I’ve been puzzling for a while about why the areas with the most power plants aren’t the areas with the worst levels of mercury pollution. Why aren’t the areas downwind from the power plants heavily polluted? I keep running across curious statements like “There was no obvious relationship between large-mouth bass or yellow perch fish tissue mercury concentrations and their locations relative to prevailing wind patterns and the incinerators” (source). In that regard I came across a critically important paper. The paper starts with what to me is a most surprising statement.
But before I get to that, a short digression. There are a couple kinds of mercury emitted by power plants, by forest fires, and by your automobile, for that matter. Well, actually three kinds, but there’s very little particulate mercury coming from any of those sources. The two kinds are “divalent” and “elemental”.
Elemental mercury (written as “Hg0”) means what you’d think, atoms of mercury vapor. Because it doesn’t bind with much and it is insoluble, it has a fairly long atmospheric half-life, on the order of a year or so. Elemental mercury is what forms the background mercury levels that are present everywhere in the atmosphere.

Figure 1. Areas in the US where fish have high levels of mercury. White areas have not been tested. EPA threshold as safe to eat is 0.30 ppm (two lightest shades of red). From the EPA’s Mercury Maps (PDF)
The other kind of mercury, divalent mercury (written as HgII), exists in the form of compounds like mercuric chloride (HgCL2). Because these compounds are both water-soluble and chemically reactive, they come out of the atmosphere quickly through deposition by precipitation. In addition, they come out slowly as elemental mercury is slowly changed into divalent mercury in the atmosphere. And as a result of all of these kinds of atmospheric mercury, plus mercury naturally in the soils, we end up with mercury in the fish.
To summarize: elemental mercury is added to the background mercury and doesn’t settle out near the power plant. Divalent mercury is reactive and water-soluble, so it rains and precipitates out near the power plant. And the problem is that analysis of the emissions from the smokestack of coal-fired power plants show on the order of 25% more divalent mercury than elemental mercury. Which sounds like bad news for those living downwind from our power plants.
With that as prologue, here’s the opening statement that I found so surprising, from a paper called “Modeling Mercury in Power Plant Plumes”.
First, the Mercury Deposition Network (MDN) data (1) along a west-to-east transect from Minnesota to Pennsylvania show no significant spatial gradient in annual mercury (Hg) concentrations in atmospheric precipitation although the Ohio Valley includes several large Hg emission sources located, under prevailing wind conditions, upwind of Pennsylvania.
Say what? No hot spots for mercury downwind of several large power plant mercury emission sources? How come I haven’t heard of that?
So I wandered off to the Mercury Deposition Network, where I found a couple more surprising maps.
 Figure 2. Total Mercury concentration in the atmosphere in 2010. Units are nanograms per litre. The red “hot spot” in the center of the US reflects the natural mercury coming from deserts and croplands, as I discussed in “The EPA’s Mercurial Madness” SOURCE
Figure 2. Total Mercury concentration in the atmosphere in 2010. Units are nanograms per litre. The red “hot spot” in the center of the US reflects the natural mercury coming from deserts and croplands, as I discussed in “The EPA’s Mercurial Madness” SOURCE
As an aside, the EPA and other scientists claim that much of the mercury in the atmosphere is “recycled” anthropogenic mercury. They say the natural emissions are in large part just man-made emissions being re-emitted. I certainly would hope that Figure 2 would put a stop to those claims. The main and overwhelming source of atmospheric mercury in the US is the natural mercury in the soils.
OK, another surprise for me. We’ve seen where the sources are and what’s in the air. Now, let’s see what gets deposited in the rain and snow. Figure 3 shows the wet deposition map for the US in 2010.
 Figure 3. Mercury wet deposition rates for the US. Units are micrograms per square metre of surface. SOURCE
Figure 3. Mercury wet deposition rates for the US. Units are micrograms per square metre of surface. SOURCE
Note that strangely, this is kind of a weather map. Why is it a weather map? Well, for example I live on the coast not far north of San Franciso Bay. I was amazed to see that I live in an area of relatively high mercury deposition. Why?
The answer is, because when the moist air sweeps in off the Pacific and hits the coastal mountains, it rains. And when it rains, I get showered with natural mercury from the ocean. Further inland on the east side of California, you can see the western slopes of the Sierra Nevada mountains painted in red. They get the moisture that doesn’t fall on the coastal ranges. And since they are much higher, they pretty much wring the moisture (and the mercury) out of the air, leaving Nevada with little mercury deposition.
The main flow of air in the US is from west to east. As a result, the hot spot over the southwestern US precipitates out in the central US. It is aided by moist air flowing in from the Gulf of Mexico during some months. This can be seen all along the Gulf Coast. Florida, like where I live, is another victim of oceanic mercury poisoning.
Finally, to return to the surprising statement I started with, in Figure 3 the blue arrow shows the prevailing winds blowing over the power plants in the Ohio River Valley towards Pennsylvania. If it is the case that the majority of the mercury emissions are divalent mercury, then why is there no trace of them raining out along the way as we’d expect?
The authors look at several different possibilities. Their final conclusion? (emphasis mine)
A sensitivity study of the impact of the Hg dry deposition velocity shows that a difference in dry deposition alone cannot explain the disparity. Similarly, a sensitivity study of the impact of cloud chemistry on results shows that the effect of clouds on Hg chemistry has only minimal impact. Possible explanations include HgII reduction to Hg0 in the plume, rapid reduction of HgII to Hg0 on ground surfaces, and/or an overestimation of the HgII fraction in the power plant emissions.
We propose that a chemical reaction not included in current models of atmospheric mercury reduces HgII to Hg0 in coal-fired power plant plumes.
This has large implications for the regulation of power plants. All the big power plants in the Ohio River Valley aren’t increasing the mercury deposition towards Pennsylvania. The mercury is being converted into elemental mercury along the way somewhere, so it’s added to the background mercury rather than raining out near the power plants.
In fact, where I live, on the pure clean Pacific coast with lots of sea breeze, I get more mercury pollution than they get around Pennsylvania despite all the coal-burning power stations just upwind from them.
And that, dear friends, was a very big surprise … when’s the EPA gonna step in to save me?
Does this mean mercury is not a poison? By no means, mercury is a bad thing, and it’s everywhere … it just shows the story has lots of tricks and turns.
Always more to learn,
w.
Willis said, “I certainly would hope that Figure 2 would put a stop to those claims.”
Would that be the same as the second figure one?
[Indeed it would … fixed, thanks. -w.]
Your second figure is labeled figure 1.
This is an excellent adjunct to your earlier post. Sampling density could be an issue when trying to describe the footprint of mercury fallout from coal-burning plants. If they have a tall stack then dispersion widens but I suspect the footprint is still very small relative to the regional sampling evident from your smooth-looking images (Figure 1 especially). What happens, for example, when you plot a small (200 km by 200 km) region centered on a coal plant? Is there no clear pattern or are there only one or two sample points for mercury concentration?
You will notice a very deep red patch just South of San Francisco Bay on that first map. The fish here are so contaminated with mercury in our fresh water lakes in Santa Clara County that they can not be eaten. The reason isn’t “pollution”, the reason is natural mercury in the soil. During the gold mining days there were many mercury mines in Santa Clara County. The place is just full of it. Fish in Stevens Creek Reservoir are so full of it that there are signs everywhere telling you not to eat the fish.
Mercury does occur naturally and some of these environmental commissars need to be aware of that fact.
“or an overestimation of the HgII fraction in the power plant emissions”??
With all that budget they can not measure accurately and do not know?
George says:
April 1, 2012 at 5:19 pm
Mercury does occur naturally and some of these environmental commissars need to be aware of that fact.
Fact is they don’t care. It’s not about pollution, it’s about political objectives.
Always more to learn,
w.
=================
Not enough people shouting it from their rooftops.
SO … what is this interstate basis for the recent EPA rulings again?
When’s the ocean going to be subjected to EPA Administrative Law edicts; I want to see fines levied and cease and desist orders issued to Gaia …
.
I’ve gotten the impression much of the impetus behind the assault on mercury from coal power plants was the mistaken assumption that since autism levels are high near power plants, mercury must be causing it. This is based on the false assumption that mercury causes autism (science has found no statistically significant connection between mercury preservatives in vaccines and autism rates, and this specific false assumption first connected mercury and autism.)
You are likely to see a spike in autism rates near coal fired power plants, but it is caused not by mercury, but by genetics; if the power plant is one of a rural area’s largest employers, and a significant number of engineers and technicians settle in the area, local autism rates go up, just have they have in other areas of high concentrations of engineers like Silicon Valley and Rochester Minnesota. A recent Dutch study contrasted autism rates in Dutch cities known as technology centers versus cities not known for technology and found a similar autism spike in the technology centers. My personal observation is that the highest rates of severe autism are associated with graduates of Midwest land grant colleges. Engineering majors meet Nursing majors in chemistry class, get married, and have a high incidence of severely autistic kids.
It’s called assortive mating.
obviously you are science hating racist …
Our river (Shenandoah in Virginia) has old mercury from Dupont (at least 50 years old). The mercury levels have actually increased a bit which attributed to the cleaner water which allows more plant growth. The plants get uprooted in floods and out comes the mercury.
I think metallic mercury is somewhat demonized, though I don’t have any good data other than anecdotal. Apparently Thomas Edison and his crew working on the light bulb had a little trouble with the mercury vacuum pump they used. Near as I could tell, it entailed taking a bucket/flask/container of mercury up a ladder, pouring it through glass piping and that would entrain bubbles of air to be dumped outside the apparatus.
I suspect they spilled a lot of mercury.
Then there was the time in 2nd grade where I had some mercury in a shallow dish (I think an old makeup container) that I was showing around the class for show & tell. I had a penny in it showing how the mecury wetted it. Not a very stable way to carry mercury, and some spilled on to the floor. That was 1958 or so, I think it’s evaporated by now. (Dad worked for Baily Meter, and they used mercury in manometers, so he’d bring a little home from time to time.)
It’s really a pity that the EPA would freak out these days if they heard some kid was holding a large drop of mercury in his hand, even if he got it mostly back in the container.
One of my favorite mercury stories is only ten years old. My daughter came home from school and said her science homework was to bring in five samples of different metals. I first considered if I should give her copper pipe or wire, when it dawned on me we could make a lasting impression.
In the basement, in a box labeled “heirloom chemicals” that I inherited from Dad, I had added a plastic film can that has a little glass jar that has a little mercury from a broken thermometer. Obviously, this would have to be one of the five, but given the current era, I took out the glass container with the mercury, wrote “Hg” on the top of the film container and had Hannah take the empty film can to school.
I gave her instructions to no open the can, but be sure the teacher saw the “Hg.” Worked great – teacher saw it, commanded “Hannah, give that to me right now!” and quickly figured out she’d just been pwned. Except we didn’t call it that because the word hadn’t been invented.
Eventually, I gave the teacher one of my two mercury wetted switch modules from the heirloom chemical box. Each module has about 500g of Hg and electrical contacts in a glass module. I make a point of not dropping that box….
While metallic mercury is so safe that even a child can survive exposure, organic mercury compounds are a very different story. Monomethylmercury is worth avoiding (I think that’s what’s in fish), and dimethylmercury is worth avoiding as though your life depends on it. It soaks through skin and latex gloves offer about 15 seconds of protection. Merely 400 mg will kill you, slowly.
See http://www.nejm.org/doi/full/10.1056/NEJM199806043382305 for the best documented case. It ends with “Before she lapsed into a vegetative state, the patient [Karen Wetterhahn at Dartmouth College] requested that her case be presented to the general medical community, to scientists working with mercury, and to toxicologists, in the hope of improving the recognition, treatment, and prevention of future cases of mercury poisoning.”
Willis:
This is outstanding. You have done a very good job and have scientific reasons with your analysis. Thank you Sir!
Those are merely facts. Facts are of no importance when you are establishing a Socialist state.
Might I suggest some background information about the toxicology of mercury, including it’s organic forms, ethyl- and methylmercury: http://www.nejm.org/doi/full/10.1056/NEJMra022471
After all, the health impacts depend on this.
