get the same answer. I think the way climate scientists do it makes more sense to them (and to me) because the climate modelers understand that the correct way to look at things is to consider the TOA radiation budget and then think about the lapse rate to figure out what happens at the surface. David’s way is wedded to thinking about the radiation budget at the surface which is usually not very helpful because for the surface energy budget, convection is very important. Trying to reason things out just on the basis of the radiative part of the equation tends to be very incomplete.
TimTheToolMan says:
The models and theories of radiative transfer in the atmosphere are backed up by a wealth of experiments. Basically, the entire field of remote sensing would cease to exist if it were wrong. If you are going to be consistent in your skepticism, you would also have to disbelieve Spencer and Christy’s entire body of work on the temperature record of the troposphere. Heck, you probably couldn’t even really believe the IR weather satellite photos that you see on TV.
Greg House says:
July 23, 2012 at 10:07 am
Phil. says:
July 23, 2012 at 9:21 am
…but there are products based on this, …Light bulbs with an IR reflective coating which causes the filament to heat up and emit more visible.
http://hirheadlights.com/USPTO%20HIR%20bulbs.pdf
http://www.pegasuslighting.com/par38-halogen-ir-light-bulbs-48w-flood-25-degree.html
=================================================
Look, just multiplying the claim is no proof of the claim being correct.
The manufacturer CLAIMS his bulb is more efficient, the same goes for the patent. Now, WHERE IS THE SCIENTIFIC PROOF FOR THAT? Nowhere, apparently.
We are talking about science here, not about marketing.
No some of us are talking about science you are not!
What did your last slave die of? Don’t be so lazy and get off your ass and google the items I referred you to and learn something about physics and engineering. I’m not going to do all the work for you, there’s enough information there for to find data about the increase of output/watt.
You want a verifiable experiment, place a heated filament at the focus of an uncoated, transparent spherical mirror (i.e. one that doesn’t reflect), measure the output at a certain power setting. Coat the mirror with a dichroic coating which reflects 95% of the IR above 1 micron and passes 95% of the light below 1 micron, now measure the output at the same power setting. You’ll find the temperature of the filament will go up with the corresponding change in light output.
Or if that’s too hard for you, immerse a small, bare Pt/Rh thermocouple in the flame of a Meker burner and measure the output of the Th/C. Then place a quartz tube around the Th/C to act as a radiation shield, measure the output again. You should find a increase in voltage consistent with a temperature increase of ~100ºC.
joeldshore;
So, what David Hoffer is arguing about comes down to just a matter of definition:
>>>>>>>>>
Indeed it does.
The definition in AR3 is that CO2 doubling = 3.7 w/m2 = +1 degree AT THE EFFECTIVE BLACK BODY TEMPERATURE OF EARTH which is -20C and considerably colder than average earth surface temps. This definition is carried over into AR4. If you are arguing that this definition tanslates 1:1 at earth surface, then I challenge you to explain why the definition was worded in the context of effective black body temperature in the first place. I challenge you also to explain how 3.7 w/m2 modeled at TOA can punch through the atmospheric column and result in not just getting through unimpeded, which would be a challenge unto itself, but to arrive at earth surface at 5.5 w/2. Where exactly did it pick up the extra 1.8 w/m2? Why was none of absorbed by water vapour and CO2 on the way down?
You can’t explain this away with lapse rate. The definition relies on the temperature at -20 C which is effective black body temperature of earth for a reason. If the value scaled 1:1 at earth surface there never would have been a reason to define it that way in the first place, and it is the only definition that provides for an energy balance as per SB Law.
So sorry, your lapse rate arm waving doesn’t cut it. The definition of CO2 doubling = +1 degree was made in reference to the effective black body temperature of earth for a reason. If it scaled 1:1 to earth surface then the definition would have been at earth surface, there would have been no reason to complicate it further.
The only reason for using effective black body temperature of earth that I can see as the reference point is because it does result in a higher number to present to the public.
Tim Folkerts says:
July 23, 2012 at 8:13 am
This is simply semantics, not science. ……
>>>>>>>>>>>>>>>>>>
Thanks for replying Tim.
You’re right that it is only semantics but you have to remember who the intended audience is. You and I could talk all day about the Greenhouse Effect and various Atmospheric Effects and understand the differences perfectly well. People in authority often use semantics to deliberately mislead people who do not understand. Use of the word greenhouse to describe this effect lends itself to misinterpretation. Proponents of CAGW love to use the word because most laypeople link greenhouses to warming, even though they may not know how a greenhouse actually works. On the other side, opponents of CAGW should avoid using the word precisely because their intended audience (laypeople) automatically link it to warming. – Just my own little rant 😉
Maybe I am taking too cynical a view of the level of understanding of climatologists. My viewpoint is skewed by reading about the unscientific and unethical misdeeds of a few who are creating a stereotypical view of their profession. This also accounts for the growing public distrust of scientists as evidenced by public opinion polls.
—-
” ‘If a CO2 molecule (or any molecule for that matter) is absorbing a photon of a specific frequency and re-emitting a molecule of the same frequency, where is the energy transfer?’
That is subtle, but let me try an explanation.
* A CO2 molecule can gain vibrational energy by absorbing a photon
* A CO2 molecule can gain vibrational energy by colliding with nearby molecules
* A CO2 molecule can lose vibrational energy by emitting a photon
* A CO2 molecule can lose vibrational energy by colliding with nearby molecules
It is important to note that a CO2 molecule could gain energy from a photon and lose that energy via a collision, or vice versa.”
I see I made a little mistake substituting the word molecule where I intended to say photon. Nevertheless, your explanation ignores my mistake and answers my question. Unfortunately I should have clarified what I am actually trying to understand.
Most of your explanation involves the conductance of energy away from the molecule, not the radiation of energy away from the molecule. If you were to take a single CO2 molecule in isolation and subject it to a stream of photons of the correct frequency for it to absorb, what happens? Does the vibrational energy of the molecule continue to increase, does the radiative energy convert into another form of energy, does the rate of photons emitting increase, or does something else happen?
RACookPE1978 says:
July 23, 2012 at 10:35 am
….A question not addressed yet.
Assume 1370 watts are present at the top of atmosphere at the equator.
Approximately 360 watts are absorbed by the atmosphere before reaching the ground. What absorbed that 360 watts that are NOT transmitted, and where does that energy “go” if O2 and N2 do not radiate or absorb IR energy?
_______________________________
Just looking at the diagram at the top of the page. Incoming solar insolation is absorbed by CO2, H2O and O3. It is also reflected by H2O in the form of clouds.
This also goes back to what Sleepalot and I were pointing out. (See my comment)
I would like to add that in looking again at Sleepalot’s data
He picks May which is midway between the vernal equinox and the summer solstice and therefore the sun would be midway between the equator and the Tropic of Cancer (the latitude line at 23.5° North) so the solar insolation at both locations would be roughly equal with a bit more expected in Barcelos, Brazil.
ALTITUDE:
Barcelos, Brazil elevation ~ 30 meters (100 ft)
Adrar, Algeria ~ Elevation: 280 metres (920 feet)
One would expect a drop in temperature of ~ 4C due to altitude for Adrar, Algeria so the difference between locations, taking into account altitude is ~ 8C higher in Adrar which is further north but with much lower humidity.
Albedo?
Photos Adrar, Algeria and Barcelos, Brazil
A quick search shows some work has been done on clouds, humidity and solar insolation but it is not something I (or my computer) could handle. I found this quite interesting since it is obvious from the chart at the top of this page that water vapor (not clouds) does effect the amount of surface insolation and I would expect to easily find information on it… info based on real life data collection and not models.
There is also the International Satellite Cloud Climatology Project (ISCCP)
And this interesting graph of top of the atmosphere vs surface vs air variation in net shortwave energy over time. link from NASA The link also contains a similar longwave radiation graph.
RE: davidmhoffer: (July 23, 2012 at 11:09 am)
“No, it is the value presented by the sun to the earth if earth was a flat plane directly facing the sun”
Thanks for pointing that out. You are correct, it was not a tropical forcing value. I see the example I cited seems to have been a rough estimate for a cloud-free surface.
From the Kiehl and Trenberth diagram above it looks like the ‘official’ forcing value is 235 W/m² which seems to imply a net albedo of about 31 percent and a 25 percent ratio of the Earth’s solar cross-section area to its spherical surface area.
Reed Coray says:
July 23, 2012 at 9:33 am
Phil. says: July 23, 2012 at 7:53 am
“Because the outgoing radiation increases as the fourth power of temperature whereas the effect of CO2 is logarithmic. Consequently the stationary point where incoming energy equals outgoing energy goes up but with bounds.”
Phil, thank you for your response.
However, I’m not sure I follow your answer. You say the outgoing radiation increases as the fourth power of the temperature whereas the effect of CO2 is logarithmic. It’s not clear what you mean by effect. I’ll assume you mean: “Temperature is proportional to the logarithm of the amount of CO2 in the atmosphere.” If my assumption is incorrect, please let me know; and clarify your meaning.
Outgoing flux=Incoming flux
∴ Surface radiation – absorption by GHGs = Incoming flux (constant)
So k*T^4 – f*ghg([CO2]) = constant
so if ghg([CO2]) goes up then T^4 must go up by the same amount. So if T^4 goes up by 4% then T goes up by ~1%..
davidmhoffer says:
I think you are confused here. Nothing is punching through the atmospheric column to get to the surface. The idea is that an increase in greenhouse gases lowers the Earth’s emission back out into space by ~3.7 W/m^2 and the temperature at the effective radiating level has to rise by ~1 C to compensate. If this were to occur in such a way that the lapse rate remained constant (which it doesn’t), the temperature at the surface would rise by the same amount and that means that the surface itself would emit an additional ~5.5 W/m^2.
Look, you can complain all you want about definitions because the point is that, at the end of the day, what matters is the total temperature rise that occurs at the surface, which includes all of the feedbacks. The theoretical surface temperature rise that occurs in the absence of feedbacks is a theoretical concept which depends on what you define as the zeroth-order change and what you define as the feedbacks. It is useless to argue what its value is in the absence of some arbitrary choice of definitions as to how you are going to define it.
However, what you are definitely not allowed to do is do what you did in your original post on the matter ( http://wattsupwiththat.com/2012/07/21/some-thoughts-on-radiative-transfer-and-ghgs/#comment-1040463 ), which was to argue that they got the zeroth order temperature rise wrong and thus that they got the temperature rise in the presence of feedbacks wrong (i.e., you claimed that they got 2.6 C but should have gotten 1.8 C).
The actual fact is that they did the calculation in a totally consistent way. (In fact, I don’t think they ever calculate a zeroth-order surface temperature rise. They just calculated the actual temperature rise that occurs including all the feedbacks.) You are doing something that is inconsistent (putting in your definition of the zeroth-order effect with their definition of the feedbacks) to arrive at a lower value for the predicted temperature rise that is just plain wrong. It’s as simple as that.
David: To summarize my point simply (and assuming the sensitivity number that you have claimed that AR5 will use is correct), what the climate scientists have essentially done is said, “Our models show on average that doubling CO2 produces a temperature rise of 2.6 C. This can be thought of as a ‘bare’ response of about 1 C multiplied by a feedback factor of about 2.6.”
What you now have come along and said is, “No…I am going to look at things in a way I think is more natural and that gives a bare response of 0.7. Then when I multiply by your feedback factor of 2.6, I get a 1.8 C rise.”
Unfortunately, it doesn’t work that way.
davidmhoffer says:
July 23, 2012 at 11:03 am
So, if we have a warm object at say 100 C exposed directly to space, it cools rather rapidly. If we insert a “cold” object at say -20 C in between the warm object and space, the warm object will obviously cool more slowly.
======================================================
That is the same apparently experimentally unproven narrative.
You really should make an effort and understand that telling people what you think is real and proving that it is real are 2 different things.
It looks very bad for you, guys. You key assertion does not appear to have any basis in real science.
Buy the way, that does not mean automatically that this key assertion is false, it only means that it is baseless. At the same time, given what an enormous amount of money the warmists have at their disposal, the apparent lack of the key proof is a very strong indication that their key assertion and thus their built on this assertion “greenhouse effect” are simply false.
Nevertheless, I am still open to any experimental scientific proof. Who knows, maybe the guys here on this thread are just not competent enough to present it.
[Moderator’s Note: This has gone on long enough. Greg, you have managed to pick a fight with half the people commenting on this blog and have gotten insulting in the process. You are suggesting that Dr. Shore, Dr. Brown and Dr. Felton are incompetent or worse. They have all answered, with great patience, your requests for evidence and proof and you’ve blown it off. You can submit a post explaining your position, citing Dr. Wood if you wish, and it will be published here. For most of us, the “Greenhouse Effect” is not controversial. If you think otherwise, defend it in a post and the comments you wil get or bug off. -REP]
Phil. says: July 23, 2012 at 1:48 pm
Outgoing flux=Incoming flux
∴ Surface radiation – absorption by GHGs = Incoming flux (constant)
So k*T^4 – f*ghg([CO2]) = constant
so if ghg([CO2]) goes up then T^4 must go up by the same amount. So if T^4 goes up by 4% then T goes up by ~1%..
Thanks again for your response.
I agree with your math–i.e., I agree that in an expression x – y = constant, if x goes up then y goes up by the same amount. I want to make sure, however, that we’re not talking at cross purposes regarding what you mean by “flux”. In the context of our discussion, the Stefan-Boltzmann equation, the k*t^4 term in your equation is “energy rate” per unit area. [If you want to include the surface energy in the constant “k”, then the term in your equation corresponds to energy rate. However, in neither case does it correspond to energy itself.] That is, (a) “surface radiation” is the rate (not the amount of) energy leaves a surface (either per unit area or for the total surface, which for his discussion I’ll take to mean “for the total surface”), (b) “absorption by GHGs” is the rate GHGs absorb energy, and “Incoming flux” is the rate energy enters the system comprised of the surface and the GHGs. Furthermore, I assume your equation applies to a state of “energy-rate” equilibrium–i.e., sufficient time as elapsed that the individual terms in your equation do not change with time.
If this interpretation is correct, then according to your equation as greenhouse gas levels increase, the temperature of the surface will increase to keep the equation in balance. So far so good. However, your equation implies that the rate energy is absorbed by greenhouse gases is never zero–in fact is a non-zero constant. So if your equation represents something physical, then isn’t it legitimate to ask: where does the energy that over time keeps being absorbed by (i.e., keeps accumulating) in the GHGs go? For energy rate equilibrium, if energy is absorbed by GHGs at a non-zero constant rate, doesn’t that energy have to leave the greenhouse gases at the same rate? If this is the case, shouldn’t your equation have in addition to a non-zero term for the “rate of energy aborption by GHGs” also have a non-zero term for the “rate of loss of energy by GHGs?”
Spector;
From the Kiehl and Trenberth diagram above it looks like the ‘official’ forcing value is 235 W/m² which seems to imply a net albedo of about 31 percent and a 25 percent ratio of the Earth’s solar cross-section area to its spherical surface area.
“”””””””””””””
To be precise, take the post albedo number and multiply by 0.5 for curvature of the earth and then multiply by 0.5 yet again to accomodate day-night.
[Moderator’s Note: This has gone on long enough. Greg, you have managed to pick a fight with half the people commenting on this blog and have gotten insulting in the process. You are suggesting that Dr. Shore, Dr. Brown and Dr. Felton are incompetent or worse. They have all answered, with great patience, your requests for evidence and proof and you’ve blown it off. …For most of us, the “Greenhouse Effect” is not controversial.
==============================================
OK, yes they did answer my comments but just for the record: none of them did present what I asked for: a link to a real genuine falsifiable scientific experiment proving the key assertion the notion of “greenhouse effect” is based on, namely that a colder body can reduce cooling of a warmer body by means of radiation.
I do not quite understand, how after all that the “greenhouse effect” can still be considered “not controversial”.
REPLY: The moderators have called me in on this. Let me make this simple, the greenhouse effect is a well established property of radiative physics in our atmosphere, one that I have observed firsthand through experimentation. There is no controversy over its existence, for did it not exist, neither would we. There is controversy however, over the magnitude of forcings and induced feedbacks. Take a 48 hour time out and leave the issue alone – Moderating this is tiresome for all involved. – Anthony
I have a very simple multiple choice question for those still commenting on this thread.
I have two 1m spheres of thin LDPE plastic (transparent to LWIR) in a sunless vacuum in deep space. Both spheres contain gas at an initial temperature of 100C. Sphere 1 contains 100% O2. Sphere 2 contains 99% O2 and 1% CO2. Which sphere cools faster? Is the answer :
A. Sphere 1 cools faster.
B. Sphere 2 cools faster.
C. Sphere 1 and 2 cool at the same rate.
Or
D. The spherical chicken found in the same region of space has “Property of Pierrehumbert” tattooed on its butt.
joeldshore;
Look, you can complain all you want about definitions because the point is that, at the end of the day, what matters is the total temperature rise that occurs at the surface, which includes all of the feedbacks.
“””””””””””””””””
And once again Joel, my point remains that IPCC AR3 defined the temperature increase as being at the effective black body temperature of earth and NOT at the surface. AR4 uses the same values at AR3, but doesnèt specify the definition, so we are left to assume that it is the same. There is no reasoning nor evidence provided in AR3 or AR4 to support the notion that the temperature change at effective black body temperature of earth (-20 C) will be equal to the temperature change at the surface (+15 C). I find it doubtful from many perspective to assume that it would be! That would require a consistant 1 degree change from TOA to surface, and that is simply illogical on multiple fronts. Further, you say
Joel Shore;
The idea is that an increase in greenhouse gases lowers the Earth’s emission back out into space by ~3.7 W/m^2 and the temperature at the effective radiating level has to rise by ~1 C to compensate.
“”””””””””””””””
Sorry, but that is not the case, and since you and I have discussed this before, Im going to assume that you have misworded it. Unless doubling CO2 increases the absorption of SW in the first place, at equilibrium, nothing changes.
240 w/m2 comes in
240 w/m2 is radiated out.
CO2 doubles
240 w/m2 comes in
240 w/m2 is radiated out.
What changes is the temperature gradient from TOA to surface. How many watts going “up” and from WHERE. Will the lower reaches of the atmosphere and earth surface be warmer as a result. I believe so. Will it be linear from surface to TOA? I doubt it. Some parts more, some parts less. To suggest that there will be the same temperature rise at surface where the greatest number of w/m2 is required to generate it and due to preponderance of water vapour, CO2’s effects are minimized, as it does at 14,000 feet where (roughly) the effective black body temperature of earth occurs and where CO2 effects are more pronounced due to lack of water vapour just doesn’t make sense.
If there is one thing we know about the system as a whole, it is ugly complex. Assuming that the effects of CO2 at effective black body temperature of -20 and at surface at +15 are both 1 degree is nonsensical, and if it were true, then there would have been no need to define it that way in the first place.
Reed Coray:”If this interpretation is correct, then according to your equation as greenhouse gas levels increase, the temperature of the surface will increase to keep the equation in balance. So far so good. However, your equation implies that the rate energy is absorbed by greenhouse gases is never zero–in fact is a non-zero constant. So if your equation represents something physical, then isn’t it legitimate to ask: where does the energy that over time keeps being absorbed by (i.e., keeps accumulating) in the GHGs go? For energy rate equilibrium, if energy is absorbed by GHGs at a non-zero constant rate, doesn’t that energy have to leave the greenhouse gases at the same rate? If this is the case, shouldn’t your equation have in addition to a non-zero term for the “rate of energy aborption by GHGs” also have a non-zero term for the “rate of loss of energy by GHGs?””
First off, GH gases do not absorb energy in the terms you speak of it above, rather they make it so energy leaves the atmosphere more slowly than OTW. A consequence of this is that at any given time more energy will be in the system (and it will be warmer on average). All energy ultimately leaves the atmosphere *just the same as it always did* it just leaves more slowly on average. This relative slowness combined with the fact that gravity pulls heavy, warm air towards the surface (causing a lapse rate) accounts for the GH effect at the surface. Secondly, you math/logic is flawed above. Just because the difference btw the surface temperature and the GH effect is constant does not imply anything in particular about the GH effect. A change in the strength of GH (whether constant, linear, logarithmic or exponential) will cause a change in the surface temperature but nowhere does this require that change to be constant.
Cheers, 🙂
joeldshore says:
July 23, 2012 at 2:35 pm
“The idea is that an increase in greenhouse gases lowers the Earth’s emission back out into space by ~3.7 W/m^2 and the temperature at the effective radiating level has to rise by ~1 C to compensate. If this were to occur in such a way that the lapse rate remained constant (which it doesn’t), the temperature at the surface would rise by the same amount and that means that the surface itself would emit an additional ~5.5 W/m^2.”