Source http://www.naturalhub.com/natural_food_guide_seafood.htm
For years, we have probably eaten tuna and swordfish with mercury levels above FDA’s limit without harmful effects. Analysis of museum specimens of tuna caught from 1879 to 1909 reveal that they contain levels of mercury as high as those in fish being caught today. Scientists therefore conclude that mercury levels in tuna, and probably swordfish, have not changed in the past 100 years…Researchers found that some fish, including tuna, can block and reduce the toxicity of mercury in their tissues. This research may explain how we have safely eaten fish containing levels of mercury higher than allowed by FDA
Next thing you know, the greenies and the EPA will be banning banana’s because of radioactive potassium. Always a new boogie man under the bed to scare us with. Screw ’em.
Where you have rainfall, Hg2+ will dissolve and end up underground (and eventually trapped as HgS), in plant material (such as the lignin of tree rings) or carried away. Low rain fall results in very poor mobilization, so deserts will have higher surface levels of Hg0, CH3HgCl and HgCl2.
You have to examine the influx rate, airborne delivery, and efflux rates, water transport below the surface, into the sea or into plants.
Coal burning increases the airborne influx, however, if there is a lot of water, the area is scrubbed clean, and the mercury is carried away, until it gets to a lake or estuary.
here is a nice paper which covers the fluxes in the US:-
http://cdn.intechopen.com/pdfs/32914/InTech-Hydrology_and_methylmercury_availability_in_coastal_plain_streams.pdf
Gold miners would use mercury to make gold amalgams, sucking the gold out of rock. They would then heat the amalgam, vaporizing the mercury and leaving the gold behind. This introduced huge amounts of mercury into the environment.
You can examine tree ring for chronology and mercury, using X-rays spectroscopy. This is often used to track changes in local changes in mobile mercury levels.
The odd thing about this is that deposition of mercury, and other heavy metals, precedes exposure, as the metals are chelated in soft lignin, so the mercury diffuses into the rings that are in the process of hardening.
There is a large body of literature on using tree rings and X-Ray spectroscopy to monitor heavy metal toxicity. I have always suspected that the ‘divergence’ in X-Ray derived ring density temperature is due to the various clean air acts and the removal of lead from gasoline.
With regard to fish, methyl mercury is perhaps a bigger issue. MeHg is produced by bacteria, and is very soluble in water. Dimethyl mercury is produced in high pH environments and is more volatile. Mercury can be sequestered in sediments in aquatic environments and mobilized depending on various factors including dissolved oxygen, pH, and temperature. The biological implications have more to do with biology than the rather simplistic chemistry being discussed.
EW-3 says:
April 1, 2012 at 5:26 pm
George says:
April 1, 2012 at 5:19 pm
Mercury does occur naturally and some of these environmental commissars need to be aware of that fact.
===============
Fact is they don’t care. It’s not about pollution, it’s about political objectives.
Someone in Congress ought to question the EPA on this. Let everyone be aware that the EPA is purely looking for grounds to shut down baseload power generation, to ensure that the power companies are bankrupted as predicted. This is an end-run around Congress after all.
Steve from Rockwood’s comment about sample density is well taken. Willis showed where the coal stations are, but the sample stations will have uneven weight on areas in the contour maps.
Here are two maps that show 2009 sample stations:
Total Mercury:
http://nadp.sws.uiuc.edu/maplib/archive/mdn/2009/2009MDNconc.gif
Lots of green east of the Mississippi River from dozens of stations. Only S. Florida is moderately high surrounded by ocean.
But that hot spot in the SW USA, is controlled by only 5 stations: 1 on all of AZ, 1 in SLC, 1 in Durango, CO, 2 in N. Nevada (gee, I wonder what is going on there!) There are only 2 stations along 1-10 from California to East Texas.
No data for Oregon. 3 in all of California.
Wet Mercury:
http://nadp.sws.uiuc.edu/maplib/archive/mdn/2009/2009MDNdep.gif
This one cries out for examination. If those are indeed the control points, just how does one justify the hot spots in NW Nevada and SW Colorado?? Yes, Durango is at 11, but the uncontrolled, extrapolated(!) hot spot high 100 miles NE of Durango is almost double that! Likewise southern AR is extrapolated at least 50% higher than any nearby control points. This contouring algorithm stinks!
Show the control before showing the interpretation!
Just think about all the black soot from diesel truck emissions caused by moving all the garbage from New York City, Long Island, and New Jersey to Pennsylvania and West Virginia.
What caused this insane bucket brigade of 18 wheelers? Why the theoretical mercury emissions from garbage to energy incinerators which were effectively banned.
It is estimated that between 5 and 10 percent of the diesel fuel use in the CT, NY, NJ metropolitan area is consumed moving trash which could otherwise be incinerated, thereby MAKING electricity rather than buring diesel.
And, the theoretical deaths due to emission from never to be built incinerators pales in comparison to the actual deaths caused by long haul garbage truck accidents.
Errata: I mistakenly said that Willis showed the coal stations, when in fact, the dots were the sample stations. However, Figure 3 shows far higher spatial frequency than can be justified by the control points. Just look at NE Calif, n of Yosemite. Somehow, Figure 3 is some modeled interaction of control points and terrain. Both Fig 1 and Fig 3 do not show the values for the control points, so who knows what the contouring algorithm and model are doing to the real data.
It is way more complex that that too. When you get into the far north right across the continent we inf water shed hot spots what are mostly related to natural Hg from the rocks. Hg is often used as pathfinder trace element in base and precious metals exploration. That is not to say we need not be careful with how we dispose and handle it. We should be careful and prudent not paranoid. We also need to learn that just because we can measure things in concentrations of three or four decimal points does not automatically equal bad.
“And that, dear friends, was a very big surprise … when’s the EPA gonna step in to save me?”
Be careful what you wish for…..
Yep, in every home too. Dozens of little 5mg Hg hand grenades. Compact fluorescent light globes, whoever came up with that idea has had more than a background dose.
The elevated concentrations of mercury about the west coast of the US might also be coming from the soils, as the young volcanoes in the region would also increase the amount of natural mercury. Mercury is higher in various fluids and rocks within volcanic terranes.
Not surprised, plenty more “everyone knows”.
Good post.
Mods: an update pls
Another masterly bit of work by Willis.
Which needs to be published far more widely.
The EPA has turned into some sort of ravenous monster. I was always afraid of the UN taking over the world, but their incompetence always reassured me.
But now I fear the EPA! With unlimited power, answering to no-one, flinging national economies this way and that with joyful abandon, and with only a token hat tip to logical thought processes, it is a far more powerful and dangerous beast.
Thank you very much for this contribution.
I’d heard sometime back about more than one “kind” of mercury and that one is dangerous but the other isn’t (within reason). I’ve also overdosed on EPA stupidity. JunkScience.com had a really great one about a Chinese pollution study with grunge numbers an order of magnitude higher than the new EPA danger numbers but that completely gutted the EPA’s estimated death count from particulates.
Now, is there a case for long term suspension of the mercury? could the stuff be going to Africa? In fact, some dust and pollen apparently cross the Atlantic, so I’m wondering if we’re not just shipping the stack garbage further than might be thought. Anyone got any info on that idea?
Steve from Rockwood says:
April 1, 2012 at 5:19 pm
Good point that the national sampling grid shown in these plots is not of sufficient density to detect any hot spots that might occur near large coal-fired power plants. However, I have reviewed a few studies that looked at soil and vegetation samples in a dense pattern around power plants. These studies found no hot spots, but that was a few years ago, so perhaps later studies found some evidence of hot spots. I have not had time to look through EPA’s references for the rulemaking. Presumably if there were convincing data showing hot spots near power plants, EPA would have presented it as support for the rulemaking.
The EPA with its linear, no-threshold concept of heavy metal toxicity. Plus, postage-stamp the mapped area, take the pattern out of context, and you get as much alarm as you wish. And change the colour scheme to emphasize the highest level, however low it is.
You cannot get the whole country behind a national program unless the whole country feels threatened. The EPA with its 50-year message of pollution threatening the nation (now the world), is trying to get the national response it needs for federal power and regulation. Everyone pays for some people’s problem or some people’s potential problem. It’s a war mentality gone viral.
Or they are intentionally trying to poison us with mercury so we become infertile. http://en.wikipedia.org/wiki/Young%27s_syndrome
The maps above have a mid range value of 10 micrograms/m^3. if a Compact Fluorescent Bulb has 5 mg, that is 5000 micrograms. To dilute that to below 10 ug/m^3, you need about 500 m^3 of air, but that would be a room of 3 m by 6 m by 28 m, or a room (or house!) with 1800 sq ft with 10 foot ceilings.
Which points out one of the major problems with the EPA and their pollution control efforts. Modern technology can detect various chemicals at orders of magnitude smaller quantities than can be shown to have any medical consequences. Those low thresholds of detections also must include error bars.
If you are detecting a contaminate at nano grams / liter, what is the precision of that measurement? At those low concentrations the difference between being detectible and nothing is very small. That suggests that the inherent noise in the data is probably very large.
How many samples were taken at each location, over what time period? What was the scatter in those measurements? Are those values the peak value detected, the median or average of multiple measurements or just a single measurement at some point in time?
Just because it is detectable and measurable, does not necessarily mean it is hazardous. Just like nuclear radiation, many assume a linear relationship with exposure and harm for chemical exposure, but what if toxic chemicals have a threshold below which the body copes with no negative consequences? What if like selenium at very low doses mercury is harmless or even essential. Without understanding those aspects of biological action the gross concentration may or not mean anything at all.
How far below the minimum threshold of detectable clinical biological consequences are these measurements? Are these levels 10x or 100x or 1000x times smaller than doses that are indistinguishable from no exposure?
With many heavy metals the method of absorption is as or more important than the gross level of exposure. A metal as a citrate for example might be orders of magnitude more soluble and biologically more reactive than the same metal in the form of an oxide or other compound form.
Some forms might be easily excreted with no biological action and other easily absorbed and captured by the tissue and biological processes.
Larry
By way of some reassurance, we all have a heavy metal detoxification system in our livers. It’s a set of proteins called metallothioneins, that are rich in sulfur amino acids. Sulfur is very effective at attaching permanently to mercury.
So, most of the mercury we eat ends up being trapped by the metallothioneins in the liver and permanently hidden away. The mercury never moves and never leaves the liver (until we die), and doesn’t get into the rest of our body.
Metallothioneins are also very effective at trapping other toxic heavy metals, such as cadmium.
The fact that we all have well-developed metabolic heavy metal traps shows that these metals have always been in our diets. The metallothioneins are evolutionary answers to perpetual heavy metal exposure over long evolutionary times.
Plants have similar proteins, developed for the same reason: heavy metals are in soils and have always been in soils. Plants evolved the proteins to defend themselves against heavy metal toxicity, from time immemorial.
That’s not to say we shouldn’t be careful about emissions. But it is to say that we’re well-equipped to protect ourselves from small chronic exposures.
Actually, Willis, if you live north of SF Bay and east of Clear Lake, you’re living in cinnabar country. Cinnabar is mercuric sulfide (HgS). There are large surface outcroppings all over that area. Plus hot springs. Some of the creeks are full of dissolved mercury that has washed off the outcrops.
I’ve been to Wilbur Hot Springs a number of times, in that area. The local hot creek washes down off the hills and the shores are rimed yellow with elemental sulfur. Bacteria live on the sulfur, and scads of black flies graze on the bacteria. Old cinnabar mine shafts dot the hills, with poorly blockaded entrances. Some of them still sport rusty equipment.
South of San Jose are the old Almaden mines, where cinnabar was mined for the mercury to use for gold production. Lot’s of surface outcrops there, too.
Apart from the cinnabar, of course, there’s the poison oak. Of the two, I tend to be more cautious of the poison oak. One should never, ever, take a leak after even a hint of a bare possibility of maybe having touched a plant. Lady-folk have a distinct advantage there.
I was a kid back before the advent of fluoridation, so by the time I was a teen I had a bunch of silver-mercury amalgam fillings. After fluoridation set in, nationally the number of cavities declined, and thus the number of amalgam fillings went down. As modern resin fillings improved, even fewer amalgam fillings, less mercury to leach from our teeth into the body.