————————————————————————————————————————
Many of you would probably consider what follows nit-picking, but I feel it’s worth pointing out, to avoid even more confusion as to how the radiative GHE is supposed to work. The temperature at the effective radiating level will NOT rise. The level itself will rise. The emission temperature must remain the same. That’s the whole point. If energy OUT is to balance energy IN (which is assumed to be constant) then the energy will have to be radiated off at the same effective temperature.
http://www.climatetheory.net/wp-content/uploads/2012/05/greenhouse-effect-held-soden-2000.png
From Soden & Held, 2000.
What happens with a doubling of atmospheric CO2 is that there’s a radiative imbalance imposed on the system – more IR is held back per unit of time (Earth’s rate of heat loss to space is reduced, amounting to the general 3.7 W/m^2 at TOA). This will force the radiating level to rise (by approx. 150 meters) for it to maintain its S-B radiating temperature. With a fixed lapse rate down to surface level, this would induce a ~1K rise in equilibrium temperature.
kristian;
Yeah, I get that. But the diagram is completely linear. Where is the justification for that. If water vapour was evenly distributed through the air column, then I could see that. But it isnt.
David Hoffer says:
Fair enough. We don’t need to talk about what the no-feedback value of the temperature change is at the surface. My point was simply that your claim that some new estimate of yours of this value lowered the estimate from the models of the sensitivity under doubling (you said from 2.6 to 1.8 C) is wrong. I am perfectly fine with leaving the estimate in the “no-feedback” case ambiguous since it depends on how the notion of “no feedbacks” is defined.
Yes…If you mean once all the adjustment has occurred, i.e. the Earth has come back into radiative balance. However, if you instantaneously double CO2 and then look, there were be only ~236 W/m^2 going out. Over time, that amount will rise as the climate system warms.
Yes…And, there is no assumption that it does change uniformly. However, you still seem to be stuck in a purely radiative view of things. In fact, in much of the troposphere, especially the tropics, the way the temperature rise tends to occur has more to do with maintaining the environmental lapse rate close to the appropriate (moist or dry) adiabatic lapse rate.
By they way, since we last talked about such things, Monckton made me aware of a paper that has helped me understand better why the estimates for the (conventionally-defined no-feedback) warming due to a doubling of CO2 tend to run a bit higher (by maybe 10% or so) than one gets from naively applying the Stefan-Boltzmann Equation using the effective emitting temperature, i.e., about 1.2 C rather than 1.05 C. And, the reason, as I now understand it, is exactly that the atmosphere will warm non-uniformly. If you consider the distribution of radiation from the different parts of the atmosphere that successfully escapes to space and it turns out that the colder parts warm more than the warmer parts, then this can actually result in a bit of a higher estimate of the warming that will occur relative to assuming that all of the emission comes from a level at a temperature of 255 K, which indeed is what the models predict.
Again, it is not worth spending a lot of time arguing about since it is essentially arbitrary, i.e., it depends on what you include as a feedback and what you include as a zeroth-order effect.
What is not arbitrary, however, is the total amount of warming that you get from a doubling, which is why I took issue with your argument that reduced that number by using inconsistent definitions of things.
Joeldshore write “The models and theories of radiative transfer in the atmosphere are backed up by a wealth of experiments. Basically, the entire field of remote sensing would cease to exist if it were wrong.”
Absolutely incorrect Joel. This statement implicitely starts of with the assumption that increasing the concentration of CO2 in the atmosphere has no other effects in the atmosphere that lead to a different result.
Science is fundamentally about observations and not modelling theories.
Kristian says:
Fair enough. What I should probably say is that the temperature at what was formerly the effective radiating level will rise. (Or, to put it another way, the rise in effective radiating level occurs immediately if you imagine an instantaneous increase in GHGs and then one finds oneself in a situation where this layer is now higher and colder and so the Earth is no longer emitting as much radiation as it is absorbing. Then, over time, the entire system warms [but certainly not uniformly] until the new effective radiating level is at 255 K and the radiative balance is re-established.)
By they way, since we last talked about such things, Monckton made me aware of a paper
>>>>>>>>>>>>>>
link?
If you consider the distribution of radiation from the different parts of the atmosphere that successfully escapes to space and it turns out that the colder parts warm more than the warmer parts
>>>>>>>>>>>>>>>>
But do they? We need to look at it from both an altitude and latitude perspective, plus probably a seasonal one. In high altitude winter temps, there is far less water vapour, so the effects of CO2 become more pronounced at lower altitudes, one reason we would expect to see polar amplification. Or should we?
At -40C, earth surface is radiating 167 w/m2. At +40 C it is radiating 548 w/m2. So, we’d expect to see CO2 effects being more pronounced in lower water vapour concentrations, but if there’s 1/3 the w/m2 to absorb and re-radiate in the first place, we get a completely different effect. Run that same thought from an altitude basis with water vapour declining with temperature…. so, do the cold parts warm more than the warm parts? My guess is still yes, but the warming would be a lot more uniform than one might otherwise expect. My point being that even from a strictly radiative physics perspective, one would not expect a linear relationship across altitudes, latitudes, and seasons.
In fact, if you refer to ERBE, you’ll find that the poles have a net loss of energy to space, and the tropics a net gain. The tropics move their excess heat to the poles via convection, water currents, etc. So, how much of the 500 w/m2 that CO2 is in a position to intercept goes into warming the surface and how much results in increased convection moving energy to the poles? Now we measure polar amplication and attribute it to what? CO2 effects directly? Or increased energy being moved from tropics to poles? Or some combination?
To be honest, I don’t have a clue. But that’s only scratching the surface of the complexity involved, and I just don’t see the response being linear. Its going to be less at the warmest places and most in the coldest places, hence the surface response will be less than the “average” response by some amount. My amount is a rough guestimate I will admit. But linear just doesn’t cut it.
That said, I’m still looking for an answer to my original question. If it makes no difference, then why did AR3 specify that they meant as measured at the effective black body temperature of earth? Further, AR4 goes on to specifically say that the temperature change may be muted at surface and may not follow the effective black body temp change (yes, they actually said that, part of why I started thinking about how they defined it in the first place, and sorry don’t have a link for that, but itz in there)
RE: davidmhoffer: (July 23, 2012 at 4:51 pm)
“To be precise, take the post albedo number and multiply by 0.5 for curvature of the earth and then multiply by 0.5 yet again to accomodate day-night.”
The nominal cross-sectional area of the Earth for intercepting solar radiation is approximately pi times the radius squared. There may be some fuzziness about this radius because of the atmosphere and increasing reflectivity at the fringes, and also the slight remaining curvature of solar radiation striking the earth. All that energy being intercepted must be radiated back out from the Earth’s surface having an area of four pi times the square of another fuzzy radius of the Earth. If we ignore the fuzziness, we get an *average* required surface referenced ‘terrestrial’ constant of 25 percent of the albedo reduced solar constant. (multiplied by one minus the albedo factor.) The net result is the same as you say above.
Side Note: Because energy radiated is proportional to the fourth power of the absolute temperature, the average radiated energy equivalent temperatures, often cited, are equivalent to the fourth root of the average of absolute temperatures raised to the fourth power.
Konrad “I have a very simple multiple choice question for those still commenting on this thread.
I have two 1m spheres of thin LDPE plastic (transparent to LWIR) in a sunless vacuum in deep space. Both spheres contain gas at an initial temperature of 100C. Sphere 1 contains 100% O2. Sphere 2 contains 99% O2 and 1% CO2. Which sphere cools faster? Is the answer :
A. Sphere 1 cools faster.
B. Sphere 2 cools faster.
C. Sphere 1 and 2 cool at the same rate.
Or
D. The spherical chicken found in the same region of space has “Property of Pierrehumbert” tattooed on its butt.”
Common sense says “C” but from by “argument from authority” , CO2 cools 100’s or thousands times faster than O2 so “B” . Konrad , rest assured, you can safely put your hand right up next to sphere1 and you won’t get burnt because O2 doesn’t radiate much.
http://www.ipcc.ch/publications_and_data/ar4/wg1/en/ch2s2-8.html
From AR4 WG1 2.8.1
It should be noted that a perturbation to the surface energy budget involves sensible and latent heat fluxes besides solar and longwave irradiance; therefore, it can quantitatively be very different from the RF, which is calculated at the tropopause, and thus is not representative of the energy balance perturbation to the surface-troposphere (climate) system. ”
RF here stands for radiative forcing. Note that it actually calls out that the surface energy budget is likely to be quantitatively different from the RF change. What they DON’T say is if it will be higher or lower. I spent a lot of hours trying to get that answer, and all that is in there is vague statements upon vague statements. There’s a couple of other sections where they tackle this in other ways and it becomes clear that they think the value will be lower at surface, or at least that is my read. One of my big issues with AR4 is that they are in fact so freaking vague on things like this, and the way the numbers are represented is always in worst possible context.
You can also refer to section 2.2
http://www.ipcc.ch/publications_and_data/ar4/wg1/en/ch2s2-2.html
check out figure 2.2 where they discuss different ways to model the effects of RF change, with temp changes much lower at surface than at higher altitudes. It has been a long time since I read through this, but I went through this section in some detail and it was clear to me that AR4 was written to mean that surface temps would change LESS than the value they were calculating for equilibrium response. How much less? I dunno, but the fact is they represent the change as being +1 degree, then quietly bury in the detailed analysis that the surface temperature change will be less than that.
joeldshore says:
July 23, 2012 at 7:32 pm
“What I should probably say is that the temperature at what was formerly the effective radiating level will rise. (Or, to put it another way, the rise in effective radiating level occurs immediately if you imagine an instantaneous increase in GHGs and then one finds oneself in a situation where this layer is now higher and colder and so the Earth is no longer emitting as much radiation as it is absorbing. Then, over time, the entire system warms [but certainly not uniformly] until the new effective radiating level is at 255 K and the radiative balance is re-established.)”
Only nitpickers will argue with that. That is the basic story. We should move on now.
In reply to joeldshore says: July 23, 2012 at 7:32 pm “What I should probably say is that the temperature at what was formerly the effective radiating level will rise…” But the satellite data for the Pacific in Griggs and Harries (JoC 2007) show NO rise in temperature at ANY altitude between 1970 and 2003. How do you explain that? When you say “Or, to put it another way, the rise in effective radiating level occurs immediately if you imagine an instantaneous increase in GHGs and then one finds oneself in a situation where this layer is now higher and colder and so the Earth is no longer emitting as much radiation as it is absorbing” does not help as the data on OLR I previously linked to from NOAA-NCEP reanalysis also belie this claim, as OLR INCREASED by 6 W/sq.m. between 1970 and 2003 despite the increase in CO2 of over 50 ppmv.
ahem… was I just noticed that figure 2.2 actually shows the opposite of what I said, my mistake. I was skimming and linked to it thinking it was something else. There’s a whole pile of junk in this section that is contradictory, and I don’t have time to go through it in detail again to find the specific sections I was thinking of. Really not worth it anyway since AR5 is on the horizon.
davidmhoffer says:
July 23, 2012 at 8:16 pm
ahem… was I just noticed that figure 2.2 actually shows the opposite of what I said, my mistake. I was skimming and linked to it thinking it was something else. There’s a whole pile of junk in this section that is contradictory, and I don’t have time to go through it in detail again to find the specific sections I was thinking of. Really not worth it anyway since AR5 is on the horizon.
Surely you do not mean to imply that for all of the billions spent since AR1, all the IPCC has created with its thousands of climate scientists is …. worthless illiterate papers that cannot be understood by nor explained by even the supporters of the 1.5 trillion dollar-per-year UN effort to kill millions and harm billions of innocents?
RACookPE1978 says:
July 23, 2012 at 8:46 pm
>>>>>>>>>>>>>>>
I respond with the following quote which I trust summarizes the matter nicely:
“One must say clearly that we redistribute de facto the world’s wealth by climate policy. One has to free oneself from the illusion that international climate policy is environmental policy. This has almost nothing to do with environmental policy anymore.”
~ Ottmar Edenhofer, Co-Chair, UN/IPCC WG-3
paulinuk says:
July 23, 2012 at 8:06 pm
Konrad “I have a very simple multiple choice question for those still commenting on this thread.
I have two 1m spheres of thin LDPE plastic (transparent to LWIR) in a sunless vacuum in deep space. Both spheres contain gas at an initial temperature of 100C. Sphere 1 contains 100% O2. Sphere 2 contains 99% O2 and 1% CO2. Which sphere cools faster? Is the answer :
A. Sphere 1 cools faster.
B. Sphere 2 cools faster.
C. Sphere 1 and 2 cool at the same rate.
Or
D. The spherical chicken found in the same region of space has “Property of Pierrehumbert” tattooed on its butt.”
Common sense says “C” but from by “argument from authority” , CO2 cools 100’s or thousands times faster than O2 so “B” . Konrad , rest assured, you can safely put your hand right up next to sphere1 and you won’t get burnt because O2 doesn’t radiate much.
Well, first of all the LDPE doesn’t radiation much in the IR, because it doesn;t absorb there (not quite 100% true, but let us assume that. Since the sphere’s are in a vacuum, the only way to lose energy is by radiation (hey, it’s your problem, don’t complain). Sphere 2 can radiate faster, it cools faster. and btw, Eli was under the impression that you ain;t supposed to do the D thing here.
Glad to be of service otherwise.
Most of your explanation involves the conductance of energy away from the molecule, not the radiation of energy away from the molecule. If you were to take a single CO2 molecule in isolation and subject it to a stream of photons of the correct frequency for it to absorb, what happens? Does the vibrational energy of the molecule continue to increase, does the radiative energy convert into another form of energy, does the rate of photons emitting increase, or does something else happen?
One of the things you have to know to think about this question is that there are slight anharmonicities in vibrational quantum states and that transitions are only allowed that change the rotational quantum numbers by 1 or zero, so pumping in a single frequency doesn’t do much once absorption and stimulated emission balance. Get a nasty enough light source and you can do interesting stuff (look up, for example IR multiphoton dissociation, a ton of papers in the late seventies and early eighties).
Shawnhet says: July 23, 2012 at 5:26 pm
I must not be communicating well. I agree that the presence of greenhouse gases (or for that matter any material) in the Earth’s atmosphere will likely alter, both in a transient sense and in a steady-state sense, the temperature profile (i.e., the temperature as a function of position) of the Earth’s surface and atmosphere. I make no quantitative statements regarding how greenhouse gases affect the temperature profile.
What I object to are (a) the statement that “greenhouse gases slow down the rate of cooling” and (b) the argument that the statement justifies the claim that “greenhouse gases” in the Earth’s atmosphere warm the Earth’s surface and its atmosphere.”
Before going further, from something you wrote it occured to me that as applied to the Earth/Earth-atmosphere system in energy-rate-equilibrium, we might be interpreting the phrase “slow down the rate of cooling” in different ways. You wrote: First off, GH gases do not absorb energy in the terms you speak of it above, rather they make it so energy leaves the atmosphere more slowly than OTW. From this I infer that you might interpret the phrase “slow down the rate of cooling” to mean: “if X is the TIME it takes an identifiable unit of heat (whatever that is) to travel from the Earth’s surface to space in the absence of greenhouse gases in the Earth’s atmosphere, and Y is the TIME it takes a comparable identifiable unit of heat to travel from the Earth’s surface to space in the presence of greenhouse gases in the Earth’s atmosphere, then the phrase “slow down the rate of cooling” means that X and Y are both positive and that Y is greater than X.
As applied to the Earth/Earth-atmosphere system in energy-rate-equilibrium, the way I interpret the phrase is that if X is the RATE energy leaves the Earth/Earth-atmosphere system in the absence of greenhouse gases in the Earth’s atmosphere, and Y is the RATE energy leaves the Earth/Earth-atmosphere system in the presence of greenhouse gases in the Earth’s atmosphere, then the phrase “slow down the rate of cooling” means that X and Y are positive and that Y is less than X.
If neither of the above reflects what you mean by the phrase “energy leaves the atmosphere more slowly than OTW”, please clarify.
Using my definition of the phrase, if after adding greenhouse gases to the Earth’s atmosphere and waiting until all transients have died out, the rate energy ENTERS the Earth/Earth atmosphere system is unchanged, then the addition of greenhouse gases to the Earth’s atmosphere will, after all transients have died out, leave the rate energy LEAVES the Earth/Earth atmosphere system unchanged. Otherwise, the Earth/Earth atmosphere system will either accumulate thermal energy without bound or lose thermal energy until there is none left.
Thus I believe that after all transients have died out, the rate of cooling won’t have changed; and if the rate of cooling hasn’t changed, how can it be said either that (a) the rate of cooling has slowed down or (b) it is this slow down that causes the Earth’s surface temperature to increase? Again, I’m not saying that greenhouse gases in the Earth’s atmosphere won’t alter the Earth’s temperature profile. What I am saying is the argument that “a cooling rate slow down” exists and “the cooling rate slow down” CAUSES the temperature profile to change cannot be justified.
I’ll give my point of view one more try. I ask a series of questions. All questions apply to steady-state conditions–i.e., after the transients introduced by any changes in atmospheric greenhouse gases have died out–and to the situation that all energy sources other than energy coming from the sun are negligible.
First Question: Does the addition of greenhouse gases to the Earth’s atmosphere alter the RATE the Earth/Earth-atmosphere system absorbs solar energy? Yes or No.
If “Yes”, please explain your answer and stop–there’s no sense going forward with the questions. If “No” continue.
Second Question: Is the RATE energy enters the Earth/Earth-atmosphere system the same as the RATE energy leaves the Earth/Earth-atmosphere system? Yes or No.
If “No”, please explain your answer and stop–there’s no sense going forward with the questions. If “Yes” continue.
Third Question: If we get to this question, we are in agreement (but we may both be wrong) that (a) the RATE energy enters the Earth/Earth-atmosphere system is unaffected by the presence of greenhouse gases in the Earth’s atmosphere and (b) the RATE energy leaves the Earth/Earth-atmosphere system equals the RATE energy enters the Earth/Earth-atmosphere system. Can we then conclude that the RATE energy leaves the Earth/Earth-atmosphere system is unaffected by the presence of greenhouse gases in the Earth’s atmosphere?
If “No”, please explain your answer and stop–there’s no sense going forward with the questions. If “Yes” continue.
Fourth Question: If we get to this question, we are in agreement that the rate energy leaves the Earth/Earth-atmosphere system is unaffected by the presence of greenhouse gases in the atmosphere. Is the RATE energy leaves the Earth/Earth-atmosphere system the same thing as the RATE OF COOLING OF THE EARTH/EARTH-ATMOSPHERE SYSTEM?
If “No”, please explain your answer and stop–there’s no sense going forward with the questions. If “Yes” continue.
Fifth Question: If we’ve reached this question, we are in agreement that the “rate of cooling of the Earth/Earth-atmosphere system” is unaffected by the presence of greenhouse gases. Is it then logical to argue that the presence of greenhouse gases in the Earth’s atmosphere “slow down the rate of cooling” and it is the “slow down of the rate cooling” that causes the Earth/Earth-system temperature to be different in the presence/absence of atmospheric greenhouse gases?
If “No”, stop–we are in agreement. If “Yes”, feel free to add a comment; but then we’ve probably reached a point where further discussion is useless.
Cheers
Well I’m sure glad I didn’t waste my time on this thread.
Luckily I was short circuited at a very early stage by the suggestion that black body emission was equal to: k T^4
(k) is Boltzmann’s constant; (sigma) is the Stefan-Boltzmann constant used in total integrated black body emission; so I exited stage left immediately on reading that, along with the earth cooling by losing heat to space. The earth neither receives from, nor loses to “space”, ANY HEAT. well not any measurable amount.
And it’s nice to read a citation to a source that agrees with me, that all matter above zero Kelvins, including neutral gases emits thermal radiation. And the spectrum of thermal radiation for any material Temperature, has no lower bound, and no upper bound of frequency or wavelength, although 98% of the energy is contained in a 16 to one wavelength (or frequency) range.
No we don’t call it black body radiation, because there simply aren’t big enough masses of neutral gases around near us, to completely absorb ALL electromagnetic radiation that impinges on them.
But there are large amounts of gases containing vast numbers of molecules in collision, thereby exhibiting a measurable Temperature, and therefore able to radiate and absorb EM radiation of ANY frequency or wavelength, including the the range from 0.7 microns to 100 microns, commonly referred to as Infra-red.
That entire contraption known as the large Hadron collider, is so ruddy big, as is the Stanford Linear Accelerator (SLAC) because particle Physicists, along with Radio Astronomers; and other Radio Physicists (such as me), are mindful of the fact that accelerated electric charges; aka variable electric currents travelling any non zero distance must radiate EM waves as shown eons ago, by the likes of Heinrich Hertz, and James Clark Maxwell.
Nils Bohr aided by Arnold Sommerfeld, tried to veto that requirement, in their quite arbitrary (but ingenious) Bohr/Sommerfeld atom model; until quantum mechanics came along and bailed them out of their predicament, so sanity remains, and accelerated charges, including atoms in collision still radiate EM waves.
I’m sorry for those hardy souls who kept feeding those pesky trolls, on this thread; I’m outta here.
That last thing you said, Reed. Go with that. This thread has been over for some time.