We’re told that mercury affects intelligence, and yet, the national IQ has declined since the 50’s. Could it be that a little mercury is like a little radiation, essentially harmless?
Willis says “Does this mean mercury is not a poison? By no means, mercury is a bad thing, and it’s everywhere … it just shows the story has lots of tricks and turns.”
First of all Willis, excellent article in my opinion… very well done.
However in regards to what is in the “”. While it is clear that Hg, especially organic mercury, can lead to poor health and/or be fatal in high enough concentrations, I think what your data clearly shows is that at the current levels of environmental Hg concentrations, Hg is not poisonous. Are you sick? Your neighbors? Do you know ANYONE in your area that has died or is sick due to Hg poisoning from air or eating to much fish? I also think it clearly shows that the Hg EPA rules in effect for the past 20 years are quite sufficient for dealing with Hg emmissions from power plants. The new rules being implented will not save lives or make people healtthier. In fact they will have just the opposite effect as it drives up the cost of energy (which increases the cost of EVERYTHING) and lowers the standard of living for all. Lower standards of living leads to more sickness and death.
As one who enjoys gold dredging as a hobby the part realy missing from all the other studies I have seen is a inventory of natural deposets of what ever they are doing a study on. In SW WA USA they would like to blame on the old minors but the only time we pick up Mercury is when dredging on a low grade Cinnabar ore vains or deposit of sands from that ore body. The natural geological events of erosion and large events like the Missoula flouds have spread sands of ore deposits hundreds of miles away with out even looking at where anchent rivers once where that are no long around. Man made may be more because of road cuts through ore bodies and the rock used to build roads and pave them. The hazmat from road cuts and building makes the EPA wories a BIG JOKE with a little understanding of mining geology.
Hazard Summary-Created in April 1992; Revised in January 2000
Mercury exists in three forms: elemental mercury, inorganic mercury compounds (primarily mercuric chloride), and organic mercury compounds (primarily methyl mercury). All forms of mercury are quite toxic, and each form exhibits different health effects.
Acute (short-term) exposure to high levels of elemental mercury in humans results in central nervous system (CNS) effects such as tremors, mood changes, and slowed sensory and motor nerve function. Chronic (long-term) exposure to elemental mercury in humans also affects the CNS, with effects such as erethism (increased excitability), irritability, excessive shyness, and tremors. Human studies are inconclusive regarding elemental mercury and cancer.
Acute exposure to inorganic mercury by the oral route may result in effects such as nausea, vomiting, and severe abdominal pain. The major effect from chronic exposure to inorganic mercury is kidney damage. Animal studies have reported effects such as alterations in testicular tissue, increased resorption rates, and abnormalities of development. Mercuric chloride (an inorganic mercury compound) exposure has been shown to result in forestomach, thyroid, and renal tumors in experimental animals.
Acute exposure of humans to very high levels of methyl mercury results in CNS effects such as blindness, deafness, and impaired level of consciousness. Chronic exposure to methyl mercury in humans also affects the CNS with symptoms such as paresthesia (a sensation of pricking on the skin), blurred vision, malaise, speech difficulties, and constriction of the visual field. Methyl mercury exposure, via the oral route, has led to significant developmental effects. Infants born to women who ingested high levels of methyl mercury exhibited mental retardation, ataxia, constriction of the visual field, blindness, and cerebral palsy.
DocM;
Evidently your mercenary nature overcame your editorial senses:
“They would then heat the amalgam, vaporizing the mercury and leaving the gold behind. This introduced huge amounts of gold into the environment.”
I think you said the vapors were Hg, not Au??
🙂
[Very good. I figure it wasn’t his mercenary nature that did it, it was the mercury fumes. I fixed DocM’s original for him, many thanks. -w.]
I wonder if this topic contains any clues as to the origins of Willis’ mercurial nature …
|8-0 !
John B., M.D. says:
April 1, 2012 at 6:30 pm
Thanks, Dr. John. Indeed organic mercury is highly toxic, and we should not forget that.
We should also not forget that with respect to the US, the sources (see Figure 2) are overwhelmingly natural.
w.
thingadonta says:
April 1, 2012 at 8:12 pm
Figure 3 shows where the mercury is precipitating from the atmosphere. It does not show mercury in the soil, from the volcanoes, or from mercury mine tailings.
w.
aired says:
April 1, 2012 at 8:23 pm
A good point indeed, aired. The sampling density is not enough to show an individual power plant. But the point of the scientific article I quoted from is that the sampling network should pick up Hg deposition downwind from the large number of coal-burning power plants and industrial furnaces in the Ohio River Valley. The fact that it does not argues strongly for the authors’ conclusion, that the HgII is getting reduced to Hg0 shortly after leaving the stack, and thus is widely diluted and added to the atmospheric background elemental mercury levels, rather than coming back to the downwind surface fairly quickly as divalent mercury.
w.
stpaulchuck says:
April 1, 2012 at 8:19 pm
A good question, chuck. The “residence time” for something in the atmosphere is generally measured as the length of time an average atom or molecule stays suspended in the atmosphere.
For elemental mercury (Hg0) that’s thought to be on the order of a year. So indeed, in that year it will circle the earth many, many times.
You are correct that we are indeed just shipping it far afield. The red area in Figure 3 where I live on the California coast and in the Sierra Nevada mountains shows that the ocean is a large source of mercury. This means mercury has been falling from the sky in those areas for millions of years. Florida has been the unlucky recipient of oceanic mercury poisoning for as long as the peninsula has existed.
And in addition to that mercury from the ocean, some of that red area in Figure 3 where I live is from the background atmospheric elemental mercury. So some of it is from diesel Mercedes in Brussels, some is from Chinese coal seam fires, and some is undoubtedly from the power plants in the Ohio River Valley. They don’t deposit their mercury between there and Pennsylvania as we thought. They deposit it on my house.
But look at the airborne concentration in Figure 2. As air passes over North America, it picks up a load of mercury, enough to turn the whole southwestern US red. Why? Wind over the desert. There’s natural mercury in those soils. When there is no vegetation to hold it down, wind-blown dust is common, and that dust puts mercury into the air.
Is that a good thing? Neither the mercury from the power plants nor the mercury from the Southwest deserts and arid regions are good things. No, absolutely not, hey, it’s my house they’re raining out on.
But will the EPA regs make a difference? Less than 1%. The amount of red in the picture will change by maybe half a percent … that’s lost in the noise. Look at Figure 2, the mercury load in the atmosphere. You’d have to pave from the Mojave desert in California right through to Texas to make a meaningful dent in the amount of mercury coming from the wind in the US deserts, the US power plants are nowhere near that league …
Meaningful change in power plant emissions, sadly, will have to occur in other countries than the US. Despite the huge amount of power generated from coal, we’re only 8% of global power plant mercury emissions.
w.
Willis:
This is ‘déjà vu’ again. And history shows the scare poses a long-term threat.
I was working at the UK’s Coal Research Establishment (CRE) in the 1980s when ‘Acid Rain’ was Europe’s ‘big scare’. It was being claimed that sulphur oxides (SOx) emitted from coal-fired power stations in the UK and Germany were causing ‘waldsterben’ (i.e. forest death in Germany and Scandinavia). SOx is very water soluble and the solution forms a dilute acid.
It was argued that flue gas desulphurisation (FGD) was needed to prevent emissions of SOx from coal-fired power stations which caused ‘acid rain’ that was killing the forests. (We now know the forests were expanding at the time, but that was not then known).
FGD adds about 20% to the capital cost and about 10% to the operating cost of a coal-fired power station. This is a major increase to the cost of coal-fired electricity.
The matter gained great importance because coal was the major source of electricity generation in the UK and Germany, but France had adopted nuclear power as its major electricity generation source. So, enforced addition of FGD to coal-fired power stations would improve the commercial and industrial competitiveness of France relative to the UK and Germany (i.e. France’s major competitors).
But I observed that the spatial distribution of the acidity of rain was not consistent with the scare. There was no evidence of ‘waldsterben’ near power stations which is where ‘acid rain’ should have been greatest (SOx is VERY water soluble). Importantly, greatest acidity of rain was near outflows from major rivers. Emissions from UK and German power stations would be required to cross high-deposition zones near river outflows if they were to reach Scandinavia.
I suggested that the increased acidity of rain and its spatial distribution of deposition were consistent with an acceleration of the natural sulphur cycle in the North Sea. Excess nitrogenous and phosphorous agricultural fertiliser was being transported to the North Sea where it was increasing growth of phytoplankton such that they emitted more dimethyl sulphide (DMS). This would explain the observed spatial distribution of the increased acidity of rain. And the explanation was supported by the fact that North Sea phytoplankton had increased to such a degree that the phytoplankton were forming toxic algal blooms that were affecting shores.
Upon investigation it was discovered that the agricultural explanation was correct. France had a powerful farming lobby so the ‘acid rain’ scare was quietly forgotten. But by then Europe’s Large Combustion Plant Directive (LCPD) had been adopted as legislation to prevent SOx emissions from European power stations, and the jobs to impose and enforce the LCPD had been created.
The people fill those jobs need to justify their jobs so they keep reducing the emission limits decreed by the LCPD. The latest of these reductions is closing the UK’s remaining coal fired power stations in the next four years although the reason for the LCPD was disproved a generation ago.
Richard
PS
Please note that SOx emissions can be a problem especially near the emission sources. But the ‘acid rain’ scare and the LCPD are not relevant to this.
Jon Alldritt says:
April 1, 2012 at 10:38 pm
We’re nothing if not a full-service website. I can’t find such a map for the US, but here’s the cinnabar mines (crossed pick and shovel) in the Coastal Range of California.

SOURCE
Indeed, that’s what makes the large red area in the center of Figure 2, natural sources.
And if you look at Figure 3, you can see that like where I live in California, in Washington and Oregon the air from the ocean rains and snows out the mercury in the first two mountain ranges. So a main source of mercury in the soil and water is from the ocean.
This doesn’t let the old miners off the hook, though. From the source of the map above, it says:
Not a pretty picture …
Good luck with your gold dredging. When I was a young man I spent far too much of a summer underwater, wearing a hookah and running the business end of a 12 inch gold dredge in the Yuba river in the Sierra Nevada mountains. Found about an ounce … gold was $32 an ounce then … a 12 inch dredge, I just look back and shake my head, I was young and foolish.
w.
Heystoopidone says:
April 1, 2012 at 11:30 pm
Indeed, I can only agree and emphasize, all forms of mercury are toxic. Liquid mercury can be handled without harm, many of us did it as kids, although I wouldn’t advise it and you’d want to wash your hands well.
But the exact same elemental mercury we handled, when inhaled, is what drove the hatter mad …
w.
PS—If you don’t mind my saying, that’s an off-putting screen name. Seems like you are either calling me or yourself “stoopid”, and neither option is very pleasant. You might consider taking another name if you want your words to carry more weight.
Willis: “Elemental Mercury” is Hg, not HgO. HgO is Mercury Oxide. I guess you wanted to put Hg° (Hg0 with the “zero” as superscript, which is a correct way to describe metals in their elemental form). Just for clarification, it was misleading for me in the first moment.
Might I respectfully suggest that non-chemists be careful about the use of chemical nomenclature. Hg toxicology is heavily dependent on chemical speciation and there is no such chemical as either methylmercury or ethylmercury despite the gay abandon with which such terms are used.
These entities are ions better expressed as MeHg+ or EtHg+. The toxicology of these ionic species is entirely dependent on the counterion to which they will always be attached in nature. If this is a simple halogen such as chlorine or bromine then you have methylmercuric chloride which is highly toxic readily absorbed through the skin and has a very high bioaccumulation potential. This is the substance that was released from the acetaldehyde plant at Minamata in Japan which accumulated in fish and resulted in the poisoning of the Japanese population in 1956. Similar organomercury compouds were used as seed dressings in the 1960s and 70s and misuse of which produced the much larger death toll in Iraq.
However, as mentioned in a previous comment Hg is very easily bound to S’ groups in proteins and this is how MeHg+ is found in normal fish tissues i.e as MeHgS-Protein compounds. As such the toxicology is very different to that of simple organomercury compounds such as MeHgCl. Breaking the Hg-S bond in the protein in order to release the toxic MeHg+ ion is very dificult and reference to Westoo’s original analytical method to determine the organic mercury content of fish clearly demonstrates the highly aggressive chemical treatment of the tissue taht was necessary to release the Hg in a form that could be measured.
So beware a little knowledege can be a dangerous thing……
Willis, this article would make more sense to newcomers if you changed the “I” to “Anthony” below Article 3 where it mentions “I live on the coast not far north of San Franciso Bay.” It is not immediately obvious that this post has two authors. — John M Reynolds
The estimated lifetime of elemental mercury in the atmosphere is around a year as another poster has noted. That means there is a global dispersion of airbourne mercury and local hotspots are due to deposition of soluble mecuric compounds from oceans/lakes more than from coal fired power plants.
Anthropogenic sources emitt around twice as much mercury (elemental and soluble) as natural surces overall. This is most notable where local industrial sources have generated Hg II compounds with beluga tusks now containting over 10x as much mercury now as they did in pre-industrial times.
It would be very hard to make a case for NOT regulating industrial mercury emissions. They are NOT harmless, they are exceeding natural exposure by around 100% and are cummulative in the food chain. This essay does nothing to refute the need to regulate mercury emissions. The vauge ‘handwaving’ about the sampled deposition rates are arguements for the complexity of the deposition process and the inadequacy of the monitering system, not a basis for the industry to deny the need for emission controls.
Figure 2 shows an improvement in the mercury levels around quite a few of the power stations, or is this just an artifact in the digitisation of the map?
Arthur Dent, alas, there is a little problem with your thiol trapping thesis. It is true that methylmercury(1+) does react with thiols (and selenols), displacing a proton. However, it is not locked away. Cysteine is the normal protein thiol species, and when the protein is digested within or outside the cell, you still have Cys-S-Hg-CH3 or Cys-S-Hg(+). Our amino acid transporters are unable to spot the difference between Cys and Cys-S-Hg-CH3 or Cys-S-Hg(+).
These two conjugated cysteine conjugates are also toxic, the former disrupting methionine pathways and the latter attacking mitochondria.
Willis Eschenbach says:
April 2, 2012 at 1:58 am
That’s what I thought too, but I looked into it a couple years ago and it turns out they used HgNO3 (or would it be Hg(NO3)2? Haven’t checked.)
Page with properly serious tone: http://www.cas.org/newsevents/connections/hatter.html
Page with Lewis Carroll tone: http://corrosion-doctors.org/Elements-Toxic/Mercury-mad-hatter.htm
The chemical triggers connection along hair protein cause it to kink and shrink, an important step of the felting process.
jmrsudbury says:
April 2, 2012 at 3:25 am
Clue 1: Anthony lives in Chico. That’s inland.
Clue 2: Willis was at the dinner yesterday.
One aspect to consider is microbial transformation. It’s not suprising to me to find that the majority of HgII is found is areas upstream from water bodies, as it is in water where microbial transformation of Mercury occurrs. Bacteria play a crucial role in methylating mercury, thus converting it from inorganic to organic. The organic form is what is incorporated into the food chain.
There is no doubt that Coal Fired plants increase Mercury load, but this load is mistakenly being identified as “natural” or elemental, simply because it is not raining out as HgII. As the elemental mercury slowly precipitates out of the atmosphere, a good portion deposits in water, and it is in water where the problems occurr. After methylation, the newly formed HgII is taken up in evaporation and then rains out. This is why your pristine west coast has such high mercury deposition, because the transformation occurs in the pacific.
I haven’t looked at the studies, but the more relevant marker would be the change in Hgii presipitation or change in methyl mercury concentrations in fish populations. I would expect, given the slow deposition rate of Hg0 that is becomes well mixed in the atmosphere, and thus, any increases resulting from coal fired plants would be noticed on a time scale.
From my ever useful “Nature’s Building Blocks” by John Elmsley . .
Minamata Bay in Japan was a case of mercury poisoning in the 1950’s. A local chemical company had discharged about 100 tonnes of mercury a year for 30 years into the bay. The bugs in the silt were eaten by the fish resulting in them containing 0.2% of their mass in mercury.
People ate the fish and about 10 000 folk were crippled with Minamata disease. Once the cause was figured out the factory implemented a mercury recovery process and banned fishing in the bay. No new cases were reported.
The real number here is 3000 tonnes of mercury resulting in fish being 0.2% mercury. Nanograms don’t really measure up.
Humans take up about 3 micrograms a day by way of food that gathers mercury from the soil. This is not considered harmful.
We have in our system . .
Blood 8 p.p.b.
Bone 0.5 p.p.m
Tissue 0.2-0.7 p.p.m.
Giving about 6 milligrams per person.
I guess the take away message is “keep calm and carry on”.
izen:
Your post at April 2, 2012 at 3:27 am consists solely of unjustified assertions that are denied by data in the above article. If you have contrary data then please state it. Otherwise, please withdraw your comment.
The important point is that Hg emissions are trivial when compared to natural sources. This is clearly demonstrated in the above article where it says;
“In fact, where I live, on the pure clean Pacific coast with lots of sea breeze, I get more mercury pollution than they get around Pennsylvania despite all the coal-burning power stations just upwind from them.”
Richard
PS
You may wish to consider changing your screen name from Izen. It always makes me think of Izal which was a cheap, abrasive and rather unpleasant toilet paper that used to common in the UK. Unless, of course, you also think that association is appropriate to your comments.
I find it strange that both Figure 2 and 3 show a power plant in Northwest Montana, right where Hungry Horse Dam sits. How error prone is power plant sighting? http://en.wikipedia.org/wiki/Hungry_Horse_Dam
Articles like these are the best form of informative science. I have learned so much about the various Hg chemical species, information that would have required serious study to find all of the various parts so clearly presented here.
From my point of view, most of us consider elemental Mercury dangerous, and ubiquitous, and Mercury in more chemically active states, an extreme hazard. At the same time, based on the excellent maps Willis has included, as well as the research papers, the EPA’s war against Hg is as close to Don Quixote as anything I can imagine.
Thanks again Willis!
Deanster:
I write to comment on your post at April 2, 2012 at 6:27 am, and I copy that entire post to avoid others needing to find it. It says;
“One aspect to consider is microbial transformation. It’s not suprising to me to find that the majority of HgII is found is areas upstream from water bodies, as it is in water where microbial transformation of Mercury occurrs. Bacteria play a crucial role in methylating mercury, thus converting it from inorganic to organic. The organic form is what is incorporated into the food chain.
There is no doubt that Coal Fired plants increase Mercury load, but this load is mistakenly being identified as “natural” or elemental, simply because it is not raining out as HgII. As the elemental mercury slowly precipitates out of the atmosphere, a good portion deposits in water, and it is in water where the problems occurr. After methylation, the newly formed HgII is taken up in evaporation and then rains out. This is why your pristine west coast has such high mercury deposition, because the transformation occurs in the pacific.
I haven’t looked at the studies, but the more relevant marker would be the change in Hgii presipitation or change in methyl mercury concentrations in fish populations. I would expect, given the slow deposition rate of Hg0 that is becomes well mixed in the atmosphere, and thus, any increases resulting from coal fired plants would be noticed on a time scale.”
Your assertion may (or may not) be correct concerning accumulation in the air of microbially transformed Hg compounds. And you may (or may not) be right that it is would be possible to “notice” a change to atmospheric loading by anthropogenically-derived Hg compounds.
But it does not matter whether you are right or not.
Firstly, biota vary for many reasons with time so – if you are right – a change to the atmospheric loading may be observed but (as with recent increase to atmospheric CO2 loading) there would be no way to definitively “notice” the anthropgenic contribution as being other than natural.
Secondly, and very importantly, the anthropogenic contribution to Hg emissions is so small relative to natural emissions that it can be considered to be trivial. Indeed, as the above article explains, the local variations in atmospheric Hg ARE trivial compared to the local differences in natural Hg emissions.
Hence, you provide an assertion that is plain wrong when you say;
“There is no doubt that Coal Fired plants increase Mercury load, but this load is mistakenly being identified as “natural” or elemental, simply because it is not raining out as HgII.”
There is much “doubt” because your assertion is constructed from a series of assumptions that have no supporting evidence. And your assertion would be trivial even if it were right because the emissions from the coal-fired plants are trivially small.
Richard
OK, you’re convincing me, Willis. A nicely done presentation, and it does make sense. It also makes it a headache to track mercury sources — if we eliminate power plants as significant anthropogenic contributors, second in line is human spills and industrial dumping — e.g. gold prospecting (increasing levels in specific rivers, which BTW might be a potential source of your mercury excess in CA even now — otherwise it is difficult to understand the inhomogeneity of levels along the CA coast is it not), paper mills, and mercury used elsewhere in industry that makes or made its way into waterways. I can easily believe that the US southwest is a source of mercury vapor, as there is a rather lot of cinnabar (and where it concentrates, mercury mines) mixed in with the native rock throughout much of the region, IIRC. Indeed, I think there are several mercury mines (started during the Gold Rush) in central and northern CA — another potential source of your hotspot.
So if we care (if we should care, and I still would personally prefer to keep my Hg levels as low as possible in spite of your compelling presentation that it is difficult to resolve damage from background noise for moderate levels of exposure:-) we should focus on mercury ores and natural earthbound mercury and direct dumping of industrial mercury compounds and native metal, not so much power plants. Or at the very least, we should demand a complete and compelling presentation of a scientific and economic case for regulation of mercury emission from power plant smokestacks.
rgb
****
Mike McMillan says:
April 1, 2012 at 9:37 pm
As modern resin fillings improved, even fewer amalgam fillings, less mercury to leach from our teeth into the body.
****
The amalgam is a metallic alloy. The mercury atoms are as “locked” in it as much as a chemical compound. Tiny amounts “released” won’t be elemental mercury, but amalgam (which is stable & nonreactive).
You can die of water intoxication if you drink too much of it. Who can say that some tiny trace amount of mercury cannot possibly be beneficial?
If the FDA existed before the discovery of the value of vitamin A is there any doubt they would have just labeled it a poison?
According to Wiki (a sometimes reliable source), liquid Hg is not particularly dangerous:
http://en.wikipedia.org/wiki/Mercury_poisoning
This accounts for how all kids used to handle quicksilver with no perceptible ill effects. Is the Hg in curly lightbulbs liquid and hence not particularly dangerous unless incinerated? Does it become vapor when lit, so that an accidentally broken lit bulb would be the biggest concern? Why does the EPA allow these things?
But then Wiki says that human sources are responsible for half of atmospheric Hg:
I would wonder about the effect of southwestern dust storms, most famously shown by the recent haboobs in Phoenix, on the prevalent mercury in the local soils. In La Paz county in southeast Arizona. Mercury bearing veins in the quartz around Quartzite, such as the Cinnabar Mine, have been a source of concentrated mercury predating European colonization of the area. Cinnabar ore was used as a pigment prior to be used in gold separation. The mercury was extracted from the ore by heating. I wonder how much mercury was released from surface deposits by the recent forest fires in northern Arizona…..
beng says: April 2, 2012 at 7:55 am
“The amalgam is a metallic alloy. The mercury atoms are as “locked” in it as
much as a chemical compound. Tiny amounts “released” won’t be elemental
mercury, but amalgam (which is stable & nonreactive).”
The chemical bond between mercury and silver is very weak – unlike most
chemical bonds in compounds.
The silver does greatly slow evaporation, etc. of the mercury. I think what
is useful is data on concentrations of mercury in people with lots of amalgam
fillings, vs. none.