What an intense thread. I read Reed’s piece 3 times and 90% of all the posts here and I think I get the gist. I see we have a physicist or two and I have read Dr. Brown’s posts here as well.
I will get to the discussion of the radiative transfer but here is some necessary background discussion:
First of all Reed well done: well written, explained and defended. I read all of your response comments. Even if some of your claims/possible occurrences do not hold to be true, the central themes will hold up, I am certain.
To begin with, Anthony Watts, among others have already conclusively shown that the weather stations have shown a heating bias via UHI, and Steve McIntyre and the paper from have shown warming biases due to improper homogenization procedures not well verified/validated within the statistical or scientific community at large. There is Koutsoyannis as well. Thus, before I mention anything about physics of greenhouse gases, whether it acts as a blanket, warms/cools/does nothing, we already have enough data and statistical analysis to obliterate, if not all so called recorded global warming, on the instrumental record, then >95% of it. Statistically W.M. Briggs who is less harsh about Mann et al., and GHG forcings also destroys the idea of using homogenization of weather stations, the statistics used in the constructions of the hockey stick using smoothing methods is shoddy, even if not performed on purpose.
Okay now on to the physics, thermodynamics fun stuff:
First in simple words greenhouse gases CANNOT induce increased work on the system, so that the colder air/atmosphere/greenhouse gas transmits heat to a warmer object. Air conditioners and refrigerators do this but not without exerting work through the compressor. Adiabatic temperature changes do occur in nature via work being exerted by external forces like atmospheric pressure, and here is where the basic underpinnings of the lapse rate discussion. However, keep in mind GHG’s do not add any energy or WORK to the system in any way. In addition GHG’s act more as thermal buffers heating and cooling the planet, rather than just “slowing the cooling process” “trapping heat/heated air/gases” etc…
Thus borrowing from my own comments and direct quotes elsewhere:
“The real question is: is the science sound based upon the immutable laws of heat transfer and the answer is a resounding no:
“Clausius Statement of the Second Law
Clausius Statement of the Second Law
The Clausius statement of the second law states
It is impossible for any system to operate in such a way that the sole result would be an energy transfer by heat from a cooler to a hotter body.
Heat can transfer from a cooler body to a hotter body if other effects accomplishing the heat transfer occur within the system or its surroundings, or both. Air conditioners and refrigerators are devices to transfer heat from a cool space to its hot surroundings. But both of them need power input. The Clausius statement says that an air conditioner cannot cool a room without power input.” Taken from: http://www.ecourses.ou.edu/cgi-bin/ebook.cgi?doc=&topic=th&chap_sec=05.2&page=theory.
Then there is excellent research on negative feedbacks as Reed alludes to:
http://wattsupwiththat.com/2011/09/20/new-peer-reviewed-paper-clouds-have-large-negative-feedback-cooling-effect-on-earths-radiation-budget/
And preliminary findings:
http://wattsupwiththat.com/2012/07/18/new-paper-on-global-water-vapor-puts-climate-modelers-in-a-bind/
Of course cloud cover with water vapor holds in heated air, or as we know heated gases, but adding more C02 and CH4 does not automatically mean we are going to get a hotter planet. As all of the IPCC reports show and as do the numerous cloud physics/atmospheric studies too, we poorly understand cloud cover, micro-physics and dynamic formation. Sure clouds at night block the leaving of some radiation/heat flow and during the day albedo reflects back radiation, but the system is so complex that the margin of error and depth of uncertainties are still far too large. Add (again, to make it clear, regardless of what letters you have after your name) to that the second law prohibits movement of heat transfer from a colder object to hotter, and , the saturation bands of C02/logarithmic activity and we see why the level of certainty and statistical clustering of 3 degrees for a doubling of C02 just cannot be, and this applies exactly because the planet obeys laws of non-equilibrium thermodynamics in an open or if you prefer semi-open system.” Yes, that is right we are not dealing with equilibrium thermodynamics but non-equilibrium, however, some researchers have been trying over the past couple of years to reconcile that fact and produce complex models utilizing non-equilibrium thermodynamics to push the agenda of C02 = linear relationship to global warming, and usually the agenda is one of CAGW. If this thread stays alive and their are responses to this post then we can get more technical/mathematical.
The planet is not a closed or semi closed system and the behavior of clouds and their micro-physics leave many known unknowns and unknown unknowns… Next time I should say what is wrong with the so called climate sensitivity as well.
References
http://wmbriggs.com/blog/?p=1459
http://climateaudit.org/?s=homogenization
http://www.ecourses.ou.edu/cgi-bin/ebook.cgi?doc=&topic=th&chap_sec=05.2&page=theory
Concepts in Thermal Physics Chapter 13, 2006. Blundell, Stephen J. Blundell, Katherine M.
Peter Atkins Physical Chemistry: All over the textbook.
http://greenhouse.geologist-1011.net/
http://www4.uwsp.edu/geo/faculty/ritter/geog101/textbook/atmospheric_moisture/lapse_rates_1.html
Reed,
I’ll try to answer your questions, please bear in mind though that I don’t think that we necessarily mean the same things with some of our terminology.
“First Question: Does the addition of greenhouse gases to the Earth’s atmosphere alter the RATE the Earth/Earth-atmosphere system absorbs solar energy? Yes or No.”
I would rephrase this as saying that GH gases do not block the radiation arriving from the sun allowing it to hit the Earth and warm it.
“Second Question: Is the RATE energy enters the Earth/Earth-atmosphere system the same as the RATE energy leaves the Earth/Earth-atmosphere system? Yes or No.”
It depends on when you measure this precisely. Consider a tap attached to a garden hose. Before you attach the hose the water hits the ground at the same rate that it leaves the tap. Then you attach the hose and the water leaves the tap at a greater rate than it hits the ground (until the hose is full of water). Then, once the hose is full of water, the water hits the ground at exactly the same rate as it leaves the tap.
If one imagines that there is a property is dependent on the amount of water that has left the tap but has yet to hit the ground then we could legitimately claim that the length of hose btw the tap and the ground caused more of this property without altering the *long-term* equivalency btw water-in and water-out or magically creating water or whatever.
The rest of your questions rest on this fundamental misunderstanding in terms of the rate of cooling of the Earth. It would not make sense to argue that because the water out of the hose is exactly equal to the the water hitting the ground in the steady state that it is impossible for a hose to allow (magically) the water from the tap to move 20 feet out from the tap before hitting the ground. Likewise, the fact that at equilibrium, energy in = energy out regardless of the presence of GH gases does not mean that GH gases cannot raise the Earth’s temperature.
I hope the above can help you frame your questions a bit better.
Cheers, 🙂
Communicating any kind of science to non-scientists requires simplification. Attacking the simplified explanations of science because you don’t like the solutions that you imagine they are supposed to be provoking people into demanding is pretty weird. If you want to prove the science wrong, you need to understand the science itself.
Eli Rabett says:
July 23, 2012 at 9:21 pm
One of the things you have to know to think about this question is that there are slight anharmonicities in vibrational quantum states and that transitions are only allowed that change the rotational quantum numbers by 1 or zero, so pumping in a single frequency doesn’t do much once absorption and stimulated emission balance. Get a nasty enough light source and you can do interesting stuff (look up, for example IR multiphoton dissociation, a ton of papers in the late seventies and early eighties).
>>>>>>
Thanks Eli, I think that I found most of my answers under Wikipedia’s description for ‘Spontaneous Emission’. Your direction to IR multiphoton dissociation is interesting because it confirms a thought that I had that if you exceed the absorption and stimulated emission balance you can break the molecular bonds – unless I miss the mark, this can occur naturally but the chances of it happening are about the same as the odds of me winning the next lottery without buying a ticket 😉
paulinuk says:
July 23, 2012 at 8:06 pm
Eli Rabett says:
July 23, 2012 at 9:10 pm
———————————–
You are both correct. The answer is of course B. The gas sphere with 99% O2 and 1% CO2 cools quicker. The question was asked to illustrate the point that was raised by Reed Corays post. The cooling effect of added CO2 in our atmosphere appears neglected in CAGW discussions.
The warming effect of increasing CO2 is an inverse logarithmic function of its concentration in the atmosphere.
The cooling effect of increasing CO2 is a linear function of its concentration in the atmosphere.
At some point the warming logarithmic curve must cross the linear slope of cooling. This could be called the “Point of no concern”
Hopefully this graph illustrates the issue. http://i48.tinypic.com/2pp0hed.jpg
Tim Folkerts writes “Your model seems to be that “average radiating height” is a fundamental feature of the earth, independent of where the molecules emitting the IR radiation are found”
I dont have a model Tim. I simply look at what we know and what we dont know and see the disconnects and there is a massive disconnect between more CO2 = higher average radiating altitude because it implicitely begins with “all else being equal” which is a nonsense.
Hi Reed
I am never comfortable with statements such as “greenhouse gases slow down the rate of cooling”. It is not a description I would use because it is not clear what it means and leads to more confusion. I prefer to say – Greenhouse gases in the atmosphere make the SURFACE of the planet warmer than it would be without them.
As for your questions, I have some problem with wording of them and rather than bicker about that let’s get to the point. The amount of energy the earth receives from the sun is always going to be balanced by the amount that the earth radiates back to space. This is called the Radiation Balance. No matter how much greenhouse gas is put into the atmosphere this balance will always be restored. The amount of energy leaving the earth will equal the amount of energy coming in.
The important point is that the radiation balance applies at the top of the atmosphere (TOA). We do not live at the top of the atmosphere, we live on the surface. It is the temperature on the surface which is important to us, not the temperature or radiation balance at TOA. Greenhouse gases do not affect the rate at which radiation comes in or goes out, that is why I find statements like “greenhouse gases slow down the rate of cooling” can be confusing.
However, greenhouse gases DO affect the temperature at the surface. They help to keep the planet warm and habitable – and there are many descriptions of how they do that.
trcurtin says:
davidmhoffer says:
I just tried to find the post where this came up but couldn’t…There’s too many posts here on WUWT. And, as I think I tried to say from the wording, this is my understanding of what they said since the wording itself was a bit cryptic. However, they were clearly addressing the issue of why the temperature change one calculates naively from using the S-B Law with T=255 K and a forcing of about 3.7 W/m^2 or so gives a temperature that is a bit lower than what the models find for a doubling of CO2 (absent feedbacks).
The statement isn’t so much that it makes no difference but rather that it depends carefully on how one is defining the zero-feedback state. And, while one might prefer one definition to another, I don’t think there is any right or wrong one.
I’ll probably have at best only sporadic web access over the next several days, so I may be slow to respond to future discussions.
Konrad says:
Unfortunately, what it illustrates is only that you still don’t understand the science of radiative transfer in the atmosphere. Your linear function cooling effect is totally fictional. The NET effect of the fact that CO2 molecules absorb and emit radiation is an approximately logarithmic radiative forcing (in the current concentration regime) with concentration.
”
Phil. says: July 23, 2012 at 1:48 pm
Outgoing flux=Incoming flux
∴ Surface radiation – absorption by GHGs = Incoming flux (constant)
So k*T^4 – f*ghg([CO2]) = constant
so if ghg([CO2]) goes up then T^4 must go up by the same amount. So if T^4 goes up by 4% then T goes up by ~1%..
Thanks again for your response.
I agree with your math–i.e., I agree that in an expression x – y = constant, if x goes up then y goes up by the same amount. I want to make sure, however, that we’re not talking at cross purposes regarding what you mean by “flux”. In the context of our discussion, the Stefan-Boltzmann equation, the k*t^4 term in your equation is “energy rate” per unit area. [If you want to include the surface energy in the constant “k”, then the term in your equation corresponds to energy rate. However, in neither case does it correspond to energy itself.] That is, (a) “surface radiation” is the rate (not the amount of) energy leaves a surface (either per unit area or for the total surface, which for his discussion I’ll take to mean “for the total surface”), (b) “absorption by GHGs” is the rate GHGs absorb energy, and “Incoming flux” is the rate energy enters the system comprised of the surface and the GHGs. Furthermore, I assume your equation applies to a state of “energy-rate” equilibrium–i.e., sufficient time as elapsed that the individual terms in your equation do not change with time.
If this interpretation is correct, then according to your equation as greenhouse gas levels increase, the temperature of the surface will increase to keep the equation in balance. So far so good. However, your equation implies that the rate energy is absorbed by greenhouse gases is never zero–in fact is a non-zero constant. So if your equation represents something physical, then isn’t it legitimate to ask: where does the energy that over time keeps being absorbed by (i.e., keeps accumulating) in the GHGs go? For energy rate equilibrium, if energy is absorbed by GHGs at a non-zero constant rate, doesn’t that energy have to leave the greenhouse gases at the same rate? If this is the case, shouldn’t your equation have in addition to a non-zero term for the “rate of energy aborption by GHGs” also have a non-zero term for the “rate of loss of energy by GHGs?”
”
**********************************
WRONG!
incoming flux is not a constant! It contains variables with variational effects greater than that of a co2 doubling from variables that are greater in magnitude than all co2 contributions. While the sun is rather constant, Earth’s albedo is not and the incoming flux that stays is the difference between this relative solar constant and that power returned to space as albedo reflection. Also, the actual solar incomming power varies by several times the effect of a co2 doubling as Earth travels through its orbit – as I recall about 90 W/m^2 peak to peak difference between perihelion and aphelion. Note these are two items are different factors. The net result of the latter is that the TSI reaching the southern hemisphere TOA is significantly greater than that reaching the northern hemisphere compared with the equivalent power effect of a co2 doubling, Combine that with the realization that most of Earth’s land mass is in the northern hemisphere and surface albedo of water tends to be under 0.04 as compared to Earth’s average of around 0.30 and voila, simple explanations would demand that the southern hemisphere be much hotter than the northern hemisphere – which it isn’t.
One of the ‘tells’ (giveaways where gamblers inadvertently show their position to their competition) I saw early on concerning the CAGW literature was the treatment of albedo as essentially a constant along with the lip service about albedo change being due to human induced land use changes being another threat to earth (mother goddess).
While one can do rather well using averages and stefan’s law to understand things, to assume albedo has little to no variation is to utterly fail to understand the more important factors involved in the whole problem.
It’s actually quite easy and rather accurate to determine Earth’s sensitivity to changes in W/m^2 incoming power being absorbed and the need for temperature variations to make up for the changes to achieve a balance. It’s also quite easy to see the ongoing presence of net negative feedback where the Earth’s change in temperature to achieve balance is less than that of a simple black body. All can be done by using a few known values and running stefan’s law forward and backwards a few times.
Incoming power average = 341 w/m^2, Earth’s average T = 288.2k , Earth’s albedo as we’ve measured it = 0.30. Stefan’s law gives us 391 w/m^2 for a bb at 288.2k. Incoming absorbed power is (1-albedo)*341w/m^2 = 239w/m^2. For balance, what escapes from the surface and atmosphere to space must equal what is absorbed = 239w/m^2 which gives us 255k which is what a bb with 0.30 albedo located at Earth’s orbit would be without an atmosphere blocking the escape of some of the IR. The difference is 33k or 33 deg C warming for all of the absorbed outgoing power that doesn’t escape to space. Take the difference of what leaves the surface and what escapes = 391-239 = 152 w/m^2 is captured in the atmosphere and doesn’t escape. Note that only about 2/3 of this is ghgs and clouds and aerosols etc. make up the other 1/3 and also that only 0.61 or 61% (239/391) of what leaves the surface escapes to space.
We have atmospheric effects blocking 152W/m^2 and providing warming of 33 deg C. 33/152 = ~0.22 deg C / W/m^2 change. This is actually the real average for Earth with all the feedbacks present. Note it is not the politically defined co2 doubling sensitivity that contains supposed additional feedbacks and assume co2 has a specific amount of w/m^2 for a doubling.
The straight calculation for a bb blanketed by the atmosphere would be to assume that for a 1 W decrease in outgoing power, 239 to 238 W/m^2, the Earth’s surface would have to heat up by enough for stefan’s law to return that 238 to 239 – but remember – only 61% of what is radiating from the surface escapes so we’d need to increase the T by enough so that 1/0.61 = 1.6 W/m^2 or 391 + 1.6 = 392.6W/m^2 which gives us (reversing stefan’s law equation) 288.47 or 0.27 deg C over the original T of 288.2k. Comparing this to the 0.22deg C rise, you can see it takes less warming to regain balance than that of the simple black body – which indicates that there is actually net negative feedback present instead of positive feedback.
Since the accepted increased power absorption for a co2 doubling is 3.7W/m^2, our sensitivity for a co2 doubling without additional feedbacks due to the rise in temperature would be 3.7 * 0.22 = 0.8 deg C. Positive feedback is extremely limited in values for there to be a stability and considering that the Earth has quite a variation in parameters that affect our balance, it is nuts to talk about high values. Let’s put a number or two to this though.
Atmospheric ghg absorption depends on the actual number of molecules of a gas in the air column. Absolute humidity fits this bill but relative humidity, RH, does not. If we increase T by 5 deg C in the entire atmospheric column where water vapor is present and if we hold RH constant – a common assumption for climatologists, we can consult the table that shows we get a 30% increase in absolute humidity. Like co2, h2o vapor is in the log region and has been for many doublings. It has roughly a little over twice the effect of co2 in the atmosphere and is roughly contributing a little over twice the doubling effect of co2. Since a 30% increase is a far cry from a full doubling, one finds there to be about 3.1 W/m^2 contribution for a full 5 degree increase in T which gives us 3.7w/m^2 (co2) +3.1w/m^2(h2o) = 7.8w/m^2 increase in our assumed 5 deg C rise due to a co2 doubling. Using our sensitivity of 0.22 deg C/W/m^2, we find we have enough power absorption to raise T by 0.22 x 7.8 = 1.7 deg C using our co2 forcing and our h2o ipcc proclaimed “primary” feedback. This leaves only 5-1.7 = 3.3 deg C of feedback missing from our consideration and it means that h2o vapor cannot be primary. Also, it means that this other feedback is far greater than stability limits allow. One can go back and assume 2 deg C rise from a co2 doubling + feedback but that substantial contribution from h2o vapor was due to the assumption of a full 5 deg C rise in the entire atmospheric column. If you drop that to 2 deg C, you lose most of that 3.1w/m^2 and it becomes a small fraction of the co2 forcing value
A final question or comparison shows the ridiculous nature of things when it comes to high sensitivity/effects. Co2 has been essentially a log function for almost a dozen doublings to get to our present value. Put another way, a co2 doubling now has the effect of about 10% of the total effect of co2 . A 5 deg C rise in temperature would require 5/0.22 = 22.7 W/m^2 of added forcing and co2 directly only can contribute 3.7w/m^2 and h2o could only contribute around 3.1 w/m^2. Considering that ghgs contribute to only around 100 w/m^2 total at present time, to claim that a co2 doubling could affect the total contribution by 20% is utterly and totally ridiculous.
Reed Coray says:
July 23, 2012 at 9:40 pm
Fifth Question: If we’ve reached this question, we are in agreement that the “rate of cooling of the Earth/Earth-atmosphere system” is unaffected by the presence of greenhouse gases. Is it then logical to argue that the presence of greenhouse gases in the Earth’s atmosphere “slow down the rate of cooling” and it is the “slow down of the rate cooling” that causes the Earth/Earth-system temperature to be different in the presence/absence of atmospheric greenhouse gases?
Yes, because it is the rate of loss from the surface that is reduced by the presence of GHGs, however the loss from the TOA is unaffected once steady state is achieved.
Reed Coray says: July 23, 2012 at 9:40 pm …
Reed, I like what you said.
Perhaps the biggest misconception you address is how “fast” or “slow” energy enters and leaves. As you quite properly state, we are interested in the RATE that energy enters or leaves, dE/dt (in Watts), not the SPEED it enters or leaves, dv/dt (in m/s). To be more accurate, people should say the rate “increases” or “decreases”. While the the term “speed up energy loss” could be correctly interpretted to me “increase dE/dt”, others can easily interpret it to mean something about the “speed of the photons” or “the time it takes a give ‘dE’ of energy to get from the ground to the top of the atmosphere”. Too many people seem to be hung up on the idea that the speed matters, when in fact it is irrelevant.
Kudos.
~~~~~~~~~~~~~~~~~~~~~~~~~~~~~~~~~~~~~~~~~~~~~~~~~~~~
In the series of questions you ask, I think you could clarify your ideas a bit by throwing in the terms “average” and “steady-state” and “net” occasionally, but overall I agree with what you said.
Basically, after the series of rhetorical questions, you have established that 341 W/m^2 arrive at the earth from the sun, and 341 W/m^2 leave again to space (on average after steady-state conditions have been achieved). You have the information from the top edge of Trenberth’s energy flow diagram: http://www.agci.org/classroom/images/trenberth_energy.png.
NOW comes the interesting part — the rest of the diagram!
1) What happens between the TOA (top of atmosphere) and the ground, and how does this affect surface temperature?
2) How does continually changing the CO2 level affect things and how far from steady-state does that make the earth?