I’ve no ideas about other states, but in California there are both large natural and historic sources for mercury in the soil, water, and fish. Major mercury mines are or were located in the Northern Coast Range for just one factoid. One of the them is just north of SR 20 on the way to Clear Lake from the Central Valley. There are also significant amounts of arsenic and selenium coming out of the ground in both the North and Southern Coast ranges. The primary historic source of mercury in the Central Valley is from the massive placer tailings from the California gold rush. Mercury was used to amalgamate gold in sluice boxes, and the major hydraulic mining companies used tons of mercury each year of operation. The mercury and coarse gold were collected from the sluices and the mercury was then distilled off the gold (and reused if it was properly done) to concentrate the fine gold. Metallic mercury is still washing out of those tailings. In the 1950s it was possible to collect mercury from the Sacramento River sand bars by panning (as in gold panning, but we were kids and mercury was far easier to find and more fun).
Nothing like starting the week off by getting my BP raised – which is what happens whenever I am forced to think about the EPA and what they call science.
Thanks Willis.
(For helping remind people of the importance of being at least somewhat scientifically literate, so as to recognize when our nannys in government are overreaching.)
Sorry but the policy train has left the station. There is no stopping it now and the chase cars of lawyers and dealing-making Sierra Club will not be diverted by details and facts. It’s a storyline management thing.
Willis, EPA has already stepped in to save you. Just earlier this year, they released the “Utility MACT” rule that restricts emissions of mercury, trace acid gases (like HF and HCl) and fine particulate matter from power plants. It has been characterized as the most expensive environmental regulation ever issued. One source, http://junkscience.com/2011/12/15/utility-mact-600-million-in-costs-for-0-2-emissions-reduction/) says it will cost $600 million, EPA (http://www.usclimatenetwork.org/resource-database/assessment-of-epa2019s-utility-mact-proposal) says it will cost $10.9 billion per year. Per year! After a while you start talking about some real money, here. And coal plants are being shut down all across the country.
Although mercury is usually described in the media as a “potent, brain destroying neurotoxin,” actual EPA studies (from models, of course) say this rule prevents an average 0.9 I.Q. point decrease. This is not even measurable. So all of the benefit is from particulate emission reduction that has already been regulated, double counting the benefit. They do this all the time for the “sake of the children.”
Richard
“Your assertion may (or may not) be correct concerning accumulation in the air of microbially transformed Hg compounds. And you may (or may not) be right that it is would be possible to “notice” a change to atmospheric loading by anthropogenically-derived Hg compounds.
But it does not matter whether you are right or not.
Firstly, biota vary for many reasons with time so – if you are right – a change to the atmospheric loading may be observed but (as with recent increase to atmospheric CO2 loading) there would be no way to definitively “notice” the anthropgenic contribution as being other than natural.
Secondly, and very importantly, the anthropogenic contribution to Hg emissions is so small relative to natural emissions that it can be considered to be trivial. Indeed, as the above article explains, the local variations in atmospheric Hg ARE trivial compared to the local differences in natural Hg emissions.
Hence, you provide an assertion that is plain wrong when you say;
“There is no doubt that Coal Fired plants increase Mercury load, but this load is mistakenly being identified as “natural” or elemental, simply because it is not raining out as HgII.”
There is much “doubt” because your assertion is constructed from a series of assumptions that have no supporting evidence. And your assertion would be trivial even if it were right because the emissions from the coal-fired plants are trivially small.”
Richard
It’s true Richard, I have no way of separating out anthropogenic mercury from natural mercury, as there is no way to assertain the contribution of natural and anthropogenic to the inorganic pool of Mercury in the water.
All I’m saying, is that according to the paper, there apparently is some unknown mechansim wherein HgII gets converted to HgO. Once that conversion occurrs, the ability to track anthropogenic contributions becomes rather difficult. The premise of Anthony’s post is … hey .. look here … the high levels of HgII precipitation is not where the coal fired plants are, . therefore, the contribution of the coal fired plants must be negligible. I don’t necessarily agree with that. HgO, per the paper, has a resident time of 1 year. That’s plenty time for the emissions of coal fired plants to mix in the atmosphere, resulting in a pattern of deposition that will be unbiquitous, as opposed to correlating to the location of coal plants. Considering that the natural contribution of mercury to the system would be expected to be somewhat constant, a rise in HgII precipitation in places such as San Fransisco could very well be carrying a signal of increased HgO deposition in the ocean resulting from a power plant in Ohio. LIke I said, I haven’t looked at the data .. for all I know, HgII precipitation in San Fran has been constant for centuries … but if there is indeed a spike in HgII precipitation, there is a possibility that it is a response resulting from increased HgO deposition in the Pacific with origins in power plants thousands of miles away.
The main point I was making, is that Microbial Transformation of HgO to methyl mercury should not be ignored in this issue, as increases in HgO deposition will contribute to the HgII concentrations via microbial transformation.
@ Robert Brown.
Sir, you are almost there, but when you state “we should focus on mercury ores and natural earthbound mercury” are you suggesting we sent the EPA in haz-mat suits to scrub the natural environment? Join us in the intellectual shelter of rational natural science, and recognize that mercury ores and natural earth-bound mercury are part of the normal fabric of planet earth and that the inhabitants of this planet have over billions of years learned to adapt and exist with these natural chemical fluxes.
We should of course make sure our industrial processes do not impact on the natural process of Hg in the environment, but when we reach the point that our anthropogenic emissions (in the United States) are an insignificant contribution, then Sir it is time to focus on more pressing matters.
That was a great read. I never knew mercury could be so complex. It is bad stuff and its deposition along the coastal areas from the oceans was a complete surprise to me.
One of the many reasons I love this site. I learn so much that I never expected. One question concerning this statement:
“The mercury is being converted into elemental mercury along the way somewhere, so it’s added to the background mercury rather than raining out near the power plants.”
If it is being added to the background levels do we have any historical or proxy measures for mercury background levels? At what level is the background levels going to be an issue?
Arthur Dent says:
April 2, 2012 at 2:28 am
I fear that you are fighting a losing fight with that one, Arthur. A search of Google Scholar, not regular Google but science Google, turns up some 79,700 pages of scientific documents that us the term methylmercury. A search of Google Scholar shows another 14,600 pages with the variant “methyl mercury”. Ethylmercury brings up another 8.700 pages.
So I tell you what, Arthur. How about you take your crusade for proper nomenclature to the blogs AFTER you’ve gotten the other 100,000 of your fellow chemists and scientists, writing in journals like the Journal of Applied Toxicology and the British Journal of Industrial Medicine and the Journal of Neurochemistry and the Journal of Chromatography, to agree that you are right and to stop using the term.
You do that, and I assure you, the blogs will fall right in line … because when I see the term “methylmercury” used in places from the journal Environmental Toxicology and Chemistry to Nature magazine, I gotta assume that the agreement on the terminology is by no means as solid as you seem to think.
Thanks,
w.
PS—I don’t even find that your claim about the nomenclature is correct. You say there is “no such chemical as … methylmercury”. But in “Advances in Molecular Toxicology“, they point to a completely different issue:
So in contrast to your claim, they say there IS a chemical called “methylmercury” …
federico says:
April 2, 2012 at 2:04 am
Thanks, federico. I had written Hg0 with a zero, not HgO with the letter O, and I’m aware of the difference. WordPress doesn’t do superscripts except in Latex,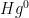 , but I try to avoid Latex because it prints small for some reason and is hard to read.
, but I try to avoid Latex because it prints small for some reason and is hard to read.
w.
jmrsudbury says:
April 2, 2012 at 3:25 am
Thanks, John, but it’s unclear what you are saying. The post only has one author, me. I live on the coast. Anthony lives in Chico, in the Central Valley. Not sure why you think he’s involved.
w.
izen says:
April 2, 2012 at 3:27 am
Thanks, izen. You’ll have to come up with a citation for both those claims. My previous post cited a paper which clearly shows that some 5,200 tonnes of mercury come from natural sources, and some 2,250 tonnes come from anthropogenic sources. So you’ve got the stick by the wrong end. Natural emissions are twice anthropogenic emissions, not the other way around.
And regarding pre-industrial beluga tusks … well, I find nothing.
Take a deep breath, my friend, you’re going to hyperventilate. The point of this article is not that we should not regulate mercury emissions. I did not say that, nor do I believe that.
What I do believe is that the regulations we have in place now are sufficient, and that the recently promulgated new EPA regulations are overkill.
Finally, I believe the findings that I highlight here, that the mercury from powerplant stacks is added to the background rather than depositing out near the power plants, means we have to look at HOW we regulate mercury. It says nothing about WHETHER we should regulate mercury, most reasonable people agree on that question, and the answer is yes.
w.
Deanster says:
April 2, 2012 at 6:27 am
That doesn’t make sense. Why would divalent mercury be found in areas “upstream from water bodies”? Who says it’s found there anyways?
Here I’ve lost you completely. No one here or elsewhere mistakes elemental mercury for natural mercury. I pointed out in the head post that elemental mercury is emitted from power plants, so that’s all being identified anthropogenic mercury, not natural mercury.
w.
Willis;
try Alt-248(numeric keypad). Hg°. Should work.
Greylar says:
April 2, 2012 at 7:00 am
Sorry for the lack of clarity. The dots are sampling stations, not power plants.
w.
Mercury in the Pacific coast ares makes perfect sense.
Where do you find Hg?
Tuna.
And where are tuna? In the Pacific.
I think I will apply for a grant.
Robert Brown says:
April 2, 2012 at 7:25 am
Thanks, Robert. A couple of comments. First, I put a map upstream which shows a host of cinnabar mines in the coastal ranges.
However, they are not the source for the hotspot of mercury raining down over my head for a couple reasons. First, they’re downwind of the coast. Second, in Fig. 3 you see the oceanic mercury all along the northernwestern coast of the US. Third, what is being measured in Fig. 3 is atmospheric deposition, not ground levels.
I have no problem with reasonable limitations of mercury from smokestack emissions. And in some countries it’s obviously necessary. The US generates 25% of the worlds electricity, and burns 21% of the world’s coal, but only puts out 8% of the global mercury emissions from power plants. And for overall emissions its even worse, we only emit about 6% of the world’s mercury.

In the US, I’d argue we’re up against diminishing returns in terms of stack emissions, particularly when you include the most recent regulations. As a result, we should focus (as you suggest) on the other sources. As a reminder, from my previous post, here’s what those other sources are:
Total US mercury emissions are less than the mercury emissions from the burning of the rest of world’s trash …
w.
Deanster says:
April 2, 2012 at 10:00 am
Not true. Anthropogenic emissions are about half natural emissions, which is smaller but by no means “trivial” when you are talking about a poison.
w.
Brian H says:
April 2, 2012 at 12:14 pm
Of course, the degree symbol, Hg°. On my Mac that’s shift-option-8, never thought of using that.
w.
@- richardscourtney says: April 2, 2012 at 6:59 am
“Your post at April 2, 2012 at 3:27 am consists solely of unjustified assertions that are denied by data in the above article. If you have contrary data then please state it. Otherwise, please withdraw your comment.