3) How do OTHER factors (variable sunlight, variable orbit, variable albedo, variable humidity …) affect the average, steady-state temperature?
4) Does it even make sense to talk about “steady state” when there are changes constantly occurring on scales from minutes to millions of years?
I have been following the comments here, smiling and LOL, sometimes, and I have come to the same conclusion that I came after my initial investigations,
on whether my carbon footprint is harmful to earth,
http://www.letterdash.com/HenryP/more-carbon-dioxide-is-ok-ok
As a scientist who not only follows the truth, but also knows the Truth, what I notice is that the pro AGW camp increasingly wants to make it seem that atmospheric chemistry and physics is so difficult that they themselves hardly understand it – and indeed they often have to consult each other on questions being posed -their income depends on it!!!
Yet, what I have found, is, that all you need is a basic understanding of probability theory and sampling technigues, which is taught at most third year university level statistics.
You are being all deluded into looking at AVERAGE temps. which has an awful lot of weather NOISE when you should be looking at MAXIMA, which gives you a proper look at the energy input by the sun.
http://www.letterdash.com/henryp/global-cooling-is-here
cba:”Atmospheric ghg absorption depends on the actual number of molecules of a gas in the air column. Absolute humidity fits this bill but relative humidity, RH, does not. If we increase T by 5 deg C in the entire atmospheric column where water vapor is present and if we hold RH constant – a common assumption for climatologists, we can consult the table that shows we get a 30% increase in absolute humidity. Like co2, h2o vapor is in the log region and has been for many doublings. It has roughly a little over twice the effect of co2 in the atmosphere and is roughly contributing a little over twice the doubling effect of co2. Since a 30% increase is a far cry from a full doubling, one finds there to be about 3.1 W/m^2 contribution for a full 5 degree increase in T which gives us 3.7w/m^2 (co2) +3.1w/m^2(h2o) = 7.8w/m^2 increase in our assumed 5 deg C rise due to a co2 doubling.”
I must say I don’t follow the derivation of your 3.1 W/m2 number above so let me show how I look at these numbers in the past. According to most of the numbers I have the seen, the total magnitude of the WV portion of the GH effect is supposed to be 75W/m2 and each additional degree of warming should put enough WV into the air to increase the WV-GHE by 2W/m-2. Assuming that the WV GH effect is basically logarithmic (like CO2) gives you something around 23 or 24W/m2 for a doubling of WV(GH of doubling/ln(2)=2Wm^-2/ln(1.06)).
However, this amount per this calculation, we can see that WV has only had the equivalent of a bit more than 3.2 doublings of WV (75w/m2/24W/m2). OTOH, CO2 has apparently had about 8.5 ~ 32W/m2 of CO2 GH effect/3.7 W/m2 of GH effect per doubling. Given that WV is supposed to be a better GH absorber and there is more WV for the vast majority of the atmosphere below the effective radiating level only logical way I can match these two numbers is to assume that WV has a negative feedback associated with it that reduces its net warming effect by at least 1/2.
Cheers, 🙂
Sorry about the last paragraph above – a couple of missing words may make it hard to understand. Here it is as I meant to write it.
However, given the 24W/m2 per the above calculation, we can see that WV has only had the equivalent of a bit more than 3.2 doublings of WV (75w/m^2/24W/m^2). OTOH, CO2 has apparently had about 8.5 ~ 32W/m2 of CO2 GH effect/3.7 W/m2 of GH effect per doubling. Given that WV is supposed to be a better GH absorber and there is more WV than CO2 for the vast majority of the atmosphere (below the effective radiating level) only logical way I can match these two numbers is to assume that WV has a negative feedback associated with it that reduces its net warming effect by at least 1/2. I believe that assuming WV has had the equivalent of about 6 doublings where each doubling has (on average) a 12W/m2 effect than arguing that it has had about three doublings where each doubling has(on average) a 24W/m2 effect.
Cheers, 🙂
Reed, I believe I have a more succint way to phrase the point I was trying to make above. IMO, the addition of GH gases slow down the rate of cooling while heating the surface speeds it up again. At equilibrium then, the rate *the Earth as a whole* cools with GH gases is the same rate at which it would cool without them. It is still accurate to say both that GH gases slow the rate of cooling and that the Earth as a whole cools at the same rate.
Cheers, 🙂
Michael Tremblay says:
July 24, 2012 at 1:05 am
Thanks Eli, I think that I found most of my answers under Wikipedia’s description for ‘Spontaneous Emission’. Your direction to IR multiphoton dissociation is interesting because it confirms a thought that I had that if you exceed the absorption and stimulated emission balance you can break the molecular bonds – unless I miss the mark, this can occur naturally but the chances of it happening are about the same as the odds of me winning the next lottery without buying a ticket 😉
You can’t beat the house, but if you provide other paths into or out of the system (e.g. feeding the upper level from reaction or a third state or providing an empty state you can emit to), you can get the same effect. This is basically what lasers do. The neatest one Eli knows is the CO2 laser in the upper atmosphere of Mars that Mike Mumma discovered.
joeldshore says:
July 24, 2012 at 5:08 am
“Unfortunately, what it illustrates is only that you still don’t understand the science of radiative transfer in the atmosphere. Your linear function cooling effect is totally fictional.”
—————————————————————————————————————-
Given your claim about my lack of understanding I believe you owe me an answer to the following multiple choice question –
I have two 1m spheres of thin LDPE plastic (transparent to LWIR) in a sunless vacuum in deep space. Both spheres contain gas at an initial temperature of 100C. Sphere 1 contains 100% O2. Sphere 2 contains 99% O2 and 1% CO2. Which sphere cools faster? Is the answer :
A. Sphere 1 cools faster.
B. Sphere 2 cools faster.
C. Sphere 1 and 2 cool at the same rate.
Or
D. None of the above.
A simple one letter answer is all that is required A, B, C or D. No hand waving, no “putting in context”, no reframing the question. Simply A, B, C or D.
Konrad says: July 24, 2012 at 2:24 pm
“I have two 1m spheres of thin LDPE plastic … “
I’m trying to decide if there is some “trick” that I am missing, since the answer seems glaringly obvious. The sphere with CO2 can radiate IR, the sphere without CO2 cannot (to any noticeable extent). Thus the sphere with CO2 can cool effectively, while the other can’t.
B. Sphere 2 with some CO2 will cool faster. Final answer. I am 99.9% sure Joel would agree (we tend to agree on basic physics like this).
I’m curious what the NEXT question is and why this question is important.
A number of commenters claim the so-called location of outgoing radiation is moved up due to GHGs
1. Where is the observational evidence of this?
2. Why do theoretical calculations on MODTRAN show the temperature profile of the atmosphere in the troposphere and stratosphere does not change at all between CO2 concentration inputs of 350 and 3500?
http://forecast.uchicago.edu/Projects/modtran.html
Konrad says: July 24, 2012 at 2:24 pm
“I have two 1m spheres of thin LDPE plastic … “
I believe the balloon surface of LDPE plastic would radiate to space at the same rate in both scenarios with equivalent starting temperatures, and therefore answer C
Hockey Schtick asks “Why do theoretical calculations on MODTRAN show the temperature profile of the atmosphere in the troposphere and stratosphere does not change at all between CO2 concentration inputs of 350 and 3500?”
As I understand it, MODTRAN is designed simply to calculate the radiation from given initial conditions, not to actually predict those initial conditions. So concentrations of gases, amount of cloud cover, and temperature profiles (among other things) are INPUTS to this program. If you want a different profile, you would program it into the computer.
Hockey Schtick says: “I believe the balloon surface of LDPE plastic would radiate to space …”
Since the plastic was postulated to be transparent, then I assumed it was 100% transparent. This means it can neither absorb nor emit IR => emissivity = 0. (In reality, the plastic might well emit IR better than such a small volume of CO2, but that is not what the question was focusing on, I think).
Tim,
The correct answer is of course B. The CO2 in the sphere acquires energy conductivly from the O2 and then radiates it to space. The point of the question was to get Joel to acknowledge that CO2 in our atmosphere can radiate energy to space that it has acquired conductivly from Earths surface and atmosphere. CO2s ability to cool in this manner should be a linear function of its concentration in the atmosphere.
Joel Shore: it is all too clear that you did not have time to read the Grigg Harries (GH, JoC, 2007) paper properly before rushing to judgment on me. What you quote from the careless Abstract to their paper is misleading as nearly the whole paper deals only with their data on differences in brightness temperatures by wave number in the spectra between 1970 and 2003. The brightness temperatures within the IR spectrum are NOT the same as the temperature at any given altitude, and while they claim to have shown that bT increased between 1970 and 2003, their own data in their Tables A1-5 show no correlation between actual temperatures by altitude in the atmosphere, which range from 192 K to 299 K and their brightness temperatures (by wave number) in their Fig.6, which range only between 288 and 296 K in Fig.6c and 240 to 278 K in Fig. 6f.
I should not have to tell you that brightness temperature is the temperature a black body in thermal equilibrium with its surroundings would have to have in order to duplicate the observed specific intensity of an object at a frequency v, and has little to do with the GH observed temperatures by altitude in the troposphere and stratosphere. Similarly the wave numbers in the GH Figures bear no relation to the altitudes in the atmosphere in Tables A1-6.
The GH discussion of the NCEP and ECMWF data in Tables A1-A6 in their Appendix does not mention the temperatures at each altitude (in hPa), so they failed to notice either that the temperatures in 1970, 1997, and 2003, are virtually identical in each year at each hPa, or that there are declining trends between 1970 and 1997, and between 1997-2003, so NO warming at all.
Unfortunately Griggs and Harries do not provide the raw data for the brightness temperatures shown in their Figures, but to judge from their Fig.6’s c and f panels, there is really no visible difference between their brightness temperatures for 1970, 1997 and 2003 in the wave numbers between 800 and 940 cm/1, or wave numbers 1280 to 1400 c/1.
Konrad, I am not sure which Tim you are referring to, but I would certainly also have answered (B). Has Joel rsponded?
You are missing something.
Please compare the following:
1. The radiation spectrum of a body with a temperature of roughly 5,500 K
2. The radiation spectrum of a body with a temperature of roughly 280 K
3. The absorption spectrum of CO2
Konrad says: “The point of the question was to get Joel to acknowledge that CO2 in our atmosphere can radiate energy to space that it has acquired conductivly from Earths surface and atmosphere. CO2s ability to cool in this manner should be a linear function of its concentration in the atmosphere.”
If that was your point, then you haven’t succeeded.
We agree that …
* Yes, CO2 can receive energy from O2.
* Yes, CO2 can radiate energy to space.
* Yes, Sphere B will cool faster.
But …
1) There is no reason to expect this to be linear. For small enough concentrations and short enough distances, the effect would be approximately linear. But as the length and/or concentration increased you get a “saturation” effect (and you should (at least approximately) exponentially approach some maximum emission of IR). At some point, the CO2 can’t radiate to a greater degree because CO2 molecules near the outside block IR that was emitted by the inner CO2 molecules. For something as small as a 1 m sphere, this saturation effect would (I strongly suspect) be minimal, but for the earth’s atmosphere, the IR that gets emitted to space by CO2 comes from near the top of the troposphere. Adding more CO2 will not make significantly more IR come out.
2) This is more subtle, but you can only say that CO2 in the atmosphere has an “ability to cool” the earth in a very restrictive sense. It’s like having a bank of bright lights and setting some dimmer lights in front of that panel. Yes, the dim lights are still providing some light, but they block the light that would have been coming from the brighter lights behind them. The net effect is of these “extra lights” to make things darker!
The warm earth is the “bank of bright (IR) lights” (which is missing in your analogy with the spheres, and hence invalidates many of the conclusions you might try to draw). The cool CO2 is the “dimmer lights in the way”. Yes, the CO2 emits IR which “cools” the earth, but the net effect is to make the earth “darker” for outgoing IR radiation (as you can clearly see in satellite IR measurements). In the case of the earth, the “total IR emitted” must eventually equal the total sunlight absorbed. So on the earth, the “bright panel of lights” have to get turned up (ie get warmer) until the outgoing light is the same as it would have been with out the “dim bulbs” blocking the light.
Or put another way, the CO2 in the atmosphere cools the earth, but it does that cooling so poorly (compared to the warmer surface behind it), that the net effect is to warm the earth!
”
Hockey Schtick says:
July 24, 2012 at 7:38 pm
A number of commenters claim the so-called location of outgoing radiation is moved up due to GHGs
1. Where is the observational evidence of this?
2. Why do theoretical calculations on MODTRAN show the temperature profile of the atmosphere in the troposphere and stratosphere does not change at all between CO2 concentration inputs of 350 and 3500?
http://forecast.uchicago.edu/Projects/modtran.html
”
************************
at the simplest level, one would expect it to. In reality, under clear skies most of it originates from the surface and the rest is smeared across the altitudes. Under cloudy skies, most originates from the cloud surface and the rest from the altitudes above that.
I believe the project giving observational information is ARM. It provides rather interesting graphs that show blackbody curves of various temperatures and shows where the absorption occurs is really just emission at lower temperatures.
MODTRAN calculator is a simple program that provides limited abilities to alter the inputs. I doubt they do a conservation of energy calculation for the air column nor would it be accurate without the inclusion of convection. for what it is, the MODTRAN calculator is a nice program that can provide a nice bit of information. However, it does not convey any serious information one way or the other when it comes to GW, much less prove AGW or CAGW.
just remember, all charletons that peddle their electric or magnetic cures for dropsy, cancer, and the common cold have some sort of actual scientific trappings or demo equipment to generate electricity and magnetism to convince the rubes that it’s not just talk. The first digital computer I ever saw as a kid was at a carnival side show – telling fortunes. Mine was that I’d grow up tall rich and famous. It was only 25% accurate. It got the part right about growing up. LOL
Hockey Schtick says:
July 24, 2012 at 7:38 pm
2. Why do theoretical calculations on MODTRAN show the temperature profile of the atmosphere in the troposphere and stratosphere does not change at all between CO2 concentration inputs of 350 and 3500?
Because you omitted to adjust your initial conditions. Try again but this time adjust the ‘ground T offset’ until you get the same ‘Iout’, otherwise you’re forcing it to have the same profile.
In light of the discussions re: TOA, WRT: CO2, temperature, thermosphere, etc. I will drag this out again, since it never seems to be part of the discussion, yet is observationally based and makes some interesting points, eg. cooling thermosphere (2°K attributable to CO2), shrinking of thermosphere due to low UV output, etc.
Shrinking atmospheric layer linked to low levels of solar radiation
AGU Release No. 10–28
26 August 2010
For Immediate Release
WASHINGTON—Large changes in the Sun’s energy output may cause Earth’s outer atmosphere to contract, new research indicates. A study published today by the American Geophysical Union links a recent, temporary shrinking of a high atmospheric layer with a sharp drop in the Sun’s ultraviolet radiation levels.
The research indicates that the Sun’s magnetic cycle, which produces differing numbers of sunspots over an approximately 11-year cycle, may vary more than previously thought.
“Our work demonstrates that the solar cycle not only varies on the typical 11-year time scale, but also can vary from one solar minimum to another,” says lead author Stanley Solomon, a scientist at the National Center for Atmospheric Research’s High Altitude Observatory. “All solar minima are not equal.” Researchers from the University of Colorado at Boulder (CU) also contributed to the project.
The findings may have implications for orbiting satellites, as well as for the International Space Station. The fact that the layer in the upper atmosphere known as the thermosphere is shrunken and less dense means that satellites can more easily maintain their orbits. But it also indicates that space debris and other objects that pose hazards may persist longer in the thermosphere.
“With lower thermospheric density, our satellites will have a longer life in orbit,” says CU professor Thomas Woods, a co-author. “This is good news for those satellites that are actually operating, but it is also bad because of the thousands of non-operating objects remaining in space that could potentially have collisions with our working satellites.”
The Sun’s energy output declined to unusually low levels from 2007 to 2009, a particularly prolonged solar minimum during which there were virtually no sunspots or solar storms. During that same period of low solar activity, Earth’s thermosphere shrank more than at any time in the 43-year era of space exploration.
The thermosphere, which ranges in altitude from about 90 to 500 kilometers (55 to more than 300 miles), is a rarified layer of gas at the edge of space where the Sun’s radiation first makes contact with Earth’s atmosphere. It typically cools and becomes less dense during low solar activity. But the magnitude of the density change during the recent solar minimum appeared to be about 30 percent greater than would have been expected by low solar activity.
The study team used computer modeling to analyze two possible factors implicated in the mystery of the shrinking thermosphere. They simulated both the impacts of solar output and the role of carbon dioxide, a potent greenhouse gas that, according to past estimates, is reducing the density of the outer atmosphere by about 2 percent to 5 percent per decade.
Their work built on several recent studies. Earlier this year, a team of scientists from the Naval Research Laboratory and George Mason University, measuring changes in satellite drag, estimated that the density of the thermosphere declined from 2007–2009 to about 30 percent less than that observed during the previous solar minimum in 1996. Other studies by scientists at the University of Southern California and CU, using measurements from sub-orbital rocket flights and space-based instruments, have estimated that levels of extreme-ultraviolet radiation—a class of photons with extremely short wavelengths—dropped about 15 percent during the same period.
However, scientists remained uncertain whether the decline in extreme-ultraviolet radiation would be sufficient to have such a dramatic impact on the thermosphere, even when combined with the effects of carbon dioxide.
To answer this question, Solomon and his colleagues used a computer model to simulate how the Sun’s output during 1996 and 2008 would affect the temperature and density of the thermosphere. They also created two simulations of thermospheric conditions in 2008—one with a level that approximated actual carbon dioxide emissions and one with a fixed, lower level.
The results showed the thermosphere cooling in 2008 by 41 kelvins (about 74 degrees Fahrenheit) compared to 1996, with just 2 K attributable to the carbon dioxide increase. The results also showed the thermosphere’s density decreasing by 31 percent, with just 3 percent attributable to carbon dioxide. The results closely approximated the 30 percent reduction in density indicated by measurements of satellite drag.
“It is now clear that the record low temperature and density were primarily caused by unusually low levels of solar radiation at the extreme-ultraviolet level,” Solomon says.
Woods says the research indicates that the Sun could be going through a period of relatively low activity, similar to periods in the early 19th and 20th centuries. This could mean that solar output may remain at a low level for the near future.
“If it is indeed similar to certain patterns in the past, then we expect to have low solar cycles for the next 10 to 30 years,” Woods says.
The study, published in Geophysical Research Letters, was funded by NASA and by the National Science Foundation.
paulinuk says:
July 23, 2012 at 9:41 am
Tim Folkerts “Yes, if you look closely enough you can find IR from room temperature N2 and O2, but it is orders of magnitude less intense than the IR from CO2, O3, CH4, etc”.
Thanks Tim, but you havn’t given me experimental evidence to back that up.The experiment I was considering was impracticable but this is more manageable: create a vacuum in a metal chamber cooled down to near absolute zero (like deep space); introduce a jet of diatomic N2 at 15c into the chamber and measure the watts of IR coming off the the jet . Do the same for triatomic Co2. You say CO2emission of IR is orders of magnitude higher, could be, I’m not sure about that.
I am, Tim’s right. Go to Hitran, look at the line browser for N2, you’ll see a solitary band just below 4 microns with a peak intensity ~10^-28. Do the same for CO2 you’ll see bands covering all wavelengths from 0.5-50 microns many of which exceed 10^-25 and some (including the 15 micron band) exceed 10^-19, i.e. nine orders of magnitude!
Your experiment has a flaw however, the N2 will most likely emerge as a liquid spray!
I saw a very dramatic illustration of this when a large tank of N2 at 3500psi outside my building blew its safety valve sending a very impressive, supersonic spray of liquid N2 tens of feet in the air, it lasted long enough for the fire brigade to arrive. 🙂
cba says:
July 24, 2012 at 6:19 am
Note that only about 2/3 of this is ghgs and clouds and aerosols etc. make up the other 1/3 and also that only 0.61 or 61% (239/391) of what leaves the surface escapes to space.
Your whole analysis is flawed because it assumes that the marginal effect of adding the next ppm of CO2 is the same as the average effect of all the existing CO2 which is clearly not correct.
It would be the same as my assuming that my retirement fund will earn at the same rate next year as the average of the last 30 years!
Steve Keohane says
http://wattsupwiththat.com/2012/07/21/some-thoughts-on-radiative-transfer-and-ghgs/#comment-1042947
that is interesting, especially in the light of my own results
http://wattsupwiththat.com/2012/07/21/some-thoughts-on-radiative-transfer-and-ghgs/#comment-1040910
\
if you can follow what my results mean:
we are on a path of (natural) global cooling, most probably due to increasing ozone (due to that shrinkage?)
I guess it will last until 2045, give or take one or two years
I HOPE NONE OF YOU GUYS HERE ARE GOING TO CLAIM NOW THAT THIS COOLING MUST BE AGC?
Phil: “Go to Hitran, look at the line browser for N2, you’ll see a solitary band just below 4 microns with a peak intensity ~10^-28. Do the same for CO2 you’ll see bands covering all wavelengths from 0.5-50 microns many of which exceed 10^-25 and some (including the 15 micron band) exceed 10^-19, i.e. nine orders of magnitude!”
Hitran is a computer program used to calculate spectral line emissions due to internal changes in a molecule or atom? Does it calculate broadband thermal emission solely due to the temperature of the gas as well ( ie the motion of molecules, containing charge, relative to one another, the blackbody type)? That’s the type I’m interested in, the temperature dependent type .
cba says “I believe the project giving observational information is ARM”
Do you or anyone have a link to a published paper of observations that show increased GHGs raise the “location” and thereby lower the temp of outgoing radiation?
paulinuk says:
July 25, 2012 at 10:45 am
Phil: “Go to Hitran, look at the line browser for N2, you’ll see a solitary band just below 4 microns with a peak intensity ~10^-28. Do the same for CO2 you’ll see bands covering all wavelengths from 0.5-50 microns many of which exceed 10^-25 and some (including the 15 micron band) exceed 10^-19, i.e. nine orders of magnitude!”
Hitran is a computer program used to calculate spectral line emissions due to internal changes in a molecule or atom? Does it calculate broadband thermal emission solely due to the temperature of the gas as well ( ie the motion of molecules, containing charge, relative to one another, the blackbody type)? That’s the type I’m interested in, the temperature dependent type .
Gases don’t emit as blackbodies at atmospheric conditions, spectral line emissions are temperature dependent however.
”
Phil. says:
July 25, 2012 at 9:43 am
cba says:
July 24, 2012 at 6:19 am
Note that only about 2/3 of this is ghgs and clouds and aerosols etc. make up the other 1/3 and also that only 0.61 or 61% (239/391) of what leaves the surface escapes to space.
Your whole analysis is flawed because it assumes that the marginal effect of adding the next ppm of CO2 is the same as the average effect of all the existing CO2 which is clearly not correct.
It would be the same as my assuming that my retirement fund will earn at the same rate next year as the average of the last 30 years!
”
**************
No! you have missed something critical. This has nothing to do with ppm of co2 other than the doubling from the rise of the industrial results in a change of power absorption of 3.7 W/m^2 – using the standard accepted value. What it does assume is the same assumption as made by the ipcc which is 1 W/m^2 of forcing is the same as some other cause of a 1 W/m^2 change. I am using the averages for sensitivity but suffice to say that if the next 1 W/m^2 change in absorption does not cause roughly the same change in temperature as any other, then there is no such thing as a sensitivity and that the ipcc is fatally flawed in a fundamental assumption which negates their whole theory’s foundation. Also, since adding W/m^2 to the total is associated with increasing T, then the fractional changes of both will become smaller as more W/m^2 are added and the temperature rises which suggests that any changes now should be less than those changes at lower temperatures and power absorption levels. And, finally, if there is significant variation from this average such that there is higher sensitivity, it means there are a lot of W/m^2 related temperature increases that must be LESS sensitivity than the average and as mentioned in the last sentence, it is more believable that the most recent changes in W/m^2 wll result in the least effect. That is essentially that the last added W/m^2, if different from the average should be lower in sensitivity than that of the first few or middle few.
As for your retirement fund, it is producing artificially low results due to conscious decisions from governments to screw you in order to spend more money they do not have. Or put another way, the socialists are running out of other people’s money.
“””””…..paulinuk says:
July 25, 2012 at 10:45 am
Phil: “Go to Hitran, look at the line browser for N2, you’ll see a solitary band just below 4 microns with a peak intensity ~10^-28. Do the same for CO2 you’ll see bands covering all wavelengths from 0.5-50 microns many of which exceed 10^-25 and some (including the 15 micron band) exceed 10^-19, i.e. nine orders of magnitude!”
Hitran is a computer program used to calculate spectral line emissions due to internal changes in a molecule or atom? Does it calculate broadband thermal emission solely due to the temperature of the gas as well ( ie the motion of molecules, containing charge, relative to one another, the blackbody type)? That’s the type I’m interested in, the temperature dependent type ……”””””
PaulinUK, as you said, Hitran is a computer program used to calculate “spectral line emissions” (absorptions too) due to atomic or molecular structure; ergo it is based on quantum mechanics.
Black body radiation on the other hand has nothing whatsoever to do with quantum mechanics, or for that matter with atomic or molecular structure, or even with atoms or molecules.
For starters, a “Black Body” as used in science discussions, is a completely fictitious and fictional object, that does not exist anywhere, nor have any experimental data been gathered of such a contraption since none exist, or ever have existed.
So the mathematical theories of black body radiation, going back to Sir James Jeans, and Lord Raleigh, and of course Wiens, and probably earlier, and eventually Max Planck, are statistical mechanical classical physical discussions of a hypothetical object that totally absorbs ALL electromagnetic radiation that falls on it, in terms of a structure of that body simply consisting of “particles” which are in mechanical collision with each other under the laws of Newtonian mechanics (dynamics); which processes manifest themselves as the “Temperature” of that collection of particles. Concepts such as translational and rotational degrees of freedom of such particles (three of each), as well as the concept of equipartition of energy (average), assigning 1/2 kT to each such DOF, and the statistical distributions of such, were studied to create the various theories of how the spectrum of emission and absorption from such bodies should look. There isn’t even any implied mechanism for why energy should be absorbed or emitted; well because the device is totaly fictional anyway.
Planck of course put the icing on the cake, by simply insisting that emission or absorption at ANY frequency or wavelength must consist of an integral number of units of hf (or nu) at that specific frequency.
Note that this does NOT quantize the energy into discrete spectral lines; ANY frequency is possible, so ANY PHOTON ENERGY is possible, so there is nothing to relate to any physical atom or molecule or its electronic structure.
As you point out this hypothetical emission is entirely a consequence of TEMPERATURE which requires only that there be a very large number of PARTICLES IN CONSTANT COLLISIONS WITH EACH OTHER, which is after all why we call it THERMAL RADIATION when talking about actual real physical emissions of EM radiation from real bodies INCLUDING ORDINARY NEUTRAL GASES..
Despite the protestations of Myrrh and others; “Thermal Radiation” has nothing to do with the human sensory mechanism of feeling warm. The 2.7 Kelvin microwave background radiation is “Thermal radiation” and it isn’t going to warm diddley squat, except maybe somebody’s cryostat at say 5 microKelvins.
The magnificence of black body radiation is that something so non existent, can form one of the most important planks of modern Physics.
But no, HITRAN cannot compute thermal radiation even from atmospheric gases, because thermal radiation doesn’t have anything to do with quantum mechanics, or electron energy levels, and molecular structure; it is purely a consequence of Hertz/Maxwellian theory of electromagnetism, and plain ordinary Newtonian mechanics of electric charge carrying bodies in collision.
I explained how all that happens some time ago somewhere here at WUWT. As I recall, I made a screwup, in that which even Phil, did not call me on. I mentioned the Biot-Savart law as being germane to the subject (maybe it is), but that was a misconstrusion due to advancing old age, and was an incorrect reference. The law which I intended to refer to, which I mistakenly referred to as the BS law (the real one) is the law of electrostatics, that says there is no net effect inside a closed conductor carrying an electric charge. The same principle aplies to gravitation, so as you go deeper into the earth, the shell of mass external to you (earth radius wise) produces no net gravitational effect on you, so you get lighter as you dig deeper.
The electric effect applies to thermal radiation origins, because neutral atoms in free flight (between collisions) look roughly like a spherical electron charge cloud, similar to the charged conductor, and inside that cloud of course you have an atomic nucleus which is also charged; but which must be essentially unaware of the existence of that electron cloud, since there is no net electric field due to the electrons, inside the cloud to affect the nuclear charge.
But once atoms come into collision range, then of course all hell breaks loose, and both the electron clouds, and the nuclei, undergo accelerations and decelerations, and of course distortion in the case of the electron cloud, and per Hertz/Maxwell, that is going to create a giant radio station that will broadcast EM programming for eons, until the two colliding atoms take leave of each other, and go their separate ways, and then the station goes off the air to eventually fire back up again on another channel, when the next collision takes place.
Compared to the first 10^-43 seconds after the big bang, when all the interesting archeo-Physics happened, the broadcasting era of a couple of atoms in collision at +Kelvin Temperatures, is almost an eternity of interesting phenomena.
George E. Smith; says:
July 25, 2012 at 12:42 pm
I will quibble a small amount with your comment in two areas. In heat transfer, thermal radiation is defined and microwaves, UV, X-rays etc are not thermal radiation. Second solids such as metals which give off thermal radiation don’t have particles in constant collisons. They are in a lattice structure that vibrates, but the particles don’t necessarily collide. Electrons can move from lattice to lattice within the solid.
Although I don’t follow Myrrh his comment about thermal radiation was seconded by an astrophysist at NASA on an episode of “Universe”. So he can be forgiven for that.
”
Hockey Schtick says:
July 25, 2012 at 11:22 am
cba says “I believe the project giving observational information is ARM”
Do you or anyone have a link to a published paper of observations that show increased GHGs raise the “location” and thereby lower the temp of outgoing radiation?
”
************************
They do show differences between areas with little and lots of h2o vapor and different surface temperatures. What one sees is bands of wavelengths which are lower in magnitude than the theoretical power curve for the surface where the surface basically matches the power curve in areas not subject to ghg absorption. The lower magnitudes for the bands correspond to a temperature curve that is for a lower temperature such as at some altitude. The whole thing is not a bb curve at some temperature and hence some altitude. These can be calculated and constructed that look rather close to the measured values. Good luck trying to find very small changes like a 3.7 W/m^2 increase in absorption due to a co2 doubling (using the calculated ones). I doubt the measurements exists to permit one to look at two measured curves with enough difference in co2 concentrations to really see anything.
The whole thing about ghgs raising the location and lowering the T of the outgoing radiation is a nonreal hansen dellusion with no meaning. For clear sky you will have a bb curve with ground temperature and with some missing chunks that are actually reduced temperature emissions. For cloudy skies you will have the surface temperature of the cloud tops with much smaller amounts of absorption chunks missing because most of the ghg effects are below the cloud tops.
Rough balance dictates there be about 239 W/m^2 escaping from the Earth/atmosphere to match the 239 W/m^2 of incoming solar power absorbed by the Earth/atmosphere system. Looking at clear skies, one finds about 265 W/m^2 escaping from the average surface T of 288.2k. You’ll note that cloudy skies have to be somewhat below 239 W/m^2 in order for the average to come up as 239 W/m^2 with about 62% cloudy skies. On average, this would be about 223 W/m^2 which would correspond to about -22 deg C if there were no ghg absorption occurring about here. It’s prbably closer to around 273k for the average cloud top and that there is probably around 80 to 100 w/m^2 of absorption – but these are extremely crude guesses just to complete the rough idea.
George E. Smith +2
George E. Smith says: “Black body radiation on the other hand has nothing whatsoever to do with quantum mechanics … “
Say what!?
Black body radiation was the START of quantum mechanics! Planck’s efforts to understand BB radiation lead him to propose quantization of energy. Without quantum mechanics, you invariably get the “ultraviolet catastrophe”.
“For starters, a “Black Body” as used in science discussions, is a completely fictitious and fictional object, that does not exist anywhere, nor have any experimental data been gathered of such a contraption since none exist, or ever have existed.”
I suppose you could say this, in the same way you could say there is no “uniform sphere” ( they are made up of discrete atoms) or “frictionless surface” (there is always SOME friction) or “AC current in the form of a sine wave” (sine waves are infinite, but all currents have to start and stop sometime). But these are still extremely useful approximations.
The fact is, there are surfaces that are close enough to black bodies that there is no experimental way to measure the imperfections — small openings into large cavities . You might look up “cavity radiation”.
“it [BB radiation] is purely a consequence of Hertz/Maxwellian theory of electromagnetism, and plain ordinary Newtonian mechanics of electric charge carrying bodies in collision.”
Not even close. Classical physics utterly fails to explain BB radiation. It leads to the “ultraviolet catastrophe”.
“As you point out this hypothetical emission is entirely a consequence of TEMPERATURE which requires only that there be a very large number of PARTICLES IN CONSTANT COLLISIONS WITH EACH OTHER, which is after all why we call it THERMAL RADIATION when talking about actual real physical emissions of EM radiation from real bodies INCLUDING ORDINARY NEUTRAL GASES.
No. The emission of photons is NOT “entirely” a consequence of temperature. Temperature is only half of the story. The other half is the quantum mechanical properties of the material.
Materials can only emit or absorb photons with energies corresponding to energy changes within the material.
For hydrogen atoms, these are the quantized energies of the electron jumping between levels, as given by the Bohr Model (as predicted by quantum mechanics).
For other monatomic gases with more electrons, the electrons can jump in to lots of different levels allowing lots of different wavelengths (as predicted by quantum mechanics).
For molecules, you add in bending and spinning, giving a whole new set of energy levels (you guessed it — as predicted by quantum mechanics).
For solids, the the discrete energy levels get spread out into bands, allowing them to potentially absorb photons over a wider set of frequencies (based once again on quantum mechanics).
* The material determines WHICH energies of photons can be emitted.
* The BB radiation curve determines the MAXIMUM intensity of photons that can be emitted at each frequency.
You need to know BOTH the temperature AND the the quantum mechanics of the material to predict the thermal radiation.
For example, CO2 emits and absorbs pretty well near 15 um. So warm CO2 will emit 15 um photons at rates approaching those of an ideal BB. CO2 emits poorly near 8 um. So warm CO2 will emit 8um photons at rates much below those of an ideal BB.
N2 emits poorly throughout the IR wavelengths, so it will emit photons much at rates much below those of an ideal BB at ANY IR wavelengths. Yes, N2 (or H2 or O2) emits IR “thermal radiation”, but at rates so much below those of CO2 or H2O or the ground so as to be insignificant.
I still can’t understand why anyone with common sense would say a big ball of nitrogen,hydrogen or oxygen gas in space at say 1,000c wouldn’t burn your backside off if you were daft enough to get close to it but CO2 would.
Tim Folkerts,
You asked about the next question. Here is Question 2. Imagine an alternate Earth. Earth 2 has the same atmosphere as Earth 1, but one small difference at the surface. The surface of Earth 2 emits no IR at 15 microns.
At TOA, will Earth 2 either-
A. Emit no IR in the 15 micron band.
B. Emit the same amount of 15 micron IR as Earth 1.
C. Emit a smaller amount of 15 micron IR than Earth 1.
D. None of the above.
paulinuk says: July 25, 2012 at 2:17 pm “I still can’t understand why anyone with common sense …”
As someone who has studied and taught physics over the years, I can assure you that people’s “common sense” is notoriously poor and should not be counted on for discerning anything but the simplest of physics problems.
Even basic Newtonian mechanics defied common sense until a few hundred years ago (and still defies the common sense of many people). Throw in quantum mechanics (of molecular vibrations and photons) and you can pretty much figure that common sense is of no use.
“Here is Question 2….”
B. The outgoing IR would be the same (at least to a very good approximation). No 15 um photons from the surface escape to outer space. All of the 15 um photons that do escape are already coming from cool CO2 in the upper troposphere, so the spectrum as seen from high above the earth would be the same.
To extend the “bank of lights” analogy from above, you turned off one of the lights (ie the light that shines 15 um photons) that was already being blocked by a dimmer light above it. Whether or not that particular “light” at the surface is on or off makes no difference.
“””””…..mkelly says:
July 25, 2012 at 1:34 pm
George E. Smith; says:
July 25, 2012 at 12:42 pm
……”””””
Electromagnetic radiation is involved in HEAT TRANSFER in exactly the same manner that grocery shopping carts are involved in HEAT TRANSFER. Both are processes for transporting ENERGY. In the first case the energy is conveyed by an electromagnetic field wave, which knows absolutely nothing about TEMPERATURE, which occurs precisely nowhere in Maxwell’s equations, so EM radiation can’t transport “heat”, only ENERGY. We get NO HEAT from the sun; we make it ALL here on earth by wasting the incoming EM energy .
The grocery cart transports HEAT in the form of presto logs to throw in your fireplace, or steak, to munch on, or alcohol to wake you up. Those things themselves are not heat; they just contain energy which can be converted into waste heat. EM radiation isn’t heat either, it’s energy that can be wasted in many ways to result in heating.
By the way, what is it that locates the atoms in those solids, so they stay in those nice lattices, and stops them from colliding with each other. ?
“””””…..Tim Folkerts says:
July 25, 2012 at 2:12 pm
George E. Smith says: “Black body radiation on the other hand has nothing whatsoever to do with quantum mechanics … “
Say what!?
Black body radiation was the START of quantum mechanics!…..”””””
Well Tim , you got into this by yourself, so I’ll let you enjoy it.
Seems to me that MATTER which IS ENERGY was quantized, long before Max Planck ever heard of quantum mechanics, which had to wait till after wave mechanics came along.
So we see that Max was the CO2 of black body radiation. He used quantum mechanics to explain BB radiation, even before quantum mechanics existed.
How many quantum numbers are involved in the Planck quantum mechanics of black body radiation ?
“””””…..No. The emission of photons is NOT “entirely” a consequence of temperature. …..”””””
Well you have never heard or read me EVER saying that Tim; to recapitulate,
from Tim Folkerts…..”No. The emission of photons is NOT “entirely” a consequence of temperature. ”
from George E. Smith…..”As you point out this hypothetical emission is entirely a consequence of TEMPERATURE which requires only that there be a very large number of PARTICLES IN CONSTANT COLLISIONS WITH EACH OTHER…”
Tim, “>>>THIS<<>>THE<<< EMISSION OF PHOTONS". Words have meaning; use MY words; they mean what I say.
The first is a restricted special case; even a hypothetical one. The second is an all inclusive general case.
The first (black body radiation) is entirely a consequence of Temperature; the second that you cite of molecular and atomic electron energy level changes, is first order quite unaffected by Temperature; but is dependent on material properties.
BB radiation is quite independent of material properties; being fictional there is no prescribed material it applies to.
Planck's quite arbitrary hypothesis that the energy at some radiated frequency be in chunks of some integer multiple of THAT frequency, did nothing to restrict the possible radiated frequencies (or absorbed); all frequencies are possible, as are all energies.
Quantum mechanics restricts both the frequencies and the energies to only certain allowed values. All other values are forbidden.
I make it a rule to never stand between someone and the edge of the cliff they want to charge over.
@HenryP says, July 25, 2012 at 10:44 am
http://www.agu.org/journals/gl/gl0419/2004GL021042/2004GL021042.pdf
http://asd-www.larc.nasa.gov/~kratz/ref/p28jgr.pdf
http://www.agu.org/journals/gl/gl1115/2011GL048061/2011GL048061.pdf
“”””” u need to know BOTH the temperature AND the the quantum mechanics of the material to predict the thermal radiation……”””””
So we have one of these experimental near black body radiators Tim; you recommended I look up cavity radiation (I didn’t bother), and it is so close to a perfect black body that we can’t measure the discrepancy; I think you said something along those lines. So we have Planck’s exquisitely perfect formula for the black body radiation spectrum, containing nothing but fundamental constants of classical Physics, and an actual real faux black body made of actual quantum mechanical materials, which we haven’t even specified; apparently don’t need to know it’s so close to perfect. So now when do the quantum mechanical properties of these quite unknown and unspecified materials get to play their part in determining the radiation spectrum of our near but not exact simulated black body radiator.
How many possible ways, and material lists are there for building a near perfect black body radiator, all of which seem to behave the same regardless of their quite different quantum mechanical properties. Well it’s a rhetorical question Tim; no need to answer
I might come back to see who wants to join you.
Classical Physics/thermodynamics/chemistry is all that is required to analyze and explain: weather, global climate systems, heat flow, temperature differentials, and so forth. No blackbody exists in natrure anywhere period, and even with corrections to gray bodies, more are needed for the more true colored bodies. QM is not needed to be invoked at all for climate science or meteorology. Now for some of the cool underpinnings, wacky counter-intuitiveness of the small constituent world, or to make lasers for that matter, QM is then necessary. Nothing George E. Smith said is wrong. I would add mkelly’s minor corrections especially regarding solids like metals.
And of course there are many unknowns with climate because it would take tens of thousands of thermodynamics equations to truly understand the complexities/dynamic turbulent flows to actually arrive a true “global mean temperature” which we CANNOT do.
Hmm, not sure why WordPress eat the YouTube link. Here is the same link as https so WordPress hopefully won’t try to expand it: https://www.youtube.com/watch?v=EEFQHDSYP1I
Tim Folkerts, thanks for answering. Yes , B should be the answer to question 2. (C could also be correct if the small amount of 15 um IR that makes it directly to space from the surface of Earth 1 is taken into account).
Question 3 references Question 2. An alternate Earth 3 is the same as Earth 2, except that it has double the amount of O2 in the atmosphere.