The important point is that Hg emissions are trivial when compared to natural sources. This is clearly demonstrated in the above article where it says; …”
If the above article did ‘clearly demonstrate’ that HG emissions are trivial when compared to natural sources it would not only deny my assertions but overturn all the contary data that I will be happy to present. I think you might have nis-read the above article. It does not claim that anthropogenic Hg is trivial when compared to natural sources. Just that the airbourne emissions from coal-fired plants are not detected (with the given sampling system and Hg/bioconversion cycle) locally.
ftp://info.mcs.anl.gov/pub/tech_reports/reports/CGC-003.pdf
Atmospheric Mercury – Global Biogeochemical
Cycles, Sources, and Sinks
Richard D. Doctor, 1 John A. Taylor, 2 and Jack D. Shannon3
1. Overview
Measurements of atmospheric mercury (Hg) at remote locations, such as the North Atlantic, confirm that the levels of mercury in the global atmosphere are increasing. In spite of considerable effort, estimating the global movement of mercury through the environment is difficult. However, anthropogenic emissions of mercury are at least double those from natural sources and are of such magnitude that they cannot be attributed to natural sources alone [Fitzgerald et al. 1998].
http://www.mercurynetwork.org.uk/wp-content/uploads/2011/10/IKIMP_Workshop_Report_Mercury_Cycle_2011.pdf
While the magnitude of the natural mercury flux (e.g., volcanic, evasion from oceans) is uncertain, it is clear from many studies of historical mercury accumulations in peat bogs, lake sediments, ice cores and biological samples that the global atmospheric mercury burden has increased on average by a factor of 3 ± 1 since the industrial revolution[1]. In some regions localised Hg pollution in soils has increased 10-fold. The impact of this increase on biota is considerable: Fig. 2 shows the steep rise in mercury in animal tissues from the Arctic since 1900.
http://www.agu.org/pubs/crossref/2008/2007GB003040.shtml
The spatial distribution of soil concentrations is derived from local steady state between land emission and deposition in the preindustrial simulation, augmented for the present day by a 15% increase in the soil reservoir distributed following the pattern of anthropogenic deposition. Mercury deposition and hence emission are predicted to be highest in the subtropics. Our atmospheric lifetime of mercury against deposition (0.50 year) is shorter than past estimates because of our accounting of Hg(0) dry deposition, but recycling from surface reservoirs results in an effective lifetime of 1.6 years against transfer to long-lived reservoirs in the soil and deep ocean. Present-day anthropogenic enrichment of mercury deposition exceeds a factor of 5 in continental source regions. We estimate that 68% of the deposition over the United States is anthropogenic, including 20% from North American emissions (20% primary and <1% recycled through surface reservoirs), 31% from emissions outside North America (22% primary and 9% recycled), and 16% from the legacy of anthropogenic mercury accumulated in soils and the deep ocean.
———————————–
Hope that clarifies why there is certainty that anthropogenic sources of Hg are known to exceed natural ones at present Richard.
Note that this may be why there is not an obvious local deposition pattern around power plants. local soil deposition may result in a re-release as bioconversion occurs and allows the Hg0 to spread globally.
PS – I will continue to use the name izen even if it does remind you of a toilet paper.
I expect you will continue to use yours, even if I am remineded of its diminutive – Dick.
[Moderator’s Note: Fine. Tit for tat. Now enough. -REP]
Gee, I guess there is some air dam that blocks mercury from reaching Mississippi and east TN when you get to the big muddy(?). Either that or the fishing is really bad there, or EPA staff balked at going there, or they forgot to air brush red onto that state.
Willis, RE the difference between Ocean and Anthropogenic emissions
There is a big difference between the 5,200 tonnes of mercury come from natural sources and some 2,250 tonnes come from anthropogenic sources. The natural sources constitute cycling in the system, the input into the system is the 90 tonnes from volcanoes and geothermal activity, the sink is the mineralizeation deep underground or at the bottom of the oceans in the sludge. At steady state, the natural input matches the sink rate, and the steady state concentration in the oceans, plants, estuaries, e.t.c. are stable. Adding mercury into this closed system increases the steady state level in all reservoirs.
So the rule of thumb is that pre-anthropogenic influx into the biosphere was 90 tonne per year and mineralization was 90 tonnes per year; adding 2,250 tonnes per year from anthropogenic sources will raise the steady states in the various reservoirs by ((2,250 +90)/90) or about 26 times, assuming the sink rate remains constant.
izen:
I agree, I did say;
“anthropogenic emissions are trivial when compared to the natural sources.”.
I meant;
“THE anthropogenic emissions FROM US POWER STATIONS are trivial when compared to the natural sources.”.
I thought it was obviousfrom context, but clearly not. I apologise for my lack of clarity.
Richard
DocMartyn says:
April 2, 2012 at 2:40 pm
So it is your claim that until humans hit the planet, there was no flow of mercury into and out of the atmosphere other than via volcanoes?
My friend, look at Figure 2. The red spot in the US Southwest is mercury entering the atmosphere. It will eventually end up in the ocean, as it has done for millions of years. Note that the mercury is NOT returned to the US Southwest, so it is NOT “cycling in the system”.
The idea that the only “input into the system” is volcanoes ignores a host of other land (and likely sea-floor) based sources of mercury.
w.
Also, DocMartyn, you discount the oceanic mercury as simple “cycling in the system” … but it is still the major source of the mercury that is raining down on my head. How is my body supposed to tell the difference in the local fish I eat?
Does the fact that the mercury in the fish is from “natural cycling” somehow make it irrelevant?
w.
Willis, we have influx, reservoirs and efflux; demineralization (mostly geothermal/vulcanoes by some chemolithotropic bacteria), reservoirs (oceans, atmosphere, dust, plants, fish, lakes, mud, e.t.c.) and efflux (mineralization).
You have to understand that transport from oceans is going to equal oceans in the steady state.
@Izen
I went to check your references in your last post and remain unconvinced.
You quote an abstract (doi:10.1029/2007GB003040) and go on to say “Hope that clarifies why there is certainty that anthropogenic sources of Hg are known to exceed natural ones at present Richard.”
If you read the paper, you will see there is less certainty than you claim. For example the paper states that they have rewritten a global ocean-atmospheric model for mercury. In their rewrites they have added to the anthropogenic tally 600 tonnes/yr from biomass burning and another 450 from artisanal mining. Think about that – do you really believe that every single volcano on earth (supposedly estimated at 500 tonnes/yr) puts out practically the same amount of Hg as shovel and sluice-box miners? Nobody else in the world prior to this thought so until the authors of this paper decided to Enron the Hg budget. Do you believe it reasonable that a forest fire should be classified as an anthropogenic source of Hg? Understand what the paper is about – the authors develop a model to explain observed Hg deposition rates with the model sources. They choose to define the far larger percentage of the global Hg budget to anthropogenic sources and hey presto their model proves an anthropogenic source in Hg deposition. If you were, say a young faculty member publishing this sort of science, probably it would help you with continuing funding from the EPA. Oh wait for it, the EPA did fund this research and one of the co-authors works for the EPA. Fancy that.
Your 2011 Mercury Workshop reference was equally flabby. It contains statements like this “Current mercury emissions to the atmosphere are estimated to be equally distributed among anthropogenic, legacy and natural emissions (Fig. 1).” The trouble is that there are no quantifiable arguments as to how they estimated the various reservoirs. Read a little farther and legacy is defined as a mixture between natural and old anthropogenic sources, but they admit it is not possible to distinguish what percentage of legacy is natural and anthropogenic. Essentially what this does is acknowledge that they cannot prove anthropogenic sources are responsible for observed Hg values, but they can claim that “legacy” reservoirs are contributing anthropogenic Hg. How convenient.
Your words: “In some regions localized Hg pollution in soils has increased 10-fold. The impact of this increase on biota is considerable: Fig. 2 shows the steep rise in mercury in animal tissues from the Arctic since 1900.” What Figure 2 shows is a suspiciously “hockey-stick” shaped graph with not actual Hg concentrations but with Hg in “% of present day Hg concentrations”. Doesn’t that strike you as slightly misleading? Is this a verifiable, standard unit of measurement? Whatever happened to good old ppm or ppb Hg, you know something we can actually check. But it gets worse, notice the sample density variations. From 1550 to 1880 there are no samples, of any sort, and yet from 1880 to the present the researchers are able to find 15 Gyrfalcon and 7 Peregrine feathers from the Arctic. [One feather has a reported value of 0 “% of present day Hg concentrations”]. Similarly the 20th Century has 13 samples of polar bear fur and only one other cluster at year 1300. Notice how most of the “hockey-stick” blade is made up of falcon feathers and polar bear hair samples. There should be an equal number of samples from the same animal collected through time. I understand that it may be difficult getting ancient samples but notice how human hair and teeth are used to define the ancient baseline, but there are no modern human hair or teeth samples. Do you really think modern samples of human hair are hard to get? This figure is not the basis to make important scientific interpretations – don’t you think that if the case was so strong, then a more compelling figure could be produced?
Willis Eschenbach says:
April 2, 2012 at 12:47 pm
> Brian H says:
> April 2, 2012 at 12:14 pm
>> Willis;
>> try Alt-248(numeric keypad). Hg°. Should work.
> Of course, the degree symbol, Hg°. On my Mac that’s shift-option-8, never thought of using that.
You can (in WordPress’s HTML subset) up ⁰ for a superscript 0, I think: Hg⁰. The problem with substituting a degree sign is that it’s a circle, zeroes are not circles.
Ric,
On a Mac, opt + 0 [zero] gives: º
shift + opt + 8 = °
Hmm, what did I type there? I forgot that WordPress is more agressive about conversions than HTML is. What I meant to say was you can use ⁰ for a superscript 0.
Willis ….
Your losing me here. What the heck are you talking about???
Your very first line in the article says, “I’ve been puzzling for a while about why the areas with the most power plants aren’t the areas with the worst levels of mercury pollution.”
Then you have this quote from some mercury guys … “We propose that a chemical reaction not included in current models of atmospheric mercury reduces HgII to Hg0 in coal-fired power plant plumes”
OK .. so you’re puzzled, as to why mercury pollution is not “upstream” of the power plant. WELL .. between your article, and what I just gave you, you have your pathway. I see nothing puzzling about it at all.
Pathway — HgII emiitted from power plant –> converted to HgO by some reduction process –> atmospheric mixing over a years period of HgO residency in atmosphere –>results in ubiquitous deposition, including deposition in the oceans –>conversion to watersoluble organic mercury by Microorgansims —> evaporation into weather systems –> rains out in places upstream of water system .. like San Francisco and Florida.
Thus .. is probable that some of that HgII raining on your head from storms comming off the pacific originated as released Hg from a power plant in Ohio. Like I said, I have no idea if there’s been an increase in HgII precipitation in San Francisco .. but if there has been, it would not suprise me in the least. .. ie., nothing puzzling about it to me at all.
Thanks for the push, Ric, I looked up what HTML wordpress allows, I think I’ve got it now:
Hg0 and Hg++ and CO2.
I seem to vaguely recall that these may cause trouble when they appear in the middle of a paragraph because they screw up line spacing Hg0 and CO2. Let me see what that does to the spacing of the lines. … Nope, seems like that’s no problem, I used the HTML instructions “sup” and “sub” for those.
Always more to learn, much appreciated.
w.
DocMartyn says:
April 2, 2012 at 5:27 pm
I do understand that, Doc. I’m just not sure what that means to you, given that we are not in a steady state. More mercury is going into the oceans than is coming out.

Overall, natural sources put thousands of tonnes of mercury into the air every year. Some comes from the deserts in the Southwest US. Some comes from volcanoes. Some comes from forests, which pull it up from the soil and add it to the air. Deserts add about 500 tonnes of mercury to the atmosphere every year. This desert and volcano mercury and the like is not recycled mercury. It is new mercury, which previously was in the ground, which is added to the air. Much of it will go into the ocean. Some of it will never (in human terms) come out.
If (as you claim) transport into the oceans equals transport out, every year we should see a huge increase in the atmospheric load. We don’t see that.
So how is that “steady state”?
Thanks,
w.
Deanster says:
April 2, 2012 at 7:39 pm
Nope. I was puzzled, as were the authors of the study I cited, as to why mercury pollution is not found downwind of power plants. For example, the very large number of power plants and industrial coal burning in the Ohio River Valley don’t have a corresponding downwind increase in mercury deposition. Turns out the mercury is added to the background circulating airborne mercury rather than being deposited out in the downwind area. Good news for Pittsburg. Bad news for me on the Pacific Coast, because that mercury comes down on me.
I hope that’s clearer.
w.
Mercury concentration of US coals is 50-130 ppb (billion), which translates into electric generating unit (EGU) stack gas concentrations of 5-13 ppb, assuming that no mercury is captured by EGU’s pollution control equipment (approx. 10 kg of stack gas is generated by burning 1 kg of coal). Mercury is vaporized in the furnace of coal-fired boilers and then recondenses as a fume as flue gas is cooled as heat is transfered from the flue gas to steam in the boiler tubes. All coal-fired EGUs are equipped with high efficiency particulate removal equipment (either electrostatic precipitators or fabric filters) which preferentially collects larger particles (because it is easier). At a minimum particulate control equpment collect 10% of the mercury. Coal fired EGU stacks (i.e. chimneys) are typically 500+ ft tall and flue gas stack exit velocities are typically a minimum of 3,000 ft/min. Given the extremely low concentrations of mercury, its sub-micron size consist, height of stacks, and high stack exit gas velocity, it shouldn’t come as a suprise that mercury ‘hot spots’ downwind of coal fired power plants are not found.