At Earth 3 TOA, is the amount of outgoing 15 um photons –
A. The same as Earth 2.
B. Greater than Earth 2.
C. Less than Earth 2.
George, I suspect a lengthy debate about quantum mechanics will not be productive here. I suspect we both understand it pretty well. Let’s start by what I think we can agree on.
I think we can agree that “ideal” BB thermal radiation (eg in an infinitely large, perfectly conducting cavity) is an abstract idea, that leads to thermal radiation with a specific mathematical form that depends only on temperature. (And if I misread your statements and thought you were discussing actual thermal radiation when you were thinking about Ideal BB thermal radiation, I apologize.)
I think we can agree that any actual materials (or actual cavities) will emit thermal radiation that is strictly less than the BB radiation at any every wavelength.
I hope we can agree that the ground is pretty close to the the ideal BB curve for thermal IR; CO2 is close to the ideal BB curve for a few bands; N2 is never close to the the deal BB curve. (This is all that really matters for the climate discussions.)
There are several finer points where I would disagree with you (eg the wavelengths are indeed quantized in Plank’s theory but the size of the cavity, but they are typically so closely spaced that it doesn’t really matter; or that the mere fact that atoms are quantized implies that they are part of “quantum machanics”). These sorts of subtle ideas would require a face-to-face meeting to explain just what we mean and what assumptions we are making, and then to iron out any remaining differences.
And none of these fine points are particularly critical.
~~~~~~~~~~~~~~~~~~~~~~~~~~~~~~~~~~~~~~~~~~~~~~~~~~~
There IS one other place where do I take exception to your writing. It is mostly a matter of semantics, but you say “We get NO HEAT from the sun; we make it ALL here on earth by wasting the incoming EM energy .”
“Heat” is a very problematic concept, but traditionally in thermodynamics “heat” means “energy in transit due to a temperature difference” or “the processes that transfer energy from one system to another system due to differences in temperature” — which includes conduction, convection, AND radiation.
* There is no heating of the earth by the sun via conduction.
* There is no heating of the earth by the sun via convection.
* There IS heating of the earth by the sun via radiation.
So thermal photons would traditionally be considered “heat” (whereas fluorescence or lasers would not).
I’ll play one more round anyway … “An alternate Earth 3 is the same as Earth 2, except that it has double the amount of O2 in the atmosphere. “
C: At Earth 3 TOA, is the amount of outgoing 15 um photons is LESS THAN Earth #1 or #2. The CO2 will remain reasonably well mixed, so the “N” moles of CO2 that make up the “top layer” and do most of the radiating will be be higher than before. Assuming the lapse rate is such that this new higher altitude is colder than the old, lower altitude, then the new, cooler “top layer” of CO2 will radiate less.
Presumably the other wavelengths will get more intense to make up the difference (ie the ground will get a little warmer.
(Now we get into potential secondary but important issues. For example, the inversion due to Ozone in the stratosphere. I am ignoring such other issues.)
Tim Folkerts, at post before your latest, you said:”The CO2 will remain reasonably well mixed, so the “N” moles of CO2 that make up the “top layer” and do most of the radiating will be be higher than before. Assuming the lapse rate is such that this new higher altitude is colder than the old, lower altitude, then the new, cooler “top layer” of CO2 will radiate less.”
Here’s some data on lapse rates from Griggs & Harries JoC 2007, Appendix Tables.
hPa T’ Ko 1970T Ko 1997T Ko 2003
1000 299.234 299.463 299.078
925 293.872 294.585 293.833
850 290.151 290.967 289.44
700 283.428 283.511 283.67
600 276.122 275.883 276.554
500 268.464 268.284 268.411
400 257.439 256.92 258.319
300 242.044 241.594 242.514
250 232.201 232.005 232.594
200 220.713 220.895 221.39
150 207.153 207.425 207.834
100 192.001 192.001 195.882
56.866 204.475 204.756 203.165
29.5643 217.385 219.476 215.503
14.792 228.791 228.063 227.923
4.60588 246.354 239.277 239.19
The Temps at tropsheric altitudes at and above 300 seem slightly warmer in 2003 than in 1970 and 1997, but cooling at hPa less than 100. Within each series the trends per hPa are negative at a faster rate in 2003 than in the earlier years:
1970: y = -6.126x + 299.56
R² = 0.7052
1997: y = -6.2822x + 300.59
R² = 0.7391
2003: y = -6.296x + 300.72
R² = 0.7538
What’s your take?
George says: “So we have one of these experimental near black body radiators Tim; you recommended I look up cavity radiation (I didn’t bother),”
But “cavity radiation” IS the way Planck derived the equations for BB radiation. I can’t decide if you feel you already know this topic (in which case you should know that the frequencies ARE quantized by the cavity) or that you feel it is not worth your time to understand the topic you are trying to explain.
“and it is so close to a perfect black body that we can’t measure the discrepancy; I think you said something along those lines.”
Yep .. i said that.
“So we have Planck’s exquisitely perfect formula for the black body radiation spectrum, containing nothing but fundamental constants of classical Physics…”
What? Planck’s constant is the EPITOME of non-classical physics! It is the key parameter that universally shows up in modern quantum physics. Planck was expecting that “h” would be a mathematical trick that he could eventually eliminate by letting it go to zero. It turned out that the constant named after him had to have one particular value, demolishing classical physics and ushering in quantum mechanics.
“and an actual real faux black body made of actual quantum mechanical materials, which we haven’t even specified; apparently don’t need to know it’s so close to perfect. ”
The whole point of cavity radiation is that the materials DON’T matter! Here are some pictures of “cavity radiators”:
* a beverage can: http://www.hoax-slayer.com/images/soda-can-top.jpg
* sewer grate: http://fc09.deviantart.net/images/i/2003/43/f/6/Sewer_Grate_01.jpg
* a hole in a pipe: http://www.mcgee-flutes.com/images/ClintHole.JPG
* an electric outlet: http://www.greenbang.com/wp-content/uploads/2008/11/electric-outlet.jpg
Note that in every case, the opening looks perfectly matte black, independent of the material on the interior of the cavity. Light can’t reflect from the “surface of the opening” Since there is nothing physically there to reflect light, so all incoming light is “absorbed” into the cavity –> emissivity = 1. Even with fairly reflective aluminum, the hole looks black –> emissivity close to 1. For large holes (compared to the cavity) and reflective materials, the hole won’t look truly black; for a large cavity, a small opening, and a rough/black surface, you can easily get the emissivity arbitrarily close to 1. This has been the “gold standard” for thermal radiation for over a century.
“How many possible ways, and material lists are there for building a near perfect black body radiator, all of which seem to behave the same regardless of their quite different quantum mechanical properties. Well it’s a rhetorical question Tim; no need to answer. “
There should be no need to answer, since the answer obviously is “infinite”. A small hole into a cavity constructed out of any material will approximate a blackbody.
Tim Folkerts,
You answered C for question 3. In question 1 sphere 2 cooled faster due the presence of CO2. In question 2 the alternate Earth 2 atmosphere was able to cool more effectively due to the conductive transfer of energy to CO2 that then radiated it to space. Yet for Earth 3 you suggest that increased CO2 would reduce the atmospheres cooling ability.
The correct answer to question 3 is B. Given the imaginary absence of outgoing 15 um IR from the surface of Earth 3 the increased quantity of CO2 in the atmosphere increases the amount of outgoing photons at the 15 um energy level.
On Earth 1 however the surface emits 15 um IR. On Earth 1 CO2 can both cool and warm. Its initial effect should be warming. The original question posed by Reed Corays post heading this thread is when do the secondary cooling effects exceed these initial warming effects.
Konrad:”On Earth 1 however the surface emits 15 um IR. On Earth 1 CO2 can both cool and warm. Its initial effect should be warming. The original question posed by Reed Corays post heading this thread is when do the secondary cooling effects exceed these initial warming effects”
I haven’t really been following your discussion with Tim, nor the original questions in your discussion but I think it is clear the effect of CO2 is *always* warming under *our Earth’s* conditions when the surface is being heated *and* there is a gravitationally driven lapse rate. Cooling effects would be from the increased SB emission at the warmer temps plus any other negative feedbacks that are beyond the scope of this discussion.
It is fine to argue that CO2 has a cooling effect under certain (non-Earthlike) circumstances but doing so doesn’t establish that CO2 will have a cooling effect under Earthlike conditions.
Again, the GH effect is proposed to work because radiation from the Earth’s surface (after being warmed from the sun) cannot leave the atmosphere as quickly or easily as it would without the presence of GH gases because that energy on leaving the Earth’s surface is continually absorbed and re-emitted. This relative inefficient means of radiating energy to space *combined* with the fact that a gravitationally induced lapse rate pulls hot air towards the surface implies the existence of a GH effect (of some magnitude). It is all these properties of the Earth acting together that produce a GH effect.
Cheers, 🙂
AJB says
http://wattsupwiththat.com/2012/07/21/some-thoughts-on-radiative-transfer-and-ghgs/#comment-1043301
Henry says
sorry ,
very bad internet connection here (in South Africa)
I don’t yet have a clue as to what you are trying to say
trccurtin says
http://wattsupwiththat.com/2012/07/21/some-thoughts-on-radiative-transfer-and-ghgs/#comment-1043667
0.7 is not good enough
try getting it >0.95
http://www.letterdash.com/henryp/global-cooling-is-here
Konrad, you originally said “except that it has double the amount of O2 [not “CO2″] in the atmosphere. ”
But now you are saying “Yet for Earth 3 you suggest that increased CO2 would reduce the atmospheres cooling ability.”
So we are comparing apples and oranges. We have Question 3a -> Doubling O2; and Question 3b –> Doubling CO2. (Although the same answer still holds).
For Question 3a (O2) I stand by my conclusion stated previously.
For question 3b, there will STILL be warming. You incorrectly conclude The correct answer to question 3 is B. Given the imaginary absence of outgoing 15 um IR from the surface of Earth 3 the increased quantity of CO2 in the atmosphere increases the amount of outgoing photons at the 15 um energy level.”
The key point you overlooked is that a relatively short distance of CO2 will block all (>99.9%) of the 15 um photons.So the photons that leave the surface are all blocked by the first km of CO2.
* The photons that are heading up at 1 km altitude were created by CO2 ~ 1 km up, where the temperature will be ~ 10 C cooler than the surface.
*The photons that are heading up at 2 km altitude were created by CO2 ~ 2 km up, where the temperature will be ~ 20 C cooler than the surface.
…
There will be some top of atmosphere level (call it “TOA2” corresponding to Question 2) where there are no longer enough CO2 molecules higher up to block the outgoing 15 um photons. Looking down from above this altitude we will see the BB curve from the ground (somewhere around 300K), with “bites” missing where CO2 has blocked the ground’s IR. The “depth” of those bites will tell you the “TOA” temperature.
If you put in more CO2, you are not blocking any more of the ground’s 15 um photons. But you are putting more CO2 above the previous “TOA2” level, blocking some of those IR photons from that cold TOA2 level. The NEW TOA3 will be a bit higher up, and (due to the lapse rate) will be even COLDER than TOA2. Thus the 15 um photons emitted to space will be even weaker.
I know it sounds backwards, but MORE CO2 leads to LESS outgoing IR (because the IR is coming from colder places that radiate poorly)!
PS. There is a SECOND effect that will also reduce the outgoing IR. The “ragged edges” of the absorption band allow some ground IR photons near 15 um to escape. With more CO2, the “ragged edges” absorb more of the plentiful IR photons from the warm ground. These will be replaced by sparse IR photons from the cooler atmosphere..
PPS. At some point in a real atmosphere there could well be an inversion in the lapse rate. I am assuming the temperature continues to drop with altitude above TOA2. If not, then adding more CO2 would have little effect. (And given that the actual TOA on earth is not that far from the Tropopause, this is a significant concern in my opinion.)
PPPS. You can model all this yourself with MODTRAN. http://geoflop.uchicago.edu/forecast/docs/Projects/modtran.html I would suggest removing the water vapor to make the effects of CO2 more distinct. Try looking down from various altitudes from 0 to 20 km. You can see the “bites” getting deeper as the air gets colder. (You can also see the “ragged edge” and “tropopasue” effects if you pay attention.)
“””””…..Tim Folkerts says:
July 25, 2012 at 9:39 pm
George says: “So we have one of these experimental near black body radiators Tim; you recommended I look up cavity radiation (I didn’t bother),”
But “cavity radiation” IS the way Planck derived the equations for BB radiation. I can’t decide if you feel you already know this topic (in which case you should know that the frequencies ARE quantized by the cavity) or that you feel it is not worth your time to understand the topic you are trying to explain. …..”””””
Tim, rather than us slinging around a lot of chaff, I would like to simply present some assorted facts and notions for the consideration of anyone who wants to think openly about this question.
First MY position is that Blackbody Radiation is classical Physics, and is NOT quantum Mechanics.
You assert the exact opposite; that Blackbody radiation is the epitome of Quantum Mechanics.
So some demonstrable facts. I recently purchased a very complete Textbook Treatise on Quantum Mechanics, since ALL of my own personal high school and University Textbooks, were lost in shipping about 51 years ago; sad occurrence.
This modern very complete text on Quantum Mechanics Contains NOWHERE in its 400 or so pages, the word BLACKBODY, nor the combination BLACKBODY RADIATION. Odd that a highly regarded Reference Textbook, would contain not a word about something that is the epitome of its subject matter.
You mention cavity radiation, and assert that there are an “infinite” number of ways of building a very reasonable facsimile (I concur). Odd, that we both agree that a very good approximation to ideal blackbody radiation, is obtainable from an arbitrary shaped and dimensioned cavity made out of quite arbitrary real physical world materials, each of which has an entirely different set of electronic energy levels, and associated quantum numbers associated with it; none of which seem to have ANY influence on the absorbed or emitted radiation or its spectrum; yet those quantum number lists and tables are an essential part of the calculable molecular or atomic absorption/emission spectrum of those real materials, with programs like HITRAN for example.
Speaking of HITRAN, where in that program does one find the knob for TEMPERATURE, or PRESSURE, or DENSITY ? Can I use HITRAN to calculate the black/grey/blue/pink emission spectrum of say a solid piece of Cobalt, which is a metal solid, that Mr Kelly asserts does not contain atoms/molecules that can collide with each other.
I can recommend to anyone the two volume set of books called “The Constitution of Binary Alloys.” Which provides complete phase diagrams of every possible binary composition of the known stable elements; specially including, the Fe/C phase diagram, sometimes known as the “Steel” diagram, which is a well known sold metal, and contains multitudes of crystalline lattice types, all of which are actually chemical compounds of various composition; which normally would indicate that the atoms are already in permanent contact with each other so their electron clouds, are already mingled; to the point where many of those electrons simply take leave of their atom, and drift freely anywhere in those crystalline lattices.
Yes as a result of the intimate proximity of those atoms in a solid, their individual electron structures and energy levels interract to form bands of energy levels, such as the Valence Band or the Conduction Band in which the free electrons can roam. And yes, those band structures in both metals, and semi-conductors do result in energy and wavelength specific absorptions and emssions of DISCRETE SPECTRAL LINES, whose frequencies are first order quite Temperature independent. The do vary with Temperature to some extent, simply because the Temperature does affect the lattice constant, so the crystal can grow or shrink (thermal expansion) and that results in detail alteration of the energy band levels. There is NO WAY that such Temperature variable band structure shifts can result in very broad band black body or thermal radiation emission.
Sorry \, but solids DO emit line spectra (more like bands)that are only slightly second order Temperature dependent, but they also emit a complete continuum of quite non-quantized Thermal (black body like) radiation, as do all other materials that have a non zero Temperature.
Plasma Physicists, are well aware that plasmas (like the sun) emit continuum spectra, that result from the fact that free electrons of quite arbitrary and non-quantized energy (in the lab co-ordinate frame) can drop into an ionised atom, and end up at one of its defined electron quantized energy levels, and when you add (x) a continuous value to (n) an integer value, you still end up with (x+n) being a continuous value; NOT a quantized value.
It must seem odd to readers, that blackbody radiation; the epitome of quantum mechanics is quite independent of the specific quantum numbers of ANY real material, including that used to form a “Cavity Radiator”, and depends entirely on the Temperature of that source or cavity; while at the same time, the resonance line/band spectra of real materials, including both the GHGs, as well as the ordinary neutral gases, are intrinsically independent of the Temperature of the source material, and vary from the intrinsic line frequencies, simply as a result of ordinary classical Physics phenomena, such as the Doppler effect, of the lab space velocity of the emitting or absorbing molecules (Temperature broadening) and the mean time between collisions of the molecules (Pressure/Density Broadening).
It is important for the lay readers to understand the fundamental difference between the “GHG mode” of energy absorption or emission, that IS a direct consequence of the specific molecular/atomic electron structure; AND IS QUANTUM MECHANICS, and the quite different absorption and emission of a continuum of frequencies, that are not related in ANY way to the specific quantized energy level structures of the source materials, and ARE absorbed/emitted by ALL physical materials that have a Temperature, by means of processes that depend only on that Temperature, and not on the material or its quantum energy level properties.
Yes Planck DID propose that the radiated energies of “radiation” at ANY specific frequency (f) of radiation should be in integral multiples of hf, h being a constant he introduced, to be determined experimentally, BUT he did NOT specify that those FREQUENCIES themselves be quantized; they can be of ANY value, and hence so can the emitted energy packet energy, it is still a continuum spectrum, and in particujlar it is NOT a picket fence of closely spaced spectral lines, as are the real high resolution molecular band spectra of GHG absorptions/emissions.
So Tim, you assert that cavity radiation is quantized by the cavity. What specific physical properties of the cavity determine what those quantized energy levels are, since we apparently are in agreement that NOTHING about the specific nature of the cavity, either size or shape or material properties, seems to have ANY influence on the spectrum of the BB radiation, or its amount; the Temperature alone dtermines the entire spectrum.
Personally, I have a VERY limited knowledge of QUANTUM MECHANICS. I am fortunate to have the counsel of some folks, who are steeped in QM. In deference to those individuals, some known to almost everybody by sight (although unknowingly) , but not by name; I am not going to drop any names; but I did ask one such person with whom I am acquainted, just what QM could add to my simple classical Newtonian/Hertzian model of how BB radiation is emitted during atomic/molecular collisions due to Temperature; and his response was almost one of shock and horror. QM could add nothing, and only muddy the waters further. You see QM simply replaced causal deterministic classical Physics, with a set of possible outcomes, of which only the statisitical probabilities of each could be found, and that simply trying to observe the existence of any one of those happenstances, immediately caused all of the alternative possibilities to simply evaporate; no matter which of the possibilities one chose to test for.
Frankly, I have not found giggle/wiki/yoohoo/ faceback/whatever, nor their lookalikes to be very rigorous or useful, in studying any of these subject matters. I defy anyone to find on the web ANY fundamental Physical explanation for the actual originating mechanism for the emission of thermal radiation; excluding of course simple one liners like “acceleration of charge”; and no don’t read back to me, my own description of how it happens, that has been already posted here at WUWT.
But I’m not going to waste any more of Anthony’s valuable bandwidth, on this subject. I could run over to some Physics specific blog, and chat with others there (mostly academics); but I’d much rather try to help even one other person, to try and grasp some of this stuff; it’s all interesting, and mostly all understandable by anyone. As I have said; Ignorance is NOT a disease; we are all born with it. But stupidity has to be taught; and sadly the web is full of those willing and able to teach it. Fortunately there is still a good supply of those trying to help others understand; and Anthony has managed to attract many of those folks, who come here, and try to educate the rest of us.
George E. Smith; says on July 25, 2012 at 4:28 pm:
“I might come back to see who wants to join you.”
============
George E, will you come back to join us even if I am not going to join Tim?
I am the idiot or “super idiot” even – who does not believe radiation can be seen by the “human eye” or indeed by Mellony’s thermopile, or any modern derivative thereof. –
I have performed many experiments – in order to prove, or disprove – this “pesky” chameleon that goes under the name of “AGW”. My “experiments”, I must admit, are not always performed under “Ideal Laboratory Condition” (ILC). – However, I have for many years, proved – to myself at least. – That “cooling by radiation” – ( that is what we shall have to call it,) is “quite insignificant”
I do not know much about IR radiation and the “wave-bands” that locks it in, or makes it convenient for us to measure it, but by now I do understand that “Thermodynamics” and “Thermoradiationics” is/are not interchangeable sciences.
George E. Smith,
Just FYI, Wiki’s page for Planck’s law: “Classical physics provides an account of some aspects of the Planck distribution, such as the Stefan–Boltzmann law, and the Wien displacement law. Other aspects of the Planck distribution cannot be accounted for in classical physics, and require quantum theory for their understanding.”
http://en.wikipedia.org/wiki/Planck%27s_law
“So Tim, you assert that cavity radiation is quantized by the cavity. What specific physical properties of the cavity determine what those quantized energy levels are, since we apparently are in agreement that NOTHING about the specific nature of the cavity, either size or shape or material properties, seems to have ANY influence on the spectrum of the BB radiation, or its amount; the Temperature alone dtermines the entire spectrum.”
I’m not an expert here by any means but I believe it is its *shape* that accounts for the ability of a cavity to approximate a blackbody. Again from the wiki page: “Though perfectly black materials do not exist, in practice a black surface can be accurately approximated.[4] As to its material interior, a body is completely black to a certain wavelength if it is completely opaque to that wavelength; that means that it absorbs all of the wavelength that penetrates the interface to enter the body; this is not too difficult to achieve in practice. On the other hand, a perfectly black interface is not found in nature. The best practical way to make an effectively black interface is to simulate an ‘interface’ by use of a small hole in the wall of a large cavity in a completely opaque body, with a controlled temperature. Radiation entering the hole has almost no possibility of escaping the cavity without being absorbed by multiple impacts with its walls.[13]”
Cheers, 🙂
“””””…..Shawnhet says:
July 26, 2012 at 2:12 pm
George E. Smith,
Just FYI, Wiki’s page for Planck’s law: “Classical physics provides an account of some aspects of the Planck distribution, such as the Stefan–Boltzmann law, and the Wien displacement law. Other aspects of the Planck distribution cannot be accounted for in classical physics, and require quantum theory for their understanding.”
http://en.wikipedia.org/wiki/Planck%27s_law …..”””””
Total and utter balderdash. The Stefan-Boltzmann “law” is nothing more than the complete integral over all wavelengths, of the Planck black body spectrum equation.
How can one obtain SB from classical Physics, if one cannot obtain the spectrum except with quantum mechanics; and you can tell wiki I said that.
Tim Folkerts says:
July 26, 2012 at 11:05 am
Konrad, you originally said “except that it has double the amount of O2 [not “CO2”] in the atmosphere. ”
But now you are saying “Yet for Earth 3 you suggest that increased CO2 would reduce the atmospheres cooling ability.”
——————————————————————————————–
Tim,
My apologies, your original answer to question 3 as posted was correct. I had typed that comment from my “smart” phone and it had “corrected” CO2 to O2. Question 3 should have read –
“Question 3 references Question 2. An alternate Earth 3 is the same as Earth 2, except that it has double the amount of CO2 in the atmosphere.”
However you state “For question 3b, there will STILL be warming.”
There could be less cooling than Earth 2, but it is inconceivable that there could be warming on Earth 3 when 15 um photons are still escaping to space.
Imagine and alternate Earth. Earth 1.5 has no 15 um IR radiated from the surface and no CO2 in the atmosphere. You appear to agree that increasing CO2 concentration between Earth 1.5 and Earth 2 will initially allow CO2 to acquire energy conductively and radiate it to space, however you appear to be claiming further increases in CO2 toward the conditions of Earth 3 will reduce this ability. When adding CO2 to the atmosphere of an alternate earth such as earth 1.5 at how many ppm would CO2 reach its peak ability as a coolant and start to lose effectiveness?
All 15 um IR photons transport the same energy. If more escape to space then conductive to radiative cooling increases.
Uh, George, I think you misunderstood what the wiki page is saying. It is saying that some aspects of Planck’s law (and, hence, blackbodies) are explicable in classical terms and some are not.
For instance, see here:
http://en.wikipedia.org/wiki/Ultraviolet_catastrophe
IAC, there are ways to derive S-B that do not reference Planck’s law (which makes sense because SB came first).
http://en.wikipedia.org/wiki/Stefan%E2%80%93Boltzmann_law
Cheers, 🙂
“Total and utter balderdash. … How can one obtain SB from classical Physics if one cannot obtain the spectrum except with quantum mechanics?”
Its laid out here, if you want to try to understand. It is actually a rather elegant proof using thermo and E&M. http://en.wikipedia.org/wiki/Stefan%E2%80%93Boltzmann_law
It is not uncommon for a net result (eg the value of an integral) to be know when the exact nature of the the details (the function being integrated) are not know. It is also not uncommon for the classical limit of a problem is known without understanding the QM details.
In this case, a relationship dealing with radiation pressure is known without knowing the actual distribution of photon energies
“You see QM simply replaced causal deterministic classical Physics …”
That is ONE of the things QM did, but that is certainly not all.
“So Tim, you assert that cavity radiation is quantized by the cavity. What specific physical properties of the cavity determine what those quantized energy levels are?”
The dimensions of the cavity determine the possible standing waves. The math is simplest if you assume a rectangular box. Check your QM book for “particle in a box”. Apply the principles to massless bosons.
“It must seem odd to readers …”
QM and modern physics does indeed often seem odd. That doesn’t mean it is wrong.
“This modern very complete text on Quantum Mechanics Contains NOWHERE in its 400 or so pages, the word BLACKBODY, nor the combination BLACKBODY RADIATION. Odd that a highly regarded Reference Textbook, would contain not a word about something that is the epitome of its subject matter.”
It is your turn to quote me accurately. I said “Planck’s constant is the EPITOME of non-classical physics! It is the key parameter that universally shows up in modern quantum physics. ”
For your complaint to be valid, your QM text would have to have “not a word” about h or h-bar.
In any case, BB radiation is not the simplest nor the most fundamental application of QM. It is a starting point historically of QM, but very few text books take a historical approach to teaching science (thank goodness!). You can learn a LOT of QM before ever seeing BB radiation as an application of QM principles.
Konrad says “Imagine and alternate Earth. Earth 1.5 has no 15 um IR radiated from the surface and no CO2 in the atmosphere. You appear to agree that increasing CO2 concentration between Earth 1.5 and Earth 2 will initially allow CO2 to acquire energy conductively and radiate it to space, however you appear to be claiming further increases in CO2 toward the conditions of Earth 3 will reduce this ability. When adding CO2 to the atmosphere of an alternate earth such as earth 1.5 at how many ppm would CO2 reach its peak ability as a coolant and start to lose effectiveness?”
Interesting! The short answer is I don’t know the optimal value, but I am sure it exists. But now we have all sorts of details that would start to come into play to get a specific number.
* With NO CO2, then there will be no radiation in the missing band. Or put another way:
>>the radiation near 15 um is like a BB with a temperature of 0 K
* With a TINY bit of CO2, there is not enough CO2 higher up to really block any of the IR from the lower CO2. The outgoing 15 um IR will come from throughout the atmosphere (but mostly from the warm lower atmosphere, since there is more CO2 there). The “TOA” for 15 um IR might be only 200 m up.
>>The radiation near 15 um is like a BB with a temperature just slightly cooler than the surface.
* With LOTS of CO2, then the IR from the warm, lower layers of the atmosphere will be blocked, only the weak stream of 15 um photons from the cold upper atmosphere can escape.
>>The radiation near 15 um is like a BB with a temperature much cooler than the surface.
So as we add CO2, we will go smoothly from no 15 um IR to much 15 um IR and then back to little 15 um IR.
This would mean that
>a world with NO CO2 would be warmest,
>a world with A LITTLE CO2 would be coolest, and
>a world with LOTS OF CO2 would be almost as warm as a world with no CO2.
(A reminder to all: this is all predicated on the surface not radiating any 15 um photons, which is not how the earth behaves.)
The following quotation is from “Computational Blackbody Radiation” by Claes Johnson:
“A blackbody thus absorbs and emits frequencies below cut-off without getting warmer, while absorbed frequencies above cut-off are not emitted but are instead stored as heat energy increasing the temperature.
A blackbody is thus like a high-pass filter, which re-emits frequencies below a cut-off frequency while capturing frequencies above cut-off as heat.”
Antennas work by absorbing energy, converting radiation into electrical signals which have POWER. At room temperatures, radio frequencies are well below cut-off. So the first phrase of this quotation seems is in error, making the second paragraph untrue.
Tim Folkerts says on July 26, 2012 at 4:20 pm:
““Total and utter balderdash. … How can one obtain SB from classical Physics if one cannot obtain the spectrum except with quantum mechanics?”
Its laid out here, if you want to try to understand. It is actually a rather elegant proof using thermo and E&M. http://en.wikipedia.org/wiki/Stefan%E2%80%93Boltzmann_law
It is not uncommon for a net result (eg the value of an integral) to be know when the exact nature of the the details (the function being integrated) are not know. It is also not uncommon for the classical limit of a problem is known without understanding the QM details””
C.mon Tim, grow up “The Stefan–Boltzmann law, also known as Stefan’s law, states that the total energy radiated per unit surface area of a black body per unit time (also known as the black-body irradiance or emissive power), j*, is directly proportional to the fourth power of the black body’s thermodynamic temperature T (also called absolute temperature):”
The Earth, that we live on is not a “black body” – It can be seen from space. – OK, so North Korea may be a perfect black body but if so, – it is so, only in “jokes”
Please classify the Earth exactly where it belongs in the scale between a “Black” and a “White” body before you make your calculus.
I.E. The Earth can be seen from space, so we know it is not a “Black Body”
Therefore “Blackbody Calculations” have no place whatsoever in our world of “climate calculations”
I’m confused by your response, O H Dahlsveen. I am going to assume that you were confused by a missing close-quote. The first paragraph (“Total and utter balderdash. … “) was George’s opinion on classical derivations of SB. The second paragraph (“It is not uncommon for a net result …”) was my reply, stating that you can indeed derive S-B classically (but not the wavelength distribution – that requires QM). Unfortunately, I left off the close-quote ( and this time I also didn’t include italics) at the end of George’s words, so it would be easy to not notice which parts were mine and which were George’s. Hopefully that clarifies things a little.
I don’t disagree with the quote you included about S-B, but it doesn’t really address the issue you quoted about classical vs QM derivations of S-B.
I do, however, disagree with what you wrote later.
“I.E. The Earth can be seen from space, so we know it is not a “Black Body”
Therefore “Blackbody Calculations” have no place whatsoever in our world of “climate calculations”
1) Yes, we all know the earth is only approximately a black-body; it is not a “true blackbody”.
2) In this context, we are discussing the absorption/emission of IR for the earth, so reflection of visible light is not germane and tells us nothing about the earth as a blackbody for thermal IR. Many materials reflect visible light quite well, but can be considered “blackbodies” for thermal IR.
3) The fact is that BB calculations put an absolute upper limit on thermal radiation (at every individual wavelength) from a surface at a given temperature, which is quite useful.
4) Furthermore, ideal BB radiation is indeed a very good approximation for the radiation from the much of the ground, from the oceans, and from clouds, so BB do indeed have a major place in climate calculations. Of course, for accurate calculations for real surfaces, actual emissivity values should and would be included (possibly as a function of wavelength), but the theoretical BB equation is still needed as a starting point.
~~~~~~~~~~~~~~~~~~~~~~~~~~~~~~~~~~~~~~~~~~~~~~~~~~~~~~~~~~~~
And now, I think it may be time to drop out of this discussion. I suspect we have all learned about as much as we can from this discussion and it is time to move on.
Just for Reference: I believe a black-body is assumed to have so many electromagnetic resonant states that radiation at any wavelength is uniformly possible and Planck’s Law is an accurate representation of the power spectrum that will be emitted on that assumption.
Ref: http://en.wikipedia.org/wiki/Planck%27s_law
The transparent gases of the atmosphere do not qualify as black-bodies, but their spectra are punctuated by a limited number of resonances.
If anyone wants to see what the 15 micron absorption-emission spectrum of CO2 really looks like, there is a NASA Virtual Planetary Laboratory website based on the HITRAN 2008 database that will plot it out for you:
http://vplapps.astro.washington.edu/vplselectmicro
select:
wavelength_low:=13 µ m
wavelength_high=17 µ m
choose molecules=CO2 in the first box and click ‘findplots’
I believe the multiple lines are from various possible quantum states of the molecule. As stated, this is only for the most abundant isotope combination for CO2–that containing no abnormal isotopes of carbon or oxygen.
This has been a very interesting thread, thank you Reed.
Thanks also to Tim Folkerts and rgbatduke for battling against the tide.
Thanks to Spector for the superb VPL link.
George E. Smith; says: July 26, 2012 at 12:52 pm
“Personally, I have a VERY limited knowledge of QUANTUM MECHANICS”
I knew I would eventually agree with something you said George.
And, on the entertainment side, thanks for the amusement features provided by Mr. Dahlsveen. “The Earth can be seen from space, so we know it is not a Black Body”. Great. Was this part of an attempt to say the most stupidest things in a single blog?
So I decided to take a look at Tim’s Wiki reference in detail to see for myself their “rather elegant proof” ? I also spent some time looking into the Quantum Mechanics Textbook I referenced.
They did say this in their introduction to QM:- “”The important parameter in quantum mechanics is Planck’s constant h = 6.626 x 10^-34 Js …….Classical Physics treated electromagnetic radiation as waves. It is particles called photons whose quantum of energy is hbar.omega where omega is the classical angular frequency””.
They then launch into Schroedinger/s equation and so on, with nary a mention of Planck or black bodies or blackbody radiation.
No argument from me as to the importance of (h) to QM; as to being “The important parameter”, I dunno, since as I have said, I know very little of QM.
Notice how all this talk of BB radiation refers to frequencies, and wavelengths; which are quite clearly properties of WAVES, as used in classicl Physics. Anybody got a Planck spectrum formula using the language of quantum mechanics, and not mentioning the frequencies or wavelengths of classical physics; some photonic language perhaps ?
The constant that Planck introduced (h) is of course nothing more than the energy contained in any single cycle of any frequency of the waves of classical physics.
But off to wikiland, where they tell us Planck’s radiation law:-
L = 2hc^2.lambda^-5 / ( exp hc/lambda.kT – 1)
Well it’s got an (h) in it so it must be quantum mechanics.
They also give us the Stefan Boltzmann law, which is just classical physics.
E = pi^2.k^4 / (60h^3c^2) T^4
I see it also has an (h) in it. Must be the energy per cycle version of (h), since it was derived years before the Planck formula.
But I diligently read the entire wiki post on S-B and some of the linked subjects as well.
But I never did find the part where it says that only certain frequencies (spectral lines) and energies are allowed, because they are quantized; you know the discrete modes that are quantized by the cavity; you know, the ones that you get our of a soda pop can or drainage grate, or even an electric power socket.
If you;re a ham, good luck at getting the signal from your 2 metre rig into the pop can or electric power socket; might get some of it into the drain grate black body cavity radiator.
“””””…..George E. Smith; says: July 26, 2012 at 12:52 pm
“Personally, I have a VERY limited knowledge of QUANTUM MECHANICS”
I knew I would eventually agree with something you said George……”””””
Well MikeB; don’t be so bashful; come to think of it, this is about the very first time YOU have EVER made any reference to ANYTHING, I have ever posted; so I must presume that most of it simply went over your head. Really it is not all that hard to understand.
And I’m still waiting for someone to point out where the Temperature knob is in HITRAN or MODTRAN, so we can use it to do Blackbody Radiation calculations;, but I also noticed on wiki, that they do actually have a reference to “Thermal Radiation”, and what it is, so all you folks in Myrrh’s club, can stop pretending, that you have never heard of it.
And if anybody has a good table of all the discrete spectral line frequencies, for the black body cavity radiator (all those quantized modes), it would be nice to get a copy of them.
Bottom line Earth is not a black body and not even a real gray body. By the time all the corrections are made we are not even approximating at all. I support George E. Smith’s statement and I DO know alot of QM and not just ‘about’. All you need is classical physics for temperature which is AVKE of molecules, no need to even go to atoms in most cases, unless you want to know more underpinnings in a layered abstraction model.
“The constant that Planck introduced (h) is of course nothing more than the energy contained in any single cycle of any frequency of the waves of classical physics.”
Not even close. Classically “any single cycle ” of EM waves could have any conceivable energy by having arbitrary large or small E&B fields.
“But I never did find the part where it says that only certain frequencies (spectral lines) and energies are allowed”
You must not have recognized that part, since any derivation of Planck’s Law will list these. For example, you could look in Wikipedia. http://en.wikipedia.org/wiki/Planck%27s_law. Look in the section labeled “Derivation”. The allowed wavelengths will also certainly be in your QM textbook in a section about a “particle in a box” but you might need to generalize to 3D if the book only does the 1D case. (The photon is the particle; the cavity is the box.)
“They also give us the Stefan Boltzmann law, which is just classical physics.
E = pi^2.k^4 / (60h^3c^2) T^4
I see it also has an (h) in it. Must be the energy per cycle version of (h), since it was derived years before the Planck formula.”
1) The FORM of S-B can be derived classically, ie P ∝ T^4. Deriving the constant in the equation requires QM.
2) As already noted, the “energy per cycle” interpretation is wrong.
3) That is the equation for P/A = E/(t*A) = the power per unit area, not the equation for energy. If anything, the equation would have to be for “energy per unit time per unit area per cycle”, whatever that might mean.
4) Minor point: that should be h-bar^3, not h^3. That is an easy mistake to make.
“But I diligently read the entire wiki post on S-B and some of the linked subjects as well.”
Wikis and webpages are not bad ways to review advanced physics topics, but clearly they are not effective ways to learn it for the first time.
“… so I must presume that most of it simply went over your head. Really it is not all that hard to understand.”
Can you truly not see the irony here? You admit you don’t understand QM and have made repeated fundamental mistakes, but you feel qualified to judge the understanding of others. Two Nobel prize winning physicists said “Nobody understands quantum mechanics” (Feynman) and “Anyone not shocked by quantum mechanics has not yet understood it.” (Bohr) yet you feel it not hard to understand.
Wikipedia is pure garbage, and this is especially true for physics and chemistry. Wikipedia is only good for things like what color hat did Paris Hilton wear while on vacation in Europe.
The problem with understanding of physics:
http://staff.science.uva.nl/~eberg/Antwerpen/Diagnostische%20toetsen/Heat%20and%20TemperatureTPT%20YeoZadnik.pdf
Power is energy flow. That is why these words are sometimes used interchangeably. One watt per square meter is the same as one joule (the meter-kilogram-second (MKS) unit of energy) per second per square meter.
If one were in a closed room where all surfaces were the same temperature, each surface would emit the same number of photons at that temperature as it would in outer space. In the fixed temperature room, however, each surface would also be receiving photons that would replace the energy being emitted. The photons emitted all these surfaces are real and detectable even though there is no net loss of energy from any surface.
The real reason that CO2 cannot be the serious global warming hazard that many fear is that its absorption band stands like a one-foot diameter tree in the middle of a ten foot wide stream. Almost all of the effect of additional CO2 is to absorb photons already being totally absorbed at a slightly lower altitude.
Here, again, is a curve showing almost no difference between the calculated energy (or power) escaping the Earth’s troposphere (‘Radiative Forcing’) for CO2 concentrations of 300 PPM and 600 PPM.
http://en.wikipedia.org/wiki/File:ModtranRadiativeForcingDoubleCO2.png
Note that most of this radiation originates in the troposphere as a result of thermal energy convected up from the surface. That is why the surface absorption plot shown in the main article can be very misleading as only (390-324)=66 of the 235 joules per second per square meter shown escaping the troposphere on the classic Kiehl-Trenberth diagram actually is radiated directly from the surface.
http://wattsupwiththat.files.wordpress.com/2009/11/trenberth-color-best.jpg
George E. Smith; says on July 27, 2012 at 4:26 pm:
“So I decided to take a look at Tim’s Wiki reference in detail to see for myself their “rather elegant proof” ?
but I also noticed on wiki, that they do actually have a reference to “Thermal Radiation”, and what it is, so all you folks in Myrrh’s club, can stop pretending, that you have never heard of it.”
==============
I do usually read your comments and, mainly, find nothing that pops out worth screaming about. In this comment however there is just one small thing, which may be important, therefore:
I too have typed “Thermal Radiation” into my search engine and looked up Wikipedia as I do like elegant explanations or even better still elegant proof.
– Well I did not find any of that there, as when we talk about “Thermal Radiation” – here on WUWT, I always assume that it is radiation from the Earth’s surface where T = (or averages) 288K we do discuss, unless, of course, Solar or any other form of “radiation” is specifically mentioned.
As you know, according to the “correct script”, there is an “Atmospheric Window” that lets a certain amount of “IR radiation” from the surface pass straight through the GHGs on its way to outer space. Therefore I recon IR radiation (from T = 288K) at ground level must be powerful stuff.
Or that idea may be completely wrong.
Well, I am not exactly in “Myrrh’s club”, but I have never been able to prove by experimentation that IR radiation from an object even with a temperature (T) = 440K, or say 150K above T ambient is significant enough to take into consideration. (Temps. mentioned here are approximates and do not really matter as long as they are not hot enough for visible light to be emitted)
Take “radiation” from a thermostatically controlled hotplate, which, when set on low has a T range of, say 125 – 150 deg. Celsius, can not reach much further than a few centimeters through, or into, the air. (Invert the hotplate and measure T just below the hot surface, convection is then bypassed and “Thermal air expansion” and radiation is all you are left with). – The heat just gets absorbed by the air, or possibly mainly by the atmospheric water vapor content.
The heat then, as I see it, is conducted and convected away.