According to the EPA, US coal-fired power plants are responsible for approximately 4% of mercury deposition in the US. However, this 4% is based on mercury emissions before the $50+ billion retrofits of selective catalytic reduction (SCR) NOx control equpment and SO2 scrubbers that occurred in 2007-2010 as a result of the Clean Air Interstate Rule (CAIR). A collateral benefit of installing SCRs and SO2 scrubbers is that mercury emissions are reduced by approximately 30%, though this figure varies widely depending on the coal that the EGU is burning (the SCR catalyst converts mercury to forms that are more easily captured by the SO2 scrubber). Thus, today coal-fired power plants are probably responsible for 3%, not 4%, of US mercury deposition.
The reason there is uncertainty about the mercury species distribution in coal-fired EGU stack gases is that the relative concentrations of mercury species is affected by a number of factors, not all of which are understood. It is known that the chlorine content of coal and whether or not the EGU is equipped with an SCR affects mercury species distribution, but it appears that the variation in mercury species distribution is not explained by these two variables alone. A significant issue complicating this analysis is the accuracy of stack gas mercury concentration measurement, which is estimated to be 2 ppb +/- 1 ppb as well as the natural variability in coal mercury content.
Generally eastern US coals have higher mercury content that western coals. However, one cannot assume that coal-fired EGUs located in the East burn eastern coals as low sulfur western coal, especially coal from the Powder River Basin (located in northeastern Wyoming and southeastern Montana), is transported great distances. PRB coal has been transported to coal fired EGUs located on the east coast.
@- Willis Eschenbach says: April 2, 2012 at 8:08 pm
“Overall, natural sources put thousands of tonnes of mercury into the air every year. Some comes from the deserts in the Southwest US. Some comes from volcanoes. Some comes from forests, which pull it up from the soil and add it to the air. Deserts add about 500 tonnes of mercury to the atmosphere every year. This desert and volcano mercury and the like is not recycled mercury. It is new mercury, which previously was in the ground, which is added to the air. ”
Correct, the paper you link to that claims 5207Mg comes from ‘natural’ sources and 2320Mg new HG from anthropogenic sources.
But the 5207Mg is a combination of new HG and existing HG in oceans and biomass. The amount of new Hg from primary natural sources as opposed to natural PROCESSES that are sources of emission is probably about half the anthropogenic sources of new Hg.
Historical studies indicated that at least half of the Hg which we are exposed to at present is from anthropogenic sources. Although some of that is caused by re-emission by natural processes.
Of course all of this is highly uncertain, Mercury as the trickster God has a habit of vanishing or accumulating in unpredictable ways. As a result the error bounds for estimates of natural and anthropogenic primary sources of new Hg are around 30%.
This could mean that things are 30% better than research indicates.
Or 30% worse.
izen says:
April 2, 2012 at 11:07 pm
I’m sorry, but this “re-emission” argument strikes me as just another way that people want to load everything bad onto humans. If that “re-emission” analysis were valid, how come it’s not used for say CO2, since the ocean must be “re-emitting” carbon from humans?
For me, the question is this:
If we shut off every human emission, what would be the total amount emitted this year?
That amount, whether it is cycled or recycled, is what remains when we have turned off the human emissions. So it is the natural emissions.
Is some of this “re-emitted” by the ocean? Assuredly, just as some of the anthropogenic carbon is re-emitted by the ocean … but so what? It’s all part of the natural cycle at that point.
Again I invite you to look at FIgure 2. The overwhelming majority of the Hg in the air over the US comes from the wind blowing over the desert. Human activities in the US are responsible for almost none of that. And I’m sorry, but I’m not buying that the mercury in the desert is recycled anthropogenic mercury in any sense.
Indeed they could …
Izen, for me the issue is the slow rain of mercury coming down from the sky onto my house. By and large it comes from the ocean. It has been raining mercury here for thousands and thousand of years, since long before humans were doing anything to mercury …
w.
Willis, Izen, Deanster, et al.:
This discussion seems to have lost its focus.
The subject of the above article (and, therefore, discussion of it) is stated by Willis in the article’s first sentence that says;
“why the areas with the most power plants aren’t the areas with the worst levels of mercury pollution.”
The article and subsequent discussion make clear that the effect of those emissions is swamped by the larger variations in other emissions of mercury that are mostly natural.
Those other emission sources (and sinks) are subject of an interesting scientific discussion, but detailed consideration of them deflects attention from the reason why the article was written at this time; viz.
the intended EPA regulations to constrain mercury emissions from the power plants.
But reducing those emissions (from power plants) to zero would have no effect of any consequence because their effect is swamped by the larger variations in other emissions of mercury that are mostly natural. And that is all that matters in practical reality.
The imposition of the EPA mercury emission constraints on the power stations would be very expensive, would probably close some power stations, and would provide no benefit because the emissions have trivial effect when compared to other emission sources.
As I explained in my post at April 2, 2012 at 1:48 am, history indicates that the focus of the discussion needs to be kept because when such emission constraints are imposed the bureaucracy to impose the constraints will continuously lower the permissible limits regardless of any scientific reasoning.
Richard
From your last post on mercury, I was wondered why a state like Maine, devoid of human life forms, was so high in mercury.
EPA ruling:
Willis, perhaps you could take the ideas from this comment and whip it into a decent thread post. I do not have your ability of getting the point across unfortunately, nor the computer skills. It would be nice to put the recent EPA Final Rule into historical context with the other Presidential Executive Orders, laws, resolutions not to mention the maneuvers of NGOs and the bureaucrats in D.C.
The Intersection of Control Food and Control Energy from Henry Kissinger’s famous 1970 quote.
In the spirit of, “All animals are equal, but some animals are more equal than others,” the EPA is going to have separate emission limits for different types of boilers.
Troutman Sanders LLP was kind enough to explain what that actually means.
From those quotes and the push for Sustainable Development by the USDA, we can make the assumption that bio-mass fired boilers are going to be treated to far less stringent emission standards than coal because they are Sustainable.
This goes along with the House Concurrent Resolution 25 ~ 110th Congress, 2007–2009 A resolution that passed in the House but died in the Senate according to GovTrack.US. The resolution reads:
As usual just because the resolution failed to pass both Houses does not mean the idea died. Nor does it mean the information cannot be twisted into the following Press Release.
So now we have NGOs such as 25X25.org pushing the expanded idea.
@- Richard
“The article and subsequent discussion make clear that the effect of those emissions is swamped by the larger variations in other emissions of mercury that are mostly natural.”
Actually the emissions we are exposed to are mostly of human produced Hg. It may have been released by natural processes re-emitting historical Hg deposits. but as the links I gave indicated, Hg levels are around double pre-industrial levels because new Hg has been added to the ‘natural’ processes of emission by the ~2000Mg/yr from human sources.
You are right that emissions from US coal fied power plants are relatively small BECAUSE the FGD and other polution measures also reduce Hg emissions, but the fact that other sources which have not yet been controlled are greater contributers to the increasing total global load of Hg is hardly an arguement to relax or avoid further possible regulatory reductions where they can be made.
@-“As I explained in my post at April 2, 2012 at 1:48 am, history indicates that the focus of the discussion needs to be kept because when such emission constraints are imposed the bureaucracy to impose the constraints will continuously lower the permissible limits regardless of any scientific reasoning.”
Not quite.
The scientific reasoning is that ANY emissions of harmful material that CAN be prevented by a technological solution is worthwhile. Especially with a very persistant, bio-accumulative heavy metal poison.
So even though the US coal fied power plants are not the major sources of Hg, the fact that Hg emissions from them CAN be reduced means they should be reduced. Regulation may be required to ensure that industry does not avoid a technically possible reduction in harmfull emissions in favour of higher profitability. That idea that a little pollution was acceptable if it increased profits went out with the Victorians.
Curiousgeorge says:
April 1, 2012 at 6:33 pm
Next thing you know, the greenies and the EPA will be banning banana’s because of radioactive potassium. Always a new boogie man under the bed to scare us with. Screw ‘em.
____________________________
You forgot the radioactive carbon 14 in the bananas and our salads too. Oh my Gosh, run in circles scream and shout the sky is falling. (quote from Chicken Green)
stpaulchuck says:
April 1, 2012 at 8:19 pm
stpaulchuck says:
April 1, 2012 at 8:19 pm
.. JunkScience.com had a really great one about a Chinese pollution study with grunge numbers an order of magnitude higher than the new EPA danger numbers ..
….Now, is there a case for long term suspension of the mercury? could the stuff be going to Africa? In fact, some dust and pollen apparently cross the Atlantic, so I’m wondering if we’re not just shipping the stack garbage further than might be thought. Anyone got any info on that idea?
_______________________________________
I have a friend living in Alaska who complains bitterly about the smog blown into her area from China.
The dirty side of economic booms: smog knows no borders (About NOAA’s studies of cross-border air pollutants.)
Richard, I agree with your comment.
My previous post that, according to the EPA US coal fired electric generating units (EGUs) are responsible for 4% of mercury deposition is based on an EPA assumed total US mercury deposition rate of 1,000-1,100 MT/year. If the estimates of 5,207 MT/year from ‘natural’ sources and 2,320 MT/year from anthropogenic sources are correct, then US coal fired EGUs are responsible for only 0.6% of US mercury deposition. Adjusting coal fired EGU mercury emission rates for collateral mercury emission reductions associated with installing SCR NOx control and SO2 scrubbers, the contribution of US coal-fired EGUs drops to <0.5% of US mercury deposition.
izen says:
April 2, 2012 at 3:27 am
The estimated lifetime of elemental mercury in the atmosphere is around a year as another poster has noted. That means there is a global dispersion of airbourne mercury and local hotspots are due to deposition of soluble mecuric compounds from oceans/lakes more than from coal fired power plants.
Anthropogenic sources emitt around twice as much mercury (elemental and soluble) as natural surces overall. This is most notable where local industrial sources have generated Hg II compounds with beluga tusks now containting over 10x as much mercury now as they did in pre-industrial times.
It would be very hard to make a case for NOT regulating industrial mercury emissions….
____________________________________________
You are comparing Apples and Oranges. I really hate that type of scare tactics used to manipulate the public. The USA has been regulating mercury emissions for years and we are at about 10% or less compared to the unregulated times.
Mercury Study Report to Congress: Overview published in 1997
Notice how all that information is hidden in the newest version as they now go after the Coal industry.
izen:
Your post at April 3, 2012 at 6:57 am is twaddle.
Either constraining the emissions from power stations will have a discernible effect or will have no discernible effect.
THE EVIDENCE PROVES BEYOND ANY DOUBT THAT THE CONSTRAINTS WILL HAVE NO DISCERNIBLE EFFECT.
And your waffle about other things does not change that.
But one of your pieces of irrelevant twaddle is so wrong that I will bother to refute it.
You assert;
“The scientific reasoning is that ANY emissions of harmful material that CAN be prevented by a technological solution is worthwhile. Especially with a very persistant, bio-accumulative heavy metal poison.”
That is NOT “scientific reasoning”. It is stupid nonsense.
If the effect of applying the “technological solution” is net negative then it should not be applied. And applying the “technological solution” has cost, so its application uses money that could be spent on other things. So, for example, spending money on constraining Hg emissions from the power stations is net negative because the constraints would have no discernible effect but prevents use of the money for demonstrably beneficial things.
Please explain why you think such idiocy is an example of “scientific reasoning”.
Richard
izen says:
April 3, 2012 at 6:57 am
Izen, is it possible that you really believe that?
Any scientist who follows that reasoning has their head a long ways up their ivory tower.
In the real world, an intelligent person makes such a decision using a cost-benefit analysis. They don’t say EEEK, there a nano-gram of mercury, Izen says that it “CAN be prevented by a technological solution” even though the solution will cost millions, so since it CAN be prevented Izen says it’s worthwhile to prevent it …
That’s a schoolkid’s kind of reasoning. Adults realize that one always, always has to balance cost and benefit. There’s always a tradeoff.