– IMHO it is a fact that those “Atomic/Molecular vibrations” that produce EM waves do not change one iota just because two, or more, solid and gas molecules are in “Thermal Contact” with each other and the “radiation” for that reason turns into “conduction”
I could bring in “The Zeroth Law of Thermodynamics” here, but this posting is getting long enough as it is.
Wiki shows a diagram and explain:
“This diagram shows how the peak wavelength and total radiated amount vary with temperature according to Wien’s displacement law. Although this plot shows relatively high temperatures, the same relationships hold true for any temperature down to absolute zero. Visible light is between 380 and 750 nm.”
Did you omit to look at that particular diagram?
It is interesting that their highest T is 5500K and the curve, of course is red, and quite steep. As T is lowered so the accompanying curve looses its “steepness” all the way down to 3500K which is the lowest T involved. The curve for T = 3500K is quite flat.
Therefore I feel it is only fair to ask you: ”What do you estimate the curve from T = 288K will look like?” – Should extrapolate down to “very flat indeed”.
I think. I may check it out one day though.
I believe the term “radiation” used in these discussions is short for “electromagnetic radiation” so that “thermal radiation” is short for “thermally induced electromagnetic radiation” where thermal or vibrational energy is converted to the photons that actually leave a surface. When those photons are absorbed by another surface, they will yield their energy to that surface even if it is warmer than the surface that emitted them. The laws of thermodynamics only apply to mutual exchanges of photons, not to the individual photons themselves.
If you and and a friend had a pointless arrangement for each to send the other a $100 check every month, neither of you would profit by this deal but your banks would still have to process the transactions. The individual checks would be equivalent to the photons emitted in ‘thermal radiation.’
Spector says:The laws of thermodynamics only apply to mutual exchanges of photons, not to the individual photons themselves.”
That is very wrong. I see that someone made such a statement on lofi.forum.physorg.com/Information-Carried-By-A-Photon_2
but this is a very misleading statement, but am not attacking you for the mistake, but just providing the necessary correction.
I think you maybe thinking of temperature not applying to a single particle.
O H Dahlsveen says:
“Well I did not find any of that there, as when we talk about “Thermal Radiation” – here on WUWT, I always assume that it is radiation from the Earth’s surface …
You should include radiation from the air and clouds, etc. but otherwise I agree. Spector said it pretty well in his previous post.
“Therefore I recon IR radiation (from T = 288K) at ground level must be powerful stuff.”
“Strength” is the wrong way to think about it. Visible light gets thru the atmosphere pretty well; some IR gets thru pretty well; some radio gets thru pretty well. It is not a question of “strength” but rather a question of the properties of the air.
Would you say that blue light has to be strong to get thru a blue piece of glass, but the red photons are weak? Or red light has to be strong to get thru a red piece of glass, but the blue photons are weak? No — those particular photons are simply “lucky enough” no to be absorbed by those particular materials.
Some wavelengths of IR are simply “lucky enough” not to get absorbed by the molecules in the atmosphere.
“Take “radiation” from a thermostatically controlled hotplate, which, when set on low has a T range of, say 125 – 150 deg. Celsius, can not reach much further than a few centimeters through, or into, the air.”
IR photography refutes this claim. Google “thermal images” to see lots of photons that traveled feet (or miles) from the hot source to the camera. It may no longer be intense enough for you to “feel” the heat, but the photons can indeed travel long distances.
”What do you estimate the curve from T = 288K will look like?”
In many ways, it will look the same as the curve at any other temperature. The curve will rise rapidly, then fall off slowly as you increase the wavelength. Of course, you would have the “rescale the axes” to get it to look similar, but the general shape stays the same.
http://en.wikipedia.org/wiki/Planck%27s_law#Percentiles
Spector says:
“Here, again, is a curve showing almost no difference between the calculated energy (or power) escaping the Earth’s troposphere (‘Radiative Forcing’) for CO2 concentrations of 300 PPM and 600 PPM.”
True enough, but there is also “almost no difference” between 288 K and 289 K. Indeed, that 1 K temperature change is about what the doubling of CO2 would produce (absent feedbacks). If there was a bigger difference in the curves, there would be a bigger difference in temperature.
” That is why the surface absorption plot shown in the main article can be very misleading as only (390-324)=66 of the 235 joules per second per square meter shown escaping the troposphere on the classic Kiehl-Trenberth diagram actually is radiated directly from the surface.”
You are, I am afraid, comparing apples and oranges. Think in terms of your cash analogy.
* Mr Surface “pays” Miss Atmosphere $350 (in IR radiation, and $78 + $24 in other forms). Mr. Surface pays only $40 directly to Mrs. Space. Any money Atmosphere pays to Surface doesn’t (directly) change the Surface–>Space exchange.
Only 40 W/m^2 escaping from the earth as a whole is radiated directly from the surface.
* Indirectly, you could say Miss Atmosphere receives $350 from Surface IR, $78 from Surface Evaporation, $24 Surface Thermals, and $67 from Mr Sun. So you could say 350/519 = 67% of any outgoing flow from Atmosphere came from Surface IR — ie $132 out of $195 leaving to Space came indirectly from from the the Surface IR (or $170 total came indirectly from the surface (counting thermals & evaporation)).
* For the surface when we focus on IR only, there is $390 coming in, and $324 heading out, so the $66 you mention in has nothing to do with space, but relates only the surface/atmosphere exchange of IR. The 66 W/m^s is the net IR flow of 66 W/m^2 thru the boundary between the Surface and the Atmosphere. It is NOT energy “escaping from the troposphere”.
BOTTOM LINE: the 66 W/m^2 you calculated and the 235 W/m^2 that you compare it to are not directly related.
“””””…..Tim Folkerts says:
July 27, 2012 at 10:17 pm
“The constant that Planck introduced (h) is of course nothing more than the energy contained in any single cycle of any frequency of the waves of classical physics.”
Not even close. Classically “any single cycle ” of EM waves could have any conceivable energy by having arbitrary large or small E&B fields.
Tim we are talking about the (h) that occurs in the E = h (nu) ” F” Planck revolution that started off “Quantum mechanics”.
So if a “black body” emits a photon of energy E and frequency = (nu) then since by Planck which you agree with (as do I), the photon energy is hnu so if I divide the Total photon energy hnu, by the number of cycles of the EM wave in one second which is (nu), I get
Energy per cycle = hnu / nu = h Joules per cycle.
Remember that (h) is the quantum of “action” ; it is NOT the quantum of “energy”; and its units are Joule.seconds.
So now tell me again how I’m “not even close”.
I wonder why you don’t consider the start of “Quantum Mechanics” to be back when the alchemists discovered that matter (stuff) was “quantised” and that the quanta, of stuff, the smallest units it could come in were atoms and molecules; so of course chemistry must also be Quantum Mechanics , and not simply applied Physics. Matter would seem to be even more quantized than Black Body radiation, since matter quanta consist of only a handful of allowable vaues; 92 or so; well until they discovered the stuff fine structure of isotopes; whereas the photon quanta of the black body radiation are allowed to have ANY value of energy whatsoever without restriction, as is the frequency (f or nu) so long as that energy is hnu.
Blackbody radiation is somewhat like a string of sausages; I’m a sausage knitting expert. You can have ANY amount of sausage you want, so long as you twist it up into individual connected “links”, and you can make smaller links and make more of them and still have the same amount of sausage, in that case h is the amount of sausage per link; well actually sausages are sort of the inverse of blackbody radiation in thathat the total amount of sausage is fixed, rather than the energy per cycle as in BB radiation.
But I’m still waiting for someoe to give the frequencies of some of the best known quantised frequencies of black body radiation ; or any sort of thermal radiation.
wikipedia.org/wiki/Thermal_radiation is the first wiki reference to thermal radiation I found; didn’t have to look far. And I’m the very worst giggler on the planet; well I use Bing anyway.
I’m quite happy to accept your assertion Tim, that Black Body Radiation was the beginning of quantum mechanics; that’s not the same as saying BB radiation IS quantum mechanics, any more than atoms and molecules are quantum mechanics. Yes QM is useful in explaining the properties or behavior of atoms and molecules; but the alchemists/chemists who discovered them weren’t quantum mechanics experts.
And my compliments to jcbmack; I often need all the help I can get. After only 52 years as a paid working Physicist, I sometimes forget stuff, and sometimes totally goof, and when I do, I tend to admit it, rather than dig the hole deeper.
And no I DON’T have a PhD; which is why as I said, I know VERY LITTLE about quantum mechanics.
Also the expression for The Stefan Boltzmann law is not quite correct, since it has a (c^2) term in it The correct expression should have the velocity of light in the particular medium, and the c^2 is only true for the vaccuum case. So c^2 needs to be replaced by (c/n)^2; where n is the refractive index of the medium in which the emission or absorption occurs. ( a classical Physics concept.). So it would seem that the BB energy depends more on the wavelength than the frequency, since the frequency is unchanged, when you change them medium, but the energy or power is not.
that is ow Roland Winston made a solar image that was brighter than the sun, because his image was in a YAG crystal.
“””””…..Did you omit to look at that particular diagram?
It is interesting that their highest T is 5500K and the curve, of course is red, and quite steep. As T is lowered so the accompanying curve looses its “steepness” all the way down to 3500K which is the lowest T involved. The curve for T = 3500K is quite flat.
Therefore I feel it is only fair to ask you: ”What do you estimate the curve from T = 288K will look like?” – Should extrapolate down to “very flat indeed”……”””””
I think. I may check it out one day though……”””””…..O H Dahlsveen says:
July 26, 2012 at 1:19 pm
George E. Smith; says on July 25, 2012 at 4:28 pm:
“I might come back to see who wants to join you.”
============
George E, will you come back to join us even if I am not going to join Tim?…..”””””
Why is it that some like to assume than anything not mentioned is something not klnown.
I don’t need to look at ANY black body radiation spectrum graph; because I already know what it looks like. ALL BB radiation spectra curves look the same, because they ARE all the same.
If you plot the spectrum (hopefully on log scale paper) with an x scale of wavelength divided by the spectral peak wavelength, and a y scale of the spectral radiance divided by the peak spectral radiance, you get a universal spectrum with a peak (y) of 1.000, and an x scale centered on 1.000, perhaps going from 0.1 to about 50.
The Planck formula is a function of a single variable: wavelength x Temperature (lambda.T)
And if you plot it the way I said, the one graph serves for any situation. It is common to plot the vertical axis, as both a linear 0 —> 1 scale, and a log scale perhaps 1E-4 (or -5) to 1.000. Not a lot of point going outside a 0.1 to 50 range on the x axis. The linear y scale is only readable for x from about 0.3 to 8, and the y log scale is down to 10^-5 at x = 0.2 and 40.
I don’t need to ping wiki to find out ANYTHING about BB radiation.
If you want a universal BB spectrum go look up Warren J Smith (the late, sadly) “Modern Optical Engineering,” page 218 in the second editionMcGraw Hill. A really great book if you want to learn anything about Optics.
Konrad: The long and the short of it is that while you may be able to imagine alternate bodies with weird spectral emission patterns such that increasing CO2 can cool it, the surface of the Earth that we live on is pretty close to being a blackbody emitter. For such an Earth and a lapse rate so that temperature decreases with altitude, the effect of increasing CO2 is to decrease, not increase, emission because as CO2 is added, the emissions that escape to space move to higher altitudes where it is colder and hence less radiation is emitted.
The approximately-logarithmic dependence of radiative forcing on CO2 concentrations is the net effect of additional CO2, i.e., the fact that CO2 both absorbs and emits. There is additional linear cooling effect…That is simply a figment of your imagination.
Of course, this picture accepted by all serious scientists in the field including Richard Lindzen and Roy Spencer and in accord with the modern radiative transfer calculations that are well-verified by an entire technical field (remote sensing), is verified by spectra of the Earth’s emissions as seen from space by satellites.
George E. Smith:”I wonder why you don’t consider the start of “Quantum Mechanics” to be back when the alchemists discovered that matter (stuff) was “quantised” and that the quanta, of stuff, the smallest units it could come in were atoms and molecules; so of course chemistry must also be Quantum Mechanics , and not simply applied Physics.”
I must admit I don’t understand the point of your most recent lines of discussion with Tim. Apparently, you are quite concerned with whether blackbodies are classical or quantum physics. I personally cannot see how this matters one bit except to say that the conception of how blackbodies function is different under classical and quantum frameworks(again see the ultraviolet catastrophe) and the view informed by QM is held to be the *correct* one. I don’t believe that you will disagree with this and I can’t see the point of trying to argue that blackbodies are 85% classical and 15% quantum or whatever.
IMO, you will be better served by focussing on what, if any, differences you may have with current GH theory.
Cheers, 🙂
I said:
Of course, that should read:
Shawnhet, I think you are right when you say ‘IMO, you will be better served by focussing on what, if any, differences you may have with current GH theory.” Partly George & I have a challenge with terminology (eg “energy per cycle” vs “energy per (cycle per unit time) ); partly I think different levels of understanding of QM is making the discussion difficult. I find the underlying physics fascinating, but it is not a simple topic. In any case, you are right that this doesn’t matter much other than for academic interest.
* Theoretically, blackbodies are ideal radiators that can only be adequately explained by QM
* Experimentally, blackbodies can be approximated extremely well by an opening to a large cavity.
* Practically, many parts of the land, the oceans, and the clouds are quite close to BBs (with respect to thermal IR). GHGs are close to BB *for specific wavelengths* but are close to “white bodies” or “clear bodies” for other wavelengths.
At this level of discussion, that is all that really needs to be said. The details matter for detailed calculations, but that is way beyond the scope of this discussion.
“””””…..Shawnhet says:
July 31, 2012 at 9:35 am
George E. Smith:”I wonder why you don’t consider the start of “Quantum Mechanics” to be back when the alchemists discovered that matter (stuff) was “quantised” and that the quanta, of stuff, the smallest units it could come in were atoms and molecules; so of course chemistry must also be Quantum Mechanics , and not simply applied Physics.”…..”””””
Well to each his own I guess. I believe that Quantum Mechanics, and its offspring QED, and QCD are generally regarded as the current best description of how the REAL world works; made of REAL materials, that have REAL electron structures, and arrays of energy levels.
It is beyond me to see how that relates to a very UNREAL notion, that has no REAL existence, and requires NO knowledge of ANY real world materials, or of the properties of any REAL materials.
In fact knowledge of ANY real materials immediately negates the possibility of a REAL black body existing, since ANY real material has a refractive index (possibly complex) which means that ANY such surface MUST have a polarised Fresnel reflection coefficient that is wavelength dependent, so NO REAL surface can absorb 100% of ALL wavelengths of EM radiation that falls on it, which is the very essence of a Black Body.
So that leaves the “Cavity Absorber/Radiator” that Tim referred to; which I am quite familiar with.
Here again reality sets in; since the cavity must be at a single (any one) Temperature > 0 and must NOT lose ANY energy from anywhere save the cavity aperture. So that requires that the walls of the cavity must be made of some material , that has a ZERO thermal conductivity; or an infinite thermal insulation property; again not anything in the REAL universe.
And the whole point of my initial comment, was the enormous importance to Physics of the investigations of the ultimate thought experiment; the non-existent hypothetical black body.
Yes Planck suggested that EM radiation must consist of discrete “quanta”; same as the alchemists realized that the simple numerical ratios of chemicals reacting in a chemistry experiment, were easiest to explain by assuming that there must be a dscrete quantum of matter for each of the “elements” of their knowledge.
A little contemplation of the notion of matter or anything else being infinitely divisible, with no minimum size for any sample of anything, is clearly even sillier, than the idea of them all being a collection of quanta.
The difference between atoms and BB photons (or THERMAL RADIATION PHOTONS), is that the former only have a very limited set of quantum sizes and compositions, while the BB/Thermal photons have no such constraint and can have ANY energy at all, so long as it is given by hF. (h) is fixed in value, but (F) is NOT, and also can have any value. This distinguishes BB or Themal radiation (Plasmas also) from the spectrally discrete lnes, of either atomic or molecular absorptions/emissions, that are the main factors in the GHG atmospheric theories. Those discrete spectral line/band spectra ARE the consequence of quantized energy levels in REAL MATERIALS.
As for current Green House theory; I’m not much interested in it, since (a) I don’t believe the data sampling regimen is valid, in the light of modern information theory, including sampled data theory, so I don’t believe the purported “data”, and Anthony’s new paper tends to bear out the glaring problems of present weather data gathering. Furthermore, I believe that negative feedback by absorption or scattering/reflection of INCOMING solar energy by H2O in all three phases, has far more control of earth’s Temperature, than the “greenhouse” effects.
Let me say categorically, I do NOT dispute the so-called green house effect; although some totally stupid experimental “demonstrations” of it are performed by idiots who deserve to be tarred and feathered; such as substituting a 2800 K 100 Watt lamp as a LWIR radiation source, that is 10,000 times as bright, as the REAL earth surfaces (or a bottle of water) at 288 K, that is the ACTUAL source of the LWIR that is intercepted by GHGs such as H2O and CO2.
But you and ANY reader are quite free to believe whatever you want. Here in the 21st century, people on earth still have some odd beliefs.
George:”It is beyond me to see how that relates to a very UNREAL notion, that has no REAL existence, and requires NO knowledge of ANY real world materials, or of the properties of any REAL materials.”
I agree that a BB is an unreal entity but it seems to approximate certain portions of the real world relatively well. To quote Tim above who says it better than me: “* Practically, many parts of the land, the oceans, and the clouds are quite close to BBs (with respect to thermal IR). GHGs are close to BB *for specific wavelengths* but are close to “white bodies” or “clear bodies” for other wavelengths.”
If what Tim says above is valid (and it seems to me to be), then that answers your question, doesn’t it? Only if someone disagrees that some portions of the real world are pretty close to BBs would it make sense to say that BB behavior has no relevance to the real world.
“And the whole point of my initial comment, was the enormous importance to Physics of the investigations of the ultimate thought experiment; the non-existent hypothetical black body.”
Yes, I agree. This is why I am having trouble with your argument. If the unreal BB doesn’t relate at all to the real world(your first comment), how can it also be of “enormous importance” to physics?
Cheers, 🙂
Well Shawn, I think we generally think of our scientific theories and models, as being our best or at least a useful description of how we believe the real world behaves; whereas, these models and their descriptions are actually all made up; as is the mathematics we use to describe the behavior of the models. The point is we can quite accurately (in most cases) describe how the model will work, because we endowed it with properties which ar under our control. The trick is to try and construct our models so that they EMULATE the real world, as closely as we know how. When they deviate from that, then we alter, reconstruct or abandon the models in favor of something that is a closer image of reality.
WUWT readers might be convinced that in “climate science” we make our computer models, and then we try to construct the data to match what our computers tell us is the behavior we want; well I exaggerate of corse.
The thing about a Black Bodyis that it was well known and realized that no such thing could exist; yet it was a contrivance about which we could say: This is how it would work if there was such a thing. And as you And Tim Folkerts too point out, we can make very good approximations to that fictitious animal, and they do behave closely like the BB, Planck theory describes, which makes them of great use. As Leif Svalgaard has pointed out several times TSI is commonly measured using such a cavity “radiation trap”. The reason that BB approximations are of such use, is that we know that ony 1% of the total energy, lies beyond each end of a 0.5 to 8.0 times the peak wavelength range, so for many purposes, the faux BB only has to be a good absorber over that restrcted range.
Just think the 6,000 K sun surface radiation peaks circa 0.5 microns wavelength, while the about 300 k earth surface emissions peak at about 10 microns. The mysterious 2.7K big bang echo, being 1/100th the temperature of the earth, is radiating around 1 mm peak wavelength.
So all of our radio and TV and other comunications traffic, must be truly cold when viewed as BB emissions. Just calculate what the Temperature would be for a BB that is radiating at 60 Hertz frequency, like our power transmission lines are.
I pretty much agree with your post above, George. Sometimes in these discussions you will run into people who use the fact that “the Earth is not a real blackbody” as an argument against the GH effect. Regardless of the truth/magnitude of the GH effect, Earth is reasonably close to a blackbody over the relevant “GH” range of wavelengths.
Cheers, 🙂
joeldshore says:
July 31, 2012 at 8:41 am
—————————————–
Joel, I was interested enough in Tim Folkerts responses to my questions to take the time to design and build an empirical experiment to test Reed’s hypothesis. Tim Folkerts is partially correct, REED CORAY IS RIGHT and you are wrong. My graph ( http://i48.tinypic.com/2pp0hed.jpg ) while dimensionless is correct.
Instead of relying on the BS (blackboard scribblings) of Pierrehumbert I would suggest you design and build your own experiments. NO. Don’t run to the web to find someone else’s work. Design it and build it yourself. An ancient Chinese proverb may be relevant here – “Tell me, I’ll forget. Show me, I’ll understand. Let me do it, I will KNOW.”
And for future reference I have empirically confirmed that the answer to my original question on this thread was both B and “ D. The spherical chicken found in the same region of space has “Property of Pierrehumbert” tattooed on its butt.”