Not only is there always a tradeoff, but as the amount of the harmful material in a waste stream decreases, the cost of removing more of it goes up very rapidly. A rule of thumb is that whatever it cost to cut the amount of unwanted material in half, it will cost double that amount to cut it in half again, and double that larger amount to cut it in half a third time.
And as anyone but a scientist in an ivory tower would know, at some point in that process, it’s no longer worth it. At some point, the costs will exceed the benefits.
And when the only justification the EPA can come up for their mercury rule is claiming that the benefit is that the IQ of fetuses whose mothers eat lots of fish will go up by two thousandths of an IQ point, they have seriously jumped the shark. This is from their Toxics Rule RIA:
Right … like they can measure or predict IQ losses to the nearest HUNDRED THOUSANDTH OF AN IQ POINT …
The goofy thing is, the analysis doesn’t even consider the effects of mercury on the population of the US. It doesn’t even consider the effects of mercury on the part of the population that eats locally caught freshwater fish in areas known to contain mercury.
Their population of interest, the ones that they are analyzing, are pregnant women who eat locally caught freshwater fish in areas known to contain mercury. That’s the population that they are trying to “protect”, Izen … wouldn’t it be cheaper to educate those women that eating fish from lakes that have contained mercury for the last million years might be a bad idea?
Because spending billions so that their children will be smarter by 2/1000 of an IQ point … well, anyone proposing that kind of madness has inhaled too much mercury in my opinion, their IQ is seriously dropping.
Izen, you seem to think I’m arguing against the regulation of mercury. I’m not. I’m against ludicrous, hugely expensive, and most important, meaningless reductions in mercury based on bogus science and inchoate fears.
w.
izen says:
April 3, 2012 at 6:57 am
Yes, and CO2 levels are heading towards double pre-industrial levels, but nobody claims that humans are exposed mostly to human generated CO2. Nobody claims that there are “re-emissions” of CO2.
 SOURCE
SOURCE
Your assumption seems to be that mercury, once it is emitted by humans, is never removed from the atmosphere. But it is removed, and on a fairly short timescale.
As a result, the reason that mercury levels are high is that we are emitting lots of mercury NOW, about 30% of the total emissions. That’s why we have excess mercury in the atmosphere, not emissions from the gold mining days.
Are levels higher now than they were historically? Certainly. Are human emissions higher than they were historically? Well, no, they were higher fifteen years ago. Are we “re-emitting” mercury from back then? No, the levels have dropped, not stayed constant. Here’s the ice core data.
As you can see from the volcanic eruptions, the is NO long-term effect from the addition of even massive amounts of mercury to the atmospheric load. We’re not “re-emitting” mercury from Krakatoa, that was gone a few years after the eruption. Similarly, we’re not re-emitting mercury from WWII, that was sequestered long ago.
The whole concept of “re-emission” is just a way for you to try to shovel more blame onto humans. It makes no scientific sense (although I’m sure you could find “scientists” who make the same claim.
w.
@- Willis Eschenbach says: April 3, 2012 at 2:44 am
“I’m sorry, but this “re-emission” argument strikes me as just another way that people want to load everything bad onto humans. If that “re-emission” analysis were valid, how come it’s not used for say CO2, since the ocean must be “re-emitting” carbon from humans?”
Well actually…
That IS the analysis used for CO2 by Revelle in the 1950s when the objection to AGW theory was that the oceans would easily absorb any extra anthropogenic CO2. He showed that the buffering bicarbonate caused the re-emission of a large proportion of the CO2.
It is also the explanation for the long persistance of the extra anthropogenic CO2 in the carbon cycle. While the residence time for an individual CO2 molecule in the atmosphere is only a few years (or less!) the extra anthro CO2 is rapidly distributed in all the parts of the active carbon cycle, biomass, oceans and atmosphere.
The long-term geological sinks for CO2, and the primary natural sources (mainly volcanic) are somewhat smaller, and slower, than the rate of addition of anthro CO2 which is why it accumulates as shown by the Keeling curve.
I would agree that the figures for pre-industrial levels of Hg are a lot more uncertain than for CO2. Also that the rate and magnitude of the primary natural sources and sinks are approximate estimates. But within those uncertainties it is clear from the comparison with Hg levels in pre-industrial samples that there has been something like a doubling (or more) of Hg flux levels. (Mason et al 1994) The best estimates have at least twice as much Hg moving between ocean/land/atmosphere.
@-“For me, the question is this:
If we shut off every human emission, what would be the total amount emitted this year?
That amount, whether it is cycled or recycled, is what remains when we have turned off the human emissions. So it is the natural emissions.”
Well according to the Pirrone(?) and Mason paper you cite the total amount would drop by around 30% or 2320Mg in the first year.
How much it would decrease after that, and how fast is speculative. The much lower past Hg levels indicate that the natural steady state maintained by the rate of emission from natural primary sources and ocean/geological sequestration maintained a lower level than exists at present. This indicates that the rate at which anthropogenic sources add Hg is exceeding the natural sequestration rate. Your guess is as good as mine as to how fast the global Hg load would decrease if the anthropogenic sources were eliminated. It really depends on the rate of premanent removal from the system of fluxes between atmosphere/ocean/land.
As with CO2, the residence time of any additional, new, dollop of the molecule added to the natural cycle depends significantly on the rate at which the molecule is geologicaly removed from the active surface cycle.
I gather from a scan of the literature on this that there are modeling attemnpts to get a handle on these factors, using the past emission rates and historical levels to (guess)estimate the rate at which Hg is abstracted from the cycle. But given the multiple uncertainties for the rate of emission from primary natural sources, and the rate/magnitude of increase in Hg levels since industrial sources became significant I doubt that anyone would put much reliance on them.
The one unavoidable conclusion is that as with sulfur and CO2 the global levels, in the land/ocean/atmosphere cycles, have risen significantly over the last century as a result of anthropogenic sources. Any significant reduction in the emissions from those human sources would reduce the global level, any increase, or maintenance of those anthropogenic emissions will continue to raise the global levels in all parts of the cycle because in each case it is clear that present emission levels exceed the amount and rate that natural processes can sequester the molecule out of the cycle.
Willis:
At April 3, 2012 at 10:35 am you say to Izen;
“you seem to think I’m arguing against the regulation of mercury. I’m not. I’m against ludicrous, hugely expensive, and most important, meaningless reductions in mercury based on bogus science and inchoate fears.”
Excellent! Well said! Yes, that is exactly the point.
Thankyou.
Richard
@-Willis Eschenbach
Ah.
We seem to have cross-posted. I did not see your most recent replies until after my last post…
Okay, quick response, I am reassured to see the fast response time in the ice-cores after major volcanic eruptions, it seems to return to the pre-industrial level of ~5ng/L in around a decade. The rapid drop in the last few decades which is attributed to the clean air act is also a cause for optimism. Your question of how much lower your exposure to Hg would be without anthropogenic sources looks like it could return to the ‘natural level’ of 5ng/L within a decade. It is now three times that level at 15ng/L.
The large response to the Mt St Helens eruption is interesting. It probably represents the impact of a local addition and would not be reflected in a global increase as is likely from the Tambora and Krakatoa events.
This would seem to increase the value of any reduction, especially locally because the indications are the cycle would return quite rapidly to the baseline level of 5ng/L.
I guess I would not defend the idea of TOTAL elimination of human emissions, I am aware of the law of diminishing returns (grin)!
But I think the evidence does support any reduction in human sources because the payoff appears to be rapid and locally enhanced. Yes, there are arguments to be made about economic viability. But there are also good reasons if the evidence does support a rapid and significant response to reduction, to impose the costs of negative externalities on human sources.
Or perhaps you consider it cheaper to treat any negative effects on human health or biological systems that a level above the baseline may cause ?
Izen:
Your post at April 3, 2012 at 11:56 am shows you still ‘do not get it’. I will try to ‘spell it out’.
Is the atmospheric load of Hg higher than pre-industrial level?
Yes.
Will the proposed constraints on Hg emissions from the power stations make a discernible effect on the atmospheric loading of Hg?
No.
Will the proposed constraints on Hg emissions from the power stations have a significant cost?
Yes.
In the light of the above three answers, is there any point in the proposed constraints on Hg emissions from the power stations?
No, there is no point, only cost.
Do the above facts mean that significant anthropogenic sources of Hg should not be identified and possibly reduced?
No.
Would the imposition of the proposed constraints on Hg emissions from the power stations inhibit identification of anthropogenic sources of Hg that could be reduced to obtain a discernible reduction of the atmospheric loading of Hg?
Probably.
Do you ‘get it’ now?
I doubt it.
Richard
izen says:
April 3, 2012 at 11:21 am
Interesting thoughts.
Revelle showed that if you add a single large pulse of carbon to the atmosphere, when that pulse is finally re-absorbed by the natural systems including the ocean, the eventual atmospheric equilibrium CO2 level is slightly above the pre-pulse equilibrium level. I don’t find anyone describing that as “re-emission of anthropogenic carbon”.
Revelle is talking about a single specific equilibrium oceanic reaction. Are you making the argument that such a reaction exists for mercury?
Let me explain my position in another way. I find no one saying that, because CO2 levels are higher, that humans are responsible for “re-emission” of CO2. I find nothing in the literature saying that the ocean is “re-emitting” anthropogenic CO2 and that therefore humans are responsible for far greater emissions than the ~ 9 GtC we emit annually.
Because by your logic, since the mercury levels are about double what they were, humans are responsible for about half of all the mercury that is emitted by the ocean and the volcanoes and the land.
But I don’t hear a single person saying that because CO2 levels are headed towards double preindustrial levels, that humans are responsible for say 40% of all the CO2 emitted by the ocean and the volcanos and the land. That’s a bridge too far.
Look at the atmospheric response after Tambora in the figure I just posted. There’s no long-term effect. We’re responsible for part of the emissions, but the oceanic emissions are just that. They are a long-term unending source of atmospheric mercury.
Gotta run, thanks for your comments, more to follow,
w.
Nice chart. Too bad it does not go back farther. Since some of us assume that CO2 increases because of the warming since the LIA, it would be nice to see Hg charted for a few more years. Perhaps the current background has increased for the same reason as CO2.
This reference indicates that there was another anthropogenic Hg peak in the Andes around either 1500AD or 700BC, depending on the date calibration model used. Also, the volcanoes so easily visible in the USGS image don’t show up at all in the Arctic cores. Actually, since the 4 cores do not agree, it is most likely that the signal, if any, is about the same size as the noise.
Another paper clearly shows that Hg deposition peaked around 5000 BCE and is lower now than it has been for most of the last few thousand years.
Hello im in middle school and i was looking for the roman god Mercury not this random stuff i dont care about Mercury mercury in the area of states and i hate that I cant find anything about this ROMAN GOD!!!!!
What is this stuff I need information and all I get is this, like seriously, this needs A TON of work. It took me an hour to read this, and it was one hour of wasted time. Barbecue sauce!
Deb Jennie:
I have been out of contact since my last post on this thread so I have only now seen your post at April 10, 2012 at 11:32 am.
Nobody has responded to your post, and I am not surprised because it is a meaningless rant devoid of any content so there is no possible specific response. However, it is possible that you are awaiting a reply and that you did intend to make a point. Therefore, if you would care to specify
(a) the “information” you require which has not been provided by Willis and/or others,
(b) the “work” you want to see,
and
(c) what you mean by “barbecue sauce”
then I will try to provide answers.
Richard
Moderators:
My reply to Deb Jennie sewems to have vanished and I would appreciate your finding it and posting it.
Thanking you in anticipation.
Richard
[Reply: You should give us more than 3 minutes! ~dbs, mod.]
dbs mod:
Please accept my sincere apology. I usually get a view of my post with a ‘waiting moderation’ note, and I did not on this occassion so I immediately thought my post had been ‘lost’. mea culpa.
I intended no offence. Indeed, I admire the very good work of the Moderators on WUWT.
Richard